Alternative Carrier Solvents for Pigments Extracted from Spalting Fungi
Abstract
:1. Introduction
2. Materials and Methods
3. Results
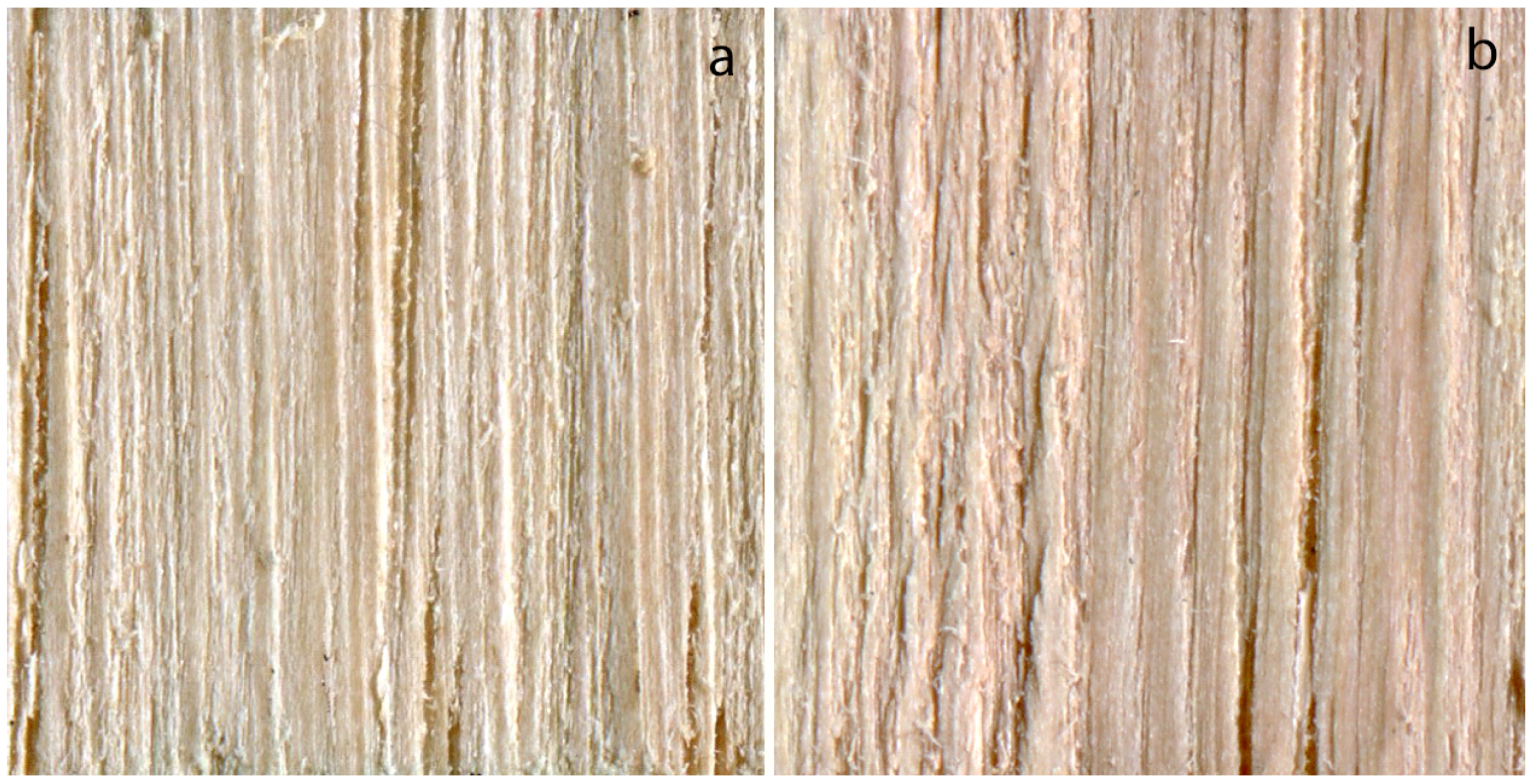
3.1. Ash
3.2. Chinkapin
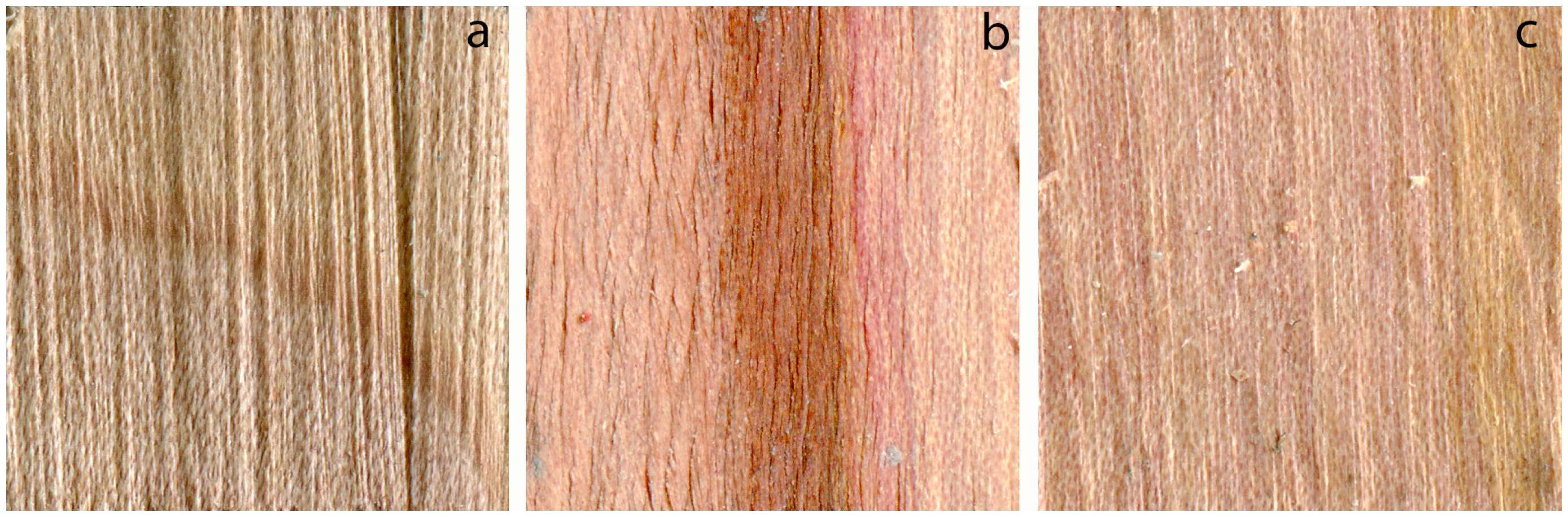
3.3. Douglas-Fir, Pacific Silver Fir and Red Alder
3.4. Madrone
3.5. Mountain Hemlock and Sugar Maple
3.6. Oregon Maple and Port-Orford Cedar
3.7. Western Larch, Lodgepole Pine, and Western Red Cedar
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Robinson, S.C.; Richter, D.L.; Laks, P.E. Colonization of sugar maple by spalting fungi. For. Prod. J. 2007, 57, 24–32. [Google Scholar]
- Robinson, S.C.; Laks, P.E.; Richter, D.L.; Pickens, J.B. Evaluating loss of machinability in spalted sugar maple. For. Prod. J. 2007, 57, 33–37. [Google Scholar]
- Zabel, R.A.; Morrell, J.J. Wood microbiology. Decay and its prevention; Harcourt Brace Jovanovich; Academic Press, Inc.: New York, NY, USA, 1992. [Google Scholar]
- Shigo, A.L. Successions of organisms in discoloration and decay of wood. Int. Rev. For. Res. 1967, 2, 237–299. [Google Scholar]
- Coates, D. The Biological Consequences of Somatic Incompatibility in Wood Decaying Basidiomycetes and other Fungi. Ph.D. Thesis, University of Bath, Newton Saint Loe, UK, 1984. [Google Scholar]
