The Preparation, Characterization and Formation Mechanism of a Calcium Phosphate Conversion Coating on Magnesium Alloy AZ91D
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
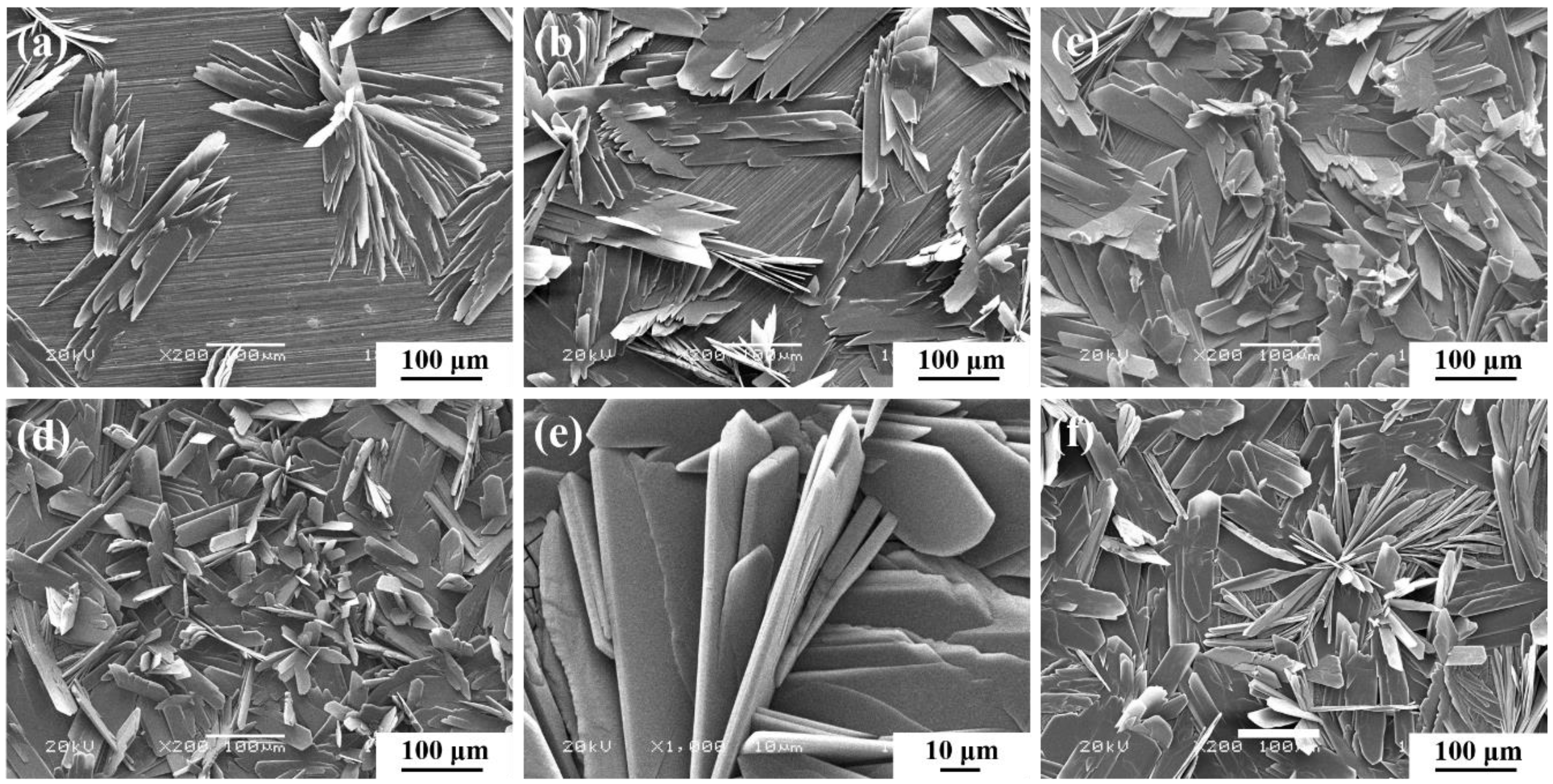
3.1. Ca-P Bath Component
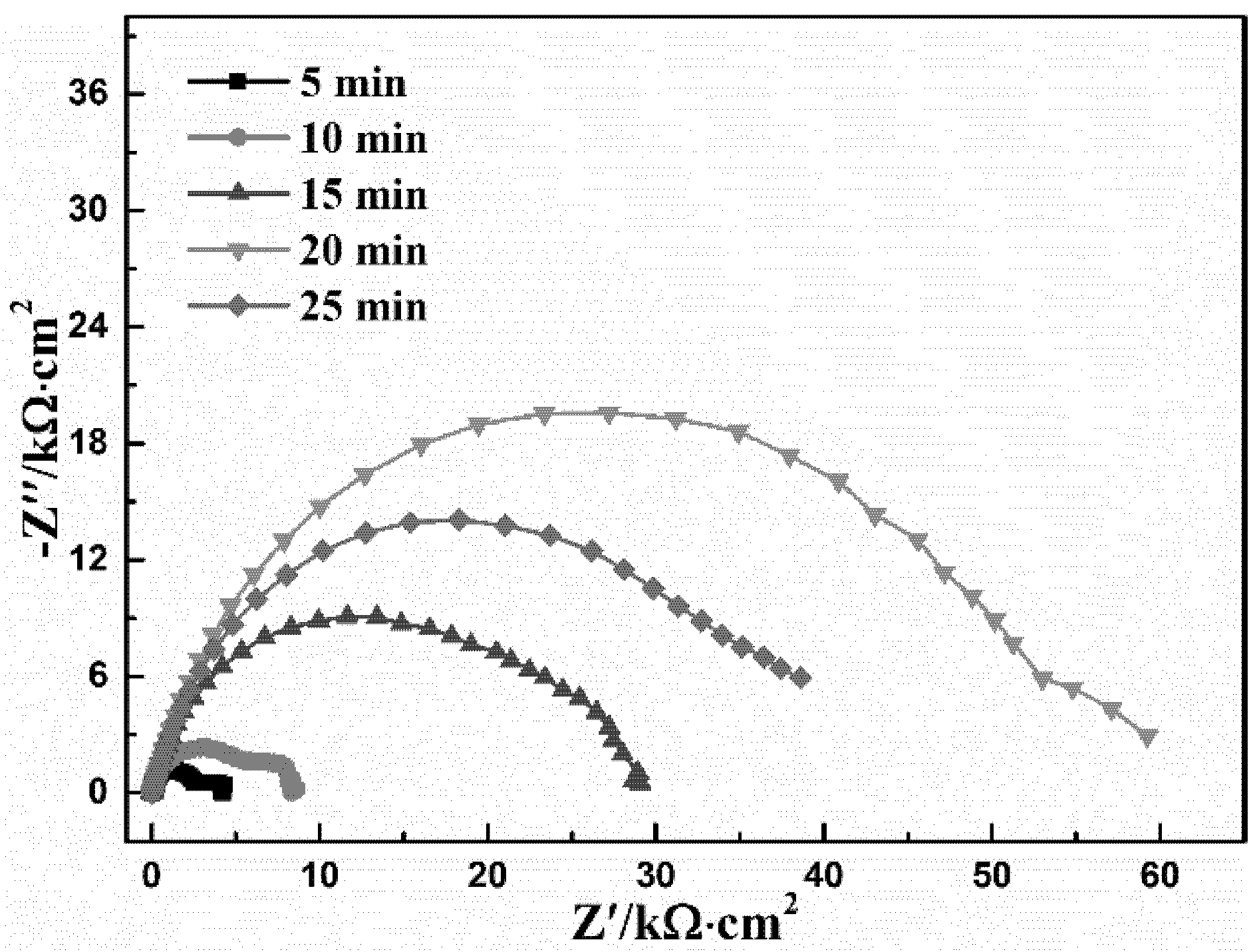
3.2. Conversion Time
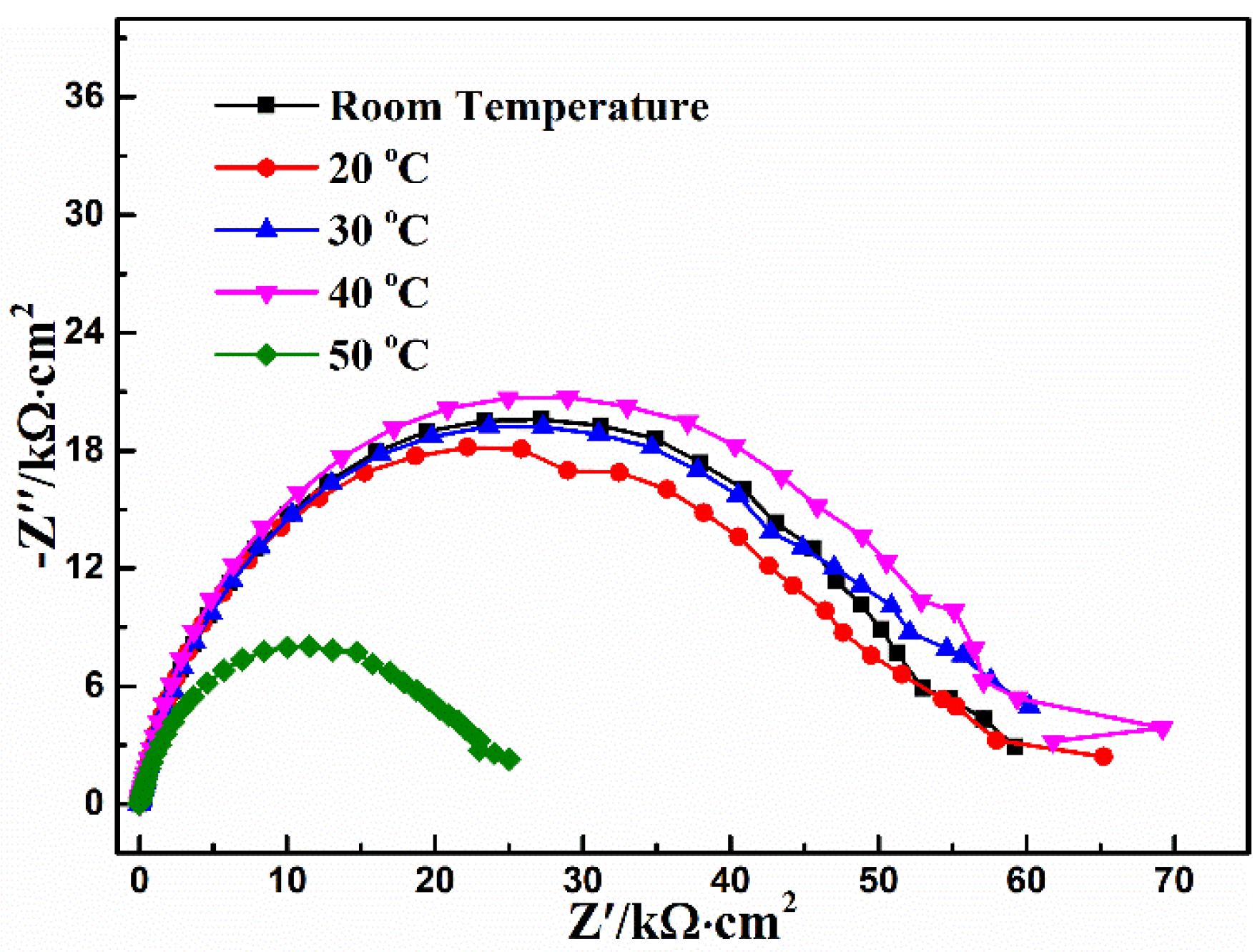
3.3. Conversion Temperature
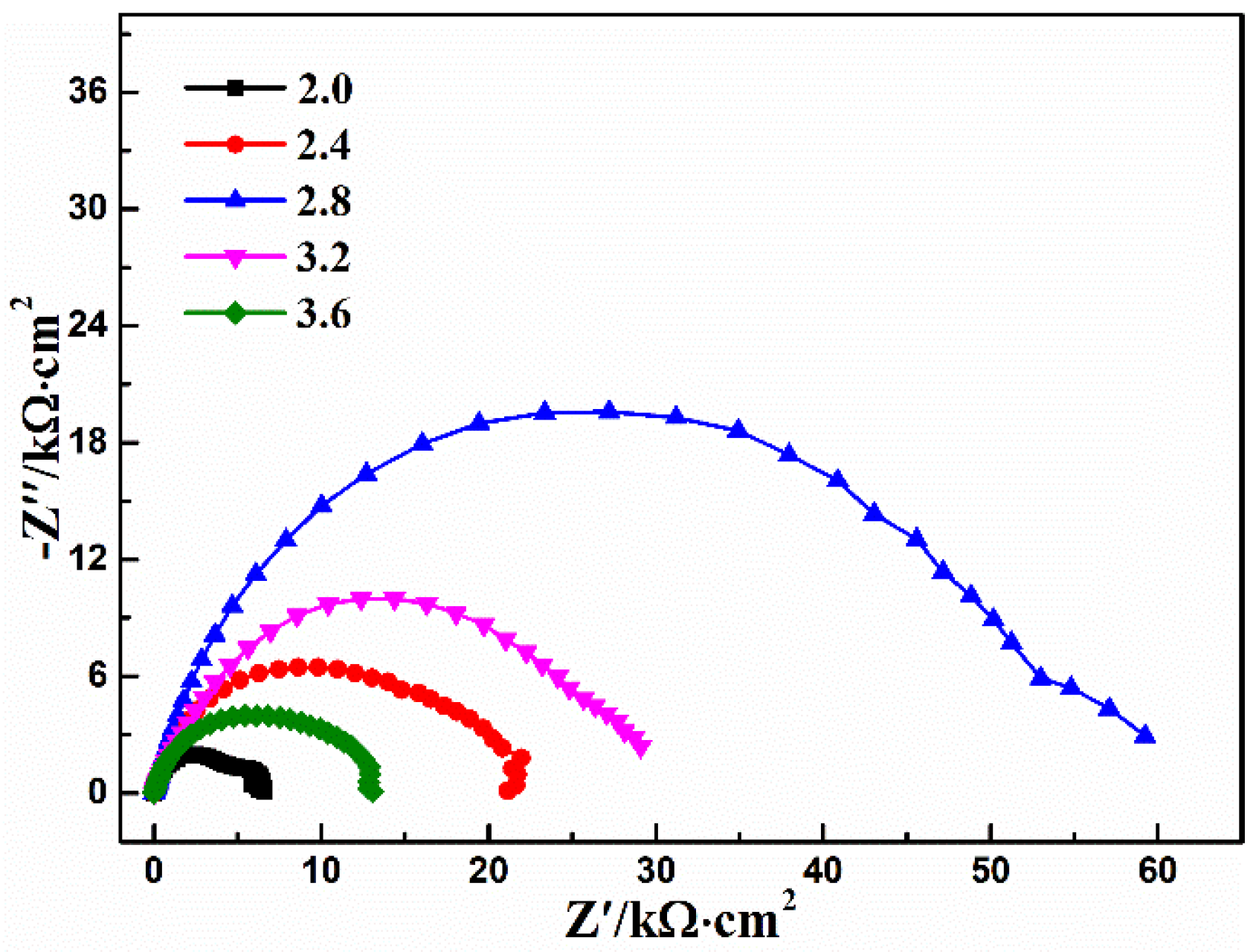
3.4. pH Value
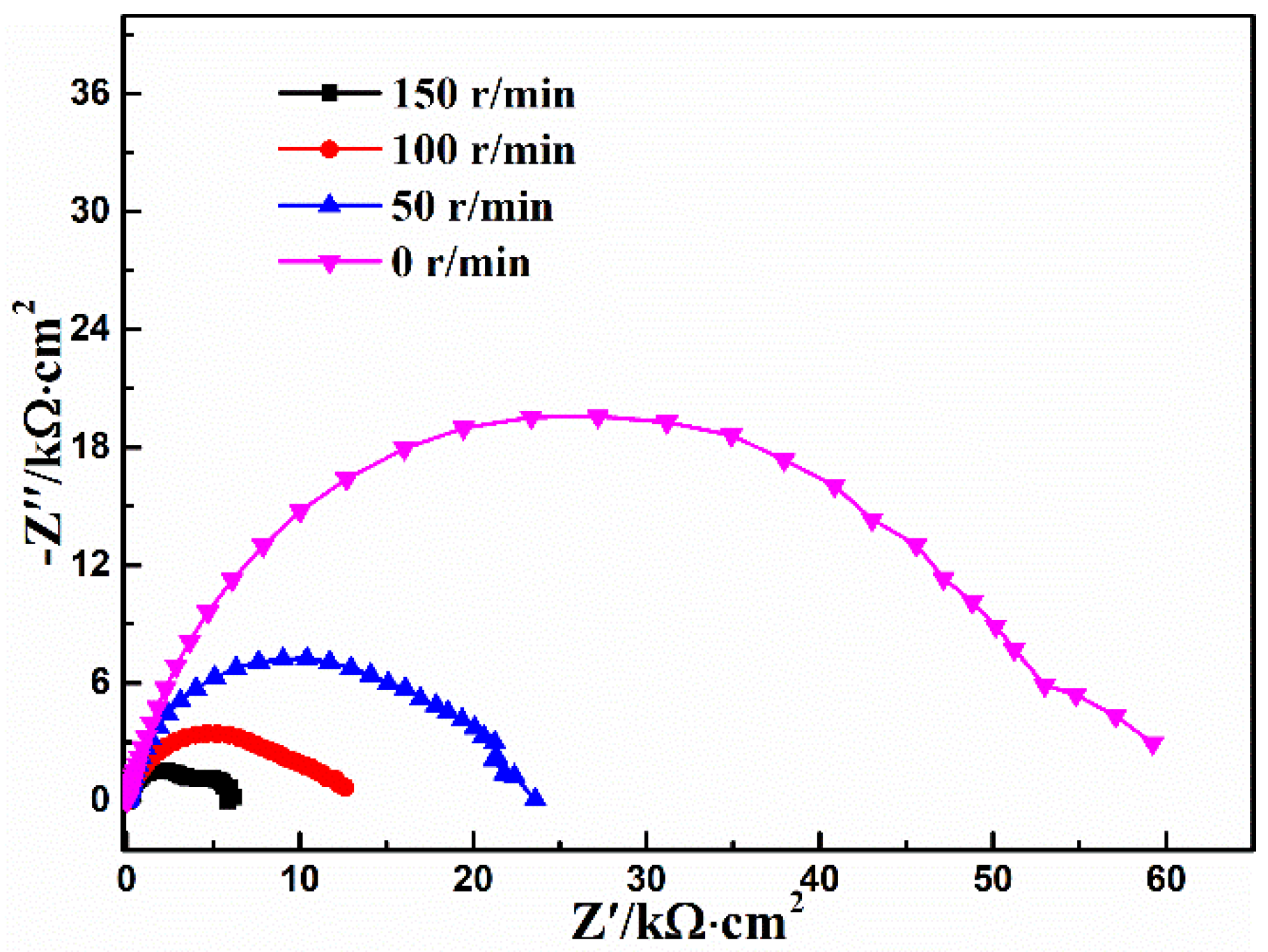
3.5. Stirring Rate
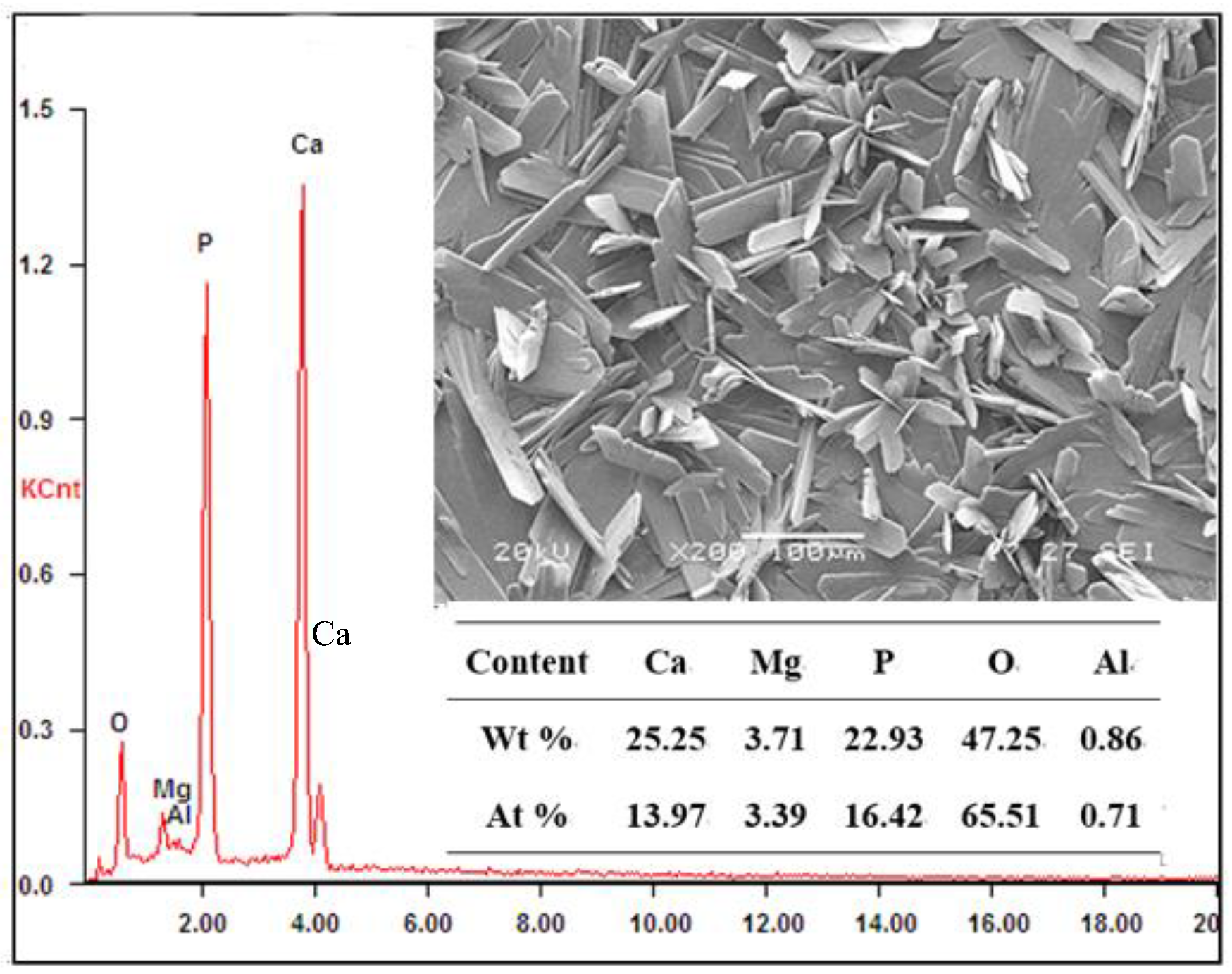
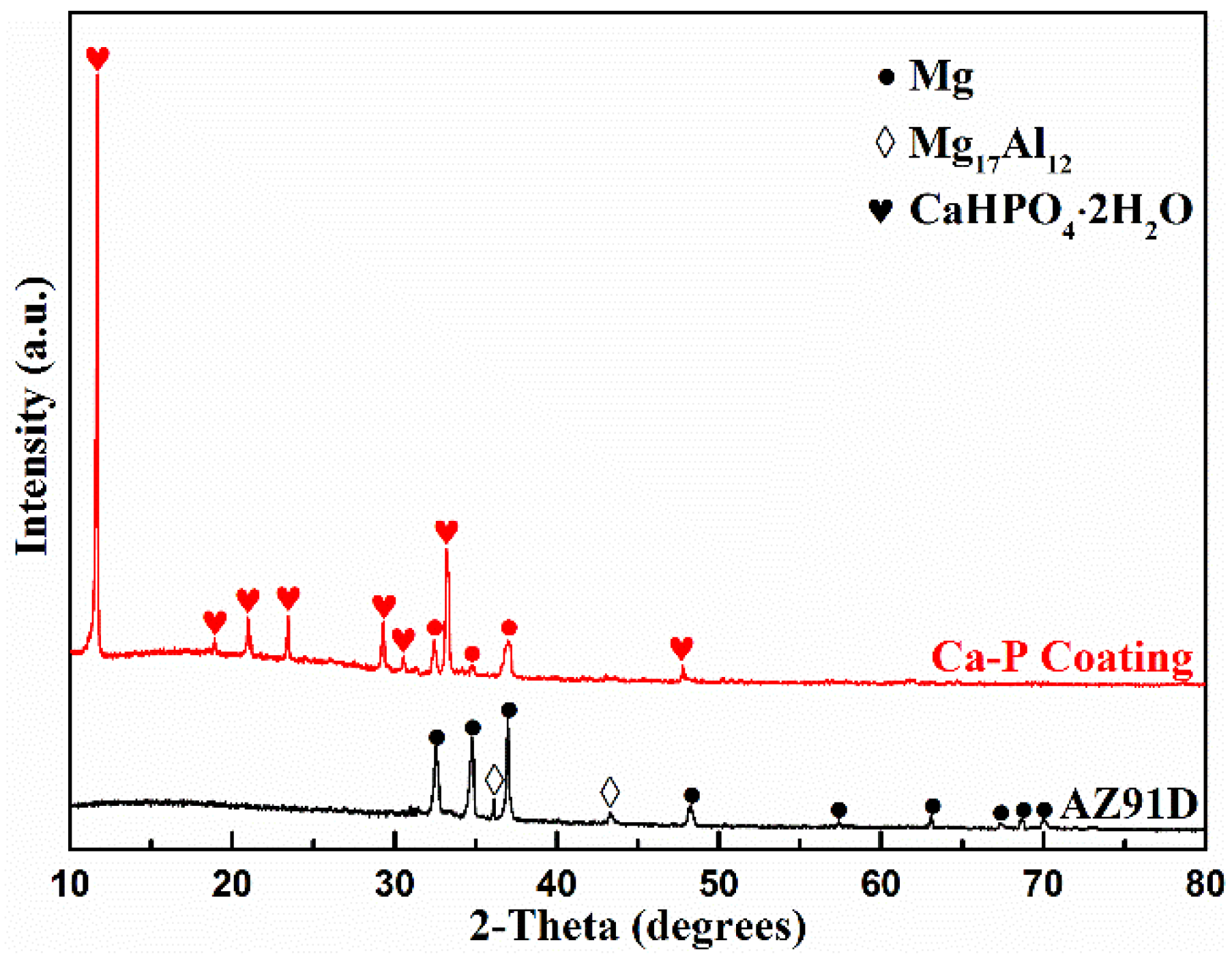
3.6. Coating Composition
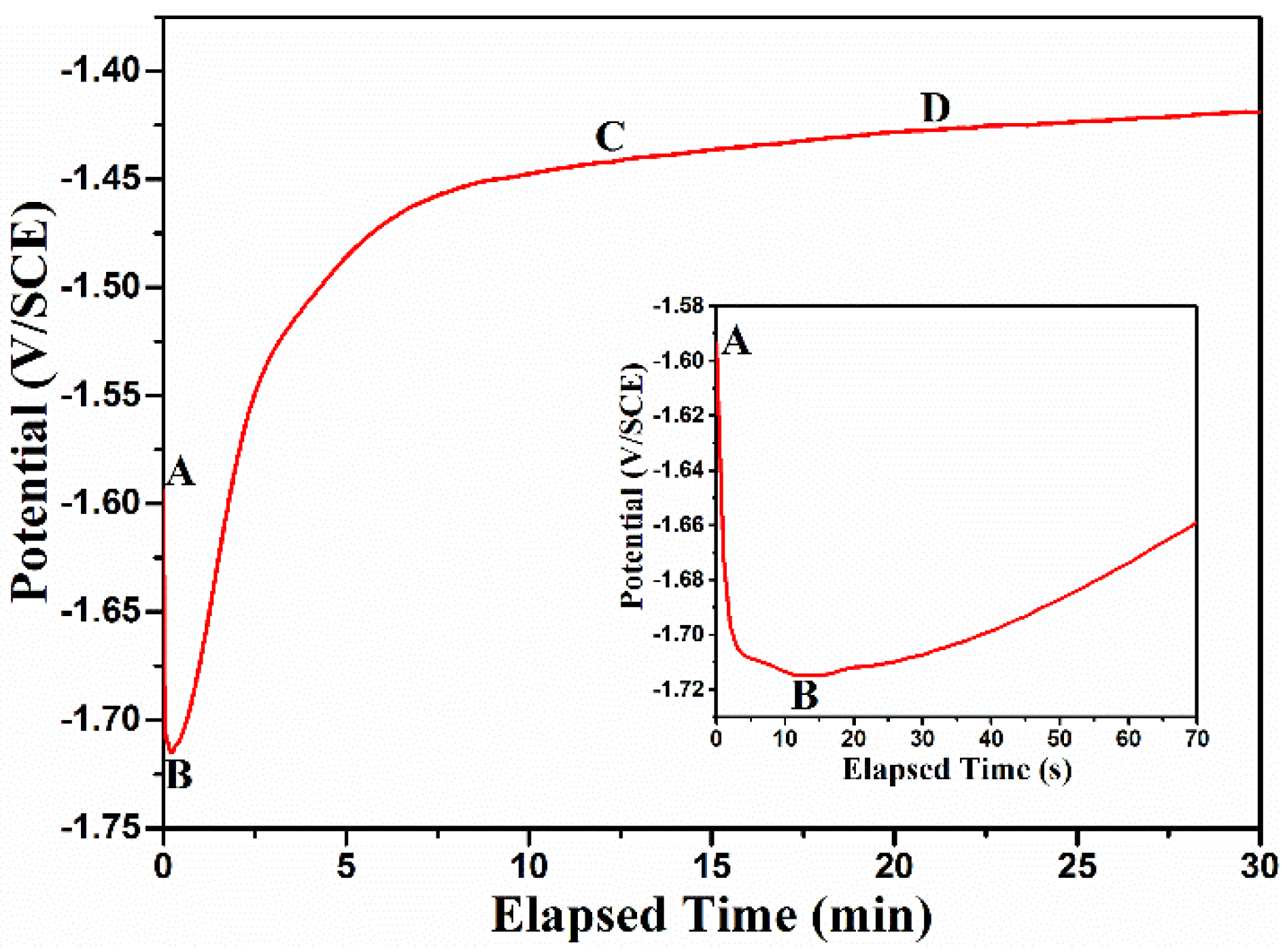
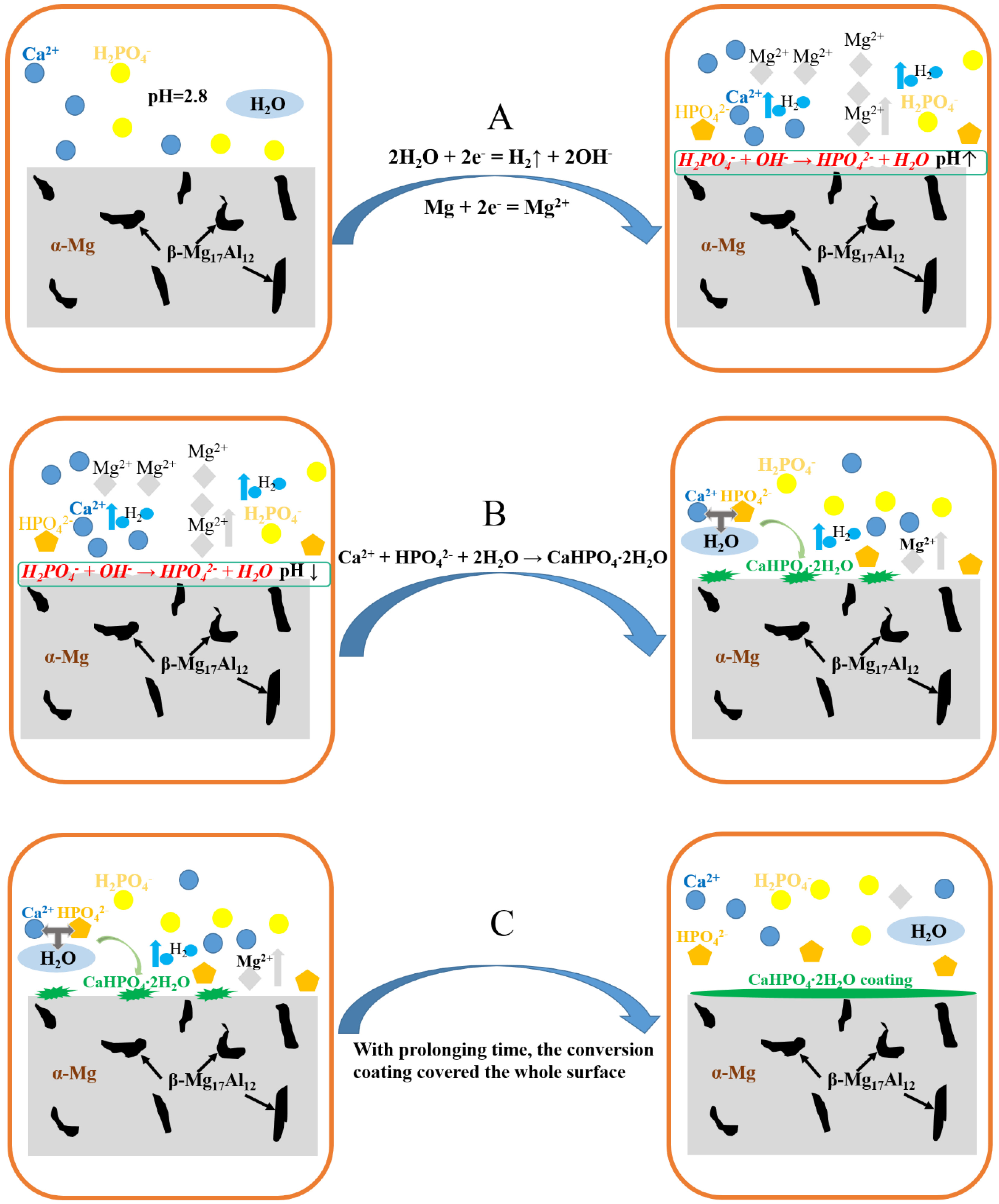
3.7. Formation Mechanism
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, F.; Shan, D.; Song, Y.; Han, E.-H.; Ke, W. Corrosion behavior of the composite ceramic coating containing zirconium oxides on AM30 magnesium alloy by plasma electrolytic oxidation. Corros. Sci. 2011, 53, 3845–3852. [Google Scholar] [CrossRef]
- Rocca, E.; Juers, C.; Steinmetz, J. Corrosion behaviour of chemical conversion treatments on as-cast Mg–Al alloys: Electrochemical and non-electrochemical methods. Corros. Sci. 2010, 52, 2172–2178. [Google Scholar] [CrossRef]
- Chen, J.; Lan, X.; Wang, C.; Zhang, Q. The Formation Mechanism and Corrosion Resistance of a Composite Phosphate Conversion Film on AM60 Alloy. Materials 2018, 11, 402. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yan, Z.; Ma, X.; Geng, T.; Wu, H.; Li, Z. Mg-MOF-74/MgF2 Composite Coating for Improving the Properties of Magnesium Alloy Implants: Hydrophilicity and Corrosion Resistance. Materials 2018, 11, 396. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Shan, D.; Han, E.-H.; Ke, W. Structure and formation mechanism of phosphate conversion coating on die-cast AZ91D magnesium alloy. Corros. Sci. 2008, 50, 329–337. [Google Scholar] [CrossRef]
