Rapid Production of Mn3O4/rGO as an Efficient Electrode Material for Supercapacitor by Flame Plasma
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Chemical
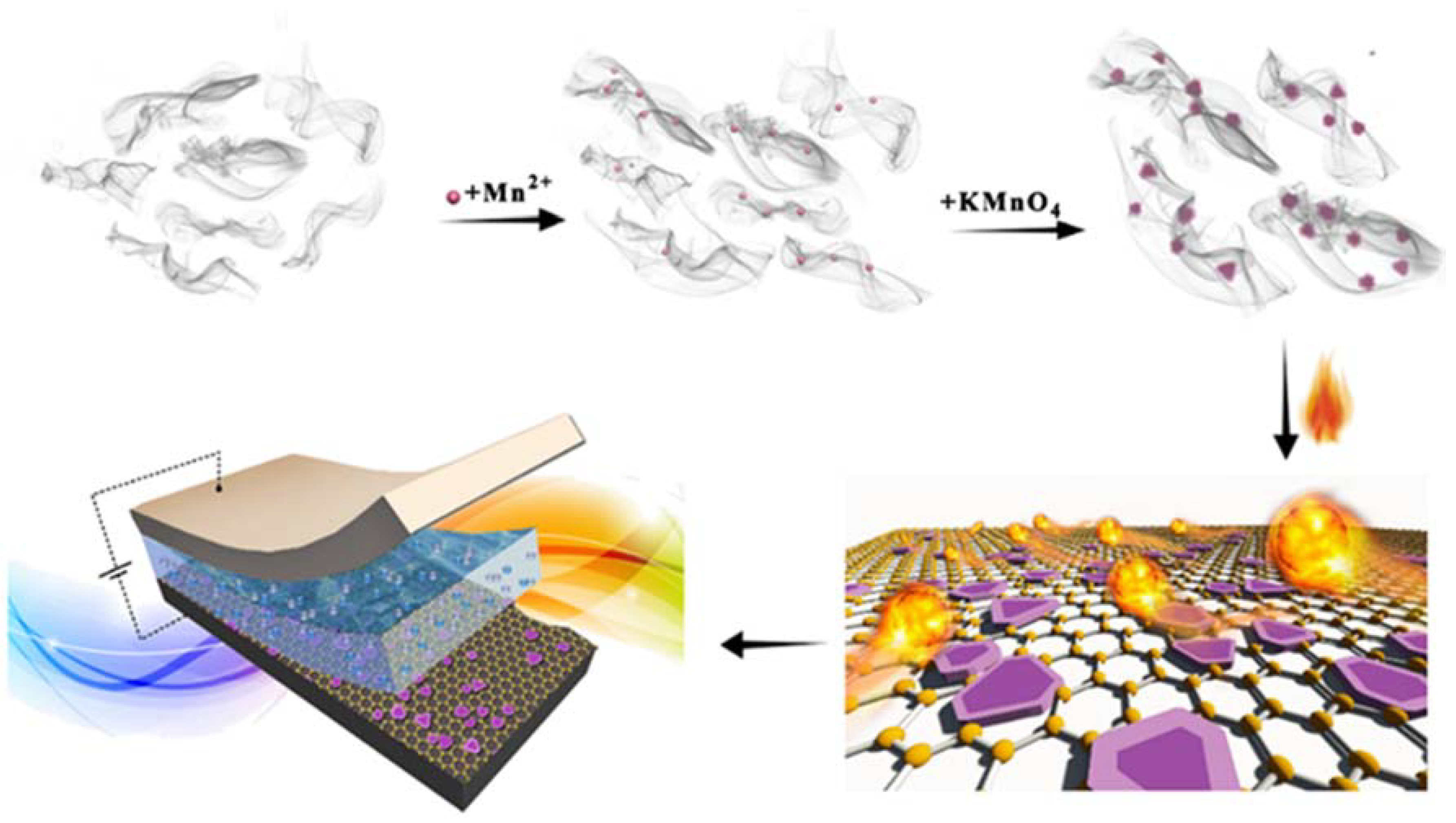
2.2. Preparation of the Mn3O4/rGO Composite
