Soft Material-Enabled, Flexible Hybrid Electronics for Medicine, Healthcare, and Human-Machine Interfaces
Abstract
1. Introduction
2. Flexible Hybrid Electronics (FHE)
2.1. Definition
2.2. Material Characteristics for Wearable and Implantable Electronics
2.3. Sensing Materials
2.4. Substrate Materials
2.5. Wearable FHE
2.5.1. Strain Sensors
2.5.2. Pressure Sensors
2.5.3. Other Types of Sensors
2.5.4. Electrodes
2.5.5. Electrical Components, Displays, and Actuators
2.5.6. Energy Storage
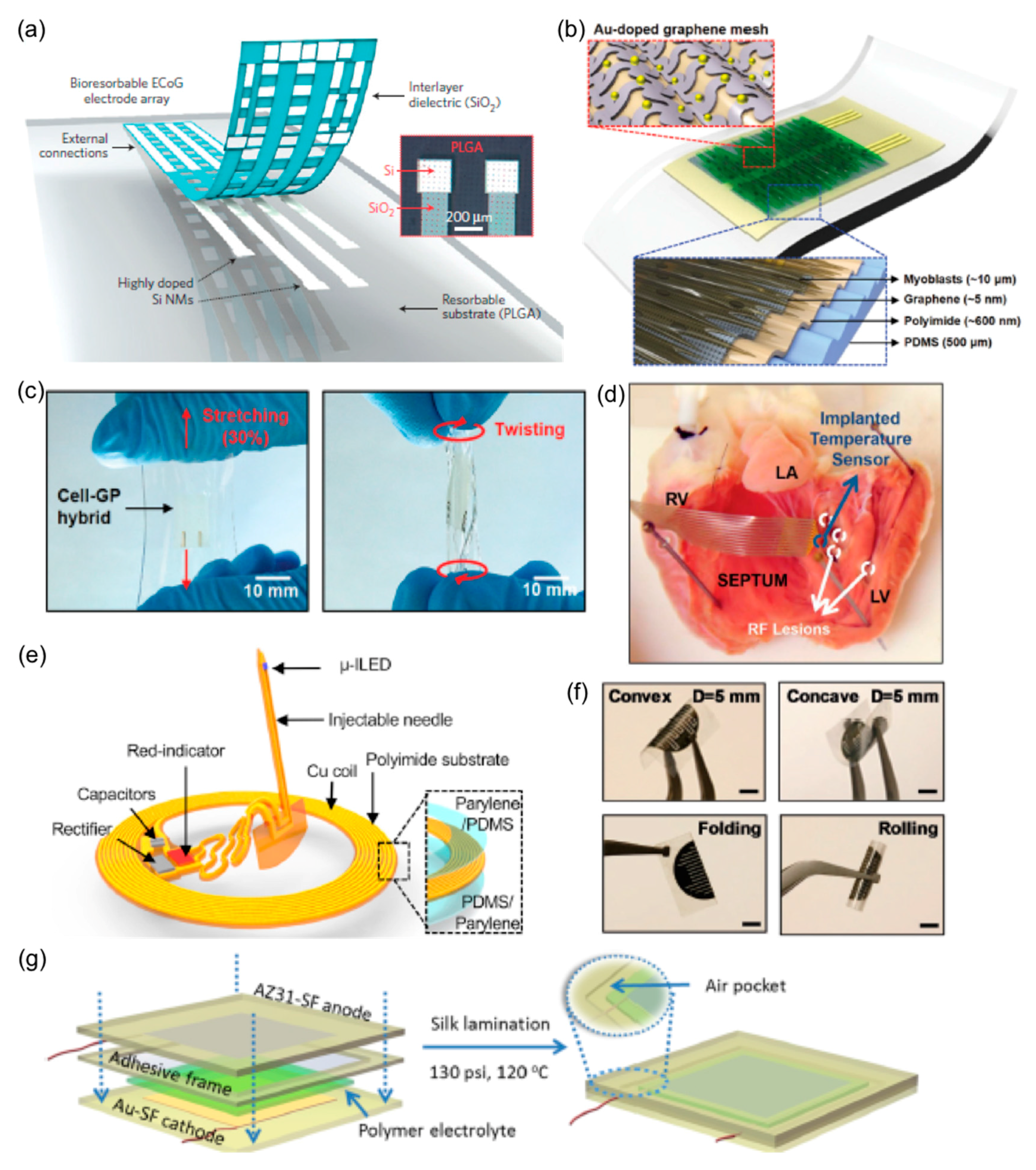
2.6. Implantable FHE
2.6.1. Implantable Electrodes and Sensors
2.6.2. Actuators
2.6.3. Energy Storage and Circuit Components
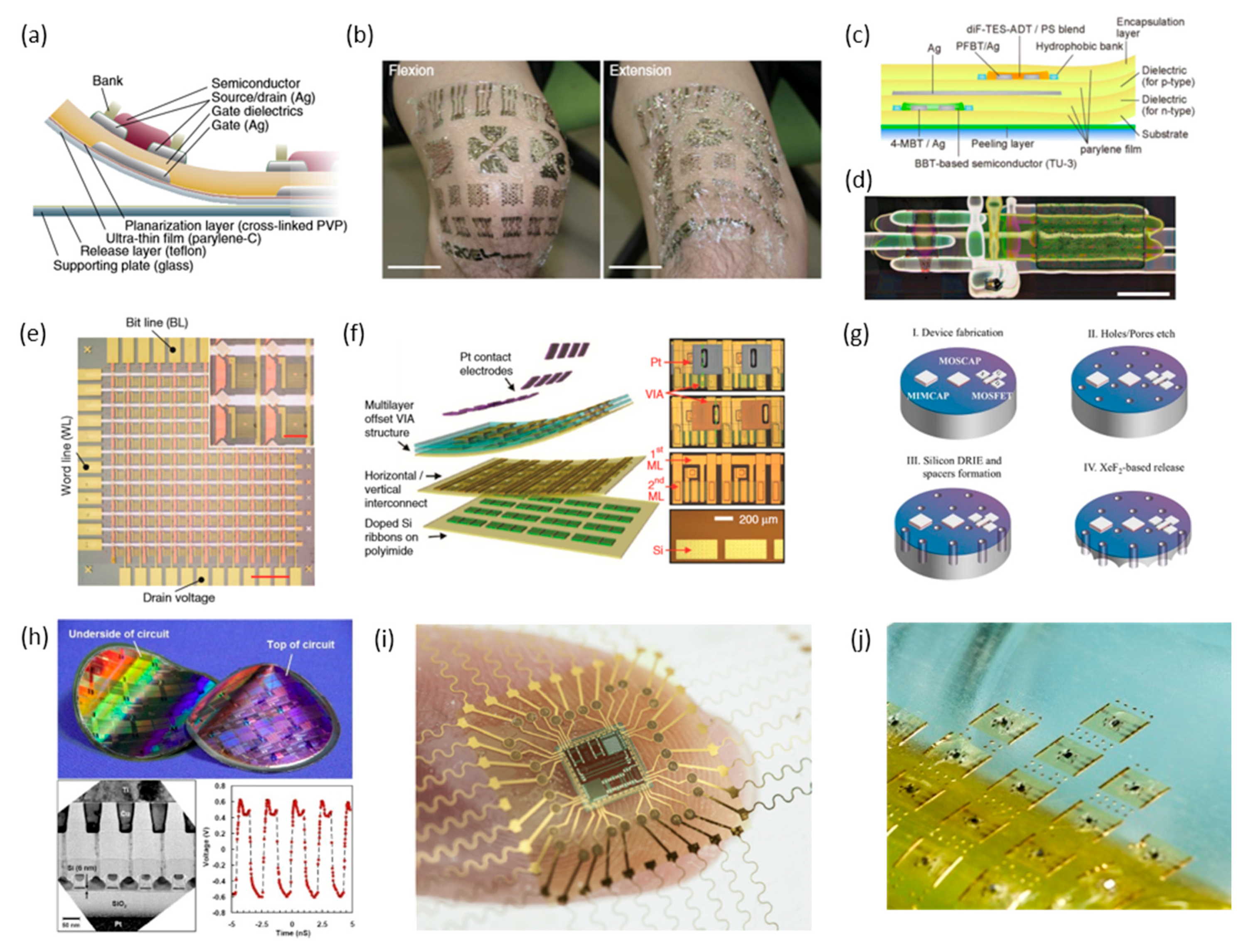
3. Integration Strategies of Electronic Circuits for FHE
3.1. Organic Electronics
3.2. Inorganic Electronics
3.3. Thinned Chips
3.4. Chip-Scale Packaging
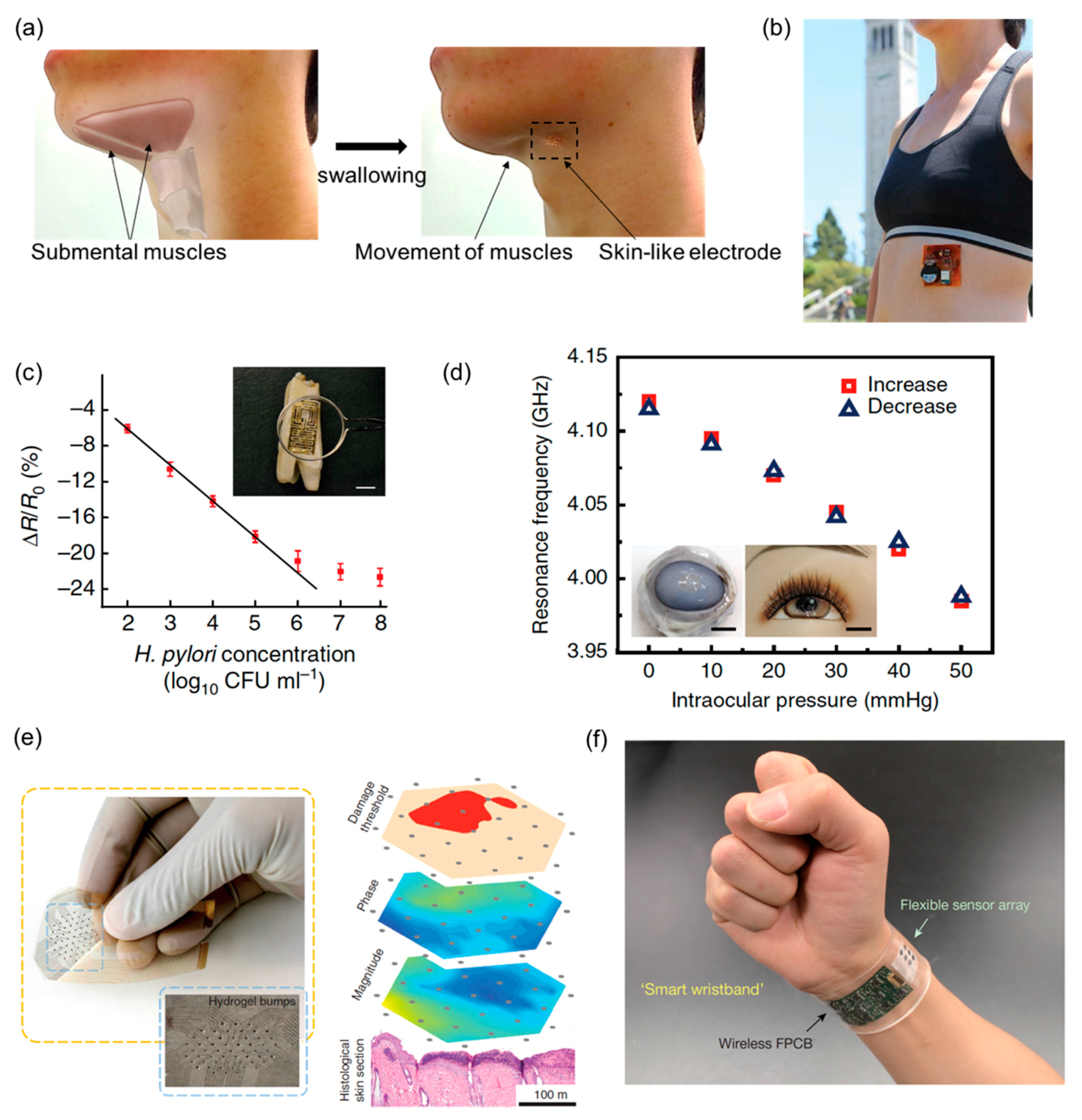
4. Health Monitoring and Disease Diagnostics
5. Human-Machine Interfaces (HMI)
6. Conclusions and Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Xu, S.; Zhang, Y.; Jia, L.; Mathewson, K.E.; Jang, K.-I.; Kim, J.; Fu, H.; Huang, X.; Chava, P.; Wang, R. Soft microfluidic assemblies of sensors, circuits, and radios for the skin. Science 2014, 344, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Hu, J.; Dai, Z.; Zhao, W.; Liu, P.; Zhao, L.; Guo, Y.; Yang, T.; Zou, L.; Jiang, K. Graphene welded carbon nanotube crossbars for biaxial strain sensors. Carbon 2017, 123, 786–793. [Google Scholar] [CrossRef]
- Stokes, A.A.; Shepherd, R.F.; Morin, S.A.; Ilievski, F.; Whitesides, G.M. A hybrid combining hard and soft robots. Soft Robot. 2014, 1, 70–74. [Google Scholar] [CrossRef]
- Valentine, A.D.; Busbee, T.A.; Boley, J.W.; Raney, J.R.; Chortos, A.; Kotikian, A.; Berrigan, J.D.; Durstock, M.F.; Lewis, J.A. Hybrid 3D printing of soft electronics. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Howe, C.; Lee, Y.; Cheon, S.; Yeo, W.-H.; Chun, Y. Microstructured thin film nitinol for a neurovascular flow-diverter. Sci. Rep. 2016, 6, 23698. [Google Scholar] [CrossRef] [PubMed]
- Roshan, Y.M.; Park, E.J. Design approach for a wireless power transfer system for wristband wearable devices. IET Power Electron. 2017. [Google Scholar] [CrossRef]
- Jeong, J.W.; Yeo, W.H.; Akhtar, A.; Norton, J.J.; Kwack, Y.J.; Li, S.; Jung, S.Y.; Su, Y.; Lee, W.; Xia, J. Materials and optimized designs for human-machine interfaces via epidermal electronics. Adv. Mater. 2013, 25, 6839–6846. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Norton, J.J.; Lee, Y.; Lee, D.S.; Agee, N.; Chen, Y.; Chun, Y.; Yeo, W.-H. Soft, conformal bioelectronics for a wireless human-wheelchair interface. Biosens. Bioelectron. 2017, 91, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Yeo, W.H.; Kim, Y.S.; Lee, J.; Ameen, A.; Shi, L.K.; Li, M.; Wang, S.D.; Ma, R.; Jin, S.H.; Kang, Z.; et al. Multifunctional epidermal electronics printed directly onto the skin. Adv. Mater. 2013, 25, 2773–2778. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.J.; Kuzum, D.; Hwang, S.-W.; Kim, B.H.; Juul, H.; Kim, N.H.; Won, S.M.; Chiang, K.; Trumpis, M.; Richardson, A.G. Bioresorbable silicon electronics for transient spatiotemporal mapping of electrical activity from the cerebral cortex. Nat. Mater. 2016, 15, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Gutbrod, S.R.; Sulkin, M.S.; Rogers, J.A.; Efimov, I.R. Patient-specific flexible and stretchable devices for cardiac diagnostics and therapy. Prog. Biophys. Mol. Biol. 2014, 115, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Gutbrod, S.R.; Bonifas, A.P.; Su, Y.; Sulkin, M.S.; Lu, N.; Chung, H.-J.; Jang, K.-I.; Liu, Z.; Ying, M. 3D multifunctional integumentary membranes for spatiotemporal cardiac measurements and stimulation across the entire epicardium. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56. [Google Scholar] [CrossRef]
- Iijima, S.; Ichihashi, T. Single-shell carbon nanotubes of 1-nm diameter. Nature 1993, 363, 603–605. [Google Scholar] [CrossRef]
- Bethune, D.; Klang, C.; De Vries, M.; Gorman, G.; Savoy, R.; Vazquez, J.; Beyers, R. Cobalt-catalysed growth of carbon nanotubes with single-atomic-layer walls. Nature 1993, 363, 605–607. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Lu, N.; Ma, R.; Kim, Y.-S.; Kim, R.-H.; Wang, S.; Wu, J.; Won, S.M.; Tao, H.; Islam, A. Epidermal electronics. Science 2011, 333, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Amjadi, M.; Kyung, K.U.; Park, I.; Sitti, M. Stretchable, skin-mountable, and wearable strain sensors and their potential applications: A review. Adv. Funct. Mater. 2016, 26, 1678–1698. [Google Scholar] [CrossRef]
- Yang, T.; Xie, D.; Li, Z.; Zhu, H. Recent advances in wearable tactile sensors: Materials, sensing mechanisms, and device performance. Mater. Sci. Eng. R Rep. 2017, 115, 1–37. [Google Scholar] [CrossRef]
