1. Introduction
The emission of greenhouse gases poses a challenge on governments, researchers, and the population around the world because of its possible effects on the planet climate change. As a result, in November 2017, COP23 was held in Germany, when strategies to reach the goals of the global action plan to combat global warming were discussed, aiming at efforts to limit the Earth’s temperature increase to below 2 °C.
Anthropogenically generated CO
2 is considered to be one of the major greenhouse gases responsible for global warming, primarily due to the combustion of fossil fuels for energy production, which accounts for more than 65% of global CO
2 emissions [
1,
2]. At this scenario, large sources of greenhouse gases (GHG) come from burning fossil fuels, like petroleum, mineral coal, and natural gas, all of them arising mainly from the energy, industry, and transportation sectors [
3]. Thereby, Carbon Capture, Utilization, and Storage (CCUS) applied to flue gases is expected to be a viable alternative to reduce the emissions of CO
2, which is a major GHG [
4,
5,
6]. Thus, preventive and remedial methods to deal with those emissions are currently under investigation, among which stand out absorption, cryogenic, and adsorption processes.
Absorption processes utilizing liquid amines show high rates of carbon capture and are widely used in industrial scale, however, there are disadvantages that are associated to their corrosive potential, such as the high amount of energy required for amine regeneration and amine losses during operation [
7,
8]. Therefore, other technologies for CO
2 separation from flue gas have been sought. Porous solid adsorbents have been widely investigated as a medium for CO
2 separation. Among these adsorbents, zeolites 4A, 13X, ZSM-5 [
9,
10,
11], activated carbons [
12,
13,
14], and Metal Organic frameworks (MOF’S) [
15,
16,
17] have been considered for low temperature applications. However, these adsorbents suffer from a rapid decline in adsorption capacities, with increases in temperature despite their high CO
2 adsorption capacities at room temperature. In addition, their selectivity for CO
2 in the presence of other gases, such as N
2, is low. The high energy input that is required to regenerate some microporous adsorbents (e.g., zeolites) is also a serious disadvantage. Therefore, more selective and efficient CO
2 adsorbents have been widely investigated, as in the case of porous supports that are functionalized with organic molecules that contain amino groups. Grafting and impregnation are commonly used techniques to incorporate organic molecules that contain amino groups on mesoporous silica supports [
18,
19,
20,
21,
22,
23,
24,
25]. The efficiency of grafting is related to the availability of OH groups on the solid surface and the density of nitrogen in the grafted moiety [
18,
19,
20]. In spite of generally reaching lower incorporated nitrogen concentration than impregnation, the pending amino groups are generally easily accessible by CO
2. In impregnation, the organic load is much higher; however, because impregnated molecules are stacked inside narrow pores, there may be diffusional limitation. Amino groups may be less accessible, which leads to lower CO
2/N molar ratios [
21]. Sanz et al. [
22] reported a double-functionalized material with CO
2/ N up to 0.48, presenting a high efficiency of the incorporated amino groups for CO
2 adsorption and claimed its stability in vacuum and temperature, which makes the regeneration process easier.
Several spectroscopic techniques have been used to study CO
2−amine interactions, with Fourier Transform Infrared Spectroscopy (FT-IR) and Nuclear Magnetic Resonance (NMR) being the most outstanding [
23,
24,
25,
26]. Although these spectroscopic experiments are able to identify the nature of the active site, they are not applicable to measuring the energy distribution of sites in adsorption; as such, additional complementary techniques need to be explored.
Previous works have demonstrated that the measurement of adsorption isotherms via method manometric device in a customized Tian-Calvet calorimeter can be used successfully to measure the heats that evolved upon CO
2 adsorption [
27,
28]. It has been found that the textural characteristics of the support and the nature/ density of the functionalized moiety have significant effects on the heat of adsorption as a function of coverage. Using calorimetry, it has been shown that there are multiple amines to interact with one CO
2 molecule under dry conditions, forming strong alkylammonium carbamate species (~90 kJ mol
−1) [
29] when the amine density is sufficiently high (>1.5 mmol Ng
−1).
In this work, the changes in site energy distribution and kinetic mechanism have been assessed by adsorption microcalorimetry for mesoporous silicas that were previously grafted with (3-aminopropyl) triethoxysilane (APTES), and then impregnated with polyethyleneimine (PEI). The double functionalized and the simply grafted sample were also tested using a magnetic suspension balance at temperatures close to post combustion scenario in order to investigate their adsorption capacity at these conditions. At the end, the adsorbent with a high Adsorbent Performance Indicator was studied in three cycles of regeneration, in order to contrast the energy consumption that is required to reach complete desorption and the new adsorption capacity in isothermal condition after each adsorption/desorption cycle was measured.
3. Results
The results of the elemental analysis are summarized in
Table 1. These data indicate that nitrogen has been effectively incorporated to the pure MSS sample after functionalization step.
In addition to nitrogen content, another important result that confirms the presence of amino groups on the grafted and double functionalized samples is the increase of carbon amount. This increase is related to the incorporation of propyl groups of the APTES molecules or/and the alkyl chains of PEI.
Some amount of nitrogen is observed on the MSS sample; a possible explanation might be residual NH
4F that remained on the material from the synthesis procedure. There is a difference between the C/N ratio measured (~3.3) and expected (3.0) for the MSG20 sample, which is possibly due to the adsorbed atmospheric CO
2 that increases the carbon amount that is detected by the equipment, as confirmed by 13C-CP-MAS NMR experiments in previous works [
26,
43].
The Maximum Theoretical Adsorption Capacity (MTAC) by chemisorption is also summarized in
Table 1. The highest theoretical chemical adsorption was for MSG20I30 sample. This fact would probably improve the attractiveness of the solid for CO
2 adsorption.
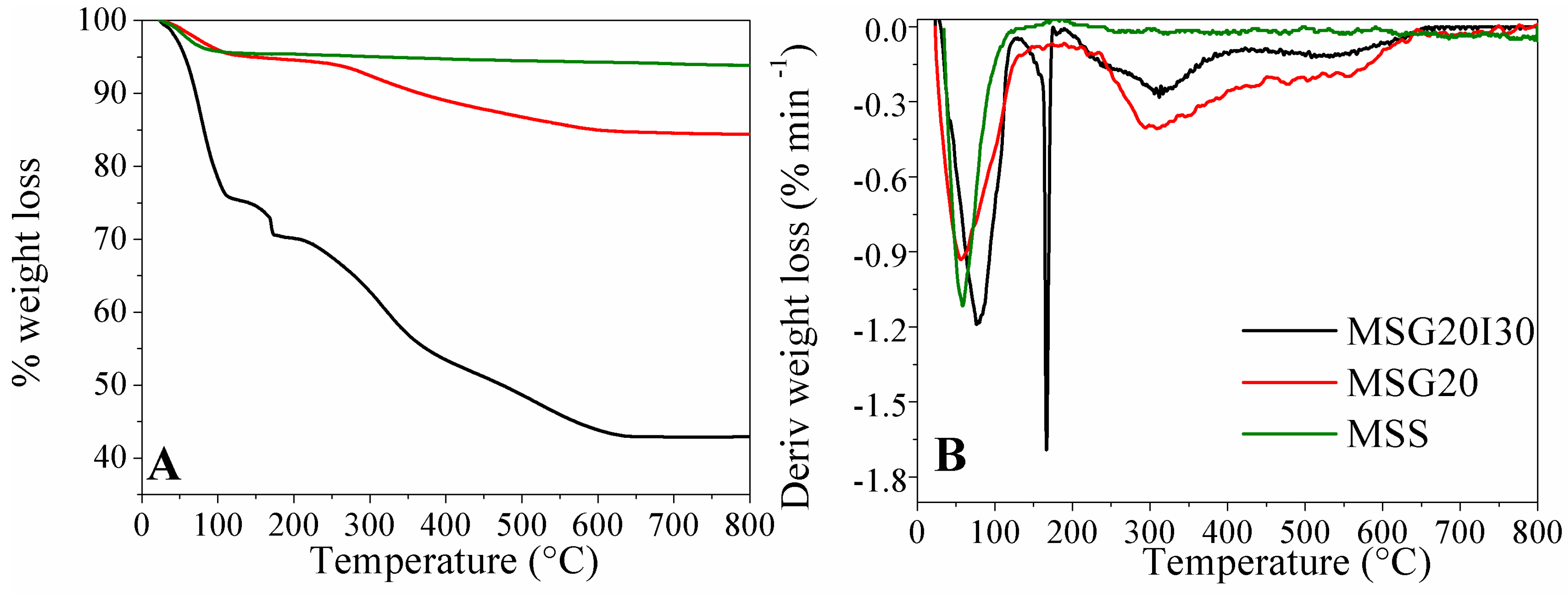
The results of the Thermogravimetric Analysis (TGA) are shown in the
Figure 1A.
