Synthesis and Characterization of “Ravine-Like” BCN Compounds with High Capacitance
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Preparation of the Precursor
3.2. Synthesis of “Ravine-Like” BCN Compounds
3.3. Characterization
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Huang, C.J.; Chen, C.; Zhang, M.W.; Lin, L.H.; Ye, X.X.; Lin, S.; Antonietti, M.; Wang, X.C. Carbon-doped BN nanosheets for metal-free photoredox catalysis. Nat. Commun. 2015, 6, 7698–8698. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Zhang, L.P.; Xia, Z.H.; Roy, A.; Chang, D.W.; Baek, J.; Dai, L.M. BCN Graphene as Efficient Metal-Free Electrocatalyst for the Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2012, 51, 4209–4212. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Lu, W.G.; Gu, C.Z.; Liu, K.H.; Bai, X.D.; Wang, E.G.; Dai, H.J. Converting Metallic Single-Walled Carbon Nanotubes into Semiconductors by Boron/Nitrogen Co-Doping. Adv. Mater. 2008, 20, 3615–3619. [Google Scholar] [CrossRef]
- Todi, V.O.; Shantheyanda, B.P.; Sundaram, K.B. Influence of annealing on the optical properties of reactively sputtered BCN thin films. Mater. Chem. Phys. 2013, 141, 596–601. [Google Scholar] [CrossRef]
- Lu, J.; Li, D.X.; Yu, D.L. Study on Electronic Properties of Single-Walled B3C10N3 Nanotubes by First Principle Calculations. Integr. Ferroelectr. 2012, 135, 17–21. [Google Scholar] [CrossRef]
- Prakash, A.; Todi, V.; Sundaram, K.B.; Ross, L.; Xu, G.H.; French, M.; Henry, P.; King, S.W. Investigation of the Dielectric and Mechanical Properties for Magnetron Sputtered BCN Thin Films. ECS J. Solid State Sci. Technol. 2015, 4, N3122–N3126. [Google Scholar] [CrossRef]
- Liu, X.B.; Jia, X.P.; Zhang, Z.F.; Zhao, M.; Guo, W.; Huang, G.F.; Ma, H.A. Synthesis and Characterization of New “BCN” Diamond under High Pressure and High Temperature Conditions. Cryst. Growth Des. 2011, 11, 1006–1014. [Google Scholar] [CrossRef]
