Self-Cleaning Ceramic Tiles Produced via Stable Coating of TiO2 Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
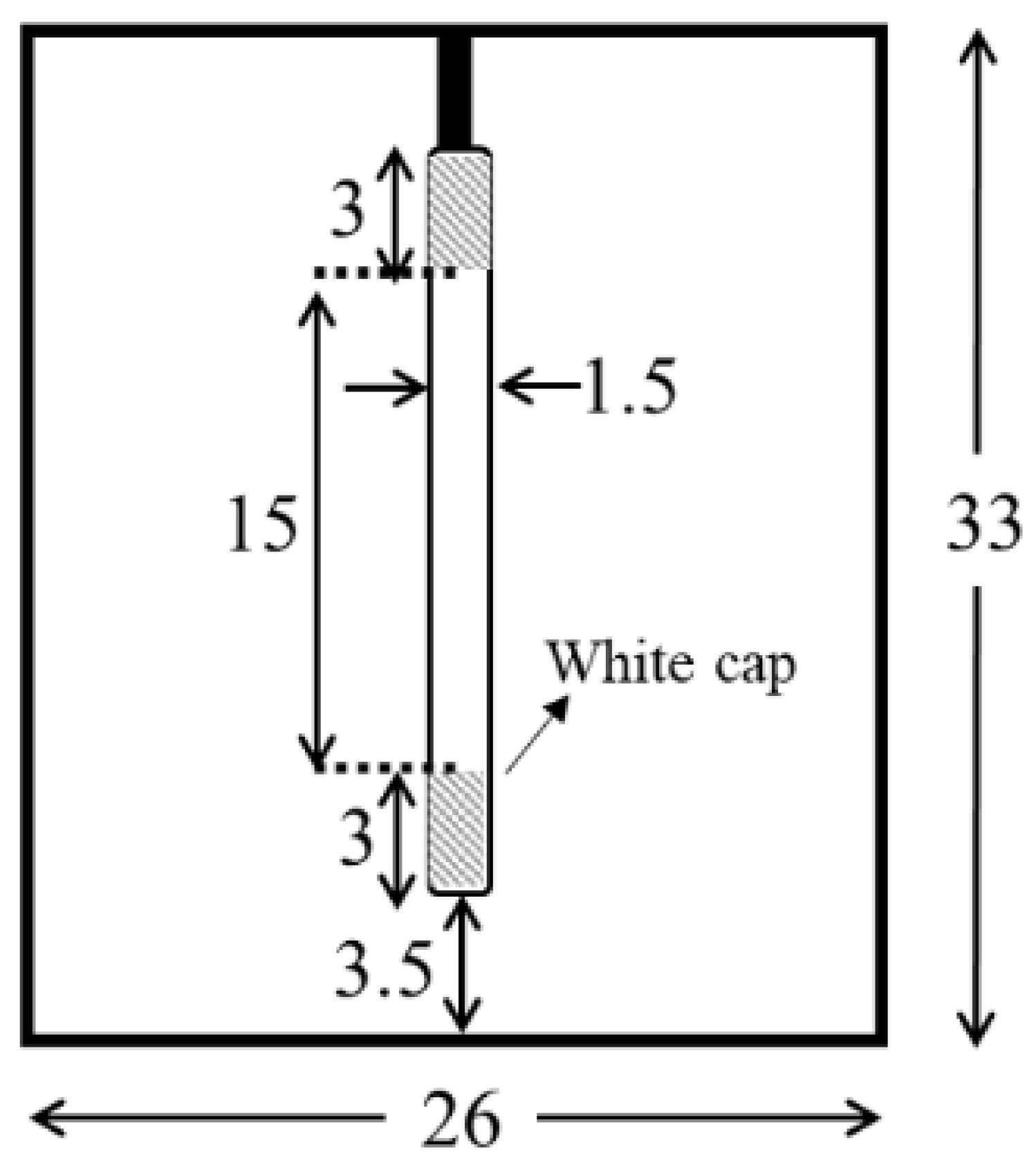
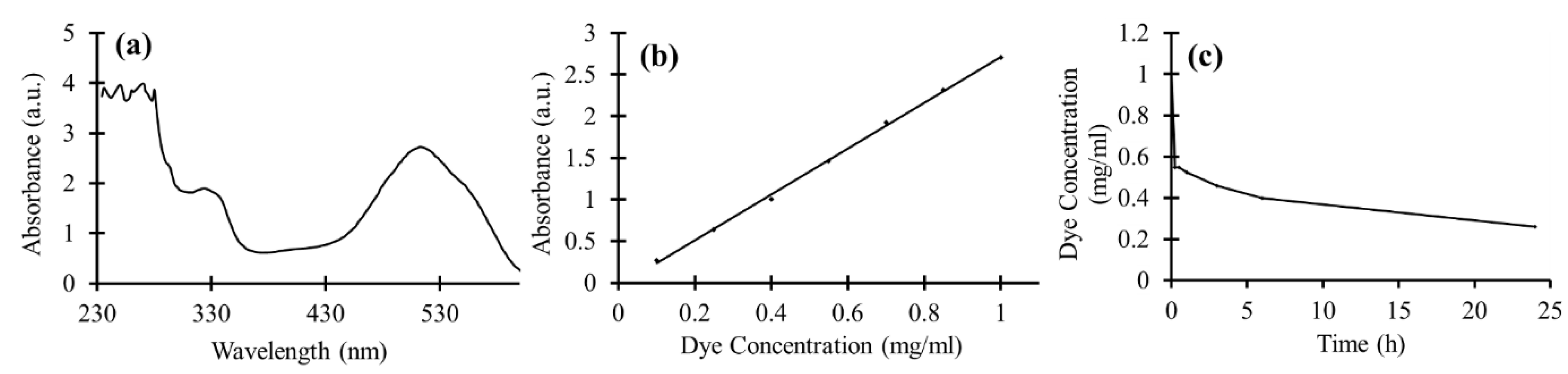
2.2. Dye-Degradation Measurements
2.3. Dye-Degradation Measurement of TiO2 Nanoparticles
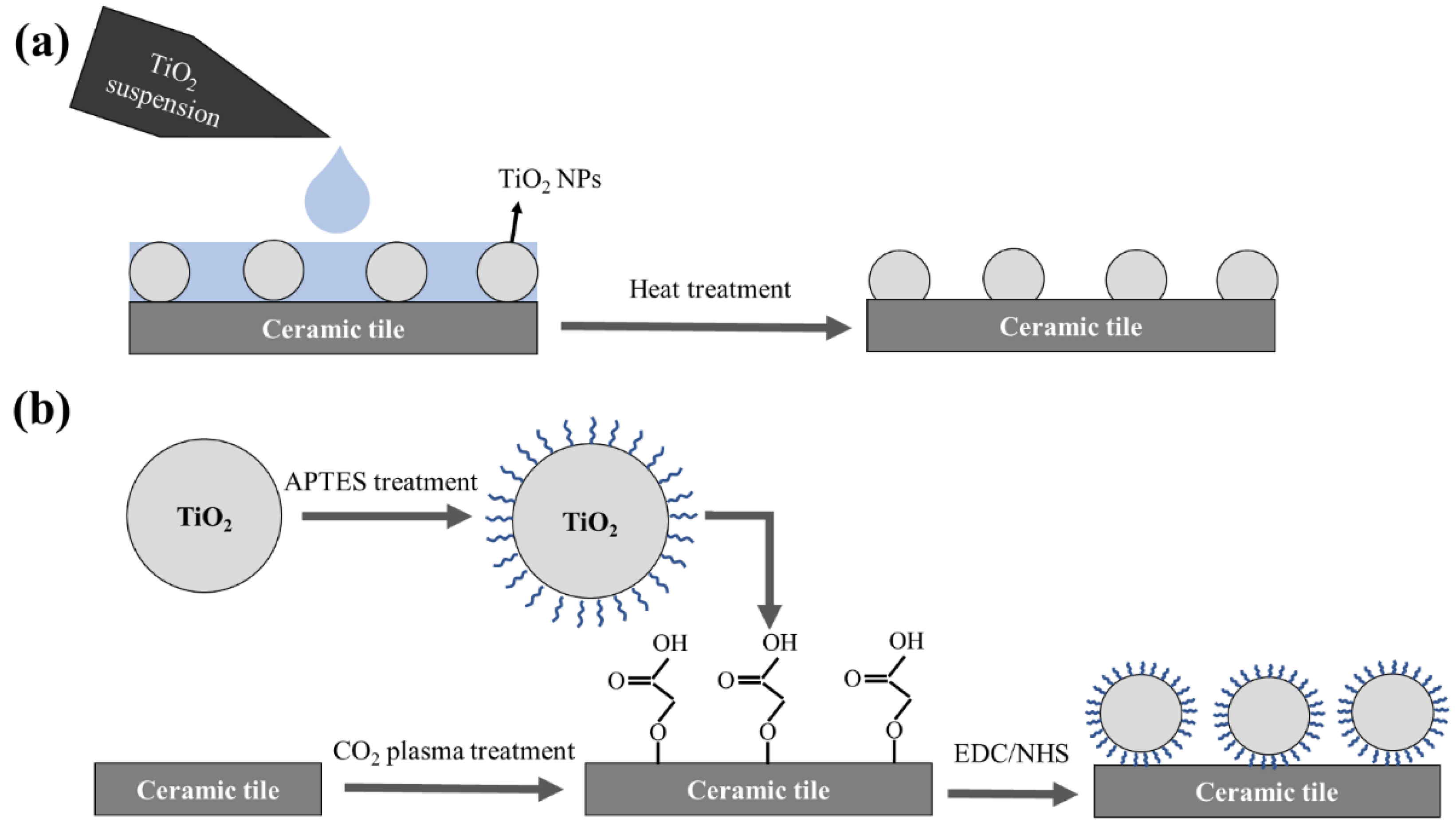
2.4. Coating TiO2 Nanoparticles Using Heat Treatment Technique
2.5. APTES Treatment Technique
2.6. Characterization Techniques
3. Results and Discussion
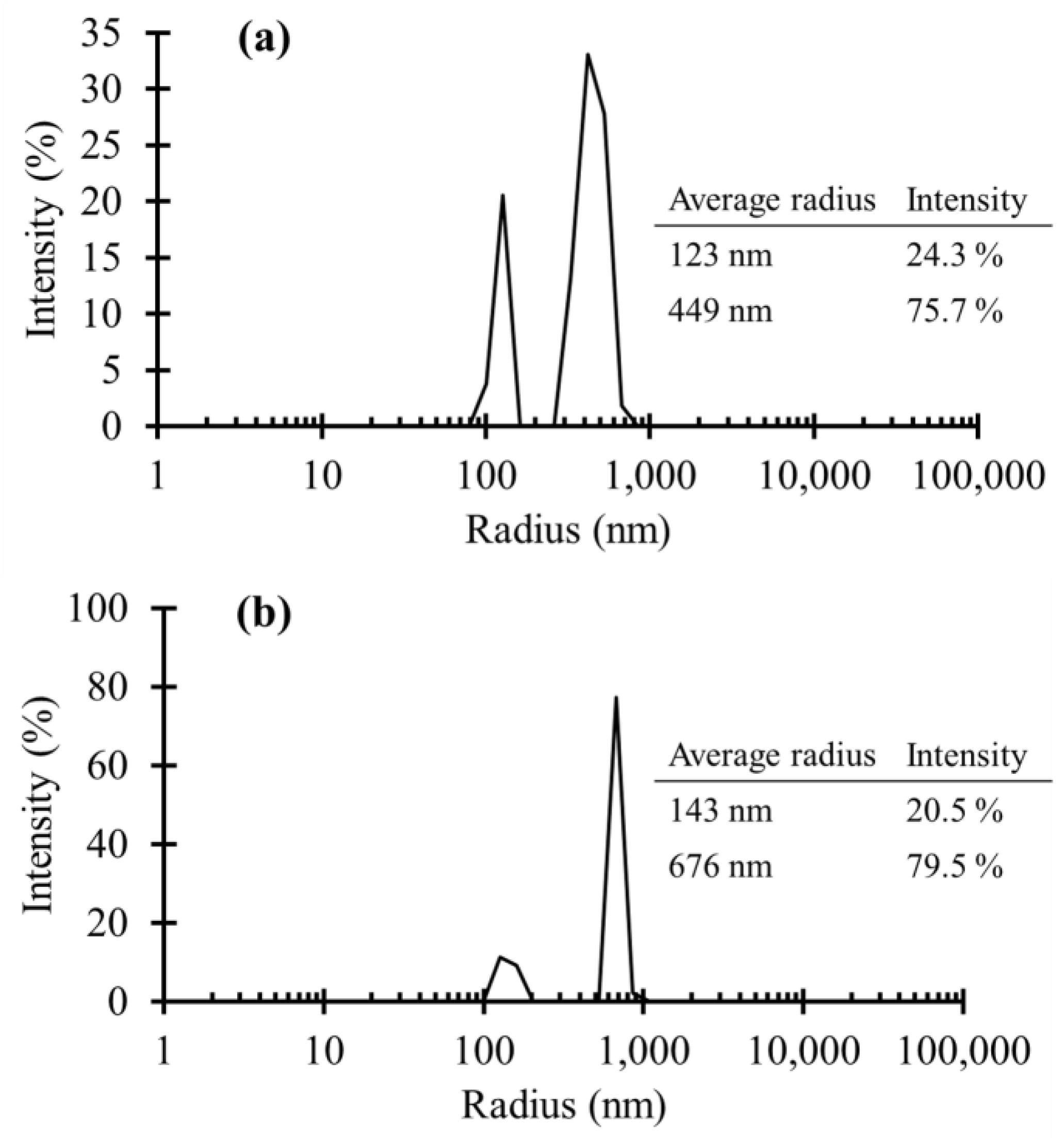
3.1. TiO2 NPs Size Measurements
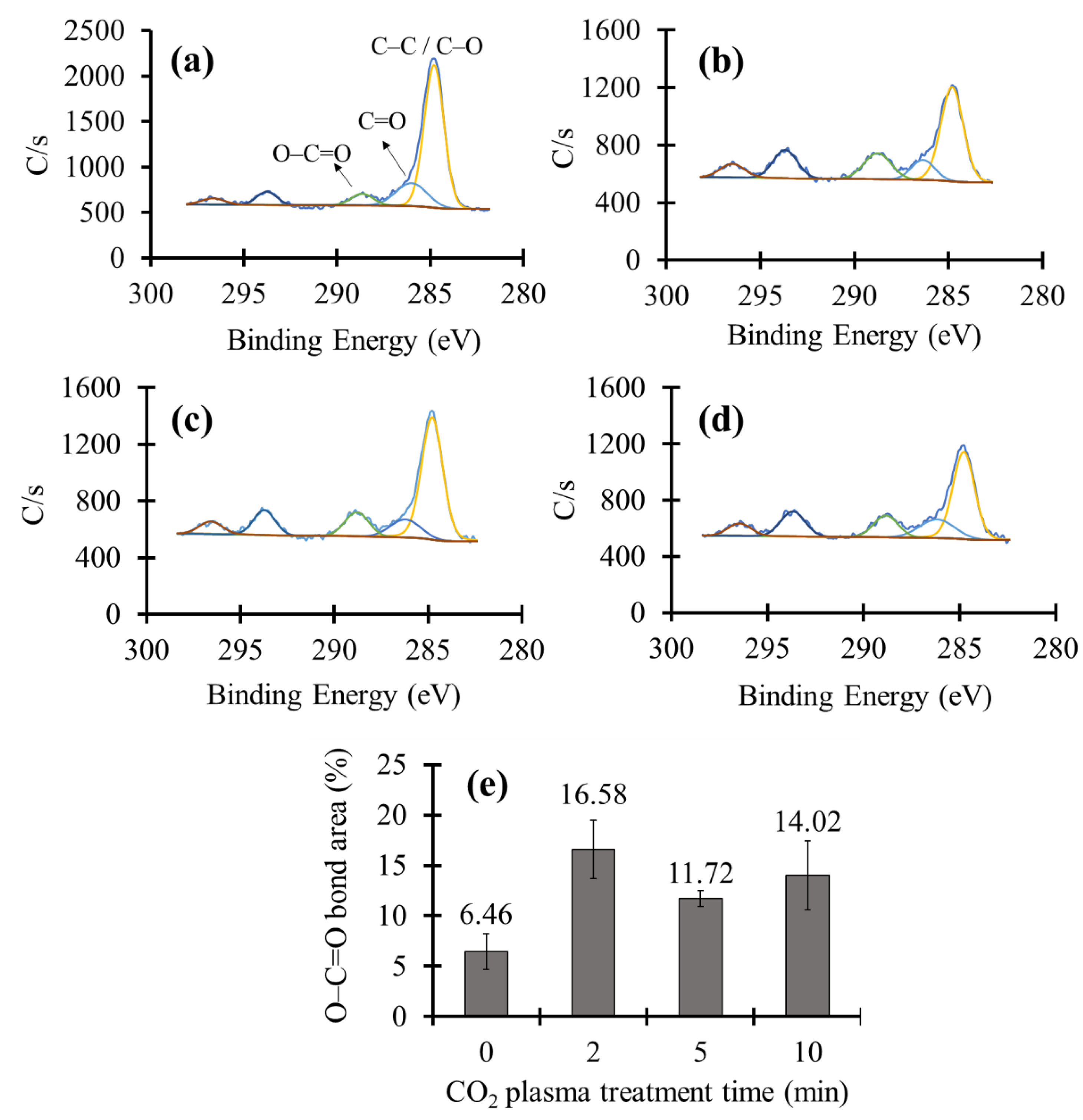
3.2. XPS Measurement of the CO2 Plasma Treated Ceramic Tiles
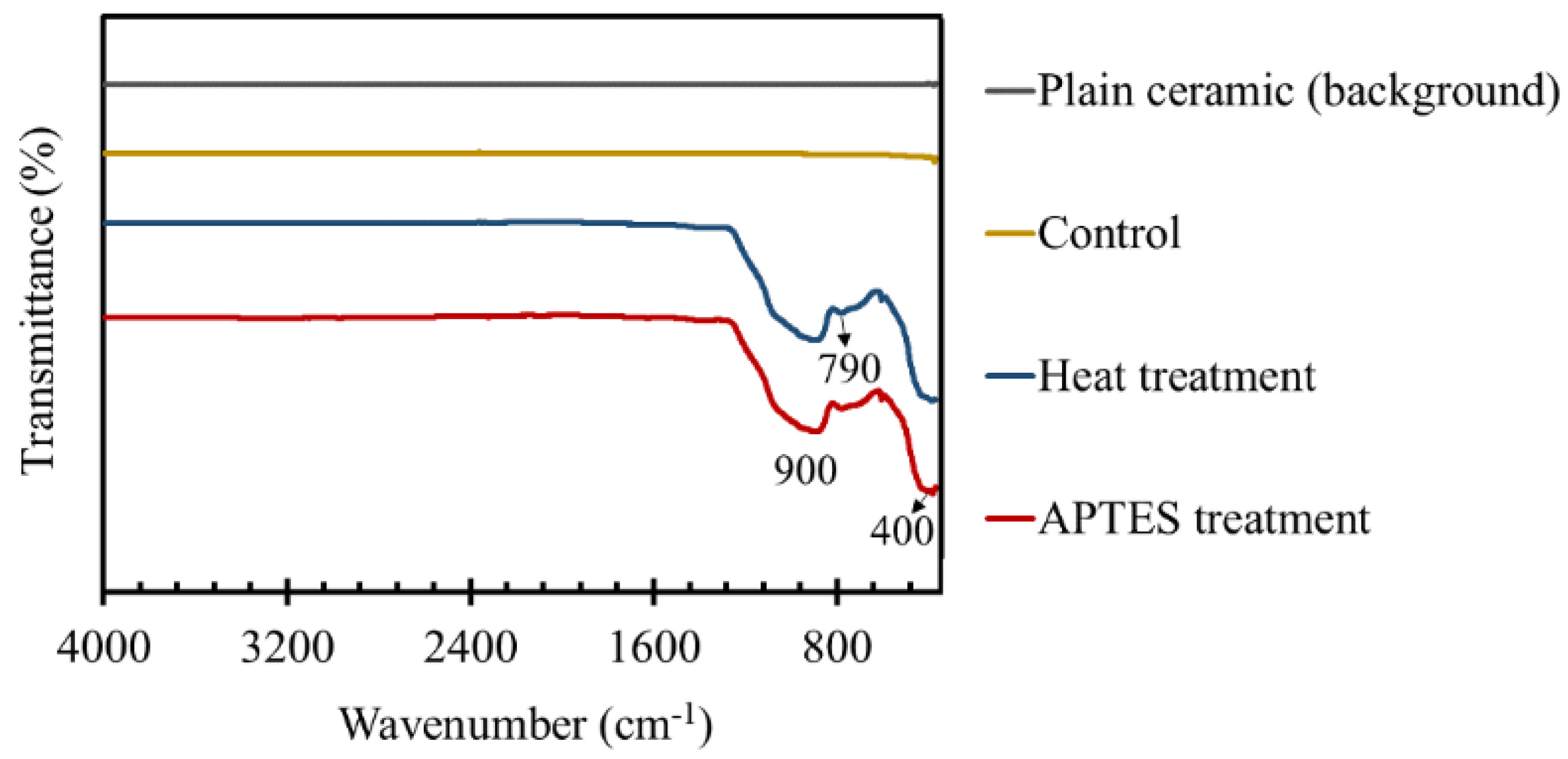
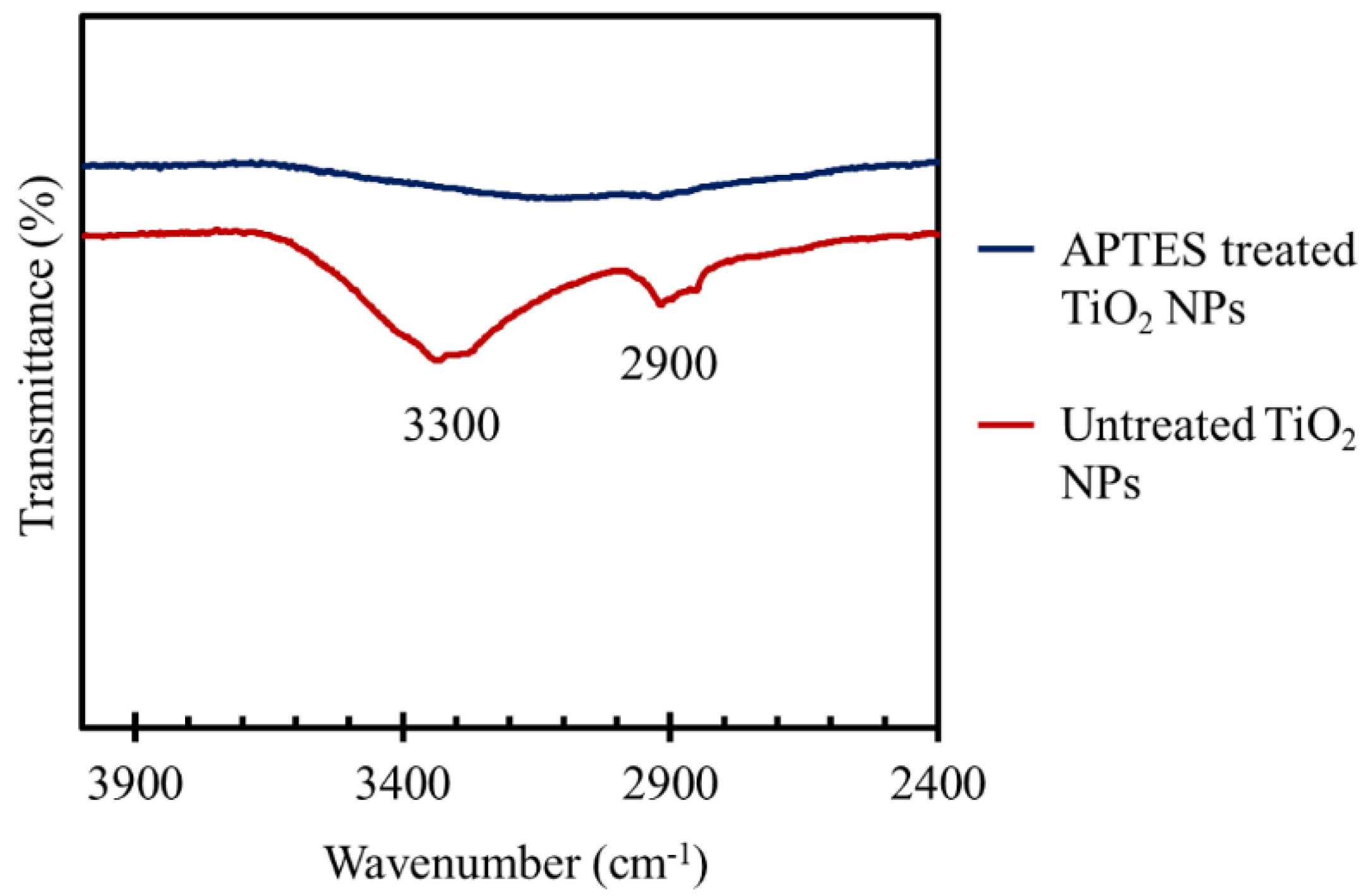
3.3. FT-IR Studies of the TiO2 Coated Tiles
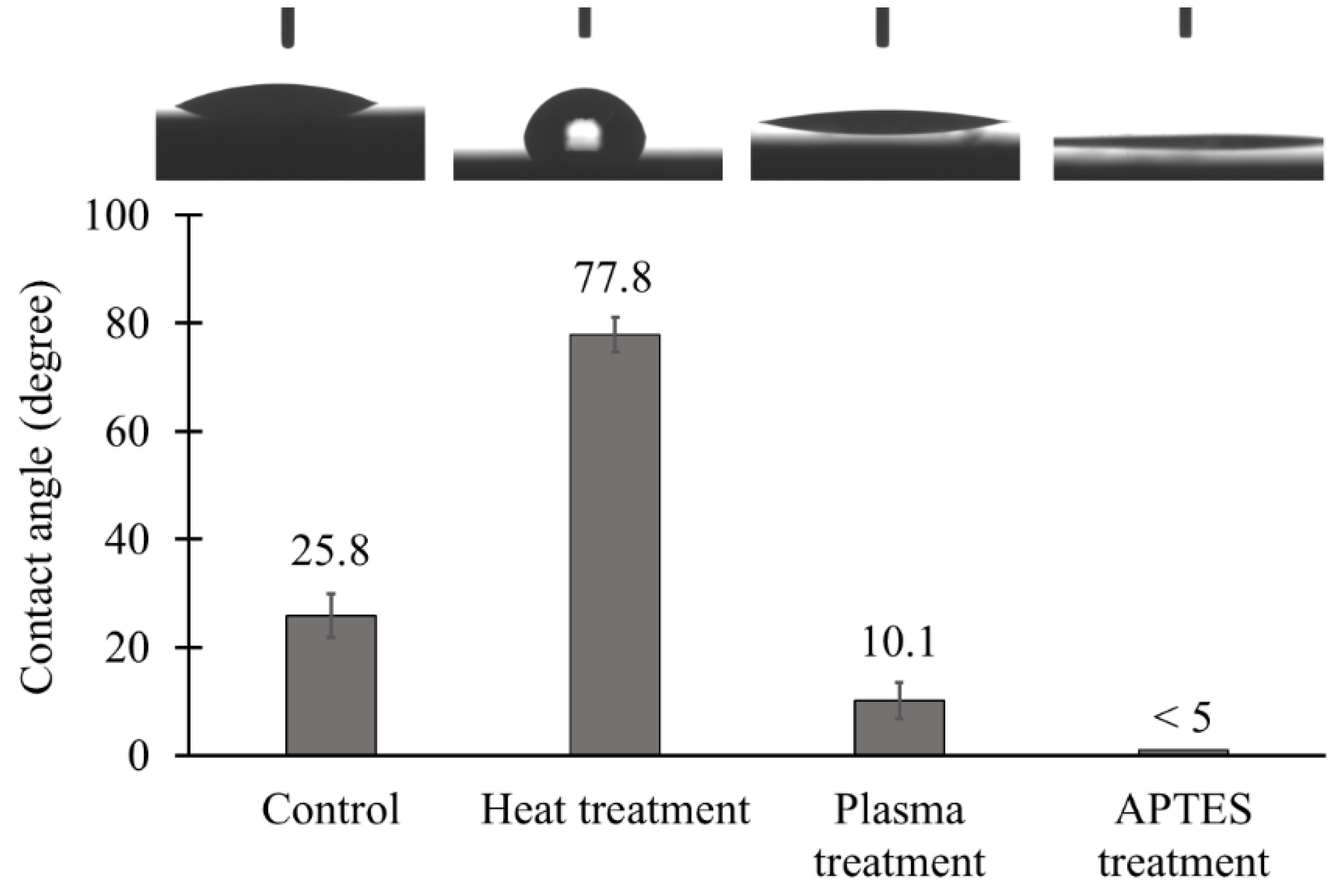
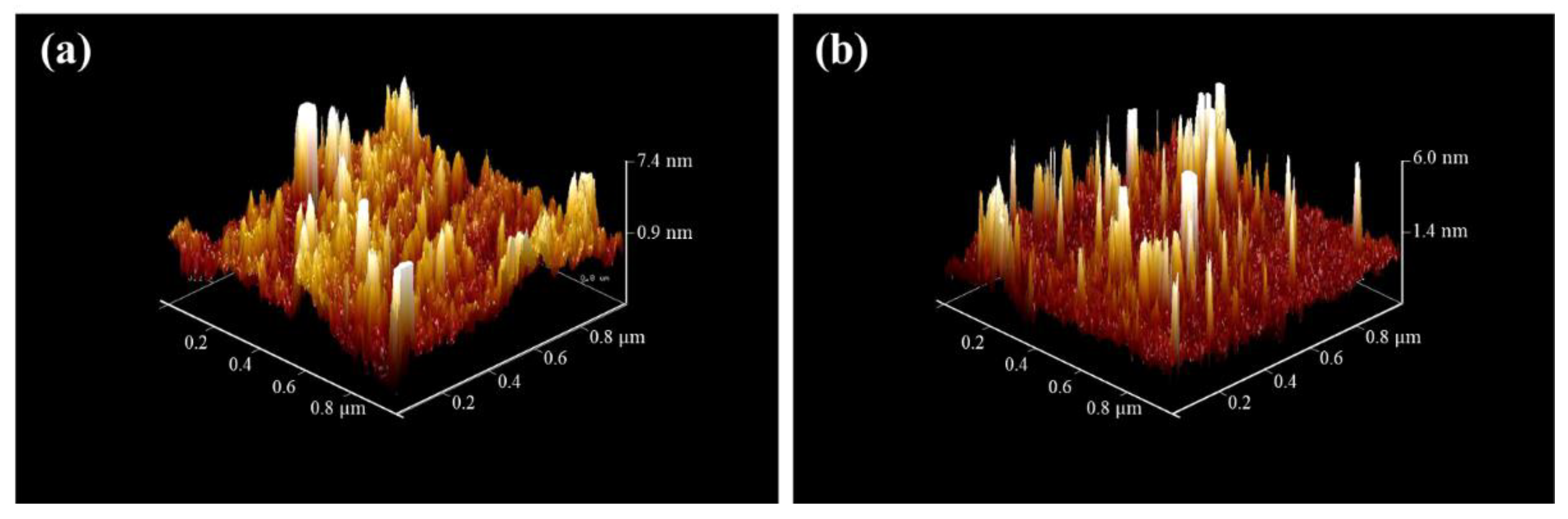
3.4. Hydrophilicity and Surface Topography of the TiO2 Coated Tiles
3.5. Dye Degradation of TiO2 NPs in Suspension
3.6. Dye Degradation on TiO2 Coated Tiles Produced Using Heat Treatment
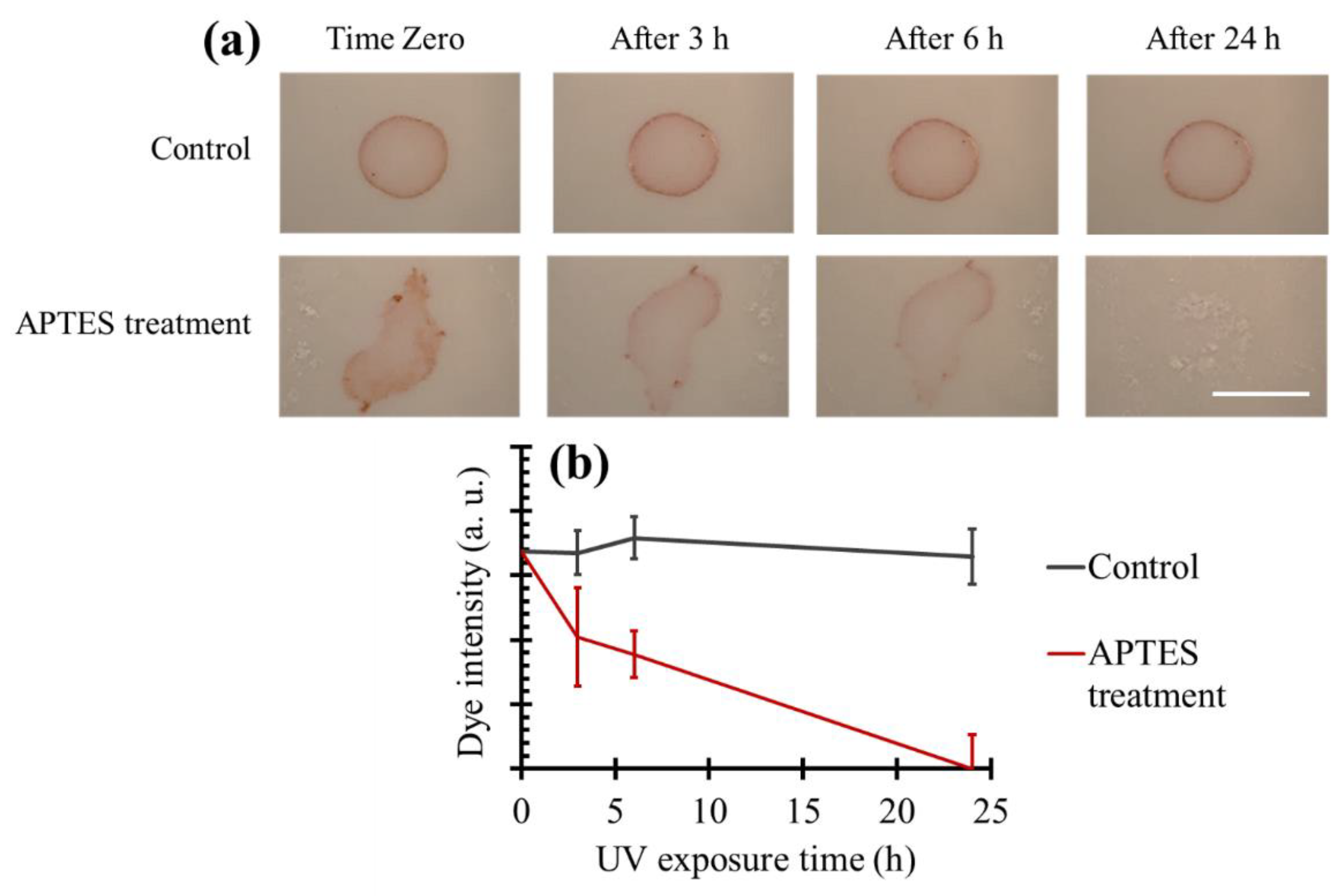
3.7. Dye Degradation on TiO2 Coated Tiles Produced Using Aminosilanization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dong, H.; Zeng, G.; Tang, L.; Fan, C.; Zhang, C.; He, X.; He, Y. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Res. 2015, 79, 128–146. [Google Scholar] [CrossRef] [PubMed]
- Weon, S.; Kim, J.; Choi, W. Dual-components modified TiO2 with Pt and fluoride as deactivation-resistant photocatalyst for the degradation of volatile organic compound. Appl. Catal. B Environ. 2018, 220, 1–8. [Google Scholar] [CrossRef]
- Li, S.; Ishikawa, S.; Huang, C.; Zhang, W.; Tanizaki, T.; Higuchi, T.; Haraga, H. Production of active intermediates and decomposition behaviors of organic compounds in the ultraviolet ray/supersonic wave reactions with TiO2 photocatalyst. Sustain. Environ. Res. 2015, 25, 67–72. [Google Scholar]