Figure 1B presents the derivate of the weight loss (DTGA) for MSS, MSG20, and MSG20I30 samples.
For all samples, the initial weight loss at around 100 °C is mainly due to the loss of physisorbed water, corresponding to 5% for MSS, 10%, for MSG20 sample, and 25% for MSG20I30 samples (point 1 in
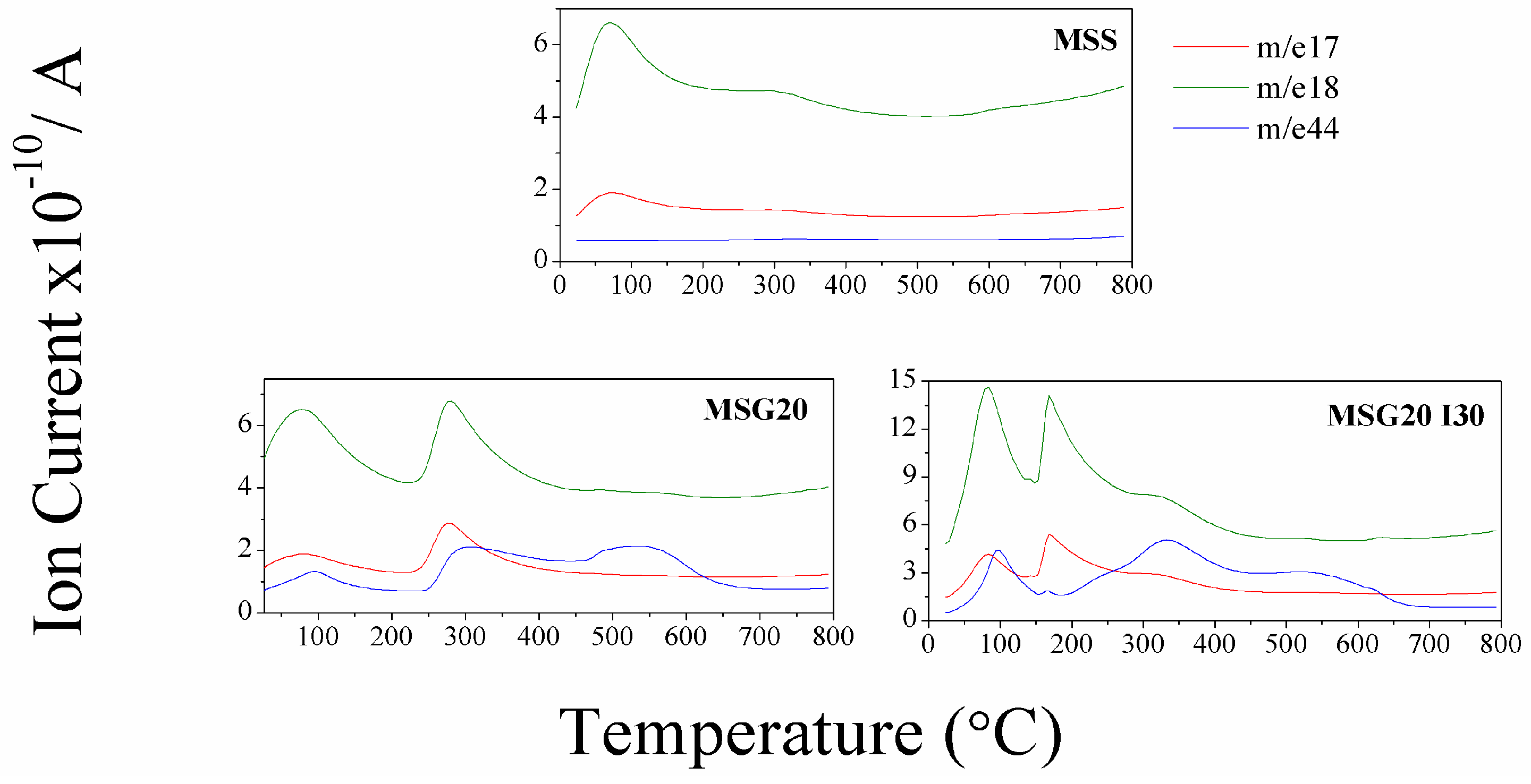
Figure 1). The mass spectra analysis, shown in
Figure 2, present the signals with
m/
z ratio of 17 and 18, which confirms the release of moisture. The difference between the weight loss in the samples can be explained with the additional
m/
z 44 that is found in the functionalized samples (MSG20 and MSG20I30), whcih is attributed to the release of the atmospheric CO
2 adsorbed in the material due to the presence of amine groups. This result is in agreement with the increase in %C observed in elemental analysis.
At 650 °C, the grafted amine was completely decomposed and was removed as volatiles. The organic content (loss weight from 150 up to 750 °C) of MSG20I30 was calculated to be about ~31 wt %, according to PEI load that was employed in the synthesis step. MSG20 has an organic content of around 10%, and this fact indicates that not all PEI dissolved was incorporated during the impregnation step. The maximum operating temperature for MSG20I30 sample would be ~150 °C, in order to avoid the decomposition of the material. This temperature is lower than that of MSG20 sample, in which case, the maximum temperature of operation is ~250 °C.
Low-angle X-ray powder patterns of mesoporous silica MSS, MSG20, and MSG20I30 samples are shown in
Figure 3A. The compiled diffractograms are contrasted with a conventional SBA-15 that was previously reported in the literature [
44].
Conventional hydrothermal SBA-15 shows a typical XRD pattern of an ordered network of mesopores with (100), (110), and (200) reflections, which are typical of a hexagonal symmetry [
44,
45]. The characteristic reflections of SBA-15 are not present in our samples. Vilarrasa et al. (2014) [
46] and Liu et al. (2012) [
47] showed similar behavior as a characteristic of Mesocellular Foam Structure (MSF). The presence of ammonium fluoride on the synthesis process might have affected the hexagonal arrangement of the solid, thus limiting the growth of the mesochannels and leading to shorter channels with low-range order. Ammonium fluoride was employed in the synthesis as a pore swelling agent in order to provide more space for surface functionalization. Many authors [
48,
49] attribute the conversion of ordered arrangement to mesocellular structure to this type of “precursors”, in which an increase in the pore size is caused by NH
4F penetration into the hydrophobic core of the surfactant micelle, thus breaking up the typical honeycomb packing of the hydrothermal SBA-15. This fact could be the reason why no noticeable diffraction signals are observed at low-angle.
