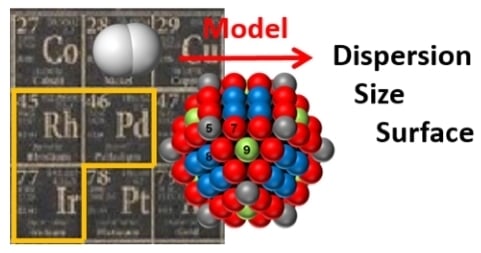
Palladium, Iridium, and Rhodium Supported Catalysts: Predictive H2 Chemisorption by Statistical Cuboctahedron Clusters Model
Abstract
:1. Introduction
2. Model Calculation
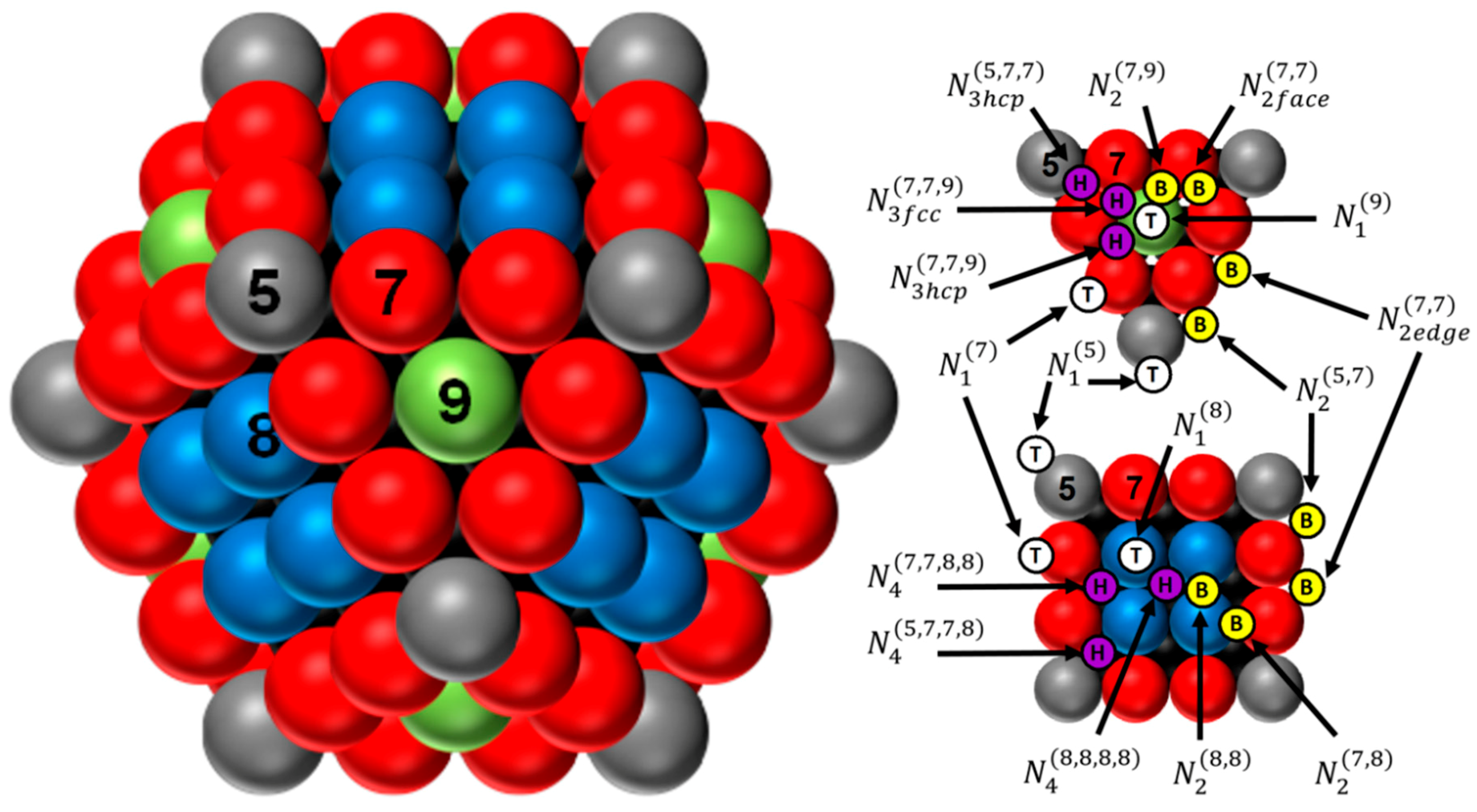
2.1. Dispersion, Size, Metallic Specific Surface Area, and Adsorption Surface Sites of the Cuboctahedron Crystallite
2.2. Surface Hydrogen Adsorption Sites on Metal Cuboctahedron Crystallite (H/M) and H Chemisorption Stoichiometric Factor (H/MS)
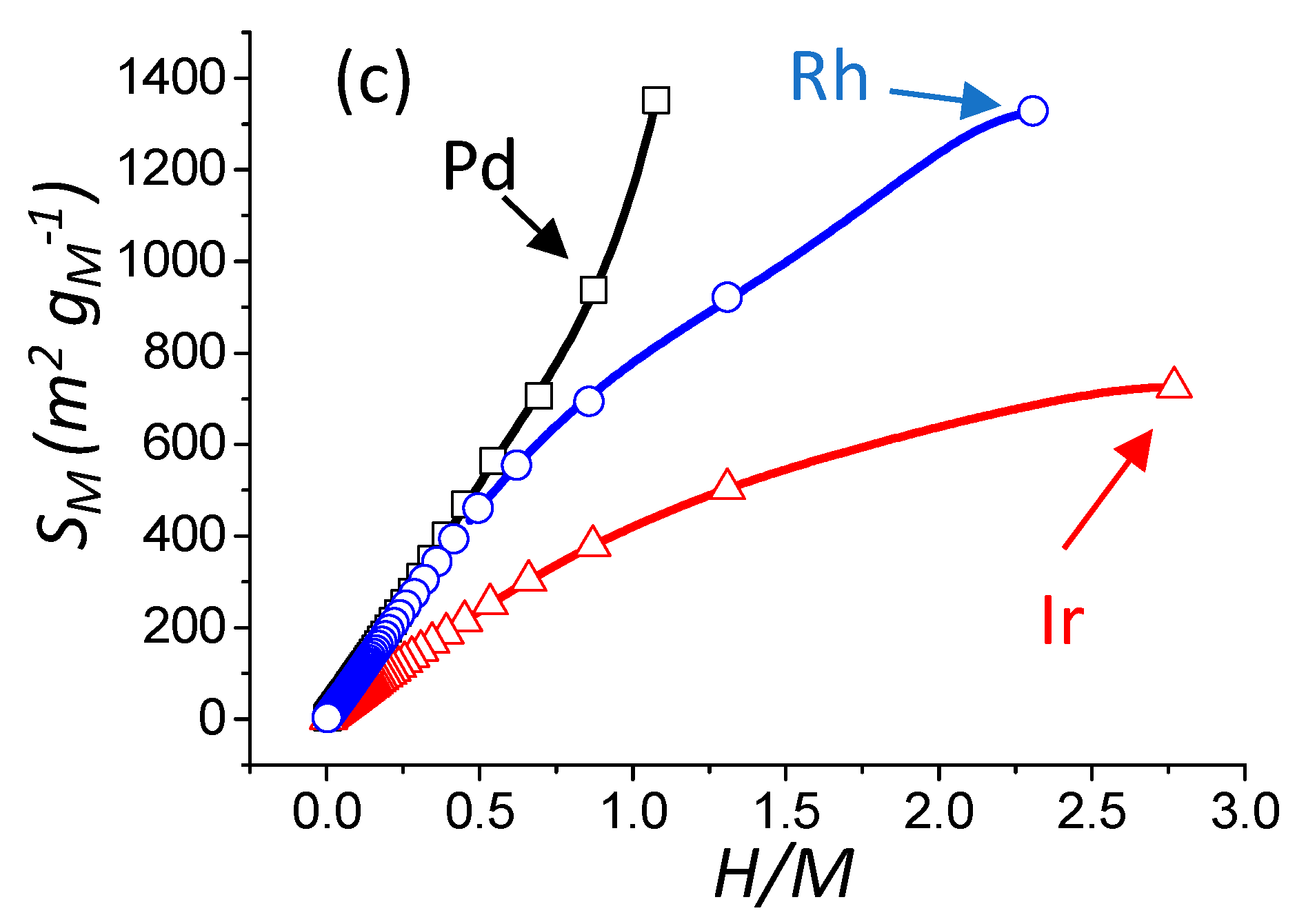
2.2.1. Case of Pd
2.2.2. Case of Ir
2.2.3. Case of Rh
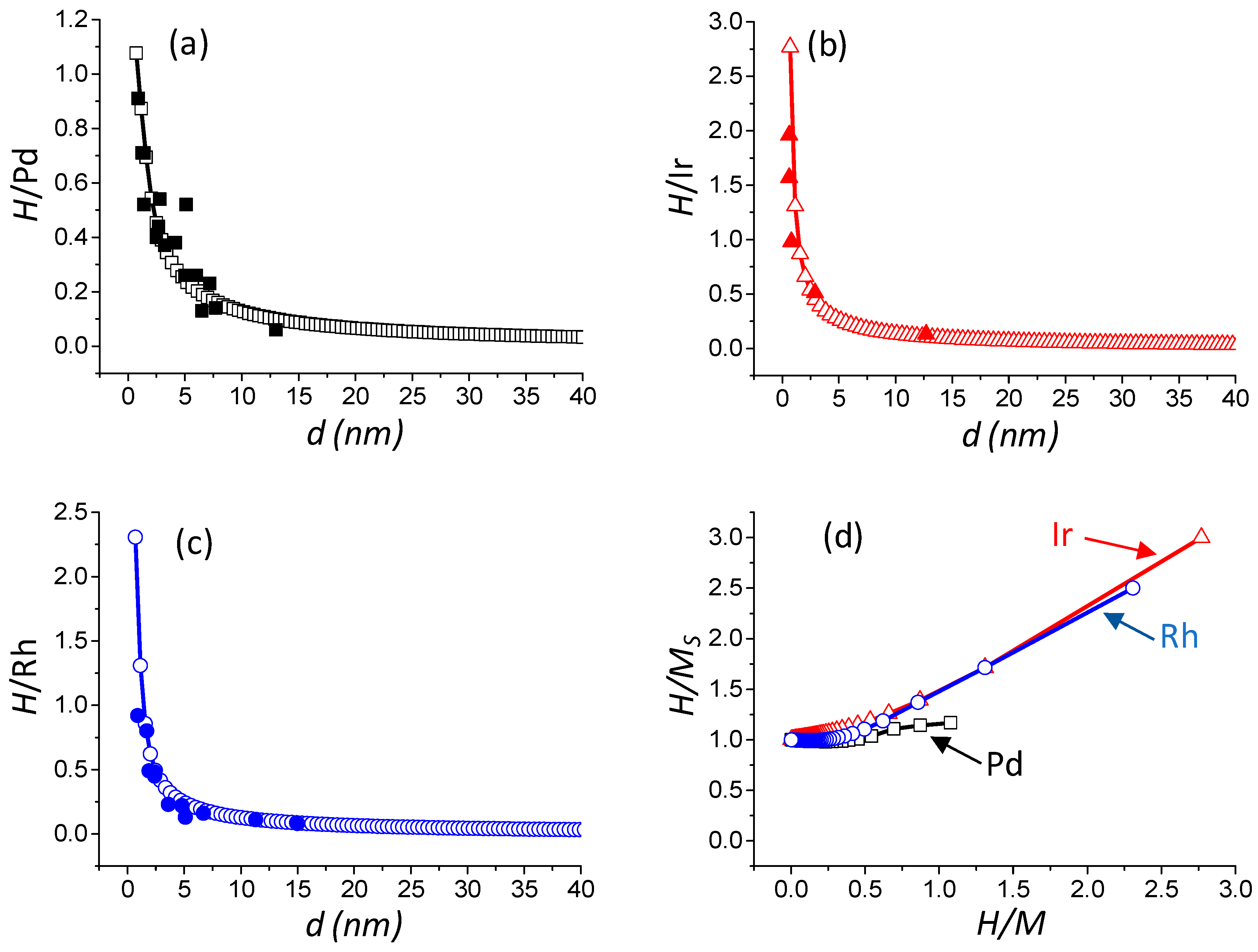
2.2.4. Determination of the Stoichiometric Factor and Correlation between Experimental and Model Calculations
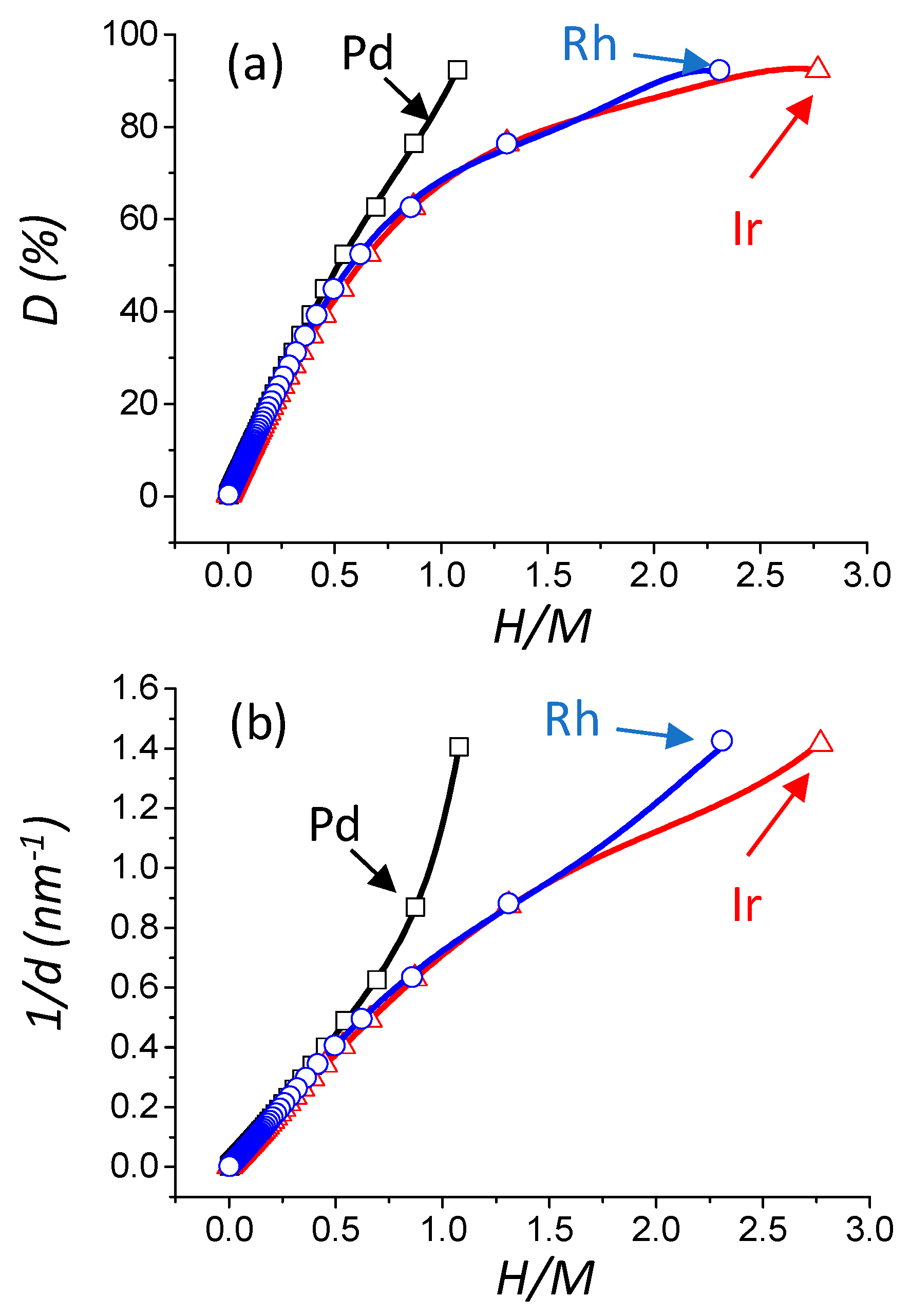
2.3. Determination of the Dispersion, Particle Size, and Metallic Specific Surface Area from H/M Ratios
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| particle size (particle diameter) | |
| metallic diameter | |
| dispersion | |
| fcc | face centered cubic |
| hcp | hexagonal close packing |
| H | hydrogen |
| number of adsorbed hydrogen per total number of metal atoms | |
| coordination number | |
| Ir | iridium |
| number of atoms lying on equivalent edge, corners atoms included | |
| metal | |
| atom on metal surface | |
| total number of bulk atoms | |
| total number of atoms of i coordination number | |
| number of hydrogen atoms adsorbed on the metal surface | |
| total number of surface atoms | |
| total number of atoms | |
| top adsorption site (for example represents the top adsorption site over a surface atom of 5 coordination number) | |
| bridge adsorption site (for example represents the bridge adsorption site between two surface atoms of 5 coordination number) | |
| hollow (3-fold) adsorption site (for example represents the hollow (3-fold) adsorption site between three surface atoms of 5 coordination number) | |
