Composite Based on Biphasic Calcium Phosphate (HA/β-TCP) and Nanocellulose from the Açaí Tegument
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Method of Obtaining the NC/HA/β-TCP Nanocomposite
2.3. Characterization of the NC/HA/β-TCP Nanocomposite
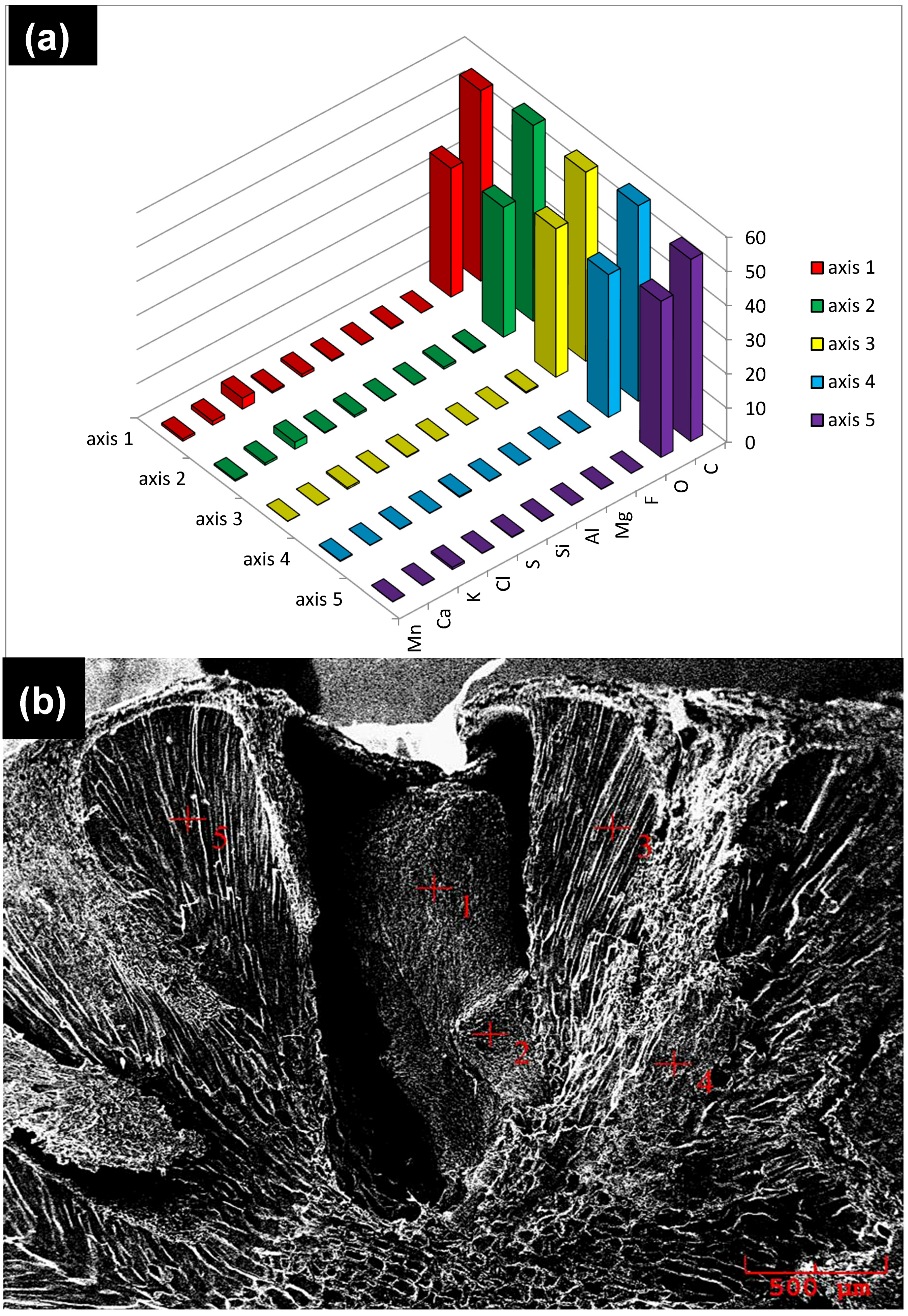
2.3.1. Scanning Electron Microscopy (SEM)/Energy Dispersive Spectroscopy (EDS)
2.3.2. X-Ray Diffraction (XRD)
2.3.3. Fourier Transform Infrared (FTIR)
2.3.4. Measurement of Size and Zeta Potential
3. Results and Discussion
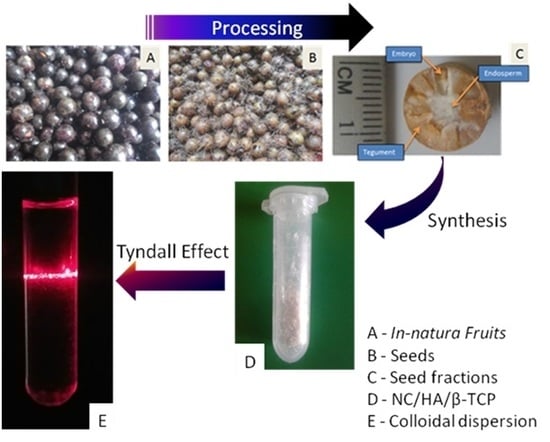
3.1. NC/HA/β-TCP Nanocomposite Synthesis
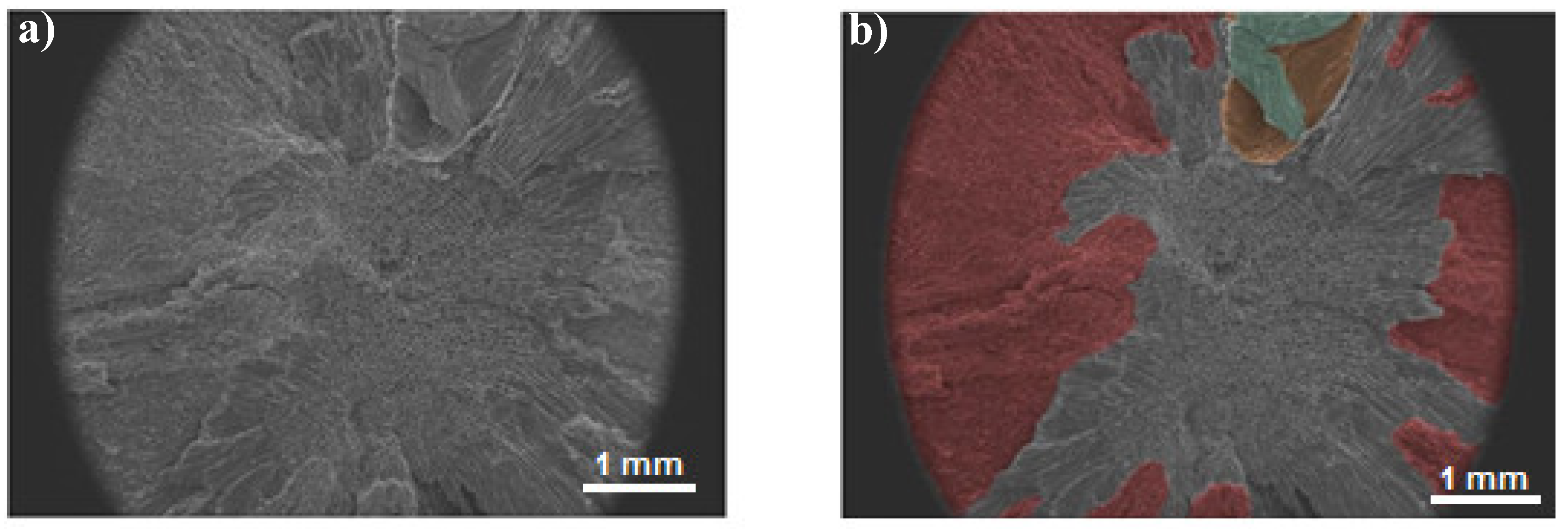
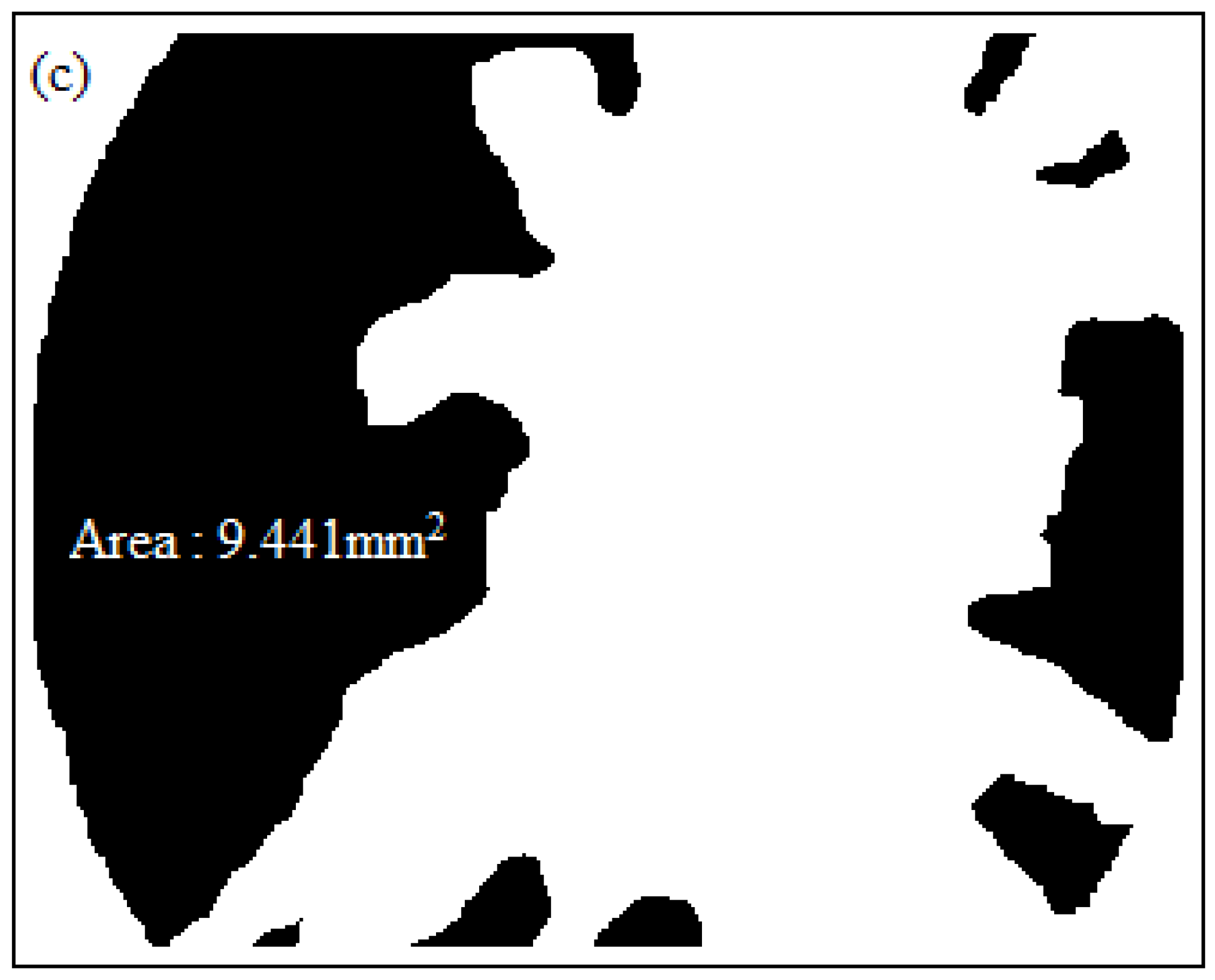
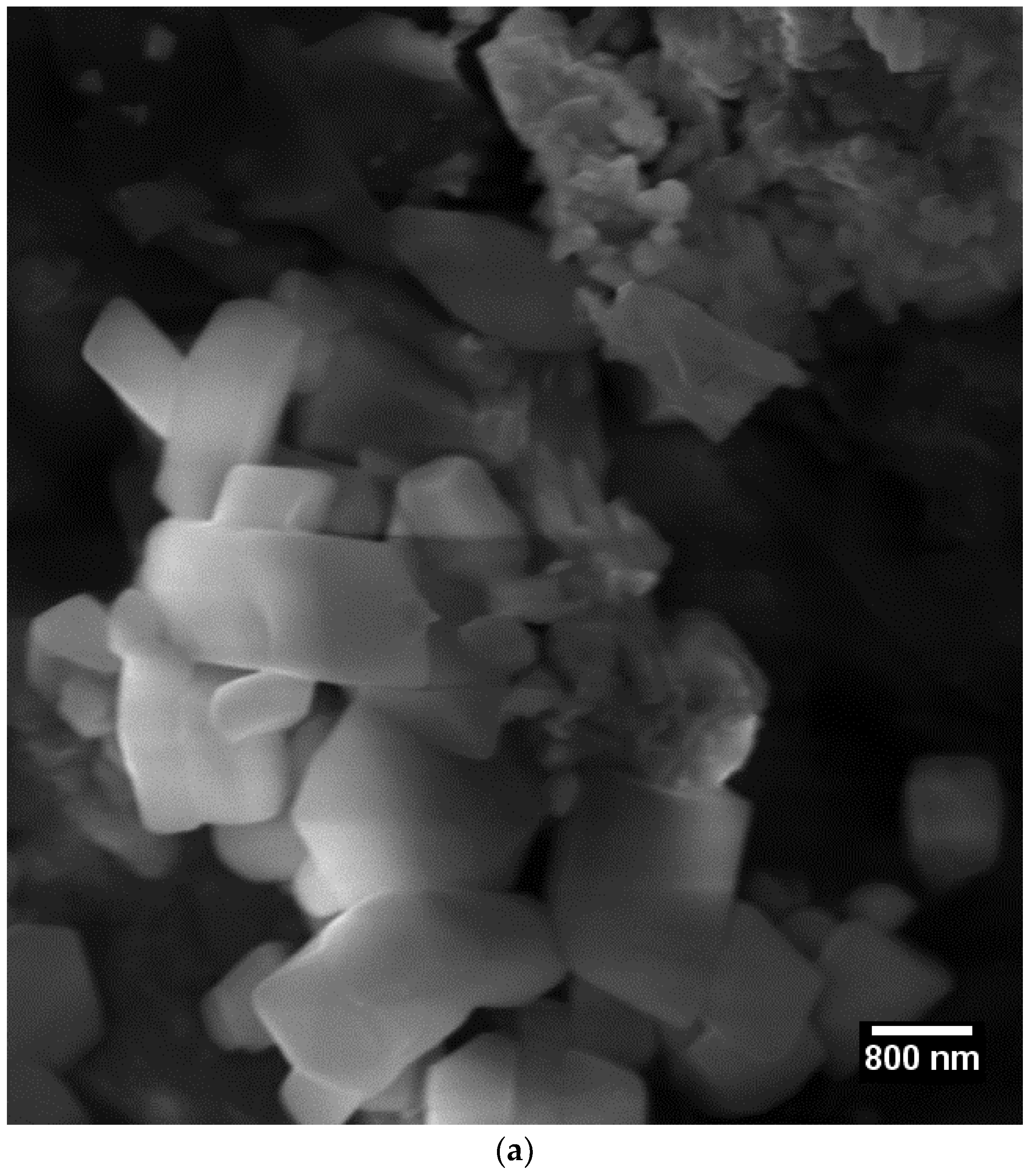
3.2. Scanning Electron Microscopy (SEM)
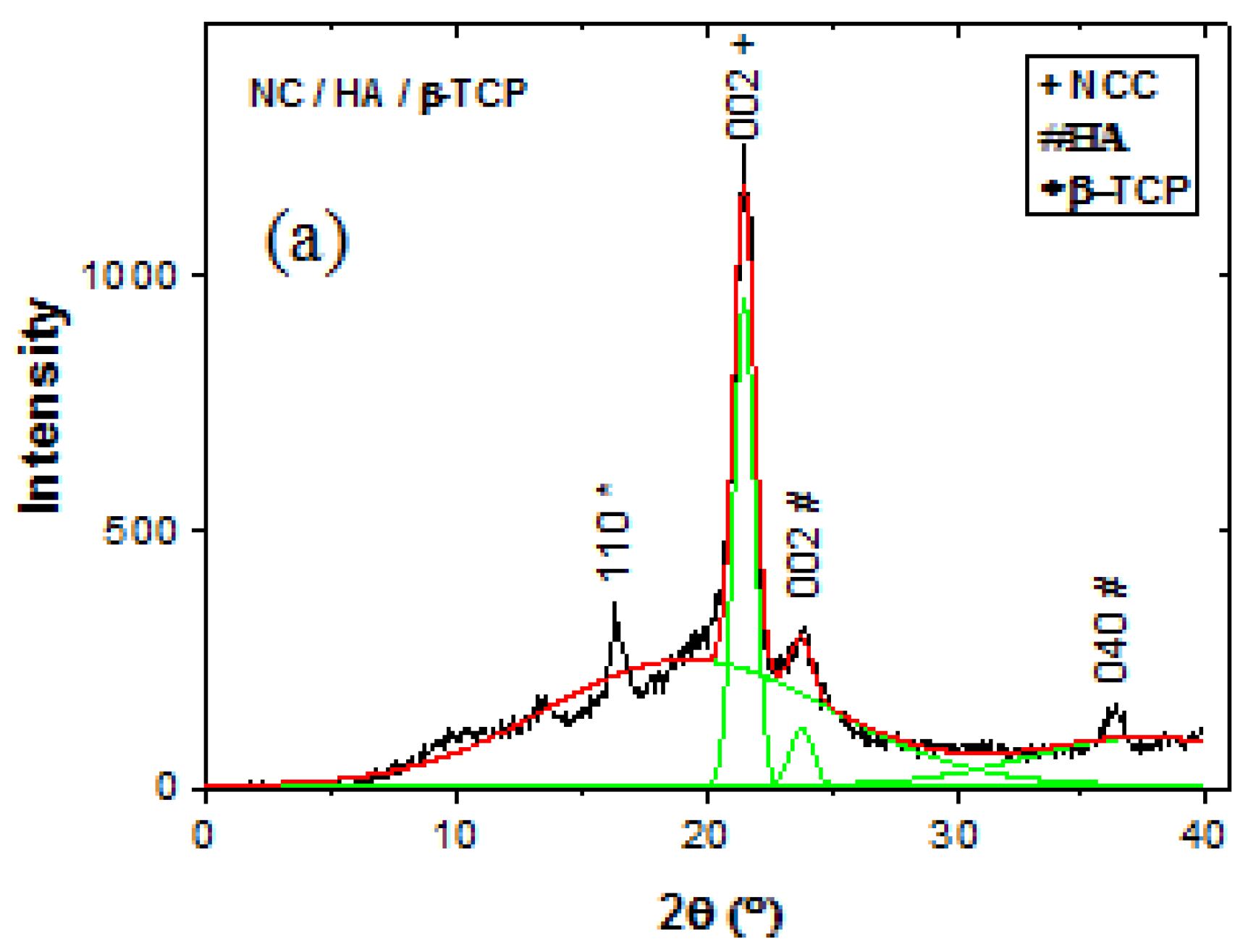
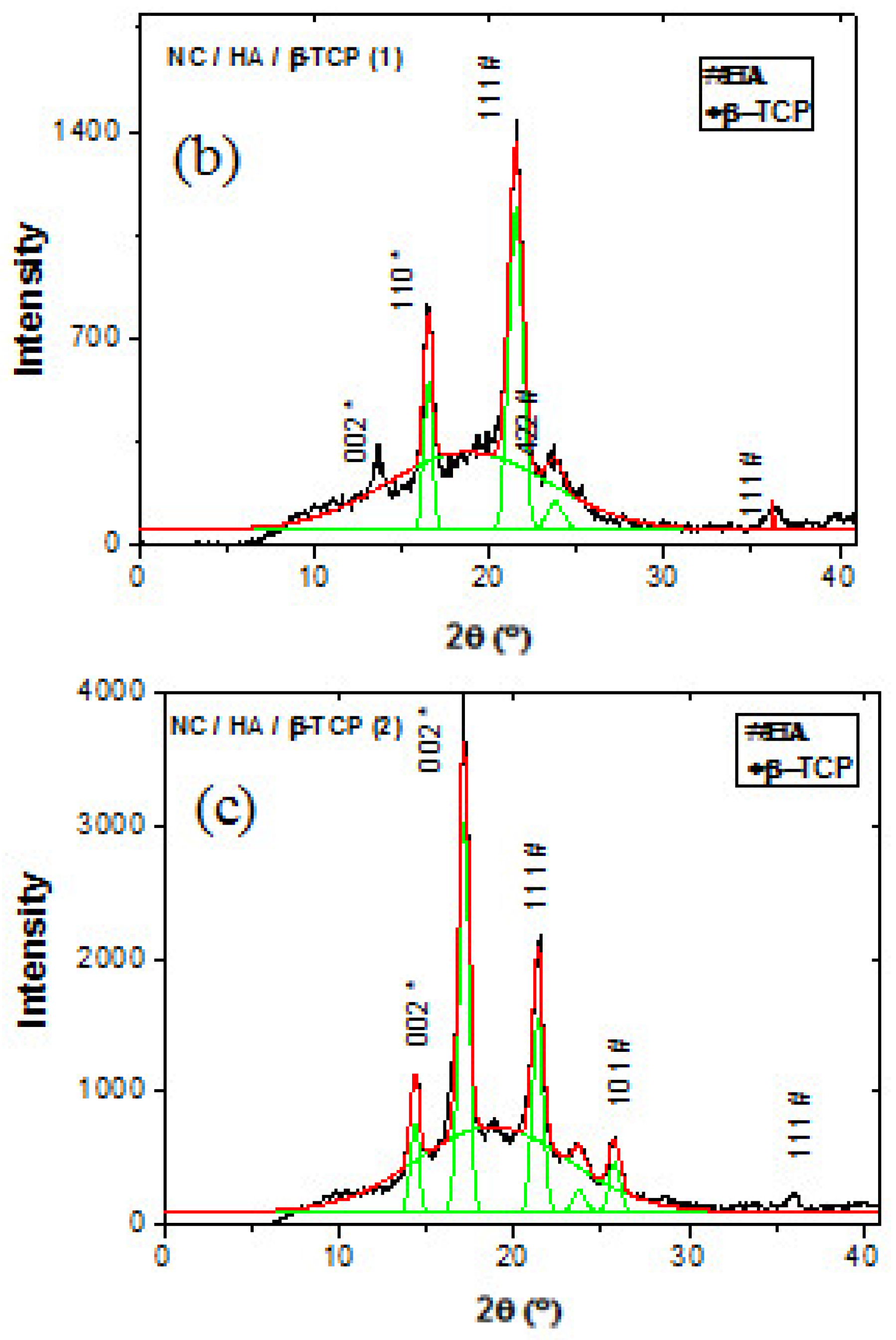
3.3. X-Ray Diffraction (XRD)
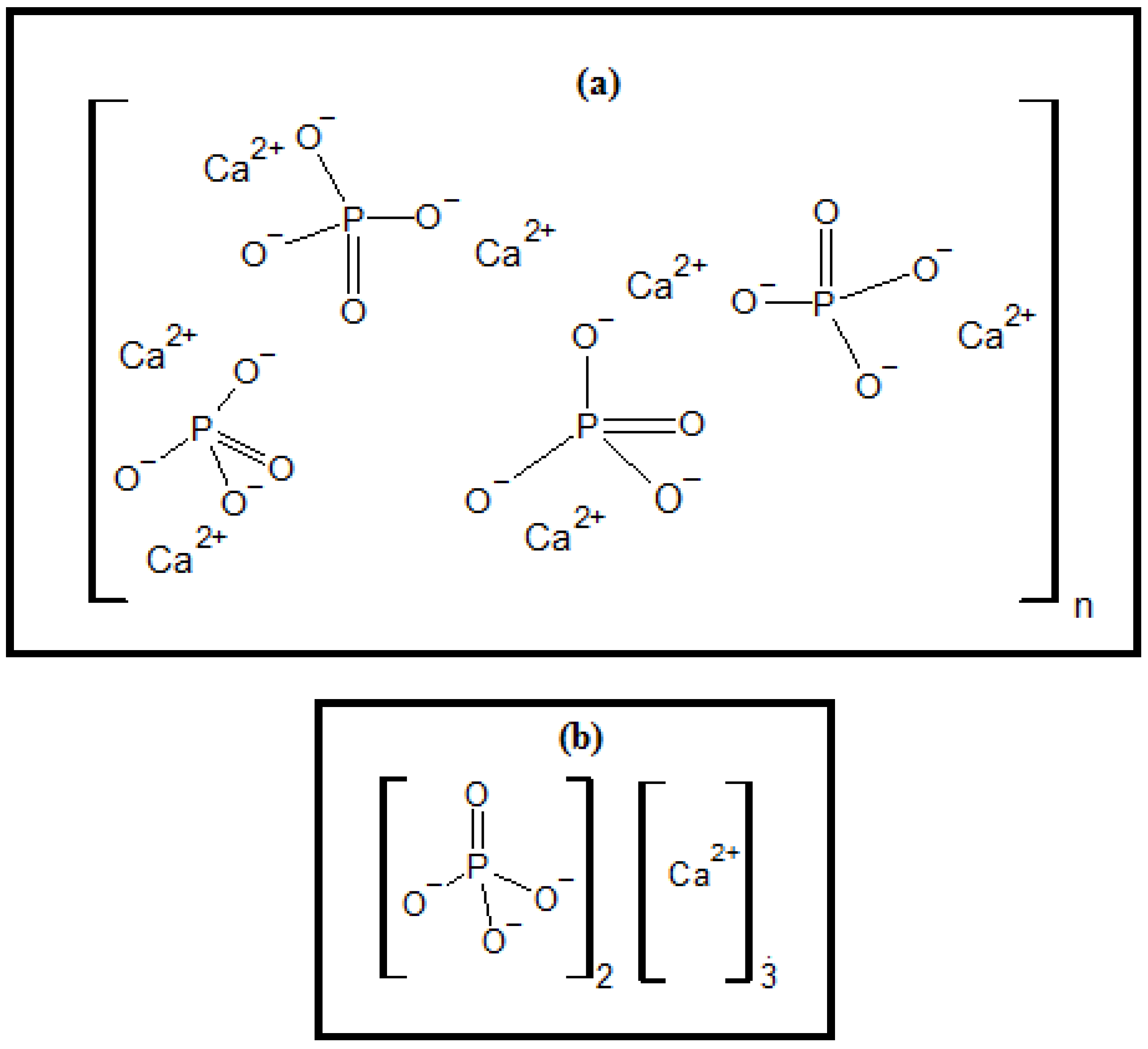
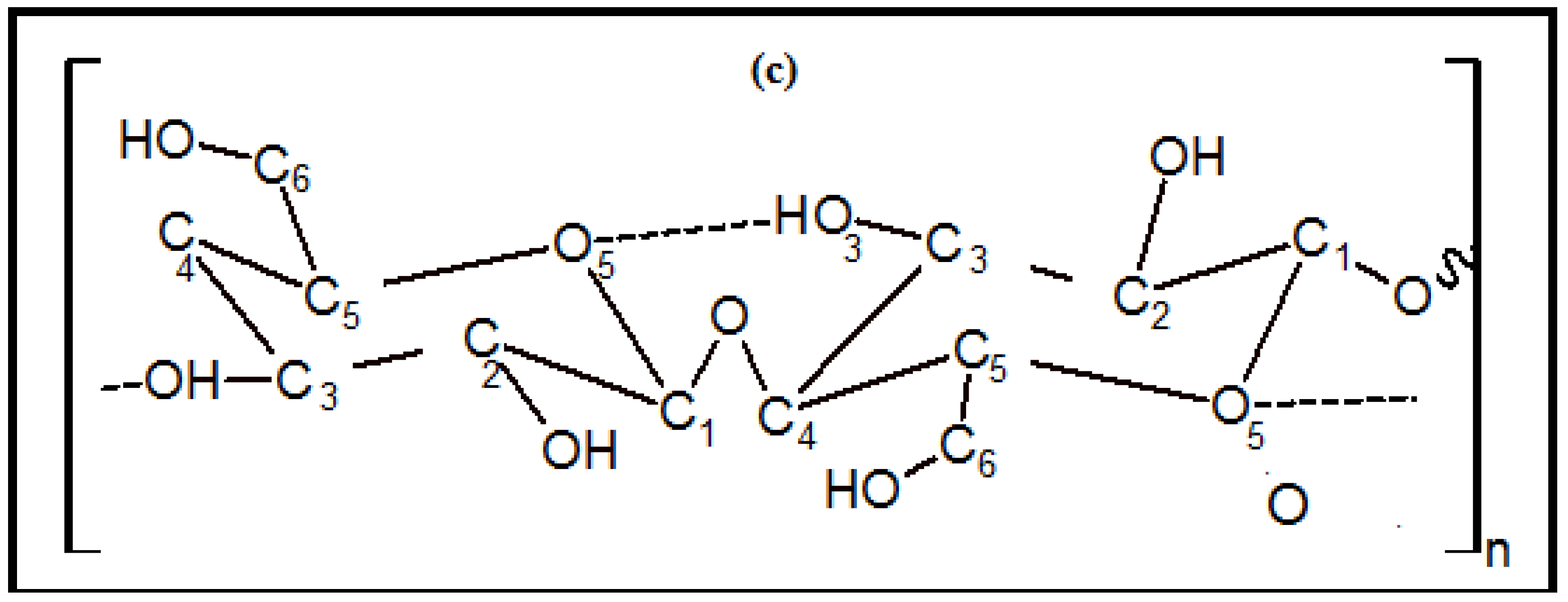
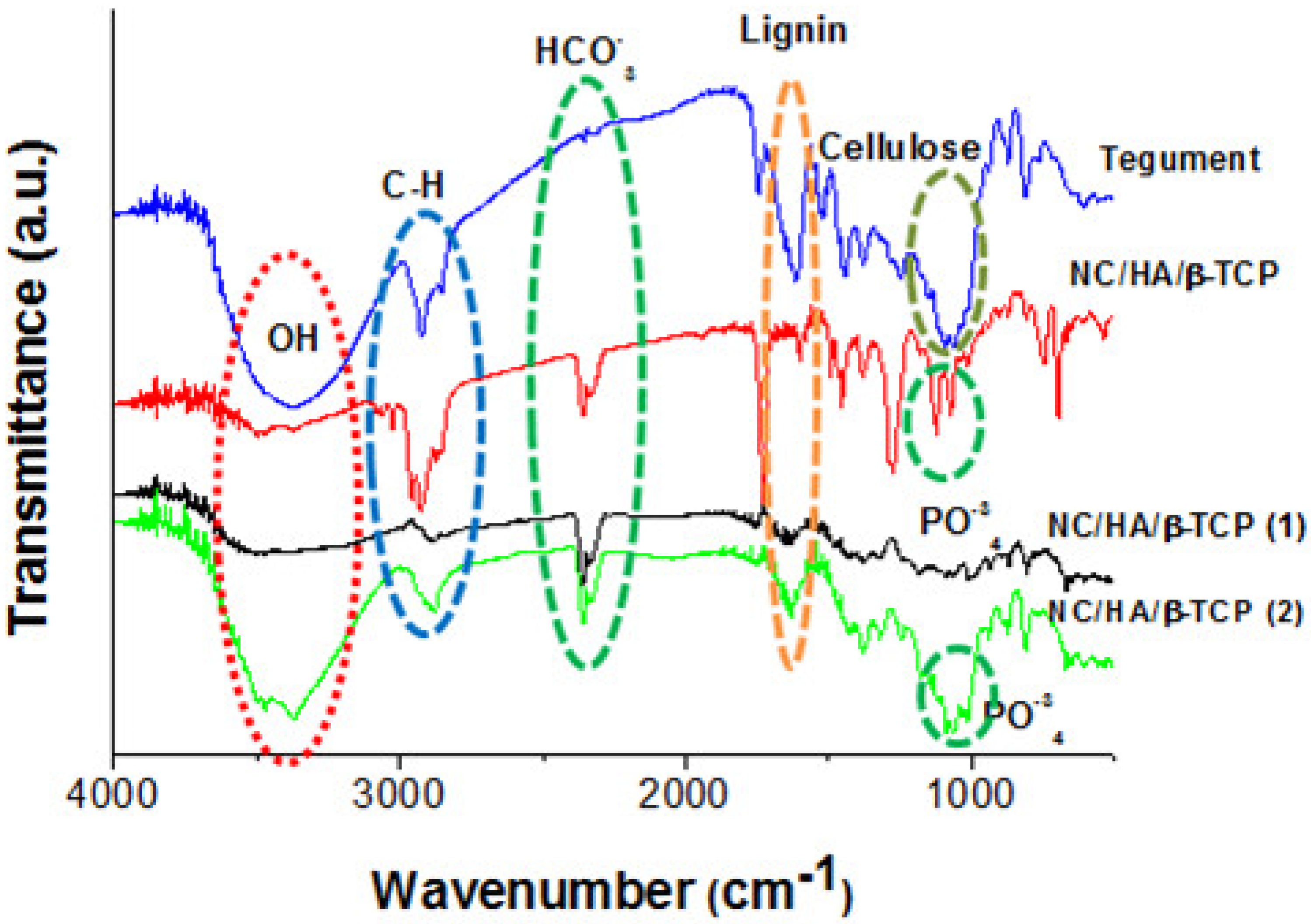
3.4. FTIR Spectrum
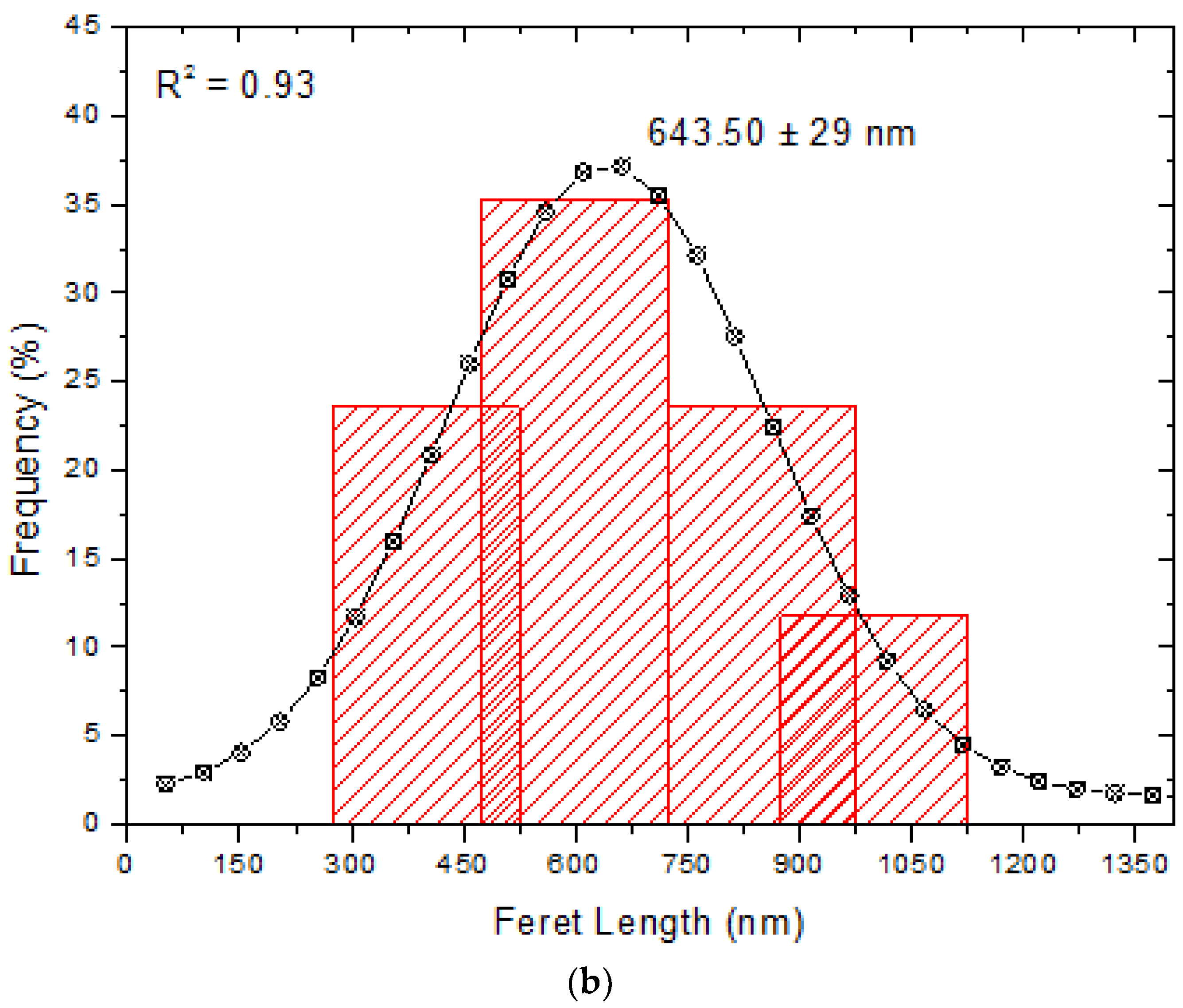
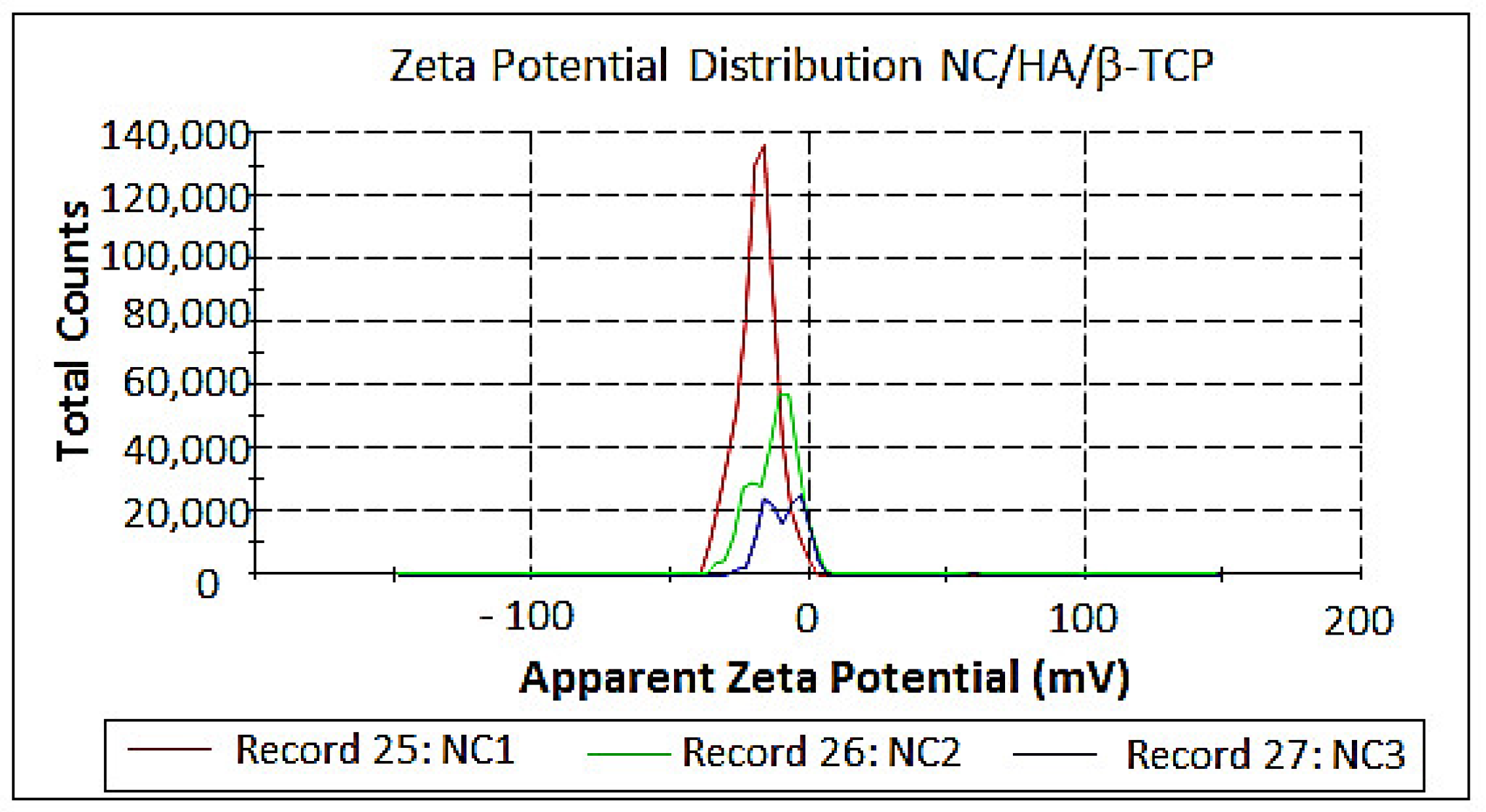
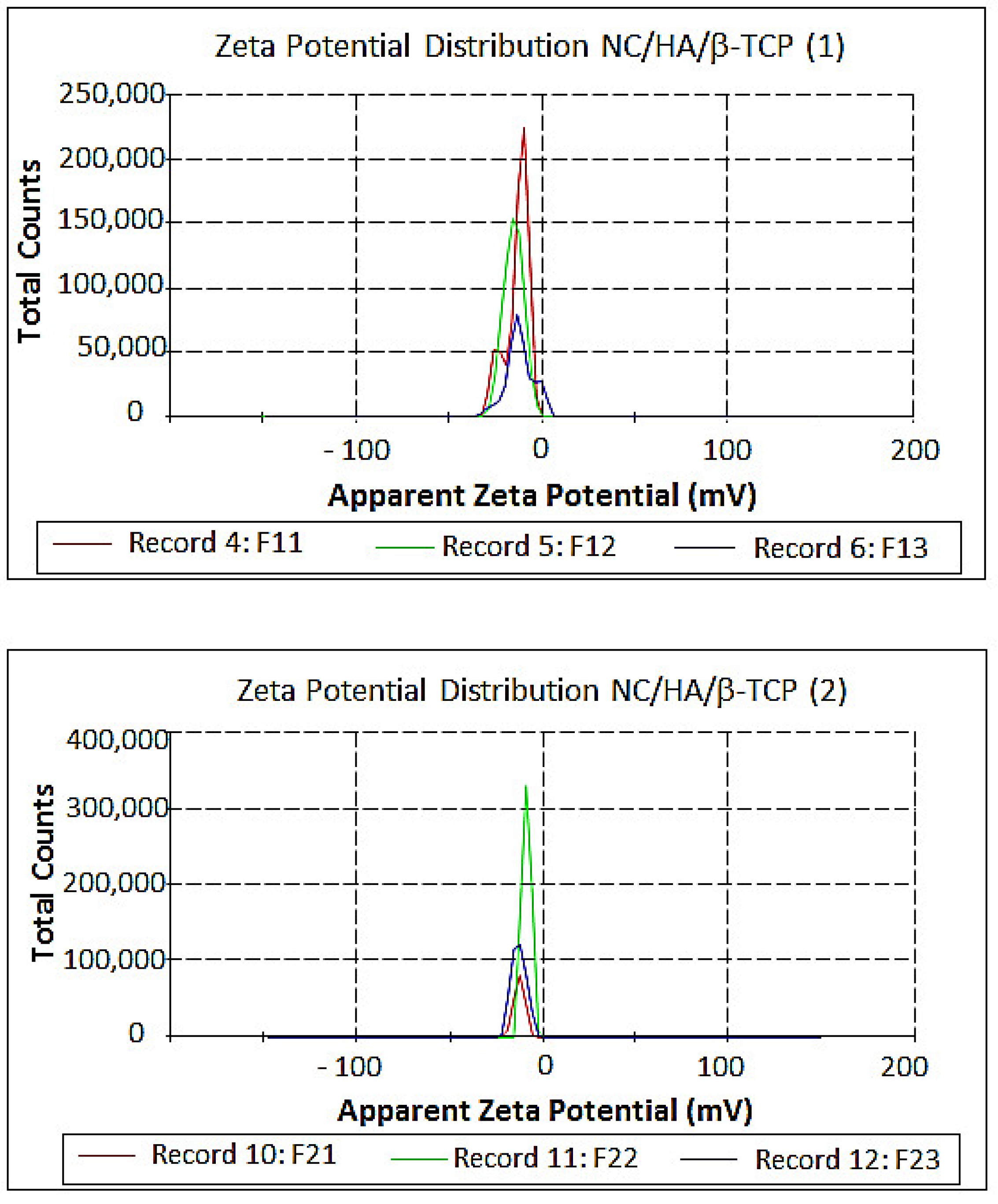
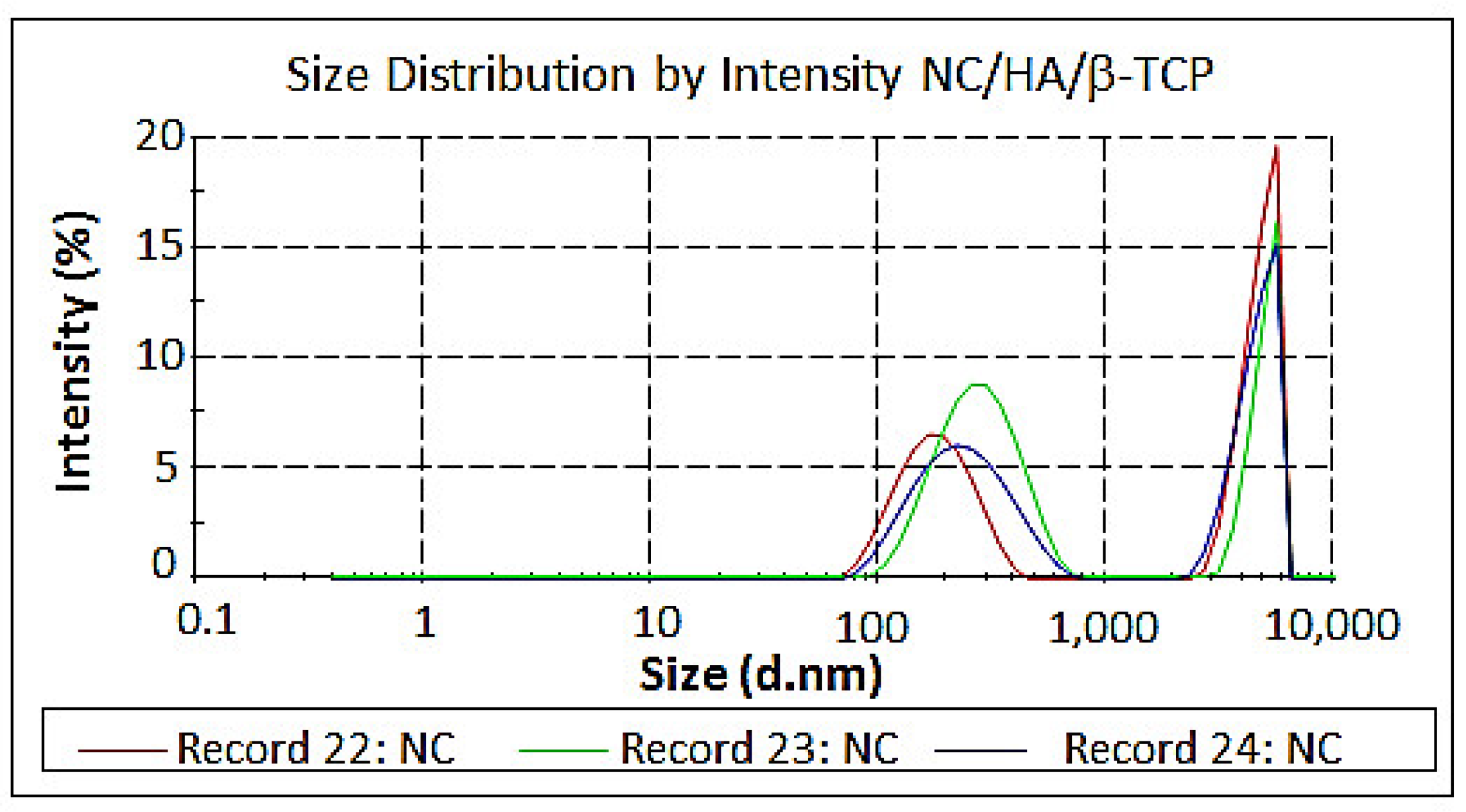
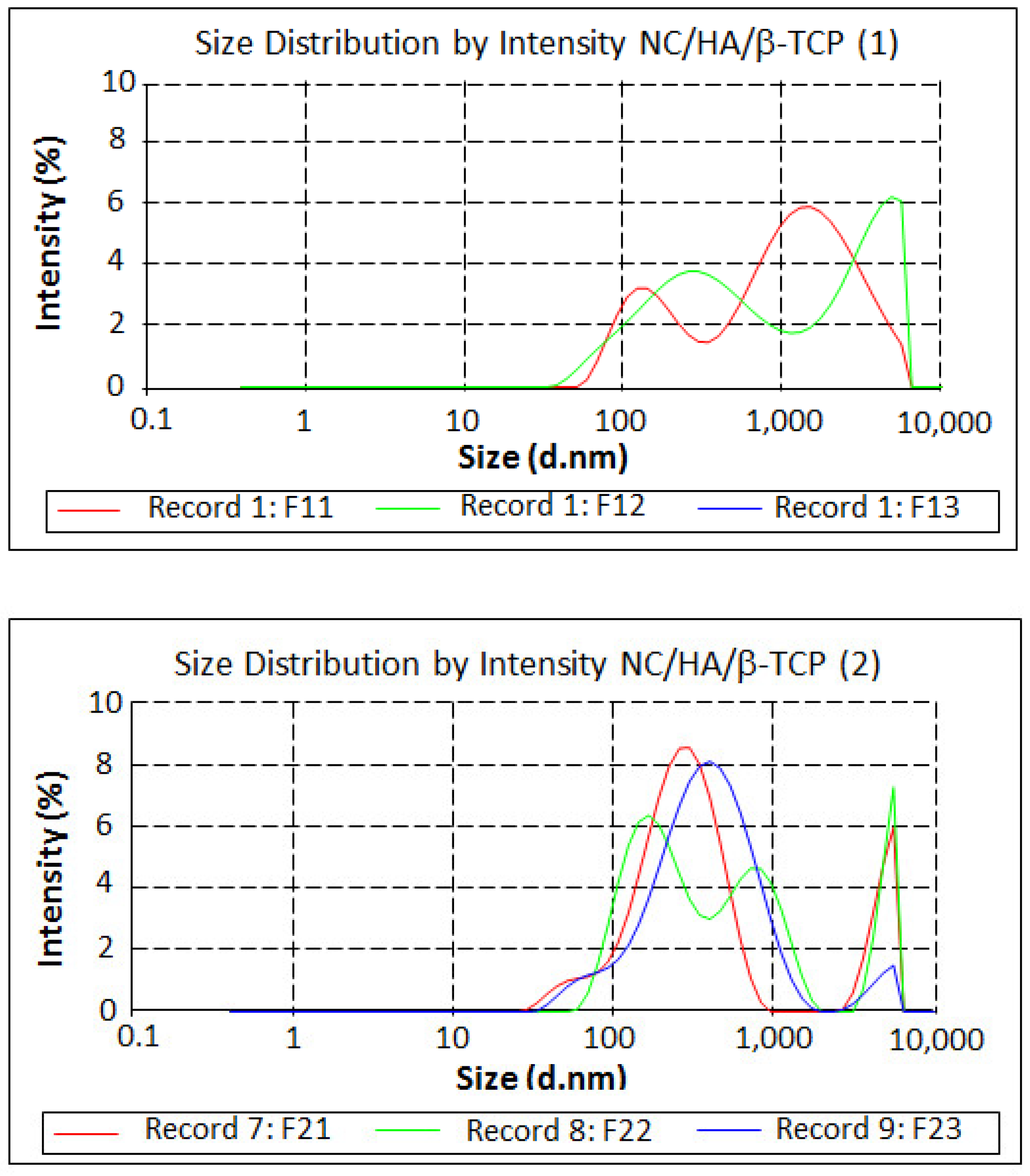
3.5. Measurement of Size and Zeta Potential
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guastaldi, A.C.; Aparecida, A.H. Fosfatos de cálcio de interesse biológico: Importância como Biomateriais, propriedades e métodos de obtenção de recobrimentos. Quím. Nova 2010, 33, 1352–1358. [Google Scholar] [CrossRef]