- She, Z.; Li, Q.; Wang, Z.; Li, L.; Chen, F.; Zhou, J. Novel Method for Controllable Fabrication of a SuperhydrophobicCuO Surface on AZ91D Magnesium Alloy. ACS Appl. Mater. Interfaces 2012, 4, 4348–4356. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.-C.; Zhang, F.; Lan, Z.-D.; Cui, H.-Z.; Han, E.-H. Corrosion resistance of calcium-modified zinc phosphate conversion coatings on magnesium–aluminium alloys. Corros. Sci. 2014, 88, 452–459. [Google Scholar] [CrossRef]
- Zhou, Y.; Yan, F.A. The Relation between Intergranular Corrosion and Electrochemical Characteristic of Carbon Steel in Carbonic Acid and Sodium Nitrite Solutions. Int. J. Electrochem. Sci. 2016, 11, 3976–3986. [Google Scholar] [CrossRef]
- Shi, Z.; Song, G.; Atrens, A. The corrosion performance of anodised magnesium alloys. Corros. Sci. 2006, 48, 3531–3546. [Google Scholar] [CrossRef]
- Song, G.-L.; Shi, Z. Corrosion mechanism and evaluation of anodized magnesium alloys. Corros. Sci. 2014, 85, 126–140. [Google Scholar] [CrossRef]
- Cui, X.J.; Lin, X.Z.; Liu, C.H.; Yang, R.S.; Zheng, X.W.; Gong, M. Fabrication and corrosion resistance of a hydrophobic micro-arc oxidation coating on AZ31 Mg alloy. Corros. Sci. 2015, 90, 402–412. [Google Scholar] [CrossRef]
- Li, M.; Cheng, Y.; Zheng, Y.F.; Zhang, X.; Xi, T.F.; Wei, S.C. Plasma enhanced chemical vapor deposited silicon coatings on Mg alloy for biomedical application. Surf. Coat. Technol. 2013, 228, S262–S265. [Google Scholar] [CrossRef]
- Gu, C.D.; Yan, W.; Zhang, J.L.; Tu, J.P. Corrosion resistance of AZ31B magnesium alloy with a conversion coating produced from a choline chloride—Urea based deep eutectic solvent. Corros. Sci. 2016, 106, 108–116. [Google Scholar] [CrossRef]