- An, B.W.; Shin, J.H.; Kim, S.-Y.; Kim, J.; Ji, S.; Park, J.; Lee, Y.; Jang, J.; Park, Y.-G.; Cho, E. Smart sensor systems for wearable electronic devices. Polymers 2017, 9, 303. [Google Scholar] [CrossRef]
- Jin, H.; Abu-Raya, Y.S.; Haick, H. Advanced materials for health monitoring with skin-based wearable devices. Adv. Healthc. Mater. 2017. [Google Scholar] [CrossRef] [PubMed]
- Lacour, S.P.; Courtine, G.; Guck, J. Materials and technologies for soft implantable neuroprostheses. Nat. Rev. Mater. 2016, 1. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, H.; Kim, J.H.; Lee, S.-H. Soft implantable microelectrodes for future medicine: Prosthetics, neural signal recording and neuromodulation. Lab Chip 2016, 16, 959–976. [Google Scholar] [CrossRef] [PubMed]
- Reina, G.; González-Domínguez, J.M.; Criado, A.; Vázquez, E.; Bianco, A.; Prato, M. Promises, facts and challenges for graphene in biomedical applications. Chem. Soc. Rev. 2017, 46, 4400–4416. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Lee, H.; Ghaffari, R.; Hyeon, T.; Kim, D.H. Recent advances in flexible and stretchable bio-electronic devices integrated with nanomaterials. Adv. Mater. 2016, 28, 4203–4218. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.; Choi, M.K.; Hyeon, T.; Kim, D.H. Nanomaterial-based soft electronics for healthcare applications. ChemNanoMat 2016, 2, 1006–1017. [Google Scholar] [CrossRef]
- Lin, S.; Yuk, H.; Zhang, T.; Parada, G.A.; Koo, H.; Yu, C.; Zhao, X. Stretchable hydrogel electronics and devices. Adv. Mater. 2016, 28, 4497–4505. [Google Scholar] [CrossRef] [PubMed]
- Someya, T.; Bao, Z.; Malliaras, G.G. The rise of plastic bioelectronics. Nature 2016, 540, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, M.; Lee, M.-S.; Kim, K.; Ji, S.; Kim, Y.-T.; Park, J.; Na, K.; Bae, K.-H.; Kim, H.K. Wearable smart sensor systems integrated on soft contact lenses for wireless ocular diagnostics. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Coffey, J.W.; Corrie, S.R.; Kendall, M.A. Early circulating biomarker detection using a wearable microprojection array skin patch. Biomaterials 2013, 34, 9572–9583. [Google Scholar] [CrossRef] [PubMed]
- Lipomi, D.J. Stretchable figures of merit in deformable electronics. Adv. Mater. 2016, 28, 4180–4183. [Google Scholar] [CrossRef] [PubMed]
- Mohan, A.; Kim, N.; Gu, Y.; Bandodkar, A.J.; You, J.M.; Kumar, R.; Kurniawan, J.F.; Xu, S.; Wang, J. Merging of thin-and thick-film fabrication technologies: Toward soft stretchable “island–bridge” devices. Adv. Mater. Technol. 2017, 2. [Google Scholar] [CrossRef]
- Lipomi, D.J.; Bao, Z. Stretchable and ultraflexible organic electronics. MRS Bull. 2017, 42, 93–97. [Google Scholar] [CrossRef]
- Kim, J.; Bae, S.H.; Kotal, M.; Stalbaum, T.; Kim, K.J.; Oh, I.K. Soft but powerful artificial muscles based on 3D Graphene-CNT-Ni heteronanostructures. Small 2017, 13. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Xu, X.; Yu, H.; Lubineau, G. Deformable and wearable carbon nanotube microwire-based sensors for ultrasensitive monitoring of strain, pressure and torsion. Nanoscale 2017, 9, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Bi, H.; Zhou, Y.; Xie, X.; Su, S.; Yin, K.; Sun, L. Graphene oxide as high-performance dielectric materials for capacitive pressure sensors. Carbon 2017, 114, 209–216. [Google Scholar] [CrossRef]
- Pang, Y.; Tian, H.; Tao, L.; Li, Y.; Wang, X.; Deng, N.; Yang, Y.; Ren, T.-L. Flexible, highly sensitive, and wearable pressure and strain sensors with graphene porous network structure. ACS Appl. Mater. Interfaces 2016, 8, 26458–26462. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Wang, Q.; Sun, S.; Zhu, W.; Wu, P. A bioinspired mineral hydrogel as a self-healable, mechanically adaptable ionic skin for highly sensitive pressure sensing. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, M.D.; Fassler, A.; Kazem, N.; Markvicka, E.J.; Mandal, P.; Majidi, C. Stretchable, high-k dielectric elastomers through liquid-metal inclusions. Adv. Mater. 2016, 28, 3726–3731. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Zhao, L.; Song, X.; Liu, J.; Zhang, P.; Gao, L. Highly conductive Mo2C nanofibers encapsulated in ultrathin MnO2 nanosheets as a self-supported electrode for high-performance capacitive energy storage. ACS Appl. Mater. Interfaces 2016, 8, 32460–32467. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Miao, X.; Fang, J.; Zhang, X.; Chen, S.; Li, W.; Feng, W.; Chen, Y.; Wang, W.; Zhang, Y. Layered-MnO2 nanosheet grown on nitrogen-doped graphene template as a composite cathode for flexible solid-state asymmetric supercapacitor. ACS Appl. Mater. Interfaces 2016, 8, 5251–5260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhang, X.; Huang, H.; Wang, J.; Li, Q.; Chen, L.Q.; Wang, Q. Toward wearable cooling devices: Highly flexible electrocaloric Ba0.67Sr0.33TiO3 nanowire arrays. Adv. Mater. 2016, 28, 4811–4816. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhu, B.; Wang, H.; Ma, X.; Hao, Y.; Chen, X. Ultra-lightweight resistive switching memory devices based on silk fibroin. Small 2016, 12, 3360–3365. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.; Kostarelos, K.; Prato, M. Making carbon nanotubes biocompatible and biodegradable. Chem. Commun. 2011, 47, 10182–10188. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.M.; Goncalves, I.C.; Magalhães, F.D. Graphene-based materials biocompatibility: A review. Colloids Surf. B Biointerfaces 2013, 111, 188–202. [Google Scholar] [CrossRef] [PubMed]