- Rodrigues, K.F.; Petrini, O.; Leuchtmann, A. Variability among isolates of xylaria cubensis as determined by isozyme analysis and somatic incompatibility tests. Mycologia 1995, 87, 592–596. [Google Scholar] [CrossRef]
- Lopez-Real, J.M.; Swift, M.J. The formation of pseudosclerotia (‘zone lines’) in wood decayed by armillaria mellea and stereum hirsutum. II. Formation in relation to the moisture content of the wood. Trans. Bri. Mycol. Soc. 1975, 64, 473–481. [Google Scholar] [CrossRef]
- Robinson, S.C. Developing fungal pigments for “painting” vascular plants. Appl. Microbiol. Biotechnol. 2012, 93, 1389–1394. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.C.; Tudor, D.; Snider, H.; Cooper, P.A. Stimulating growth and xylindein production of chlorociboria aeruginascens in agar-based systems. AMB Expr. 2012, 2, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hallsworth, J.E. Stress-free microbes lack vitality. Fungal Biol. 2018, in press. [Google Scholar] [CrossRef]
- Blanchette, R.A.; Wilmering, A.M.; Baumeister, M. The use of green-stained wood caused by the fungus chlorociboria in intarsia masterpieces from the 15th century. Holzforschung 1992, 46, 225–232. [Google Scholar] [CrossRef]
- Michaelsen, H.; Unger, A.; Fischer, C.-H. Blaugrüne Färbung an Intarsienhölzern des 16. Bis 18. Jahrhunderts. Restauro 1992, 98, 17–25. [Google Scholar]