- Luttrell, T.; Halpegamage, S.; Tao, J.; Kramer, A.; Sutter, E.; Batzill, M. Why is anatase a better photocatalyst than rutile? Model studies on epitaxial TiO2 films. Sci. Rep. 2015, 4, 4043. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Irie, H.; Fujishima, A. TiO2 photocatalysis: A historical overview and future prospects. Jpn. J. Appl. Phys. 2005, 44, 8269–8285. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Identification and roles of the active species generated on various photocatalysts. In Photocatalysis and Water Purification; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013. [Google Scholar]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Marugán, J.; van Grieken, R.; Pablos, C.; Satuf, M.L.; Cassano, A.E.; Alfano, O.M. Photocatalytic inactivation of escherichia coli aqueous suspensions in a fixed-bed reactor. Catal. Today 2015, 252, 143–149. [Google Scholar] [CrossRef]
- Liu, H.-L.; Yang, T.C.-K. Photocatalytic inactivation of escherichia coli and lactobacillus helveticus by ZnO and TiO2 activated with ultraviolet light. Process Biochem. 2003, 39, 475–481. [Google Scholar] [CrossRef]
- Padervand, M.; Vossoughi, M.; Yousefi, H.; Salari, H.; Gholami, M.R. An experimental and theoretical study on the structure and photoactivity of XFe2O4 (X = Mn, Fe, Ni, Co, and Zn) structures. Russ. J. Phys. Chem. A 2014, 88, 2451–2461. [Google Scholar] [CrossRef]
- Banerjee, S.; Dionysiou, D.D.; Pillai, S.C. Self-cleaning applications of TiO2 by photo-induced hydrophilicity and photocatalysis. Appl. Catal. B Environ. 2015, 176–177, 396–428. [Google Scholar] [CrossRef]
- Klein, M.; Grabowska, E.; Zaleska, A. Noble metal modified TiO2 for photocatalytic air purification. Physicochem. Probl. Miner. Process. 2015, 51, 49–57. [Google Scholar]
- Song, J.; Wang, X.; Yan, J.; Yu, J.; Sun, G.; Ding, B. Soft Zr-doped TiO2 nanofibrous membranes with enhanced photocatalytic activity for water purification. Sci. Rep. 2017, 7, 1636. [Google Scholar] [CrossRef] [PubMed]
