Improving the Water Resistance and Mechanical Properties of Feather Keratin/Polyvinyl Alcohol/Tris(Hydroxymethyl)Aminomethane Blend Films by Cross-Linking with Transglutaminase, CaCl2, and Genipin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the 0.1% Cross-Linking Agent Solutions
2.3. Preparation of the Blend Films
2.4. Characterization
2.5. Statistical Analysis
3. Results and Discussion
3.1. Examination of the Film Morphologies
3.2. FTIR Analysis
3.3. Mechanical Properties
3.4. Moisture Sensitivities of the Blend Films
3.4.1. Total Soluble Masses of the Blend Films
3.4.2. Contact Angles of the Blend Films
3.5. Barrier Properties of the Blend Films
3.5.1. Water Vapor Permeability (WVP)
3.5.2. Oxygen Permeability (OP)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.Q. Dissociation and Extraction of Feather Keratin by Steam Flash Explosion. Ph.D. Thesis, Jiangnan University, Wuxi, China, December 2015. [Google Scholar]
- Shavandi, A.; Bekhit, A.E.D.A.; Carne, A.; Bekhit, A. Evaluation of keratin extraction from wool by chemical methods for bio-polymer application. J. Bioact. Compat. Polym. 2016, 32, 163–177. [Google Scholar] [CrossRef]
- Brown, E.M.; Pandya, K.; Taylor, M.M.; Liu, C.-K. Comparison of methods for extraction of keratin from waste wool. Agric. Sci. 2016, 7, 670–679. [Google Scholar] [CrossRef]
- Gupta, A.; Kumar, P.; Bin Mohd Yunus, R.; Binti Kamarudin, N. Extraction of keratin protein from chicken feather. J. Chem. Chem. Eng. 2012, 6, 732–737. [Google Scholar]
- Nakamura, A.; Arimoto, M.; Takeuchi, K.; Fujii, T. A rapid extraction procedure of human hair proteins and identification of phosphorylated species. Biol. Pharm. Bull. 2002, 25, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Shui-qing, J.; Lin, Z.; Haixia, W.; Gang, C. Study on effective extraction of keratin from human hair wastes. Integr. Ferroelectr. 2017, 180, 102–107. [Google Scholar] [CrossRef]