- Ma, F.K.; Wang, M.X.; Shao, Y.L.; Wang, L.J.; Wu, Y.Z.; Wang, Z.P.; Hao, X.P. ‘Thermal substitution’ for preparing ternary BCN nanosheets with enhanced and controllable nonlinear optical performance. J. Mater. Chem. C. 2017, 5, 2559–2565. [Google Scholar] [CrossRef]
- Karbhal, I.; Devarapalli, R.R.; Debgupta, J.; Pillai, V.K.; Ajayan, P.M.; Shelke, M.V. Facile Green Synthesis of BCN Nanosheets as High-Performance Electrode Material for Electrochemical Energy Storage. Chem. Eur. J. 2016, 22, 7134–7140. [Google Scholar] [CrossRef] [PubMed]
- Dou, S.; Huang, X.B.; Ma, Z.L.; Wu, J.H.; Wang, S.Y. A simple approach to the synthesis of BCN graphene with high capacitance. Nanotechnology 2015, 26, 045402. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Huang, X.K.; Qu, L.T. Heteroatom substituted and decorated graphene: Preparation and applications. Phys. Chem. Chem. Phys. 2015, 17, 32077–32098. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.L.; Gao, Q.M. Boron and nitrogen co-doped porous carbon and its enhanced properties as supercapacitor. J. Power. Sources 2009, 186, 551–556. [Google Scholar] [CrossRef]
- Geick, R.; Perry, C.H.; Rupprecht, G. Normal Modes in Hexagonal Boron Nitride. Phys. Rev. 1966, 146, 543–547. [Google Scholar] [CrossRef]
- Li, D.X.; Lu, J.; Yu, D.L.; Tian, Y.J. Study on Raman Spectroscopy and Purification of B-C-N Compound. Metall. Mater. Trans. A 2011, 42, 2527. [Google Scholar] [CrossRef]
- Shirai, K.; Emura, S.; Gonda, S.; Kumashiro, Y. Infrared study of amorphous B1−xCx films. J. Appl. Phys. 1995, 78, 3392–3400. [Google Scholar] [CrossRef]
- Lü, J.N.; Li, H.D.; Zhu, P.W.; Lü, X.Y.; Li, Y.G. Composited BCN/carbon fibers prepared by hot-filament chemical vapor deposition. Appl. Surf. Sci. 2011, 257, 4963–4967. [Google Scholar] [CrossRef]