- Kumar, A.; Negi, Y.S.; Choudhary, V.; Bhardwaj, N.K. Microstructural and mechanical properties of porous biocomposite scaffolds based on polyvinylalcohol, nano-hydroxyapatite and cellulose nanocrystals. Cellulose 2014, 21, 3409–3426. [Google Scholar] [CrossRef]
- Frohbergh, M.E.; Katsman, A.; Botta, G.P.; Lazarovici, P.; Schauer, C.L.; Wegst, U.G.K.; Lelke, P.I. Electrospun hydroxyapatite-containing chitosan nanofibers crosslinked with genipin for bone tissue engineering. Biomaterials 2012, 33, 9167–9178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, S.Z.; Ni, P.Y.; Wang, B.Y.; Chu, B.Y.; Peng, J.R.; Zheng, L.; Zhao, X.; Luo, F.; Wei, Y.Q.; Qian, Z.Y. In vivo biocompatibility and osteogenesis of electrospunpoly(-caprolactone)-poly(ethylene glycol)-poly(-caprolactone)/nano-hydroxyapatite composite scaffold. Biomaterials 2012, 33, 8363–8671. [Google Scholar] [CrossRef] [PubMed]
- Phipps, M.C.; Clem, W.C.; Grunda, J.M.; Clines, G.A.; Belis, S.L. Increasing the pore sizes of bone-mimetic electrospun scaffolds comprised of polycaprolactone, collagen I and hydroxyapatite to enhance cell infiltration. Biomaterials 2012, 33, 524–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, F.Y.; Wang, S.G.; Wen, S.H.; Shen, M.W.; Zhu, M.F.; Shi, X.Y. Characterization and antibacterial activity of amoxicillin-loaded electrospunnano-hydroxyapatite/poly(lactic-co-glycolic acid) composite nanofibers. Biomaterials 2013, 34, 1402–1412. [Google Scholar] [CrossRef] [PubMed]
- Jazayeri, H.E.; Rodriguez-Romero, M.; Razavi, M.; Tahriri, M.; Ganjawalla, K.; Rasoulianboroujeni, M.; Malekoshoaraie, M.H.; Khoshroo, K.; Tayebi, L. The cross-disciplinary emergence of 3D printed bioceramic scaffolds in Orthopedic bioengineering. Ceram. Int. 2018, 44, 1–9. [Google Scholar] [CrossRef]