- Ji, Y.; Chen, J.; Lv, J.; Li, Z.; Xing, L.; Ding, S. Extraction of keratin with ionic liquids from poultry feather. Sep. Purif. Technol. 2014, 132, 577–583. [Google Scholar] [CrossRef]
- Xu, J.; Zhu, P.; Zhang, L.; Sui, S.Y.; Dong, Z.H.; Zuo, Y. Study of keratin extractionfrom human hair. Wool Text. J. 2015, 43, 38–42. [Google Scholar]
- Kang, D.; Herschend, J.; Al-Soud, W.A.; Mortensen, M.S.; Gonzalo, M.; Jacquiod, S.; Sørensen, S.J. Enrichment and characterization of an environmental microbial consortium displaying efficient keratinolytic activity. Bioresour. Technol. 2018, 270, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Sekar, V.; Kannan, M.; Ganesan, R.; Dheeba, B.; Sivakumar, N.; Kannan, K. Isolation and screening of keratinolytic bacteria from feather dumping soil in and around cuddalore and villupuram, tamil nadu. Proc. Nat. Acad. Sci. India 2015, 86, 1–9. [Google Scholar] [CrossRef]
- Jin, E.; Reddy, N.; Zhu, Z.; Yang, Y. Graft polymerization of native chicken feathers for thermoplastic applications. J. Agric. Food Chem. 2011, 59, 1729–1738. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhou, H.; Hua, J. New progress in utilization of feather fibers. China Leather 2011, 40, 36–40. [Google Scholar]
- Dou, Y.; Zhang, B.N.; He, M.; Yin, G.Q.; Cui, Y.D. The structure, tensile properties and water resistance of hydrolyzed feather keratin-based bioplastics. Chin. J. Chem. Eng. 2016, 24, 415–420. [Google Scholar] [CrossRef]
- Dou, Y.; Huang, X.; Zhang, B.; He, M.; Yin, G.; Cui, Y. Preparation and characterization of dialdehyde starch crosslinked feather keratin film for food packaging application. RSC Adv. 2015, 5, 27168–27174. [Google Scholar] [CrossRef]
- Dou, Y.; Zhang, B.; He, M.; Yin, G.; Cui, Y. Preparation and physicochemical properties of dialdehyde starch crosslinked feather keratin/pva composite films. J. Macromol. Sci. Part A Pure Appl. Chem. 2014, 51, 1009–1015. [Google Scholar] [CrossRef]
