High-Temperature Wettability and Interactions between Y-Containing Ni-Based Alloys and Various Oxide Ceramics
Abstract
:1. Introduction
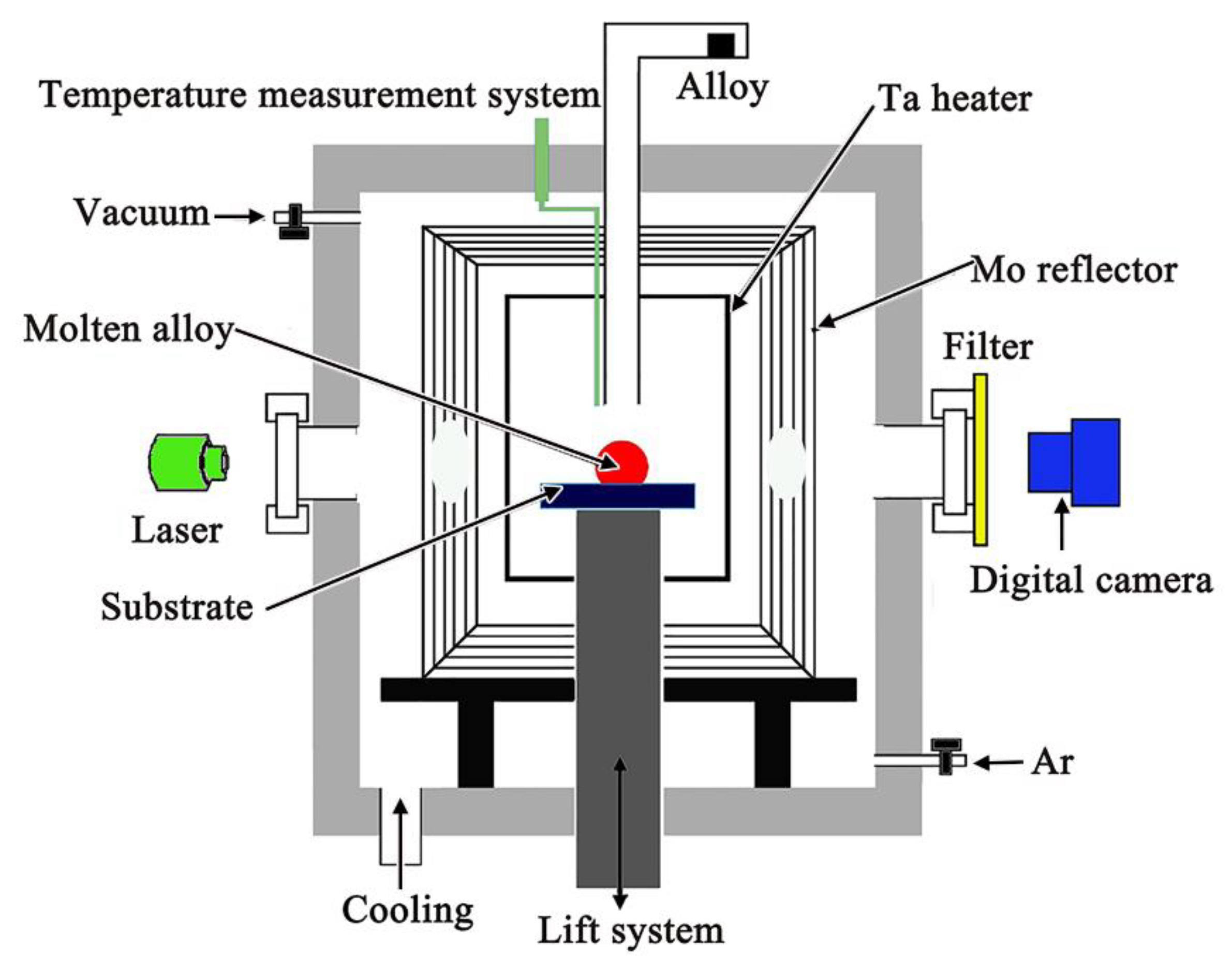
2. Materials and Methods
3. Results
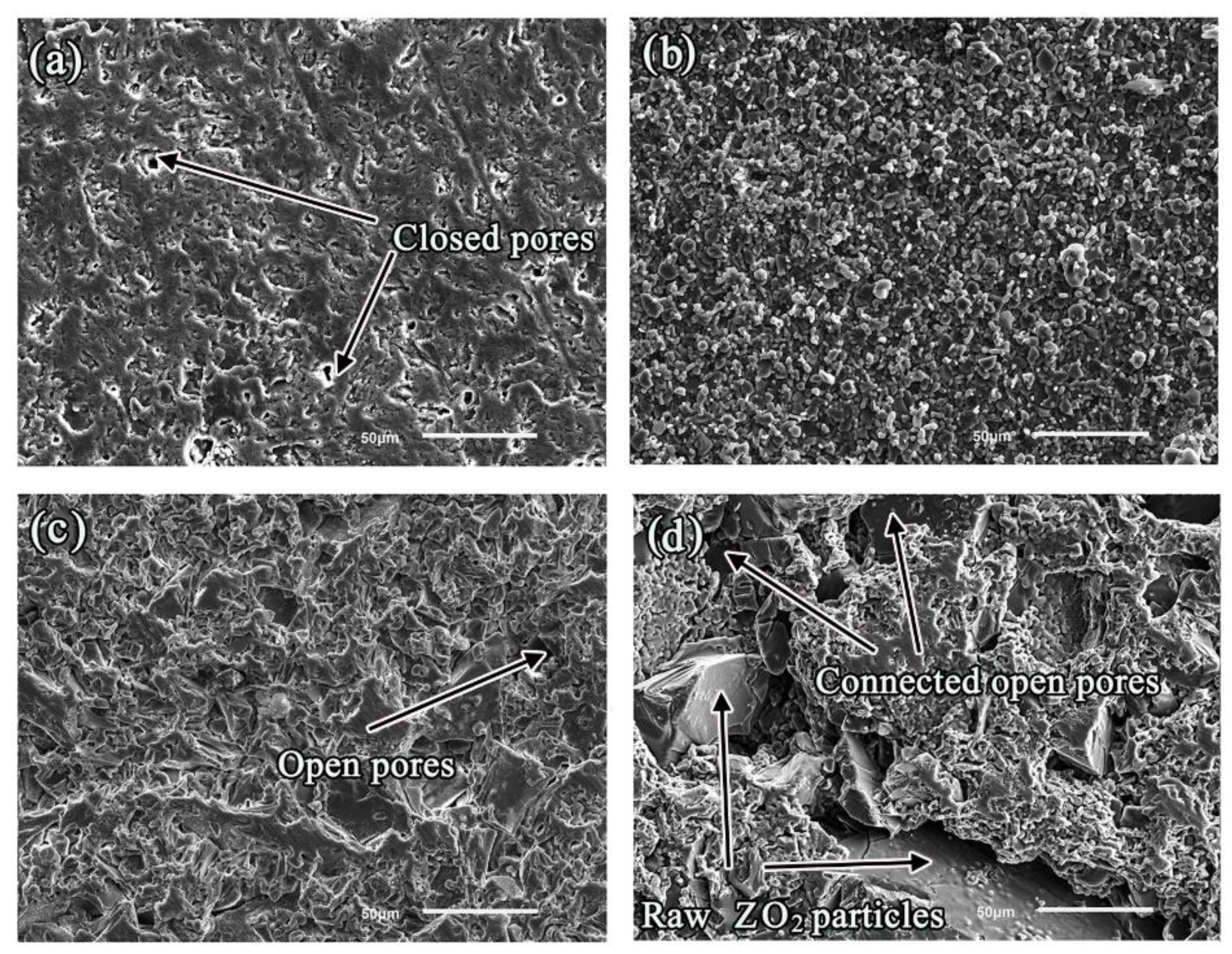
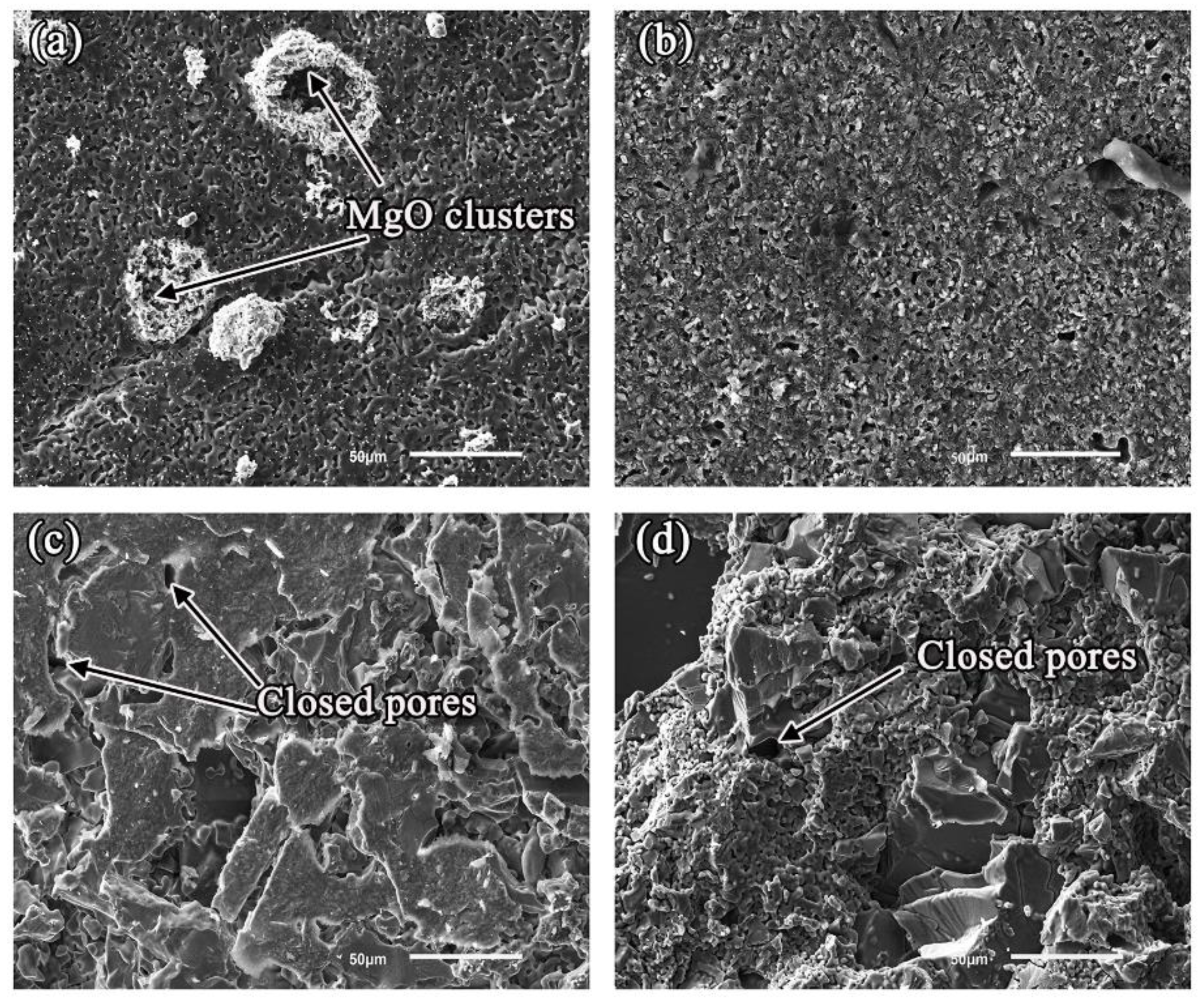
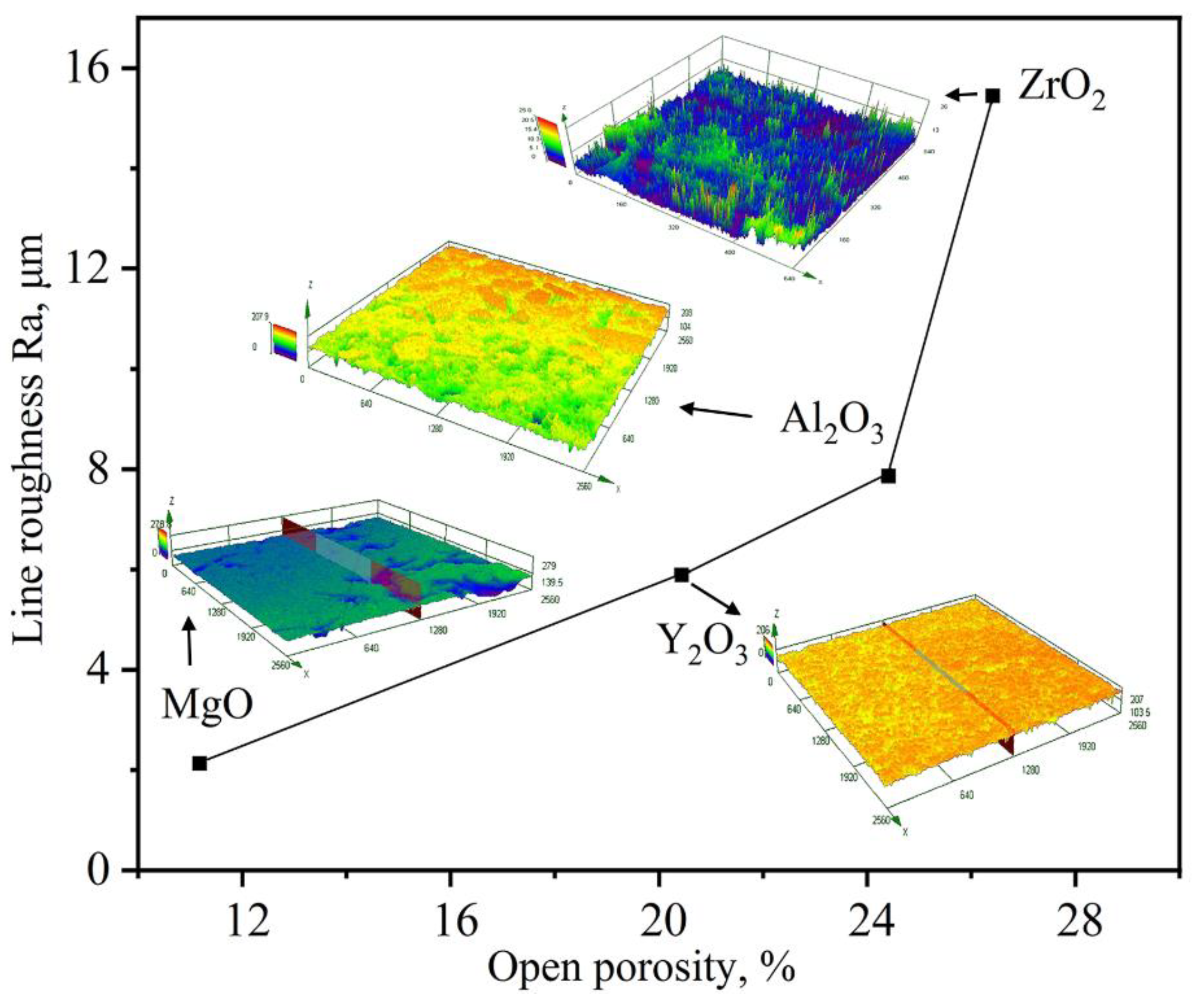
3.1. Microstructural of Ceramic Substrates
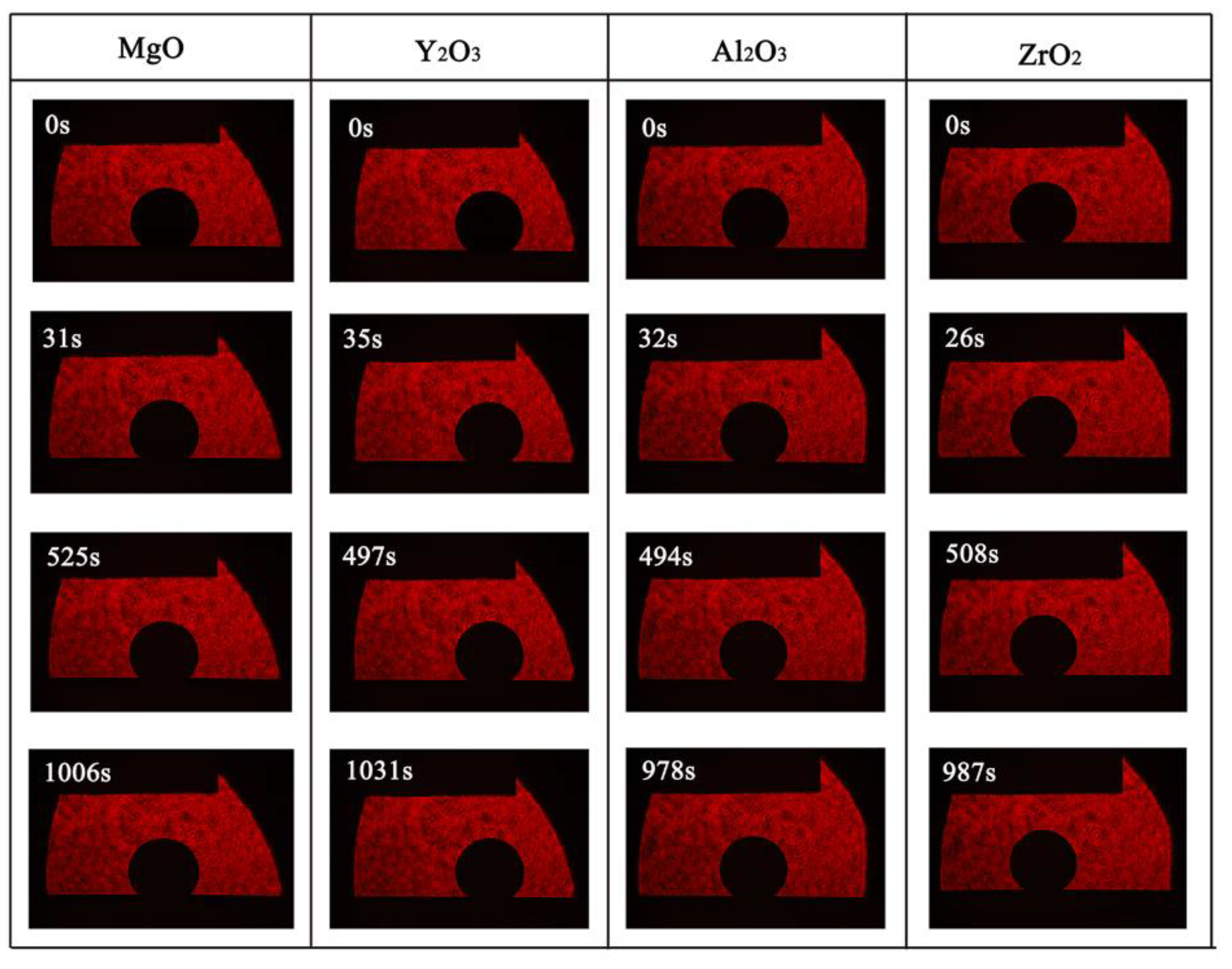
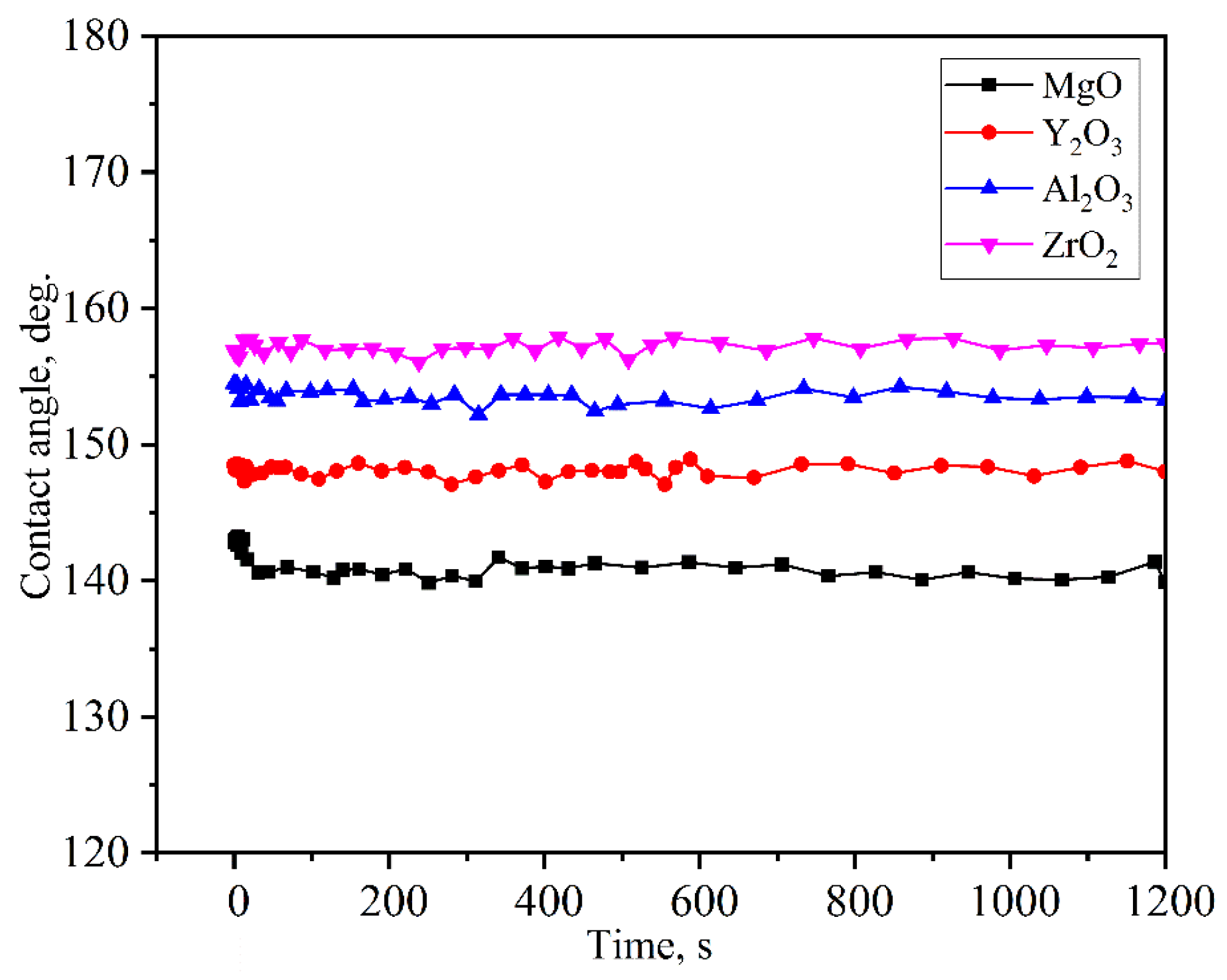
3.2. Wetting Behavior of the Alloys on the Oxide Ceramics Substrates
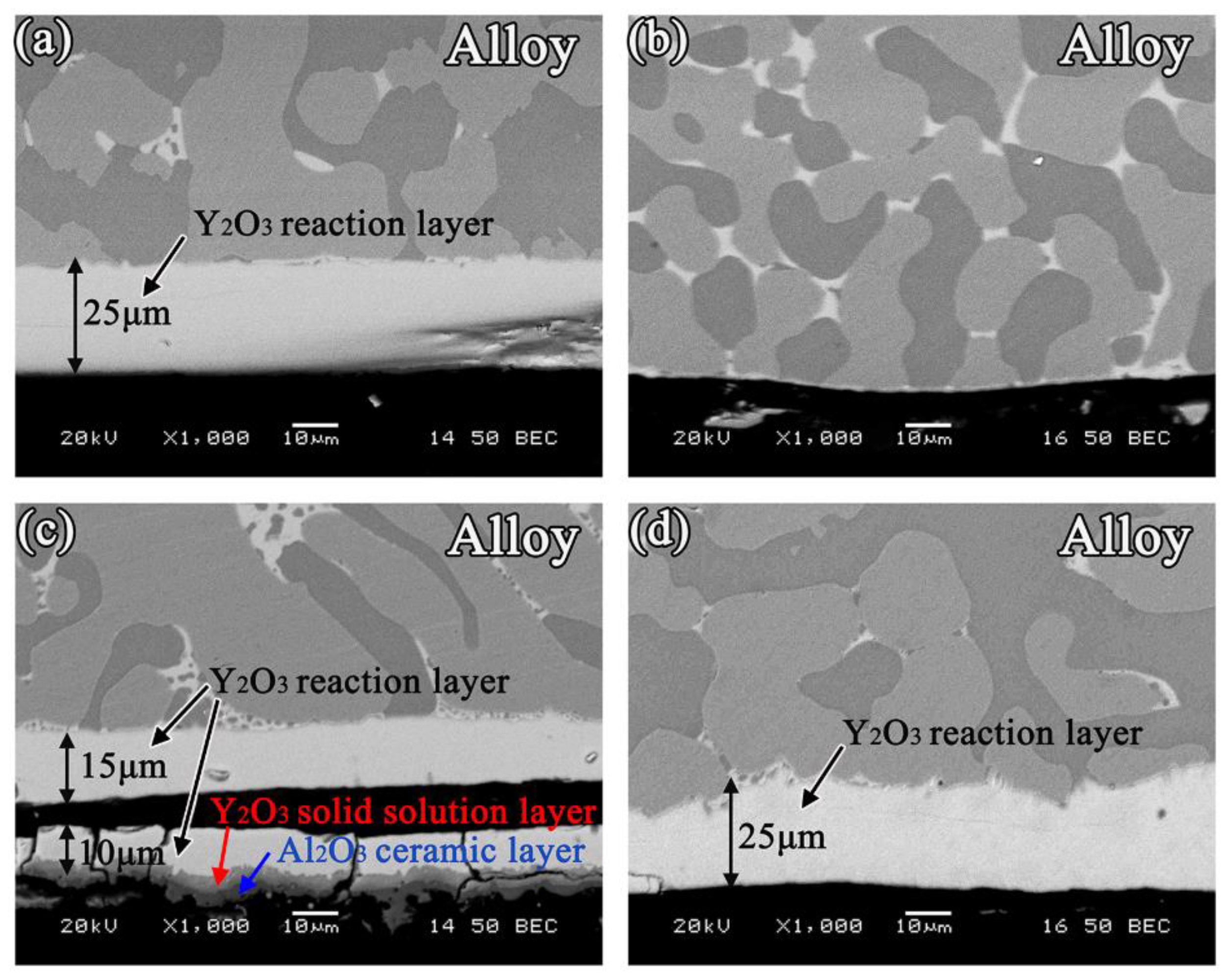
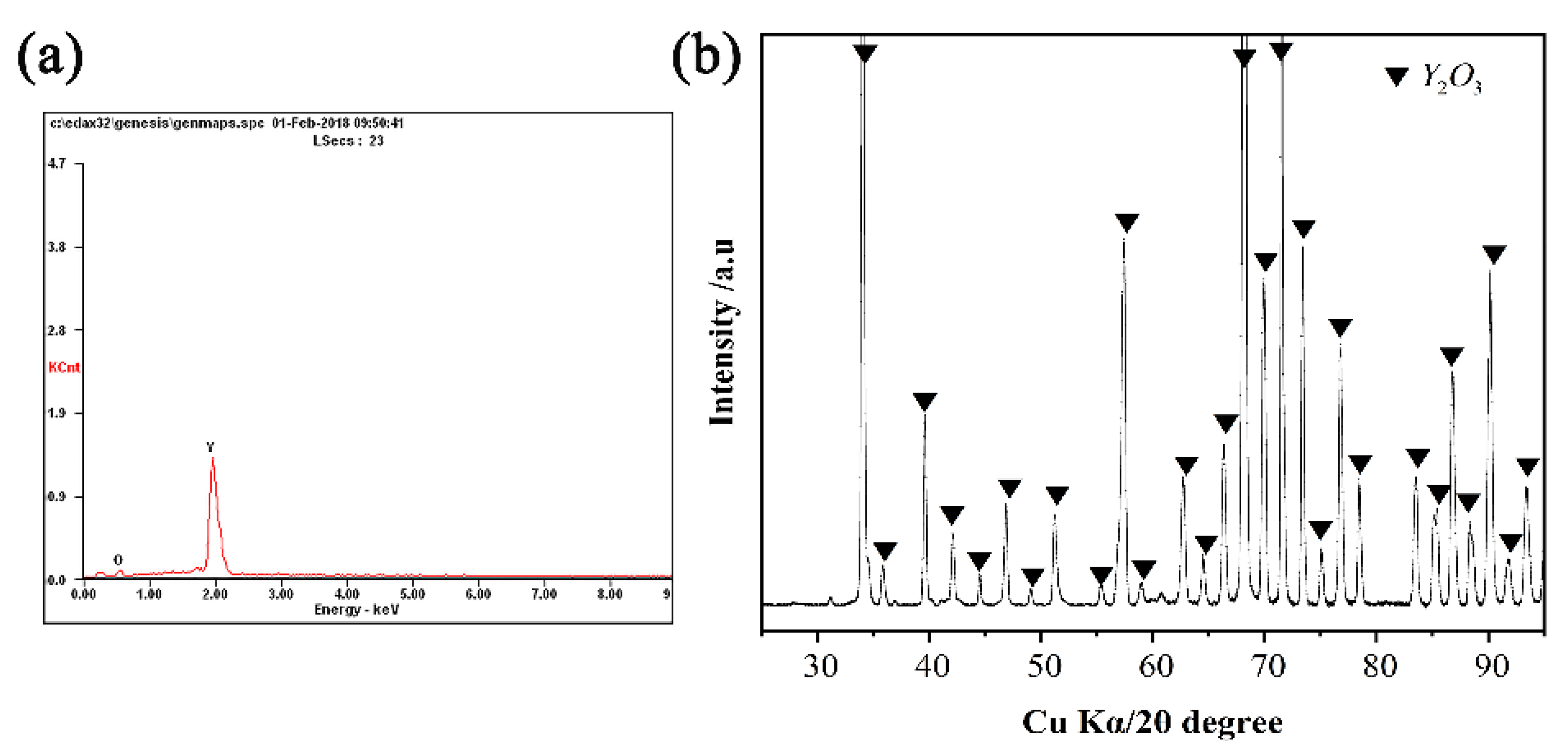
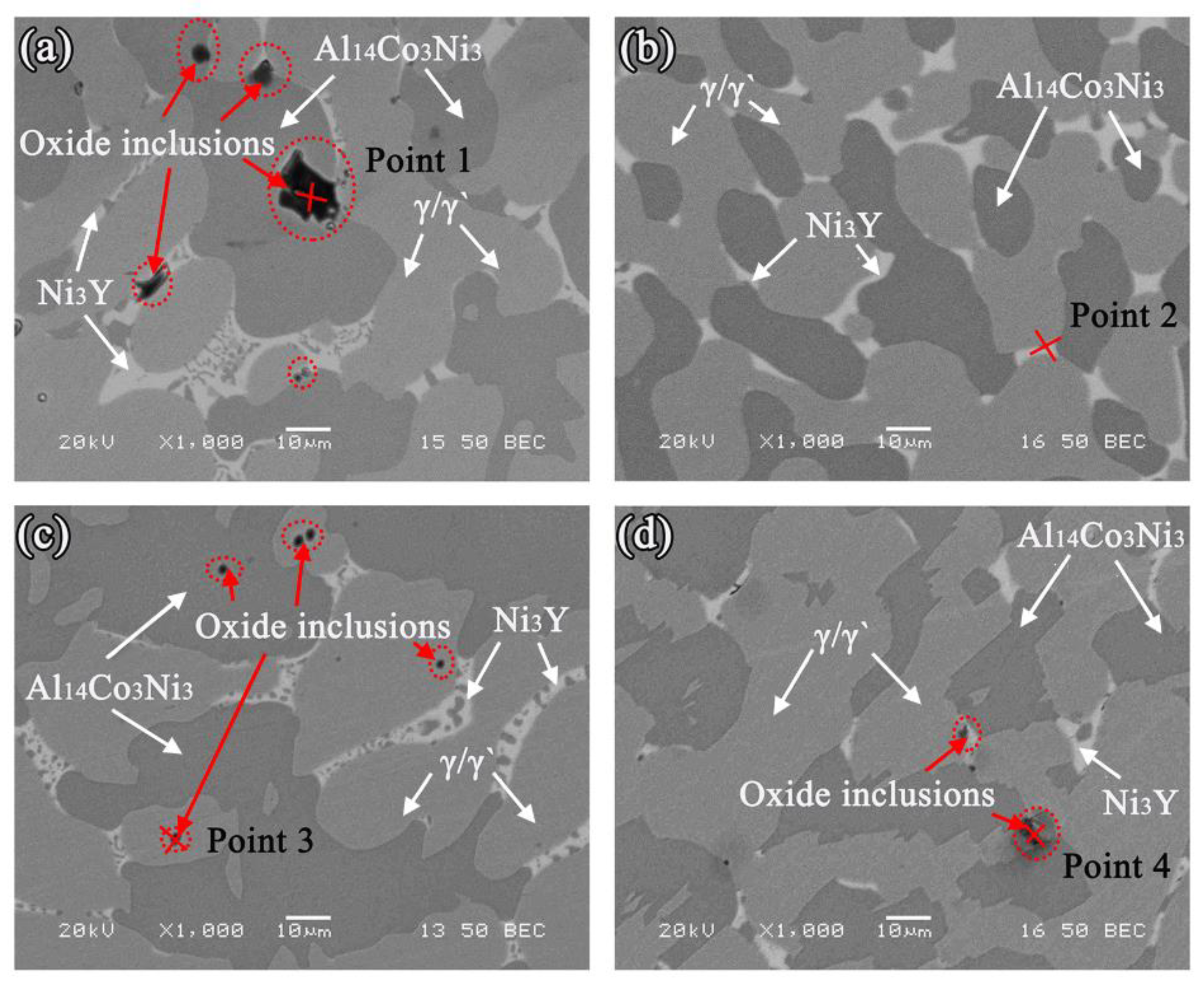
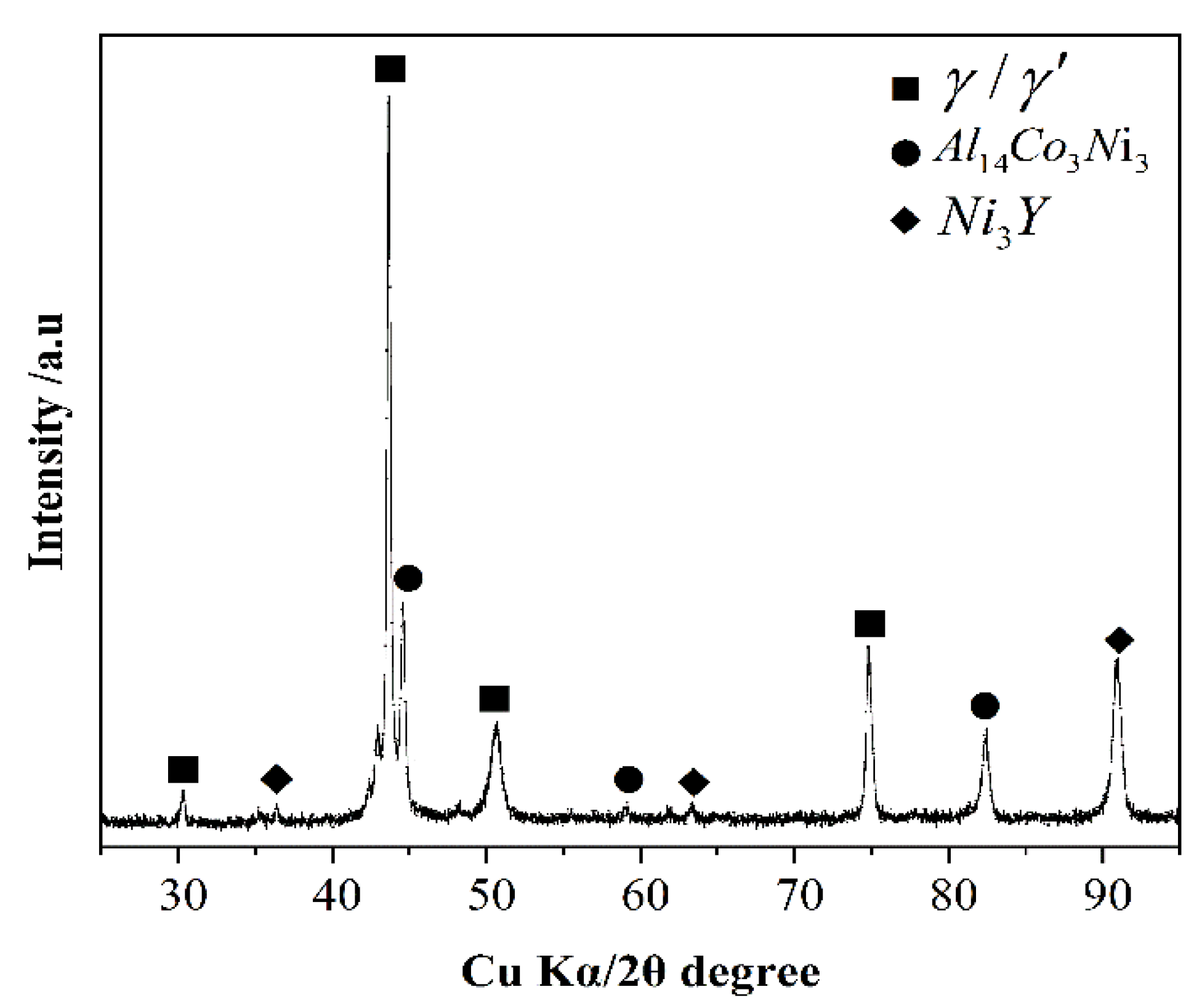
3.3. Interfacial Reactions between the Alloys and Ceramics
4. Discussion
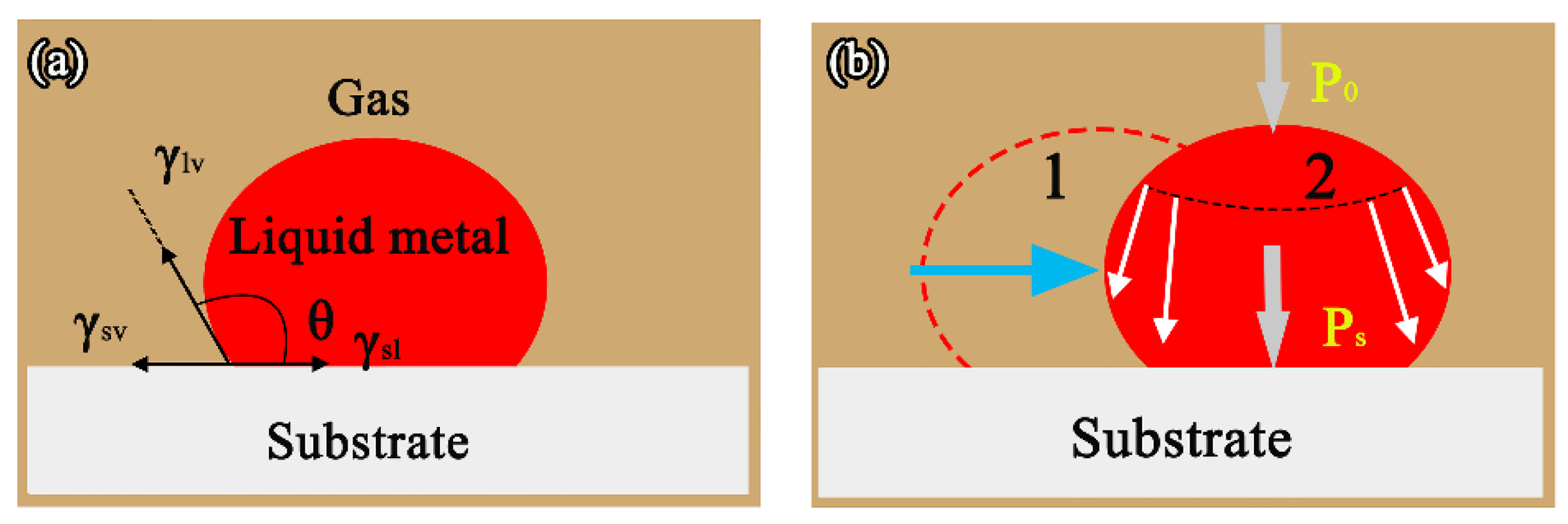
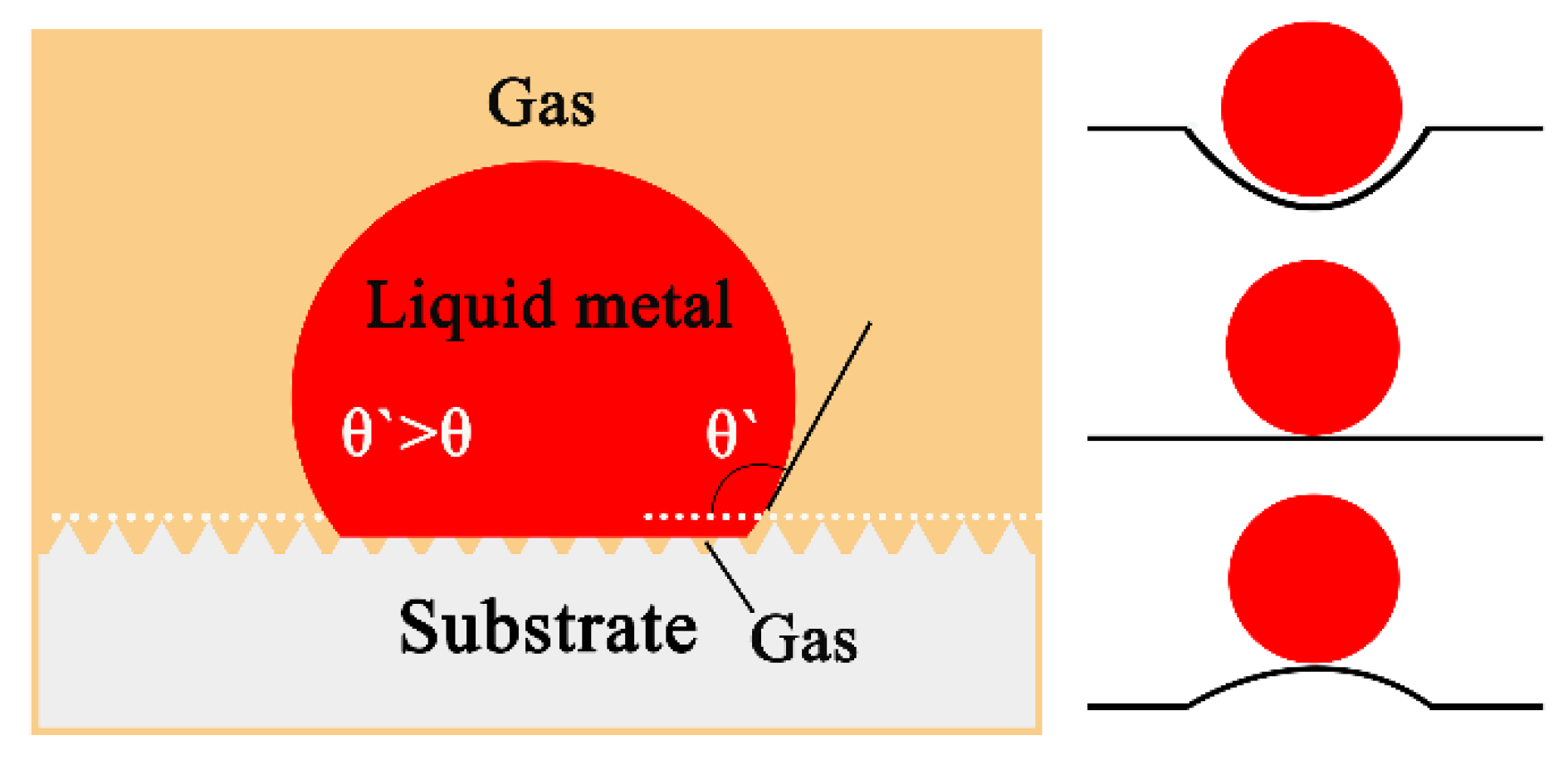
4.1. Wetting Kinetics and Driving Force
4.2. Thermodynamic Analysis of Interfacial Reactions
5. Conclusions
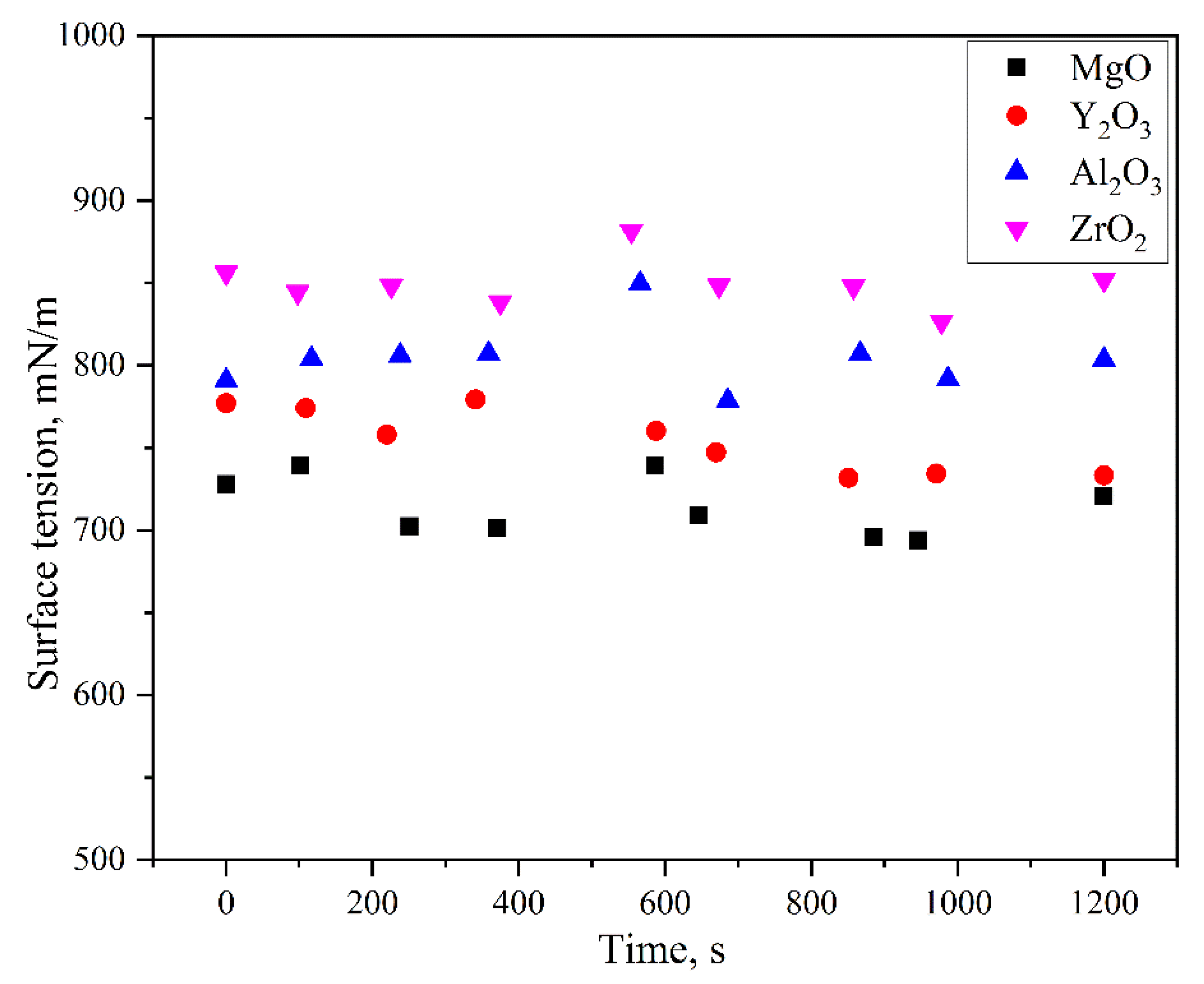
- With an increase of the experiment time, the contact angles of molten alloys on MgO, Y2O3, Al2O3, and ZrO2 substrates rapidly became stable. The equilibrium angles were 140°, 148°, 154°, and 157°, respectively. There was no characteristic transition in wettability when the type of substrates changed and no alloy liquid penetrated into the substrates. The influence of different ceramics on the wettability can be explained by the surface tension of the melts and the surface roughness of the substrates.