- Syama, S.; Mohanan, P. Safety and biocompatibility of graphene: A new generation nanomaterial for biomedical application. Int. J. Biol. Macromol. 2016, 86, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Fabbro, A.; Scaini, D.; León, V.N.; Vázquez, E.; Cellot, G.; Privitera, G.; Lombardi, L.; Torrisi, F.; Tomarchio, F.; Bonaccorso, F. Graphene-based interfaces do not alter target nerve cells. ACS Nano 2016, 10, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Sun, X.; Hargrove, D.; Chen, J.; Song, D.; Dong, Q.; Lu, X.; Fan, T.-H.; Fu, Y.; Lei, Y. A biocompatible and biodegradable protein hydrogel with green and red autofluorescence: Preparation, characterization and in vivo biodegradation tracking and modeling. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Kang, S.K.; Won, S.M.; Gutruf, P.; Jeong, Y.R.; Koo, J.; Lee, S.S.; Rogers, J.A.; Ha, J.S. Fully biodegradable microsupercapacitor for power storage in transient electronics. Adv. Energy Mater. 2017. [Google Scholar] [CrossRef]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Wang, X.; Liu, J. Recent advancements in liquid metal flexible printed electronics: Properties, technologies, and applications. Micromachines 2016, 7, 206. [Google Scholar] [CrossRef]
- Yang, K.; Zeng, M.; Fu, X.; Li, J.; Ma, N.; Tao, L. Establishing biodegradable single-layer MnO2 nanosheets as a platform for live cell microRNA sensing. RSC Adv. 2015, 5, 104245–104249. [Google Scholar] [CrossRef]
- Fan, W.; Bu, W.; Shen, B.; He, Q.; Cui, Z.; Liu, Y.; Zheng, X.; Zhao, K.; Shi, J. Intelligent MnO2 nanosheets anchored with upconversion nanoprobes for concurrent pH-/H2O2-responsive ucl imaging and oxygen-elevated synergetic therapy. Adv. Mater. 2015, 27, 4155–4161. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Shan, D.; Chen, R.; Zhang, F.; Han, E.-H. Biodegradable behaviors of AZ31 magnesium alloy in simulated body fluid. Mater. Sci. Eng. C 2009, 29, 1039–1045. [Google Scholar] [CrossRef]
- Hwang, S.-W.; Park, G.; Edwards, C.; Corbin, E.A.; Kang, S.-K.; Cheng, H.; Song, J.-K.; Kim, J.-H.; Yu, S.; Ng, J. Dissolution chemistry and biocompatibility of single-crystalline silicon nanomembranes and associated materials for transient electronics. ACS Nano 2014, 8, 5843–5851. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Movia, D.; Mahfoud, O.K.; Volkov, Y.; Prina-Mello, A. Silver nanowires as prospective carriers for drug delivery in cancer treatment: An in vitro biocompatibility study on lung adenocarcinoma cells and fibroblasts. Eur. J. Nanomed. 2013, 5, 195–204. [Google Scholar] [CrossRef]
- Ryu, M.; Yang, J.H.; Ahn, Y.; Sim, M.; Lee, K.H.; Kim, K.; Lee, T.; Yoo, S.-J.; Kim, S.Y.; Moon, C. Enhancement of interface characteristics of neural probe based on graphene, zno nanowires, and conducting polymer pedot. ACS Appl. Mater. Interfaces 2017, 9, 10577–10586. [Google Scholar] [CrossRef] [PubMed]
- Ball, J.P.; Mound, B.A.; Nino, J.C.; Allen, J.B. Biocompatible evaluation of barium titanate foamed ceramic structures for orthopedic applications. J. Biomed. Mater. Res. Part A 2014, 102, 2089–2095. [Google Scholar] [CrossRef] [PubMed]
- Chiappini, C.; Liu, X.; Fakhoury, J.R.; Ferrari, M. Biodegradable porous silicon barcode nanowires with defined geometry. Adv. Funct. Mater. 2010, 20, 2231–2239. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.W.; Lai, J.J.; Wei, K.H.; Chen, P. Studies of surface-modified gold nanowires inside living cells. Adv. Funct. Mater. 2007, 17, 3707–3714. [Google Scholar] [CrossRef]
- Byrne, F.; Prina-Mello, A.; Whelan, A.; Mohamed, B.M.; Davies, A.; Gun’ko, Y.K.; Coey, J.; Volkov, Y. High content analysis of the biocompatibility of nickel nanowires. J. Magn. Magn. Mater. 2009, 321, 1341–1345. [Google Scholar] [CrossRef]
- Berggren, M.; Richter-Dahlfors, A. Organic bioelectronics. Adv. Mater. 2007, 19, 3201–3213. [Google Scholar] [CrossRef]
- Gui, Q.; He, Y.; Gao, N.; Tao, X.; Wang, Y. A skin-inspired integrated sensor for synchronous monitoring of multiparameter signals. Adv. Funct. Mater. 2017, 27. [Google Scholar] [CrossRef]
- Kim, H.; Eom, T.S.; Cho, W.; Woo, K.; Shon, Y.; Wie, J.J.; Shim, B.S. Soft electronics on asymmetrical porous conducting membranes by molecular layer-by-layer assembly. Sens. Actuators B Chem. 2018, 254, 916–925. [Google Scholar] [CrossRef]
