Efficient Photocatalytic Degradation of Malachite Green in Seawater by the Hybrid of Zinc-Oxide Nanorods Grown on Three-Dimensional (3D) Reduced Graphene Oxide(RGO)/Ni Foam
Abstract
1. Introduction
2. Experimental Section
2.1. Preparationof ZnO/RGO@NFHybrid
2.2. Physical Characterization
2.3. Photocatalytic Activities Measurements
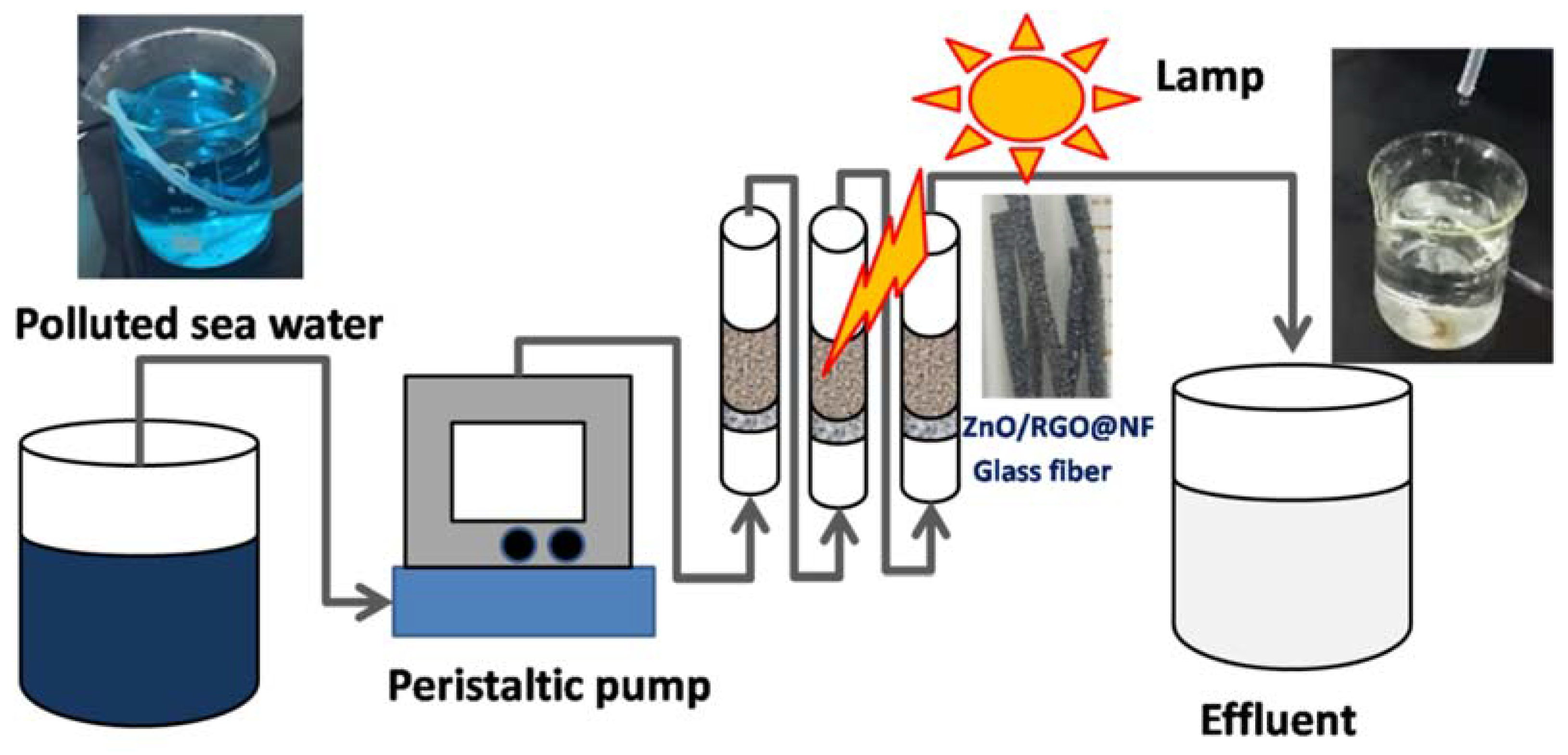
2.4. PracticalApplication in Seawater
3. Results and Discussion
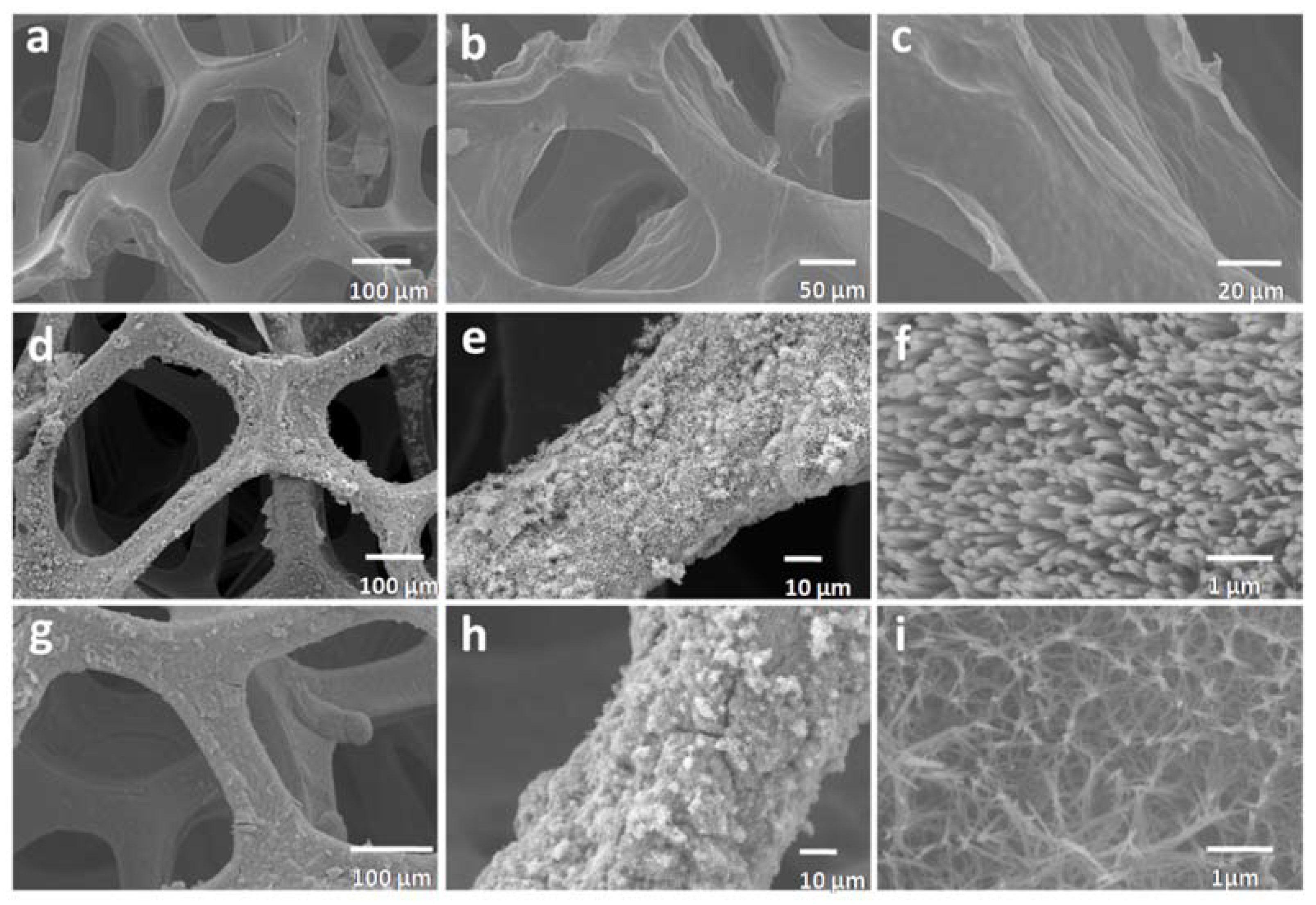
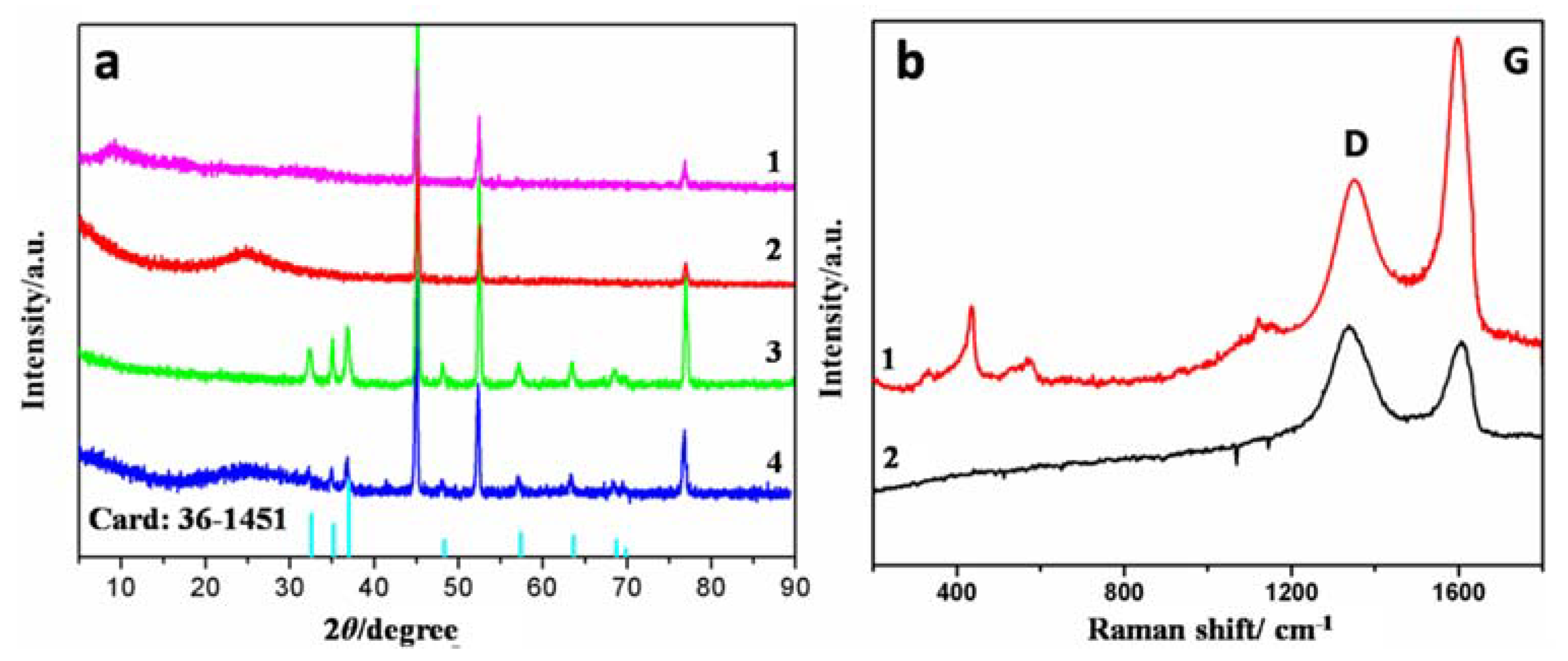
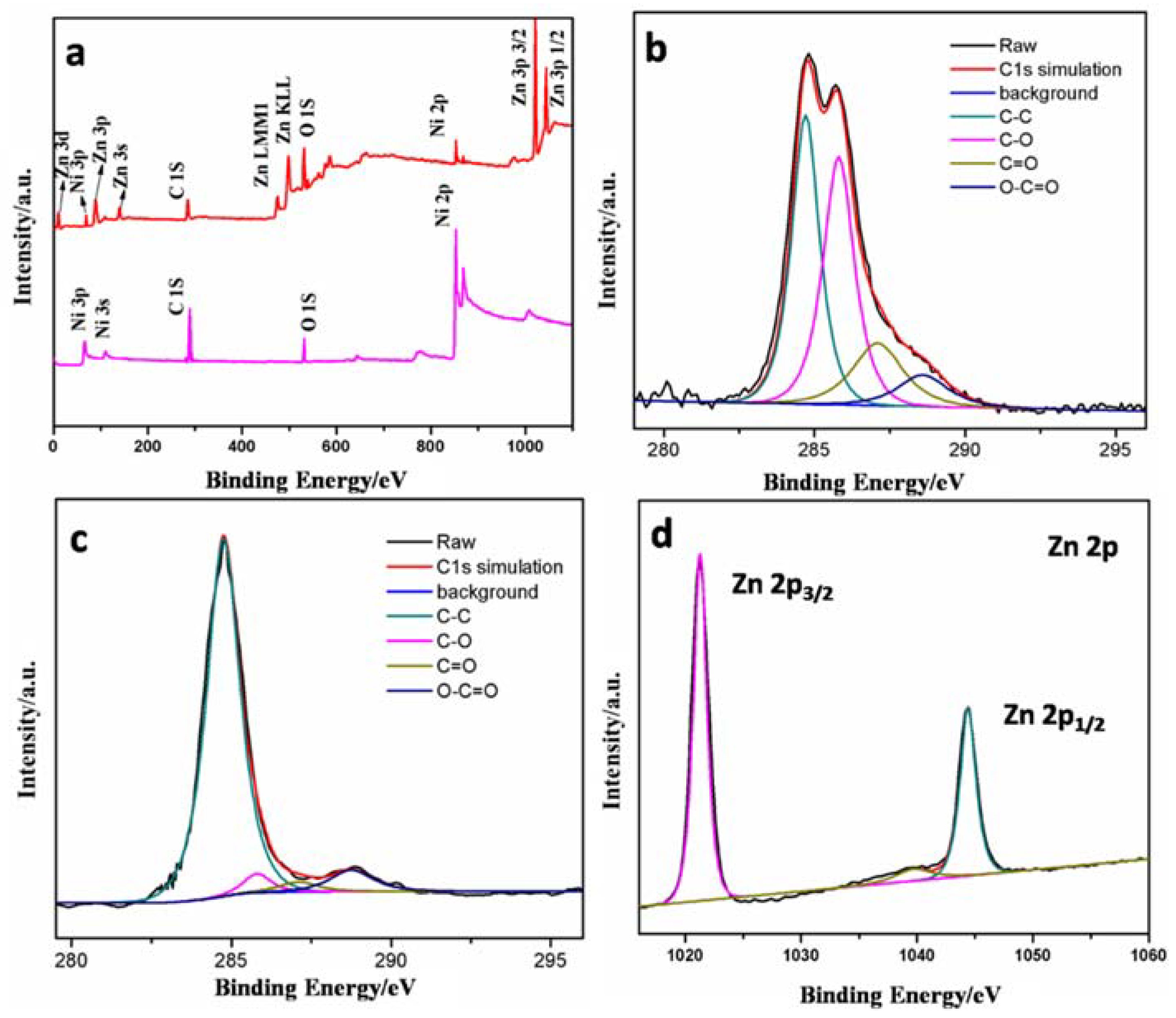
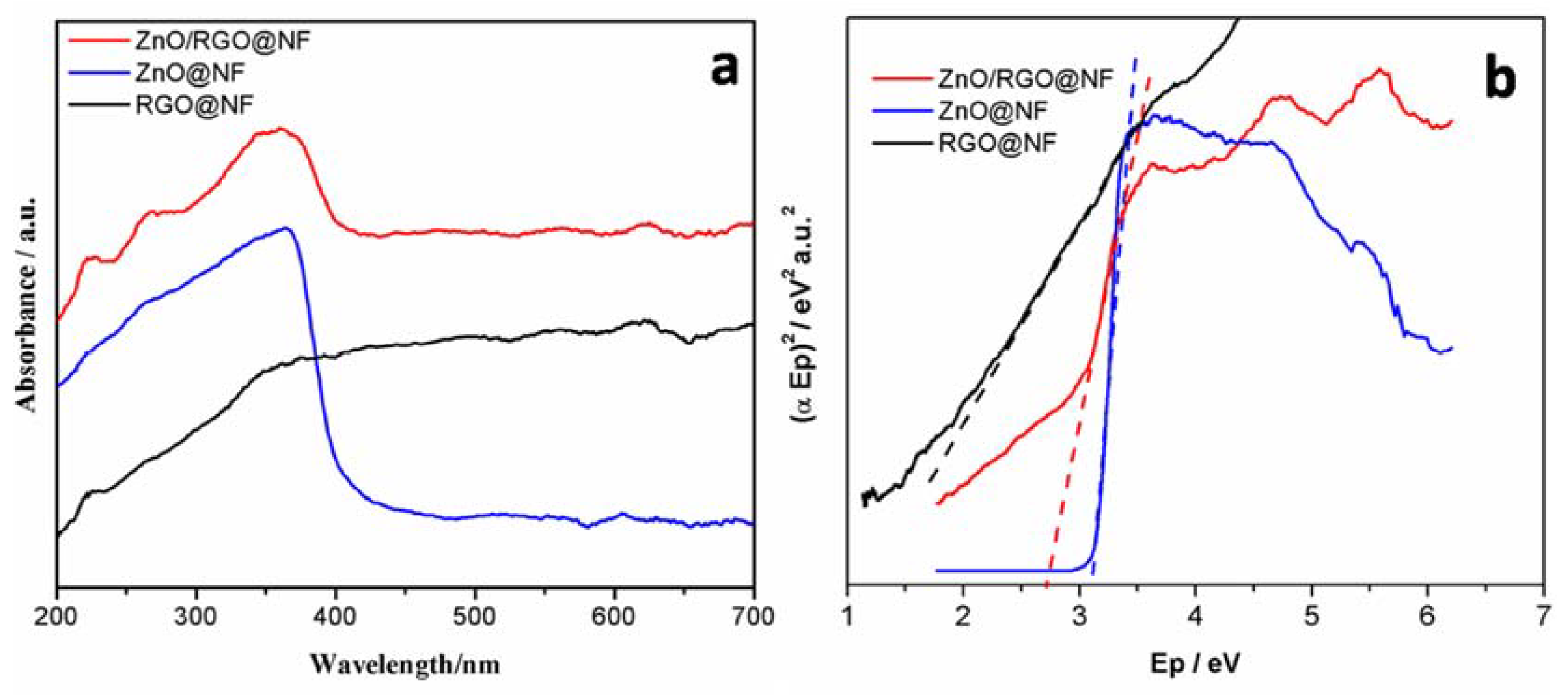
3.1. Characterization of Materials
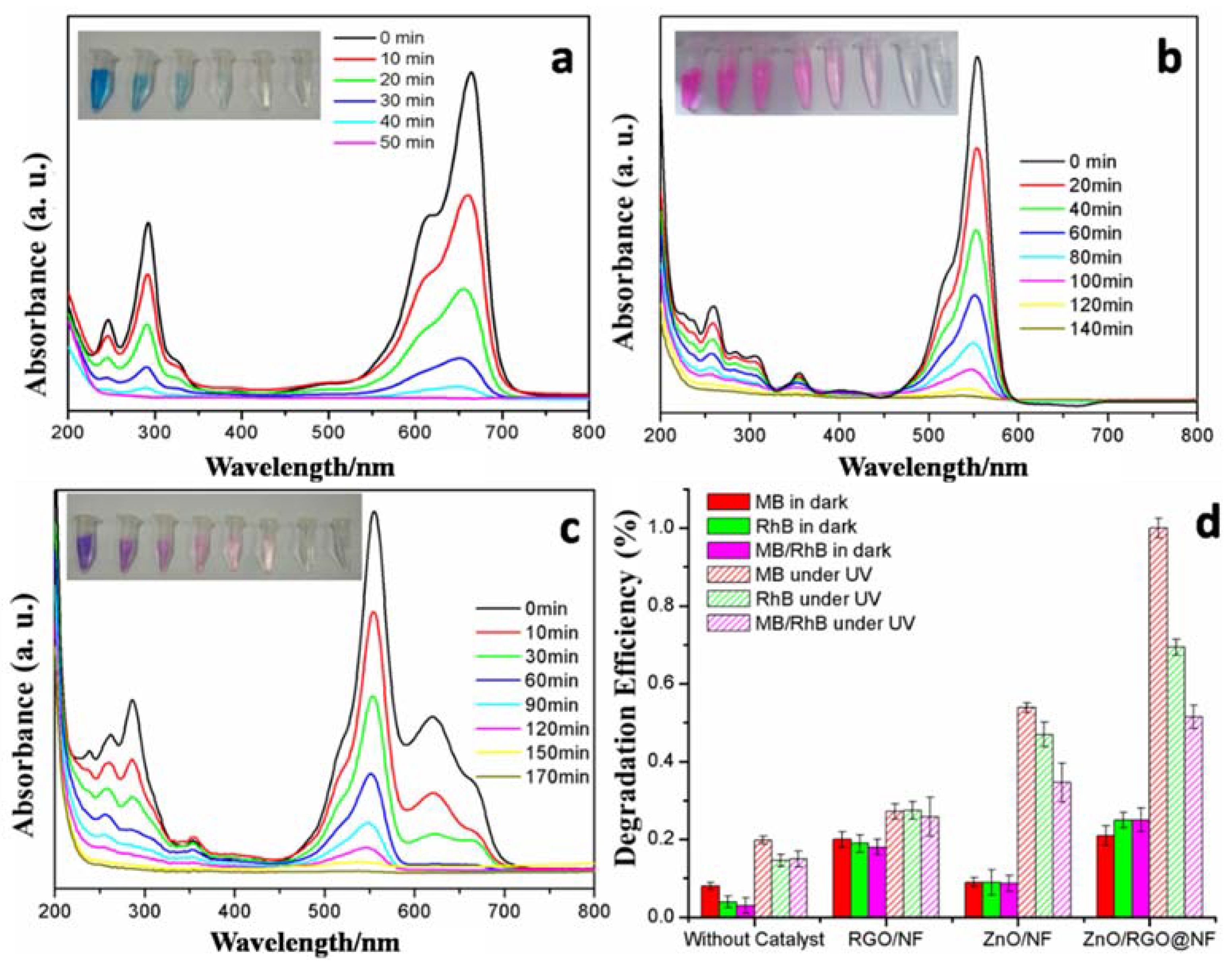
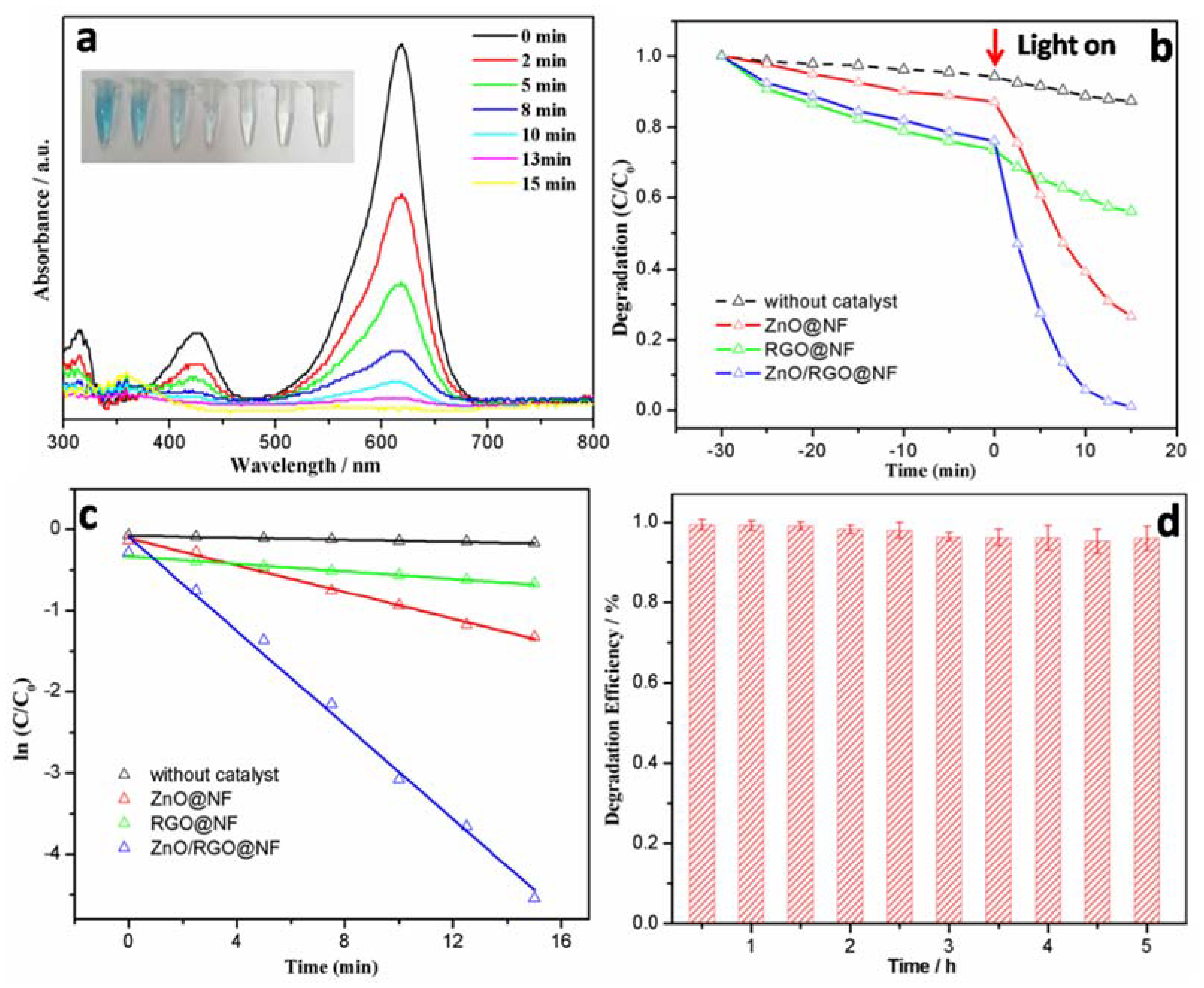
3.2. Characterization of Photocatalytic Activity
3.3. Application of ZnO/RGO@NF as Photocatalytic Catalysts of MG in Seawater
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alderman, D.J. Malachite green: A review. J. Fish Dis. 1985, 8, 289–298. [Google Scholar] [CrossRef]
- Archana, C.; Sachin, G.G.; Suresh, S.U.; Rajamma, S. Mineralization of malachite green dye over visible light responsive bismuth doped TiO2–ZrO2 ferromagnetic nanocomposites. New J. Chem. 2015, 39, 3629–3638. [Google Scholar] [CrossRef]
- María, J.M.B.; Herrera, S.; Uclés, A.; Agüera, A.; Hernando, M.D.; Shimelis, O.; Rudolfsson, M.; Fernández-Alba, A.R. Determination of malachite green residues in fish using molecularly imprinted solid-phase extraction followed by liquid chromatography linear ion trap mass spectrometry. Anal. Chim. Acta 2010, 665, 47–54. [Google Scholar] [CrossRef]
