Preparation of Assembled Carbon Soot Films and Hydrophobic Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of CS Self-Assembly Films
2.3. Characterization
3. Results and Discussion
3.1. Interface Self-Assembly of CS Solution with Different Concentration
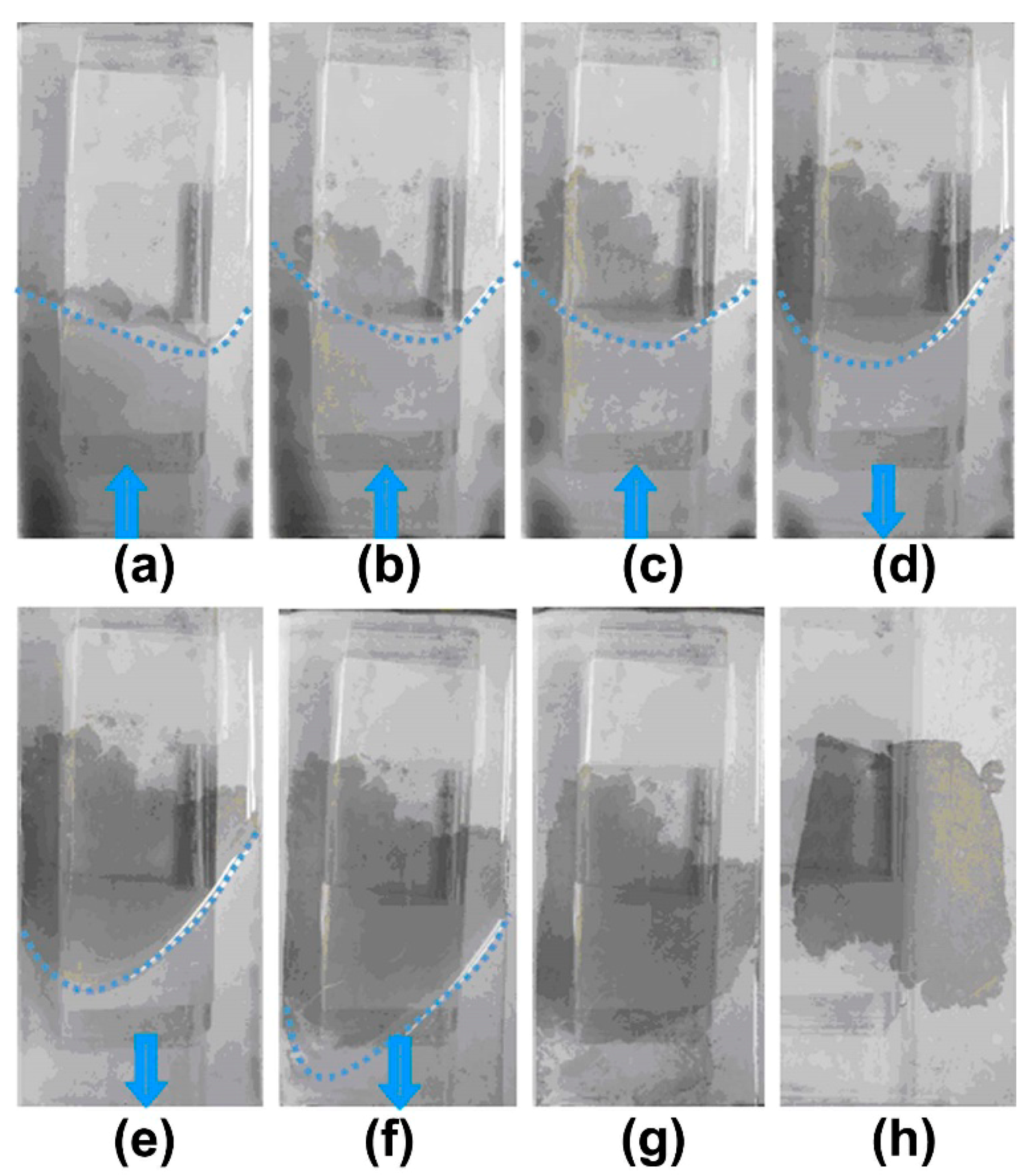
3.2. Time Evolution of CS Self-Assembly Film Formation
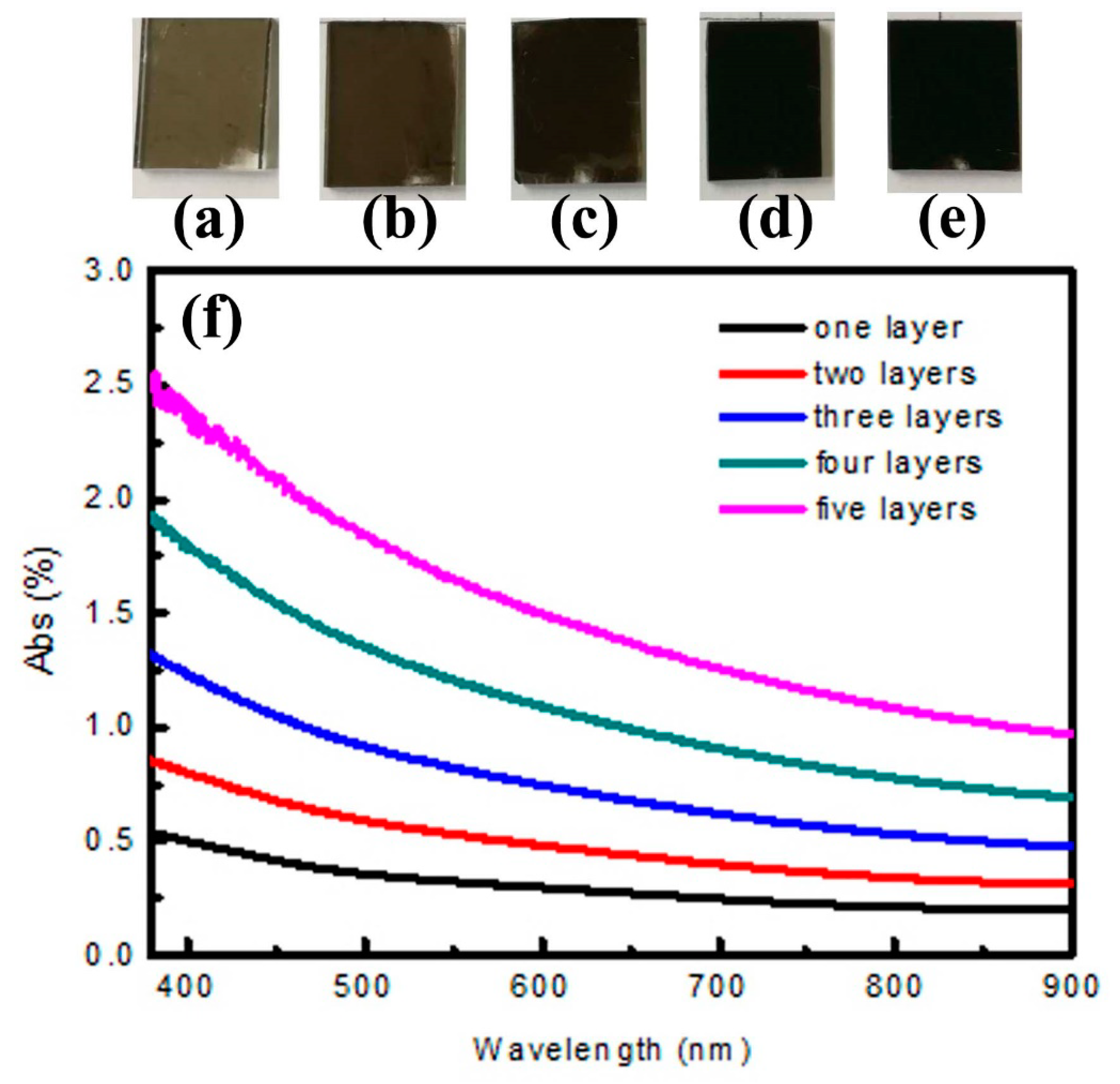
3.3. Optical Properties of CS Self-Assembly Films with Different Layers
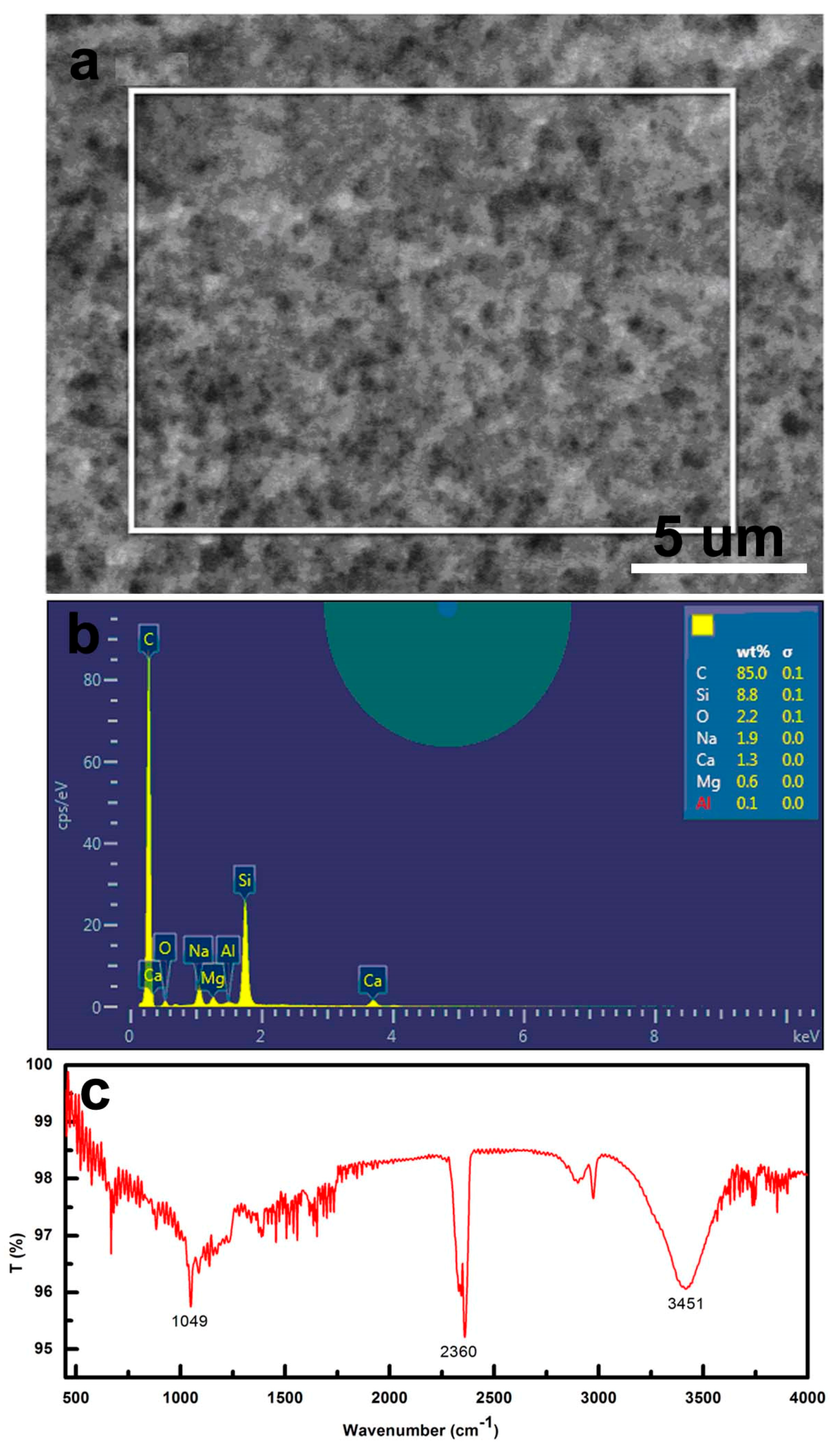
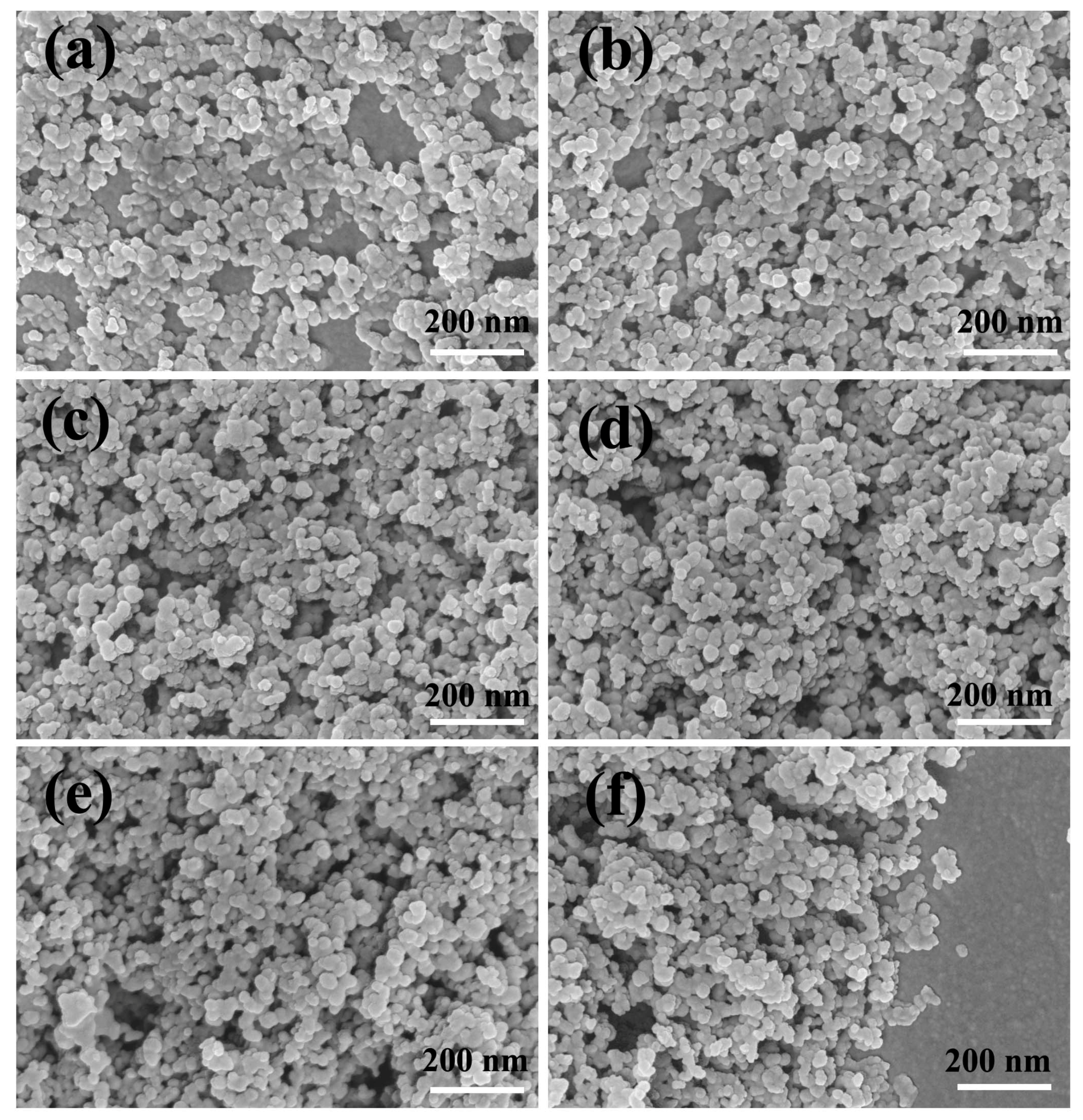
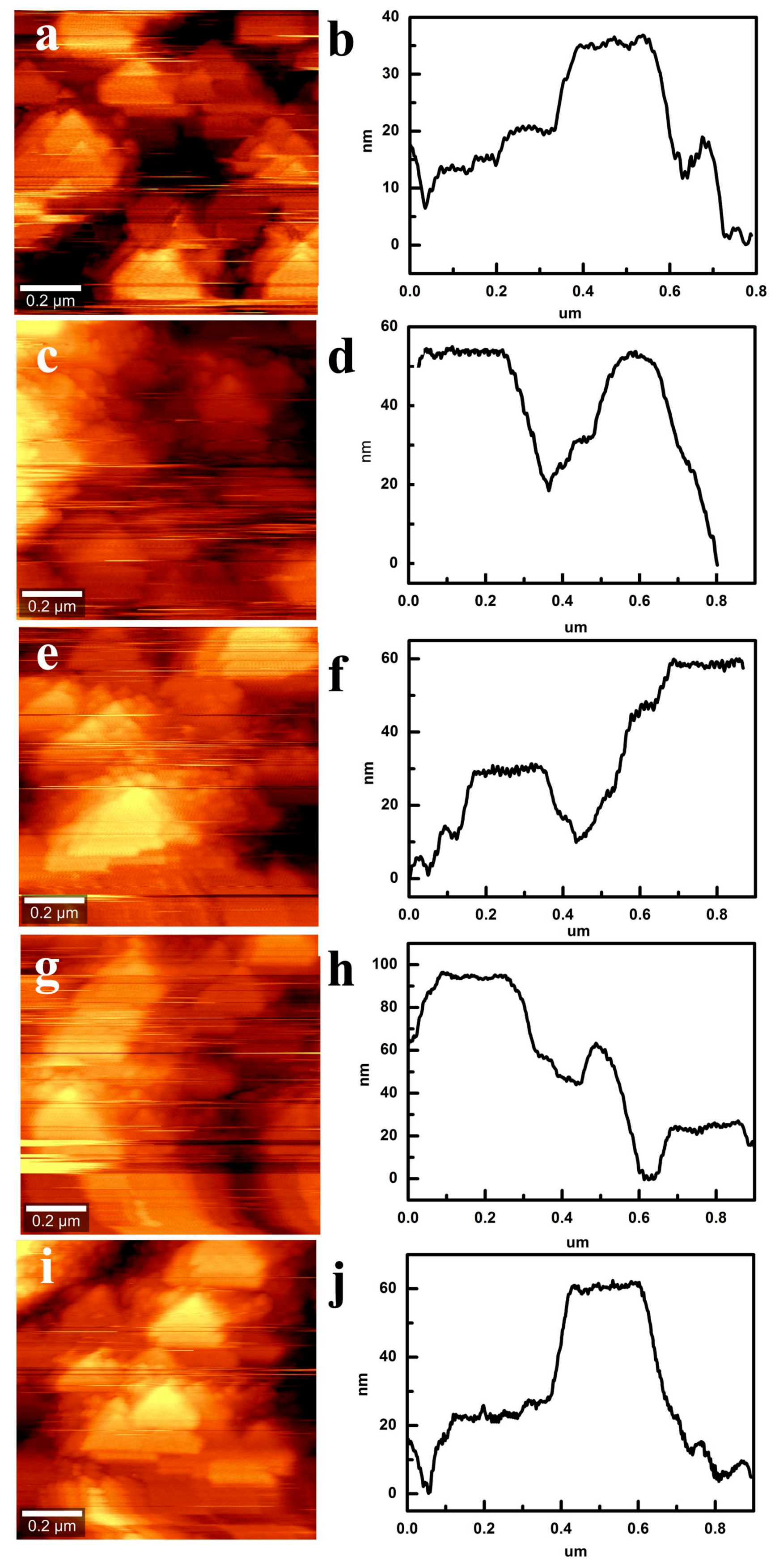
3.4. Composition and Morphology of CS Self-Assembly Films with Different Layers
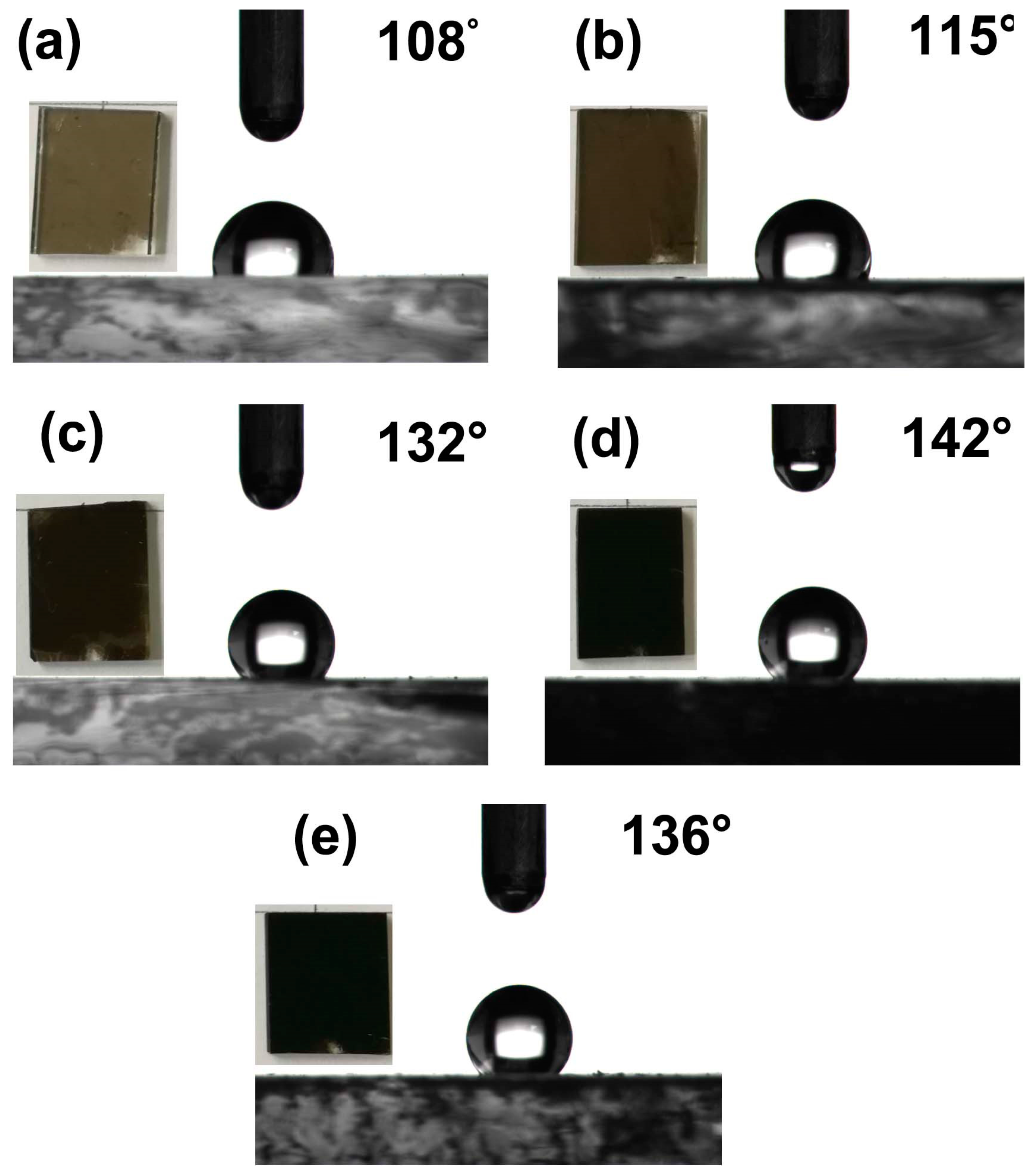