- Kireev, D.; Seyock, S.; Ernst, M.; Maybeck, V.; Wolfrum, B.; Offenhäusser, A. Versatile flexible graphene multielectrode arrays. Biosensors 2016, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, C.; Wang, Q.; Jian, M.; Zhang, Y. Sheath–core graphite/silk fiber made by dry-meyer-rod-coating for wearable strain sensors. ACS Appl. Mater. Interfaces 2016, 8, 20894–20899. [Google Scholar] [CrossRef] [PubMed]
- Yeo, J.C.; Yu, J.; Shang, M.; Loh, K.P.; Lim, C.T. Highly flexible graphene oxide nanosuspension liquid-based microfluidic tactile sensor. Small 2016, 12, 1593–1604. [Google Scholar]
- Huang, X.; Leng, T.; Zhu, M.; Zhang, X.; Chen, J.; Chang, K.; Aqeeli, M.; Geim, A.K.; Novoselov, K.S.; Hu, Z. Highly flexible and conductive printed graphene for wireless wearable communications applications. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Wang, J.; Qian, K.; Chen, J.; Li, S.; Lee, P.S. Extremely stretchable strain sensors based on conductive self-healing dynamic cross-links hydrogels for human-motion detection. Adv. Sci. 2017, 4. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.-H.; Li, Y.; Cutting, G.R.; Searson, P.C. A wearable potentiometric sensor with integrated salt bridge for sweat chloride measurement. Sens. Actuators B Chem. 2017, 250, 673–678. [Google Scholar] [CrossRef]
- Gao, Y.; Ota, H.; Schaler, E.W.; Chen, K.; Zhao, A.; Gao, W.; Fahad, H.M.; Leng, Y.; Zheng, A.; Xiong, F. Wearable microfluidic diaphragm pressure sensor for health and tactile touch monitoring. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Yeo, W.-H. Skin-like electronics for a persistent brain-computer interface. J. Nat. Sci. 2015, 1, e132. [Google Scholar]
- Ko, Y.; Kim, J.; Kim, D.; Yamauchi, Y.; Kim, J.H.; You, J. A simple silver nanowire patterning method based on poly (ethylene glycol) photolithography and its application for soft electronics. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-Q.; Zhu, W.-B.; Yu, X.-G.; Huang, P.; Fu, S.-Y.; Hu, N.; Liao, K. Multifunctional wearable device based on flexible and conductive carbon sponge/polydimethylsiloxane composite. ACS Appl. Mater. Interfaces 2016, 8, 33189–33196. [Google Scholar] [CrossRef] [PubMed]
- Takamatsu, S.; Lonjaret, T.; Ismailova, E.; Masuda, A.; Itoh, T.; Malliaras, G.G. Wearable keyboard using conducting polymer electrodes on textiles. Adv. Mater. 2016, 28, 4485–4488. [Google Scholar] [CrossRef] [PubMed]
- Roche, E.T.; Horvath, M.A.; Wamala, I.; Alazmani, A.; Song, S.-E.; Whyte, W.; Machaidze, Z.; Payne, C.J.; Weaver, J.C.; Fishbein, G. Soft robotic sleeve supports heart function. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Ecoflex 00-30. Available online: https://www.smooth-on.com/products/ecoflex-00-30/ (accessed on 23 January 2018).
- Salvatore, G.A.; Sülzle, J.; Dalla Valle, F.; Cantarella, G.; Robotti, F.; Jokic, P.; Knobelspies, S.; Daus, A.; Büthe, L.; Petti, L. Biodegradable and highly deformable temperature sensors for the internet of things. Adv. Funct. Mater. 2017, 27. [Google Scholar] [CrossRef]
- Johnston, I.; McCluskey, D.; Tan, C.; Tracey, M. Mechanical characterization of bulk sylgard 184 for microfluidics and microengineering. J. Micromech. Microeng. 2014, 24, 035017. [Google Scholar] [CrossRef]
- Product Information 184 Silicone Elastomer. Available online: https://www.galco.com/techdoc/dowc/sylgard-184_pg.pdf (accessed on 23 January 2018).
- Kim, S.-J.; Lee, D.-S.; Kim, I.-G.; Sohn, D.-W.; Park, J.-Y.; Choi, B.-K.; Kim, S.-W. Evaluation of the biocompatibility of a coating material for an implantable bladder volume sensor. Kaohsiung J. Med. Sci. 2012, 28, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Silbione LSR 4330. Available online: http://www.silbione.com/wp-content/uploads/2014/02/Silbione-LSR-4330.pdf (accessed on 23 January 2018).
- Silicones, B. Silbione Liquid Silicone Rubber (LSR) Elastomers for Healthcare and Medical Device Fabrication. Available online: http://www.silbione.com/wp-content/uploads/2014/01/Biocompatibility-LSR.pdf (accessed on 23 January 2018).
- Parylene Properties. Available online: https://vsiparylene.com/parylene-advantages/properties/#mechanical (accessed on 24 January 2018).
- Rodger, D.C.; Weiland, J.D.; Humayun, M.S.; Tai, Y.-C. Scalable high lead-count parylene package for retinal prostheses. Sens. Actuators B Chem. 2006, 117, 107–114. [Google Scholar] [CrossRef]
- Song, J.S.; Lee, S.; Jung, S.H.; Cha, G.C.; Mun, M.S. Improved biocompatibility of parylene-C films prepared by chemical vapor deposition and the subsequent plasma treatment. J. Appl. Polym. Sci. 2009, 112, 3677–3685. [Google Scholar] [CrossRef]
- Properties of Various High Performance Films. Available online: http://www.teijinfilmsolutions.jp/english/product/hi_film.html (accessed on 24 January 2018).