- Geng, Z.; Lin, Y.; Yu, X.; Shen, Q.; Ma, L.; Li, Z.; Pan, N.; Wang, X. Highly efficient dye adsorption and removal: A functional hybrid of reduced graphene oxide–Fe3O4 nanoparticles as an easily regenerative adsorbent. J. Mater. Chem. 2012, 22, 3527–3535. [Google Scholar] [CrossRef]
- Dipshika, D.; Anjali, P. Adsolubilization phenomenon perceived in chitosan beads leading to a fast and enhanced malachite green removal. Chem. Eng. J. 2016, 290, 371–380. [Google Scholar] [CrossRef]
- Sun, L.; Hu, S.; Sun, H.; Guo, H.; Zhu, H.; Liu, M.; Sun, H. Malachite green adsorption onto Fe3O4@SiO2-NH2: Isotherms, kinetic and process optimization. RSC Adv. 2015, 5, 11837–11844. [Google Scholar] [CrossRef]
- Jiang, W.; Liu, Y.; Wang, J.; Zhang, M.; Luo, W.; Zhu, Y. Hydrogels: Separation-Free Polyaniline/TiO2 3D Hydrogel with High Photocatalytic Activity. Adv. Mater. Interfaces 2016, 3, 1500502. [Google Scholar] [CrossRef]
- Wang, C.; Meng, D.; Sun, J.; Memon, J.; Huang, Y.; Geng, J. Graphene Wrapped TiO2 Based Catalysts with Enhanced Photocatalytic Activity. Adv. Mater. Interfaces 2014, 1, 1300150. [Google Scholar] [CrossRef]
- Shen, Y.D.; Deng, X.F.; Xu, Z.L.; Wang, Y.; Lei, H.T.; Wang, H.; Yang, J.Y.; Xiao, Z.L.; Sun, Y.M. Simultaneous determination of malachite green, brilliant green and crystal violet in grass carp tissues by a broad-specificity indirect competitive enzyme enzymelinked immunosorbent assay. Anal. Chim. Acta 2011, 707, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Conti, G.O.; Copat, C.; Wang, Z.; D’Agati, P.; Cristaldi, A.; Ferrante, M. Determination of illegal antimicrobials in aquaculture feed and fish: An ELISA study. Food Control 2015, 50, 937–941. [Google Scholar] [CrossRef]