| hollow (4-fold) adsorption site (for example represents the hollow (4-fold) adsorption site between four surface atoms of 5 coordination number) | |
| Pd | palladium |
| Pt | platinum |
| Rh | rhodium |
| accessible surface area of the surface atom of type | |
| metallic specific surface area | |
| density of the metal | |
| reciprocal particle size of the considered metal | |
| chemisorption stoichiometric factor of hydrogen atoms over the metal surface |
References
- Ma, Z.; Zaera, F. Heterogeneous catalysis by metals. In Encyclopedia of Inorganic Chemistry; King, R.B., Crabtree, R.H., Lukehart, C.M., Atwood, D.A., Scott, R.A., Eds.; John Wiley & Sons, Ltd.: New York, NY, USA, 2006. [Google Scholar]
- Dumesic, J.A.; Huber, G.W.; Boudart, M. Principles of heterogeneous catalysis. In Handbook of Heterogeneous Catalysis; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008. [Google Scholar]
- Nakatsuji, H.; Matsuzaki, Y.; Yonezawa, T. Abinitio theoretical study on the reactions of a hydrogen molecule with small platinum clusters: A model for chemisorption on a pt surface. J. Chem. Phys. 1988, 88, 5759–5769. [Google Scholar] [CrossRef]
- Yang, B.; Lu, Q.; Wang, Y.; Zhuang, L.; Lu, J.; Liu, P.; Wang, J.; Wang, R. Simple and low-cost preparation method for highly dispersed PtRu/C catalysts. Chem. Mater. 2003, 15, 3552–3557. [Google Scholar] [CrossRef]
- Tian, Z.Q.; Xie, F.Y.; Shen, P.K. Preparation of high loading Pt supported on carbon by on-site reduction. J. Mater. Sci. 2004, 39, 1507–1509. [Google Scholar] [CrossRef]
- Shokouhimehr, M. Magnetically separable and sustainable nanostructured catalysts for heterogeneous reduction of nitroaromatics. Catalysts 2015, 5, 534–560. [Google Scholar] [CrossRef]
- Gawande, M.; Rathi, A.; Branco, P.; Varma, R. Sustainable utility of magnetically recyclable nano-catalysts in water: Applications in organic synthesis. Appl. Sci. 2013, 3, 656–674. [Google Scholar] [CrossRef]
