Physical and Morphological Changes of Poly(tetrafluoroethylene) after Using Non-Thermal Plasma-Treatments
Abstract
1. Introduction
2. Materials and Methods
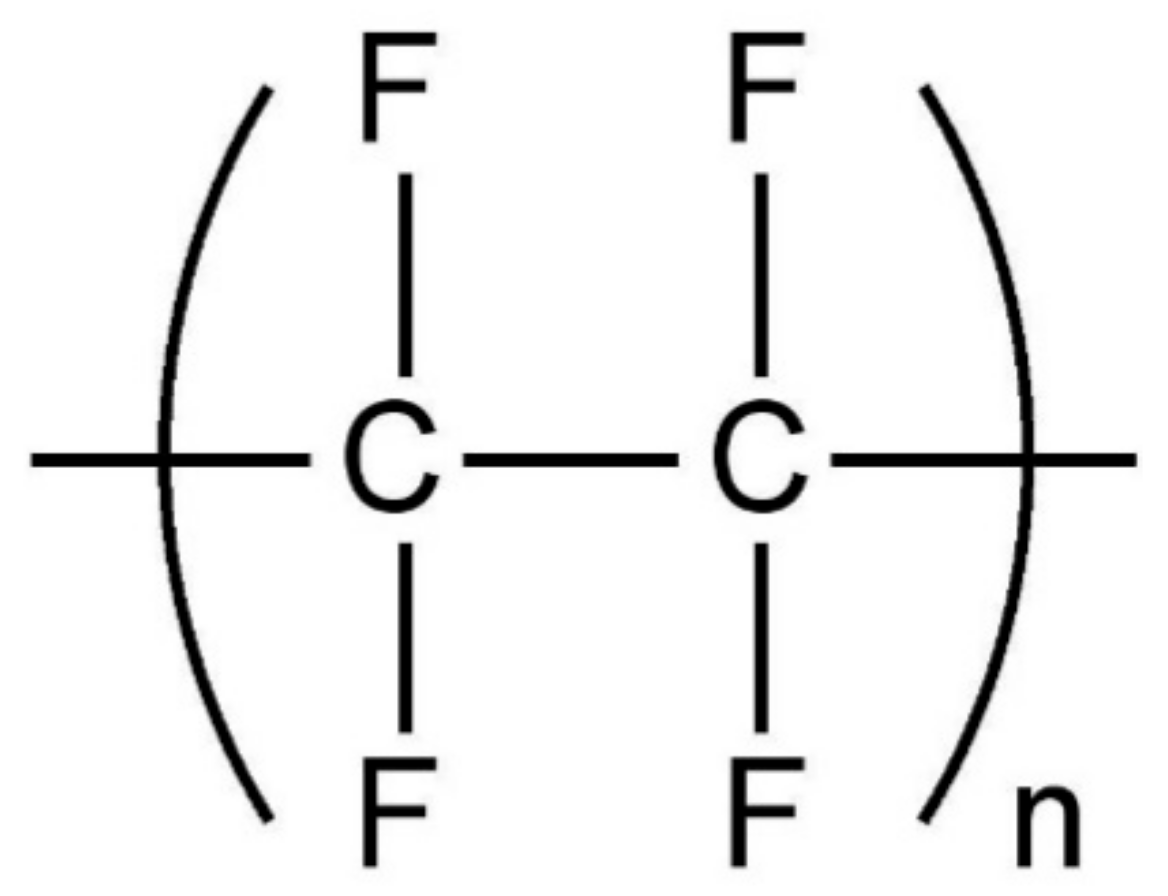
2.1. Materials
2.2. Surface Wettability Assessment
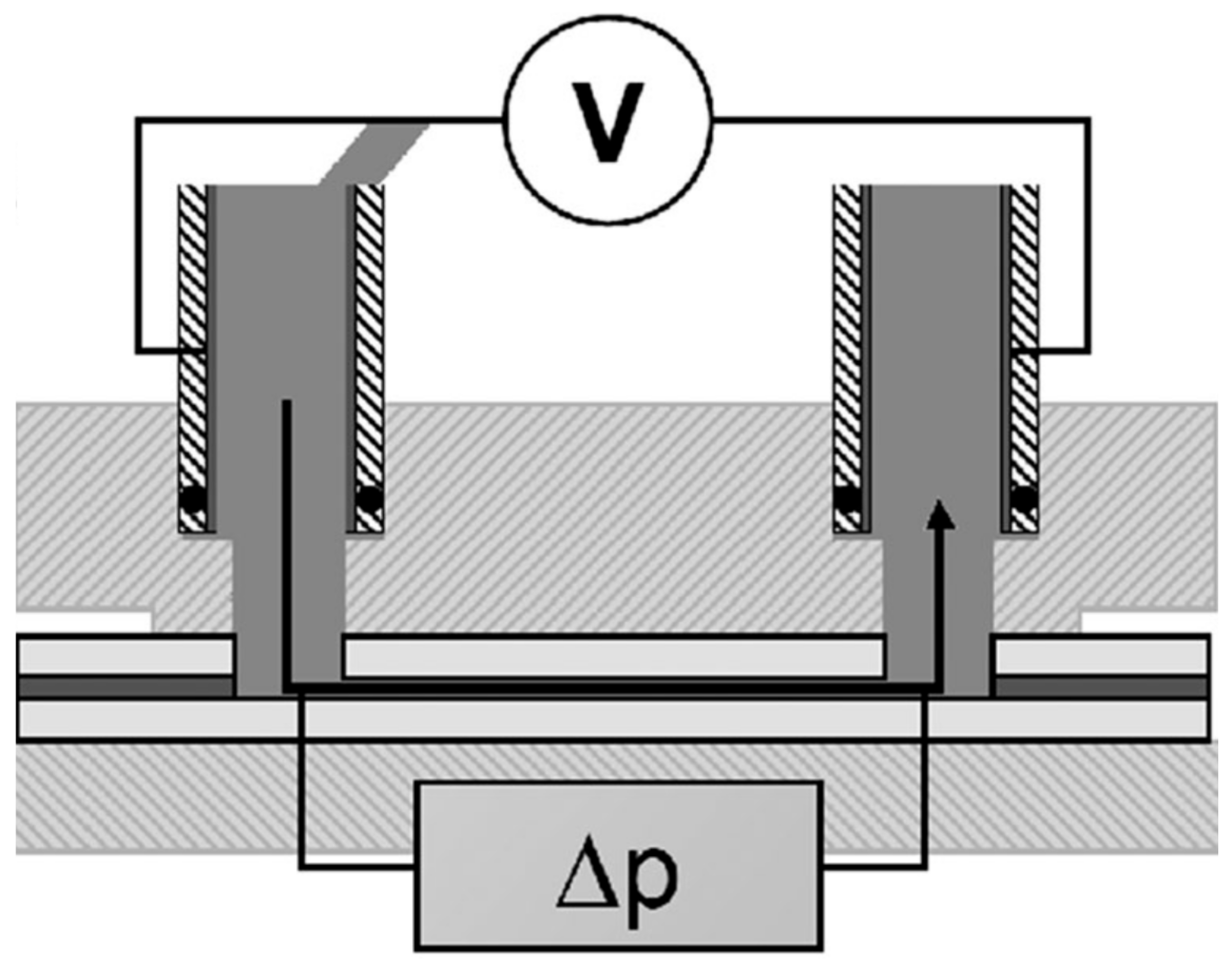
2.3. Electrokinetic Analysis
2.4. Topographical Evaluation
3. Results
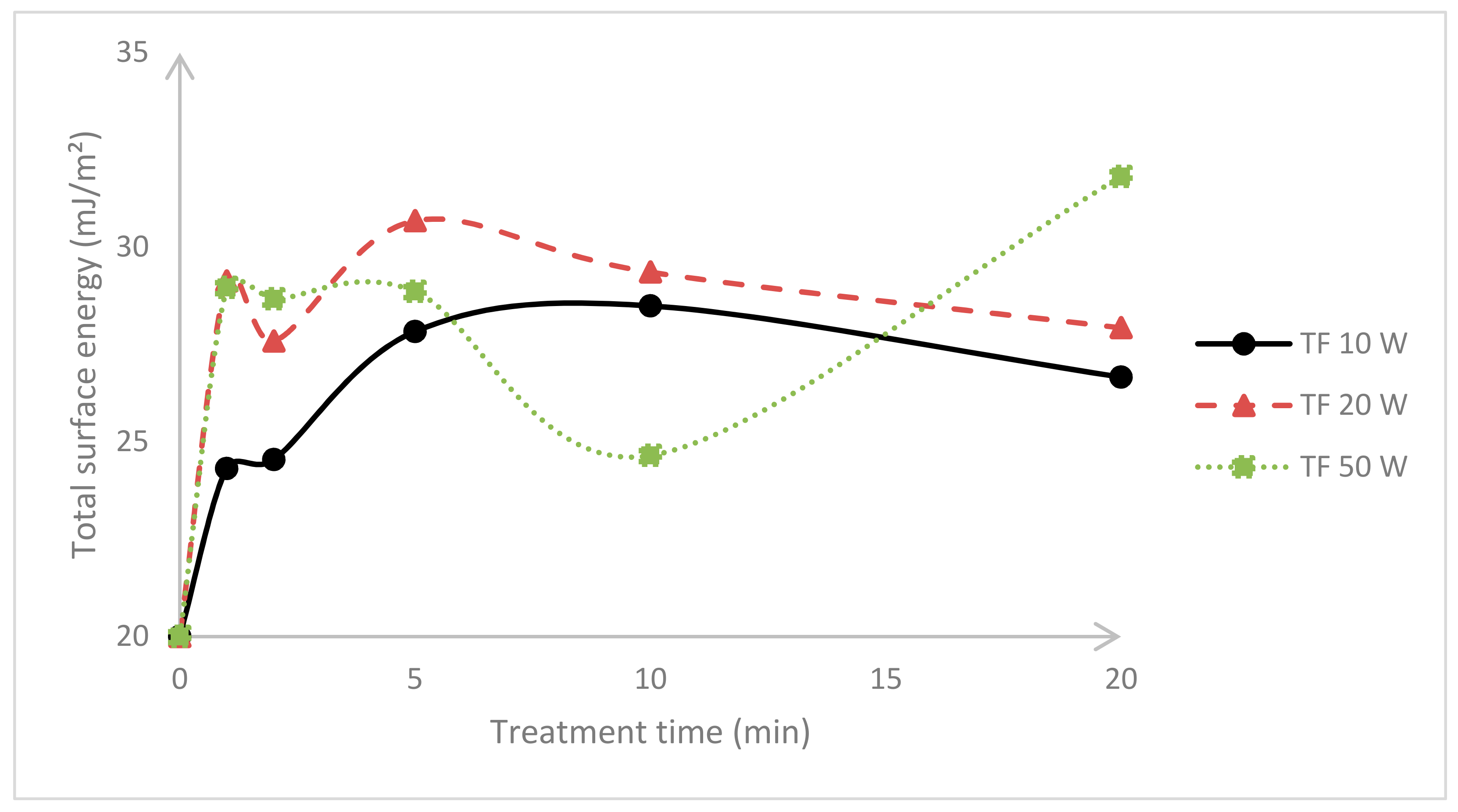
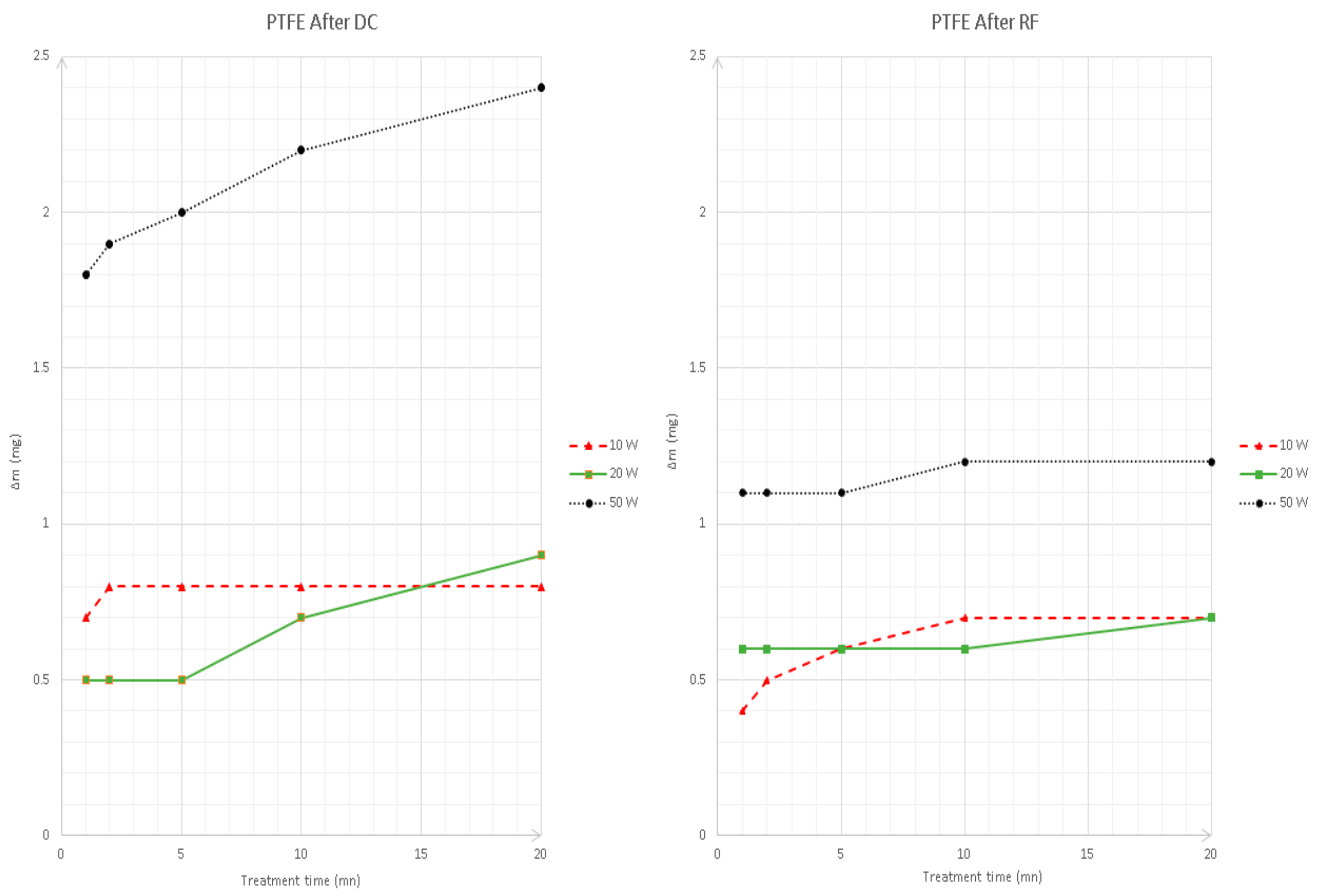
3.1. Surface Energy Evaluation
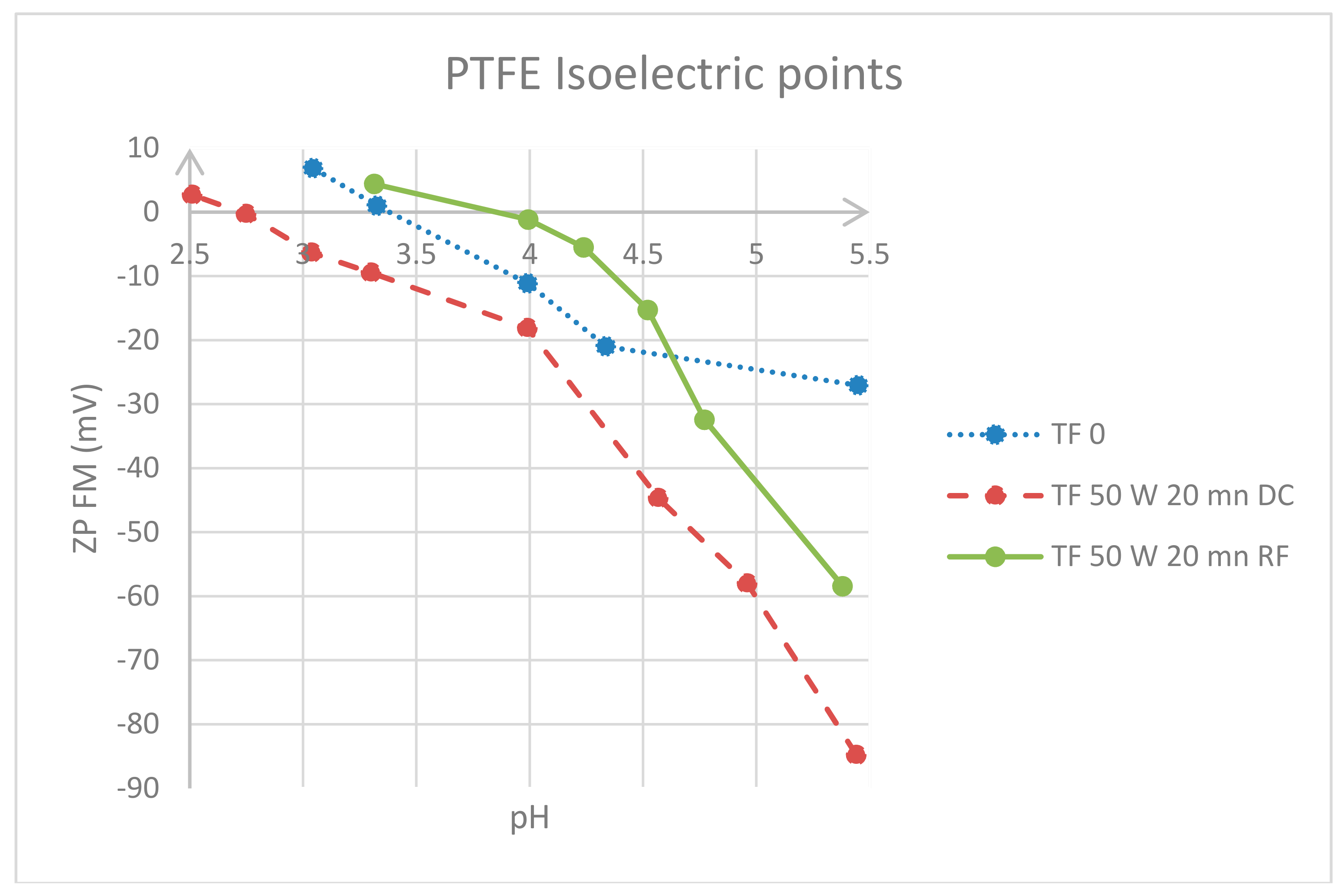
3.2. Surface Charge Appraisal
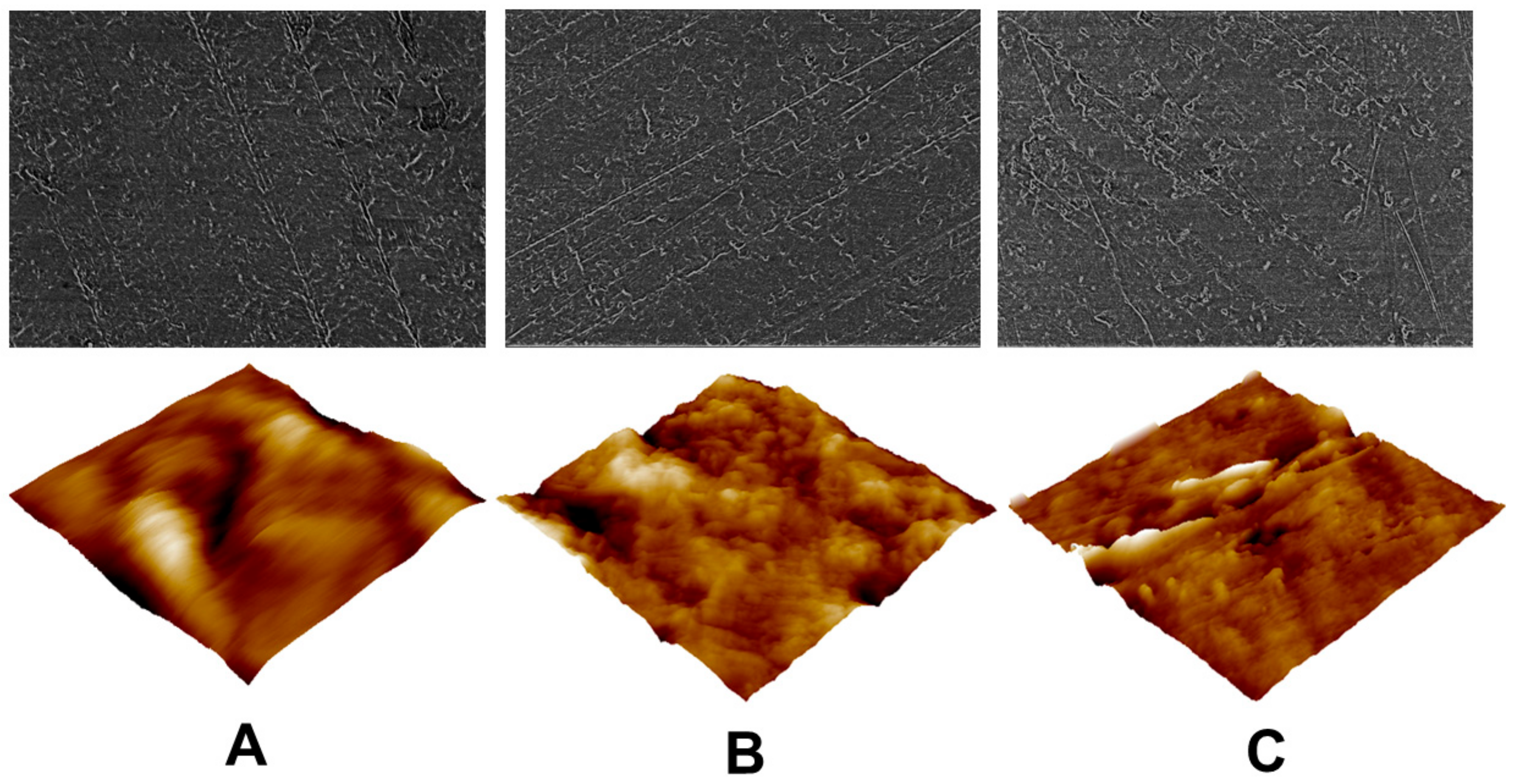
3.3. Topographical Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Desmet, T.; Morent, R.; De Geyter, N.; Leys, C.; Schacht, E.; Dubruel, P. Nonthermal plasma technology as a versatile strategy for polymeric biomaterials surface modification: A review. Biomacromolecules 2009, 10, 2351–2378. [Google Scholar] [CrossRef] [PubMed]