- With the exception of the Y2O3 system, an approximately 25 μm continuous Y2O3 reaction layer was observed along the interface of the alloys. The Y2O3 layer significantly reduced the wettability and interactions of Ni-20Co-20Cr-10Al-1.5Y alloys on MgO, Al2O3, and ZrO2.
- Oxide particles were found in the alloy matrices of all the systems. The average area percentage of oxides were 0.59% (MgO), 0.11% (Al2O3), 0.09% (ZrO2), and 0.02% (Y2O3), respectively. The formation of oxides could be attributed to the thermal dissociation of substrates, which provided sufficient [O] atoms that could dissolve into the molten alloys easily.
- The Y2O3 ceramic with open porosity levels of 20.44% was most beneficial with regard to the vacuum induction melting of high-purity Ni-20Co-20Cr-10Al-1.5Y alloys.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guo, M.H.; Wang, Q.M.; Gong, J.; Sun, C.; Huang, R.F.; Wen, L.S. Oxidation and hot corrosion behavior of gradient nicocralysib coatings deposited by a combination of arc ion plating and magnetron sputtering techniques. Corros. Sci. 2006, 48, 2750–2764. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, X.; Bai, M.; Yang, L.; Li, C.; Wang, L.; Carr, J.A.; Xiao, P. A mechanistic understanding on rumpling of a nicocraly bond coat for thermal barrier coating applications. Acta Mater. 2017, 128, 31–42. [Google Scholar] [CrossRef]