- Corbos, E.C.; Haneda, M.; Courtois, X.; Marecot, P.; Duprez, D.; Hamada, H. Nox abatement for lean-burn engines under lean-rich atmosphere over mixed NSR-SCR catalysts: Influences of the addition of a SCR catalyst and of the operational conditions. Appl. Catal. A Gen. 2009, 365, 187–193. [Google Scholar] [CrossRef]
- Le Valant, A.; Garron, A.; Bion, N.; Epron, F.; Duprez, D. Hydrogen production from raw bioethanol over Rh/MgAl2O4 catalyst impact of impurities: Heavy alcohol, aldehyde, ester, acid and amine. Catal. Today 2008, 138, 169–174. [Google Scholar] [CrossRef]
- Mary, S.; Kappenstein, C.; Balcon, S.; Rossignol, S.; Gengembre, E. Monopropellant decomposition catalysts. I. Ageing of highly loaded Ir/Al2O3 catalysts in oxygen and steam. Influence of chloride content. Appl. Catal. A Gen. 1999, 182, 317–325. [Google Scholar] [CrossRef]
- Moraes, R.; Thomas, K.; Thomas, S.; Van Donk, S.; Grasso, G.; Gilson, J.-P.; Houalla, M. Ring opening of decalin and methylcyclohexane over bifunctional Ir/WO3/Al2O3 catalysts. J. Catal. 2013, 299, 30–43. [Google Scholar] [CrossRef]
- Nordlander, P.; Holloway, S.; Norskov, J.K. Hydrogen adsorption on metal-surfaces. Surf. Sci. 1984, 136, 59–81. [Google Scholar] [CrossRef]
- Mustard, D.G.; Bartholomew, C.H. Determination of metal crystallite size and morphology in supported nickel-catalysts. J. Catal. 1981, 67, 186–206. [Google Scholar] [CrossRef]
- Bergeret, G.; Gallezot, P. Particle size and dispersion measurements. In Handbook of Heterogeneous Catalysis; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008. [Google Scholar]
- Bartholemew, C.H. H2 adsorption on supported noble metals and its use in determining metal dispersion. In Catalysis: Volume 11; Spivey, J.J., Agarwal, S.K., Eds.; The Royal Society of Chemistry: London, UK, 1994; Volume 11, pp. 93–126. [Google Scholar]
- Foger, K. Dispersed metal catalysts. In Catalysis: Science and Technology; Anderson, J.R., Boudart, M., Eds.; Springer: Berlin/Heidelberg, Germany, 1984; Volume 6, pp. 227–305. [Google Scholar]