- Veleirinho, B.; Coelho, D.S.; Dias, P.F.; Maraschin, M.; Pinto, R.; Cargnin-Ferreira, E.; Peixoto, A.; Souza, J.A.; Ribeiro-do-Valle, R.M.; Lopes-da-Silva, J.A. Foreign body reaction associated with pet and pet/chitosan electrospun nanofibrous abdominal meshes. PLoS ONE 2014, 9, e95293. [Google Scholar] [CrossRef] [PubMed]
- Ramires, P.; Mirenghi, L.; Romano, A.; Palumbo, F.; Nicolardi, G. Plasma-treated pet surfaces improve the biocompatibility of human endothelial cells. J. Biomed. Mater. Res. Part A 2000, 51, 535–539. [Google Scholar] [CrossRef]
- Swar, S.; Zajícová, V.; Rysová, M.; Lovětinská-Šlamborová, I.; Voleský, L.; Stibor, I. Biocompatible surface modification of poly(ethylene terephthalate) focused on pathogenic bacteria: Promising prospects in biomedical applications. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Kai, W.; Hirota, Y.; Hua, L.; Inoue, Y. Thermal and mechanical properties of a poly(Є-caprolactone)/graphite oxide composite. J. Appl. Polym. Sci. 2008, 107, 1395–1400. [Google Scholar] [CrossRef]
- Serrano, M.; Pagani, R.; Vallet-Regı, M.; Pena, J.; Ramila, A.; Izquierdo, I.; Portoles, M. In vitro biocompatibility assessment of poly(ε-caprolactone) films using l929 mouse fibroblasts. Biomaterials 2004, 25, 5603–5611. [Google Scholar] [CrossRef] [PubMed]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Del Valle, J.; de la Oliva, N.; Müller, M.; Stieglitz, T.; Navarro, X. Biocompatibility evaluation of parylene C and polyimide as substrates for peripheral nerve interfaces. In Proceedings of the 2015 7th International IEEE/EMBS Conference on Neural Engineering (NER), Montpellier, France, 22–24 April 2015; pp. 442–445. [Google Scholar]
- Mills, C.; Escarré, J.; Engel, E.; Martinez, E.; Errachid, A.; Bertomeu, J.; Andreu, J.; Planell, J.A.; Samitier, J. Micro- and nanostructuring of poly(ethylene-2,6-naphthalate) surfaces, for biomedical applications, using polymer replication techniques. Nanotechnology 2005, 16, 369. [Google Scholar] [CrossRef]
- Pes. Available online: https://www.plasticsintl.com/datasheets/Radel_A_PES.pdf (accessed on 23 January 2018).
- Azadbakht, M.; Madaeni, S.S.; Sahebjamee, F. Biocompatibility of polyethersulfone membranes for cell culture systems. Eng. Life Sci. 2011, 11, 629–635. [Google Scholar] [CrossRef]
- Teflon (ptfe). Available online: http://www.dielectriccorp.com/downloads/thermoplastics/teflon.pdf (accessed on 23 January 2018).
- Risbud, M.V.; Hambir, S.; Jog, J.; Bhonde, R. Biocompatibility assessment of polytetrafluoroethylene/wollastonite composites using endothelial cells and macrophages. J. Biomater. Sci. Polym. Ed. 2001, 12, 1177–1189. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An overview of poly(lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef] [PubMed]
- Ignatius, A.; Claes, L.E. In vitro biocompatibility of bioresorbable polymers: Poly(l,dl-lactide) and poly(l-Lactide-co-glycolide). Biomaterials 1996, 17, 831–839. [Google Scholar] [CrossRef]
- Cyclo Olefin Polymer. Available online: http://www.zeon.co.jp/content/200181692.pdf (accessed on 23 January 2018).
- Tubia, I.; Mujika, M.; Artieda, J.; Valencia, M.; Arana, S. Soft polymer sensor for recording surface cortical activity in freely moving rodents. Sens. Actuators A Phys. 2016, 251, 241–247. [Google Scholar] [CrossRef]
- Lu, S.; Wang, X.; Lu, Q.; Zhang, X.; Kluge, J.A.; Uppal, N.; Omenetto, F.; Kaplan, D.L. Insoluble and flexible silk films containing glycerol. Biomacromolecules 2009, 11, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Hwang, S.-W.; Marelli, B.; An, B.; Moreau, J.E.; Yang, M.; Brenckle, M.A.; Kim, S.; Kaplan, D.L.; Rogers, J.A. Silk-based resorbable electronic devices for remotely controlled therapy and in vivo infection abatement. Proc. Natl. Acad. Sci. USA 2014, 111, 17385–17389. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, B. Biodegradation of silk biomaterials. Int. J. Mol. Sci. 2009, 10, 1514–1524. [Google Scholar] [CrossRef] [PubMed]
- Do, T.N.; Visell, Y. Stretchable, twisted conductive microtubules for wearable computing, robotics, electronics, and healthcare. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-J.; Cao, W.-T.; Ma, M.-G.; Wan, P. Ultrasensitive wearable soft strain sensors of conductive, self-healing, and elastic hydrogels with synergistic “soft and hard” hybrid networks. ACS Appl. Mater. Interfaces 2017, 9, 25559–25570. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Nicholls, B.; Lee, D.S.; Chen, Y.; Chun, Y.; Ang, C.S.; Yeo, W.-H. Soft electronics enabled ergonomic human-computer interaction for swallowing training. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.H.; Jeong, S.; Shim, H.J.; Son, D.; Kim, J.; Kim, D.C.; Choi, S.; Hong, J.-I.; Kim, D.-H. Wearable electrocardiogram monitor using carbon nanotube electronics and color-tunable organic light-emitting diodes. ACS Nano 2017, 11, 10032–10041. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Shim, H.J.; Yang, J.; Choi, M.K.; Kim, D.C.; Kim, J.; Hyeon, T.; Kim, D.H. Ultrathin quantum dot display integrated with wearable electronics. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Giffney, T.; Bejanin, E.; Kurian, A.S.; Travas-Sejdic, J.; Aw, K. Highly stretchable printed strain sensors using multi-walled carbon nanotube/silicone rubber composites. Sens. Actuators A Phys. 2017, 259, 44–49. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Yamamoto, D.; Takada, M.; Naito, H.; Arie, T.; Akita, S.; Takei, K. Efficient skin temperature sensor and stable gel-less sticky ECG sensor for a wearable flexible healthcare patch. Adv. Healthc. Mater. 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Nakata, S.; Arie, T.; Akita, S.; Takei, K. Wearable, flexible, and multifunctional healthcare device with an isfet chemical sensor for simultaneous sweat pH and skin temperature monitoring. ACS Sens. 2017, 2, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xi, J.; Huang, W.; Yuen, M.M. Stretchable conductive elastomer for wireless wearable communication applications. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.; Yin, H.; Zhang, W.; Liu, Y.; Tang, Z.; Zhu, M.Q. High-performance fiber-shaped all-solid-state asymmetric supercapacitors based on ultrathin MnO2 nanosheet/carbon fiber cathodes for wearable electronics. Adv. Energy Mater. 2016, 6. [Google Scholar] [CrossRef]
- Yin, B.; Wen, Y.; Hong, T.; Xie, Z.; Yuan, G.; Ji, Q.; Jia, H. Highly stretchable, ultrasensitive, and wearable strain sensors based on facilely prepared reduced graphene oxide woven fabrics in an ethanol flame. ACS Appl. Mater. Interfaces 2017, 9, 32054–32064. [Google Scholar] [CrossRef] [PubMed]
- Chun, S.; Choi, Y.; Park, W. All-graphene strain sensor on soft substrate. Carbon 2017, 116, 753–759. [Google Scholar] [CrossRef]
- Hu, Y.; Zhao, T.; Zhu, P.; Zhang, Y.; Liang, X.; Sun, R.; Wong, C.-P. A low-cost, printable, and stretchable strain sensor based on highly conductive elastic composites with tunable sensitivity for human motion monitoring. Nano Res. 2017. [Google Scholar] [CrossRef]