Transmission electron micrographs (
Figure 3(B1,B2)) show that effectively the addition of NH
4F prevents the typical hexagonal arrangement of SBA-15, leading to a mesocellular foam structure [
46].
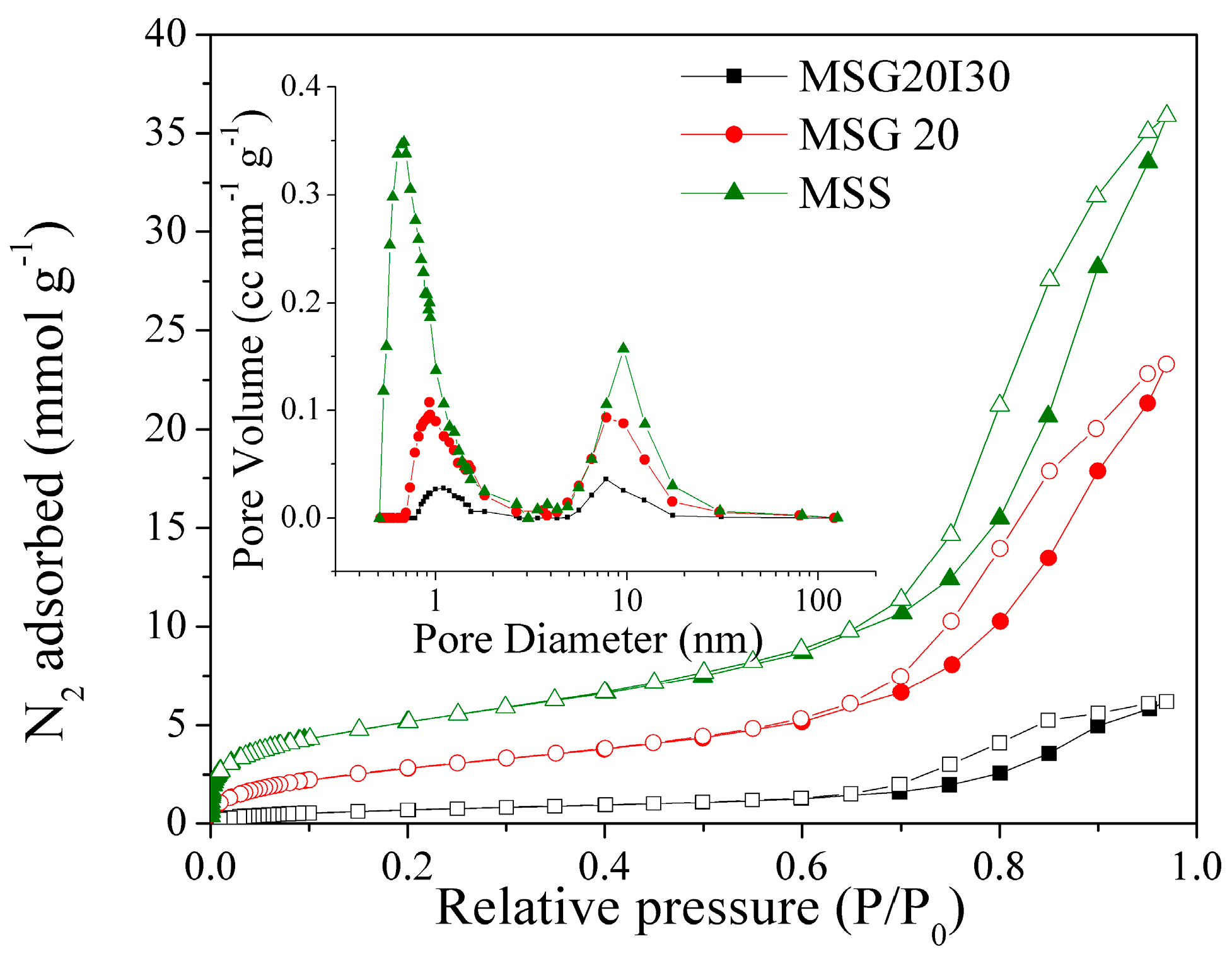
The N
2 adsorption/desorption isotherms at −196 °C are shown in
Figure 4. All of the samples have a H2(b) type hysteresis [
50], which is associated with mesocellular silica foams (MSF) [
49], leading to a shift of the hysteresis loop to a higher relative pressure. After the immobilization of PEI on MSG20, the total pore volume was reduced from 0.96 to 0.06 cm
3 g
−1 (see
Table 2). The specific surface area was also reduced dramatically from 211 to 52 m
2g
−1, which is expected due to the filling of pores with PEI, decreasing the surface area, micropore, and the total pore volume. The Pore Size Distributions (PSD) for all of the samples in logarithmic scale (inset of
Figure 4) show a bimodal distribution with a smaller pore size of ~1 nm (micropores) and larger pores with sizes of around 7.8 nm, confirming that our materials remain mesoporous after the functionalization step.
Textural properties are summarized in
Table 2. Textural properties decrease as N content increases, which is indicative that amine groups has been effectively incorporated to the bulk MSS sample.