- Bilandžić, N.; Varenina, I.; Kolanović, B.S.; Oraić, D.; Zrncić, S. Malachite green residues in farmed fish in Croatia. Food Control 2012, 26, 393–396. [Google Scholar] [CrossRef]
- He, J.; Cui, J. Malachite green and chloramphenicol in aquatic products from regions around Dongting Lake in Hunan, China. Food Addit. Contam. Part B 2016, 9, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Belpaire, C.; Reyns, T.; Geeraerts, C.; Van Loco, J. Toxic textile dyes accumulate in wild European eel Anguilla anguilla. Chemosphere 2015, 138, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Akira, F.; Kenichi, H. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Liu, B.; Sang, Y.; Liu, H. Heterostructures construction on TiO2 nanobelts: A powerful tool for building high-performance photocatalysts. Appl. Catal. B 2017, 202, 620–641. [Google Scholar] [CrossRef]
- Hadis, D.; Alireza, N. Increased photocatalytic activity of NiO and ZnO in photodegradation of a model drug aqueous solution: Effect of coupling, supporting, particles size and calcination temperature. J. Hazard. Mater. 2017, 321, 629–638. [Google Scholar] [CrossRef]
- Roya, S.; Mastaneh, S.; Mohammad, R.Z.; Ali, A.S. High-performance visible light-driven Ni-ZnO/rGO/nylon-6 & Ni-ZnO/rGO/nylon-6/Ag nanofiber webs for degrading dye pollutant and study their antibacterial properties. J. Alloy. Compd. 2017, 729, 921–928. [Google Scholar] [CrossRef]
- Sethi, Y.A.; Panmand, R.P.; Kadam, S.R.; Kulkarni, A.K.; Apte, S.K.; Naik, S.D.; Munirathnam, N.; Kulkarni, M.V.; Kale, B.B. Nanostructured CdS sensitized CdWO4 nanorods for hydrogen generation from hydrogen sulfide and dye degradation under sunlight. J. Colloid Interface Sci. 2017, 487, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Robi, S.D.; Jian, Z.; Md, M.; Benjamin, J.C.; Yue, Z.B.; Hareem, K.; Nitu, S.; Ali, Z.; Farjana, H.; Torben, D.; et al. Two dimensional PbMoO4: A photocatalytic material derived from a naturally non-layered crystal. Nano Energy 2018, 49, 237–246. [Google Scholar] [CrossRef]
- Datta, R.S.; Haque, F.; Mohiuddin, M.; Carey, B.J.; Syed, N.; Zavabeti, A.; Zhang, B.; Khan, H.; Berean, K.J.; Ou, J.Z.; et al. Highly active two dimensional α-MoO3−x for the electrocatalytic hydrogen evolution reaction. J. Mater. Chem. A 2017, 5, 24223–24231. [Google Scholar] [CrossRef]
- Xu, Z.; Li, H.; Wu, Z.; Sun, J.; Ying, Z.; Wu, J.; Xu, N. Enhanced charge separation of vertically aligned CdS/g-C3N4 heterojunction nanocone arrays and corresponding mechanisms. J. Mater. Chem. C 2016, 4, 7501–7507. [Google Scholar] [CrossRef]
- Chen, X.; Chen, H.; Guan, J.; Zhen, J.; Sun, Z.; Du, P.; Lu, Y.; Yang, S. A facile mechanochemical route to a covalently bonded graphitic carbon nitride (g-C3N4) and fullerene hybrid toward enhanced visible light photocatalytic hydrogen production. Nanoscale 2017, 9, 5615–5623. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Wang, J.; Xu, H.; Xiong, Y. Coordination chemistry in the design of heterogeneous photocatalysts. Chem. Soc. Rev. 2017, 46, 2799–2823. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.H.; Yang, P.Y.; Lai, C.M.; Lu, S.C.; Li, G.A.; Chang, W.C.; Tuan, H.Y. Synthesis of Cu/ZnO core/shell nanocomposites and their use as efficient photocatalysts. CryEngComm 2016, 18, 616–621. [Google Scholar] [CrossRef]