2.2.1. Synthesis of Graphene Oxide (GO)
2.2.2. Synthesis of MnO2/GO Film
2.2.3. Synthesis of Mn3O4/rGO Film
2.3. Electrode Preparation
2.4. Materials Characterization
2.5. Electrochemical Measurements
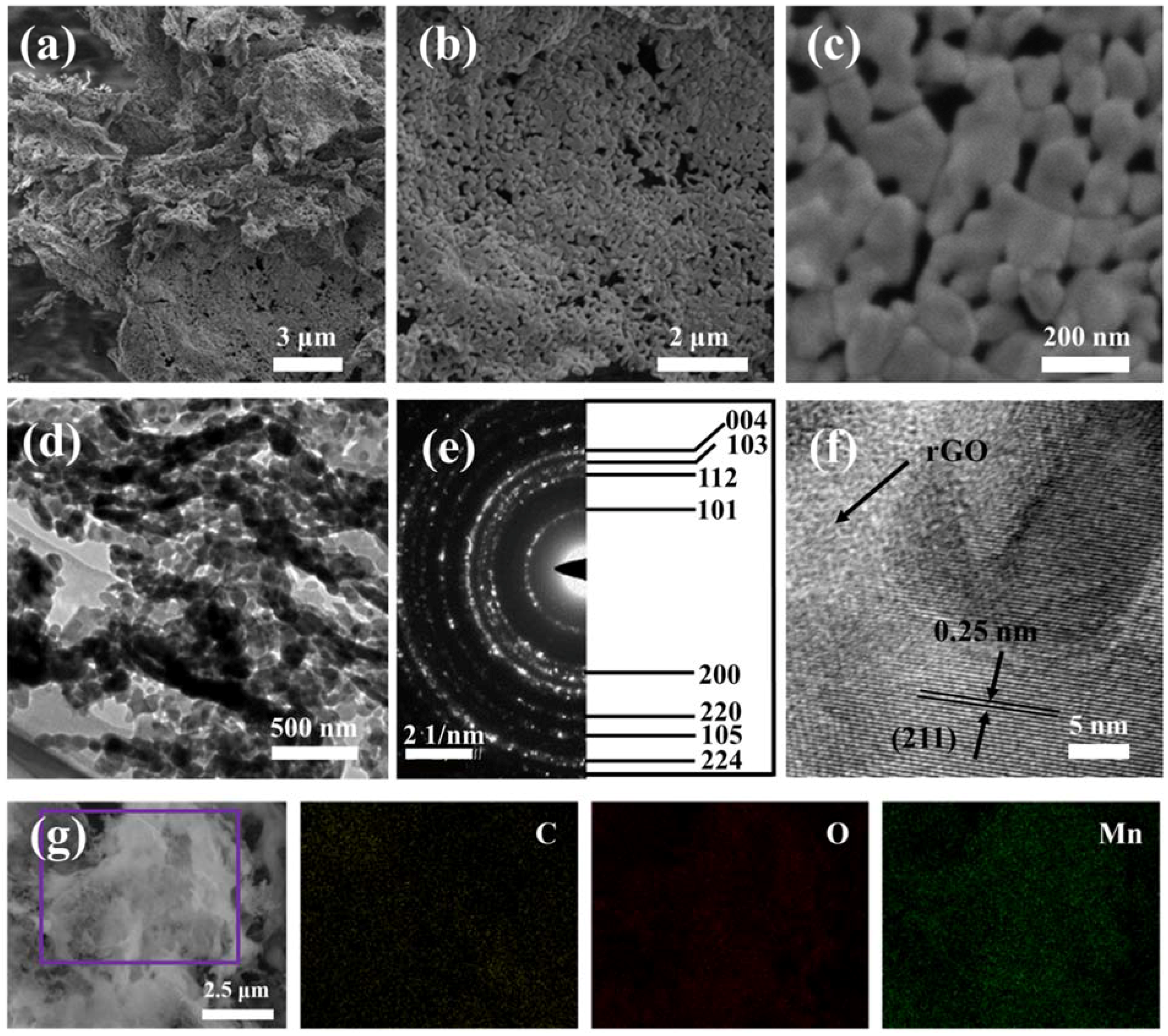
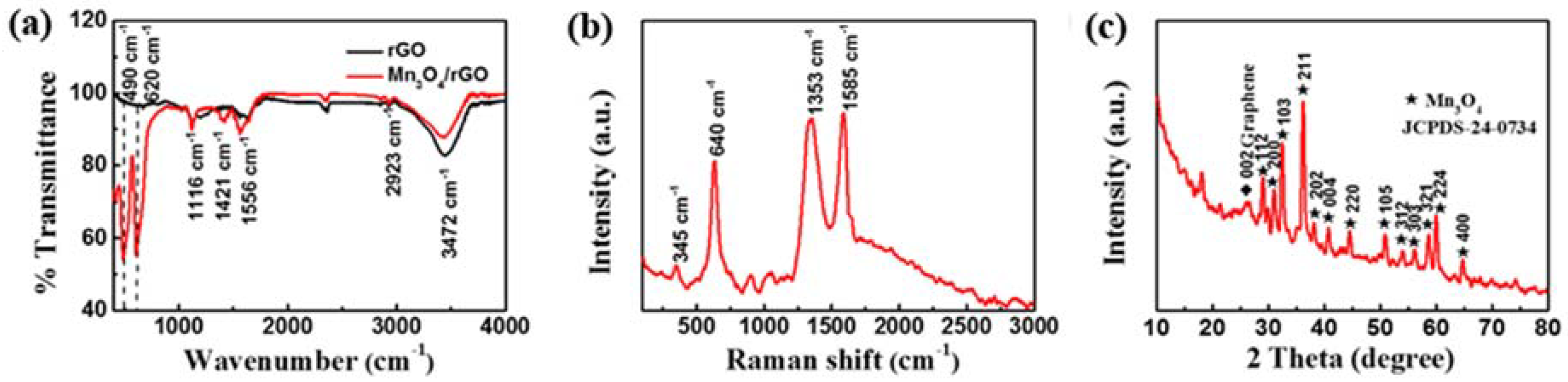