- Dou, Y.; Zhang, B.; He, M.; Yin, G.; Cui, Y.; Savina, I.N. Keratin/polyvinyl alcohol blend films cross-linked by dialdehyde starch and their potential application for drug release. Polymers 2015, 7, 580–591. [Google Scholar] [CrossRef] [Green Version]
- He, M.; Zhang, B.; Dou, Y.; Yin, G.; Cui, Y. Blend modification of feather keratin-based films using sodium alginate. J. Appl. Polym. Sci. 2017, 134, 44680–44687. [Google Scholar] [CrossRef]
- He, M.; Zhang, B.; Dou, Y.; Yin, G.; Cui, Y.; Chen, X. Fabrication and characterization of electrospun feather keratin/poly(vinyl alcohol) composite nanofibers. RSC Adv. 2017, 7, 9854–9861. [Google Scholar] [CrossRef] [Green Version]
- Isarankura Na Ayutthaya, S.; Tanpichai, S.; Wootthikanokkhan, J. Keratin extracted from chicken feather waste: Extraction, preparation, and structural characterization of the keratin and keratin/biopolymer films and electrospuns. J. Polym. Environ. 2015, 23, 506–516. [Google Scholar] [CrossRef]
- Sow, W.T.; Lui, Y.S.; Ng, K.W. Electrospun human keratin matrices as templates for tissue regeneration. Nanomedicine 2013, 8, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Shibayama, M.; Tanabe, T.; Yamauchi, K. Preparation and physicochemical properties of compression-molded keratin films. Biomaterials 2004, 25, 2265–2272. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, H.; Yuan, X.; Cui, S. Wool keratin: A novel building block for layer-by-layer self-assembly. J. Colloid Interface Sci. 2009, 336, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wu, S.; Yi, M.; Ge, J.; Yin, G.; Li, X. Preparation and physicochemical properties of blend films of feather keratin and poly(vinyl alcohol) compatibilized by tris(hydroxymethyl)aminomethane. Polymers 2018, 10, 1054. [Google Scholar] [CrossRef]
