In Situ Room Temperature Electron-Beam Driven Graphene Growth from Hydrocarbon Contamination in a Transmission Electron Microscope
Abstract
1. Introduction
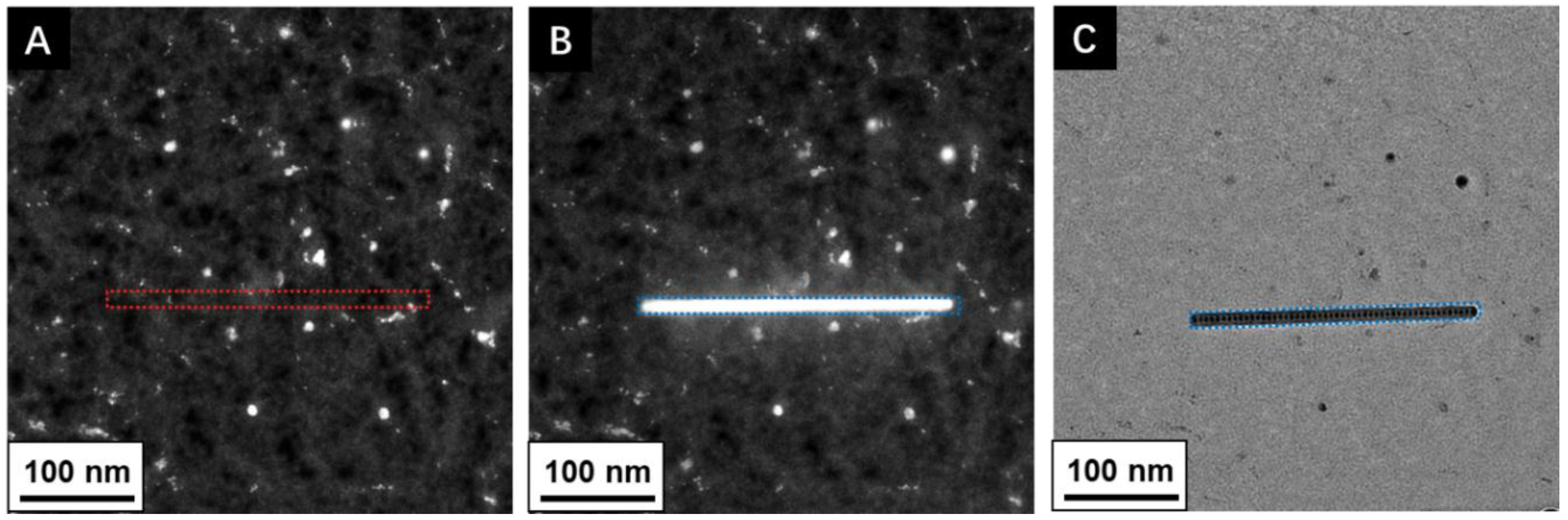
2. Sources of Contamination
3. Electron–Sample/Contamination Interactions
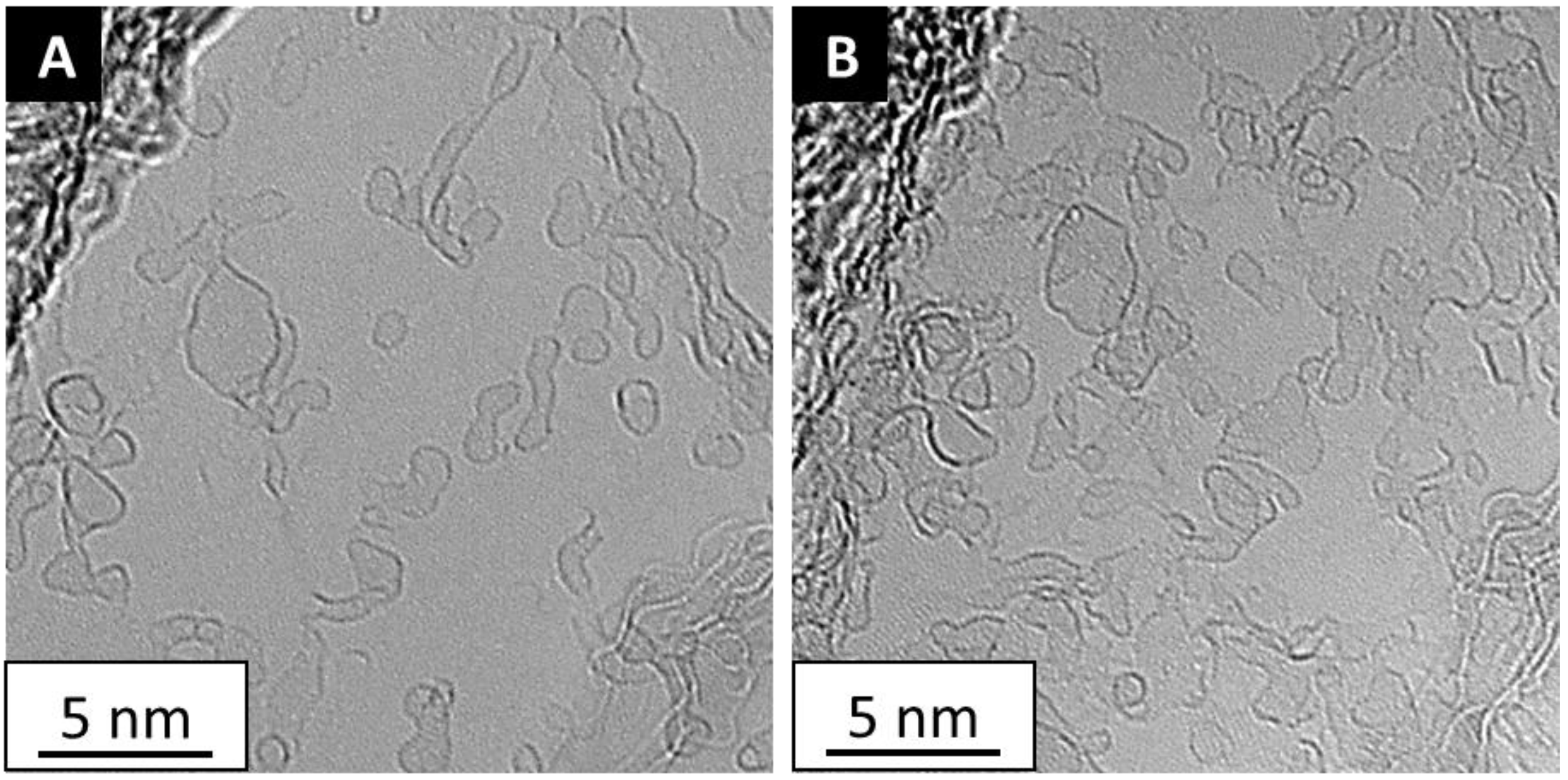
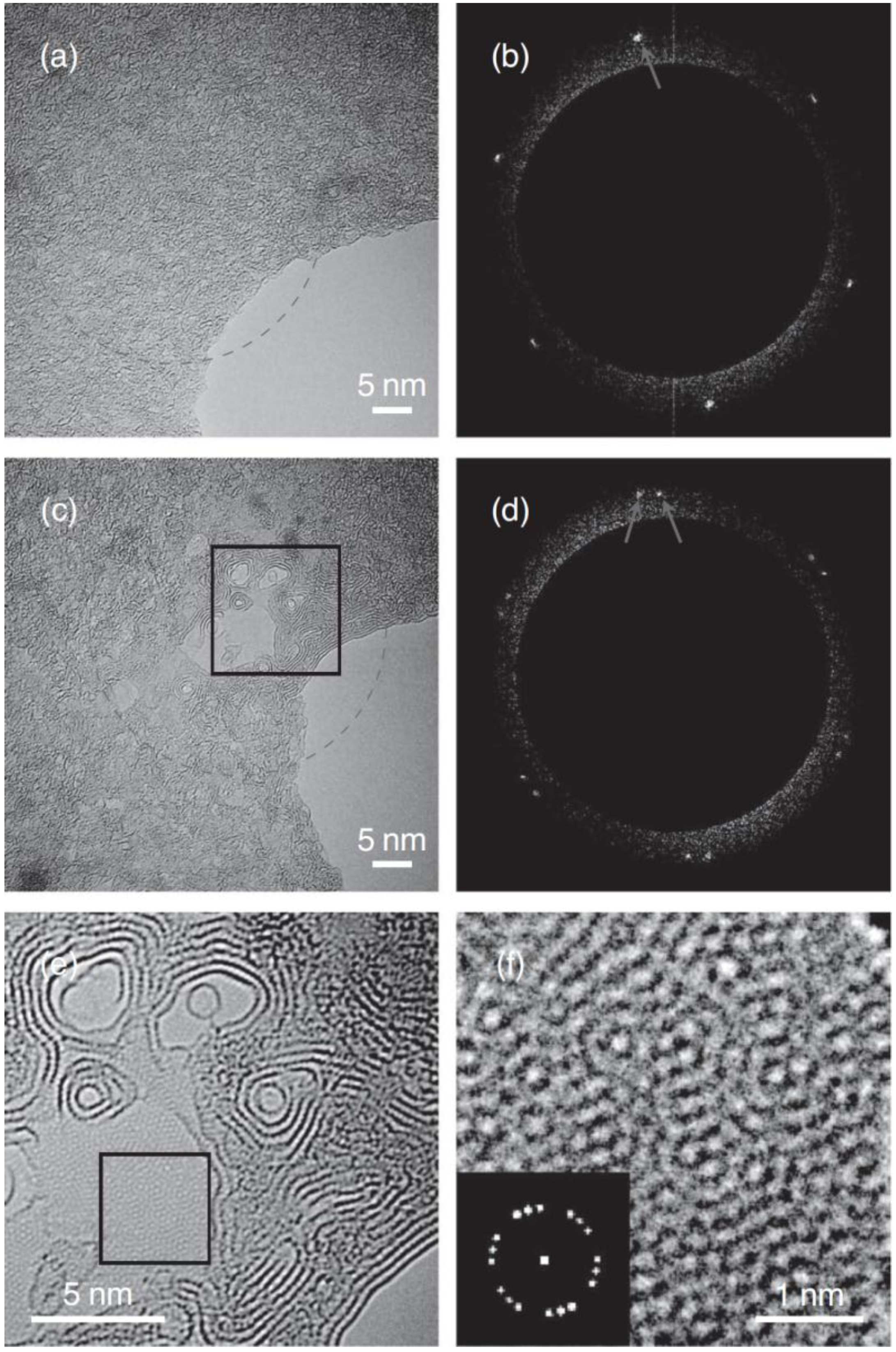
4. In Situ Graphene Growth
4.1. Catalyst Free In Situ Graphene Growth
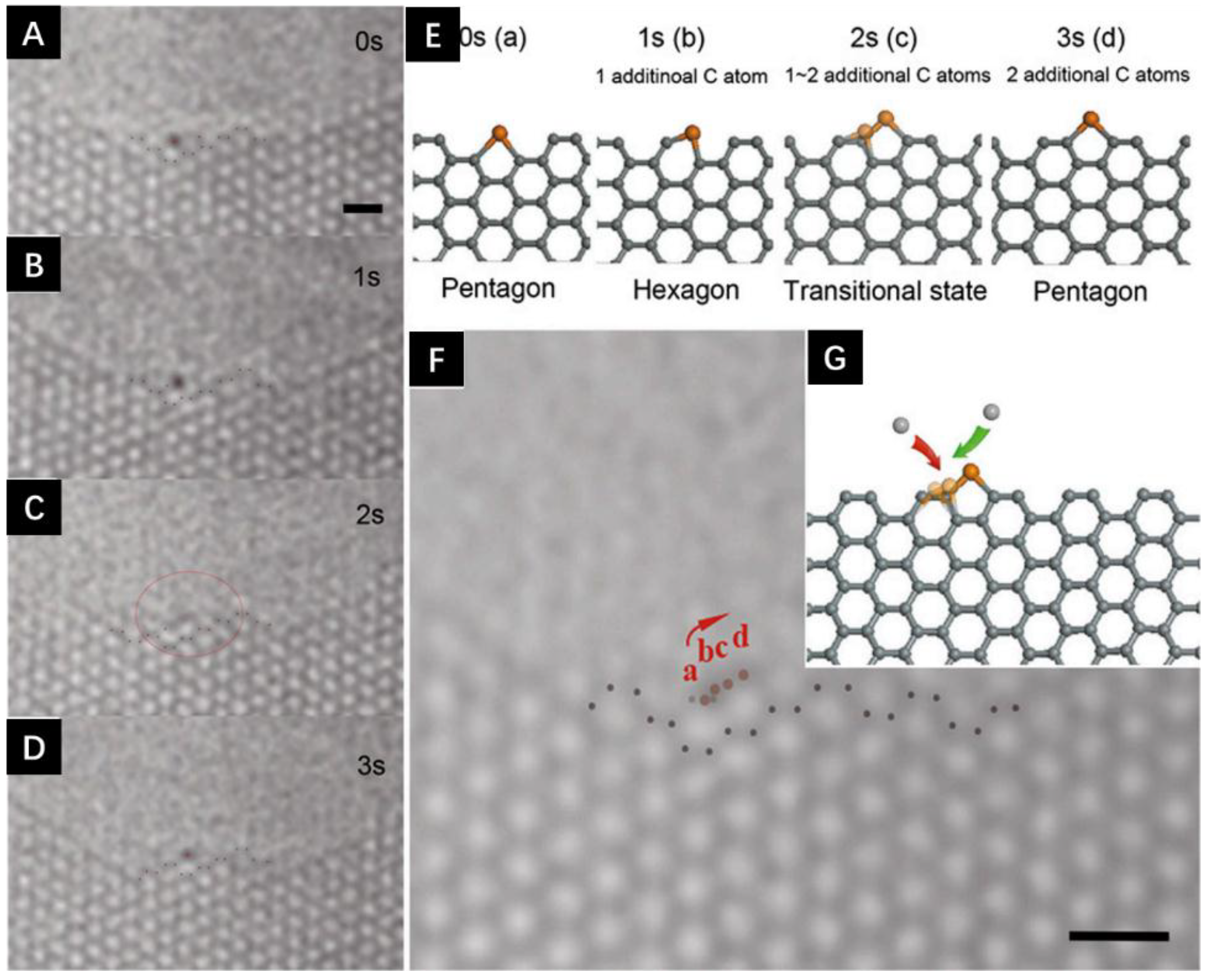
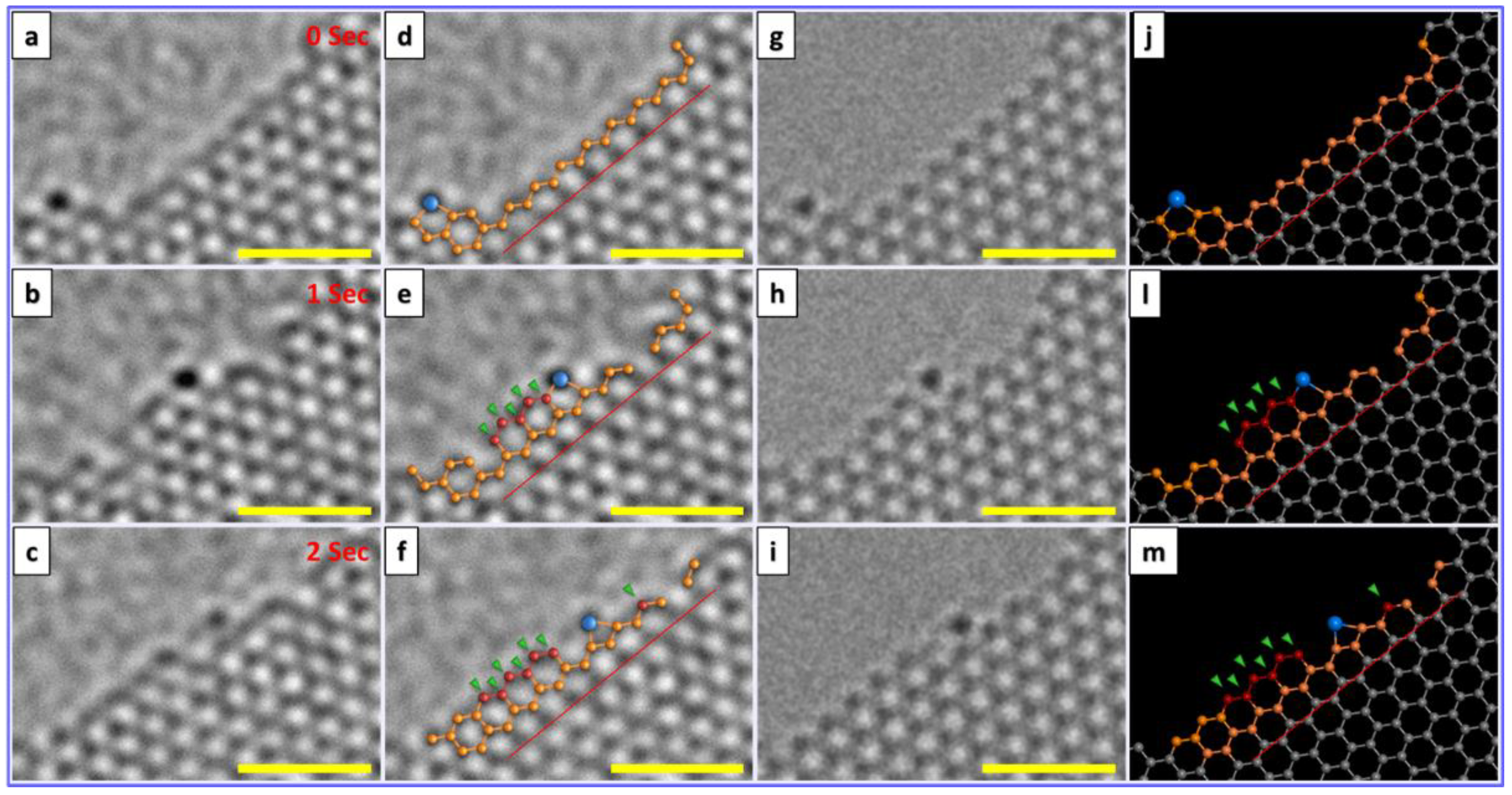
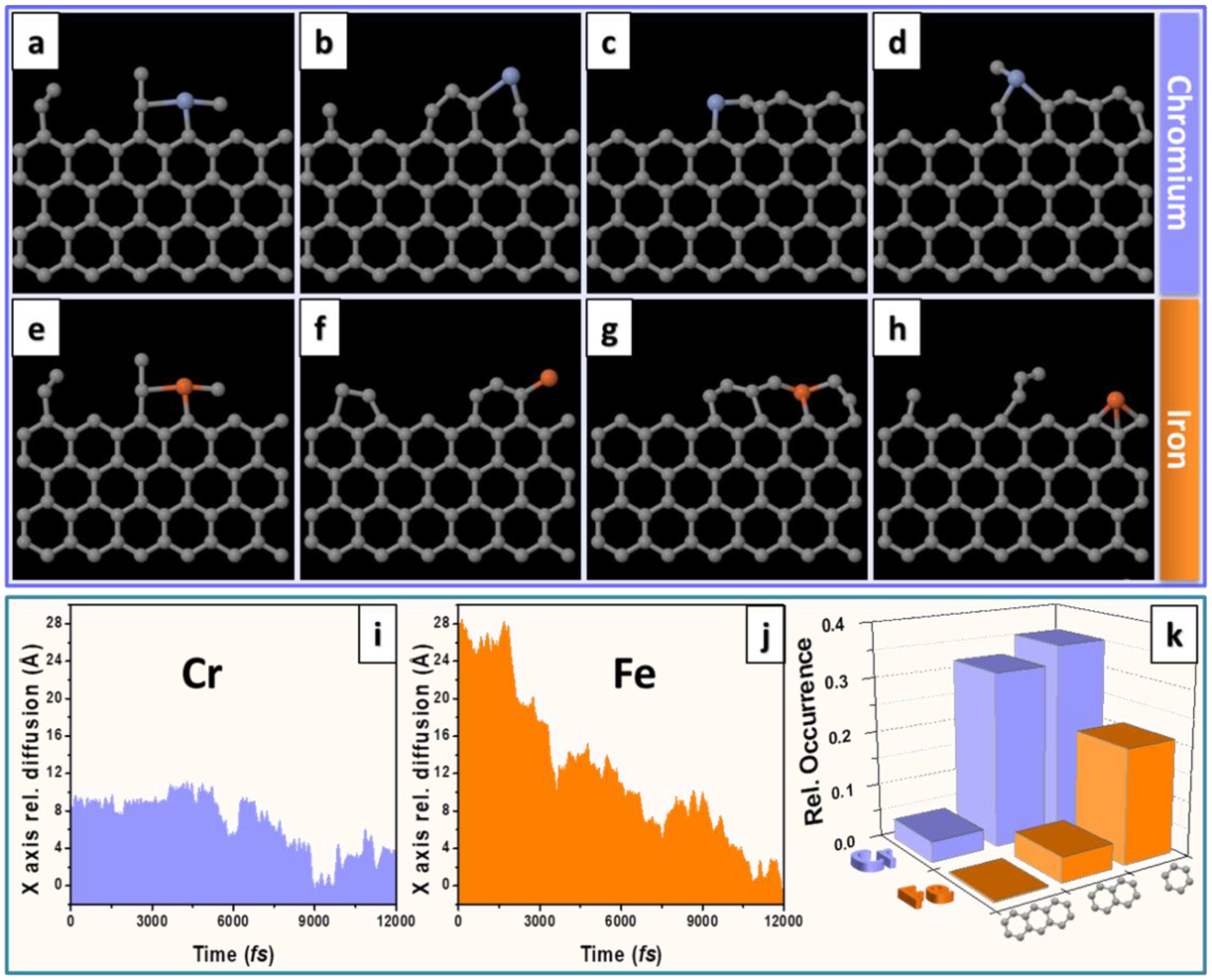
4.2. Catalyst Assisted In Situ Graphene Growth
5. Summary and Future Thoughts
Funding
Acknowledgments
Conflicts of Interest
References
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.T.; Holland, B.; Byrne, M.; Gun’ko, Y.K.; et al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol. 2008, 3, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Rümmeli, M.H.; Rocha, C.G.; Ortmann, F.; Ibrahim, I.; Sevincli, H.; Börrnert, F.; Kunstmann, J.; Bachmatiuk, A.; Pötschke, M.; Shiraishi, M.; et al. Graphene: Piecing it together. Adv. Mater. 