- Imani, R.; Dillert, R.; Bahnemann, D.W.; Pazoki, M.; Apih, T.; Kononenko, V.; Repar, N.; Kralj-Iglič, V.; Boschloo, G.; Drobne, D.; et al. Multifunctional gadolinium-doped mesoporous TiO2 nanobeads: Photoluminescence, enhanced spin relaxation, and reactive oxygen species photogeneration, beneficial for cancer diagnosis and treatment. Small 2017, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.W.; Chung, H.; Kim, M.Y.; Lee, J.; Choi, I.H.; Cheon, J. Development of water-soluble single-crystalline TiO2 nanoparticles for photocatalytic cancer-cell treatment. Small 2007, 3, 850–853. [Google Scholar] [CrossRef] [PubMed]
- Ohko, Y.; Utsumi, Y.; Niwa, C.; Tatsuma, T.; Kobayakawa, K.; Satoh, Y.; Kubota, Y.; Fujishima, A. Self-sterilizing and self-cleaning of silicone catheters coated with TiO2 photocatalyst thin films: A preclinical work. J. Biomed. Mater. Res. 2001, 58, 97–101. [Google Scholar] [CrossRef]
- Suketa, N.; Sawase, T.; Kitaura, H.; Naito, M.; Baba, K.; Nakayama, K.; Wennerberg, A.; Atsuta, M. An antibacterial surface on dental implants, based on the photocatalytic bactericidal effect. Clin. Implant Dent. Relat. Res. 2005, 7, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Kwak, S.-Y. Photocatalytic inactivation of E. coli with a mesoporous TiO2 coated film using the film adhesion method. Environ. Sci. Technol. 2009, 43, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Behpour, M.; Mehrzad, M.; Hosseinpour-Mashkani, S.M. TiO2 thin film: Preparation, characterization, and its photocatalytic degradation of basic yellow 28 dye. J. Nanostructures 2015, 5, 183–187. [Google Scholar]
- Vera, M.L.; Leyva, G.; Litter, M.I. Simple TiO2 coatings by sol–gel techniques combined with commercial TiO2 particles for use in heterogeneous photocatalysis. J. Nanosci. Nanotechnol. 2017, 17, 4946–4954. [Google Scholar] [CrossRef]
- Justicia, I.; Garcia, G.; Vázquez, L.; Santiso, J.; Ordejón, P.; Battiston, G.; Gerbasi, R.; Figueras, A. Self-doped titanium oxide thin films for efficient visible light photocatalysis: An example: Nonylphenol photodegradation. Sens. Actuators B Chem. 2005, 109, 52–56. [Google Scholar] [CrossRef]
