Fast and Cost-Effective Synthesis of High-Quality Graphene on Copper Foils Using High-Current Arc Evaporation
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Parameter Optimization
3.2. Results for Optimized Parameters
3.3. Transfer and Comparison to CVD-Graphene
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferrari, A.C.; Bonaccorso, F.; Fal’Ko, V.; Novoselov, K.S.; Roche, S.; Bøggild, P.; Borini, S.; Koppens, F.H.L.; Palermo, V.; Pugno, N.; et al. Science and technology roadmap for graphene, related two-dimensional crystals, and hybrid systems. Nanoscale 2015, 7, 4598–4810. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cai, W.; An, J.; Kim, S.; Nah, J.; Yang, D.; Piner, R.; Velamakanni, A.; Jung, I.; Tutuc, E.; et al. Large-Area Synthesis of High-Quality and Uniform graphene Films on Copper Foils. Science 2009, 324, 1312–1314. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, L.; Chen, S. Large area CVD growth of graphene. Synth. Met. 2015, 210, 95–108. [Google Scholar] [CrossRef]
- Woehrl, N.; Ochedowski, O.; Gottlieb, S.; Shibasaki, K.; Schulz, S. Plasma-enhanced chemical vapor deposition of graphene on copper substrates. AIP Adv. 2014, 4, 047128. [Google Scholar] [CrossRef]
- Li, M.; Liu, D.; Wei, D.; Song, X.; Wei, D.; Wee, A.T.S. Controllable Synthesis of Graphene by Plasma-Enhanced Chemical Vapor Deposition and Its Related Applications. Adv. Sci. 2016, 3, 1600003. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Kim, H.; Lee, Y.; Xu, X.; Park, J.-S.; Zheng, Y.; Balakrishnan, J.; Lei, T.; Kim, H.R.; Song, Y.I.; et al. Roll-to-roll production of 30-inch graphene films for transparent electrodes. Nat. Nanotechnol. 2010, 5, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Polsen, E.S.; McNerny, D.Q.; Viswanath, B.; Pattinson, S.W.; Hart, A.J. High-speed roll-to-roll manufacturing of graphene using a concentric tube CVD reactor. Sci. Rep. 2015, 5, 10257. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.W.; Raitses, Y.; Yao, N. Structural variations of the cathode deposit in the carbon arc. Carbon 2016, 105, 490–495. [Google Scholar] [CrossRef]
- Sun, D.L.; Hong, R.Y.; Liu, J.Y.; Wang, F.; Wang, Y.F. Preparation of carbon nanomaterials using two-group arc discharge plasma. Chem. Eng. J. 2016, 303, 217–230. [Google Scholar] [CrossRef]
- Subrahmanyam, K.S.; Panchakarla, L.S.; Govindaraj, A.; Rao, C.N.R. Simple Method of Preparing Graphene Flakes by an Arc-Discharge Method. J. Phys. Chem. Lett. C 2009, 113, 4257–4259. [Google Scholar] [CrossRef]
- Wang, Z.; Li, N.; Shi, Z.; Gu, Z. Low-cost and large-scale synthesis of graphene nanosheets by arc discharge in Air. Nanotechnology 2010, 21, 175602. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, Y.; Yang, H.; Shi, Z. Large-Scale Synthesis of High-Quality Graphene Sheets by Improved Alternating Current Arc-discharge Method. RSC Adv. 2016, 6, 93119–93124. [Google Scholar] [CrossRef]
- Petereit, B.; Siemroth, P.; Schneider, H.H.; Hilgers, H. High current filtered arc deposition for ultra-thin carbon overcoats on magnetic hard disks and read-write heads. Surf. Coat. Technol. 2003, 648, 174–175. [Google Scholar] [CrossRef]
- Ferrari, A.C. Diamond-like carbon for magnetic storage disks. Surf. Coat. Technol. 2004, 180–181, 190–206. [Google Scholar] [CrossRef]
- Ilie, A.; Ferrari, A.C.; Yagi, T.; Rodil, S.E.; Robertson, J.; Barborini, E.; Milani, P. Role of sp2 phase in field emission from nanostructured carbons. J. Appl. Phys. 2001, 90, 2024–2032. [Google Scholar] [CrossRef]
- Zhang, T.F.; Kim, K.-W.; Kimb, K.H. Nitrogen-Incorporated Hydrogenated Amorphous Carbon Film, Electrodes on Ti Substrates by Hybrid Deposition Technique and Annealing. J. Electrochem. Soc. 2016, 163, E54–E61. [Google Scholar] [CrossRef]