- Audic, J.L.; Chaufer, B. Influence of plasticizers and crosslinking on the properties of biodegradable films made from sodium caseinate. Eur. Polym. J. 2005, 41, 1934–1942. [Google Scholar] [CrossRef]
- Galietta, G.; Gioia, L.D.; Guilbert, S.; Cuq, B. Mechanical and thermomechanical properties of films based on whey proteins as affected by plasticizer and crosslinking agents. J. Dairy Sci. 1998, 81, 3123–3130. [Google Scholar] [CrossRef]
- Marquié, C. Chemical reactions in cottonseed protein cross-linking by formaldehyde, glutaraldehyde, and glyoxal for the formation of protein films with enhanced mechanical properties. J. Agric. Food. Chem. 2001, 49, 4676–4681. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, R.A.; Grosso, C.R.F. Characterization of gelatin based films modified with transglutaminase, glyoxal and formaldehyde. Food Hydrocolloids 2004, 18, 717–726. [Google Scholar] [CrossRef]
- Woods, K.K.; Selling, G.W. Improved tensile strength of zein films using glyoxal as a crosslinking reagent. J. Biobased Mater. Bioenergy 2007, 1, 282–288. [Google Scholar] [CrossRef]
- Bigi, A.; Cojazzi, G.; Panzavolta, S.; Rubini, K.; Roveri, N. Mechanical and thermal properties of gelatin films at different degrees of glutaraldehyde crosslinking. Biomaterials 2001, 22, 763–768. [Google Scholar] [CrossRef]
- Song, N.B.; Lee, J.H.; Mijan, M.A.; Song, K.B. Development of a chicken feather protein film containing clove oil and its application in smoked salmon packaging. LWT Food Sci. Technol. 2014, 57, 453–460. [Google Scholar] [CrossRef]
- Mahmoud, R.; Savello, P.A. Solubility and hydrolyzability of films produced by transglutaminase catalytic crosslinking of whey protein 1. J. Dairy Sci. 1993, 76, 29–35. [Google Scholar] [CrossRef]