- Morent, R.; De Geyter, N.; Desmet, T.; Dubruel, P.; Leys, C. Plasma Surface Modification of Biodegradable Polymers: A Review. Plasma Process Polym. 2011, 8, 171–190. [Google Scholar] [CrossRef]
- Puliyalil, H.; Recek, N.; Filipic, G.; Cekada, M.; Jerman, I.; Mozetic, M.; Thomas, S.; Cvelbar, U. Mechanisms of hydrophobization of polymeric composites etched in CF4 plasma. Surf. Interface Anal. 2017, 49, 334–339. [Google Scholar] [CrossRef]
- Gotoh, K.; Shohbuke, E.; Ryu, G. Application of atmospheric pressure plasma polymerization for soil guard finishing of textiles. Text. Res. J. 2018, 88, 1278–1289. [Google Scholar] [CrossRef]
- Vesel, A.; Mozetic, M. New developments in surface functionalization of polymers using controlled plasma treatments. J. Phys. D Appl. Phys. 2017, 50, 293001. [Google Scholar] [CrossRef]
- Khedkar, J.; Negulescu, I.; Meletis, E.I. Sliding wear behavior of PTFE composites. Wear 2002, 252, 361–369. [Google Scholar] [CrossRef]
- Pricea, D.M.; Jarratt, M. Thermal conductivity of PTFE and PTFE composites. Thermochim. Acta 2002, 392, 231–236. [Google Scholar] [CrossRef]
- Buerkle, M.; Asai, Y. Thermal conductance of Teflon and Polyethylene: Insight from an atomistic, single-molecule level. Sci. Rep. 2017, 7, 41898. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.; Bae, D. Mechanical properties of poly(tetrafluoroethylene) composites reinforced with graphene nanoplatelets by solid-state processing. Compos. Part 2016, 95, 317–323. [Google Scholar] [CrossRef]
- Morent, R.; De Geyter, N.; Verschuren, J.; De Clerck, K.; Kiekens, P.; Leys, C. Non-thermal plasma treatment of textiles. Surf. Coat. Technol. 2008, 202, 3427–3449. [Google Scholar] [CrossRef]