3.5. Hydrophobic Performance of CS Self-Assembly Films with Different Layers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Franklin, A.D. Nanomaterials in transistors: From high-performance to thin-film applications. Science 2015, 349, 2750–2756. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.O.; Park, J.S.; Choi, D.S.; Kim, J.Y.; Lee, S.H.; Lee, K.E.; Kim, Y.-H.; Song, M.H.; Yoo, S.; Kim, S.O. Workfunction-Tunable, N-Doped Reduced Graphene Transparent Electrodes for High-Performance Polymer Light-Emitting Diodes. ACS Nano 2012, 6, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Abdelkader, A.M.; Valles, C.; Cooper, A.J.; Kinloch, I.A.; Dryfe, R.A.W. Alkali Reduction of Graphene Oxide in Molten Halide Salts: Production of Corrugated Graphene Derivatives for High-Performance Supercapacitors. ACS Nano 2014, 8, 11225–11233. [Google Scholar] [CrossRef] [PubMed]
- Xing, F.; Meng, G.-X.; Zhang, Q.; Pan, L.-T.; Wang, P.; Liu, Z.-B.; Jiang, W.-S.; Chen, Y.; Tian, J.-G. Ultrasensitive Flow Sensing of a Single Cell Using Graphene-Based Optical Sensors. Nano Lett. 2014, 14, 3563–3569. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Mammen, L.; Butt, H.J.; Vollmer, D. Candle Soot as a Template for a Transparent Robust Superamphiphobic Coating. Science 2012, 335, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Bing, W.; Sun, H.J.; Yan, Z.Q.; Ren, J.S.; Qu, X.G. Programmed Bacteria Death Induced by Carbon Dots with Different Surface Charge. Small 2016, 12, 4713–4718. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhou, J.; Du, R.; Xie, Z.; Deng, S.; Liu, R.; Liu, Z.; Zhang, J. Robust Superhydrophobic Foam: A Graphdiyne-Based Hierarchical Architecture for Oil/Water Separation. Adv. Mater. 