- Wu, X.; Liu, A.; Wang, W.; Ye, R. Improved mechanical properties and thermal-stability of collagen fiber based film by crosslinking with casein, keratin or spi: Effect of crosslinking process and concentrations of proteins. Int. J. Biol. Macromol. 2018, 109, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, Y.; Liu, A.; Wang, W. Improved thermal-stability and mechanical properties of type i collagen by crosslinking with casein, keratin and soy protein isolate using transglutaminase. Int. J. Biol. Macromol. 2017, 98, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Arabestani, A.; Kadivar, M.; Shahedi, M.; Goli, S.A.H.; Porta, R. Properties of a new protein film from bitter vetch (vicia ervilia) and effect of cacl2 on its hydrophobicity. Int. J. Biol. Macromol. 2013, 57, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Song, M.; Hourston, D.J. Novel chitosan-based films cross-linked by genipin with improved physical properties. Biomacromolecules 2004, 5, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Jia, J.; Guo, Y.; Liu, Y.; Zhu, P. Preparation and characterization of ipn hydrogels composed of chitosan and gelatin cross-linked by genipin. Carbohydr. Polym. 2014, 99, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wu, Y.; Lin, Y.; Yu, L.; Sung, H. A novel ph-sensitive hydrogel composed of n,o-carboxymethyl chitosan and alginate cross-linked by genipin for protein drug delivery. J. Control. Release 2004, 96, 285–300. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.F.; Ng, Y.F.; Pudney, P.D.A. Mechanism and kinetics of the crosslinking reaction between biopolymers containing primary amine groups and genipin. Inc. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 3941–3953. [Google Scholar] [CrossRef]
- Motta, A.; Barbato, B.; Foss, C.; Torricelli, P.; Migliaresi, C. Stabilization of bombyx mori silk fibroin/sericin films by crosslinking with peg-de 600 and genipin. J. Bioact. Compat. Polym. 2016, 26, 130–143. [Google Scholar] [CrossRef]