- Lei, L.; Shi, J.; Wang, X.; Liu, D.; Xu, H.G. Microstructure and electrochemical behavior of cerium conversion coating modified with silane agent on magnesium substrates. Appl. Surf. Sci. 2016, 376, 161–171. [Google Scholar] [CrossRef]
- Xiong, Q.Y.; Zhou, Y.; Xiong, J.P. The Study of a Phosphate Conversion Coating on Magnesium Alloy AZ91D: II. Effects of Components and their Content in Phosphating Bath. Int. J. Electrochem. Sci. 2015, 10, 8454–8464. [Google Scholar]
- Wang, H.L.; Liu, L.Y.; Dou, Y.; Zhang, W.Z.; Jiang, W.F. Preparation and corrosion resistance of electroless Ni-P/SiC functionally gradient coatings on AZ91D magnesium alloy. Appl. Surf. Sci. 2013, 286, 319–327. [Google Scholar] [CrossRef]
- Chen, X.B.; Birbilis, N.; Abbott, T.B. Effect of [Ca2+] and [PO43−] levels on the formation of calcium phosphate conversion coatings on die-cast magnesium alloy AZ91D. Corros. Sci. 2012, 55, 226–232. [Google Scholar] [CrossRef]
- Zhou, Y.; Xiong, J.P.; Yan, F.A. The preparation and characterization of a nano-CeO2/phosphate composite coating on magnesium alloy AZ91D. Surf. Coat. Technol. 2017, 328, 335–343. [Google Scholar] [CrossRef]
- Li, Q.; Xu, S.Q.; Hu, J.Y.; Zhang, S.Y.; Zhong, X.K.; Yang, X.K. The effects to the structure and electrochemical behavior of zinc phosphate conversion coatings with ethanolamine on magnesium alloy AZ91D. Electrochim. Acta 2010, 55, 887–894. [Google Scholar] [CrossRef]
- Chen, X.B.; Zhou, X.; Abbott, T.B.; Easton, M.A.; Birbilis, N. Double-layered manganese phosphate conversion coating on magnesium alloy AZ91D: Insights into coating formation, growth and corrosion resistance. Surf. Coat. Technol. 2013, 217, 147–155. [Google Scholar] [CrossRef]
- Chen, Y.; Luan, B.L.; Song, G.-L.; Yang, Q.; Kingston, D.M.; Bensebaa, F. An investigation of new barium phosphate chemical conversion coating on AZ31 magnesium alloy. Surf. Coat. Technol. 2012, 210, 156–165. [Google Scholar] [CrossRef]