- Sainsbury, T.; Satti, A.; May, P.; Wang, Z.; McGovern, I.; Gun’ko, Y.K.; Coleman, J. Oxygen Radical Functionalization of Boron Nitride Nanosheets. J. Am. Chem. Soc. 2012, 134, 18758–18771. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Hamsen, C.; Jia, X.; Kim, K.K.; Reina, A.; Hofmann, M.; Hsu, A.; Zhang, K.; Li, H.; Juang, Z. Synthesis of few-layer hexagonal boron nitride thin film by chemical vapor deposition. Nano Lett. 2010, 10, 4134–4139. [Google Scholar] [CrossRef] [PubMed]
- Fei, H.L.; Ye, R.Q.; Ye, G.L.; Gong, Y.J.; Peng, Z.W.; Fan, X.J.; Samuel, E.L.G.; Ajayan, P.M.; Tour, J.M. Boron-and nitrogen-doped graphene quantum dots/graphene hybrid nanoplatelets as efficient electrocatalysts for oxygen reduction. ACS Nano 2014, 8, 10837–10843. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.S.; Yang, P.J.; Pan, Z.M.; Cao, X.N.; Xie, Z.L.; Wang, X.C. Carbon-Doped BN Nanosheets for the Oxidative Dehydrogenation of Ethylbenzene. Angew. Chem. Int. Ed. 2017, 129, 8343–8347. [Google Scholar] [CrossRef]