- Chen, L.C.; Zhang, C.; Yang, Z.G. Effect of pre-oxidation on the hot corrosion of conicralyre alloy. Corros. Sci. 2011, 53, 374–380. [Google Scholar] [CrossRef]
- Jiang, S.M.; Peng, X.; Bao, Z.B.; Liu, S.C.; Wang, Q.M.; Gong, J.; Sun, C. Preparation and hot corrosion behaviour of a mcraly + alsiy composite coating. Corros. Sci. 2008, 50, 3213–3220. [Google Scholar] [CrossRef]
- Otubo, J.; Rigo, O.D.; Neto, C.M.; Mei, P.R. The effects of vacuum induction melting and electron beam melting techniques on the purity of NiTi shape memory alloys. Mater. Sci. Eng. A 2006, 438–440, 679–682. [Google Scholar] [CrossRef]
- Paton, B.E.; Saenko, V.Y.; Pomarin, Y.M.; Medovar, L.B.; Grigorenko, G.M.; Fedorovskii, B.B.; Petrenko, V.L.; Chernets, A.V. Arc slag remelting for high strength steel & various alloys. J. Mater. Sci. 2004, 39, 7269–7274. [Google Scholar]
- Jiang, L.; Guo, S.; Bian, Y.; Zhang, M.; Ding, W. Effect of sintering temperature on mechanical properties of magnesia partially stabilized zirconia refractory. Ceram. Int. 2016, 42, 10593–10598. [Google Scholar] [CrossRef]
- Pirowski, Z. Evaluation of high-temperature physicochemical interactions between the H282Alloy melt and ceramic material of the crucible. Arch. Found. Eng. 2014, 14, 83–90. [Google Scholar] [CrossRef]