2011, 23, 4471–4490. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Fang, M.; Wu, F.; Wu, H.; Wang, L.; Chen, G. Preparation of graphene by exfoliation of graphite using wet ball milling. J. Mater. Chem. 2010, 20, 5817–5819. [Google Scholar] [CrossRef]
- Green, A.A.; Hersam, M.C. Emerging methods for producing monodisperse graphene dispersions. J. Phys. Chem. Lett. 2010, 1, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.C.; Manchester, S.; Sarin, L.; Gao, Y.; Kulaots, I.; Hurt, R.H. Mercury vapor release from broken compact fluorescent lamps and in situ capture by new nanomaterial sorbents. Environ. Sci. Technol. 2008, 42, 5772–5778. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A.; Novoselov, K.S. Electric Field Effect in Atomically Thin Carbon Films. Source Sci. New Ser. Gene Expr. Genes Action 2007, 306, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, X.; Zhang, L.; Lee, S.; Dai, H. Chemically derived, ultrasmooth graphene nanoribbon semiconductors. Science 2008, 319, 1229–1232. [Google Scholar] [CrossRef] [PubMed]
- Warner, J.H.; Rummeli, M.H.; Gemming, T.; Buchner, B.; Briggs, G.A.D. Direct Imaging of Rotational Stacking Faults in Few Layer Graphene. Nano Lett. 2009, 9, 102–1106. [Google Scholar] [CrossRef] [PubMed]
- Blake, P.; Brimicombe, P.D.; Nair, R.R.; Booth, T.J.; Jiang, D.; Schedin, F.; Ponomarenko, L.A.; Morozov, S.V.; Gleeson, H.F.; Hill, E.W.; et al. Graphene Based Liquid Crystal Device. Nano Lett. 2008, 8, 1704–1708. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Piner, R.D.; Chen, X.; Wu, N.; Nguyen, S.T.; Ruoff, R.S. Stable aqueous dispersions of graphitic nanoplatelets via the reduction of exfoliated graphite oxide in the presence of poly(sodium 4-styrenesulfonate). J. Mater. Chem. 2006, 16, 155–158. [Google Scholar] [CrossRef]
