Interfacial Microstructure and Properties of Si3N4 Ceramics/Cu/304 Stainless Steel Brazed by Ti40Zr25B0.2Cu Amorphous Solder
Abstract
1. Introduction
2. Materials and Methods
3. Results
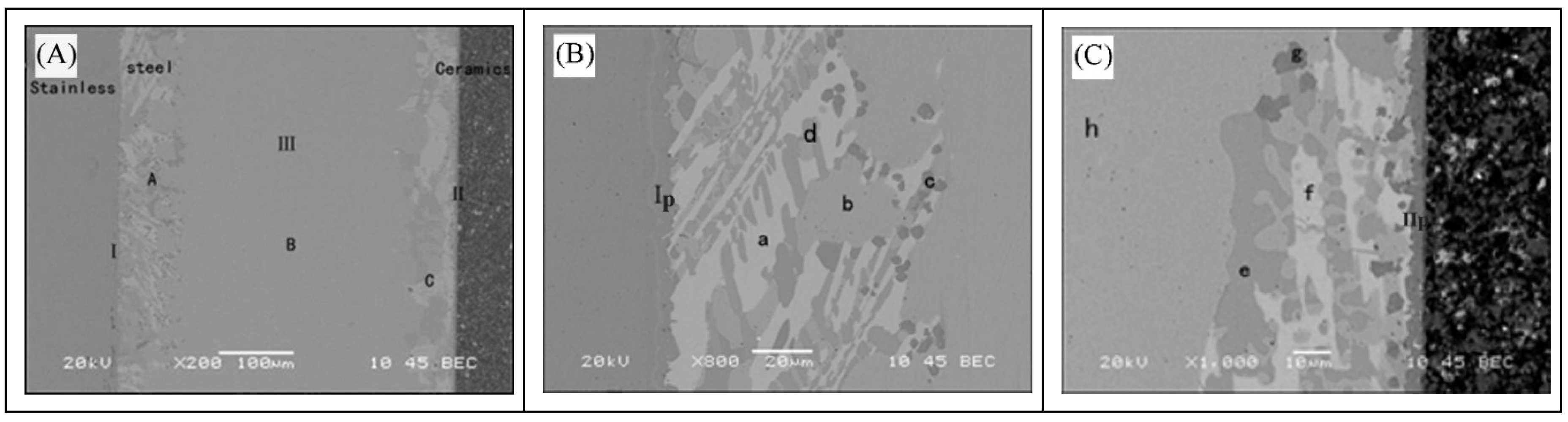