- Mi, H.Y.; Jing, X.; Salick, M.; Cordie, T.; Peng, X.F.; Turng, L.S. Morphology, mechanical properties, and mineralization of rigid thermoplastic polyurethane/hydroxyapatite scaffolds for bone tissue applications: Effects of fabrication approaches and hydroxyapatite size. J. Mater. Sci. 2014, 49, 2324–2337. [Google Scholar] [CrossRef]
- Pan, H.; Zheng, Q.; Yang, S.; Guo, X. Effects of functionalization of PLGA-[Asp-PEG]n copolymer surfaces with Arg-Gly-Asp peptides, hydroxyapatite nanoparticles, and BMP-2-derived peptides on cell behavior in vitro. J. Biomed. Mater. Res. A 2014, 102, 4526–4535. [Google Scholar] [PubMed]
- Gayathri, B.; Muthukumarasamy, N.; Velauthapillai, D.; Santhosh, S.B.; Asokan, V. Magnesium incorporated hydroxyapatite nanoparticles: Preparation, characterization, antibacterial and larvicidal activity. Arab. J. Chem. 2018, 11, 645–654. [Google Scholar] [CrossRef]
- Sunil, B.R.; Jagannatham, M. Producing hydroxyapatite from fish bones by heat treatment. Mater. Lett. 2016, 185, 411–414. [Google Scholar] [CrossRef]
- Heidari, F.; Razavi, M.; Ghaedi, M.; Forooghi, M.; Tahriri, M.; Tayebi, L. Investigation of mechanical properties of natural hydroxyapatite samples prepared by cold isostatic pressing method. J. Alloys Compd. 2017, 693, 1150–1156. [Google Scholar] [CrossRef]
- Fragal, E.H.; Cellet, T.S.P.; Fragal, V.H.; Companhoni, M.V.P.; Ueda-Nakamura, T.; Muniz, E.C.; Silva, R.; Rubira, A.F. Hybrid materials for bone tissue engineering from biomimetic growthofhydroxiapatite on cellulose nanowhiskers. Carbohyd. Polym. 2016, 152, 734–746. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Chiono, V.; Tonda-Turo, C.; Ferreira, A.M.; Ciardelli, G. Polymeric membranes for guided bone regeneration. Biotechnol. J. 2011, 6, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Sarikaya, B.; Aydin, H.M. Collagen/beta-tricalcium phosphate based synthetic bone grafts via dehydrothermal processing. BioMed Res. Int. 2015. [Google Scholar] [CrossRef] [PubMed]
- Bellucci, D.; Sola, A.; Cannillo, V. Hydroxyapatite and tricalcium phosphate composites with bioactive glass as second phase: State of the art and current applications. J. Biomed. Mater. Res. A 2016, 104, 1030–1056. [Google Scholar] [CrossRef] [PubMed]
- Sohier, J.; Daculsi, G.; Sourice, S.; De Groot, K.; Layrolle, P. Porous beta tricalcium phosphate scaffolds used as a BMP-2 delivery system for bone tissue engineering. J. Biomed. Mater. Res. A 2010, 92, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, M.; Kakagawa, K.; Asai, Y. Clinico-pathological studies on the tissue reactions of human pulp treated with various kinds of calcium phosphate ceramics. Bull. Tokyo Dent. Coll. 1991, 32, 111–120. [Google Scholar] [PubMed]
- Enkel, B.; Dupas, C.; Armengol, V.; Adou, J.A.; Bosco, J.; Daculsi, G.; Jean, A.; Laboux, O.; LeGeros, R.Z.; Weiss, P. Bioactive materials in endodontics. Expert Rev. Med. Devices 2008, 5, 475–494. [Google Scholar] [CrossRef] [PubMed]
- Larsson, P.A.; Berglund, L.A.; Wagberg, L. Ductile all-cellulose nanocomposite films fabricated from core–shell structured cellulose nanofibrils. Biomacromolecules 2014, 15, 2218–2223. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lu, A.; Zhang, L. Recent Advances in Regenerated Cellulose Materials. Prog. Polym. Sci. 2016, 53, 169–206. [Google Scholar] [CrossRef]