- Ranjith, K.S.; Rajendra Kumar, R.T. Regeneration of an efficient, solar active hierarchical ZnO flower photocatalyst for repeatable usage: Controlled desorption of poisoned species from active catalytic sites. RSC Adv. 2017, 7, 4983–4992. [Google Scholar] [CrossRef]
- Kavitha, M.K.; Gopinath, P.; John, H. Reduced graphene oxide–ZnO self-assembled films: Tailoring the visible light photoconductivity by the intrinsic defect states in ZnO. Phys. Chem. Chem. Phys. 2015, 17, 14647–14655. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Du, L.; Yu, X.; Tan, S.; Cai, X.; Yang, P.; Gu, Y.; Mai, W. Significantly Enhanced Photocatalytic Activities and Charge Separation Mechanism of Pd-Decorated ZnO–Graphene Oxide Nanocomposites. ACS Appl. Mater. Interfaces 2014, 6, 3623–3629. [Google Scholar] [CrossRef] [PubMed]
- Narendra, S.; Jai, P.; Raju Kumar, G. Design and engineering of high-performance photocatalytic systems based on metal oxide–graphene–noble metal nanocomposites. Mol. Syst. Des. Eng. 2017, 2, 422–439. [Google Scholar] [CrossRef]
- Eseoghene, H.U.; Moses, G.P.; Jane, C.N.; Omotayo, A.A. Photoelectrochemical degradation of orange II dye in wastewater at a silver–zinc oxide/reduced graphene oxide nanocomposite photoanode. RSC Adv. 2016, 6, 52868–52877. [Google Scholar] [CrossRef]
- Divya, K.S.; Marilyn, M.X.; Vandana, P.V.; Reethu, V.N.; Suresh, M.A. Quaternary TiO2/ZnO/RGO/Ag nanocomposite with enhanced visible light photocatalytic performance. New J. Chem. 2017, 41, 6445–6454. [Google Scholar] [CrossRef]
- Xu, T.; Hu, J.; Yang, Y.; Que, W.; Yin, X.; Wu, H.; Chen, L. Ternary system of ZnO nanorods/reduced graphene oxide/CuInS2 quantum dots for enhanced photocatalytic performance. J. Alloy. Compd. 2018, 734, 196–203. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Dong, F.; Huang, H. Readily attainable spongy foam photocatalyst for promising practical photocatalysis. Appl. Catal. B 2017, 208, 75–81. [Google Scholar] [CrossRef]