- Tung, V.C.; Allen, M.J.; Yang, Y.; Kaner, R.B. High-throughput solution processing of large-scale graphene. Nat. Nanotechnol. 2009, 4, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Müller, M.B.; Gilje, S.; Kaner, R.B.; Wallace, G.G. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2008, 3, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Li, X.; Ji, R.; Teng, K.S.; Tai, G.; Ye, J.; Wei, C.; Lau, S.P. Bottom-up synthesis of large-scale graphene oxide nanosheets. J. Mater. Chem. 2012, 22, 5676. [Google Scholar] [CrossRef]
- Zhang, W.; Cui, J.; Tao, C.A.; Wu, Y.; Li, Z.; Ma, L.; Wen, Y.; Li, G. A strategy for producing pure single-layer graphene sheets based on a confined self-assembly approach. Angew. Chem. Int. Ed. 2009, 48, 5864–5868. [Google Scholar] [CrossRef] [PubMed]
- Choucair, M.; Thordarson, P.; Stride, J.A. Gram-scale production of graphene based on solvothermal synthesis and sonication. Nat. Nanotechnol. 2009, 4, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Mattevi, C.; Kim, H.; Chhowalla, M. A review of chemical vapour deposition of graphene on copper. J. Mater. Chem. 2011, 21, 3324–3334. [Google Scholar] [CrossRef]
- Reina, A.; Jia, X.T.; Ho, J.; Nezich, D.; Son, H.B.; Bulovic, V.; Dresselhaus, M.S.; Kong, J. Large Area, Few-Layer Graphene Films on Arbitrary Substrates by Chemical Vapor Deposition. Nano Lett. 2009, 9, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Rümmeli, M.H.; Bachmatiuk, A.; Scott, A.; Börrnert, F.; Warner, J.H.; Hoffmann, V.; Lin, J.-H.; Cuniberti, G.; Büchner, B. Direct Low Temperature Nano-Graphene Synthesis over a Dielectric Insulator. ACS Nano 2011, 4, 4206–4210. [Google Scholar] [CrossRef] [PubMed]
- Fanton, M.A.; Robinson, J.A.; Puls, C.; Liu, Y.; Hollander, M.J.; Weiland, B.E.; Labella, M.; Trumbull, K.; Kasarda, R.; Howsare, C.; et al. Characterization of