- Dufresne, A. Nanocellulose: A new ageless bionanomaterial. Mater. Today 2013, 16, 220–227. [Google Scholar] [CrossRef]
- García, A.; Gandini, A.; Labidi, J.; Belgacem, N.; Bras, J. Industrial and crop wastes: A new source for nanocellulose biorefinery. Ind. Crop Prod. 2016, 93, 26–38. [Google Scholar] [CrossRef]
- Henrique, M.A.; Silvério, H.A.; Neto, W.P.F.; Pasquini, D. Valorization of an agro-industrial waste, mango seed, by the extraction and characterization of its cellulose nanocrystals. J. Environ. Manag. 2013, 121, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Lorenzo, L.M.; Vallet-Regí, M. Controlled crystallization of calcium phosphate apatites. Chem. Mater. 2000, 12, 2460–2465. [Google Scholar] [CrossRef]
- Aoba, T. Solubility properties of human tooth mineral and pathogenesis of dental caries. Oral Dis. 2004, 10, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Lozano, N.; Velázquez-Castillo, R.; Rivera-Muñoz, E.M.; Bucio-Galindo, L.; Mondragón-Galicia, G.; Manzano-Ramírez, A.; Ocampo, M.A.; Apátiga-Castro, L. Crystal growth and structural analysis of hydroxyapatite nanofibers synthesized by the hydrothermal microwave-assisted method. Ceram. Int. 2017, 43, 451–457. [Google Scholar] [CrossRef]
- Samir, M.A.S.; Alloin, F.; Dufresne, A. Review of recent research into cellulosic whiskers, their properties and their application in nanocomposite field. Biomacromolecules 2005, 612, 612–626. [Google Scholar] [CrossRef] [PubMed]
- Rigo, E.C.S.; Gehrke, S.A.; Carbonari, M. Síntese e Caracterização de Hidroxiapatita Obtida pelo Método da Precipitação. Revista Dental Press Periodontia Implantol. Maringá 2007, 1, 39–50. [Google Scholar]