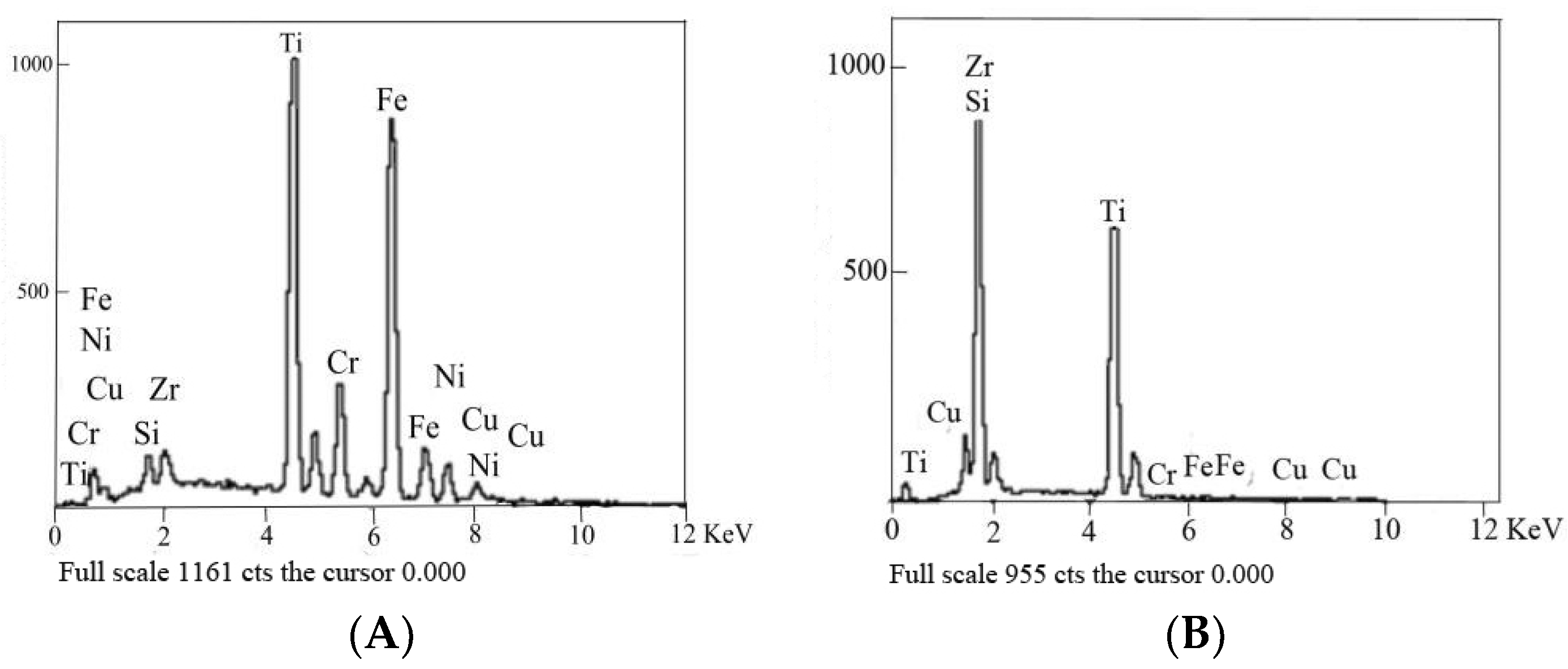
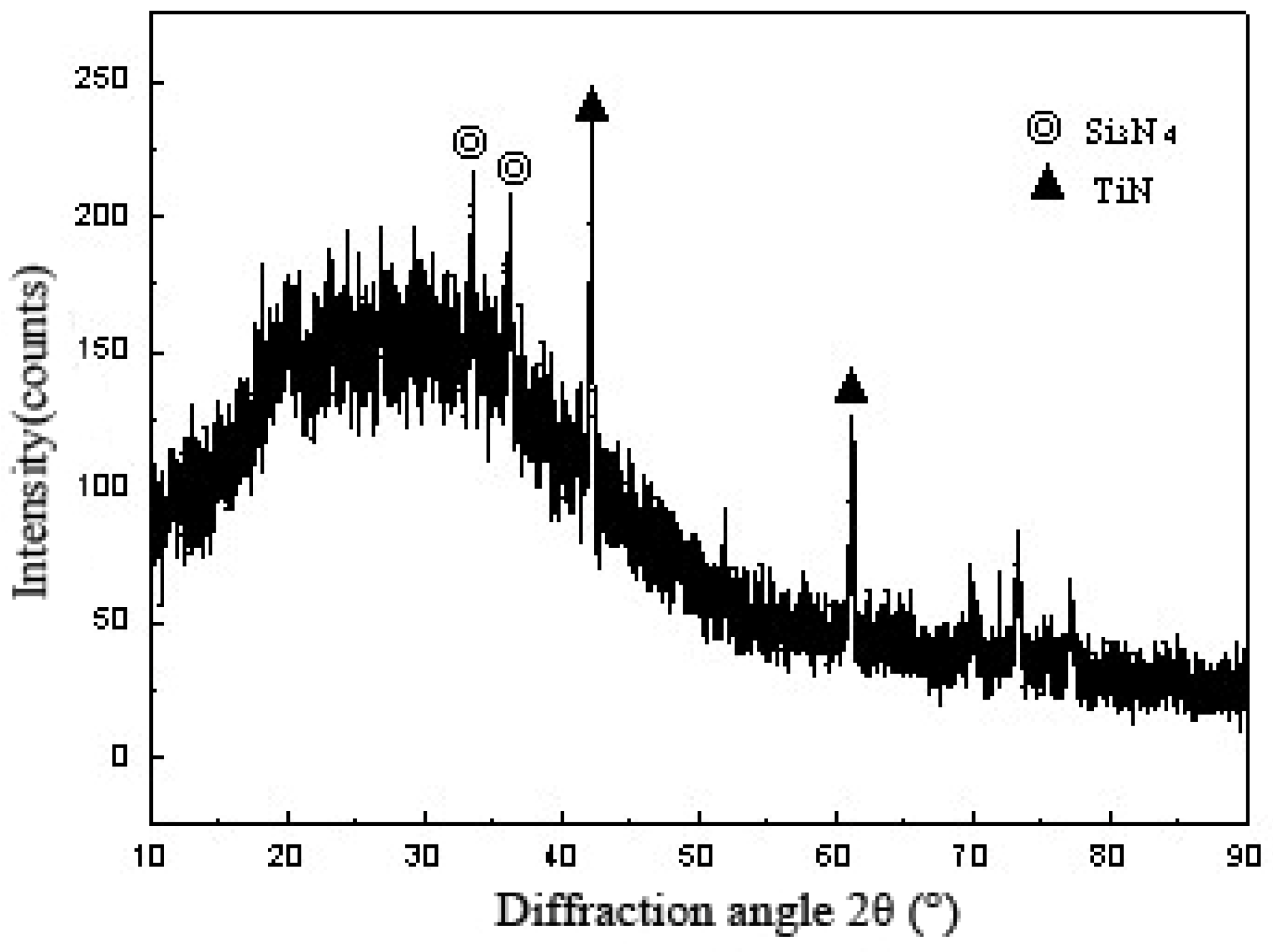
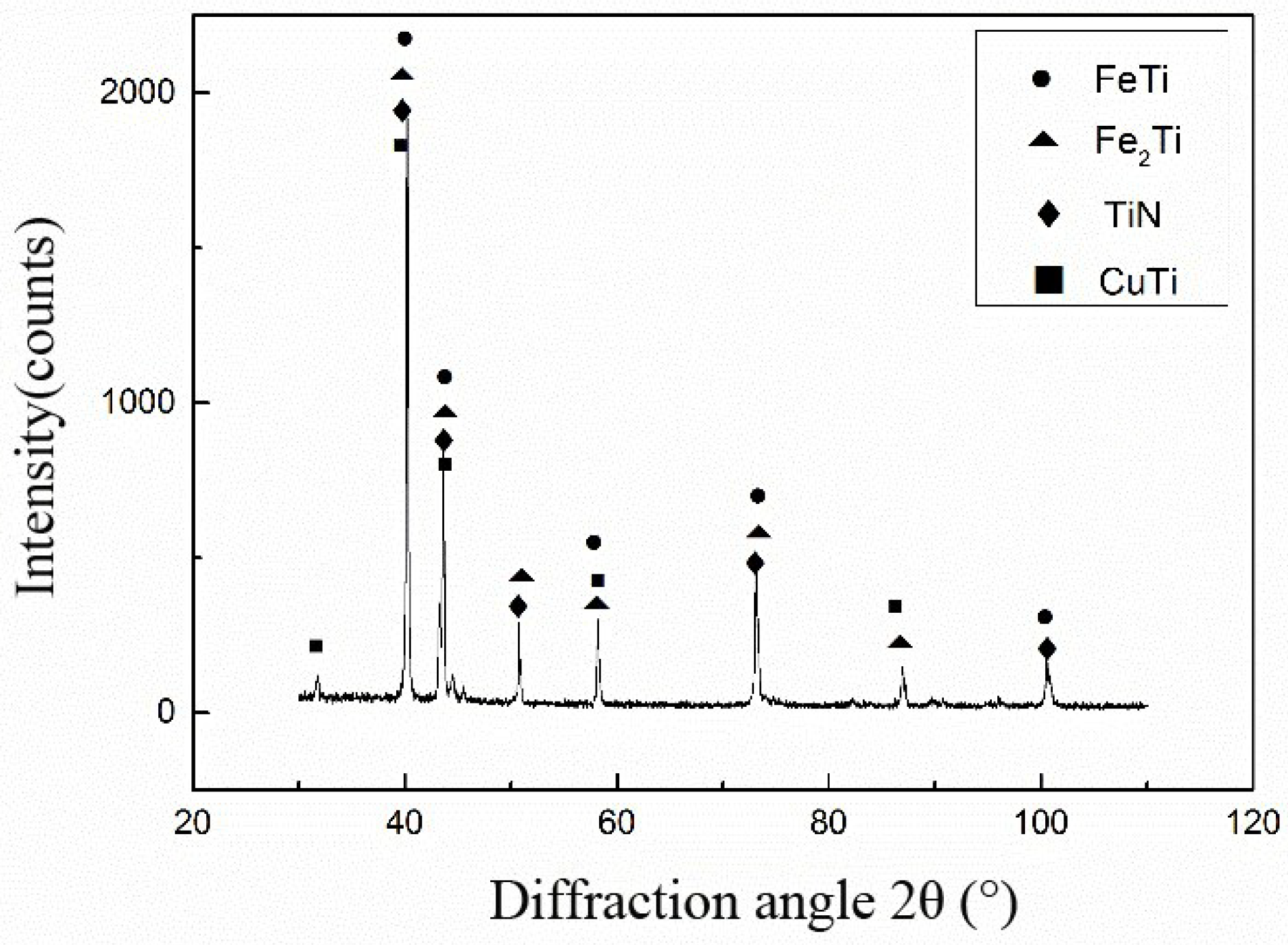
3.1. Analysis of Interface Structure
3.2. Performance of Brazed Joints
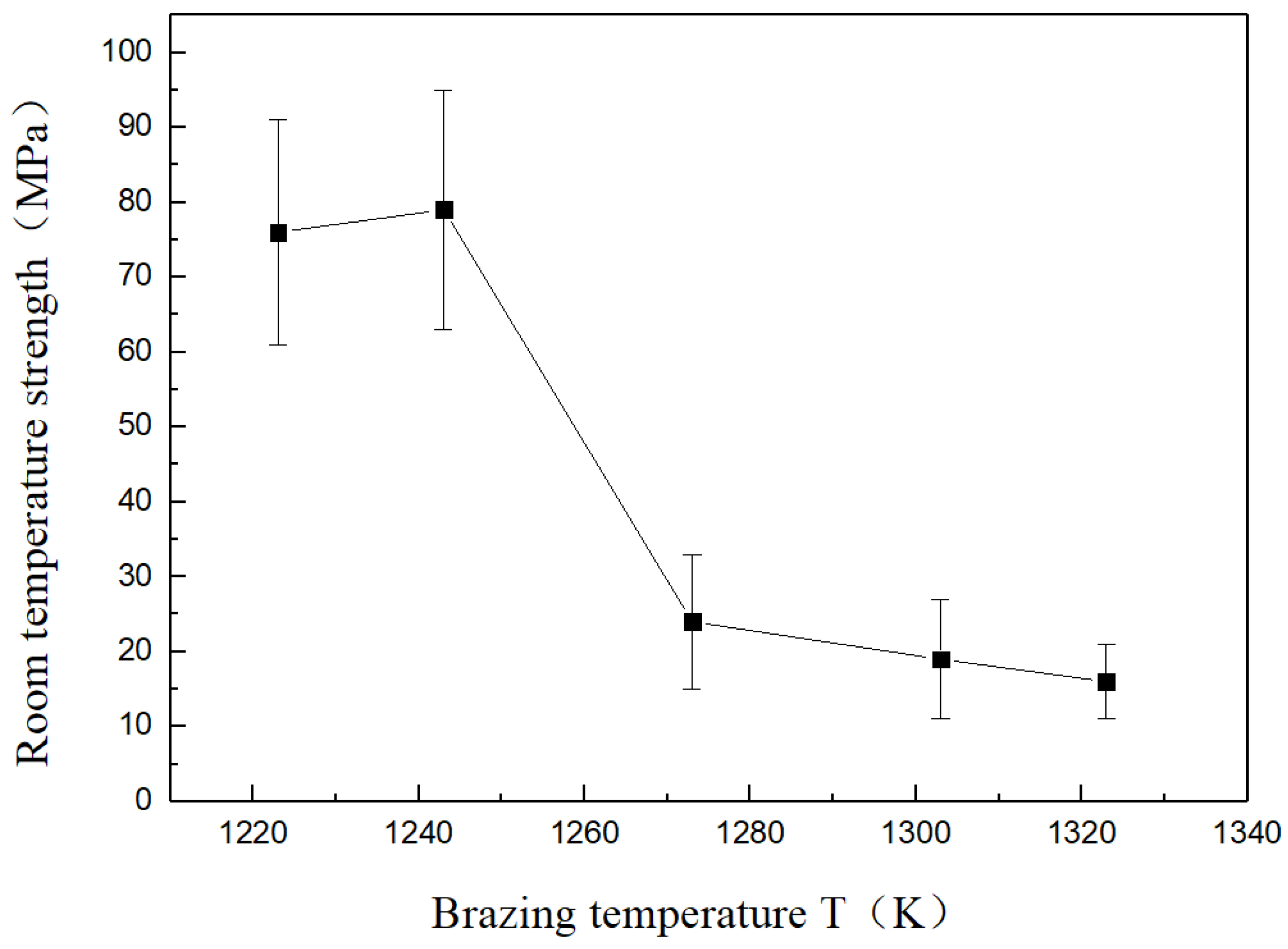