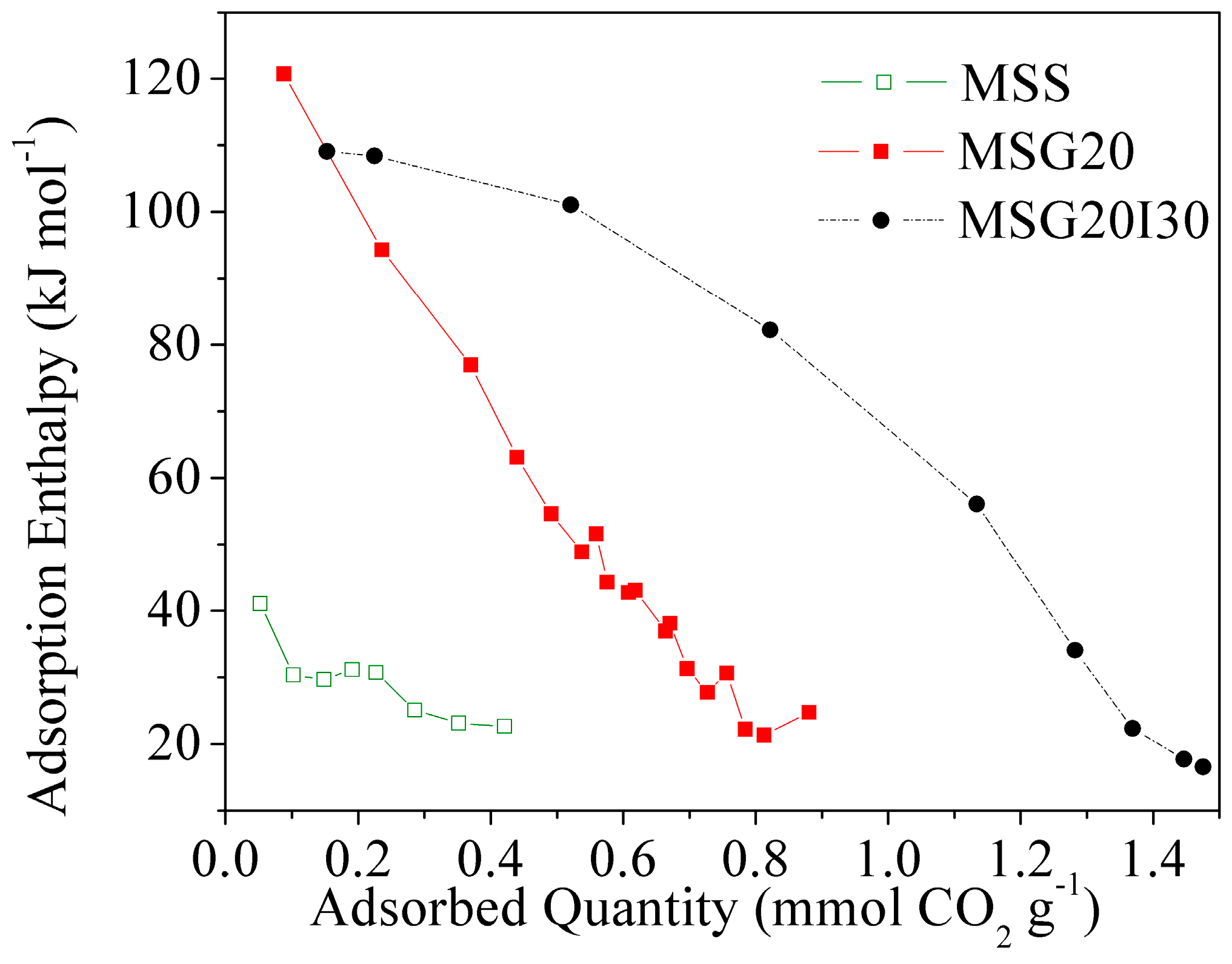
Figure 5 shows CO
2 adsorption microcalorimetric curves of samples at 25 °C under anhydrous conditions. The samples show a decrease in the differential enthalpy with an increasing CO
2 uptake, which suggests that they have a heterogeneous surface, according to the classification that was proposed by Rouquerol et al. [
39].
These curves show that, for the two functionalized samples, the initial enthalpy values are in the range of ~110–120 kJ mol
−1. Thus, we can consider that this relatively high enthalpy value is due to the interaction of CO
2 with grafted and/or impregnated amines. Namely, the chemisorption of CO
2 on amine pairs to form propyl ammonium carbamate species has an adsorption enthalpy of ~−90 kJ mol
−1 [
51].
Although the enthalpy at a low coverage is similar, there is a remarkable change of sites energy with the addition of PEI.
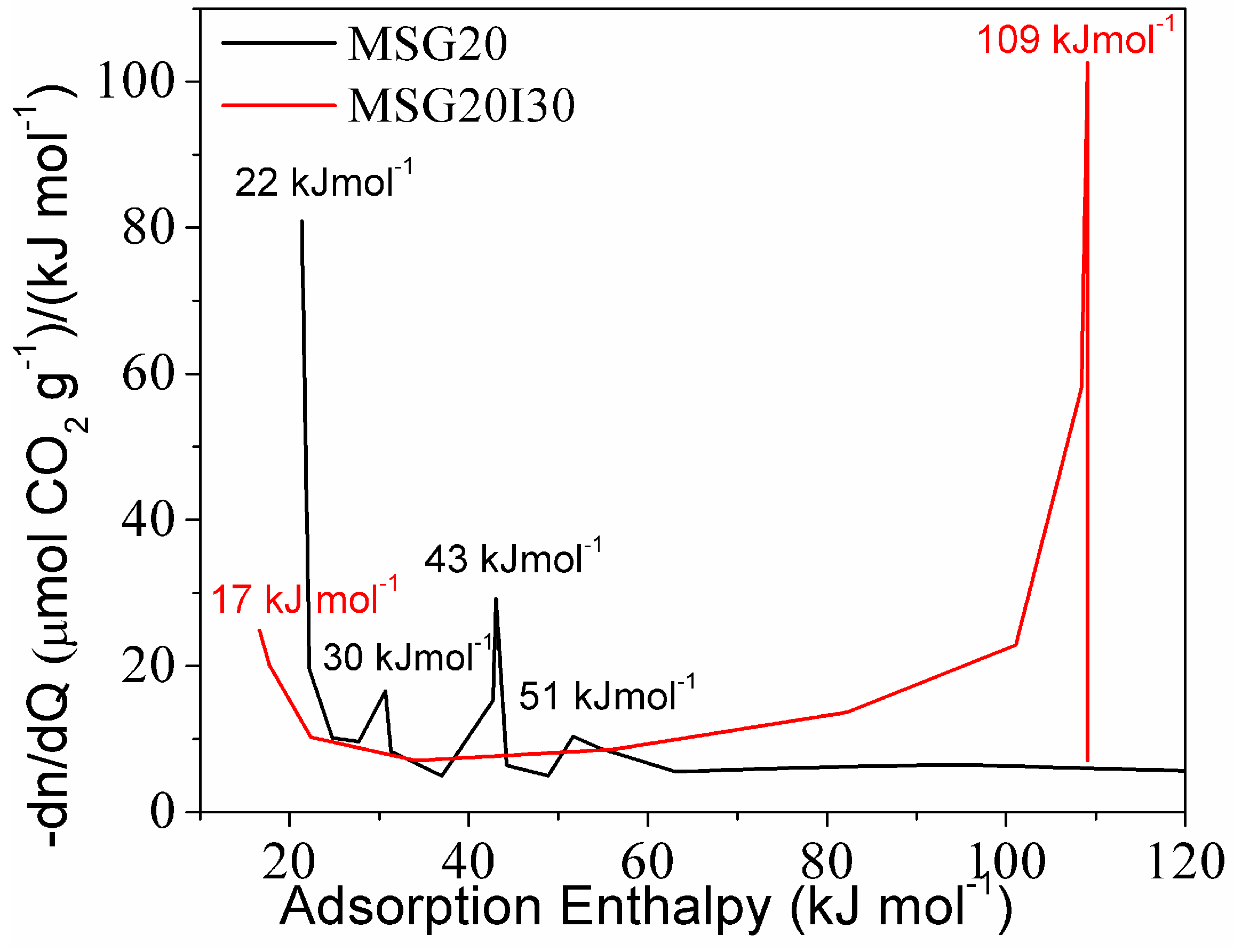
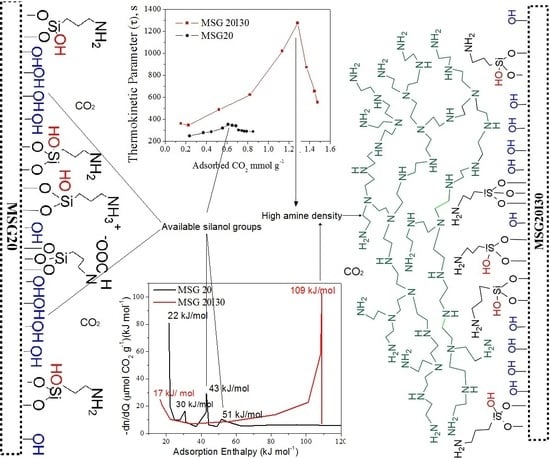
For a better appreciation of the change of adsorption mechanism on the double functionalized solid, the distribution of active sites adsorbing CO
2 on the samples is shown in
Figure 6. Peaks in the distribution represent the frequency of sites with the same energy of adsorption. In the case of chemisorption, these represent intermediate products that are formed as soon as the CO
2 pressure increases.