- Justicia, I.; Ordejón, P.; Canto, G.; Mozos, J.L.; Fraxedas, J.; Battiston, G.A.; Gerbasi, R.; Figueras, A. Designed self-doped titanium dioxide thin films for efficient visible-light photocatalysis. Adv. Mater. 2002, 14, 1399–1402. [Google Scholar] [CrossRef]
- Yemmireddy, V.K.; Farrell, G.D.; Hung, Y.C. Development of titanium dioxide (TiO2) nanocoatings on food contact surfaces and method to evaluate their durability and photocatalytic bactericidal property. J. Food Sci. 2015, 80, N1903–N1911. [Google Scholar] [CrossRef] [PubMed]
- Sopyan, I.; Watanabe, M.; Murasawa, S.; Hashimoto, K.; Fujishima, A. A film-type photocatalyst incorporating highly active TiO2 powder and fluororesin binder: Photocatalytic activity and long-term stability. J. Electroanal. Chem. 1996, 415, 183–186. [Google Scholar] [CrossRef]
- Lin, H.; Xu, Z.; Wang, X.; Long, J.; Su, W.; Fu, X.; Lin, Q. Photocatalytic and antibacterial properties of medical-grade PVC material coated with TiO2 film. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 87, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Contaldi, V.; Licciulli, A.; Marzo, F. Self-cleaning mineral paint for application in architectural heritage. Coatings 2016, 6, 48. [Google Scholar] [CrossRef]
- Gunda, N.S.K.; Singh, M.; Norman, L.; Kaur, K.; Mitra, S.K. Optimization and characterization of biomolecule immobilization on silicon substrates using (3-aminopropyl)triethoxysilane (APTES) and glutaraldehyde linker. Appl. Surf. Sci. 2014, 305, 522–530. [Google Scholar] [CrossRef]
- Landoulsi, J.; Genet, M.J.; Kirat, K.E.; Richard, C.; Pulvin, S.; Rouxhe, P.G. Silanization with APTES for controlling the interactions between stainless steel and biocomponents: Reality vs. expectation. Biomater. Phys. Chem. 2011, 5, 99–126. [Google Scholar]
- Li, G.; Yang, P.; Qin, W.; Maitz, M.F.; Zhou, S.; Huang, N. The effect of coimmobilizing heparin and fibronectin on titanium on hemocompatibility and endothelialization. Biomaterials 2011, 32, 4691–4703. [Google Scholar] [CrossRef] [PubMed]