- Kip, B.J.; Duivenvoorden, F.B.M.; Koningsberger, D.C.; Prins, R. Determination of metal particle size of highly dispersed Rh, Ir, and Pt catalysts by hydrogen chemisorption and exafs. J. Catal. 1987, 105, 26–38. [Google Scholar] [CrossRef]
- McVicker, G.B.; Baker, R.T.K.; Garten, R.L.; Kugler, E.L. Chemisorption properties of iridium on alumina catalysts. J. Catal. 1980, 65, 207–220. [Google Scholar] [CrossRef]
- Krishnamurthy, S.; Landolt, G.R.; Schoennagel, H.J. The stoichiometry of hydrogen and CO chemisorption on Irγ-Al2O3. J. Catal. 1982, 78, 319–326. [Google Scholar] [CrossRef]
- Prins, R. Hydrogen spillover. Facts and fiction. Chem. Rev. 2012, 112, 2714–2738. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.R.; Benesi, H.A.; Curtis, R.M.; Meisenheimer, R.G. Particle size determination of supported catalytic metals: Platinum on silica gel. J. Catal. 1962, 1, 336–344. [Google Scholar] [CrossRef]
- Benson, J.E.; Hwang, H.S.; Boudart, M. Hydrogen-oxygen titration method for the measurement of supported palladium surface areas. J. Catal. 1973, 30, 146–153. [Google Scholar] [CrossRef]
- Ji, Y.; van der Eerden, A.M.J.; Koot, V.; Kooyman, P.J.; Meeldijk, J.D.; Weckhuysen, B.M.; Koningsberger, D.C. Influence of support ionicity on the hydrogen chemisorption of Pt particles dispersed in Y zeolite: Consequences for Pt particle size determination using the H/M method. J. Catal. 2005, 234, 376–384. [Google Scholar] [CrossRef]
- Wilson, G.R.; Hall, W.K. Studies of the hydrogen held by solids. Xix. H2 and O2 chemisorption on silica-supported platinum. J. Catal. 1972, 24, 306–314. [Google Scholar] [CrossRef]
- Le Valant, A.; Comminges, C.; Can, F.; Thomas, K.; Houalla, M.; Epron, F. Platinum supported catalysts: Predictive CO and H2 chemisorption by a statistical cuboctahedron cluster model. J. Phys. Chem. C 2016, 120, 26374–26385. [Google Scholar] [CrossRef]