- Niu, J.P.; Yang, K.N.; Sun, X.F.; Jin, T.; Guan, H.R.; Hu, Z.Q. Denitrogenation during vacuum induction melting refining ni base superalloy using cao crucible. Mater. Sci. Technol. 2002, 18, 1041–1044. [Google Scholar] [CrossRef]
- Yang, J.; Yamasaki, T.; Kuwabara, M. Behavior of inclusions in deoxidation process of molten steel with in situ produced Mg vapor. ISIJ Int. 2007, 47, 699–708. [Google Scholar] [CrossRef]
- Merlin, V.; Eustathopoulos, N. Wetting and adhesion of Ni-Al alloys on α-Al2O3 single crystals. J. Mater. Sci. 1995, 30, 3619–3624. [Google Scholar] [CrossRef]
- Siegmund, P.; Guhl, C.; Schmidt, E.; Roßberg, A.; Rettenmayr, M. Reactive wetting of alumina by Ti-rich Ni–Ti–Zr alloys. J. Mater. Sci. 2016, 51, 3693–3700. [Google Scholar] [CrossRef]
- Yao, J.S.; Tang, D.Z.; Liu, X.G.; Xiao, C.B.; Li, X.; Cao, C.X. Interaction between two Ni-base alloys and ceramic moulds. Mater. Sci. Forum 2013, 747–748, 765–771. [Google Scholar] [CrossRef]
- Zheng, L.; Xiao, C.; Zhang, G.; Gu, G.; Li, X.; Liu, X.; Xue, M.; Tang, D. Investigation of interfacial reaction between high Cr content cast Nickel based superalloy k4648 and ceramic cores. J. Aeronaut. Mater. 2012, 32, 1283–1292. [Google Scholar]
- Li, Q.; Song, J.; Wang, D.; Yu, Q.; Xiao, C. Effect of Cr, Hf and temperature on interface reaction between nickel melt and silicon oxide core. Rare Met. 2011, 30, 405–409. [Google Scholar] [CrossRef]