- Badv, M.; Jaffer, I.H.; Weitz, J.I.; Didar, T.F. An omniphobic lubricant-infused coating produced by chemical vapor deposition of hydrophobic organosilanes attenuates clotting on catheter surfaces. Sci. Rep. 2017, 7, 11639. [Google Scholar] [CrossRef] [PubMed]
- Villegas, M.; Cetinic, Z.; Shakeri, A.; Didar, T.F. Fabricating smooth PDMS microfluidic channels from low-resolution 3D printed molds using an omniphobic lubricant-infused coating. Anal. Chim. Acta 2018, 1000, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Didar, T.F.; Cartwright, M.J.; Rottman, M.; Graveline, A.R.; Gamini, N.; Watters, A.L.; Leslie, D.C.; Mammoto, T.; Rodas, M.J.; Kang, J.H.; et al. Improved treatment of systemic blood infections using antibiotics with extracorporeal opsonin hemoadsorption. Biomaterials 2015, 67, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Didar, T.F.; Tabrizian, M. Generating multiplex gradients of biomolecules for controlling cellular adhesion in parallel microfluidic channels. Lab Chip 2012, 12, 4363–4371. [Google Scholar] [CrossRef] [PubMed]
- Didar, T.F.; Bowey, K.; Almazan, G.; Tabrizian, M. A miniaturized multipurpose platform for rapid, label-free, and simultaneous separation, patterning, and in vitro culture of primary and rare cells. Adv. Healthc. Mater. 2014, 3, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, A.; Sun, N.; Badv, M.; Didar, T.F. Generating 2-dimensional concentration gradients of biomolecules using a simple microfluidic design. Biomicrofluidics 2017, 11. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.J.; Kim, S.; Lee, B.S.; Kim, A.; Ah, C.S.; Huh, C.; Sung, G.Y.; Yun, W.S. Enhanced protein immobilization efficiency on a TiO2 surface modified with a hydroxyl functional group. Langmuir 2009, 25, 11692–11697. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Sajedin, S.M.; Kelly, S.M.; Lee, A.F.; Kornherr, A. UV-stable paper coated with APTES-modified P25 TiO2 nanoparticles. Carbohydr. Polym. 2014, 114, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Tang, D.; Guo, Y. The fabrication and self-flocculation effect of hybrid TiO2 nanoparticles grafted with poly(N-isopropylacrylamide) at ambient temperature via surface-initiated atom transfer radical polymerization. J. Mater. Chem. 2012, 22, 16872. [Google Scholar] [CrossRef]