- Gomes, L.C.; Di Lello, B.C.; Campos, J.B.; Sampaio, M. Síntese e caracterização de fosfatos de cálcio a partir da casca de ovo de galinha. Cerâmica 2012, 58, 448–452. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, A.; Tsuge, T.; Kikuch, M. Preparation of porous β-tricalcium phosphate using starfish-derived calcium carbonate as a precursor. Ceram. Int. 2016, 42, 15376–15382. [Google Scholar] [CrossRef]
- Lengowski, E.C.; Muniz, G.I.B.; Nisgoski, S.; Magalhães, W.L.E. Cellulose acquirement evaluation methods with different degrees of crystallinity. Sci. For. 2013, 41, 185–194. [Google Scholar]
- Jiang, F.; Hsieh, Y.L. Cellulose nanocrystal isolation from tomato peels and assembled nanofibers. Carbohyd. Polym. 2015, 122, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Jayaramudu, J.; Guduri, B.R.; Rajulu, A.V. Characterization of new natural cellulosic fabric Grewia tilifolia. Carbohyd. Polym. 2010, 79, 847–851. [Google Scholar] [CrossRef]
- Spiridon, I.; Teacă, C.; Bodîrlău, R. structural changes evidenced by FTIR spectroscopy in cellulose materials after pre-treatment with ionic liquid and enzymatic hydrolysis. BioResources 2010, 6, 400–413. [Google Scholar]
- Kačuráková, M.; Smith, A.C.; Gidley, M.J.; Wilson, R.H. Molecular interactions in bacterial cellulose composites studied by 1D FT-IR and dynamic 2D FT-IR spectroscopy. Carbohyd. Res. 2002, 337, 1145–1153. [Google Scholar] [CrossRef]