- Kanetkar, C.S.; Kacar, A.S.; Stefanescu, D.M. The wetting characteristics and surface tension of some Ni-based alloys on yttria, hafnia, alumina, and zirconia substrates. Metall. Trans. A 1988, 19, 1833–1839. [Google Scholar] [CrossRef]
- Valenza, F.; Muolo, M.L.; Passerone, A. Wetting and interactions of Ni- and CO-based superalloys with different ceramic materials. J. Mater. Sci. 2010, 45, 2071–2079. [Google Scholar] [CrossRef]
- Verhiest, K.; Mullens, S.; Paul, J.; Graeve, I.D.; Wispelaere, N.D.; Claessens, S.; Debremaecker, A.; Verbeken, K. Experimental study on the contact angle formation of solidified iron–chromium droplets onto yttria ceramic substrates for the yttria/ferrous alloy system with variable chromium content. Ceram. Int. 2014, 40, 2187–2200. [Google Scholar] [CrossRef]
- Virieux, X.Y.; Desmaison, J.; Labbe, J.C.; Gabriel, A. Interaction between two Ni-base alloys and oxide ceramics: SiO2, ZrO2, HfO2, Al2O3. Mater. Sci. Forum 1997, 251–254, 925–934. [Google Scholar] [CrossRef]
- Jiang, L.; Guo, S.; Bian, Y.; Zhang, M.; Ding, W. Interfacial behaviors of magnesia partially stabilized zirconia by nickel-based superalloy. Mater. Lett. 2016, 181, 313–316. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, Y.; Jin, T.; Sun, X. Effect of C and Hf contents on the interface reactions and wettability between a Ni3Al-based superalloy and ceramic mould material. J. Mater. Sci. Technol. 2016, 32, 177–181. [Google Scholar] [CrossRef]