- Otterstedt, A. Investigating green marquetry on bowed-string instruments. The leaves be greene. Galpin Soc. J. 2001, 54, 330–338. [Google Scholar] [CrossRef]
- Lieberman, C. Über Xylindein. Ber. der Dtsch. Chem. Ges. 1874, 7, 1102–1103. [Google Scholar]
- Edwards, R.L.; Kale, N. The structure of xylindein. Tetrahedron 1965, 21, 2095–2107. [Google Scholar] [CrossRef]
- Blackburn, G.M.; Ekong, D.E.; Nielson, A.H.; Todd, L. Xylindein. Chimia 1965, 19, 208–212. [Google Scholar]
- Saikawa, Y.; Watanabe, T.; Hashimoto, K.; Nakata, A. Absolute configuration and tautomeric structure of xylindein, a blue-green pigment of chlorociboria species. Phytochemistry 2000, 55, 237–240. [Google Scholar] [CrossRef]
- Maeda, M.; Yamauchi, T.; Oshima, K.; Shimomura, M.; Miyauchi, S.; Mukae, K.; Sakaki, T.; Shibata, M.; Wakamatsu, K. Extraction of xylindein from chlorociboria aeruginosa complex and its biological characteristics. Bull. Nagaoka Univ. Technol. 2003, 25, 105–111. [Google Scholar]
- Hinsch, E.M.; Weber, G.; Chen, H.-L.; Robinson, S.C. Colorfastness of extracted wood-staining fungal pigments on fabrics: A new potential for textile dyes. J. Text. Appar. Technol. Manag. 2015, 9. Available online: http://ojs.cnr.ncsu.edu/index.php/JTATM/article/view/8018 (accessed on 12 December 2017).