- Hiromoto, S.; Yamamoto, A. High corrosion resistance of magnesium coated with hydroxyapatite directly synthesized in an aqueous solution. Electrochim. Acta 2009, 54, 7085–7093. [Google Scholar] [CrossRef]
- Yanovska, A.; Kuznetsov, V.; Stanislavov, A.; Danilchenko, S.; Sukhodub, L. Calcium-phosphate coatings obtained biomimetically on magnesium substrates under low magnetic field. Appl. Surf. Sci. 2012, 258, 8577–8584. [Google Scholar] [CrossRef]
- Hornberger, H.; Virtanen, S.; Boccaccini, A.R. Biomedical coatings on magnesium alloys—A review. Acta Biomater. 2012, 8, 2442–2455. [Google Scholar] [CrossRef] [PubMed]
- Shadanbaz, S.; Dias, G.J. Calcium phosphate coatings on magnesium alloys for biomedical applications: A review. Acta Biomater. 2012, 8, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.S.; Roy, A.; Lee, B.; Kumta, P.N. Aqueous deposition of calcium phosphates and silicate substituted calcium phosphates on magnesium alloys. Mater. Sci. Eng. B 2011, 176, 1695–1702. [Google Scholar] [CrossRef]
- Su, Y.C.; Guo, Y.T.; Huang, Z.L.; Zhang, Z.H.; Li, G.Y.; Lian, J.S.; Ren, L.Q. Preparation and corrosion behaviors of calcium phosphate conversion coating on magnesium alloy. Surf. Coat. Technol. 2016, 307, 99–108. [Google Scholar] [CrossRef]
- Su, Y.C.; Su, Y.C.; Lu, Y.B.; Lian, J.S.; Li, G.Y. Composite Microstructure and Formation Mechanism of Calcium Phosphate Conversion Coating on Magnesium Alloy. J. Electrochem. Soc. 2016, 163, G138–G143. [Google Scholar] [CrossRef]
- Song, Y.W.; Shan, D.Y.; Chen, R.S.; Zhang, F.; Han, E.H. A novel phosphate conversion film on Mg-8.8Li alloy. Surf. Coat. Technol. 2009, 203, 1107–1113. [Google Scholar] [CrossRef]
- Song, Y.W.; Shan, D.Y.; Chen, R.S.; Zhang, F.; Han, E.H. Formation mechanism of phosphate conversion film on Mg-8.8Li alloy. Corros. Sci. 2009, 51, 62–69. [Google Scholar] [CrossRef]