- Kritsalis, P.; Merlin, V.; Coudurier, L.; Eustathopoulos, N. Effect of Cr on interfacial interaction and wetting mechanisms in Ni Alloy/Alumina systems. Acta Metall. Mater. 1992, 40, 1167–1175. [Google Scholar] [CrossRef]
- Majumdar, S.; Schliephake, D.; Gorr, B.; Christ, H.J.; Heilmaier, M. Effect of yttrium alloying on intermediate to high-temperature oxidation behavior of MO-Si-B alloys. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2013, 44, 2243–2257. [Google Scholar] [CrossRef]
- Yong-Taeg, O.; Fujino, S.; Morinaga, K. Fabrication of transparent silica glass by powder sintering. Sci. Technol. Adv. Mater. 2002, 3, 297–301. [Google Scholar] [CrossRef]
- Shen, P.; Fujii, H.; Nogi, K. Wetting, adhesion and diffusion in Cu–Al/SiO2 system at 1473 K. Scr. Mater. 2005, 52, 1259–1263. [Google Scholar] [CrossRef]
- Xiao, F.; Yang, R.; Zhang, C. Surface tension of molten Ni-W and Ni-Cr alloys. Mater. Sci. Eng. B 2006, 132, 183–186. [Google Scholar] [CrossRef]
- Zhu, J.; Kamiya, A.; Yamada, T.; Shi, W.; Naganuma, K.; Mukai, K. Surface tension, wettability and reactivity of molten titanium in Ti/yttria-stabilized zirconia system. Mater. Sci. Eng. A 2002, 327, 117–127. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Rumpf, D.I.B. Thermochemical data of pure substances. Vet. Immunol. Immunopathol. 1997, 55, 359–360. [Google Scholar] [CrossRef]