- Hsu, M.H.; Chang, C.J. S-doped ZnO nanorods on stainless-steel wire mesh as immobilized hierarchical photocatalysts for photocatalytic H2 production. Int. J. Hydrog. Energy 2014, 39, 16524–16533. [Google Scholar] [CrossRef]
- Mouheb, S.; Mohamed, F.N.; Ali, R.; Meenakshisundaram, S.; Ammar, H. TiO2–PANI/Cork composite: A new floating photocatalyst for the treatment of organic pollutants under sunlight irradiation. J. Environ. Sci. 2017, 60, 3–13. [Google Scholar] [CrossRef]
- Wang, M.Y.; Zhu, W.; Ma, L.; Ma, J.J.; Zhang, D.E.; Tong, Z.W.; Chen, J. Enhanced simultaneous detection of ractopamine and salbutamol—Via electrochemical-facial deposition of MnO2nanoflowers onto 3D RGO/Ni foam templates. Biosens. Bioelectron. 2016, 78, 259–266. [Google Scholar] [CrossRef] [PubMed]
- William, S.H.; Richard, E.O. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Liu, X.; Cong, R.; Cao, L.; Liu, S.; Cui, H. The structure, morphology and photocatalytic activity of graphene–TiO2multilayer films and charge transfer at the interface. New J. Chem. 2014, 38, 2362–2367. [Google Scholar] [CrossRef]
- Zhou, H.; Qiu, C.; Liu, Z.; Yang, H.; Hu, L.; Liu, J.; Yang, H.; Gu, C.; Sun, L. Thickness-Dependent Morphologies of Gold on N-Layer Graphenes. J. Am. Chem. Soc. 2010, 132, 944–946. [Google Scholar] [CrossRef] [PubMed]
- Im, H.J.; Jun, G.H.; Lee, D.J.; Ryu, H.J.; Hong, S.H. Enhanced electromagnetic interference shielding behavior of Graphene Nanoplatelet/Ni/Wax nanocomposites. J. Mater. Chem. C 2017, 5, 6471–6479. [Google Scholar] [CrossRef]