- You, R.; Xu, Y.; Liu, G.; Liu, Y.; Li, X.; Li, M. Regulating the degradation rate of silk fibroin films through changing the genipin crosslinking degree. Polym. Degrad. Stab. 2014, 109, 226–232. [Google Scholar] [CrossRef]
- Vasconcelos, A.; Gomes, A.C.; Cavaco-Paulo, A. Novel silk fibroin/elastin wound dressings. Acta Biomater. 2012, 8, 3049–3060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bigi, A.; Cojazzi, G.; Panzavolta, S.; Roveri, N.; Rubini, K. Stabilization of gelatin films by crosslinking with genipin. Biomaterials 2002, 23, 4827–4832. [Google Scholar] [CrossRef]
- Wang, K.; Luo, S.; Cai, J.; Sun, Q.; Zhao, Y.; Zhong, X.; Jiang, S.; Zheng, Z. Effects of partial hydrolysis and subsequent cross-linking on wheat gluten physicochemical properties and structure. Food Chem. 2016, 197, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Misra, N.N.; Kaur, S.; Tiwari, B.K.; Kaur, A.; Singh, N.; Cullen, P.J. Atmospheric pressure cold plasma (acp) treatment of wheat flour. Food Hydrocolloids 2015, 44, 115–121. [Google Scholar] [CrossRef]
- Avena-Bustillos, R.J.; Krochta, J.M. Water vapor permeability of caseinate-based edible films as affected by ph, calcium crosslinking and lipid content. J. Food Sci. 2010, 58, 904–907. [Google Scholar] [CrossRef]
- Hernandezmunoz, P.; Villalobos, R.; Chiralt, A. Effect of cross-linking using aldehydes on properties of glutenin-rich films. Food Hydrocolloids 2004, 18, 403–411. [Google Scholar] [CrossRef]
- Martucci, J.F.; Ruseckaite, R.A. Tensile properties, barrier properties, and biodegradation in soil of compression—molded gelatin-dialdehyde starch films. J. Appl. Polym. Sci. 2010, 112, 2166–2178. [Google Scholar] [CrossRef]
- Pereda, M.; Aranguren, M.I.; Marcovich, N.E. Effect of crosslinking on the properties of sodium caseinate films. J. Appl. Polym. Sci. 2010, 116, 18–26. [Google Scholar] [CrossRef]