- Sun, R.X.; Wang, P.F.; Zhao, D.D.; Sun, Z.Z.; Li, C.Q.; Chen, K.Z. An environment-friendly calcium phosphate conversion coating on AZ91D alloy and its corrosion resistance. Mater. Corros. 2015, 66, 383–386. [Google Scholar] [CrossRef]
- Curioni, M.; Salamone, L.; Scenini, F.; Santamaria, M.; Di Natale, M. A mathematical description accounting for the superfluous hydrogen evolution and the inductive behaviour observed during electrochemical measurements on magnesium. Electrochim. Acta 2018, 274, 343–352. [Google Scholar] [CrossRef]
- Curioni, M.; Scenini, F.; Monetta, T.; Bellucci, F. Correlation between electrochemical impedance measurements and corrosion rate of magnesium investigated by real-time hydrogen measurement and optical imaging. Electrochim. Acta 2015, 166, 372–384. [Google Scholar] [CrossRef]
- Liu, W.J.; Cao, F.H.; Chen, A.N.; Chang, L.R.; Zhang, J.Q.; Cao, C.A. Corrosion behaviour of AM60 magnesium alloys containing Ce or La under thin electrolyte layers. Part 1: Microstructural characterization and electrochemical behaviour. Corros. Sci. 2010, 52, 627–638. [Google Scholar] [CrossRef]
- Cui, L.-Y.; Gao, S.-D.; Li, P.-P.; Zeng, R.-C.; Zhang, F.; Li, S.-Q.; Han, E.-H. Corrosion resistance of a self-healing micro-arc oxidation/polymethyltrimethoxysilane composite coating on magnesium alloy AZ31. Corros. Sci. 2017, 118, 84–95. [Google Scholar] [CrossRef]
- Deng, F.G.; Wang, L.S.; Zhou, Y.; Gong, X.H.; Zhao, X.P.; Hu, T.; Wu, C.G. Effect of nanosilica content on the corrosion inhibition of composite coatings of a filled epoxy resin grafted with a hydrophobic fluoroalkylsilane: A dual critical concentrations interpretation. RSC Adv. 2017, 7, 48876–48893. [Google Scholar] [CrossRef]
- Xiong, Q.Y.; Xiong, J.P.; Zhou, Y.; Yan, F.A. The Study of a Phosphate Conversion Coating on Magnesium Alloy AZ91D: III. Nano-particles Modification. Int. J. Electrochem. Sci. 2017, 12, 4238–4250. [Google Scholar] [CrossRef]