- Freel, J. Chemisorption on supported platinum: I. Evaluation of a pulse method. J. Catal. 1972, 25, 139–148. [Google Scholar] [CrossRef]
- Freel, J. Chemisorption on supported platinum: II. Stoichiometry for hydrogen, oxygen and carbon monoxide. J. Catal. 1972, 25, 149–160. [Google Scholar] [CrossRef]
- Okamoto, Y. Comparison of hydrogen atom adsorption on Pt clusters with that on Pt surfaces: A study from density-functional calculations. Chem. Phys. Lett. 2006, 429, 209–213. [Google Scholar] [CrossRef]
- Van Hardeveld, R.; Hartog, F. The statistics of surface atoms and surface sites on metal crystals. Surf. Sci. 1969, 15, 189–230. [Google Scholar] [CrossRef]
- Eichler, A.; Hafner, J.; Kresse, G. Hydrogen adsorption on the (100) surfaces of rhodium and palladium: The influence of non-local exchange—Correlation interactions. J. Phys. Condens. Matter 1996, 8, 7659. [Google Scholar] [CrossRef]
- Greeley, J.; Mavrikakis, M. Surface and subsurface hydrogen: Adsorption properties on transition metals and near-surface alloys. J. Phys. Chem. B 2005, 109, 3460–3471. [Google Scholar] [CrossRef] [PubMed]
- Watari, N.; Ohnishi, S.; Ishii, Y. Hydrogen storage in Pd clusters. J. Phys. Condens. Matter 2000, 12, 6799. [Google Scholar] [CrossRef]
- Davis, J.B.A.; Horswell, S.L.; Piccolo, L.; Johnston, R.L. Computational study of the adsorption of benzene and hydrogen on palladium–iridium nanoalloys. J. Organomet. Chem. 2015, 792, 190–193. [Google Scholar] [CrossRef]
- Petrova, G.P.; Vayssilov, G.N.; Rösch, N. Density functional study of hydrogen adsorption on tetrairidium supported on hydroxylated and dehydroxylated zeolite surfaces. J. Phys. Chem. C 2007, 111, 14484–14492. [Google Scholar] [CrossRef]
- Mainardi, D.S.; Balbuena, P.B. Hydrogen and oxygen adsorption on Rhn (n = 1 − 6) clusters. J. Phys. Chem. A 2003, 107, 10370–10380. [Google Scholar] [CrossRef]