The energy distribution (
Figure 6) showed four signals for the MSG20 sample. Two corresponding to low enthalpies are probably related to physisorption. The other two are present at enthalpies that are higher or close to −40kJ mol
−1 (possibly related to chemisorption). Yoo et al. (2015) [
52] mentioned that the enthalpy value of −65kJ mol
−1 could be associated to the combination of CO
2 adsorbed via intramolecular interactions with silanols and/or other amine groups (when the grafted moieties are DI-TRI amines) to form carbamate. Therefore, the peak that was observed at the MSG20 energy distribution curve in the range −50–65 kJ mol
−1 may be attributed to the formation of silyl propyl carbamate on the MSG20 surface.
Moreover, formation enthalpy related to carbamic acid formation (~43 kJ mol
−1) is also present in the distribution. Bacsik et al. (2011) [
53] concluded that the ammonium carbamate ion pairs and hydrogen-bonded carbamic acid were weakly chemisorbed and could be outgassed by vacuum. Danon et al. (2011) [
25] observed that, after cell evacuation in FT-IR equipment, only the band associated with the bound (silyl propyl) carbamate kept intact, indicating that this molecule has a stronger interaction that cannot be reversed only by vacuum. For this reason, the MSG20I30 sample could be an interesting CO
2 capture adsorbent with respect to energy consumption because the irreversibly bound (silyl propyl) carbamate formation is suppressed. MSG20I30 site distribution does not show this peak. On the other hand, diffusional resistances may be increased, as mentioned by Bollini et al. (2012) [
21], for materials with high amine density, which affects adsorption kinetics.
The amine density on these samples, as well as the amount of adsorption sites and the maximum thermokinetic parameter (τ
max) in each case are summarized in
Table 3. For both functionalized solids, the active sites with strength lower than −40 kJ mol
−1 do not vary significantly as the amine density increases, in contrast with the chemisorption sites (strength higher than −40 kJ mol
−1), which consistently increase for higher amine loadings.
The MSG20 sample showed τ
max at −43 kJ mol
−1, providing additional evidence that the rate-limiting adsorption mechanism is essentially due to carbamic acid formation by hydrogen bonds [
18].
For the MSG20I30 sample, the maximum thermokinetic parameter at −34 kJ mol−1 is related to physisorption. This provides additional evidence that the adsorption rate-limiting mechanism is essentially due to physisorption on this sample (diffusional resistances).
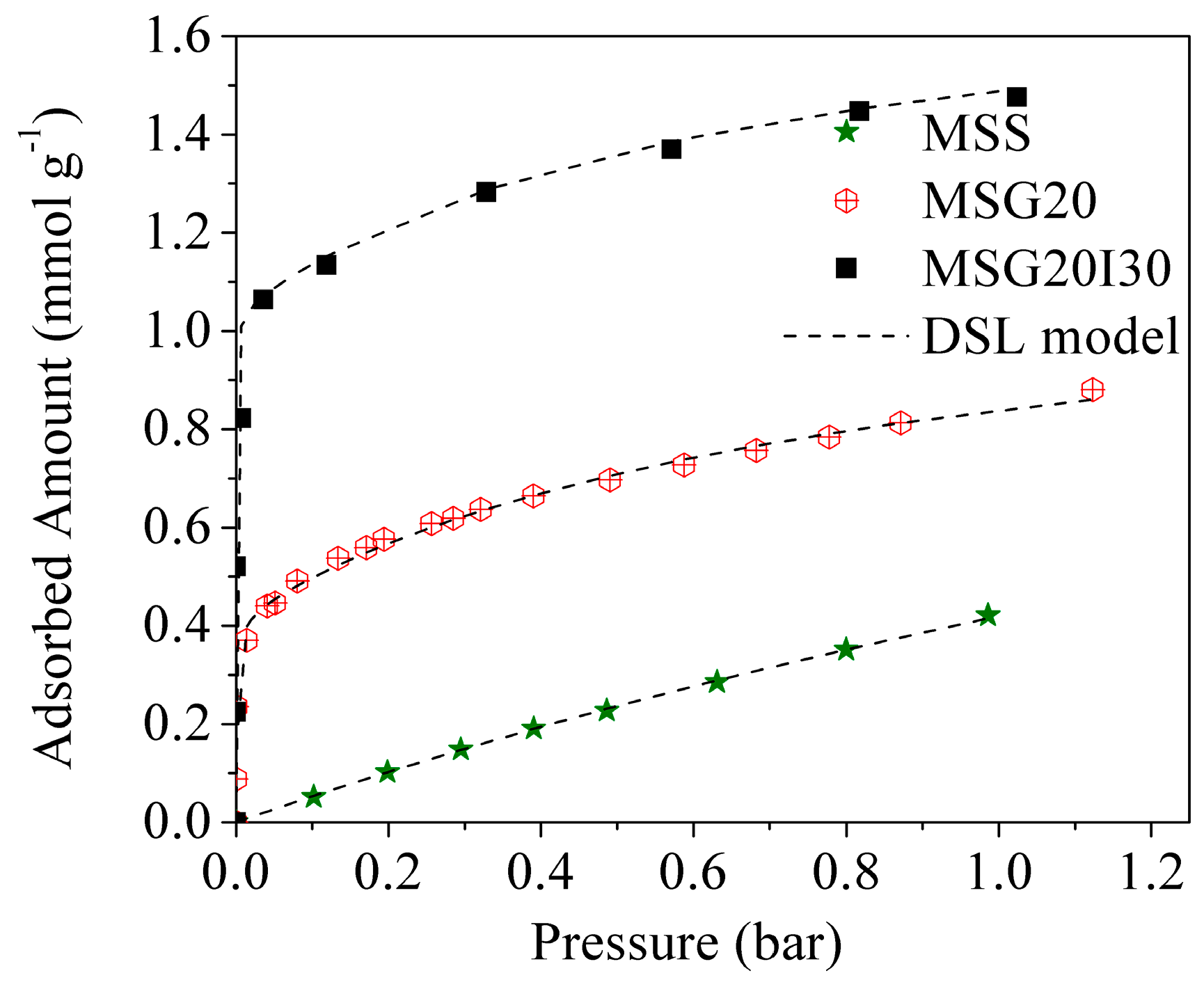
3.1. Pure CO2 Adsorption Isotherms at Low Pressures
CO
2 adsorption isotherms of all the materials at 25 °C are compared in
Figure 7. For the functionalized samples, the isotherms showed a steep increase at pressure <0.1 bar and a gradual increase from 0.1 to 1.0 bar. The high capacity and the steep nature of the CO
2 isotherm at low pressure on amine loaded silica are known as being caused by the chemical reaction between CO
2 and the primary amine groups (–NH
2), forming the products of adsorption previously discussed. The further gradual increase beyond the “knee” from 0.1 to 1.0 bar was attributed to the physical adsorption of CO
2 on the grafted mesoporous material, but it is more notorious for MSS, which does not present the primary increase knee. As expected, the CO
2 adsorption at low pressures is more favorable for the double functionalized sample, which has a higher percentage of added amines.