3.3. Effects of Brazing Temperature on Mechanical Properties
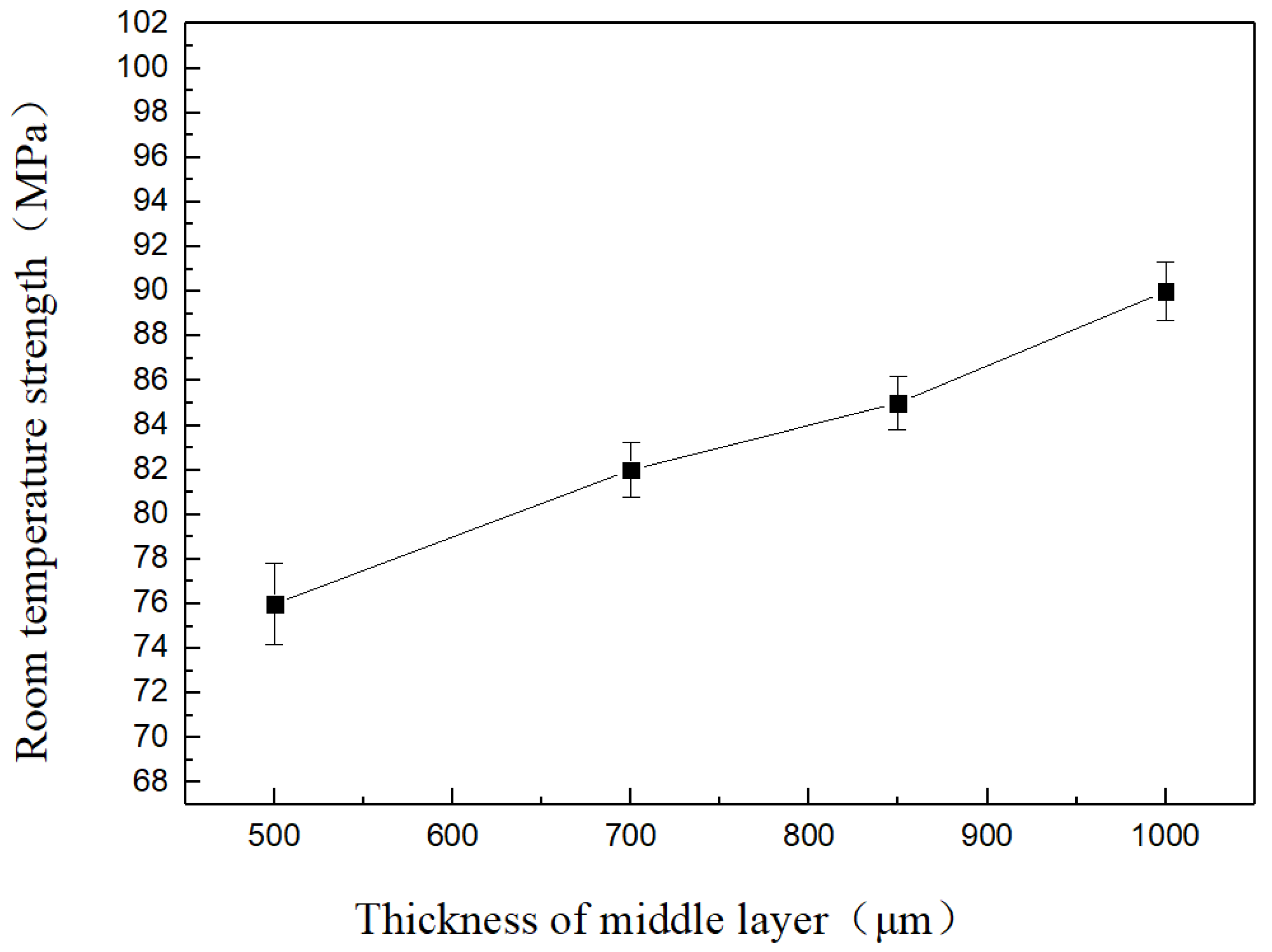
3.4. Effects of Cu Foil Thickness on Mechanical Properties
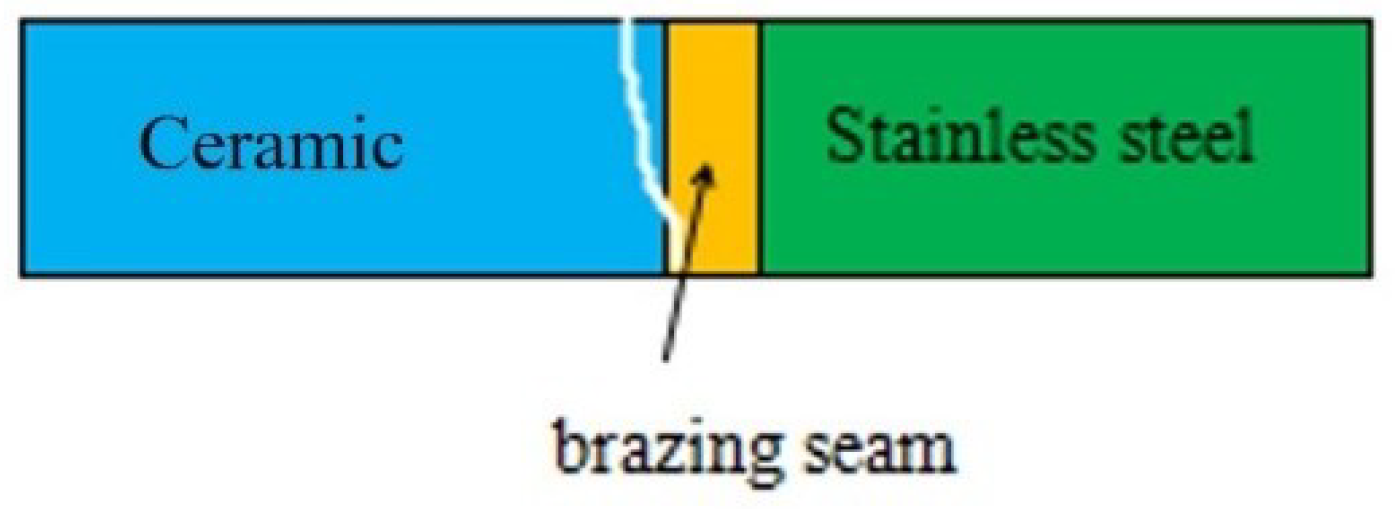
3.5. Fracture Path of Joint
4. Conclusions
- (1)
- 304/Ti40Zr25B0.2Cu/Cu/Ti40Zr25B0.2Cu/Si3N4 ceramics were brazed by Ti40Zr25B0.2Cu amorphous solder. A good brazed joint was obtained and joint interface was continuous and dense.
- (2)
- The interface structure of the 304/Ti40Zr25B0.2Cu/Cu/Ti40Zr25B0.2Cu/Si3N4 ceramics joint might be 304/FeTi/Cu-Zr+Cu-Ti+Fe-Ti/Cu(s,s)/Cu-Zr+Cu-Ti+Fe-Ti/Ti-Si+Zr-Si/TiN/Si3N4 ceramics from left to right.
- (3)
- Joint strength at room temperature rapidly decreased with increasing brazing temperature. The performance of welded joints was improved significantly with increasing Cu foil layer thickness. With a brazing temperature of 1223 K and an intermediate Cu foil layer of welded joint of 1000 μm, the strength at room temperature reached a maximum of 90 MPa.