- Saravanakumar, S.S.; Kumaravel, A.; Nagarajan, T.; Sudhakar, P.; Baskaran, R. Characterization of a novel natural cellulosic fiber from Prosopis juliflora bark. Carbohyd. Polym. 2013, 92, 1928–1933. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Guo, H.; Zhang, H.; He, X. Polymeric micelle-templated synthesis of hydroxyapatite hollow nanoparticles for a drug delivery system. Acta Biomater. 2010, 6, 2212–2218. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, R.M.; Bassler, G.C.; Morril, T.C. Identificação Espectrométrica de Compostos Orgânico, 5th ed.; LTC: Rio de Janeiro, Brazil, 1994; p. 460. [Google Scholar]
- Brito, S.L.M.; Gouvêa, D. Caracterização superficial de nanopartículas de BaTiO3 preparado pelo método dos precursores poliméricos. Cerâmica 2010, 56, 228–236. [Google Scholar] [CrossRef]
- Morais, J.P.S.; Rosa, M.D.; de Souza, M.D.M.; Nascimento, L.D.; do Nascimento, D.M.; Cassales, A.R. Extraction and characterization of nanocellulose structures from raw cotton linter. Carbohyd. Polym. 2013, 91, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Toma, H.E. Nanotecnologia Molecular-Materiais e Dispositivos; Blucher: São Paulo, Brazil, 2016; Volume 6, p. 333. [Google Scholar]
- Zarbin, A.J.G. Química de (nano) materiais. Quim. Nova 2007, 30, 1469–1479. [Google Scholar] [CrossRef]
- Beyene, D.; Chae, M.; Dai, J.; Danumah, C.; Tosto, F.; Demesa, A.G.; Bressler, D.C. Characterization of cellulase-treated fibers and resulting cellulose nanocrystals generated through acid hydrolysis. Materials 2018, 11, 1272. [Google Scholar] [CrossRef] [PubMed]
- Filipova, I.; Fridrihsone, V.; Cabulis, U.; Berzins, A. Synthesis of nanofibrillated cellulose by combined ammonium persulphate treatment with ultrasound and mechanical processing. Nanomaterials 2018, 8, 640. [Google Scholar] [CrossRef] [PubMed]