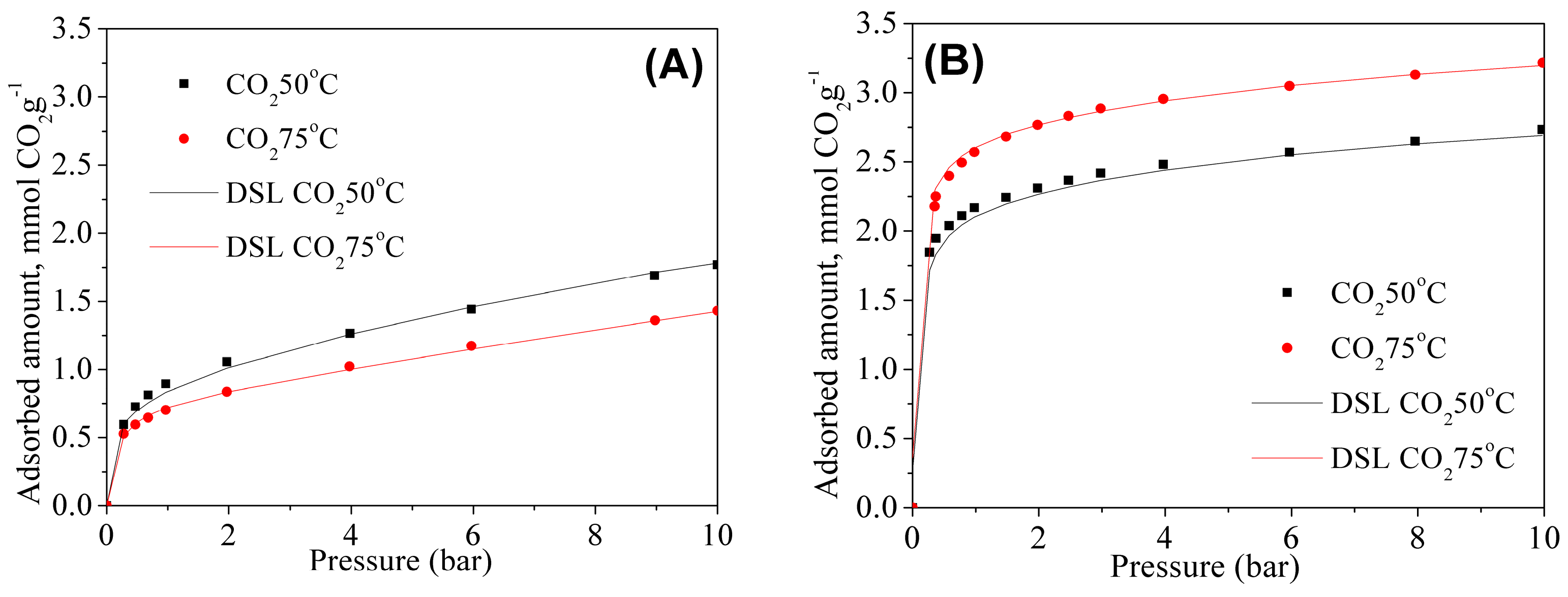
3.2. Pure CO2 and Binary CO2/N2 Adsorption Isotherms at High Pressures
High-pressure adsorption isotherms were also measured for grafted and double functionalized material for CO
2 and N
2 at 50 and 75 °C. The CO
2 isotherms are shown in
Figure 8A,B. The fitting parameters of Dualsite Langmuir model and the coefficients of determination of the fitted model are summarized in the
Table 4 and
Table 5 for the MSG20 and MSG20I30 samples, respectively.
As expected, CO
2 adsorption is enhanced with the increases of the temperature for high density amine sample (MSG20I30), particularly at low pressures. As it can be observed in
Table 5,
qm1 and
b1 are higher at 75 °C for MSG20I30 sample. This behavior is characteristic for chemisorption on samples with high percentages of functionalized amine. The coefficients of determination show for all cases that the model adequately fits experimental data, and this fact has an important impact for the selectivity estimation.
The results of adsorption capacities that is reported in the literature for mesoporous silica functionalized with APTES, MAP (3-(Methylamino)-propyltrimethoxysilane), and double functionalized with APTES/TEPA and APTES/PEI are summarized in
Table 6. The adsorption capacities that were obtained in this work are in the same range as those that are found in the literature under similar conditions.
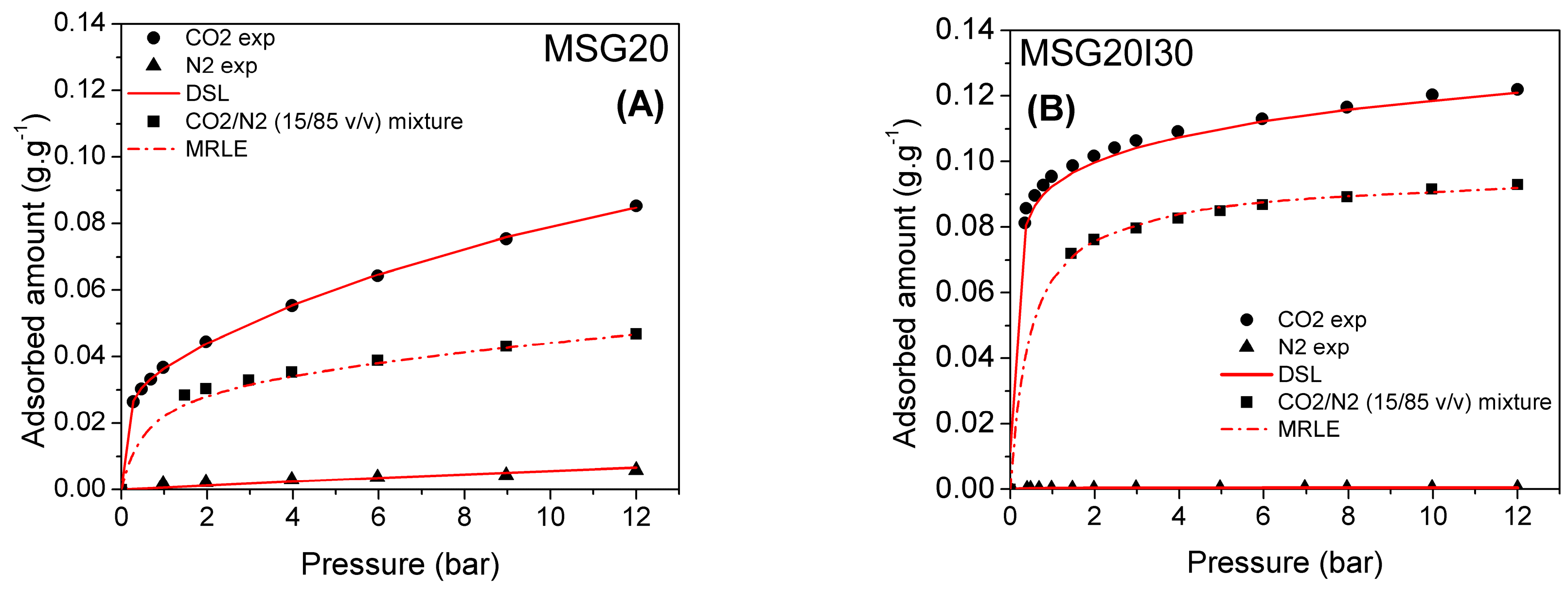
The MSG20 and MSG20I30 molar selectivities were estimated from binary isotherms using Dual Site Extended Langmuir (DSEL) in order to obtain the CO2 adsorbed under binary conditions. The highest selectivity values are reached at low pressures. This is due to the strong interaction of CO2 with the incorporated amine, which are mostly available at low pressures. N2 at low pressures does not have strong interactions with either –OH or –NH2 groups and the physisorption is weak. The highest values for selectivity, as expected, were obtained for MSG20I30 at both temperatures, stressing the advantage of this sample in contrast with the grafted one.