- Lee, H.; Kwon, D.; Cho, H.; Park, I.; Kim, J. Soft nanocomposite based multi-point, multi-directional strain mapping sensor using anisotropic electrical impedance tomography. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Kim, J.; Chu, M.; Khine, M. Highly flexible wrinkled carbon nanotube thin film strain sensor to monitor human movement. Adv. Mater. Technol. 2016, 1. [Google Scholar] [CrossRef]
- Cooper, C.B.; Arutselvan, K.; Liu, Y.; Armstrong, D.; Lin, Y.; Khan, M.R.; Genzer, J.; Dickey, M.D. Stretchable capacitive sensors of torsion, strain, and touch using double helix liquid metal fibers. Adv. Funct. Mater. 2017, 27. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, X.; Liang, J.; Lv, H.; Tong, H.; Ma, L.; Hu, Y.; Zhu, G.; Zhang, T.; Tie, Z. Versatile electronic skins for motion detection of joints enabled by aligned few-walled carbon nanotubes in flexible polymer composites. Adv. Funct. Mater. 2017, 27. [Google Scholar] [CrossRef]
- You, B.; Han, C.J.; Kim, Y.; Ju, B.-K.; Kim, J.-W. A wearable piezocapacitive pressure sensor with a single layer of silver nanowire-based elastomeric composite electrodes. J. Mater. Chem. A 2016, 4, 10435–10443. [Google Scholar] [CrossRef]
- Mannsfeld, S.C.; Tee, B.C.; Stoltenberg, R.M.; Chen, C.V.H.; Barman, S.; Muir, B.V.; Sokolov, A.N.; Reese, C.; Bao, Z. Highly sensitive flexible pressure sensors with microstructured rubber dielectric layers. Nat. Mater. 2010, 9, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.; Tee, B.C.-K.; Mei, J.; Appleton, A.L.; Kim, D.H.; Wang, H.; Bao, Z. Flexible polymer transistors with high pressure sensitivity for application in electronic skin and health monitoring. Nat. Commun. 2013, 4, 1859. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Jo, I.; Kang, S.; Jang, B.; Moon, J.; Park, J.B.; Lee, S.; Rho, S.; Kim, Y.; Hong, B.H. Smart contact lenses with graphene coating for electromagnetic interference shielding and dehydration protection. ACS Nano 2017, 11, 5318–5324. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Bandodkar, A.J.; Valdés-Ramírez, G.; Windmiller, J.R.; Yang, Z.; Ramírez, J.; Chan, G.; Wang, J. Electrochemical tattoo biosensors for real-time noninvasive lactate monitoring in human perspiration. Anal. Chem. 2013, 85, 6553–6560. [Google Scholar] [CrossRef] [PubMed]
- Imani, S.; Bandodkar, A.J.; Mohan, A.V.; Kumar, R.; Yu, S.; Wang, J.; Mercier, P.P. A wearable chemical–electrophysiological hybrid biosensing system for real-time health and fitness monitoring. Nat. Commun. 2016, 7, 11650. [Google Scholar] [CrossRef] [PubMed]
- Bandodkar, A.J.; Jia, W.; Yardımcı, C.; Wang, X.; Ramirez, J.; Wang, J. Tattoo-based noninvasive glucose monitoring: A proof-of-concept study. Anal. Chem. 2014, 87, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Bandodkar, A.J.; Nuñez-Flores, R.; Jia, W.; Wang, J. All-printed stretchable electrochemical devices. Adv. Mater. 2015, 27, 3060–3065. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Zhou, Z.K.; Li, Q.; Xue, J.; Di Falco, A.; Yang, Z.; Zhou, J.; Wang, X. Flexible nanowire cluster as a wearable colorimetric humidity sensor. Small 2017. [Google Scholar] [CrossRef] [PubMed]
- Coskun, M.B.; Qiu, L.; Arefin, M.S.; Neild, A.; Yuce, M.; Li, D.; Alan, T. Detecting subtle vibrations using graphene-based cellular elastomers. ACS Appl. Mater. Interfaces 2017, 9, 11345–11349. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Kim, J.; Jang, B.; Chae, Y.; Kim, J.-H.; Ahn, J.-H. Graphene-based three-dimensional capacitive touch sensor for wearable electronics. ACS Nano 2017, 11, 7950–7957. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Joo, M.-K.; Le, V.T.; Ovalle-Robles, R.; Lepró, X.; Lima, M.D.; Suh, D.G.; Yu, H.Y.; Lee, Y.H.; Suh, D. Ultrastretchable analog/digital signal transmission line with carbon nanotube sheets. ACS Appl. Mater. Interfaces 2017, 9, 26286–26292. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.G.; Alrowais, H.; Pavlidis, S.; Brand, O. Size-scalable and high-density liquid-metal-based soft electronic passive components and circuits using soft lithography. Adv. Funct. Mater. 2017, 27. [Google Scholar] [CrossRef]
- Chortos, A.; Zhu, C.; Oh, J.Y.; Yan, X.; Pochorovski, I.; To, J.W.-F.; Liu, N.; Kraft, U.; Murmann, B.; Bao, Z. Investigating limiting factors in stretchable all-carbon transistors for reliable stretchable electronics. ACS Nano 2017, 11, 7925–7937. [Google Scholar] [CrossRef] [PubMed]
- Yokota, T.; Zalar, P.; Kaltenbrunner, M.; Jinno, H.; Matsuhisa, N.; Kitanosako, H.; Tachibana, Y.; Yukita, W.; Koizumi, M.; Someya, T. Ultraflexible organic photonic skin. Sci. Adv. 2016, 2, e1501856. [Google Scholar] [CrossRef] [PubMed]
- Shang, Q.; Deng, W.; Zhang, X.; Wang, L.; Huang, L.; Jie, J. An inherent multifunctional sellotape substrate for high-performance flexible and wearable organic single-crystal nanowire array-based transistors. Adv. Electron. Mater. 2016, 2. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Zhao, Y.; Ge, F.; Komarneni, S.; Cai, Z. Wearable solid-state supercapacitors operating at high working voltage with a flexible nanocomposite electrode. ACS Appl. Mater. Interfaces 2016, 8, 25905–25914. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Chen, R.; Hua, L.; Li, L.; Chen, R.; Gong, Y.; Yu, C.; Zhou, J.; Liu, B.; Sun, G. Highly concentrated, ultrathin nickel hydroxide nanosheet ink for wearable energy storage devices. Adv. Mater. 2017. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Xu, X.; Liu, C.; Tekell, M.C.; Ning, J.; Guo, J.; Zhang, J.; Fan, D. Ultralight and binder-free all-solid-state flexible supercapacitors for powering wearable strain sensors. Adv. Funct. Mater. 2017. [Google Scholar] [CrossRef]
- Bandodkar, A.; You, J.-M.; Kim, N.-H.; Gu, Y.; Kumar, R.; Vinu Mohan, A.M.; Kurniawan, J.F.; Imani, S.; Nakagawa, T.; Parish, B. Soft, stretchable, high power density electronic skin-based biofuel cells for scavenging energy from human sweat. Energy Environ. Sci. 2017. [Google Scholar] [CrossRef]
- Lee, J.W.; Xu, R.; Lee, S.; Jang, K.-I.; Yang, Y.; Banks, A.; Yu, K.J.; Kim, J.; Xu, S.; Ma, S. Soft, thin skin-mounted power management systems and their use in wireless thermography. Proc. Natl. Acad. Sci. USA 2016, 113, 6131–6136. [Google Scholar] [CrossRef] [PubMed]
- Berchmans, S.; Bandodkar, A.J.; Jia, W.; Ramírez, J.; Meng, Y.S.; Wang, J. An epidermal alkaline rechargeable Ag–Zn printable tattoo battery for wearable electronics. J. Mater. Chem. A 2014, 2, 15788–15795. [Google Scholar] [CrossRef]
- Kumar, R.; Shin, J.; Yin, L.; You, J.M.; Meng, Y.S.; Wang, J. All-printed, stretchable Zn-Ag2O rechargeable battery via hyperelastic binder for self-powering wearable electronics. Adv. Energy Mater. 2017, 7. [Google Scholar] [CrossRef]
- Kim, S.J.; Cho, K.W.; Cho, H.R.; Wang, L.; Park, S.Y.; Lee, S.E.; Hyeon, T.; Lu, N.; Choi, S.H.; Kim, D.H. Stretchable and transparent biointerface using cell-sheet–graphene hybrid for electrophysiology and therapy of skeletal muscle. Adv. Funct. Mater. 2016, 26, 3207–3217. [Google Scholar] [CrossRef]