- Golinski, P.; Krick, T.P.; Blanchette, R.A.; Mirocha, C.J. Chemical characterization of a red pigment (5, 8-dihydroxy-2, 7-dimethoxy-1, 4-naphthalenedione) produced by arthrographis cuboidea in pink stained wood. Holzforsch.-Int. J. Biol. Chem. Phys. Technol. Wood 1995, 49, 407–410. [Google Scholar]
- Vega Gutierrez, S.M.; Robinson, S.C. Microscopic analysis of pigments extracted from spalting fungi. J. Fungi 2017, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Vega Gutierrez, S.M. Spalting Fungi: Genetic Identification, Material Interactions and Microscopical Characteristics of Extracted Pigments. Ph.D. Thesis, Oregon State University, Oregon, OR, USA, 2017. [Google Scholar]
- Vega Gutierrez, S.M.; Hazell, K.K.; Simonsen, J.; Robinson, S.C. Description of a novel naphthoquinonic crystal produced by the fungus Scytalidium cuboideum. Anal. Chem. In Review. 2018. [Google Scholar]
- Robinson, S.C.; Tudor, D.; Cooper, P.A. Feasibility of using red pigment producing fungi to stain wood for decorative applications. Can. J. For. Res. 2011, 41, 1722–1728. [Google Scholar] [CrossRef]
- Robinson, S.C.; Hinsch, E.; Weber, G.; Leipus, K.; Cerney, D. Wood colorization through pressure treating: The potential of extracted colorants from spalting fungi as a replacement for woodworkers’ aniline dyes. Materials 2014, 7, 5427–5437. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.C.; Weber, G.; Hinsch, E.; Vega Gutierrez, S.M.; Pittis, L.; Freitas, S. Utilizing extracted fungal pigments for wood spalting: A comparison of induced fungal pigmentation to fungal dyeing. J. Coat. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Vega Gutierrez, S.; Vega Gutierrez, P.; Godinez, A.; Pittis, L.; Huber, M.; Stanton, S.; Robinson, S. Feasibility of coloring bamboo with the application of natural and extracted fungal pigments. Coatings 2016, 6, 37. [Google Scholar] [CrossRef]
- Weber, G.; Chen, H.-L.; Hinsch, E.; Freitas, S.; Robinson, S. Pigments extracted from the wood-staining fungi chlorociboria aeruginosa, scytalidium cuboideum, and S. ganodermophthorum show potential for use as textile dyes. Color. Technol. 2014, 130, 445–452. [Google Scholar] [CrossRef]
- Hinsch, E.M.; Robinson, S.C. Mechanical color reading of wood-staining fungal pigment textile dyes: An alternative method for determining colorfastness. Coatings 2016, 6, 25. [Google Scholar] [CrossRef]
- Palomino Agurto, M.E.; Vega Gutierrez, S.M.; Chen, H.-L.; Robinson, S.C. Wood-rotting fungal pigments as colorant coatings on oil-based textile dyes. Coatings 2017, 7, 152. [Google Scholar] [CrossRef]
- Lindquist, M. Spalted wood. Rare jewels from death and decay. Fine Woodw. 1977, 7, 50–53. [Google Scholar]
- Rath, J.J. Organic Semiconductors: Incorporating Xylindein into (opto) Electronic Devices. Ph.D. Thesis, Oregon State University, Corvallis, OR, USA, 2016. [Google Scholar]
- Robinson, S.C.; Vega Gutierrez, S.M.; Cespedes Garcia, R.A.; Iroume, N.; Vorland, N.R.; McClelland, A.; Huber, M.; Stanton, S. Potential for carrying dyes derived from spalting fungi in natural oils. J. Coat. Technol. Res. 2017, 14, 1107–1113. [Google Scholar] [CrossRef]
- Raggio, O.; Wilmering, A.M. The Gubbio Studiolo and Its Conservation: Italian Renaissance Intarsia and the Conservation of the Gubbio Studiolo; Metropolitan Museum of Art: New York, NY, USA, 1999; Volume 2. [Google Scholar]
- Robinson, S.C.; Hinsch, E.; Weber, G.; Freitas, S. Method of extraction and resolubilisation of pigments from chlorociboria aeruginosa and scytalidium cuboideum, two prolific spalting fungi. Color. Technol. 2014, 130, 221–225. [Google Scholar] [CrossRef]
- Alguacil, J.; Kauppinen, T.; Porta, M.; Partanen, T.; Malats, N.; Kogevinas, M.; Benavides, F.G.; Obiols, J.; Bernal, F.; Rifà, J. Risk of pancreatic cancer and occupational exposures in spain. Ann. Occup. Hyg. 2000, 44, 391–403. [Google Scholar] [CrossRef]
- Beck, H.G.; Freitas, S.; Weber, G.; Robinson, S.C.; Morrell, J.J. Resistance of fungal derived pigments to ultraviolet light exposure. In International Research Group in Wood Protection; International Research Group/Wood Protection: St George, UT, USA, 2014. [Google Scholar]
- Weber, G.L.; Boonloed, A.; Naas, K.M.; Koesdjojo, M.T.; Remcho, V.T.; Robinson, S.C. A method to stimulate production of extracellular pigments from wood-degrading fungi using a water carrier. Curr. Res. Environ. Appl. Mycol. 2016, 6, 218–230. [Google Scholar] [CrossRef]
- Robinson, S.C.; Laks, P.E.; Turnquist, E.J. A method for digital color analysis of spalted wood using scion image software. Materials 2009, 2, 62–75. [Google Scholar] [CrossRef]
- Hansen, C.M. The three-dimensional solubility parameter-key to paint component affinities: Solvents, plasticizers, polymers, and resins. II. Dyes, emulsifiers, mutual solubility and compatibility, and pigments. III. Independent cal-culation of the parameter components. J. Paint Technol. 1967, 39, 505–510. [Google Scholar]
- Hansen, C.M. Hansen Solubility Parameters: A User’s Handbook; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Barwick, V.J. Strategies for solvent selection—A literature review. TrAC Trends Anal. Chem. 1997, 16, 293–309. [Google Scholar] [CrossRef]
- LSU. Polarity Index. Available online: http://macro.lsu.edu/howto/solvents/Polarity%20index.htm (accessed on 9 December 2017).