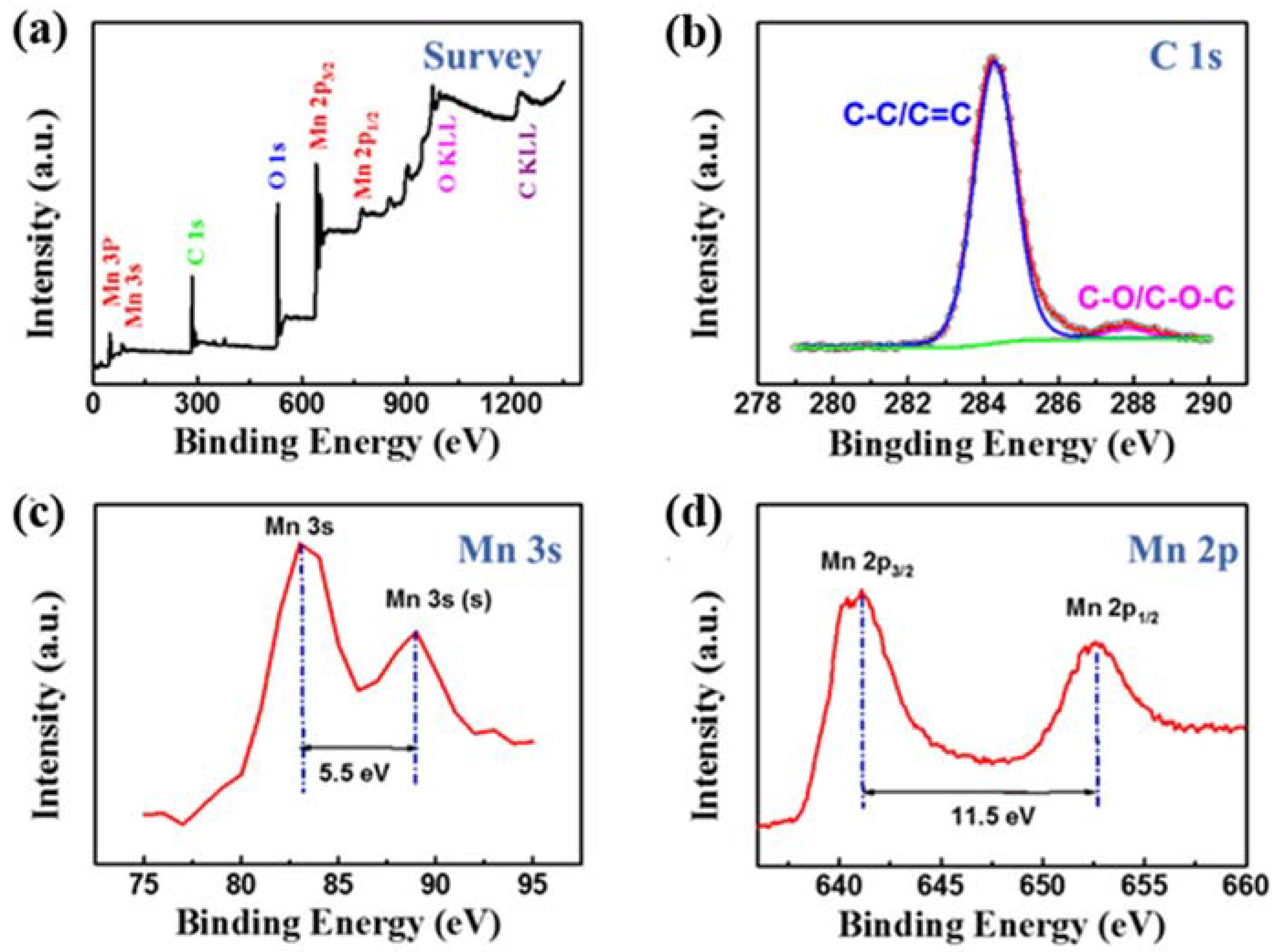
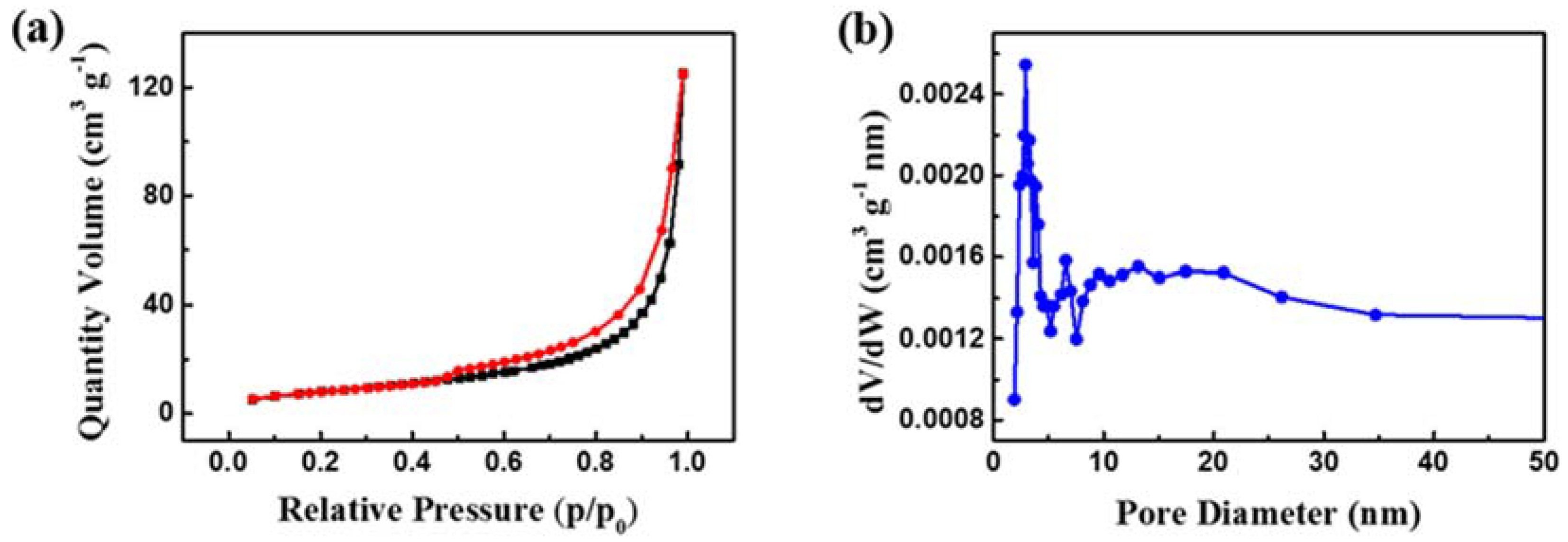
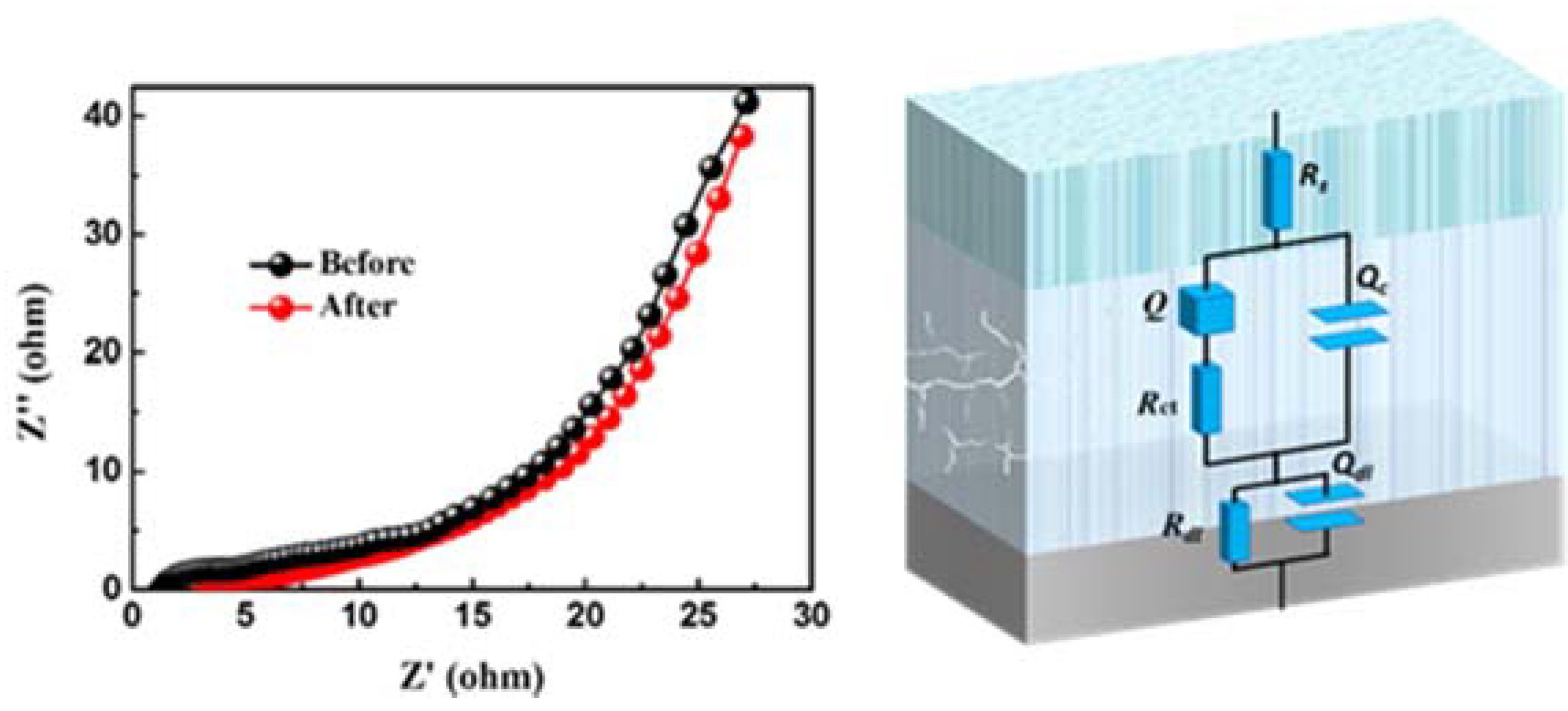
3. Results and Discussion
4. Conclusions
- (1)
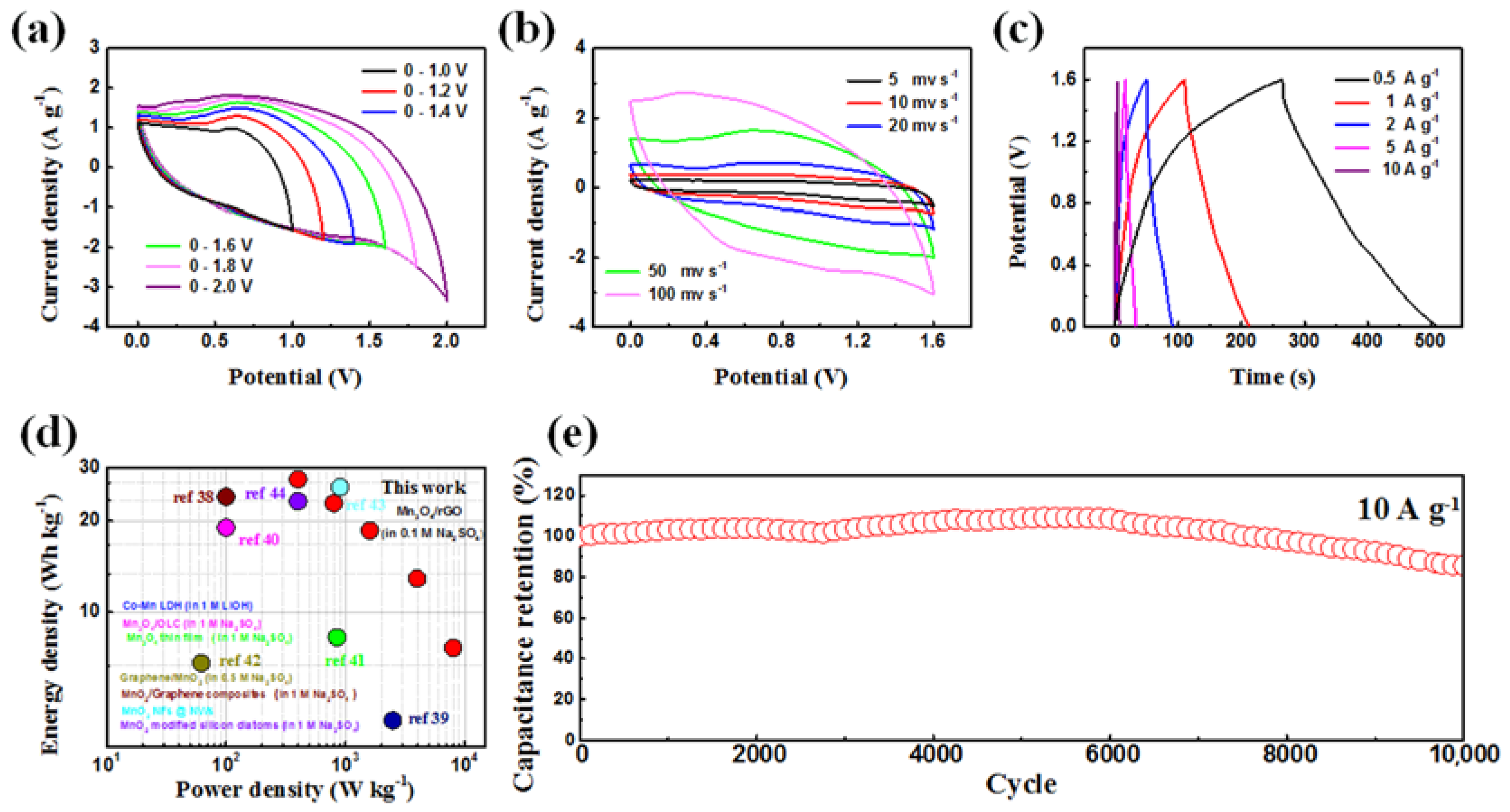