- Mager-Maury, C.; Bonnard, G.; Chizallet, C.; Sautet, P.; Raybaud, P. H2-induced reconstruction of supported Pt clusters: Metal-Support interaction versus surface hydride. ChemCatChem 2011, 3, 200–207. [Google Scholar] [CrossRef]
- Aben, P.C. Palladium areas in supported catalysts. Determination of palladium surface areas in supported catalysts by means of hydrogen chemisorption. J. Catal. 1968, 10, 224–229. [Google Scholar] [CrossRef]
- Goetz, J.; Volpe, M.A.; Sica, A.M.; Gigola, C.E.; Touroude, R. Low-loaded palladium on α-alumina catalysts: Characterization by chemisorption, electron-microscopy, and photoelectron spectroscopy. J. Catal. 1995, 153, 86–93. [Google Scholar] [CrossRef]
- Kunimori, K.; Uchijima, T.; Yamada, M.; Matsumoto, H.; Hattori, T.; Murakami, Y. Percentage exposed of supported Pt, Pd and Rh catalysts studied by gas adsorption, tpr and tem methods. Appl. Catal. 1982, 4, 67–81. [Google Scholar] [CrossRef]
- Zou, W.; Gonzalez, R.D. The preparation of silica supported Pd catalysts: The effect of pretreatment variables on particle size. Catal. Lett. 1992, 12, 73–86. [Google Scholar] [CrossRef]
- Fuentes, S.; Figueras, F. The influence of particle size on the catalytic properties of alumina-supported rhodium catalysts. J. Catal. 1980, 61, 443–453. [Google Scholar] [CrossRef]
- Grass, M.E.; Joo, S.H.; Zhang, Y.; Somorjai, G.A. Colloidally synthesized monodisperse Rh nanoparticles supported on SBA-15 for size- and pretreatment-dependent studies of CO oxidation. J. Phys. Chem. C 2009, 113, 8616–8623. [Google Scholar] [CrossRef]
| Type | m | ||||
|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | ||
| Atoms | 13 | 55 | 147 | ||
| 12 | 42 | 92 | |||
| 1 | 13 | 55 | |||
| 12 | 12 | 12 | 12 | ||
| 0 | 24 | 48 | |||
| 0 | 6 | 24 | |||
| 0 | 0 | 8 | |||
| D (%) | Pd, Ir and Rh | 92.3 | 76.4 | 62.6 | |
| d (nm) | Pd | 0.7 | 1.2 | 1.6 | |
| Ir | 0.7 | 1.1 | 1.6 | ||
| Rh | 0.7 | 1.1 | 1.6 | ||
| SM (m2 g−1) | Pd | 1352.2 | 937.3 | 705.7 | |
| Ir | 725.7 | 503.0 | 378.8 | ||