- Young, J.A. Dichloromethane. J. Chem. Educ. 2004, 81, 1415. [Google Scholar] [CrossRef]
- Britt, P.F. Pyrolysis and Combustion of Acetonitrile (ch {sub 3} cn); ORNL Oak Ridge National Laboratory (US): Oak Ridge, TN, USA, 2002.
- Young, J.A. Pyridine. J. Chem. Educ. 2009, 86, 684. [Google Scholar] [CrossRef]
- EPA. Health Effects Notebook for Hazardous Air Pollutants. Available online: http://www3.epa.gov/airtoxics/hlthef/acetonit.html (accessed on 12 December 2017).
- Robinson, S.C.; Vega Gutierrez, S.M.; Cespedes Garcia, R.A.; Iroume, N.; Vorland, N.R.; Andersen, C.; de Oliveira Xaxa, I.D.; Kramer, O.E.; Huber, M.E. Potential for fungal dyes as colorants in oil and acrylic paints. J. Coat. Technol. Res. 2018, 1–5. [Google Scholar] [CrossRef]

| Wood | Solvent | Treatment | Pigment | Mean Percent Coverage | Standard Deviation |
|---|---|---|---|---|---|
| Chinkapin | Acetone | 16w | Red | 27 (CDEFG) | 10.25 |
| Green | 6.67 (EFG) | 2.01 | |||
| 30w | Red | 26.67 (CDEFG) | 9.63 | ||
| Green | 0 (G) | 0 | |||
| 30 | Red | 92.33 (ABC) | 29.35 | ||
| Green | 0 (G) | 0 | |||
| 60w | Red | 100 (A) | 32.76 | ||
| Green | 8.33 (EFG) | 3.14 | |||
| Acetonitrile | 16w | Red | 26.33 (CDEFG) | 8.82 | |
| Green | 0 (G) | 0 | |||
| 30w | Red | 72.33 (ABCDE) | 23.8 | ||
| Green | 0 (G) | 0 | |||
| 30 | Red | 98.67 (AB) | 31.17 | ||
| Green | 0 (G) | 0 | |||
| 60w | Red | 62.67 (ABCDEFG) | 19.7 | ||
| Green | 0 (G) | 0 | |||
| Chloroform | 16w | Red | 1 (FG) | 0.5 | |
| Green | 11.67 (EFG) | 4.21 | |||
| 30w | Red | 24.33 (CDEFG) | 7.74 | ||
| Green | 23 (CDEFG) | 6.49 | |||
| 30 | Red | 1.33 (FG) | 0.5 | ||
| Green | 7.67 (EFG) | 2.67 | |||
| 60w | Red | 60 (ABCDEFG) | 18.15 | ||
| Green | 3 (FG) | 0.96 | |||
| THF | 16w | Red | 38.33 (ABCDEFG) | 12.88 | |
| Green | 0 (G) | 0 | |||
| 30w | Red | 65.67 (ABCDEFG) | 21.16 | ||
| Green | 0 (G) | 0 | |||
| 30 | Red | 59 (ABCDEFG) | 16.42 | ||
| Green | 28.67 (CDEFG) | 10.85 | |||
| 60w | Red | 85 (ABCD) | 25.75 | ||
| Green | 10 (EFG) | 3.67 | |||
| Pyridine | 16w | Red | 89.33 (ABCD) | 27.62 | |
| Green | 13.33 (EFG) | 4.78 | |||
| 30w | Red | 67 (ABCDEF) | 22.43 | ||
| Green | 29.33 (BCDEFG) | 11.36 | |||
| 30 | Red | 46.67 (ABCDEFG) | 14.26 | ||
| Green | 32.67 (BCDEFG) | 11.98 | |||
| 60w | Red | - | - | ||
| Green | - | - |