- Farjana, J.S.; Hemen, K.; Aslam, M.; Amartya, M. Correlations between preparation methods, structural features and electrochemical Li-storage behavior of reduced graphene oxide. Nanoscale 2017, 9, 11303–11317. [Google Scholar] [CrossRef]
- Rudolf, C.H.; Shawn, S.; Emre, E.; Stefan, W.; Jörg, J.S. Zinc diketonates as single source precursors for ZnO nanoparticles: Microwave-assisted synthesis, electrophoretic deposition and field-effect transistor device properties. J. Mater. Chem. C 2016, 4, 7345–7352. [Google Scholar] [CrossRef]
- Bilal, A.; Animesh, K.O.; Florian, H.; Ingo, F.; Donfack, P.; Arnulf, M. Tailoring of enhanced interfacial polarization in WO3 nanorods grown over reduced graphene oxide synthesized by a one-step hydrothermal method. RSC Adv. 2017, 7, 13985–13996. [Google Scholar] [CrossRef]
- Amparo, F.; Verónica, R.; Teresa, V.; Gregorio, M. Room temperature sintering of polar ZnO nanosheets: II-mechanism. Phys. Chem. Chem. Phys. 2017, 19, 16413–16425. [Google Scholar] [CrossRef]
- Aimin, W.; Jing, L.; Baodan, L.; Wenjin, Y.; Yanan, J.; Lusheng, L.; Xinglai, Z.; Changmin, X.; Xin, J. Band-gap tailoring and visible-light-driven photocatalytic performance of porous (GaN)1−x(ZnO)x solid solution. Dalton Trans. 2017, 46, 2643–2652. [Google Scholar] [CrossRef]
- Xue, B.; Zou, Y. Uniform distribution of ZnO nanoparticles on the surface of graphene and its enhanced photocatalytic performance. Appl. Surf. Sci. 2018, 440, 1123–1129. [Google Scholar] [CrossRef]
- Alaka, S.; Dipti, P.D. Transfiguring UV light active “metal oxides” to visible light active photocatalyst by reduced graphene oxide hypostatization. Catal. Today 2018, 300, 124–135. [Google Scholar] [CrossRef]