- Koh, A.; Gutbrod, S.R.; Meyers, J.D.; Lu, C.; Webb, R.C.; Shin, G.; Li, Y.; Kang, S.K.; Huang, Y.; Efimov, I.R. Ultrathin injectable sensors of temperature, thermal conductivity, and heat capacity for cardiac ablation monitoring. Adv. Healthc. Mater. 2016, 5, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Shin, G.; Gomez, A.M.; Al-Hasani, R.; Jeong, Y.R.; Kim, J.; Xie, Z.; Banks, A.; Lee, S.M.; Han, S.Y.; Yoo, C.J. Flexible near-field wireless optoelectronics as subdermal implants for broad applications in optogenetics. Neuron 2017, 93, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Wang, C.; Ranganathan, V.; Napier, B.; Yu, C.; Chao, Y.; Forsyth, M.; Omenetto, F.G.; MacFarlane, D.R.; Wallace, G.G. A biodegradable thin-film magnesium primary battery using silk fibroin–ionic liquid polymer electrolyte. ACS Energy Lett. 2017, 2, 831–836. [Google Scholar] [CrossRef]
- Fang, H.; Yu, K.J.; Gloschat, C.; Yang, Z.; Song, E.; Chiang, C.-H.; Zhao, J.; Won, S.M.; Xu, S.; Trumpis, M. Capacitively coupled arrays of multiplexed flexible silicon transistors for long-term cardiac electrophysiology. Nat. Biomed. Eng. 2017, 1. [Google Scholar] [CrossRef]
- Park, J.; Choi, S.; Janardhan, A.H.; Lee, S.-Y.; Raut, S.; Soares, J.; Shin, K.; Yang, S.; Lee, C.; Kang, K.-W. Electromechanical cardioplasty using a wrapped elasto-conductive epicardial mesh. Sci. Transl. Med. 2016, 8, 344ra386. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Shin, H.J.; Lee, H.; Jeong, C.K.; Park, H.; Hwang, G.T.; Lee, H.Y.; Joe, D.J.; Han, J.H.; Lee, S.H. In vivo self-powered wireless transmission using biocompatible flexible energy harvesters. Adv. Funct. Mater. 2017. [Google Scholar] [CrossRef]
- Lu, Y.; Lyu, H.; Richardson, A.G.; Lucas, T.H.; Kuzum, D. Flexible Neural Electrode Array Based-on Porous Graphene for Cortical Microstimulation and Sensing; Scientific Reports 6; Macmillan Publishers Limited: Basingstoke, UK, 2016; Volume 9. [Google Scholar]
- Han, L.; Lu, X.; Wang, M.; Gan, D.; Deng, W.; Wang, K.; Fang, L.; Liu, K.; Chan, C.W.; Tang, Y. A mussel-inspired conductive, self-adhesive, and self-healable tough hydrogel as cell stimulators and implantable bioelectronics. Small 2017, 13. [Google Scholar] [CrossRef] [PubMed]
- Kireev, D.; Brambach, M.; Seyock, S.; Maybeck, V.; Fu, W.; Wolfrum, B.; Offenhäusser, A. Graphene transistors for interfacing with cells: Towards a deeper understanding of liquid gating and sensitivity. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Yetisen, A.K.; Jiang, N.; Fallahi, A.; Montelongo, Y.; Ruiz-Esparza, G.U.; Tamayol, A.; Zhang, Y.S.; Mahmood, I.; Yang, S.A.; Kim, K.S. Glucose-sensitive hydrogel optical fibers functionalized with phenylboronic acid. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.; Wang, Y.; Qing, Q. Scalable fabrication framework of implantable ultrathin and flexible probes with biodegradable sacrificial layers. Nano Lett. 2017, 17, 7315–7322. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Hu, Y.; Wan, J.; Gao, Q.; Wang, Y.; Xie, S.; Qiu, L.; Wang, C.; Zheng, G.; Wang, B. Biocompatible carbon nanotube fibers for implantable supercapacitors. Carbon 2017, 122, 162–167. [Google Scholar] [CrossRef]
- Hwang, S.W.; Kim, D.H.; Tao, H.; Kim, T.I.; Kim, S.; Yu, K.J.; Panilaitis, B.; Jeong, J.W.; Song, J.K.; Omenetto, F.G. Materials and fabrication processes for transient and bioresorbable high-performance electronics. Adv. Funct. Mater. 2013, 23, 4087–4093. [Google Scholar] [CrossRef]
- Kang, S.-K.; Murphy, R.K.; Hwang, S.-W.; Lee, S.M.; Harburg, D.V.; Krueger, N.A.; Shin, J.; Gamble, P.; Cheng, H.; Yu, S. Bioresorbable silicon electronic sensors for the brain. Nature 2016, 530, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Zhang, H.; Jin, L.; Li, Y.; Li, J.; Gan, G.; Wei, M.; Li, M.; Liao, Y. Controllably degradable transient electronic antennas based on water-soluble PVA/TiO2 films. J. Mater. Sci. 2018, 53, 2638–2647. [Google Scholar] [CrossRef]
- Acar, H.; Çınar, S.; Thunga, M.; Kessler, M.R.; Hashemi, N.; Montazami, R. Study of physically transient insulating materials as a potential platform for transient electronics and bioelectronics. Adv. Funct. Mater. 2014, 24, 4135–4143. [Google Scholar] [CrossRef]
- Fukuda, K.; Takeda, Y.; Yoshimura, Y.; Shiwaku, R.; Tran, L.T.; Sekine, T.; Mizukami, M.; Kumaki, D.; Tokito, S. Fully-printed high-performance organic thin-film transistors and circuitry on one-micron-thick polymer films. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y.; Hayasaka, K.; Shiwaku, R.; Yokosawa, K.; Shiba, T.; Mamada, M.; Kumaki, D.; Fukuda, K.; Tokito, S. Fabrication of ultra-thin printed organic tft cmos logic circuits optimized for low-voltage wearable sensor applications. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Kaltenbrunner, M.; Sekitani, T.; Reeder, J.; Yokota, T.; Kuribara, K.; Tokuhara, T.; Drack, M.; Schwodiauer, R.; Graz, I.; Bauer-Gogonea, S.; et al. An ultra-lightweight design for imperceptible plastic electronics. Nature 2013, 499, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Burghartz, J.N.; Appel, W.; Harendt, C.; Rempp, H.; Richter, H.; Zimmermann, M. Ultra-thin chip technology and applications, a new paradigm in silicon technology. Solid-State Electron. 2010, 54, 818–829. [Google Scholar] [CrossRef]
- Viventi, J.; Kim, D.H.; Vigeland, L.; Frechette, E.S.; Blanco, J.A.; Kim, Y.S.; Avrin, A.E.; Tiruvadi, V.R.; Hwang, S.W.; Vanleer, A.C.; et al. Flexible, foldable, actively multiplexed, high-density electrode array for mapping brain activity in vivo. Nat. Neurosci. 2011, 14, 1599–1605. [Google Scholar] [CrossRef] [PubMed]
- Rojas, J.P.; Syed, A.; Hussain, M.M. Mechanically flexible optically transparent porous mono-crystalline silicon substrate. In Proceedings of the 2012 IEEE 25th International Conference on Micro Electro Mechanical Systems (MEMS), Paris, France, 29 January–2 February 2012; pp. 281–284. [Google Scholar]
- Bedell, S.W.; Fogel, K.; Lauro, P.; Shahrjerdi, D.; Ott, J.A.; Sadana, D. Layer transfer by controlled spalling. J. Phys. D Appl. Phys. 2013, 46, 152002. [Google Scholar] [CrossRef]
- Van den Brand, J.; de Kok, M.; Koetse, M.; Cauwe, M.; Verplancke, R.; Bossuyt, F.; Jablonski, M.; Vanfleteren, J. Flexible and stretchable electronics for wearable health devices. Solid-State Electron. 2015, 113, 116–120. [Google Scholar] [CrossRef]
- Kim, Y.S.; Lu, J.; Shih, B.; Gharibans, A.; Zou, Z.; Matsuno, K.; Aguilera, R.; Han, Y.; Meek, A.; Xiao, J. Scalable manufacturing of solderable and stretchable physiologic sensing systems. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Rojas, J.P.; Torres Sevilla, G.A.; Ghoneim, M.T.; Inayat, S.B.; Ahmed, S.M.; Hussain, A.M.; Hussain, M.M. Transformational silicon electronics. ACS Nano 2014, 8, 1468–1474. [Google Scholar] [CrossRef] [PubMed]
- Khan, Y.; Garg, M.; Gui, Q.; Schadt, M.; Gaikwad, A.; Han, D.; Yamamoto, N.A.D.; Hart, P.; Welte, R.; Wilson, W.; et al. Flexible hybrid electronics: Direct interfacing of soft and hard electronics for wearable health monitoring. Adv. Funct. Mater. 2016, 26, 8764–8775. [Google Scholar] [CrossRef]