| Rh | 1329.1 | 921.3 | 693.7 | ||
| Top sites | 12 | 12 | 12 | 12 | |
| 0 | 24 | 48 | |||
| 0 | 6 | 24 | |||
| 0 | 0 | 8 | |||
| Bridge sites | 24 | 0 | 0 | 0 | |
| 0 | 48 | 48 | 48 | ||
| 0 | 0 | 24 | |||
| 0 | 24 | 24 | 24 | ||
| 0 | 24 | 48 | |||
| 0 | 0 | 24 | |||
| 0 | 0 | 48 | |||
| 0 | 0 | 0 | |||
| Hollow sites | 8 | 0 | 0 | 0 | |
| 0 | 24 | 24 | 24 | ||
| 0 | 8 | 0 | 0 | ||
| 0 | 0 | 24 | 24 | ||
| 0 | 0 | 24 | |||
| 0 | 0 | 0 | |||
| 0 | 0 | 0 | |||
| 0 | 0 | 0 | |||
| 6 | 0 | 0 | 0 | ||
| 0 | 24 | 24 | 24 | ||
| 0 | 0 | 24 | |||
| 0 | 0 | 6 | |||
| Metal | Surface or Shape | H Adsorption Favored Sites | Ref |
|---|---|---|---|
| Pd | (100) | Hollow 4-fold | [30] |
| (111) | Hollow 3-fold fcc | [31] | |
| Cuboctahedron (Pd13) | Hollow 4-fold and 3-fold hcp | [32] | |
| Ir | (100) | Bridge | [33] |
| (111) | Top | [31] | |
| Truncated octahedron (Ir38) | Bridge (edge) | [33] | |
| Tetrahedron (Ir4) | Top (corner) and Bridge (at Ir–Ir bonds) | [34] | |
| Rh | (100) | Hollow 4-fold | [30] |
| (111) | Hollow 3-fold fcc | [31] | |
| Tetrahedron (Rh4) | Bridge (edge) | [35] | |
| Octahedron (Rh6) | Bridge (edge) | [35] |
| M/Support | H/M | d (nm) | Ref |
|---|---|---|---|
| Pd/SiO2 | 0.40 | 2.5 | |
| Pd/SiO2 | 0.13 | 6.5 | |
| Pd/Al2O3 | 0.41 | 2.5 | [37] |
| Pd/Al2O3 | 0.06 | 13 | |
| Pd/Al2O3 | 0.54 | 2.8 | |
| Pd/Al2O3 | 0.52 | 1.4 | |
| Pd/Al2O3 | 0.52 | 5.1 | |
| Pd/Al2O3 | 0.14 | 7.7 | [38] |
| Pd/Al2O3 | 0.26 | 6 | |
| Pd/Al2O3 | 0.23 | 7.2 | |
| Pd/Al2O3 | 0.91 | 0.9 | [39] |
| Pd/Al2O3 | 0.26 | 5 | |
| Pd/Al2O3 | 0.44 | 2.7 | |
| Pd/Al2O3 | 0.37 | 3.2 | [40] |
| Pd/Al2O3 | 0.38 | 4.2 | |
| Pd/Al2O3 | 0.71 | 1.4 | |
| Pd/Al2O3 | 0.71 | 1.2 | |
| Ir/Al2O3 | 1.96 | <0.6 | |
| Ir/Al2O3 | 1.57 | <0.6 | |
| Ir/Al2O3 | 0.98 | 0.81 | [18] |
| Ir/Al2O3 | 0.51 | 2.9 | |
| Ir/Al2O3 | 0.13 | 12.7 | |
| Rh/Al2O3 | 0.92 | 0.9 | [39] |
| Rh/Al2O3 | 0.22 | 4.8 | |
| Rh/Al2O3 | 0.80 | 1.7 | [41] |
| Rh/Al2O3 | 0.45 | 2.4 | |