- The Mn3O4/rGO electrode in the three-electrode system delivered high capacitance up to 342.5 F g−1 at the current density of 1 A g−1 and exhibited equally remarkable cyclic stability.
- (2)
- The ASC consisting of an AG negative and a Mn3O4/rGO positive achieved a relatively high energy density 27.41 Wh kg−1 and a power density of 8 kW kg−1.
- (3)
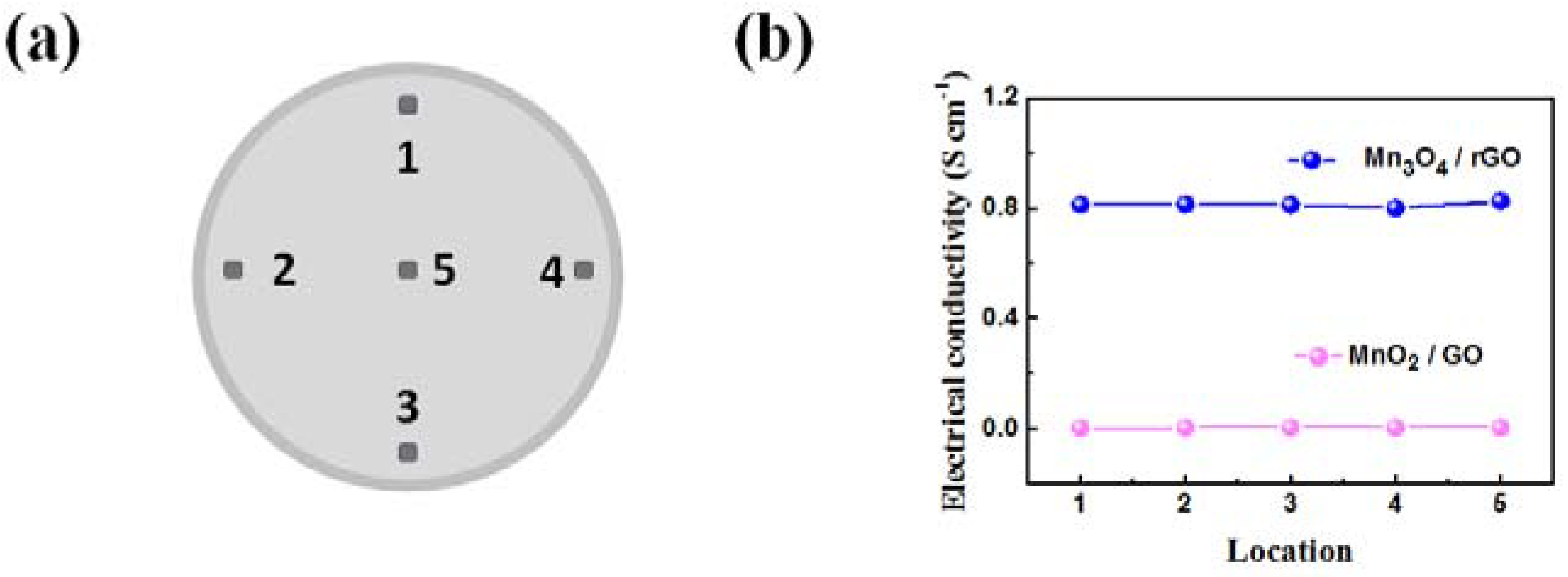
- Mn3O4/rGO material demonstrated in this paper can offer conductive porous texture for the excellent electron and ion percolation network.