graphene films and transistors grown on sapphire by metal-free chemical vapor deposition. ACS Nano 2011, 5, 8062–8069. [Google Scholar] [CrossRef] [PubMed]
- Yannopoulos, S.N.; Siokou, A.; Nasikas, N.K.; Dracopoulos, V.; Ravani, F.; Papatheodorou, G.N. CO2-Laser-Induced Growth of Epitaxial Graphene on 6H-SiC(0001). Adv. Funct. Mater. 2012, 22, 113–120. [Google Scholar] [CrossRef]
- Yu, X.Z.; Hwang, C.G.; Jozwiak, C.M.; Köhl, A.; Schmid, A.K.; Lanzara, A. New synthesis method for the growth of epitaxial graphene. J. Electron Spectrosc. Relat. Phenom. 2011, 184, 100–106. [Google Scholar] [CrossRef]
- Lee, S.; Toney, M.F.; Ko, W.; Randel, Ќ.J.C.; Jung, Ќ.H.J.; Munakata, K.; Lu, J.; Geballe, T.H.; Beasley, M.R.; Sinclair, R.; et al. Laser-Synthesized Epitaxial Graphene. ACS Nano 2010, 4, 7524–7530. [Google Scholar] [CrossRef] [PubMed]
- Mean Free Path. Available online: https://en.wikipedia.org/wiki/Mean_free_path (accessed on 25 May 2018).