- Feynman, R.P. There’s plenty of room at the bottom. J. Microelectromech. Syst. 1992, 1, 60–64. [Google Scholar] [CrossRef]
- Pais, A. Einstein on Planck: 1905. The Rayleigh-Einstein-Jeans Law. In 19b in Subtle is the Lord: The Science and the Life of Albert Einstein; Oxford University Press: New York, NY, USA, 1982; pp. 372–375. [Google Scholar]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valentim, R.M.B.; Andrade, S.M.C.; Dos Santos, M.E.M.; Santos, A.C.; Pereira, V.S.; Dos Santos, I.P.; Dias, C.G.B.T.; Dos Reis, M.A.L. Composite Based on Biphasic Calcium Phosphate (HA/β-TCP) and Nanocellulose from the Açaí Tegument. Materials 2018, 11, 2213. https://doi.org/10.3390/ma11112213
Valentim RMB, Andrade SMC, Dos Santos MEM, Santos AC, Pereira VS, Dos Santos IP, Dias CGBT, Dos Reis MAL. Composite Based on Biphasic Calcium Phosphate (HA/β-TCP) and Nanocellulose from the Açaí Tegument. Materials. 2018; 11(11):2213. https://doi.org/10.3390/ma11112213
Chicago/Turabian StyleValentim, Rachel M. B., Sabina M. C. Andrade, Maria E. M. Dos Santos, Aline C. Santos, Victor S. Pereira, Izael P. Dos Santos, Carmen G. B. T. Dias, and Marcos A. L. Dos Reis. 2018. "Composite Based on Biphasic Calcium Phosphate (HA/β-TCP) and Nanocellulose from the Açaí Tegument" Materials 11, no. 11: 2213. https://doi.org/10.3390/ma11112213
APA StyleValentim, R. M. B., Andrade, S. M. C., Dos Santos, M. E. M., Santos, A. C., Pereira, V. S., Dos Santos, I. P., Dias, C. G. B. T., & Dos Reis, M. A. L. (2018). Composite Based on Biphasic Calcium Phosphate (HA/β-TCP) and Nanocellulose from the Açaí Tegument. Materials, 11(11), 2213. https://doi.org/10.3390/ma11112213