- Saha, R.L.; Nandy, T.K.; Misra, R.D.K.; Jacob, K.T. On the evaluation of stability of rare earth oxides as face coats for investment casting of titanium. Metall. Trans. B 1990, 21, 559–566. [Google Scholar] [CrossRef]
- Eustathopoulos, N. Dynamics of wetting in reactive metal/ceramic systems. Acta Mater. 1998, 46, 2319–2327. [Google Scholar]
| Parameters | MgO | Y2O3 | Al2O3 | ZrO2 |
|---|---|---|---|---|
| Purity (%) | 99.9 | 99.9 | 99.9 | 94 |
| Particle size (mesh) | 500 | 325 | 200–300 | 200 |
| Grinding time (min) | 10 | 10 | 10 | 10 |
| PVA binder (wt %) | 5 | 5 | 5 | 5 |
| Molding pressure (MPa) | 10 | 10 | 10 | 10 |
| Open porosity (%) | 11.8 | 20.44 | 24.41 | 26.42 |
| Element | O | Al | Y | Cr | Co | Ni | Zr |
|---|---|---|---|---|---|---|---|
| Point 1 | 8.52 | 23.68 | 0.45 | 9.51 | 11.74 | 46.10 | - |
| Point 2 | 2.29 | 37.71 | 0.35 | 9.19 | 10.88 | 39.58 | - |
| Point 3 | 2.44 | 7.05 | 0.21 | 26.74 | 21.38 | 42.17 | - |
| Point 4 | 12.60 | 5.67 | 2.76 | 14.31 | 11.99 | 30.57 | 22.10 |
| Systems | Number of Oxide Particles | Average Diameter of Oxide Particles/μm3 | Area Percentage of Oxide Inclusion |
|---|---|---|---|
| MgO | 57 | 31.3 | 0.59% |
| Al2O3 | 125 | 6.8 | 0.11% |