Author Contributions
Funding
Conflicts of Interest
References
- Shi, Y.W. Materials welding engineering. In China Engineering Ceremony; Chemical Industry Press: Beijing, China, 2006; pp. 326–334. [Google Scholar]
- Ren, J.L.; Wu, A.P. Connection of Advanced Materials; Machinery Industry Press: Beijing, China, 2000; pp. 120–173. [Google Scholar]
- Li, Y.J. Weld of Special and Difficult Welding Materials; Chemical Industry Press: Beijing, China, 2004; pp. 204–224. [Google Scholar]
- Hao, S.; Sholl, D.S. Rapid prediction of hydrogen permeation through amorphous metal membranes: An efficient computational screening approach. Energy Environ. Sci. 2013, 6, 232–240. [Google Scholar] [CrossRef]
- Li, J.; Sheng, G.M.; Huang, L. Ti-Nb-Cu Stress Buffer Layer for TiC Cermet/304 Stainless Steel Diffusion Bonding. Rare Met. Mater. Eng. 2016, 45, 123–126. [Google Scholar] [CrossRef]
- Hattali, M.L.; Mestati, N.; Treheux, D. Electric charge trapping, residual stresses and properties of ceramics after metal/ceramics bonding. J. Eur. Ceram. Soc. 2012, 32, 717–725. [Google Scholar] [CrossRef]
- Liu, Y.H.; Hu, J.D.; Shen, P.; Han, X.H.; Li, J.C. Microstructural and mechanical properties of jointed ZrO2/Ti-6Al-4V alloy using Ti33Zr17Cu50 amorphous brazing filler. Mater. Des. 2013, 47, 281–286. [Google Scholar] [CrossRef]
- Suanuma, K.; Okamato, T.; Koizumi, M.; Shimada, M. Method for Preventing Thermal Expansion Mismatch Effect in Ceramic-Metal Joining. J. Mater. Sci. Lett. 1985, 4, 648–650. [Google Scholar] [CrossRef]
- Xian, A.P.; Si, Z.Y. Interlayer Design for joining Pressureless Sintered Sialon Ceramic and 40Cr Steel Brazing with Ag57Cu38Ti Filler Metal. J. Mater. Sci. 1992, 27, 1560–1566. [Google Scholar] [CrossRef]
- Zou, J.S.; Jiang, Z.G.; Zhao, Q.Z.; Chen, Z. Brazing of Si3N4 with amorphous Ti40Zr25Ni15Cu20 filler. Mater. Sci. Eng. A 2009, 507, 155–160. [Google Scholar] [CrossRef]
- Chang, H.; Choi, S.C.; Park, S.W.; Kim, T.W. Effect of Residual Stress on Fracture Strength of Si3N4/Stainless Steel Joints with a Cu-Interlayer. J. Mater. Eng. Perform. 2002, 11, 640–644. [Google Scholar] [CrossRef]
- Cao, J.; Zhao, H.; Jin, G.R.; Feng, G.C. Intermediate layer of Cu influenced on Interface Structure of SiC ceramics/stainless steel brazed joint. Mater. Sci. Technol. 2009, 17, 106–109. (In Chinese) [Google Scholar]
- Lin, T.S.; Yang, M.X.; He, P.; Huang, C.; Pan, F.; Huang, Y.D. Effect of in situ synthesized TiB whisker on microstructure and mechanical properties of carbon–carbon composite and TiBw/Ti–6Al–4V composite joint. Mater. Des. 2011, 32, 4553–4558. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Shine, R.K.; Chang, C.S. Transmission electron microscopy study of the infrared brazed high-strength titanium alloy. J. Mater. Sci. Technol. 2010, 26, 311–316. [Google Scholar] [CrossRef]
- Tsunoda, T.; Shi, K.; Shohji, I.; Matsu, K.; Taguchi, Y. Joint strength and microstructures of brazed joints of stainless steel with Fe-based filler. In Proceedings of the 2014 IEEE 16th Electronics Packaging Technology Conference (EPTC), Singapore, 3–5 December 2014. [Google Scholar]