- Isaacson, M. Electron beam induced damage of organic solids: Implications for analytical electron microscopy. Ultramicroscopy 1979, 4, 193–199. [Google Scholar] [CrossRef]
- Hren, J.J. Specimen contamination in analytical electron microscopy: Sources and solutions. Ultramicroscopy 1978, 3, 375–380. [Google Scholar] [CrossRef]
- Hirsch, P.; Kässens, M.; Püttmann, M.; Reimer, L. Contamination in a scanning electron microscope and the influence of specimen cooling. Scanning 1994, 16, 101–110. [Google Scholar] [CrossRef]
- Stewart, R.L. Insulating Films Formed Under Electron and Ion Bombardment. Phys. Rev. 1934, 3649, 488–490. [Google Scholar] [CrossRef]
- Love, G.; Scott, V.D.; Dennis, N.M.T.; Laurenson, L. Sources of contamination in electron optical equipment. Scanning 1981, 4, 32–39. [Google Scholar] [CrossRef]
- Hart, R.K.; Kassner, T.F.; Matjrin, J.K. The contamination of surfaces during high-energy electron irradiation. Philos. Mag. 1970, 21, 453–467. [Google Scholar] [CrossRef]
- Ennos, A.E. The sources of electron-induced contamination in kinetic vacuum systems. Br. J. Appl. Phys. 1954, 5, 27–31. [Google Scholar] [CrossRef]
- Ennos, A.E. The origin of specimen contamination in the electron microscope. Br. J. Appl. Phys. 1953, 4, 101–106. [Google Scholar] [CrossRef]
- Hren, J.J. Barriers to AEM: Contamination and Etching BT—Introduction to Analytical Electron Microscopy; Hren, J.J., Goldstein, J.I., Joy, D.C., Eds.; Springer: Boston, MA, USA, 1979; pp. 481–505. ISBN 978-1-4757-5581-7. [Google Scholar]
- Rummeli, M.H.; Ta, H.Q.; Mendes, R.G.; Gonzalez-Martinez, I.G.; Zhao, L.; Gao, J.; Fu, L.; Gemming, T.; Bachmatiuk, A.; Liu, Z. New Frontiers in Electron-Beam Driven Chemistry in and around Graphene. Adv. Mater. 2018, 30, 1800715. [Google Scholar]
- Börrnert, F.; Avdoshenko, S.M.; Bachmatiuk, A.; Ibrahim, I.; Büchner, B.; Cuniberti, G.; Rümmeli, M.H. Amorphous carbon under 80 kV electron irradiation: A means to make or break graphene. Adv. Mater. 2012, 24, 5630–5635. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.H.; Wang, J.Y.; Chen, Q.; Peng, L.M. In situ fabrication and graphitization of amorphous carbon nanowires and their electrical properties. J. Phys. Chem. B 2006, 110, 5423–5428. [Google Scholar] [CrossRef] [PubMed]
- Banhart, F. Irradiation effects in carbon nanostructures. Rep. Prog. Phys. 1999, 62, 1181. [Google Scholar] [CrossRef]
- Urban, K.; Seeger, A. Radiation-induced diffusion of point-defects during low-temperature electron irradiation. Philos. Mag. 1974, 30, 1395–1418. [Google Scholar] [CrossRef]
- Ugarte, D. Curling and closure of graphitic networks under electron-beam irradiation. Nature 1992, 359, 707–709. [Google Scholar] [CrossRef] [PubMed]
- Yajima, A.; Abe, S.; Fuse, T.; Mera, Y.; Maeda, K.; Suzuki, K. Electron-Irradiation-Induced Ordering In Tetrahedral-Amorphous Carbon Films. Mol. Cryst. Liq. Cryst. 2002, 388, 147–151. [Google Scholar] [CrossRef]
- Barreiro, A.; Boerrnert, F.; Avdoshenko, S.M.; Rellinghaus, B.; Cuniberti, G.; Ruemmeli, M.H. Transforming Amorphous Carbon into Graphene by Current-Induced Annealing. Available online: https://nano.tu-dresden.de/pubs/reprints/cond-mat/1201.3131.pdf (accessed on 25 May 2018).