Adsorption isotherms for binary mixtures of CO
2 and N
2 (15/85
v/
v) are shown in
Figure 9 for MSG20 and MSG20I30 samples. The binary mixture mole fraction was chosen to be representative of a post-combustion scenario of flue gases (15% CO
2/85% N
2) and at high temperatures (50–75 °C). The points stand for experimental data and the lines stand for predictions from the multi region extended Langmuir (MREL) model using parameters that were obtained from the single component isotherms.
The Adsorbent Performance Indicator (API) was calculated for MSG20 and MSG20I30 samples (see
Table 7). In this computation, working capacity in the pressure range from 0.02 bar to 1 bar at 50 and 75 °C was used. The exponents A, B, and C (Equation (13)) were assumed to be 1, following the procedure that was adopted to calculate the API for purification scenarios by Wiersum et al. [
42]. The highest value of API is found for the MSG20I30 sample at 75 °C. When the temperature increases, the parameter also does. This indicates a good performance to purification on the post combustion process. The API for MSG20I30 is the highest in contrast with other materials that were studied for the post-combustion scenario. Alvarez-Gutierrez et al. (2017) [
59] calculated API for carbons on the post-combustion condition. They obtained values <1 at 50 °C, moreover API for carbons decreases as the temperature increases [
60] so that carbons would not be adequate materials for post combustion scenario. Pillai et al. (2015) [
61] calculated API on MOFs and they obtained values that were close to the MSG20I30 sample.
3.3. Stability and Energy Consumption between Adsorption Cycles
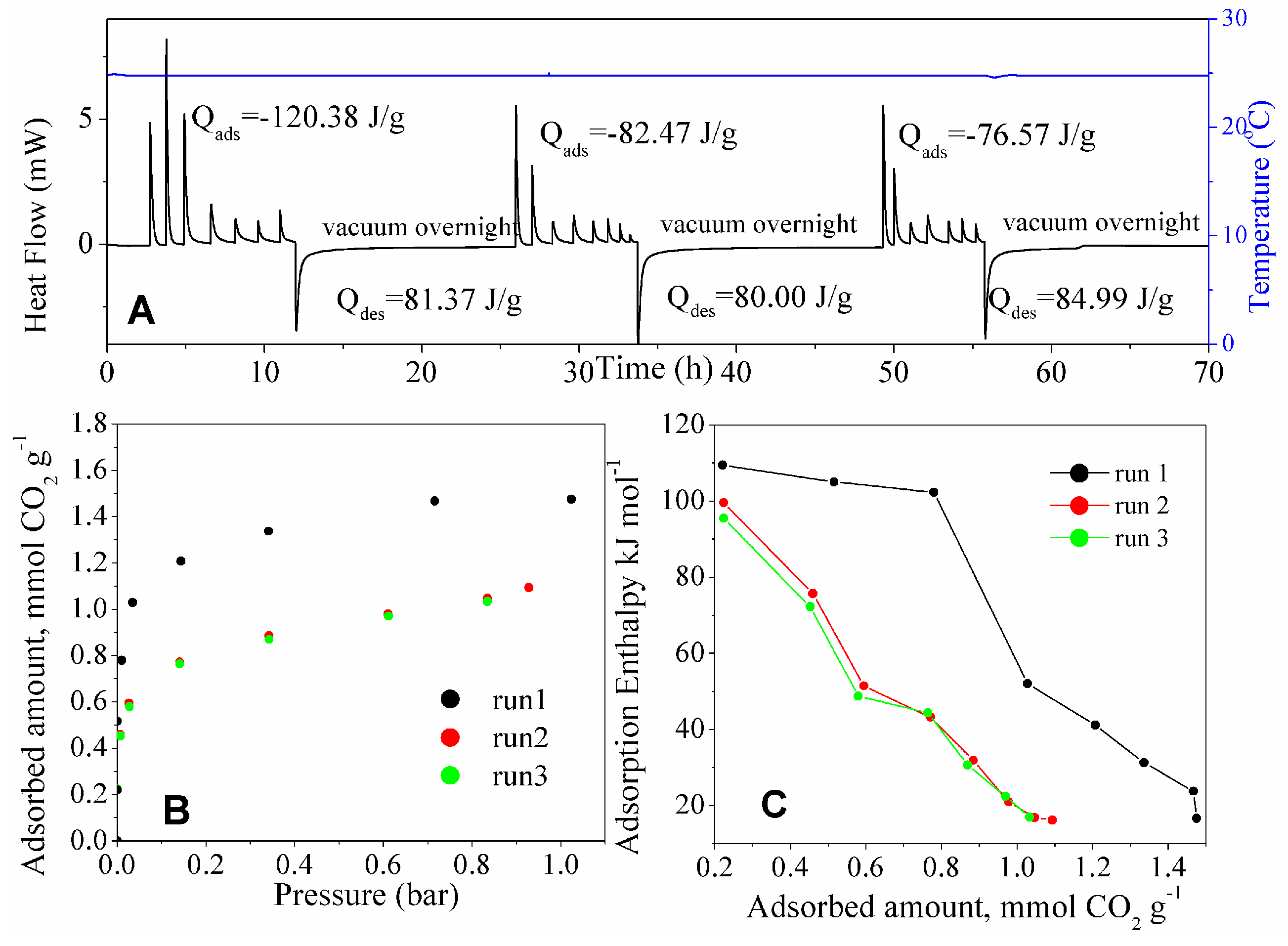
For practical use, the adsorbent should not only possess a high adsorption capacity for pure CO
2 and a high Adsorbent Performance Indicator, but it should also display a reversible adsorption–desorption pattern. Runs of CO
2 adsorption (isotherms, thermograms, and differential enthalpies) at 25 and 50 °C on the adsorbent with best performance (MSG20I30 sample) previously degassed at 120 °C for 4 h are shown in
Figure 10 and
Figure 11, respectively. The CO
2 adsorption isotherm for run 1 in contrast to run 2 at 25 °C do not follow the same path. The thermograms show a difference between adsorption and desorption enthalpies of 39.01 J per gram of solid. This observation suggests that the CO
2 molecules that were adsorbed in the first round cannot be completely desorbed, even under overnight molecular vacuum. The enthalpies of adsorption at near-zero coverage do not differ distinctly for the first adsorption and the subsequent ones. This suggests that chemisorption is still happening on the free amine groups that remain after the first evacuation, in lower intensity for adsorption sites occupation. The first occupation of available sites may be due to the irreversible reaction between CO
2 and amine on this material [
62]. On the other hand, that irreversibility may also be attributed to the diffusion limitations that are imposed by the high amine density of MSG20I30 [
22,
29]. This would result that after the first adsorption run not all CO
2 is released from the sample during the time high vacuum is applied. It is likely that both mechanisms (chemisorption and hindered diffusion) contribute to cause this irreversibility.