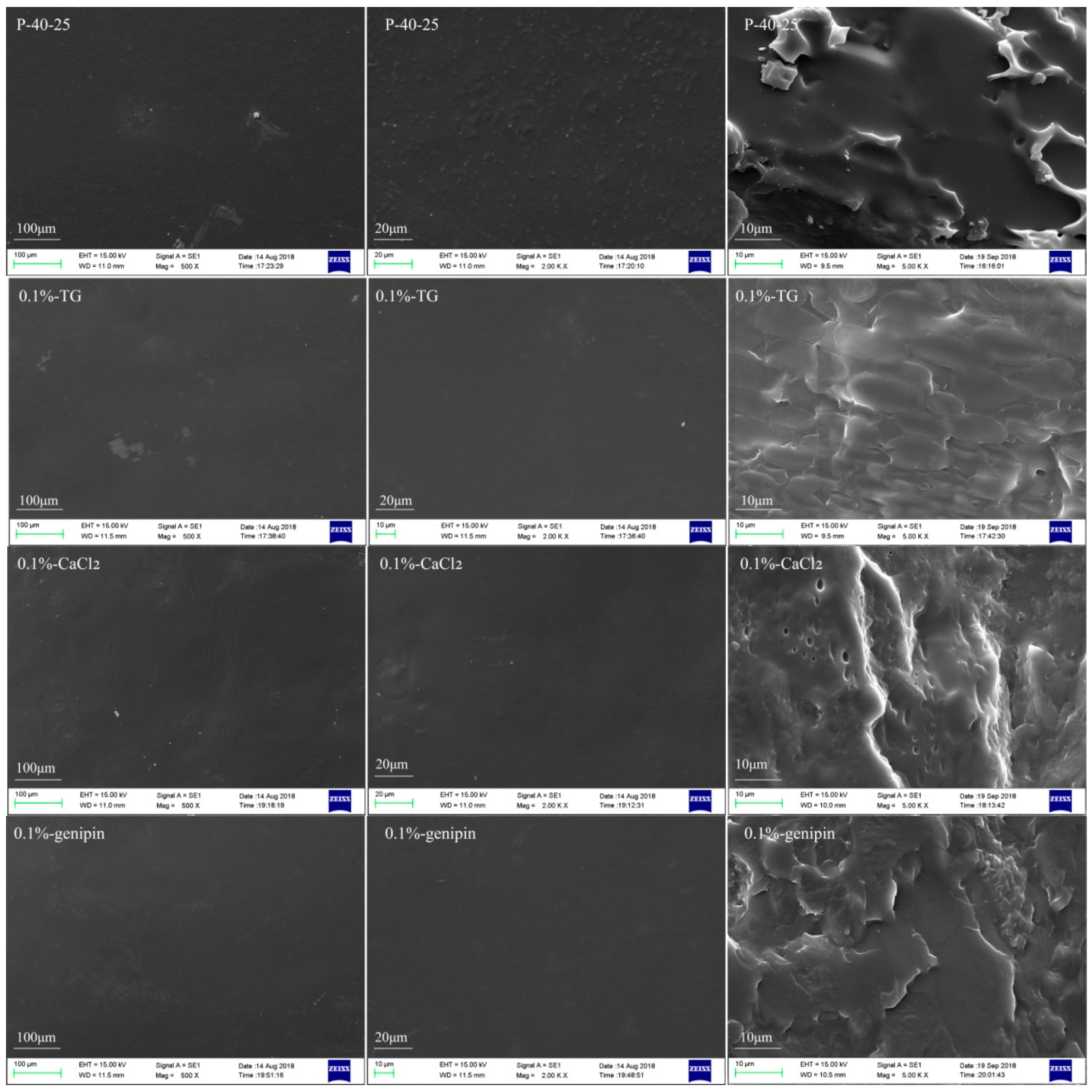
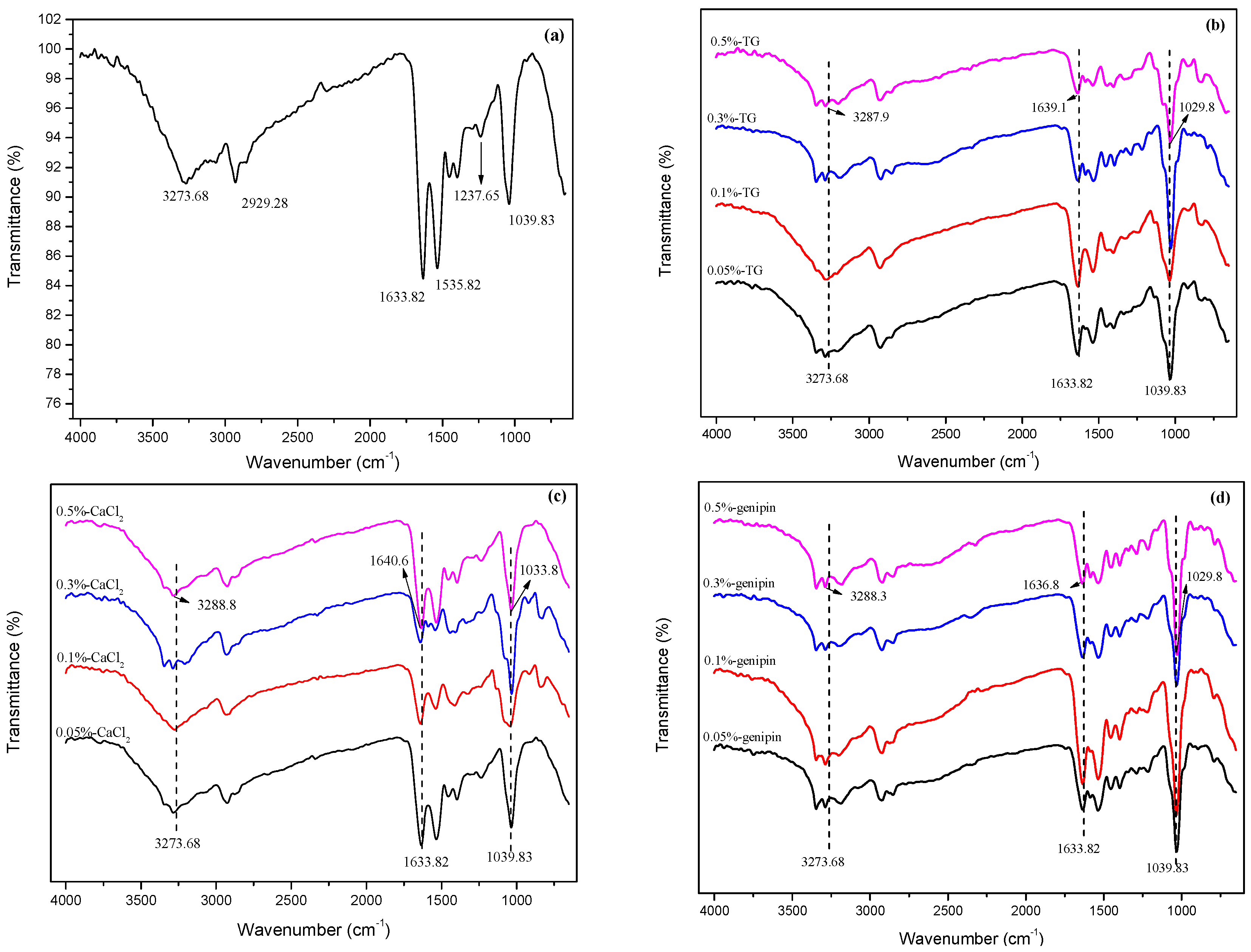
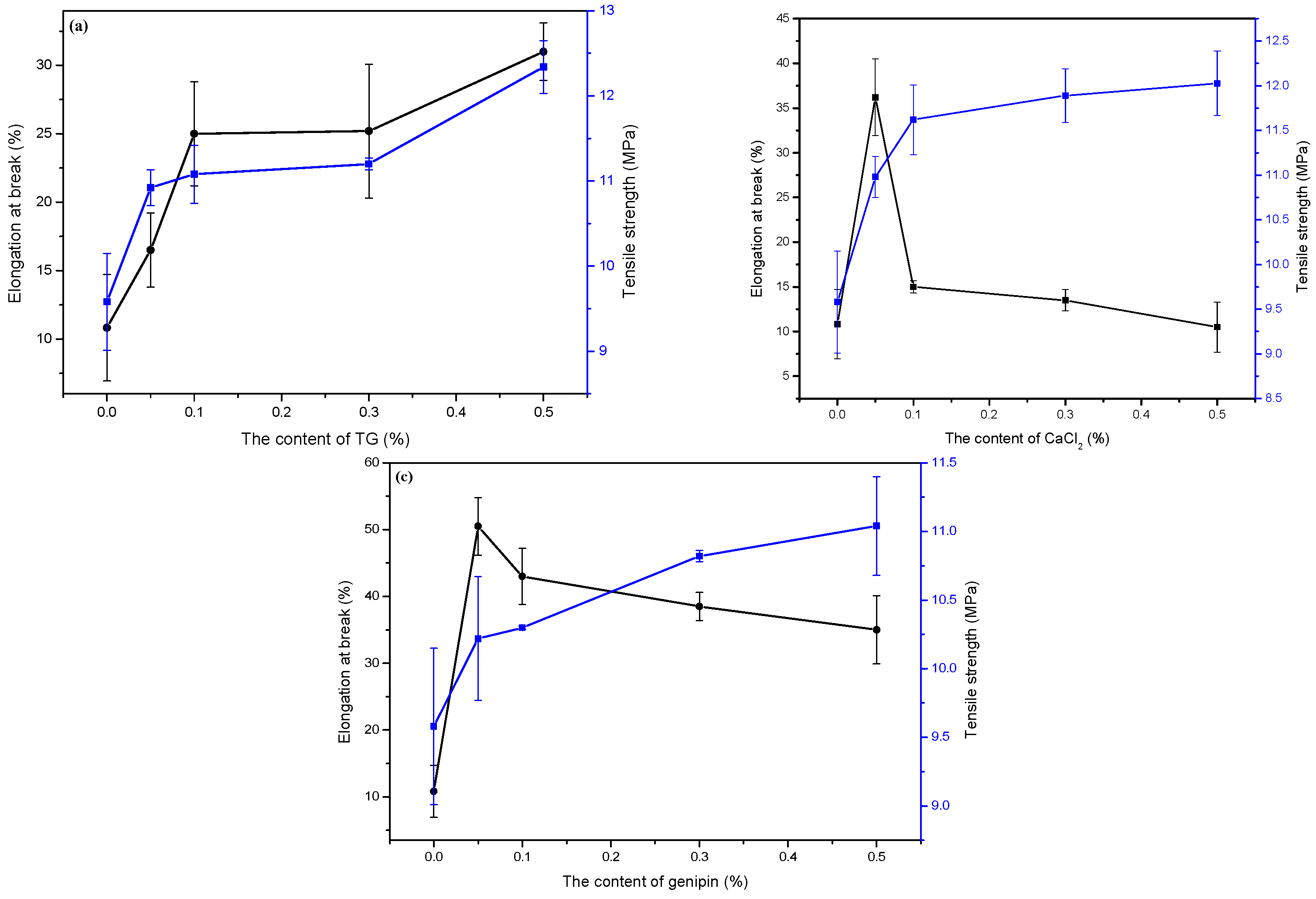
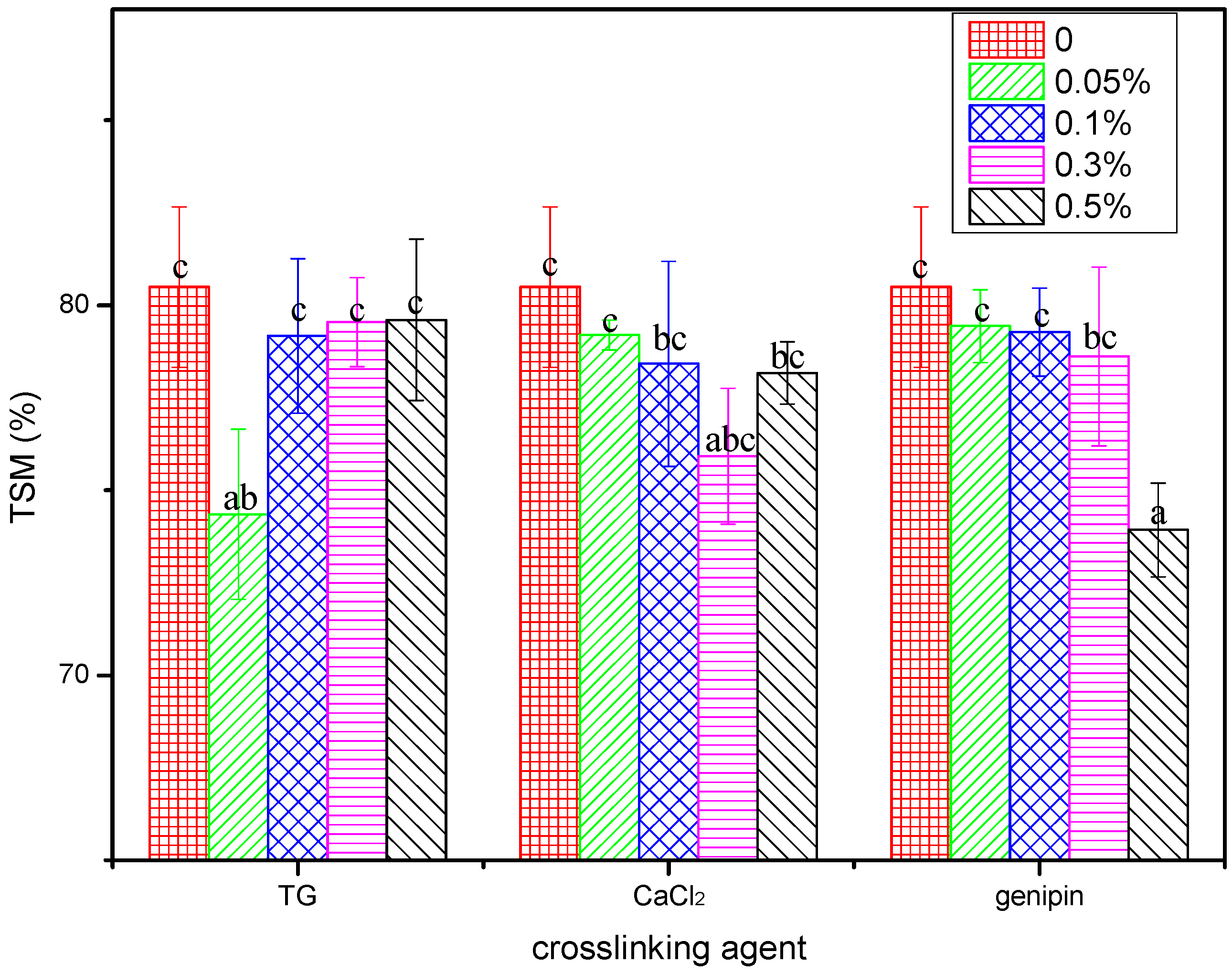
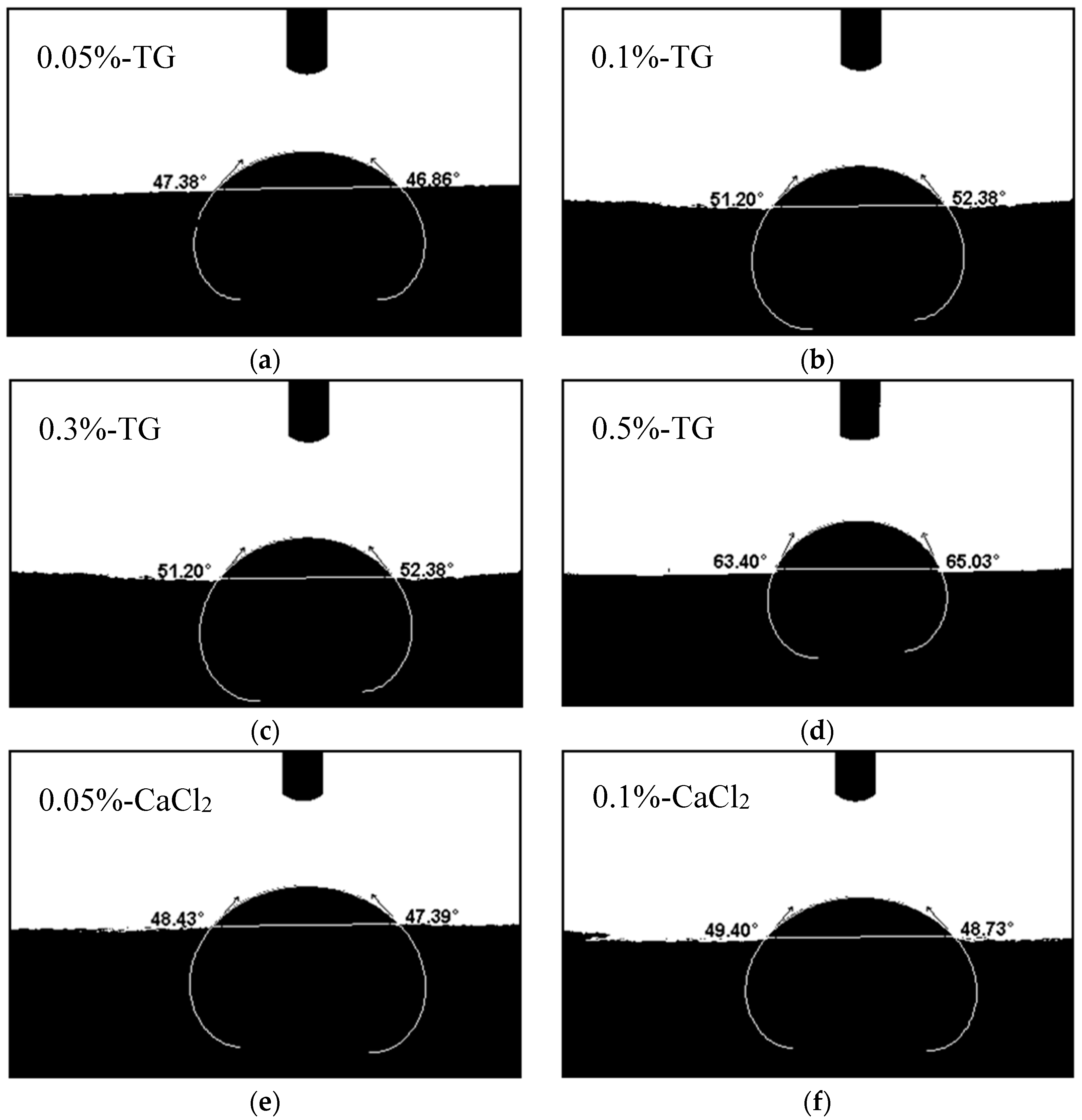
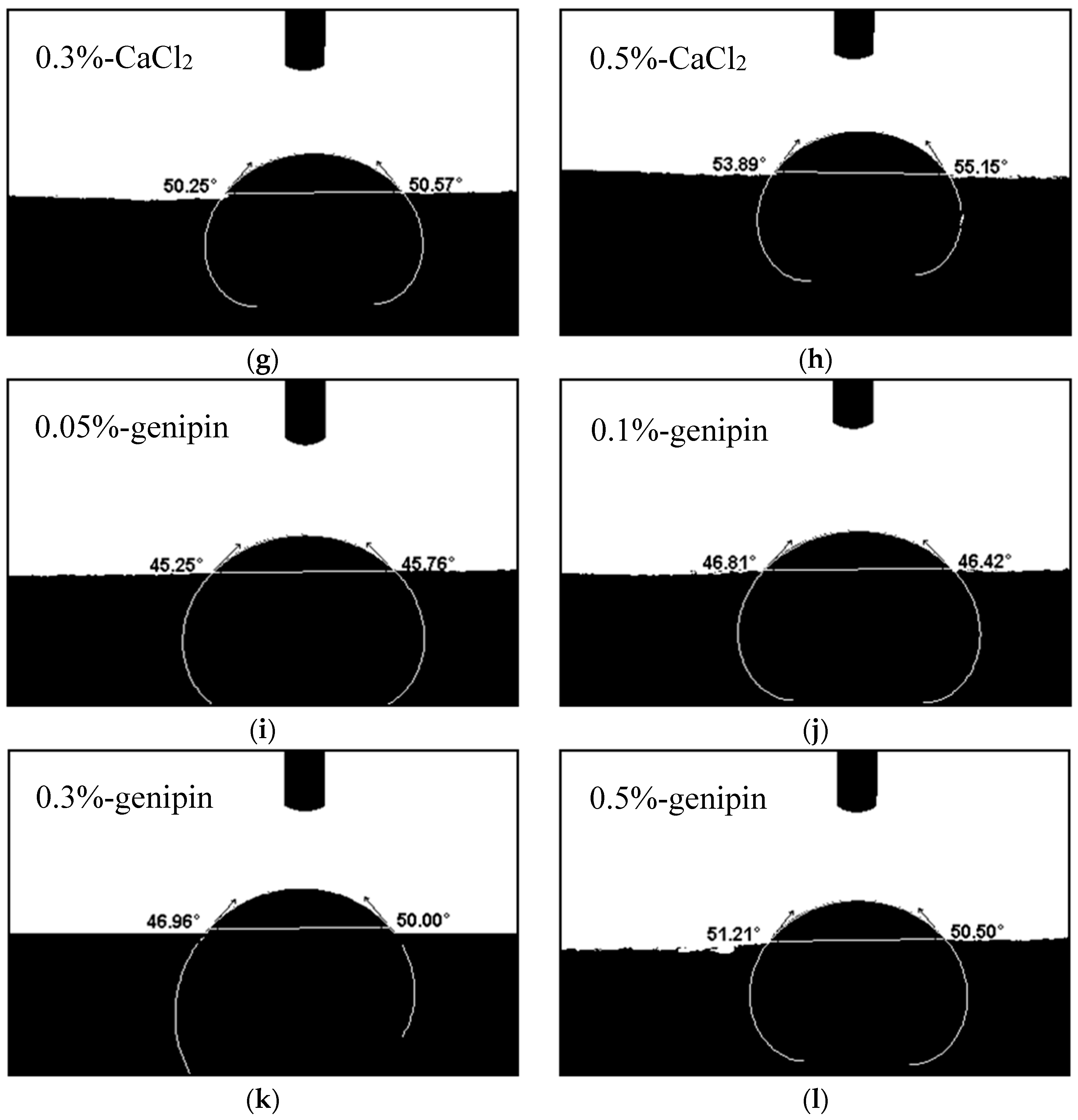
| Sample | FK Powder (g) | 6% PVA (g) | 6% Tris (g) | 0.1% TG (g) | 0.1% CaCl2 (g) | 0.1% Genipin (g) |
|---|---|---|---|---|---|---|
| P-40-25 | 1.2 | 13.33 | 8.33 | 0 | 0 | 0 |
| 0.05%-TG | 1.2 | 13.33 | 8.33 | 1 | 0 | 0 |
| 0.1%-TG | 1.2 | 13.33 | 8.33 | 2 | 0 | 0 |
| 0.3-TG | 1.2 | 13.33 | 8.33 | 6 | 0 | 0 |
| 0.5%-TG | 1.2 | 13.33 | 8.33 | 10 | 0 | 0 |
| 0.05%-CaCl2 | 1.2 | 13.33 | 8.33 | 0 | 1 | 0 |
| 0.1%-CaCl2 | 1.2 | 13.33 | 8.33 | 0 | 2 | 0 |
| 0.3%-CaCl2 | 1.2 | 13.33 | 8.33 | 0 | 6 | 0 |
| 0.5%-CaCl2 | 1.2 | 13.33 | 8.33 | 0 | 10 | 0 |
| 0.05%-genipin | 1.2 | 13.33 | 8.33 | 0 | 0 | 1 |
| 0.1%-genipin | 1.2 | 13.33 | 8.33 | 0 | 0 | 2 |
| 0.3%-genipin | 1.2 | 13.33 | 8.33 | 0 | 0 | 6 |
| 0.5%-genipin | 1.2 | 13.33 | 8.33 | 0 | 0 | 10 |
| Sample | α-Helices (%) | β-Turns (%) | β-Sheets (%) | Random Coils (%) |
|---|---|---|---|---|
| P-40-25 | 14.25 | 23.91 | 39.33 | 20.36 |
| 0.05%-TG | 15.61 | 28.64 | 33.68 | 21.26 |
| 0.1%-TG | 14.85 | 27.60 | 35.27 | 20.74 |
| 0.3%-TG | 16.19 | 28.05 | 33.55 | 22.38 |
| 0.5%-TG | 16.17 | 30.66 | 31.40 | 21.83 |
| 0.05%-CaCl2 | 14.34 | 24.08 | 38.92 | 20.75 |
| 0.1%-CaCl2 | 15.85 | 27.53 | 33.68 | 21.50 |
| 0.3%-CaCl2 | 16.29 | 32.09 | 29.80 | 21.87 |
| 0.5%-CaCl2 | 14.37 | 22.07 | 40.37 | 21.38 |
| 0.05%-genipin | 14.97 | 27.08 | 36.04 | 21.12 |
| 0.1%-genipin | 14.83 | 26.11 | 37.02 | 20.85 |
| 0.3%-genipin | 15.20 | 26.43 | 36.20 | 21.36 |
| 0.5%-genipin | 16.21 | 27.78 | 33.62 | 22.74 |
| Sample | WVP | OP |
|---|---|---|
| (×10−12 g·cm−1·s−1·Pa−1) | (×10−5 cm3·m−2·d−1·Pa−1) | |
| P-40-25 | 3.09 ± 0.1 f | 11.78 ± 0.65 a |
| 0.05%-TG | 1.68 ± 0.08 a | 826.4 ± 2.53 d |