- Xu, Z.; Shang, J.; Liu, C.; Kang, C.; Guo, H.; Du, Y. The preparation and characterization of TiO2 ultrafine particles. Mater. Sci. Eng. B 1999, 63, 211–214. [Google Scholar] [CrossRef]
- Yu, J.-G.; Yu, H.-G.; Cheng, B.; Zhao, X.-J.; Yu, J.C.; Ho, W.-K. The effect of calcination temperature on the surface microstructure and photocatalytic activity of TiO2 thin films prepared by liquid phase deposition. J. Phys. Chem. B 2003, 107, 13871–13879. [Google Scholar] [CrossRef]
- Lopez, N.; Nørskov, J.K.; Janssens, T.V.W.; Carlsson, A.; Puig-Molina, A.; Clausen, B.S.; Grunwaldt, J.-D. The adhesion and shape of nanosized Au particles in a Au/TiO2 catalyst. J. Catal. 2004, 225, 86–94. [Google Scholar] [CrossRef]
- Kang, I.-C.; Zhang, Q.; Yin, S.; Sato, T.; Saito, F. Preparation of a visible sensitive carbon doped TiO2 photo-catalyst by grinding TiO2 with ethanol and heating treatment. Appl. Catal. B Environ. 2008, 80, 81–87. [Google Scholar] [CrossRef]
- Yamada, K.; Yamane, H.; Matsushima, S.; Nakamura, H.; Ohira, K.; Kouya, M.; Kumada, K. Effect of thermal treatment on photocatalytic activity of N-doped TiO2 particles under visible light. Thin Solid Films 2008, 516, 7482–7487. [Google Scholar] [CrossRef]
- Widegren, J.; Bergström, L. The effect of acids and bases on the dispersion and stabilization of ceramic particles in ethanol. J. Eur. Ceram. Soc. 2000, 20, 659–665. [Google Scholar] [CrossRef]
- Kardys, A.Y.; Bharali, D.J.; Mousa, S.A. Amino-functionalized silica nanoparticles: In vitro evaluation for targeted delivery and therapy of pancreatic cancer. J. Nanotechnol. 2013, 2013, 768724. [Google Scholar] [CrossRef]
- Chen, L.-X.; Zheng, J.-N.; Wang, A.-J.; Wu, L.-J.; Chen, J.-R.; Feng, J.-J. Facile synthesis of porous bimetallic alloyed PdAg nanoflowers supported on reduced graphene oxide for simultaneous detection of ascorbic acid, dopamine, and uric acid. Analyst 2015, 140, 3183–3192. [Google Scholar] [CrossRef] [PubMed]