| Rh/Al2O3 | 0.082 | 15 | |
| Rh/SBA-15 | 0.49 | 1.9 | |
| Rh/SBA-15 | 0.49 | 1.9 | |
| Rh/SBA-15 | 0.48 | 2.4 | |
| Rh/SBA-15 | 0.23 | 3.6 | [42] |
| Rh/SBA-15 | 0.13 | 5.1 | |
| Rh/SBA-15 | 0.16 | 6.7 | |
| Rh/SBA-15 | 0.11 | 11.3 |
| Equation | ||||||
|---|---|---|---|---|---|---|
| Pd | −5.055 | 71.208 | −117.720 | 38.434 | 98.775 | |
| Ir | −2.116 | 13.163 | −20.633 | −23.073 | 100.361 | |
| Rh | −8.599 | 46.065 | −73.064 | 2.015 | 101.969 | |
| Pd | 1.912 | −2.665 | 0.875 | 0.288 | 0.737 | |
| Ir | 0.000 | 0.038 | −0.171 | 0.099 | 0.743 | |
| Rh | −0.063 | 0.390 | −0.771 | 0.414 | 0.753 | |
| Pd | 1053.493 | −1139.725 | −119.922 | 463.061 | 903.061 | |
| Ir | −12.169 | 81.710 | −176.563 | 39.215 | 487.871 | |
| Rh | −106.053 | 576.518 | −1005.933 | 413.062 | 900.314 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drault, F.; Comminges, C.; Can, F.; Pirault-Roy, L.; Epron, F.; Le Valant, A. Palladium, Iridium, and Rhodium Supported Catalysts: Predictive H2 Chemisorption by Statistical Cuboctahedron Clusters Model. Materials 2018, 11, 819. https://doi.org/10.3390/ma11050819
Drault F, Comminges C, Can F, Pirault-Roy L, Epron F, Le Valant A. Palladium, Iridium, and Rhodium Supported Catalysts: Predictive H2 Chemisorption by Statistical Cuboctahedron Clusters Model. Materials. 2018; 11(5):819. https://doi.org/10.3390/ma11050819
Chicago/Turabian StyleDrault, Fabien, Clément Comminges, Fabien Can, Laurence Pirault-Roy, Florence Epron, and Anthony Le Valant. 2018. "Palladium, Iridium, and Rhodium Supported Catalysts: Predictive H2 Chemisorption by Statistical Cuboctahedron Clusters Model" Materials 11, no. 5: 819. https://doi.org/10.3390/ma11050819
APA StyleDrault, F., Comminges, C., Can, F., Pirault-Roy, L., Epron, F., & Le Valant, A. (2018). Palladium, Iridium, and Rhodium Supported Catalysts: Predictive H2 Chemisorption by Statistical Cuboctahedron Clusters Model. Materials, 11(5), 819. https://doi.org/10.3390/ma11050819