- (4)
- Such fabrication technique is simple, rapid, facile and low-cost and it would give an insight into producing the similar metal oxide/graphene as potential active materials for energy storage device.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, L.L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, Z.; Huang, Y.; Ma, Y.; Wang, C.; Chen, M.; Chen, Y. Supercapacitor devices based on graphene materials. J. Phys. Chem. C 2009, 113, 13103–13107. [Google Scholar] [CrossRef]
- Jia, R.; Zhu, F.; Sun, S.; Zhai, T.; Xia, H. Dual support ensuring high-energy supercapacitors via high-performance NiCo2S4@Fe2O3 anode and working potential enlarged MnO2 cathode. J. Power Sources 2017, 341, 427–434. [Google Scholar] [CrossRef]
- Futaba, D.N.; Hata, K.; Yamada, T.; Hiraoka, T.; Hayamizu, Y.; Kakudate, Y.; Tanaike, O.; Hatori, H.; Yumura, M.; Iijima, S. Shape-engineerable and highly densely packed single-walled carbon nanotubes and their application as super-capacitor electrodes. Nat. Mater. 2006, 5, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Chiam, S.L.; Lim, H.N.; Hafiz, S.M.; Pandikumar, A.; Huang, N.M. Electrochemical Performance of Supercapacitor with Stacked Copper Foils Coated with Graphene Nanoplatelets. Sci. Rep. 2018, 8, 3093. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Z.; Xiao, Y.H.; Su, D.C.; Zhou, L.M.; Wu, S.D.; Han, L.F.; Fang, S.M.; Cao, S.K. High-quality porous cobalt monoxide nanowires@ultrathin manganese dioxide sheets core-shell nanowire arrays on Ni foam for high-performance supercapacitor. Electrochim. Acta 2016, 194, 377–384. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y.; Dunn, B. Materials science. Where do batteries end and supercapacitors begin? Science 2014, 343, 1210–1211. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhang, Y.; Qian, X. MoS2/RGO/Ni3S2 Nanocomposite in situ Grown on Ni Foam Substrate and Its High Electrochemical Performance. Electrochim. Acta 2016, 198, 135–143. [Google Scholar] [CrossRef]
- Garakani, M.A.; Abouali, S.; Xu, Z.-L.; Huang, J.; Huang, J.-Q.; Kim, J.-K. Heterogeneous, mesoporous NiCo2O4-MnO2/graphene foam for asymmetric supercapacitors with ultrahigh specific energies. J. Mater. Chem. A 2017, 5, 3547–3557. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, H.; Mu, X.; Chen, J.; Zhang, P.; Wang, Y.; He, Y.; Zhang, Z.; Pan, X.; Xie, E. Importance of polypyrrole in constructing 3D hierarchical carbon nanotube@MnO2 perfect core-shell nanostructures for high-performance flexible supercapacitors. Nanoscale 2015, 7, 14697–14706. [Google Scholar] [CrossRef] [PubMed]
- Ortaboy, S.; Alper, J.P.; Rossi, F.; Bertoni, G.; Salviati, G.; Carraro, C.; Maboudian, R. MnOx-decorated carbonized porous silicon nanowire electrodes for high performance supercapacitors. Energy Environ. Sci. 2017, 10, 1505–1516. [Google Scholar] [CrossRef]
- Deng, J.W.; Chen, L.F.; Sun, Y.Y.; Ma, M.H.; Fu, L. Interconnected MnO2 nanoflakes assembled on graphene foam as a binder-free and long-cycle life lithium battery anode. Carbon 2015, 92, 177–184. [Google Scholar] [CrossRef]
- Yuan, T.; Jiang, Y.; Sun, W.; Xiang, B.; Li, Y.; Yan, M.; Xu, B.; Dou, S. Ever-increasing pseudocapacitance in RGO-MnO-RGO sandwich nanostructures for ultrahigh-rate lithium storage. Adv. Funct. Mater. 2016, 26, 2198–2206. [Google Scholar] [CrossRef]
- Pan, C.; Gu, H.; Dong, L. Synthesis and electrochemical performance of polyaniline@MnO2/graphene ternary composites for electrochemical supercapacitors. J. Power Sources 2016, 303, 175–181. [Google Scholar] [CrossRef]
- Yao, J.; Pan, Q.; Yao, S.; Duan, L.; Liu, J. Mesoporous MnO2 nanosphere/graphene sheets as electrodes for supercapacitor synthesized by a simple and inexpensive reflux reaction. Electrochim. Acta 2017, 238, 30–35. [Google Scholar] [CrossRef]
- Li, L.; Raji, A.R.O.; Tour, J.M. Graphene-wrapped MnO2-graphene nanoribbons as anode materials for high-performance lithium ion batteries. Adv. Mater. 2013, 25, 6298–6302. [Google Scholar] [CrossRef] [PubMed]