- Praveen, P.; Viruthagiri, G.; Mugundan, S.; Shanmugam, N. Structural, optical and morphological analyses of pristine titanium di-oxide nanoparticles—Synthesized via sol-gel route. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 117, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.B.; Zhou, Y.Z.; Vongehr, S.; Tang, S.C.; Meng, X.K. Effects of hydrothermal temperature on formation and decoloration characteristics of anatase TiO2 nanoparticles. Sci. China Technol. Sci. 2012, 55, 894–902. [Google Scholar] [CrossRef]
- Watson, S.; Beydoun, D.; Scott, J.; Amal, R. Preparation of nanosized crystalline TiO2 particles at low temperature for photocatalysis. J. Nanoparticle Res. 2004, 6, 193–207. [Google Scholar] [CrossRef]
- Chen, Y.F.; Lee, C.Y.; Yeng, M.Y.; Chiu, H.T. The effect of calcination temperature on the crystallinity of TiO2 nanopowders. J. Cryst. Growth 2003, 247, 363–370. [Google Scholar] [CrossRef]
- Chellappa, M.; Anjaneyulu, U.; Manivasagam, G.; Vijayalakshmi, U. Preparation and evaluation of the cytotoxic nature of TiO2 nanoparticles by direct contact method. Int. J. Nanomed. 2015, 10, 31–41. [Google Scholar]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies, 3rd ed.; John Wiley & Sons Ltd.: Chichester, UK, 2001. [Google Scholar]
- Olurode, K.; Neelgund, G.M.; Oki, A.; Luo, Z. A facile hydrothermal approach for construction of carbon coating on TiO2 nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 89, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Kango, S.; Kalia, S.; Celli, A.; Njuguna, J.; Habibi, Y.; Kumar, R. Surface modification of inorganic nanoparticles for development of organic-inorganic nanocomposites—A review. Prog. Polym. Sci. 2013, 38, 1232–1261. [Google Scholar] [CrossRef]
- Yoshimitsu, Z.; Nakajima, A.; Watanabe, T.; Hashimoto, K. Effects of surface structure on the hydrophobicity and sliding behavior of water droplets. Langmuir 2002, 18, 5818–5822. [Google Scholar] [CrossRef]