- Chen, X.; Xiong, Q.Y.; Zhu, F.; Li, H.; Liu, D.; Xiong, J.P.; Zhou, Y. The Effects of Chloride Anions on Corrosion and Passivation Behavior of 254SMO Stainless Steel in Water Absorbed of Blast Furnace Gas (BFG). Int. J. Electrochem. Sci. 2018, 13, 1656–1665. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, P.; Zuo, Y.; Liu, D.; Yan, F.A. The Structure and Composition of Corrosion Product Film and its Relation to Corrosion Rate for Carbon Steels in CO2 Saturated Solutions at Different Temperatures. J. Braz. Chem. Soc. 2017, 28, 2490–2499. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Li, C.J.; Ohmori, A. Influence of substrate roughness on the bonding mechanisms of high velocity oxy-fuel sprayed coatings. Thin Solid Films 2005, 485, 141–147. [Google Scholar] [CrossRef]
- Elsentriecy, H.H.; Azumi, K.; Konno, H. Improvement in stannate chemical conversion coatings on AZ91 D magnesium alloy using the potentiostatic technique. Electrochim. Acta 2007, 53, 1006–1012. [Google Scholar] [CrossRef]
- Li, X.; Weng, Z.; Yuan, W.; Luo, X.; Wong, H.M.; Liu, X.; Wu, S.; Yeung, K.W.K.; Zheng, Y.; Chu, P.K. Corrosion resistance of dicalcium phosphate dihydrate/poly(lactic-co-glycolic acid) hybrid coating on AZ31 magnesium alloy. Corros. Sci. 2016, 102, 209–221. [Google Scholar] [CrossRef]
- Anicai, L.; Masi, R.; Santamaria, M.; Di Quarto, F. A photoelectrochemical investigation of conversion coatings on Mg substrates. Corros. Sci. 2005, 47, 2883–2900. [Google Scholar] [CrossRef]
- Santamaria, M.; Di Quarto, F.; Zanna, S.; Marcus, P. Initial surface film on magnesium metal: A characterization by X-ray photoelectron spectroscopy (XPS) and photocurrent spectroscopy (PCS). Electrochim. Acta 2007, 53, 1314–1324. [Google Scholar] [CrossRef]