| 0.1%-TG | 2.32 ± 0.09 cd | 820 ± 4.47 c |
| 0.3%-TG | 2.46 ± 0.06 de | 4012 ± 2.82 k |
| 0.5%-TG | 2.58 ± 0.11 e | 4038 ± 4.65 l |
| 0.05%-CaCl2 | 3.06 ± 0.06 f | 1865 ± 3.53 e |
| 0.1%-CaCl2 | 2.59 ± 0.13 e | 3243 ± 1.9 h |
| 0.3%-CaCl2 | 1.64 ± 0.05 a | 3559 ± 2.32 j |
| 0.5%-CaCl2 | 3.06 ± 0.07 f | 33970 ± 5.7 m |
| 0.05%-genipin | 2.33 ± 0.12 cd | 368.3 ± 0.77 b |
| 0.1%-genipin | 2.39 ± 0.1 cd | 2068 ± 0.12 f |
| 0.3%-genipin | 2.24 ± 0.03 c | 2708 ± 1.45 g |
| 0.5%-genipin | 2.02 ± 0.05 b | 3531 ± 3.61 i |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.; Chen, X.; Yi, M.; Ge, J.; Yin, G.; Li, X. Improving the Water Resistance and Mechanical Properties of Feather Keratin/Polyvinyl Alcohol/Tris(Hydroxymethyl)Aminomethane Blend Films by Cross-Linking with Transglutaminase, CaCl2, and Genipin. Materials 2018, 11, 2203. https://doi.org/10.3390/ma11112203
Wu S, Chen X, Yi M, Ge J, Yin G, Li X. Improving the Water Resistance and Mechanical Properties of Feather Keratin/Polyvinyl Alcohol/Tris(Hydroxymethyl)Aminomethane Blend Films by Cross-Linking with Transglutaminase, CaCl2, and Genipin. Materials. 2018; 11(11):2203. https://doi.org/10.3390/ma11112203
Chicago/Turabian StyleWu, Shufang, Xunjun Chen, Minghao Yi, Jianfang Ge, Guoqiang Yin, and Xinming Li. 2018. "Improving the Water Resistance and Mechanical Properties of Feather Keratin/Polyvinyl Alcohol/Tris(Hydroxymethyl)Aminomethane Blend Films by Cross-Linking with Transglutaminase, CaCl2, and Genipin" Materials 11, no. 11: 2203. https://doi.org/10.3390/ma11112203
APA StyleWu, S., Chen, X., Yi, M., Ge, J., Yin, G., & Li, X. (2018). Improving the Water Resistance and Mechanical Properties of Feather Keratin/Polyvinyl Alcohol/Tris(Hydroxymethyl)Aminomethane Blend Films by Cross-Linking with Transglutaminase, CaCl2, and Genipin. Materials, 11(11), 2203. https://doi.org/10.3390/ma11112203