- Xu, X.P.; Liu, Q.M.; Xia, C.Z.; Zou, J.S. Microstructure and Properties of Si3N4 Ceramics and 304 Stainless Steel Brazed Joint with Cu/Ag-Cu/Ti laminated Filler Metal. High Temp. Mater. Process. 2017, 37, 128–134. [Google Scholar] [CrossRef]
- Zou, J.S.; Liu, R.; Wang, L. Influence of Alloying Elements on Properties of Ti-Ni-Cu Brazing Alloys. Rare Met. Mater. Eng. 2010, 39, 1023–1026. [Google Scholar]
- Xia, C.Z.; Sun, W.W.; Zhou, Y.; Xu, X.P. Thermal fatigue damage and residual mechanical properties of W-Cu/Ag-Cu/1Cr18Ni9 brazed joint. J. Alloys Compd. 2018, 741, 155–160. [Google Scholar] [CrossRef]

| Project of Performance | Hot-Pressing Si3N4 Ceramics |
|---|---|
| Density (g/cm3) | 3.25–3.35 |
| Hardness (HRA) | 92–94 |
| Modulus of elasticity (GPa) | 304–330 |
| Thermal expansion coefficient (10−6/K) | 3.2–3.5 |
| Coefficient of thermal conductivity (J/(cm·s·K)) | 0.155–0.293 |
| △H0298 (KJ/mol) | −749 |
| Point | Si | Ti | Zr | Cu | Fe | Cr | Ni | Possible Phase |
|---|---|---|---|---|---|---|---|---|
| Ip | 1.25 | 28.86 | 3.01 | 3.73 | 46.51 | 9.58 | 7.06 | FeTi |
| IIp | 34.68 | 54.42 | 9.54 | 0.72 | 0.37 | 0.27 | - | TiN, Ti-Si |
| a | - | 1.95 | 22.88 | 70.17 | 0.86 | 0.24 | 3.90 | CuZr2 |
| b | - | 0.92 | - | 98.96 | 0.12 | - | - | Cu(s,s) |
| c | 0.68 | 45 | 2.84 | 12.68 | 24.4 | 5.53 | 8.88 | Fe-Ti, Cu-Ti |
| d | - | 26.67 | 2.42 | 48.14 | 2.09 | - | - | Cu-Ti |
| e | 0.04 | 32.56 | 1.18 | 64.44 | 1.62 | 0.18 | - | Cu-Ti |
| f | 0.02 | 6.04 | 21.77 | 71.65 | 0.52 | - | - | CuZr2 |
| g | 2.17 | 60.66 | 2.15 | 21.63 | 12.06 | 1.33 | - | Fe-Ti, Cu-Ti |
| h | 0.06 | 0.06 | - | 99.78 | - | 0.09 | - | Cu(s,s) |
| Brazing Temperature T (K) | Thickness of Cu Foil (μm) | Room-Temperature Strength (MPa) |
|---|---|---|
| 1223 | 500 | 76 |
| 700 | 82 | |
| 850 | 85 | |
| 1000 | 90 | |
| 1243 | 500 | 79 |
| 1273 | 500 | 24 |
| 1303 | 500 | 19 |
| 1323 | 500 | 16 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Wang, Y.; Zou, J.; Xia, C. Interfacial Microstructure and Properties of Si3N4 Ceramics/Cu/304 Stainless Steel Brazed by Ti40Zr25B0.2Cu Amorphous Solder. Materials 2018, 11, 2226. https://doi.org/10.3390/ma11112226
Xu X, Wang Y, Zou J, Xia C. Interfacial Microstructure and Properties of Si3N4 Ceramics/Cu/304 Stainless Steel Brazed by Ti40Zr25B0.2Cu Amorphous Solder. Materials. 2018; 11(11):2226. https://doi.org/10.3390/ma11112226
Chicago/Turabian StyleXu, Xiangping, Yi Wang, Jiasheng Zou, and Chunzhi Xia. 2018. "Interfacial Microstructure and Properties of Si3N4 Ceramics/Cu/304 Stainless Steel Brazed by Ti40Zr25B0.2Cu Amorphous Solder" Materials 11, no. 11: 2226. https://doi.org/10.3390/ma11112226
APA StyleXu, X., Wang, Y., Zou, J., & Xia, C. (2018). Interfacial Microstructure and Properties of Si3N4 Ceramics/Cu/304 Stainless Steel Brazed by Ti40Zr25B0.2Cu Amorphous Solder. Materials, 11(11), 2226. https://doi.org/10.3390/ma11112226