- Svorcik, V.; Reznickova, A.; Kolska, Z.; Slepicka, P.; Hnatowicz, V. Variable surface properties of PTFE foils. e-Polymers 2010, 133, 1–6. [Google Scholar]
- Preocanin, T.; Selmani, A.; Lindqvist-Reis, P.; Heberling, F.; Kallaya, N.; Lutzenkirchen, J. Surface charge at Teflon/aqueous solution of potassium chloride interfaces. Colloids Surf. A 2012, 412, 120–128. [Google Scholar] [CrossRef]
- Zanini, S.; Barni, R.; Della Pergola, R.; Riccardi, C. Modification of the PTFE wettability by oxygen plasma treatments: Influence of the operating parameters and investigation of the ageing behavior. J. Phys. D Appl. Phys. 2014, 47, 325202. [Google Scholar] [CrossRef]
- Kang, D.H.; Kang, H.W. Surface energy characteristics of zeolite embedded PVDF nanofiber films with electrospinning process. Appl. Surf. Sci. 2016, 387, 82–88. [Google Scholar] [CrossRef]
- Jia, J.; Kang, G.; Zou, T.; Li, M.; Zhou, M.; Cao, Y. Sintering process investigation during polytetrafluoroethylene hollow fibre membrane fabrication by extrusion method. High Perform. Polym. 2017, 29, 1069–1082. [Google Scholar] [CrossRef]
- Jurczuk, K.; Galeski, A.; Morawiec, J. Effect of poly(tetrafluoroethylene) nanofibers on foaming behavior of linear and branched polypropylenes. Eur. Polym. J. 2017, 88, 171–182. [Google Scholar] [CrossRef]
- Kang, D.H.; Kang, H.W. Advanced electrospinning using circle electrodes for freestanding PVDF nanofiber film fabrication. Appl. Surf. Sci. 2018, 455, 251–257. [Google Scholar] [CrossRef]
- Lopez-Garcia, J. Wettability analysis and water absorption studies of plasma active polymeric materials. In Non-Thermal Plasma Technology for Polymeric Materials: Applications in Composites, Nanostructured Materials and Bio-Medical Fields, 1st