- Zhang, G.A.; Liu, D.; Li, Y.Z.; Guo, X.P. Corrosion behaviour of N80 carbon steel in formation water under dynamic supercritical CO2 condition. Corros. Sci. 2017, 120, 107–1120. [Google Scholar] [CrossRef]
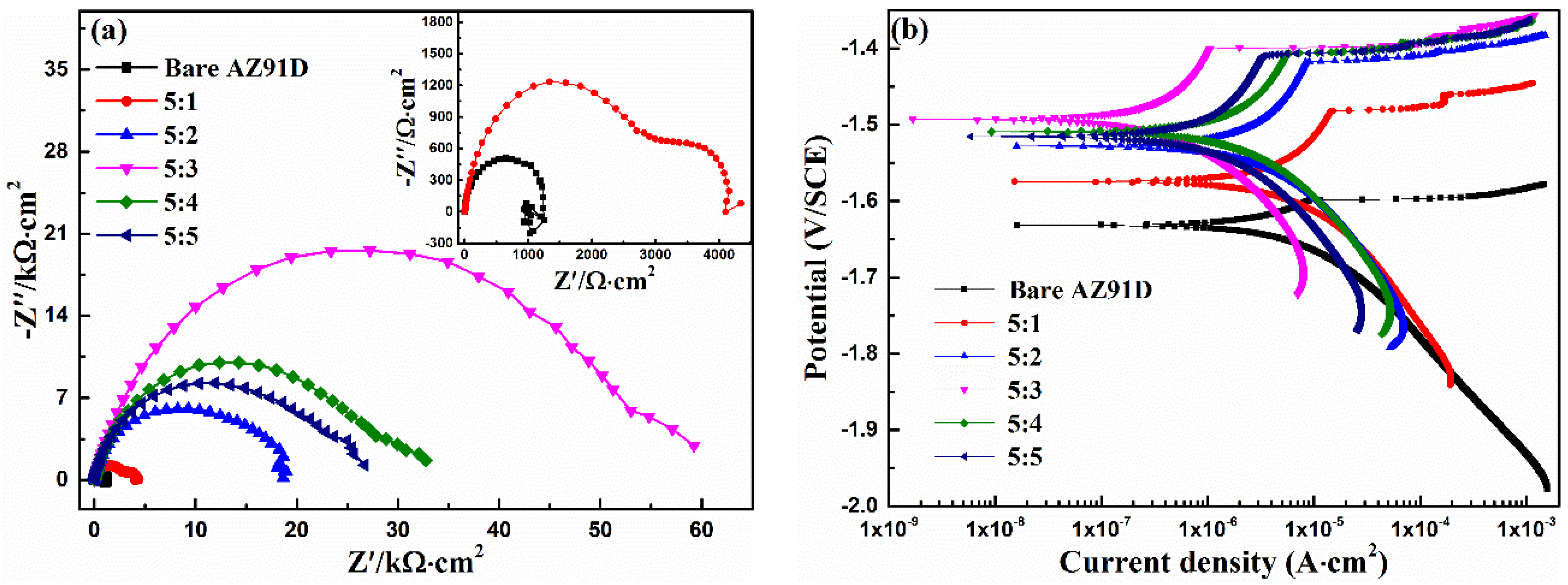
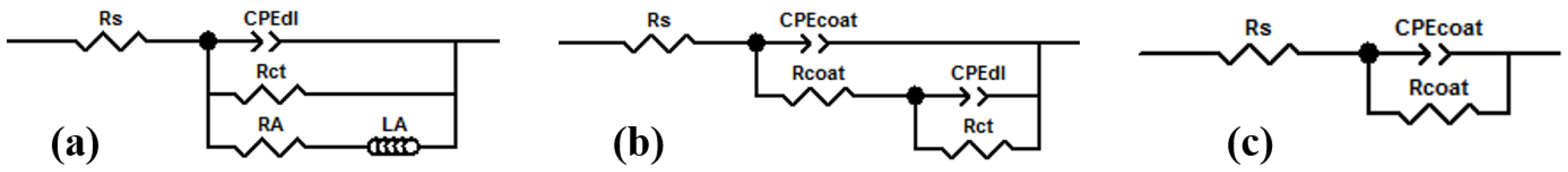
| Rs (Ω·cm2) | CPEcoat (μF·cm−2) | n1 | Rcoat (kΩ·cm2) | CPEdl (μF·cm−2) | n2 | Rct (kΩ·cm2) | LA (kΩ·cm2) | RA (H·cm2) | χ2 10−3 | |
|---|---|---|---|---|---|---|---|---|---|---|
| none | 5.86 | - | - | - | 12.7 | 0.91 | 1.26 | 4.84 | 888 | 4.8 |
| 5:1 | 7.88 | 12.4 | 0.90 | 2.87 | 707 | 0.82 | 1.38 | - | - | 0.8 |
| 5:2 | 12.4 | 16.1 | 0.82 | 18.3 | - | - | - | - | - | 4.1 |
| 5:3 | 17.8 | 13.2 | 0.80 | 56.3 | - | - | - | - | - | 4.0 |
| 5:4 | 12.3 | 17.1 | 0.80 | 29.2 | - | - | - | - | - | 2.9 |
| 5:5 | 11.9 | 15.2 | 0.82 | 25.6 | - | - | - | - | - | 3.5 |
| Ecorr (VSCE) | icorr (μA/cm2) | bc (mV/dec) | |
|---|---|---|---|
| none | −1.632 | 11.8 | −159 |
| 5:1 | −1.574 | 8.60 | −167 |
| 5:2 | −1.528 | 4.41 | −166 |
| 5:3 | −1.493 | 0.610 | −140 |
| 5:4 | −1.509 | 2.01 | −171 |
| 5:5 | −1.515 | 2.93 | −156 |
| Time (min) | Rs (Ω·cm2) | CPEcoat (μF·cm−2) | n1 | Rcoat (kΩ·cm2) | CPEdl (μF·cm−2) | n2 | Rct (kΩ·cm2) | χ2 10−3 |
|---|---|---|---|---|---|---|---|---|
| 5 | 9.28 | 0.987 | 0.92 | 2.66 | 941 | 0.82 | 1.57 | 0.8 |
| 10 | 8.57 | 16.1 | 0.84 | 6.04 | 553 | 0.98 | 2.35 | 1.4 |
| 15 | 14.2 | 14.6 | 0.81 | 27.4 | - | - | - | 3.4 |
| 20 | 17.8 | 13.2 | 0.80 | 56.3 | - | - | - | 4.0 |
| 25 | 14.2 | 16.7 | 0.81 | 39.8 | - | - | - | 2.4 |
| Temperature (°C) | Rs (Ω·cm2) | CPEcoat (μF·cm−2) | n | Rcoat (kΩ·cm2) | χ2 10−3 |
|---|---|---|---|---|---|
| Room temperature | 17.8 | 13.2 | 0.80 | 56.3 | 4.0 |
| 20 | 15.6 | 11.6 | 0.81 | 54.8 | 4.2 |
| 30 | 15.0 | 13.1 | 0.80 | 56.7 | 3.4 |
| 40 | 15.6 | 12.1 | 0.82 | 60.2 | 4.0 |