- Xiong, T.; Lee, W.S.V.; Huang, X.; Xue, J.M. Mn3O4/reduced graphene oxide-based supercapacitor with ultra-long cycling performance. J. Mater. Chem. A 2017, 5, 12762–12768. [Google Scholar] [CrossRef]
- Wang, Y.; Lai, W.H.; Wang, N. A reduced graphene oxide/mixed-valent manganese oxides composite electrode for tailorable and surface mountable supercapacitors with high capacitance and super-long life. Energy Environ. Sci. 2017, 10, 941–949. [Google Scholar] [CrossRef]
- Ke, Q.; Wang, J. Graphene-based materials for supercapacitor electrodes—A review. J. Materiomics 2016, 2, 37–54. [Google Scholar] [CrossRef]
- Wu, Z.-S.; Zhou, G.; Yin, L.-C.; Ren, W.; Li, F.; Cheng, H.-M. Graphene/metal oxide composite electrode materials for energy storage. Nano Energy 2012, 1, 107–131. [Google Scholar] [CrossRef]
- Liu, M.; Shi, M.; Lu, W.; Zhu, D.; Li, L.; Gan, L. Core–shell reduced graphene oxide/MnOx@carbon hollow nanospheres for high performance supercapacitor electrodes. Chem. Eng. J. 2017, 313, 518–526. [Google Scholar] [CrossRef]
- Hu, Y.; Guan, C.; Feng, G.; Ke, Q.; Huang, X.; Wang, J. Flexible asymmetric supercapacitor based on structure-optimized Mn3O4/reduced graphene oxide nanohybrid paper with high energy and power density. Adv. Funct. Mater. 2015, 25, 7291–7299. [Google Scholar] [CrossRef]
- Wang, L.; Ouyang, Y.; Jiao, X.; Xia, X.; Lei, W.; Hao, Q. Polyaniline-assisted growth of MnO2 ultrathin nanosheets on graphene and porous graphene for asymmetric supercapacitor with enhanced energy density. Chem. Eng. J. 2018, 334, 1–9. [Google Scholar] [CrossRef]
- Liu, L.; Su, L.; Lang, J.; Hu, B.; Xu, S.; Yan, X. Controllable synthesis of Mn3O4 nanodots@nitrogen-doped graphene and its application for high energy density supercapacitors. J. Mater. Chem. A 2017, 5, 5523–5531. [Google Scholar] [CrossRef]
- Zhang, H. Ultrathin two-dimensional nanomaterials. ACS Nano 2015, 9, 9451–9469. [Google Scholar] [CrossRef] [PubMed]
- Chhowalla, M.; Liu, Z.; Zhang, H. Two-dimensional transition metal dichalcogenide (TMD) nanosheets. Chem. Soc. Rev. 2015, 44, 2584–2586. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Wang, S.; Xia, T.; Li, X.; Jin, Q.; Wu, Q.; Wang, L.; Wei, Z.; Wang, P. Hydrothermal synthesis of a MnOOH/three-dimensional reduced graphene oxide composite and its electrochemical properties for supercapacitors. J. Mater. Chem. A 2015, 3, 20944–20951. [Google Scholar] [CrossRef]
- Park, S.K.; Suh, D.H.; Park, H.S. Electrochemical assembly of reduced graphene oxide/manganese dioxide nanocomposites into hierarchical sea urchin-like structures for supercapacitive electrodes. J. Alloy. Compd. 2016, 668, 146–151. [Google Scholar] [CrossRef]
- Zhang, W.; Zou, X.; Zhao, J. Rapid production of a bulk of porous mesh reduced graphene oxide films using a naked flame. J. Mater. Chem. C 2015, 3, 2788–2791. [Google Scholar] [CrossRef]
- Vazquez-Olmos, A.; Redon, R.; Rodriguez-Gattorno, G.; Esther Mata-Zamora, M.; Morales-Leal, F.; Fernandez-Osorio, A.L.; Saniger, J.M. One-step synthesis of Mn3O4 nanoparticles: Structural and magnetic study. J. Colloid Interface Sci. 2005, 291, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Jian, H.; Mai, K.; Ren, Z.; Wang, J.; Fu, X.; Fan, C.; Sun, C.; Qian, G.; Wang, Z. Shape- and size-controlled synthesis of Mn3O4 nanocrystals at room temperature. Eur. J. Inorg. Chem. 2014, 2014, 3023–3029. [Google Scholar] [CrossRef]
- Anilkumar, K.M.; Manoj, M.; Jinisha, B.; Pradeep, V.S.; Jayalekshmi, S. Mn3O4 /reduced graphene oxide nanocomposite electrodes with tailored morphology for high power supercapacitor applications. Electrochim. Acta 2017, 236, 424–433. [Google Scholar]
- Wang, L.; Li, Y.; Han, Z.; Chen, L.; Qian, B.; Jiang, X.; Pinto, J.; Yang, G. Composite structure and properties of Mn3O4/graphene oxide and Mn3O4/graphene. J. Mater. Chem. A 2013, 1, 8385–8397. [Google Scholar] [CrossRef]