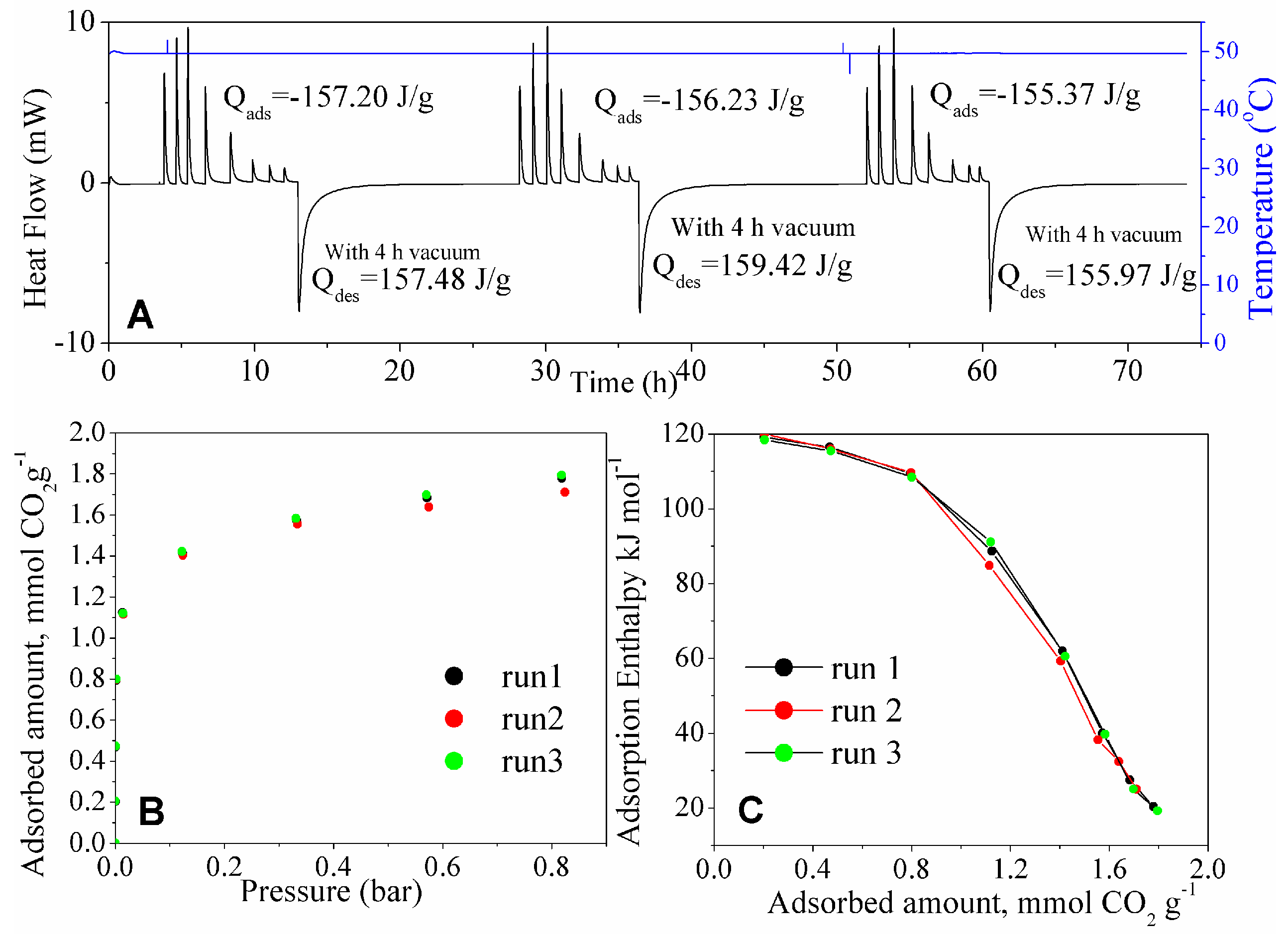
The calorimetric cycles at 50 °C for MSG20I30 are shown in
Figure 11. The thermogram integration shows reversibility at this temperature. The three adsorption isotherms and thermograms overlap at this temperature. An increase of temperature eventually would enhance intraparticle mass transfer, allowing for faster CO
2 evacuation and have the adsorption sites available again. The rupture of the strong bond formed between CO
2 and amino propyl groups can be achieved with high temperature and molecular vacuum, as these results at 50 °C suggest. At this temperature, MSG20I30 sample achieves reversibility after 4 h under vacuum.
Based on the difference between the energies of adsorption and desorption (after vacuum application), the temperature that is necessary to get complete outgassing was calculated for the experiments carried out at 25 °C, while considering a caloric capacity of 0.75 J g
−1 °C
−1. This temperature is in agreement with other works in our group [
63], where grafting materials were studied in a fixed bed calculating degassing temperatures around 90 °C with partial pressure reduction. At 50 °C the process is reversible, so this calculation was not computed. Enthalpy data and calculated temperature to complete outgassing are pointed out along with the isotherms that were collected for the three runs for MSG20I30 in
Figure 12.
Three runs at 25 °C for MSG20I30 sample are presented in
Figure 12. Both heating up to the calculated temperature and molecular vacuum were used between the runs, with the purpose of testing if adsorption-desorption is truly reversible at these conditions. The results show that the MSG20I30 has reversibility in the pressure range used (0–1 bar), thus confirming that a mild increase in temperature is required to completely desorb CO
2 at 25 °C. Under post combustion scenarios (higher temperatures), this increase is not necessary, recovering the maximum CO
2 capacity by applying only pressure swing.
4. Conclusions
In this work, the characteristics and the behavior of mesoporous silica samples functionalized by grafting and by double functionalization were analyzed, in order to evaluate in energetic terms their performance as CO2 capture material in post combustion scenarios.
The maximum value of thermokinetic parameter for the functionalized samples indicated that the dominant mechanism depends on the amine density. For the sample with low/medium amino groups density (4–5 molec·nm−2), the carbamic acid/silyl carbamate formation would be the mechanism that is dominant. For the double functionalized sample (MSG20I30, high amino density), CO2 diffusion would be the limiting phenomenon.
The microcalorimetric studies confirmed that new adsorption sites were generated by the functionalization step. For materials with higher amine density, the proportion of propyl carbamate/silyl carbamate formed is higher than for materials with low or medium amine density. This is agreement with the distribution of sites found from the differential adsorption enthalpy of MSG20I30. This sample did not present a signal of silyl formation (stronger bonds that could cause irreversibility in cycles).
CO2 adsorption capacities increased with the temperature for MSG20I30 sample, an opposite behavior than the MSG20 sample. This fact suggested a greater contribution of physisorption mechanism than CO2 chemisorption on MSG20. These properties derived in higher selectivity, higher working capacity, and also higher API values for MSG20I30 than MSG20 sample at high temperatures (50 and 75 °C).
A complete desorption of MSG20I30 at 25 °C was not possible only by molecular vacuum. The differential adsorption enthalpy at zero coverage suggests that this irreversibility is attributed to the occupation of sites that are not restored after the first adsorption round, changing the sites distribution on the sample. This occupation of sites could be caused by either diffusional limitation or strong chemical bonds of adsorption products formed. At higher temperatures, these sites become free after the first outgassing process.
Thereby, from the obtained results, the double functionalization method would be a more efficient route to incorporate amino groups on the support with views to its application on post combustion scenarios under dry conditions, taking into account the less consumption of energy to recover the maximum CO2 capacity and its higher performance at high temperatures.