| 50 | 11.9 | 20.9 | 0.79 | 23.6 | 1.7 |
| pH | Rs (Ω·cm2) | CPEcoat (μF·cm−2) | n1 | Rcoat (kΩ·cm2) | CPEdl (μF·cm−2) | n2 | Rct (kΩ·cm2) | χ2 10−3 |
|---|---|---|---|---|---|---|---|---|
| 3.6 | 8.7 | 18.7 | 0.81 | 10.7 | 345 | 0.99 | 2.40 | 0.9 |
| 3.2 | 11.3 | 20.9 | 0.77 | 29.1 | - | - | - | 0.9 |
| 2.8 | 17.8 | 13.2 | 0.80 | 56.3 | - | - | - | 4.0 |
| 2.4 | 11.5 | 15.6 | 0.81 | 20.1 | - | - | - | 3.9 |
| 2.0 | 8.66 | 14.2 | 0.85 | 4.96 | 522 | 0.96 | 1.56 | 1.3 |
| Stirring Rate (r/min) | Rs (Ω·cm2) | CPEcoat (μF·cm−2) | n1 | Rcoat (kΩ·cm2) | CPEdl (μF·cm−2) | n2 | Rct (kΩ·cm2) | χ2 10−3 |
|---|---|---|---|---|---|---|---|---|
| 150 | 6.95 | 14.5 | 0.84 | 4.02 | 448 | 0.96 | 1.93 | 1.5 |
| 100 | 9.01 | 23.6 | 0.80 | 8.97 | 810 | 0.87 | 3.32 | 0.5 |
| 50 | 11.1 | 18.3 | 0.82 | 21.3 | - | - | - | 3.5 |
| 0 | 17.8 | 13.2 | 0.80 | 56.3 | - | - | - | 4.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Li, Y.; Zhou, Y.; Ding, Y. The Preparation, Characterization and Formation Mechanism of a Calcium Phosphate Conversion Coating on Magnesium Alloy AZ91D. Materials 2018, 11, 908. https://doi.org/10.3390/ma11060908
Liu D, Li Y, Zhou Y, Ding Y. The Preparation, Characterization and Formation Mechanism of a Calcium Phosphate Conversion Coating on Magnesium Alloy AZ91D. Materials. 2018; 11(6):908. https://doi.org/10.3390/ma11060908
Chicago/Turabian StyleLiu, Dong, Yanyan Li, Yong Zhou, and Yigang Ding. 2018. "The Preparation, Characterization and Formation Mechanism of a Calcium Phosphate Conversion Coating on Magnesium Alloy AZ91D" Materials 11, no. 6: 908. https://doi.org/10.3390/ma11060908
APA StyleLiu, D., Li, Y., Zhou, Y., & Ding, Y. (2018). The Preparation, Characterization and Formation Mechanism of a Calcium Phosphate Conversion Coating on Magnesium Alloy AZ91D. Materials, 11(6), 908. https://doi.org/10.3390/ma11060908