- Lux, H.; Siemroth, P.; Sgarlata, A.; Prosposito, P.; Schubert, M.A.; Casalboni, M.; Schrader, S. Synthesis of graphene-like transparent conductive films on dielectric substrates using a modified filtered vacuum arc system. J. Appl. Phys. 2015, 117, 195304. [Google Scholar] [CrossRef]
- Scheibe, H.J.; Schultrich, B.; Ziegele, H.; Siemroth, P. Deposition of superhard amorphous carbon films by pulsed arc sources. IEEE Trans. Plasma Sci. 1997, 25, 685–688. [Google Scholar] [CrossRef]
- Petzow, G. Metallographisches, Keramographisches, Plastographiches Ätzen; Gebrüder Borntraeger Berlin-Stuttgart: Berlin, Germany, 2006; ISBN 978-3443230166. [Google Scholar]
- Chen, Y.; Gong, X.-L.; Gai, J.-G. Progress and Challenges in Transfer of Large-Area Graphene Films. Adv. Sci. 2016, 3, 1500343. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zheng, Y.; Xu, X.; Dubuisson, E.; Bao, Q.; Lu, J.; Loh, K.P. Electrochemical Delamination of CVD Grown Graphene Film: Toward the Recyclable Use of Copper Catalyst. ACS Nano 2011, 5, 9927–9933. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C.; Basko, D.M. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Bonaccorso, F.; Sun, Z.; Hasan, T.; Ferrari, A.C. Graphene photonics and optoelectronics. Nat. Photonics 2010, 4, 611–622. [Google Scholar] [CrossRef]
- Deokar, G.; Avila, J.; Razado-Colambo, I.; Codron, J.L.; Boyaval, C.; Galopin, E.; Asensio, M.-C.; Vignaud, D. Towards high quality CVD graphene growth and transfer. Carbon 2015, 89, 82–92. [Google Scholar] [CrossRef]

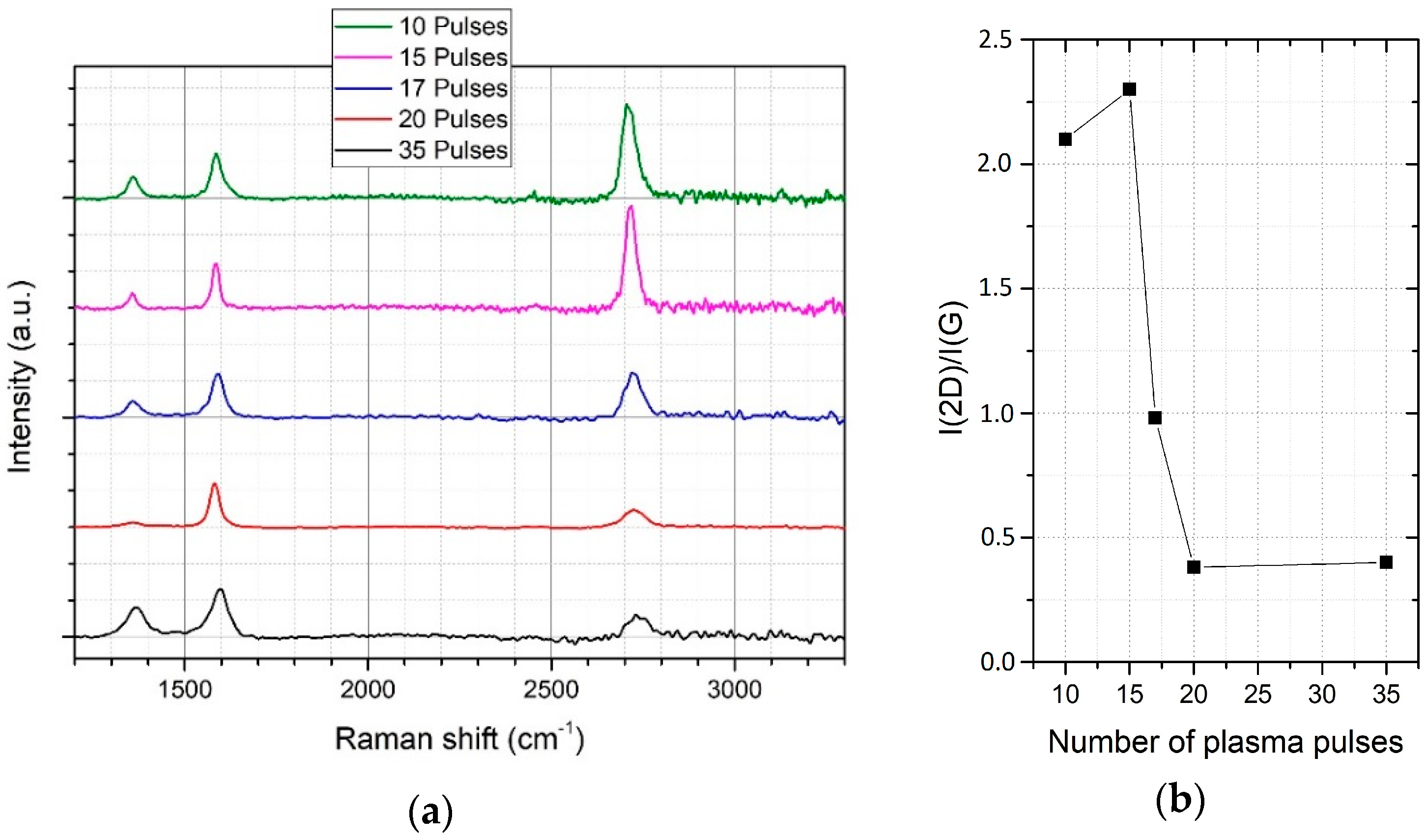
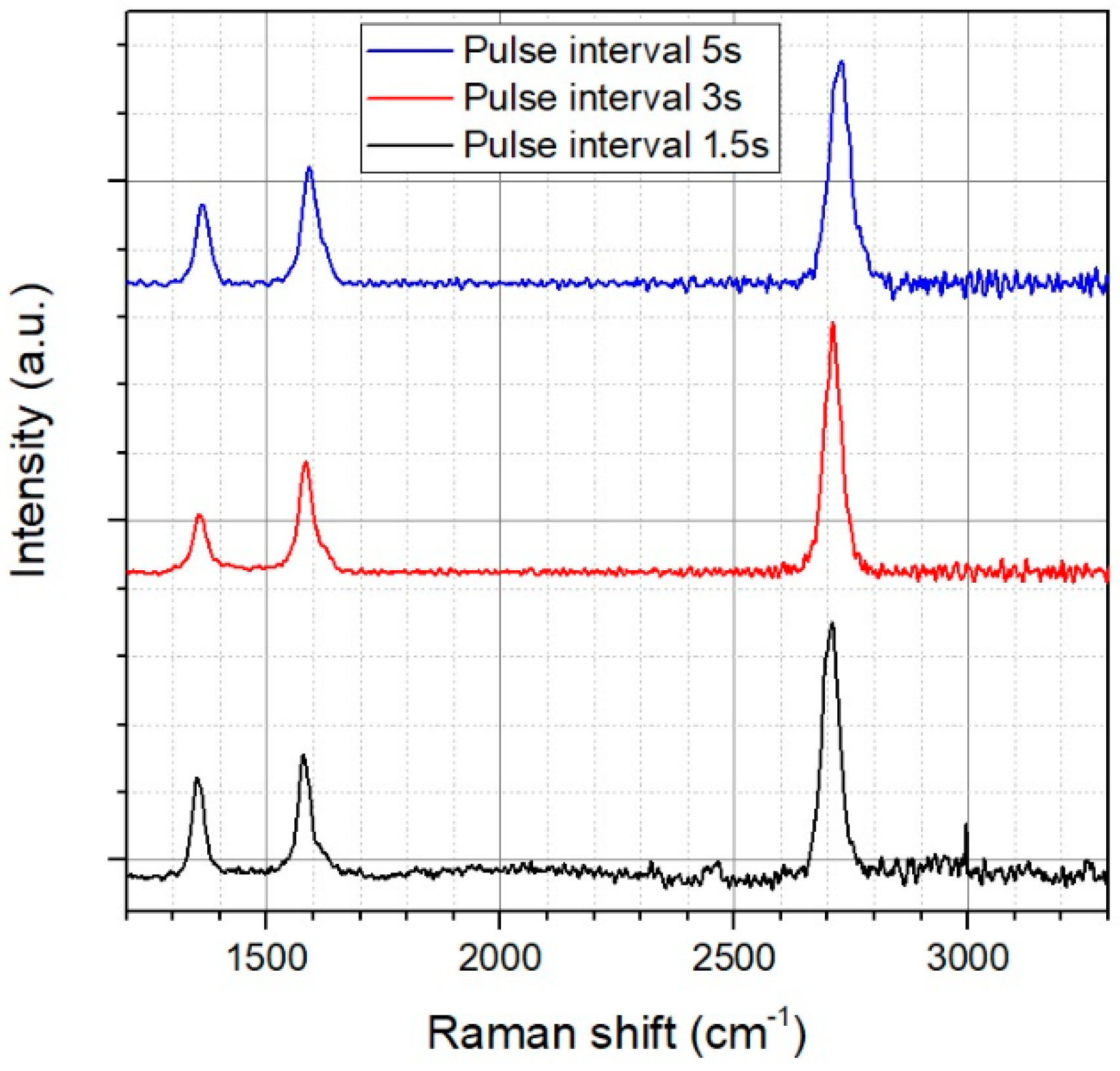
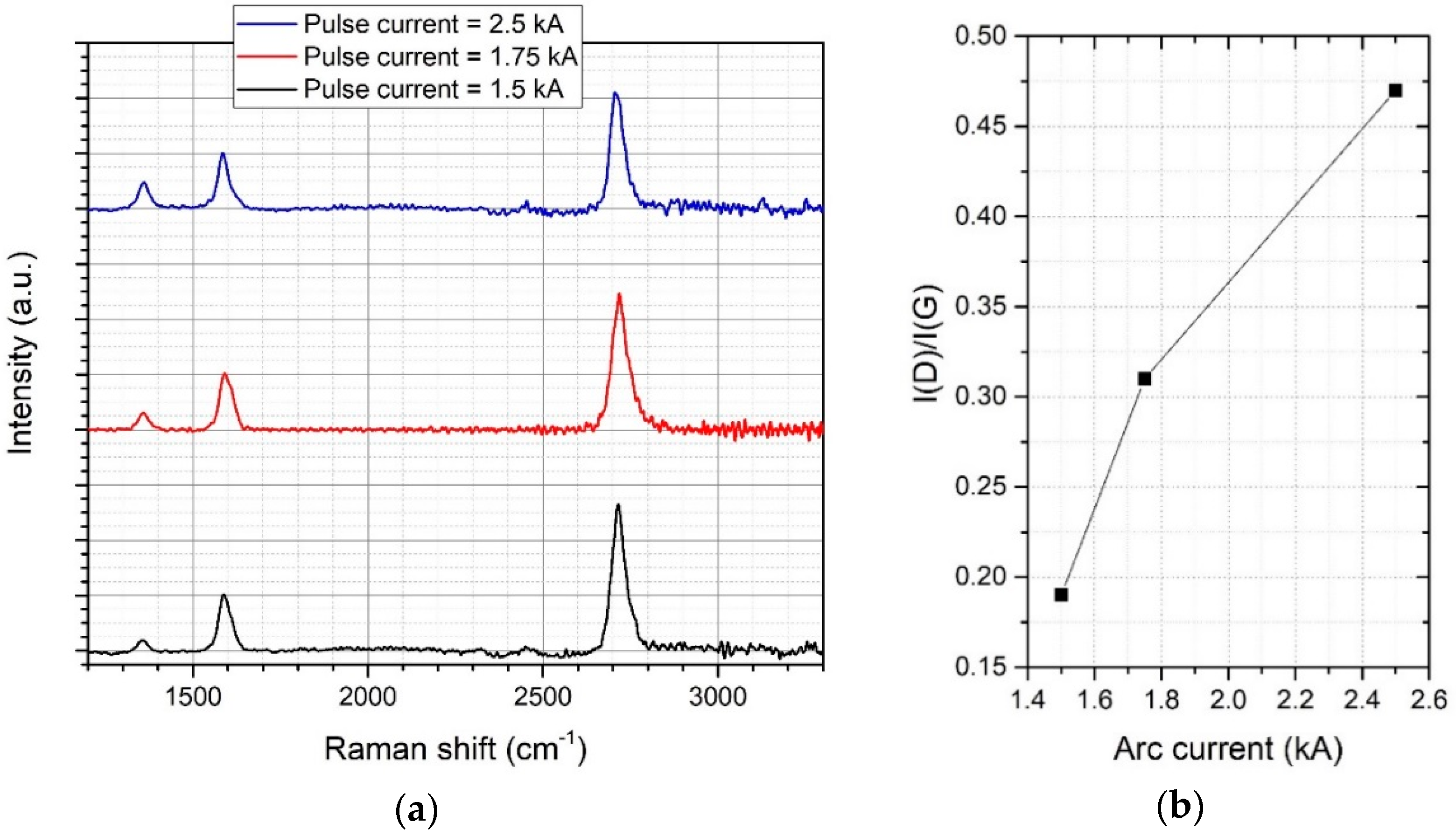
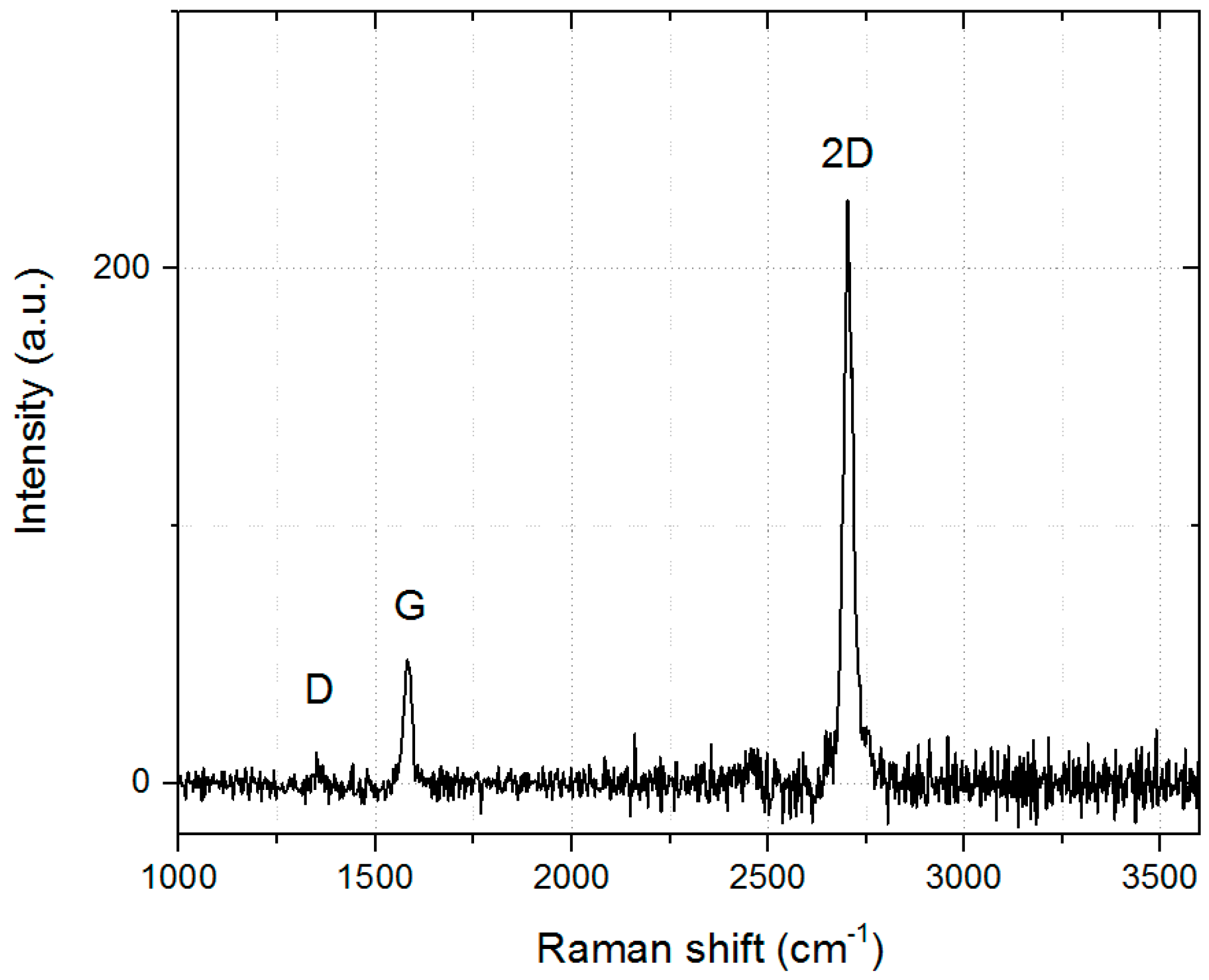


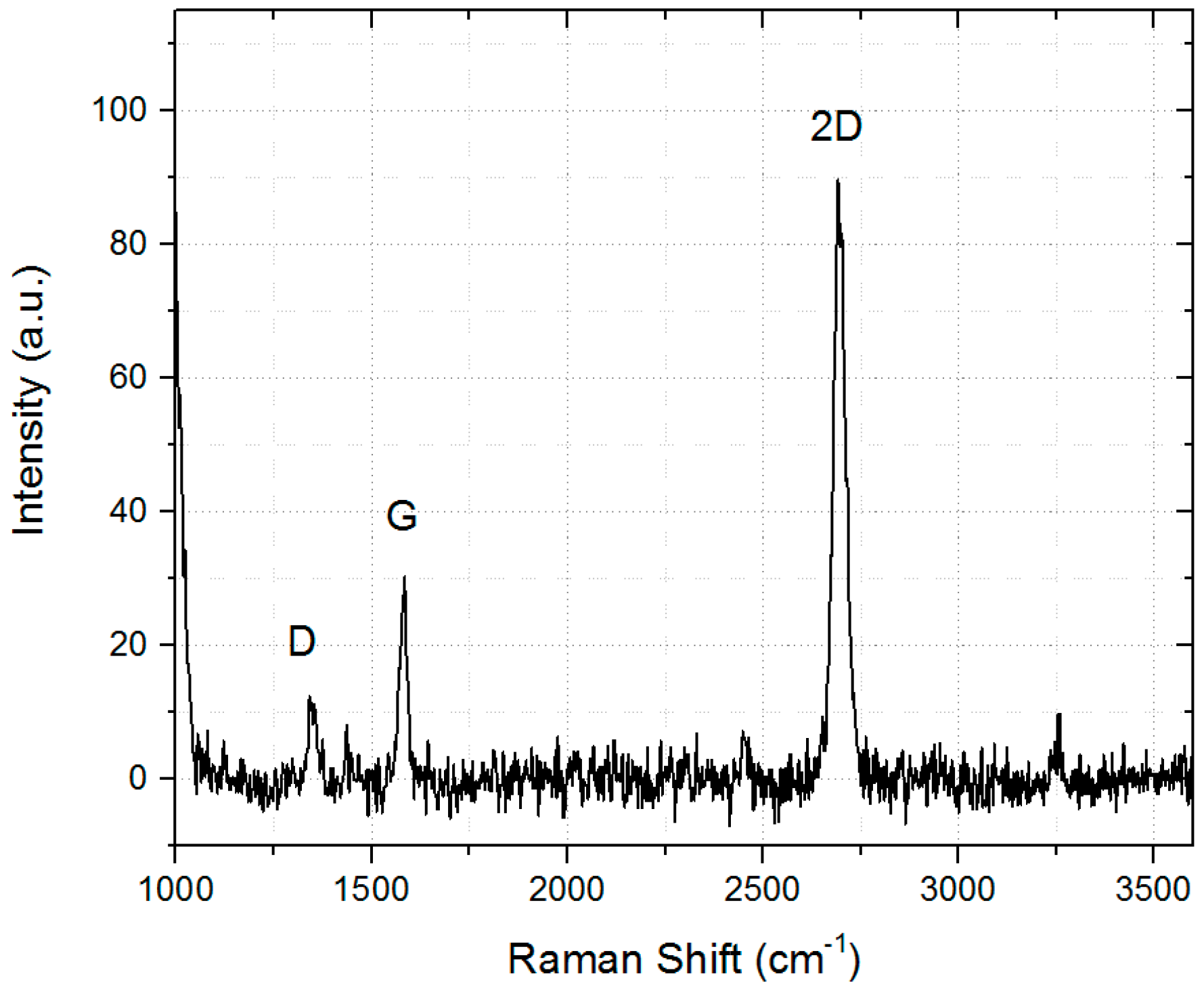

| Raman Peak | Max. Position | FWHM |
|---|---|---|
| G | 1581 cm−1 | 24 cm−1 |
| 2D | 2703 cm−1 | 28 cm−1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lux, H.; Edling, M.; Siemroth, P.; Schrader, S. Fast and Cost-Effective Synthesis of High-Quality Graphene on Copper Foils Using High-Current Arc Evaporation. Materials 2018, 11, 804. https://doi.org/10.3390/ma11050804
Lux H, Edling M, Siemroth P, Schrader S. Fast and Cost-Effective Synthesis of High-Quality Graphene on Copper Foils Using High-Current Arc Evaporation. Materials. 2018; 11(5):804. https://doi.org/10.3390/ma11050804
Chicago/Turabian StyleLux, Helge, Matthias Edling, Peter Siemroth, and Sigurd Schrader. 2018. "Fast and Cost-Effective Synthesis of High-Quality Graphene on Copper Foils Using High-Current Arc Evaporation" Materials 11, no. 5: 804. https://doi.org/10.3390/ma11050804
APA StyleLux, H., Edling, M., Siemroth, P., & Schrader, S. (2018). Fast and Cost-Effective Synthesis of High-Quality Graphene on Copper Foils Using High-Current Arc Evaporation. Materials, 11(5), 804. https://doi.org/10.3390/ma11050804