2016, 28, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kang, R.; Tang, X.; She, H.; Yang, Y.; Zha, F. Superhydrophobic meshes that can repel hot water and strong corrosive liquids used for efficient gravity-driven oil/water separation. Nanoscale 2016, 8, 7638–7645. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, Z.; Liao, D.; Tian, H.; Zha, F.; Feng, H.; Guo, L. Smart candle soot coated membranes for on-demand immiscible oil/water mixture and emulsion switchable separation. Nanoscale 2017, 9, 13610–13617. [Google Scholar] [CrossRef] [PubMed]
- Nine, M.J.; Tung, T.T.; Alotaibi, F.; Tran, D.N.H.; Losic, D. Facile Adhesion-Tuning of Superhydrophobic Surfaces between “Lotus” and “Petal” Effect and Their Influence on Icing and Deicing Properties. ACS Appl. Mater. Interfaces 2017, 9, 8393–8402. [Google Scholar] [CrossRef] [PubMed]
- Yaliwal, V.S.; Banapurmath, N.R.; Gireesh, N.M.; Tewari, P.G. Production and utilization of renewable and sustainable gaseous fuel for power generation applications: A review of literature. Renew. Sustain. Energy Rev. 2014, 34, 608–627. [Google Scholar] [CrossRef]
- Vilaro, I.; Yague, J.L.; Borros, S. Superhydrophobic Copper Surfaces with Anticorrosion Properties Fabricated by Solventless CVD Methods. ACS Appl. Mater. Interfaces 2017, 9, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Qahtan, T.F.; Gondal, M.A.; Alade, I.O.; Dastageer, M.A. Fabrication of Water Jet Resistant and Thermally Stable Superhydrophobic Surfaces by Spray Coating of Candle Soot Dispersion. Sci. Rep. 2017, 7, 7531–7538. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.-L.; Yan, W.-G.; Zhao, J.; Li, Z.