- Babasaheb, J.W.; Roby, S.; Kashinath, R.P.; Dipalee, D.M. Calixarene based nanocomposite materials for high-performance supercapacitor electrode. New J. Chem. 2017, 41, 9752–9761. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Cai, C.; Wang, M.; Guo, Q.; Wang, B.; Luo, W.; Wang, Y.; Zhang, C.; Zhou, L.; Zhang, D.; et al. Efficient Photocatalytic Degradation of Malachite Green in Seawater by the Hybrid of Zinc-Oxide Nanorods Grown on Three-Dimensional (3D) Reduced Graphene Oxide(RGO)/Ni Foam. Materials 2018, 11, 1004. https://doi.org/10.3390/ma11061004
Wang Q, Cai C, Wang M, Guo Q, Wang B, Luo W, Wang Y, Zhang C, Zhou L, Zhang D, et al. Efficient Photocatalytic Degradation of Malachite Green in Seawater by the Hybrid of Zinc-Oxide Nanorods Grown on Three-Dimensional (3D) Reduced Graphene Oxide(RGO)/Ni Foam. Materials. 2018; 11(6):1004. https://doi.org/10.3390/ma11061004
Chicago/Turabian StyleWang, Qing, Chaoyue Cai, Mingyan Wang, Qian Guo, Biao Wang, Weina Luo, Yujuan Wang, Chenyan Zhang, Lihua Zhou, Dongen Zhang, and et al. 2018. "Efficient Photocatalytic Degradation of Malachite Green in Seawater by the Hybrid of Zinc-Oxide Nanorods Grown on Three-Dimensional (3D) Reduced Graphene Oxide(RGO)/Ni Foam" Materials 11, no. 6: 1004. https://doi.org/10.3390/ma11061004
APA StyleWang, Q., Cai, C., Wang, M., Guo, Q., Wang, B., Luo, W., Wang, Y., Zhang, C., Zhou, L., Zhang, D., Tong, Z., Liu, Y., & Chen, J. (2018). Efficient Photocatalytic Degradation of Malachite Green in Seawater by the Hybrid of Zinc-Oxide Nanorods Grown on Three-Dimensional (3D) Reduced Graphene Oxide(RGO)/Ni Foam. Materials, 11(6), 1004. https://doi.org/10.3390/ma11061004