| Wood | Solvent | Treatments | Pigment | Mean Percent Coverage | Standard Deviation |
|---|---|---|---|---|---|
| Madrone | Acetone | 16w | Red | 88.33 (A) | 62.46 |
| Green | 93.33 (A) | 65.99 | |||
| 30w | Red | 20 (BC) | 14.14 | ||
| Green | 53.33 (ABC) | 37.71 | |||
| 30 | Red | 90 (A) | 63.64 | ||
| Green | 40 (ABC) | 28.28 | |||
| 60w | Red | 100 (A) | 70.71 | ||
| Green | 96.67 (A) | 68.36 | |||
| Acetonitrile | 16w | Red | 68.33 (AB) | 48.32 | |
| Green | 96.67 (A) | 68.36 | |||
| 30w | Red | 100 (A) | 70.71 | ||
| Green | 86.67 (A) | 61.28 | |||
| 30 | Red | 85 (A) | 60.1 | ||
| Green | 83.33 (AB) | 58.92 | |||
| 60w | Red | 60 (ABC) | 42.43 | ||
| Green | 43.33 (ABC) | 30.64 | |||
| Chloroform | 16w | Red | 0 (C) | 0 | |
| Green | 0 (C) | 0 | |||
| 30w | Red | 86.67 (A) | 61.28 | ||
| Green | 0 (C) | 0 | |||
| 30 | Red | 93.33 (A) | 65.99 | ||
| Green | 0 (C) | 0 | |||
| 60w | Red | 53.33 (ABC) | 37.71 | ||
| Green | 0 (C) | 0 | |||
| THF | 16w | Red | 0 (C) | 0 | |
| Green | 0 (C) | 0 | |||
| 30w | Red | 0 (C) | 0 | ||
| Green | 0 (C) | 0 | |||
| 30 | Red | 0 (C) | 0 | ||
| Green | 0 (C) | 0 | |||
| 60w | Red | 0 (C) | - | ||
| Green | 0 (C) | 0 | |||
| Pyridine | 16w | Red | 83.33 (AB) | 58.92 | |
| Green | 0 (C) | 0 | |||
| 30w | Red | 0 (C) | 0 | ||
| Green | 0 (C) | 0 | |||
| 30 | Red | 85 (A) | 60.1 | ||
| Green | 0 (C) | 0 | |||
| 60w | Red | - | - | ||
| Green | - | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pittis, L.; Rodrigues de Oliveira, D.; Vega Gutierrez, S.M.; Robinson, S.C. Alternative Carrier Solvents for Pigments Extracted from Spalting Fungi. Materials 2018, 11, 897. https://doi.org/10.3390/ma11060897
Pittis L, Rodrigues de Oliveira D, Vega Gutierrez SM, Robinson SC. Alternative Carrier Solvents for Pigments Extracted from Spalting Fungi. Materials. 2018; 11(6):897. https://doi.org/10.3390/ma11060897
Chicago/Turabian StylePittis, Lauren, Diego Rodrigues de Oliveira, Sarath M. Vega Gutierrez, and Seri C. Robinson. 2018. "Alternative Carrier Solvents for Pigments Extracted from Spalting Fungi" Materials 11, no. 6: 897. https://doi.org/10.3390/ma11060897
APA StylePittis, L., Rodrigues de Oliveira, D., Vega Gutierrez, S. M., & Robinson, S. C. (2018). Alternative Carrier Solvents for Pigments Extracted from Spalting Fungi. Materials, 11(6), 897. https://doi.org/10.3390/ma11060897