| ZrO2 | 64 | 10.1 | 0.09% |
| Y2O3 | 42 | 3.9 | 0.02% |
| Oxides | ΔGf (kJ/mol) | No. |
|---|---|---|
| MgO(s) = Mg(l) + O(g) | 339.741 | (3) |
| 1/3Al2O3(s) = 2/3Al(l) + O(g) | 355.326 | (4) |
| 1/2ZrO2(s) = 1/2Zr(l) + O(g) | 372.979 | (5) |
| 1/3Y2O3(s) = 2/3Y(l) + O(g) | 454.243 | (6) |
| Al2O3(s) ↔ Al2O(g) + O(g) | - | (7) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Zhang, H.; Gao, M.; Li, Q.; Bian, W.; Tao, T.; Zhang, H. High-Temperature Wettability and Interactions between Y-Containing Ni-Based Alloys and Various Oxide Ceramics. Materials 2018, 11, 749. https://doi.org/10.3390/ma11050749
Li J, Zhang H, Gao M, Li Q, Bian W, Tao T, Zhang H. High-Temperature Wettability and Interactions between Y-Containing Ni-Based Alloys and Various Oxide Ceramics. Materials. 2018; 11(5):749. https://doi.org/10.3390/ma11050749
Chicago/Turabian StyleLi, Jinpeng, Huarui Zhang, Ming Gao, Qingling Li, Weidong Bian, Tongxiang Tao, and Hu Zhang. 2018. "High-Temperature Wettability and Interactions between Y-Containing Ni-Based Alloys and Various Oxide Ceramics" Materials 11, no. 5: 749. https://doi.org/10.3390/ma11050749
APA StyleLi, J., Zhang, H., Gao, M., Li, Q., Bian, W., Tao, T., & Zhang, H. (2018). High-Temperature Wettability and Interactions between Y-Containing Ni-Based Alloys and Various Oxide Ceramics. Materials, 11(5), 749. https://doi.org/10.3390/ma11050749