- Mannoor, M.S.; Tao, H.; Clayton, J.D.; Sengupta, A.; Kaplan, D.L.; Naik, R.R.; Verma, N.; Omenetto, F.G.; McAlpine, M.C. Graphene-based wireless bacteria detection on tooth enamel. Nat. Commun. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Swisher, S.L.; Lin, M.C.; Liao, A.; Leeflang, E.J.; Khan, Y.; Pavinatto, F.J.; Mann, K.; Naujokas, A.; Young, D.; Roy, S.; et al. Impedance sensing device enables early detection of pressure ulcers in vivo. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Emaminejad, S.; Nyein, H.Y.Y.; Challa, S.; Chen, K.; Peck, A.; Fahad, H.M.; Ota, H.; Shiraki, H.; Kiriya, D.; et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 2016, 529. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Akhtar, A.; Liu, Y.; Chen, H.; Yeo, W.H.; Park, S.I.; Boyce, B.; Kim, H.; Yu, J.; Lai, H.Y. An epidermal stimulation and sensing platform for sensorimotor prosthetic control, management of lower back exertion, and electrical muscle activation. Adv. Mater. 2016, 28, 4462–4471. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, M.; Shim, H.J.; Ghaffari, R.; Cho, H.R.; Son, D.; Jung, Y.H.; Soh, M.; Choi, C.; Jung, S. Stretchable silicon nanoribbon electronics for skin prosthesis. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Malechka, T.; Tetzel, T.; Krebs, U.; Feuser, D.; Graeser, A. Sbci-headset—Wearable and modular device for hybrid brain-computer interface. Micromachines 2015, 6, 291–311. [Google Scholar] [CrossRef]
- Heo, J.; Yoon, H.; Park, K.S. A novel wearable forehead eog measurement system for human computer interfaces. Sensors 2017, 17. [Google Scholar] [CrossRef] [PubMed]



| Sensing Material | Type | Biocompatible/Biodegradable 1 |
|---|---|---|
| Carbon Nanotube | Organic | Yes/Yes 2 [44] |
| Graphene | Inorganic | Yes/No 3 [45,46,47] |
| Hydrogel | Organic/Inorganic | Yes/Yes 4 [48,49,50] |
| Liquid Metal (EGaIn) | Inorganic | Yes/No 5 [51] |
| Nanosheet and Thin Film (MnO2, Mn, Mg, Si) | Inorganic | Yes/Yes 6 [52,53,54,55] |
| Nanowire (Ag, ZnO, Si, Au, BaTiO3, Ni) | Inorganic | Yes/No 7 [56,57,58,59,60,61] |
| Conducting Polymer (PEDOT:PSS) | Organic | Yes/No 8 [62] |
| Substrate Material | Organic/Inorganic | Young’s Modulus/% Elongation at Break | Biocompatible/Biodegradable |
|---|---|---|---|
| Silicone elastomer (Ecoflex 00-30) | Organic | 0.07 MPa/900% [77] | Y/N 1 [78] |
| Silicone elastomer (Sylgard 184) | Organic | 1.32–2.97 MPa [79]/120% [80] | Y/N 2 [81] |
| Silicone elastomer (Silbione LSR 4330) | Organic | 1.38 MPa/750% [82] | Y/N 3 [83] |
| Parylene (VSI Parylene C) | Organic | 2800 MPa/200% [84] | Y/N 4 [85,86] |
| Polyethylene terephthalate (PET) | Organic | 230 MPa/120% [87] | Y/N 5 [88,89,90] |
| Polycaprolactone (PCL) | Organic | 340.2 MPa/853.8% [91] | Y/Y 6 [92,93] |
| Polyimide (PI) | Organic | 280 MPa/80% [87] | Y/N 7 [94] |
| Polyethylene naphthalate (PEN) | Organic | 280 MPa/90% [87] | Y/N 8 [95] |
| Polyethersulfone (PES) | Organic | 2654.5 MPa/100% [96] | Y/N 9 [97] |
| Polytetrafluoroethylene (PTFE) | Organic | 0.06 MPa/400% [98] | Y/N 10 [99] |
| Poly(lactic-co-glycolic acid) (PLGA) | Organic | 2000 MPa/3–10% [100] | Y/Y 11 [101] |
| Cyclic olefin polymer (Zeonor 1020R) | Organic | 2100 MPa/90% [102] | Y/N 12 [103] |
| Silk fibroin | Organic | 2500 MPa/2.1% (dry) 16.7 MPa/127.8% (wet) [104] | Y/Y 13 [105,106] |
| Device Type | Sensing Material | Application | Substrate Material | Target Signal | Sensitivity | Flexibility | Stretchability | Reference (Year) |
|---|---|---|---|---|---|---|---|---|
| Strain Sensor | MWCNT | Motion, Bending | Ecoflex | Resistance | 1.5 GF | - | 300% | [112] (2017) |
| EGaIn Liquid | Motion, Contact | Ecoflex Microtubules | Resistance | - | - | 750% | [107] (2017) | |
| CS-PDMS | Blood Pulse, Breathing, | PDMS | Resistance, Temperature | GF 1.78 | 180° | 228% | [74] (2016) | |
| Graphite Flake Sheath and Silk Fiber Core | Joint Motion, Multiaxial | Ecoflex | Resistance | 14.5 GF | - | 15% | [66] (2016) | |
| Self-healing SWCNT-Hydrogel | Bending | VHB Mounting Tape | Resistance | GF 0.24 (100% Strain), GF 1.51 (1000% Strain) | 540° Twisting, 150° Bending | 1000% | [69] (2017) | |
| Pressure Sensor | GPN | Blood Pressure | PDMS | Resistance | 0.09/kPa | - | 40% | [37] (2016) |
| Light Sensor | Ionic Liquid, PU fiber, SWCNT, Au film | Electronic Skin | Ecoflex | Conductivity | 2.4 mW | 90° | 50% | [63] (2017) |
| Temperature | PEIE/CNT-PDMS. Ag electrode | Healthcare Patch | PET | Resistance, Voltage | 0.85%/°C, | - | - | [113] (2017) |
| Sweat Sensor | InGaZnO ISFET, PI, CNT/PEDOT:PSS | Healthcare and Sports | PET | Current, Resistance | 51.2 mV/pH | 10 mm Radius | - | [114] (2017) |
| Hydrogel, Ag/AgCl Electrode | Fitness Monitoring | PET | Voltage | 52.8 mV/decade | - | - | [70] (2017) | |
| Electrode | Au | EOG, Eye Movement | PI | Voltage | 13.3 µV/° | 0.5 mm Radius | 30% | [8] (2017) |
| Antenna | Ag-PDMS | Wireless Communication | Conductivity | - | - | 20% | [115] (2017) | |
| QD Display | QDs | Sensor Display, Touch Sensor | Parylene | Intensity | - | 180° | - | [111] (2017) |
| Cooling Device | BaSrTiO Nanowires | Cooling | PDMS | - | - | 5 mm Radius | 25% | [42] (2016) |
| Supercapacitor | MnO2 Nanosheet, Carbon Fiber, Graphene, PVA | Energy Storage | Cotton Textile | [116] (2016) |
| Device Type | Sensing Material | Application | Substrate Material | Target Signal | Sensitivity | Flexibility | Stretchability | Reference (Year) |
|---|---|---|---|---|---|---|---|---|
| Electrode | Myoblasts, Au-Graphene | EMG, Stimulation, Therapy | PI, PDMS | Voltage | - | - | 40% | [147] (2016) |
| Si nanomembranes | Electrophysiological Mapping | PI | Voltage, Current | - | 5 mm radius | - | [151] (2017) | |
| Doped Si Nanomembranes | Monitor Brain, Muscle, Organ Activity | PLGA | Voltage | - | 1 mm radius | - | [10] (2016) | |
| LE-AgNW | ECG, Biventricular Pacing | SBS Rubber | Voltage, Contractility | - | - | - | [152] (2016) | |
| Cardiac Temperature Sensor | Au | Lesion Characterization | PET | - | 0.26%/°C | 21 N/m Bending Stiffness | - | [148] (2016) |
| Optogenetic Light Delivery | Cu | Optogenetics | PI, Parylene, PDMS | Output Power | - | 6 mm radius | - | [149] (2017) |
| Biodegradable Microsupercapacitor | W, Fe, Mo, NaCl-Hydrogel | Power Storage | PLGA | Capacitance | - | 5 mm diameter | - | [49] (2017) |
| Biodegradable Battery | Mg | Power Supply | Silk Fibroin | - | 0.06 mAh/cm2 (Specific Capacity) | - | 98% | [150] (2017) |
| Energy Harvester | PMN-PZT-Mn | ECG, Wireless Data Transmission | PET, PU | Voltage | - | 9.95−5 N/m Bending Stiffness | - | [153] (2017) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herbert, R.; Kim, J.-H.; Kim, Y.S.; Lee, H.M.; Yeo, W.-H. Soft Material-Enabled, Flexible Hybrid Electronics for Medicine, Healthcare, and Human-Machine Interfaces. Materials 2018, 11, 187. https://doi.org/10.3390/ma11020187
Herbert R, Kim J-H, Kim YS, Lee HM, Yeo W-H. Soft Material-Enabled, Flexible Hybrid Electronics for Medicine, Healthcare, and Human-Machine Interfaces. Materials. 2018; 11(2):187. https://doi.org/10.3390/ma11020187
Chicago/Turabian StyleHerbert, Robert, Jong-Hoon Kim, Yun Soung Kim, Hye Moon Lee, and Woon-Hong Yeo. 2018. "Soft Material-Enabled, Flexible Hybrid Electronics for Medicine, Healthcare, and Human-Machine Interfaces" Materials 11, no. 2: 187. https://doi.org/10.3390/ma11020187
APA StyleHerbert, R., Kim, J.-H., Kim, Y. S., Lee, H. M., & Yeo, W.-H. (2018). Soft Material-Enabled, Flexible Hybrid Electronics for Medicine, Healthcare, and Human-Machine Interfaces. Materials, 11(2), 187. https://doi.org/10.3390/ma11020187