-B.; Tian, J.-G. Surface Plasmonic Properties and Fabrication of Large Area Disordered and Binary Ordered Au Particle Arrays. Superlattices Microstruct. 2015, 85, 92–100. [Google Scholar] [CrossRef]
- Yan, W.-G.; Qi, J.-W.; Li, Z.-B.; Tian, J.-G. Fabrication and Optical Properties of Au-Coated Polystyrene Nanosphere Arrays with Controlled Gaps. Plasmonics 2014, 9, 565–571. [Google Scholar] [CrossRef]
- Luo, C.-L.; Yan, W.-G.; Han, J.; Chen, W.; Zhao, J.; Wei, X.; Qi, J.; Liu, Z. Fabrication and Photoelectric Properties of Large Area ZnO Nanorod with Au Nanospheres. Plasmonics 2016, 11, 131–137. [Google Scholar] [CrossRef]
- Sahoo, B.N.; Kandasubramanian, B. An experimental design for the investigation of water repellent property of candle soot particles. Mater. Chem. Phys. 2014, 148, 134–142. [Google Scholar] [CrossRef]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.; Zhao, K.; Yan, W.-G.; Liu, Z. Preparation of Assembled Carbon Soot Films and Hydrophobic Properties. Materials 2018, 11, 2318. https://doi.org/10.3390/ma11112318
Zhao L, Zhao K, Yan W-G, Liu Z. Preparation of Assembled Carbon Soot Films and Hydrophobic Properties. Materials. 2018; 11(11):2318. https://doi.org/10.3390/ma11112318
Chicago/Turabian StyleZhao, Lei, Kang Zhao, Wei-Guo Yan, and Zhifeng Liu. 2018. "Preparation of Assembled Carbon Soot Films and Hydrophobic Properties" Materials 11, no. 11: 2318. https://doi.org/10.3390/ma11112318
APA StyleZhao, L., Zhao, K., Yan, W.-G., & Liu, Z. (2018). Preparation of Assembled Carbon Soot Films and Hydrophobic Properties. Materials, 11(11), 2318. https://doi.org/10.3390/ma11112318