- Wang, S.Y.; Iyyamperumal, E.; Roy, A.; Xue, Y.H.; Yu, D.S.; Dai, L.M. Vertically aligned BCN nanotubes as efficient metal-free electrocatalysts for the oxygen reduction reaction: A synergetic effect by co-doping with boron and nitrogen. Angew. Chem. Int. Ed. 2011, 50, 11756–11760. [Google Scholar] [CrossRef] [PubMed]
- Weng, Q.H.; Wang, B.J.; Wang, X.B.; Hanagata, N.; Li, X.; Liu, D.Q.; Wang, X.; Jiang, X.F.; Bando, Y.; Golberg, D. Highly water-soluble, porous, and biocompatible boron nitrides for anticancer drug delivery. ACS Nano 2014, 6, 6123–6130. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.S.; Li, N.; Gao, F.M.; Zhao, Y.F.; Hou, L.; Xu, Z.M. Vertically-aligned BCN Nanotube Arrays with Superior Performance in Electrochemical capacitors. Sci. Rep. 2014, 4, 6083–6088. [Google Scholar] [CrossRef] [PubMed]
- Iyyamperumal, E.; Wang, S.Y.; Dai, L.M. Vertically Aligned BCN Nanotubes with High Capacitance. ACS Nano 2012, 6, 5259–5265. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Wang, Z.Y.; Zhang, M.D.; Yu, C.; Wang, G.; Dong, Y.F.; Liu, S.H.; Wang, Y.W.; Qiu, J.S. Sustainable Synthesis and Assembly of Biomass-DerivedB/N Co-Doped Carbon Nanosheets with Ultrahigh Aspect Ratio for High-Performance Supercapacitors. Adv. Funct. Mater. 2016, 26, 111–119. [Google Scholar]
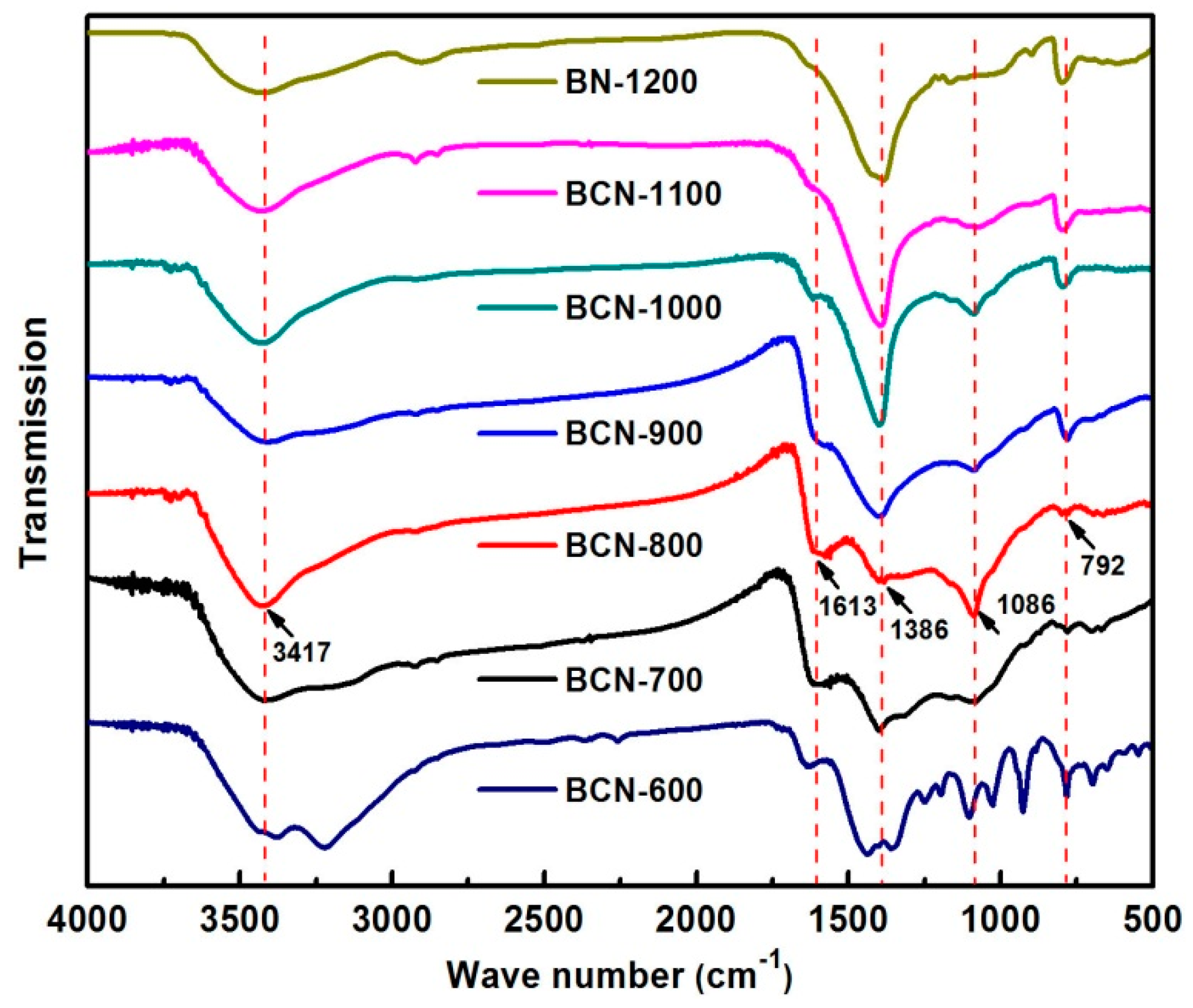
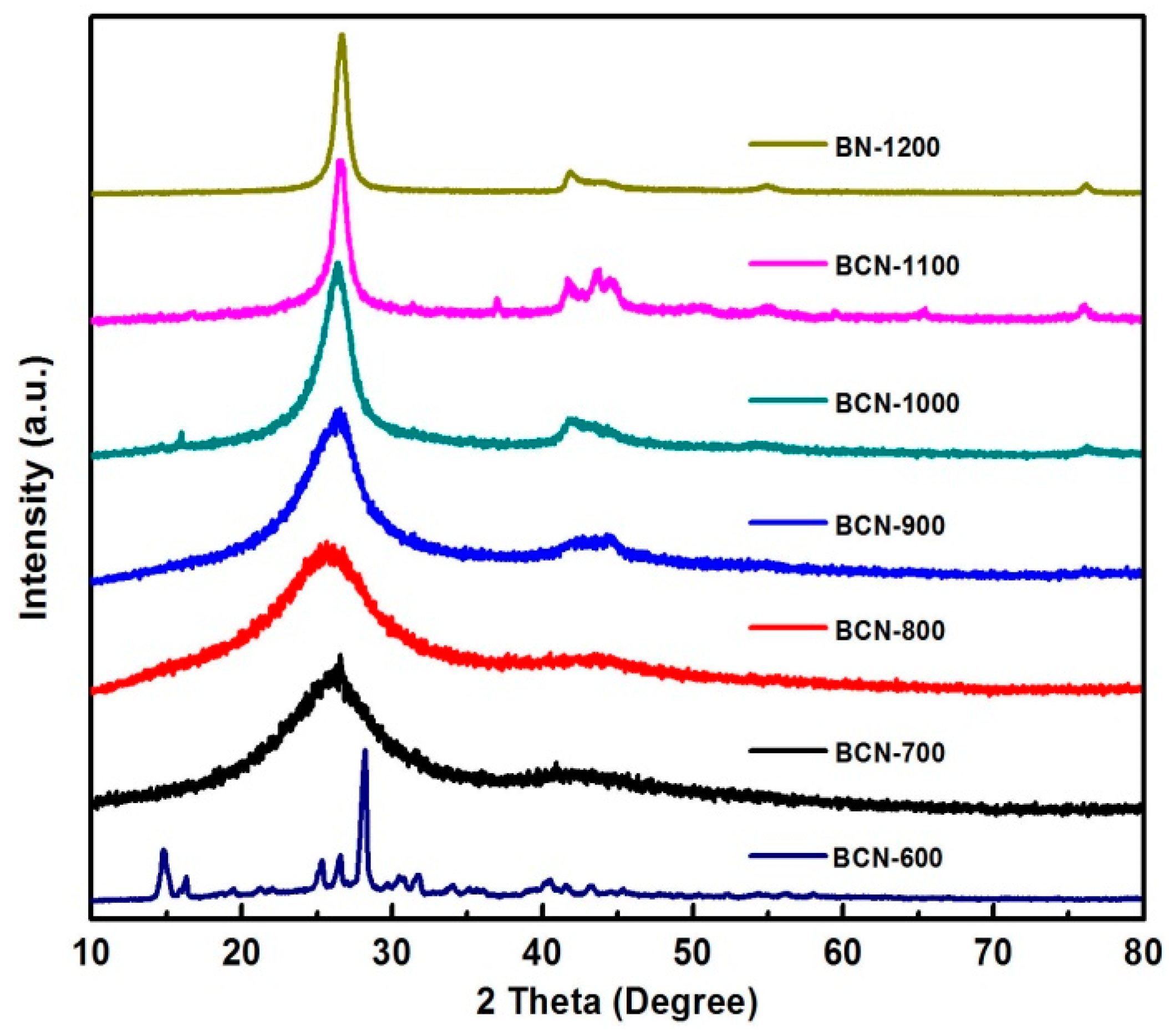
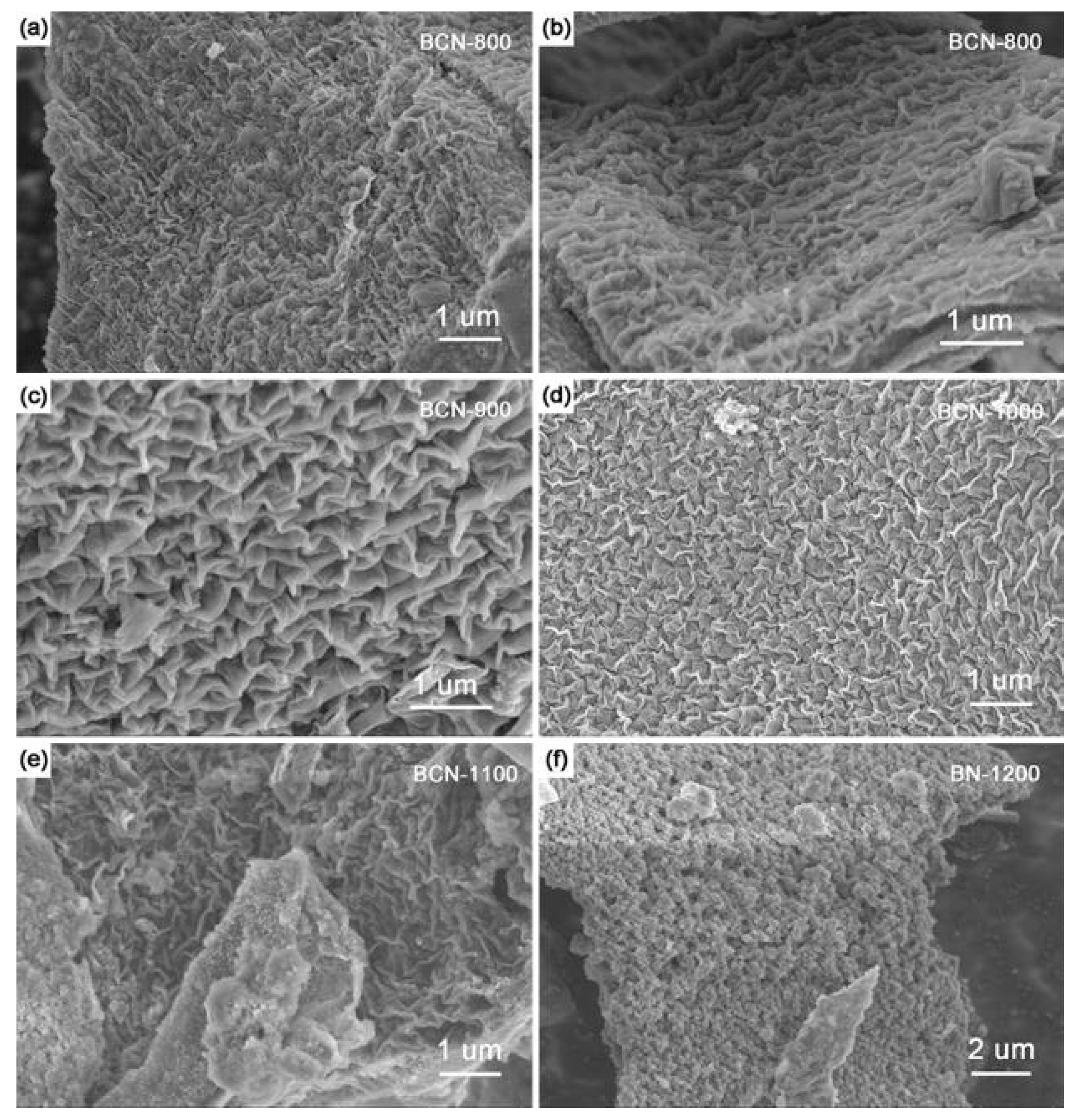
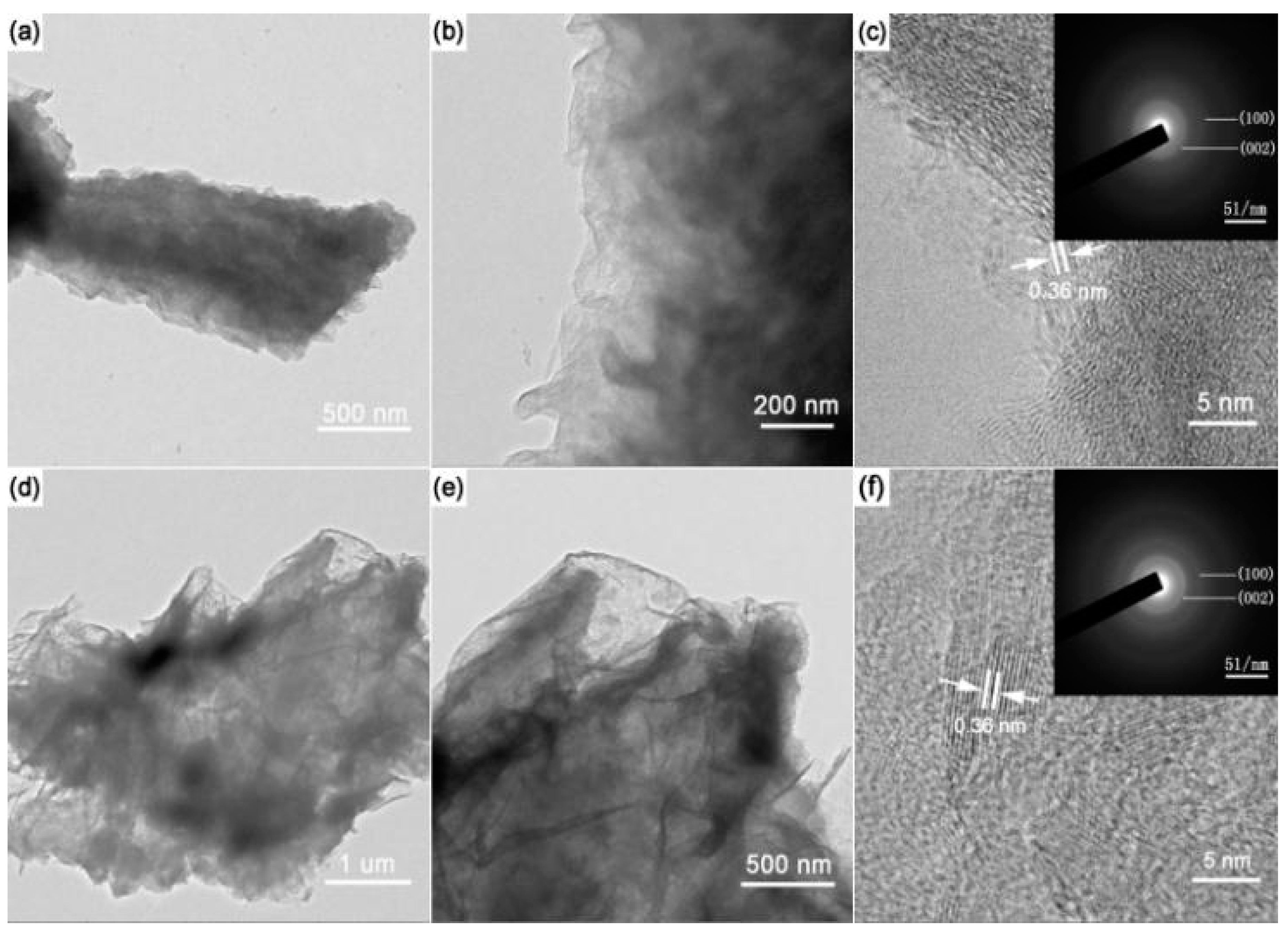
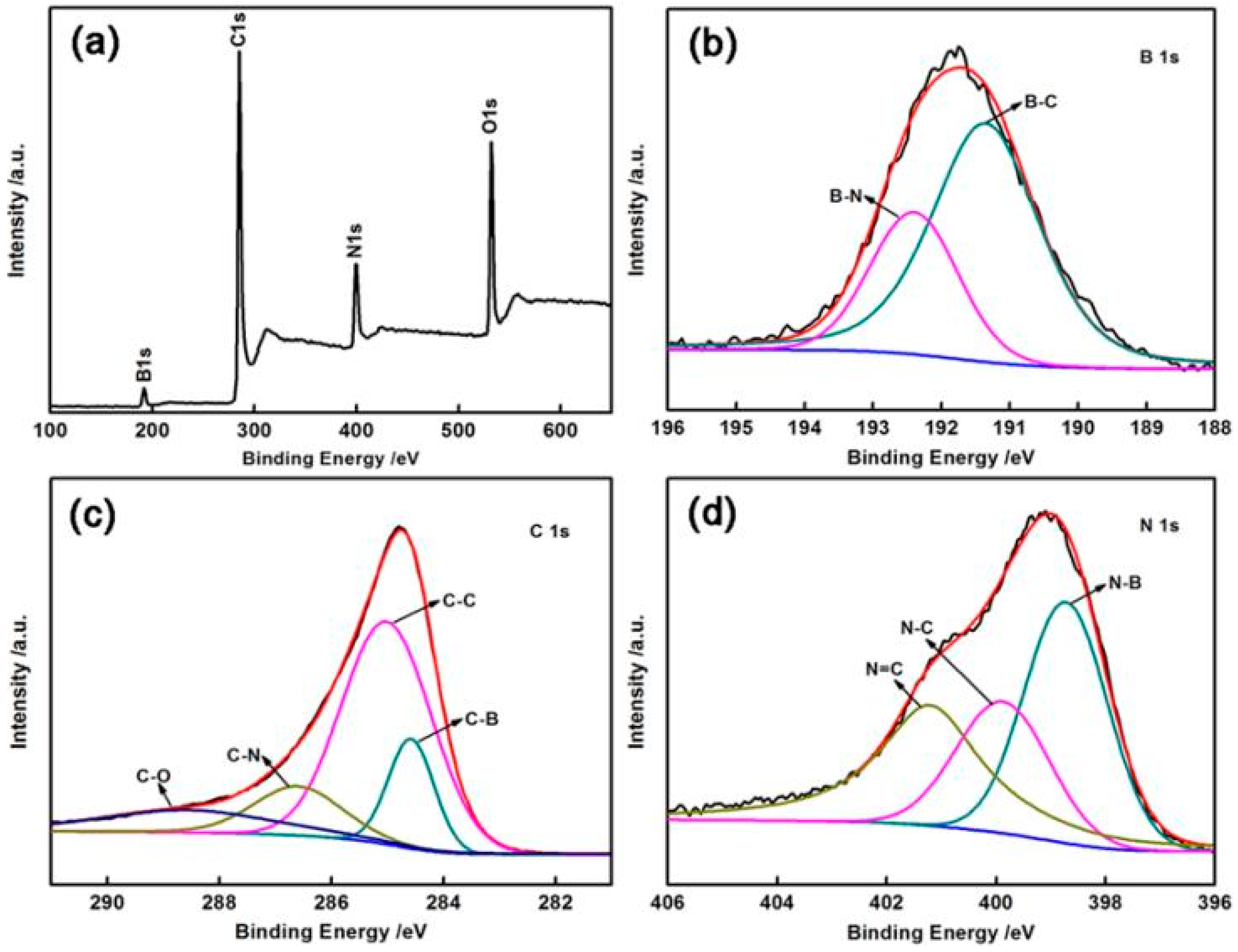
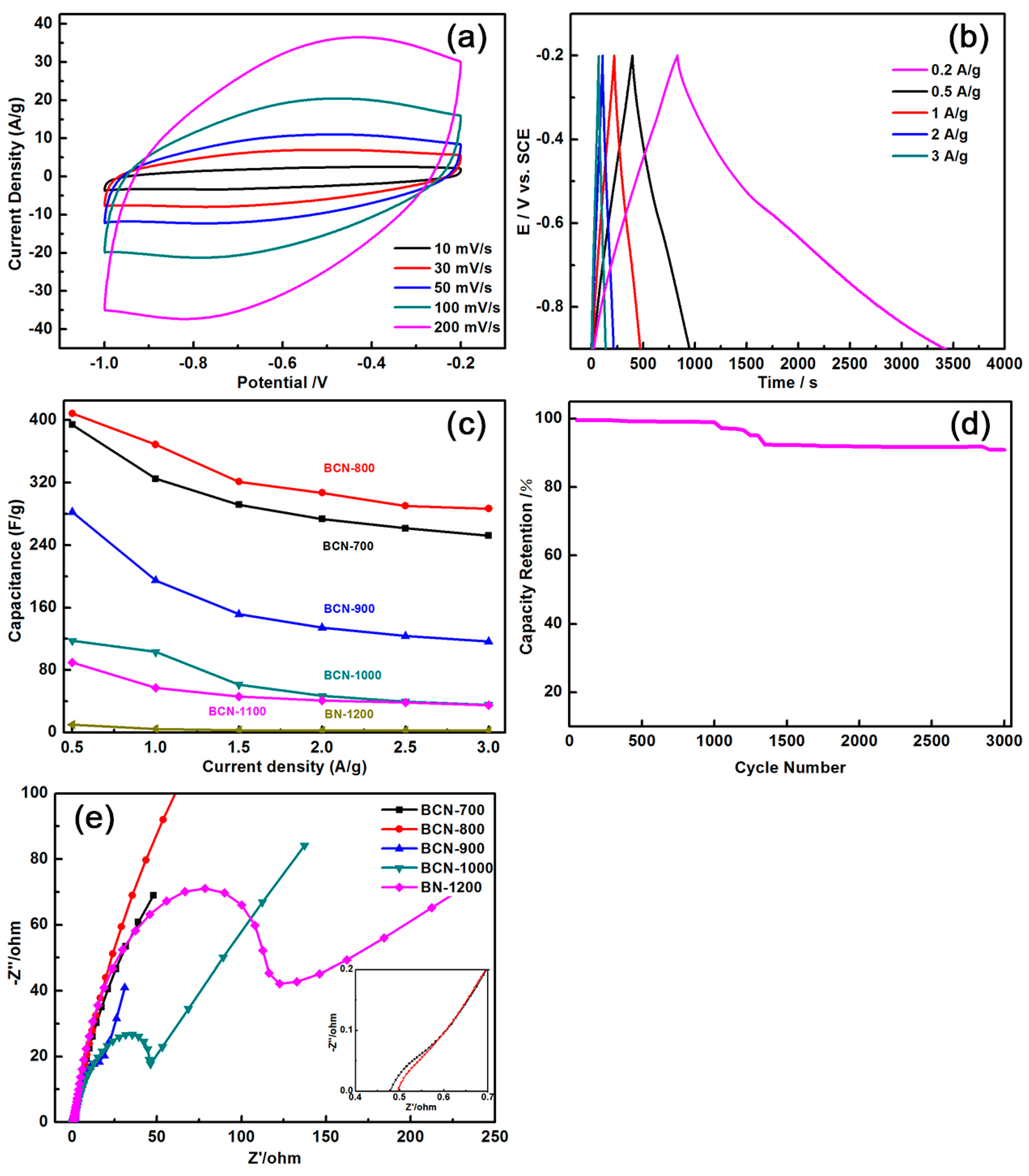
| Sample | B (at %) | C (at %) | N (at %) | O (at %) |
|---|---|---|---|---|
| BCN-700 | 13.02 | 52.54 | 13.83 | 20.61 |
| BCN-800 | 10.15 | 62.6 | 12.17 | 15.02 |
| BCN-900 | 20.94 | 48.7 | 16.23 | 14.11 |
| BCN-1000 | 43.09 | 14.0 | 32.9 | 9.97 |
| BCN-1100 | 40.87 | 14.9 | 30.9 | 13.1 |
| BN-1200 | 49.05 | 4.37 | 39.5 | 7.01 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, D.; Huang, Y.; Hu, X.; Li, R.; Qian, Y.; Li, D. Synthesis and Characterization of “Ravine-Like” BCN Compounds with High Capacitance. Materials 2018, 11, 209. https://doi.org/10.3390/ma11020209
Chen D, Huang Y, Hu X, Li R, Qian Y, Li D. Synthesis and Characterization of “Ravine-Like” BCN Compounds with High Capacitance. Materials. 2018; 11(2):209. https://doi.org/10.3390/ma11020209
Chicago/Turabian StyleChen, Dongping, Yanzhen Huang, Xinling Hu, Rongkai Li, Yingjiang Qian, and Dongxu Li. 2018. "Synthesis and Characterization of “Ravine-Like” BCN Compounds with High Capacitance" Materials 11, no. 2: 209. https://doi.org/10.3390/ma11020209
APA StyleChen, D., Huang, Y., Hu, X., Li, R., Qian, Y., & Li, D. (2018). Synthesis and Characterization of “Ravine-Like” BCN Compounds with High Capacitance. Materials, 11(2), 209. https://doi.org/10.3390/ma11020209