- Han, J.-B.; Wang, X.; Wang, N.; Wei, Z.-H.; Yu, G.-P.; Zhou, Z.-G.; Wang, Q.-Q. Effect of plasma treatment on hydrophilic properties of TiO2 thin films. Surf. Coat. Technol. 2006, 200, 4876–4878. [Google Scholar] [CrossRef]
- Nie, X.; Zhuo, S.; Maeng, G.; Sohlberg, K. Doping of TiO2 polymorphs for altered optical and photocatalytic properties. Int. J. Photoenergy 2009, 2009, 1–22. [Google Scholar] [CrossRef]
- Ren, R.; Yang, Z.; Shaw, L.L. Polymorphic transformation and powder characteristics of TiO2 during high energy milling. J. Mater. Sci. 2000, 35, 6015–6026. [Google Scholar] [CrossRef]
- Mei, Z.-G.; Wang, Y.; Shang, S.; Liu, Z.-K. First-principles study of the mechanical properties and phase stability of TiO2. Comput. Mater. Sci. 2014, 83, 114–119. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef]
- Zhao, J.; Milanova, M.; Warmoeskerken, M.M.C.G.; Dutschk, V. Surface modification of TiO2 nanoparticles with silane coupling agents. Colloids Surfaces A Physicochem. Eng. Asp. 2012, 413, 273–279. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shakeri, A.; Yip, D.; Badv, M.; Imani, S.M.; Sanjari, M.; Didar, T.F. Self-Cleaning Ceramic Tiles Produced via Stable Coating of TiO2 Nanoparticles. Materials 2018, 11, 1003. https://doi.org/10.3390/ma11061003
Shakeri A, Yip D, Badv M, Imani SM, Sanjari M, Didar TF. Self-Cleaning Ceramic Tiles Produced via Stable Coating of TiO2 Nanoparticles. Materials. 2018; 11(6):1003. https://doi.org/10.3390/ma11061003
Chicago/Turabian StyleShakeri, Amid, Darren Yip, Maryam Badv, Sara M. Imani, Mehdi Sanjari, and Tohid F. Didar. 2018. "Self-Cleaning Ceramic Tiles Produced via Stable Coating of TiO2 Nanoparticles" Materials 11, no. 6: 1003. https://doi.org/10.3390/ma11061003
APA StyleShakeri, A., Yip, D., Badv, M., Imani, S. M., Sanjari, M., & Didar, T. F. (2018). Self-Cleaning Ceramic Tiles Produced via Stable Coating of TiO2 Nanoparticles. Materials, 11(6), 1003. https://doi.org/10.3390/ma11061003