- Zobelli, A.; Gloter, A.; Ewels, C.P.; Colliex, C. Shaping single walled nanotubes with an electron beam. Phys. Rev. B Condens. Matter Mater. Phys. 2008, 77, 045410. [Google Scholar] [CrossRef]
- Girit, Ç.Ö.; Meyer, J.C.; Erni, R.; Rossell, M.D.; Kisielowski, C.; Yang, L.; Park, C.-H.; Crommie, M.F.; Cohen, M.L.; Louie, S.G.; et al. Graphene at the Edge: Stability and Dynamics. Science 2009, 666, 1705–1708. [Google Scholar] [CrossRef] [PubMed]
- Egerton, R.F.; Li, P.; Malac, M. Radiation damage in the TEM and SEM. Micron 2004, 35, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Teng, P.Y.; Yeh, C.H.; Koshino, M.; Chiu, P.W.; Suenaga, K. Structural and Chemical Dynamics of Pyridinic-Nitrogen Defects in Graphene. Nano Lett. 2015, 15, 7408–7413. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Deng, Q.; Avdoshenko, S.M.; Fu, L.; Eckert, J.; Rummeli, M.H. Direct in situ observations of single Fe atom catalytic processes and anomalous diffusion at graphene edges. Proc. Natl. Acad. Sci. USA 2014, 111, 15641–15646. [Google Scholar] [CrossRef] [PubMed]
- Ta, H.Q.; Zhao, L.; Yin, W.; Pohl, D.; Rellinghaus, B.; Gemming, T.; Trzebicka, B.; Palisaitis, J.; Jing, G.; Persson, P.O.Å.; et al. Single Cr atom catalytic growth of graphene. Nano Res. 2017, 2, 1–7. [Google Scholar] [CrossRef]
- Zhao, L.; Ta, H.Q.; Dianat, A.; Soni, A.; Fediai, A.; Yin, W.; Gemming, T.; Trzebicka, B.; Cuniberti, G.; Liu, Z.; et al. In situ electron driven carbon nanopillar-fullerene transformation through Cr atom mediation. Nano Lett. 2017, 17, 4725–4732. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rummeli, M.H.; Pan, Y.; Zhao, L.; Gao, J.; Ta, H.Q.; Martinez, I.G.; Mendes, R.G.; Gemming, T.; Fu, L.; Bachmatiuk, A.; et al. In Situ Room Temperature Electron-Beam Driven Graphene Growth from Hydrocarbon Contamination in a Transmission Electron Microscope. Materials 2018, 11, 896. https://doi.org/10.3390/ma11060896
Rummeli MH, Pan Y, Zhao L, Gao J, Ta HQ, Martinez IG, Mendes RG, Gemming T, Fu L, Bachmatiuk A, et al. In Situ Room Temperature Electron-Beam Driven Graphene Growth from Hydrocarbon Contamination in a Transmission Electron Microscope. Materials. 2018; 11(6):896. https://doi.org/10.3390/ma11060896
Chicago/Turabian StyleRummeli, Mark H, Yumo Pan, Liang Zhao, Jing Gao, Huy Q Ta, Ignacio G. Martinez, Rafael G. Mendes, Thomas Gemming, Lei Fu, Alicja Bachmatiuk, and et al. 2018. "In Situ Room Temperature Electron-Beam Driven Graphene Growth from Hydrocarbon Contamination in a Transmission Electron Microscope" Materials 11, no. 6: 896. https://doi.org/10.3390/ma11060896
APA StyleRummeli, M. H., Pan, Y., Zhao, L., Gao, J., Ta, H. Q., Martinez, I. G., Mendes, R. G., Gemming, T., Fu, L., Bachmatiuk, A., & Liu, Z. (2018). In Situ Room Temperature Electron-Beam Driven Graphene Growth from Hydrocarbon Contamination in a Transmission Electron Microscope. Materials, 11(6), 896. https://doi.org/10.3390/ma11060896