- Jin, G.; Xiao, X.; Li, S.; Zhao, K.; Wu, Y.; Sun, D.; Wang, F. Strongly coupled graphene/Mn3O4 composite with enhanced electrochemical performance for supercapacitor electrode. Electrochim. Acta 2015, 178, 689–698. [Google Scholar] [CrossRef]
- Lu, Y.C.; Xu, Z.; Gasteiger, H.A.; Chen, S.; Hamad-Schifferli, K.; Shao-Horn, Y. Platinum-gold nanoparticles: A highly active bifunctional electrocatalyst for rechargeable lithium-air batteries. J. Am. Chem. Soc. 2010, 132, 12170–12171. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Hall, A.S.; Kim, J.D.; Mallouk, T.E. A facile and template-free hydrothermal synthesis of Mn3O4 nanorods on graphene sheets for supercapacitor electrodes with long cycle stability. Chem. Mater. 2012, 24, 1158–1164. [Google Scholar] [CrossRef]
- Li, L.; Xu, J.; Lei, J.; Zhang, J.; McLarnon, F.; Wei, Z.; Li, N.; Pan, F. A one-step, cost-effective green method to in situ fabricate Ni(OH)2 hexagonal platelets on Ni foam as binder-free supercapacitor electrode materials. J. Mater. Chem. A 2015, 3, 1953–1960. [Google Scholar] [CrossRef]
- Miniach, E.; Śliwak, A.; Moyseowicz, A.; Fernández-Garcia, L.; González, Z.; Granda, M.; Menendez, R.; Gryglewicz, G. MnO2/thermally reduced graphene oxide composites for high-voltage asymmetric supercapacitors. Electrochim. Acta 2017, 240, 53–62. [Google Scholar] [CrossRef]
- Jagadale, A.D.; Guan, G.; Li, X.; Du, X.; Ma, X.; Hao, X.; Abudula, A. Ultrathin nanoflakes of cobalt–manganese layered double hydroxide with high reversibility for asymmetric supercapacitor. J. Power Sources 2016, 306, 526–534. [Google Scholar] [CrossRef]
- Makgopa, K.; Raju, K.; Ejikeme, P.M.; Ozoemena, K.I. High-performance Mn3O4/onion-like carbon (OLC) nanohybrid pseudocapacitor: Unravelling the intrinsic properties of OLC against other carbon supports. Carbon 2017, 117, 20–32. [Google Scholar] [CrossRef]
- Yadav, A.A.; Jadhav, S.N.; Chougule, D.M.; Patil, P.D.; Chavan, U.J.; Kolekar, Y.D. Spray deposited Hausmannite Mn3O4 thin films using aqueous/organic solvent mixture for supercapacitor applications. Electrochim. Acta 2016, 206, 134–142. [Google Scholar] [CrossRef]
- He, Y.; Chen, W.; Li, X.; Zhang, Z.; Fu, J.; Zhao, C.; Xie, E. Freestanding three-dimensional graphene/MnO2 composite networks as ultralight and flexible supercapacitor electrodes. ACS Nano 2013, 7, 174–182. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Wang, C.; Zhuge, F.; Deng, X.; Xu, X.; Zhai, T. Flexible and high energy density asymmetrical supercapacitors based on core/shell conducting polymer nanowires/manganese dioxide nanoflakes. Nano Energy 2017, 35, 242–250. [Google Scholar] [CrossRef]
- Le, Q.J.; Wang, T.; Tran, D.N.H.; Dong, F.; Zhang, Y.X.; Losic, D. Morphology-controlled MnO2 modified silicon diatoms for high-performance asymmetric supercapacitors. J. Mater. Chem. A 2017, 5, 10856–10865. [Google Scholar] [CrossRef]
- Li, R.; Wang, S.; Huang, Z.; Lu, F.; He, T. NiCo2S4@Co(OH)2 core-shell nanotube arrays in situ grown on Ni foam for high performances asymmetric supercapacitors. J. Power Sources 2016, 312, 156–164. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Guo, L.; Shi, W.; Zou, X.; Xiang, B.; Xing, S. Rapid Production of Mn3O4/rGO as an Efficient Electrode Material for Supercapacitor by Flame Plasma. Materials 2018, 11, 881. https://doi.org/10.3390/ma11060881
Zhou Y, Guo L, Shi W, Zou X, Xiang B, Xing S. Rapid Production of Mn3O4/rGO as an Efficient Electrode Material for Supercapacitor by Flame Plasma. Materials. 2018; 11(6):881. https://doi.org/10.3390/ma11060881
Chicago/Turabian StyleZhou, Yang, Lei Guo, Wei Shi, Xuefeng Zou, Bin Xiang, and Shaohua Xing. 2018. "Rapid Production of Mn3O4/rGO as an Efficient Electrode Material for Supercapacitor by Flame Plasma" Materials 11, no. 6: 881. https://doi.org/10.3390/ma11060881
APA StyleZhou, Y., Guo, L., Shi, W., Zou, X., Xiang, B., & Xing, S. (2018). Rapid Production of Mn3O4/rGO as an Efficient Electrode Material for Supercapacitor by Flame Plasma. Materials, 11(6), 881. https://doi.org/10.3390/ma11060881