ed.; Thomas, S., Mozetic, M., Cvelbar, U., Spatenka, P., Praveen, K.M., Eds.; Elsevier: Amsterdam, The Netherlands, 11 October 2018; Chapter 9; ISBN 978-018131527. [Google Scholar]
- Bursikova, V.; Stahel, P.; Navratil, Z.; Bursik, J.; Janca, J. Surface Energy Evaluation of Plasma Treated Materials by Contact Angle Measurement, 1st ed.; Masaryk University: Brno, Czech Republic, 1 January 2004; pp. 1–70. ISBN 80-210-3563-3. [Google Scholar]
- Garcia, J.L.; Asadinezhad, A.; Pachernik, J.; Lehocky, M.; Junkar, I.; Humpolicek, P.; Saha, P.; Valasek, P. Cell proliferation of HaCaT keratinocytes on collagen films modified by argon plasma treatment. Molecules 2010, 15, 2845–2856. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.L.; Pachernik, J.; Lehocky, M.; Junkar, I.; Humpolicek, P.; Saha, P. Enhanced keratinocyte cell attachment to atelocollagen thin films through air and nitrogen plasma treatment. Prog. Colloid Polym. Sci. 2011, 138, 89–94. [Google Scholar] [CrossRef]
- Recek, N.; Resnik, M.; Motaln, H.; Lah-Turnsek, T.; Augustine, R.; Kalarikkal, N.; Thomas, S.; Mozetic, M. Cell Adhesion on Polycaprolactone Modified by Plasma Treatment. Int. J. Polym. Sci. 2016, 1, 1–9. [Google Scholar] [CrossRef]
- Vesel, A. Modification of polystyrene with a highly reactive cold oxygen plasma. Surf. Coat. Technol. 2010, 205, 490–497. [Google Scholar] [CrossRef]
- Vesel, A.; Mozetic, M. Surface modification and ageing of PMMA polymer by oxygen plasma treatment. Vacuum 2012, 86, 634–637. [Google Scholar] [CrossRef]
- Ozaltin, K.; Lehocky, M.; Humpolicek, P.; Pelkova, J.; Saha, P. A new route of fucoidan immobilization on low density polyethylene and its blood compatibility and anticoagulation activity. Int. J. Mol. Sci. 2016, 17, 908. [Google Scholar] [CrossRef] [PubMed]
- Asadinezhad, A.; Lehocky, M.; Saha, P.; Mozetic, M. Recent Progress in Surface Modification of Polyvinyl Chloride. Materials 2012, 5, 2937–2959. [Google Scholar] [CrossRef]
- Vesel, A.; Zaplotnik, R.; Modic, M.; Mozetic, M. Hemocompatibility properties of a polymer surface treated in plasma containing sulfur. Surf. Interface Anal. 2016, 48, 601–605. [Google Scholar] [CrossRef]
- Stoleru, E.; Zaharescu, T.; Hitruc, E.G.; Vesel, A.; Ioanid, E.G.; Coroaba, A.; Safrany, A.; Pricope, G.; Lungu, M.; Schick, C.; et al. Lactoferrin-immobilized surfaces onto functionalized PLA assisted by the gamma-rays and nitrogen plasma to create materials with multifunctional properties. ACS Appl. Mater. Interfaces 2016, 46, 31902–31915. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Garcia, J.; Primc, G.; Junkar, I.; Lehocky, M.; Mozetic, M. On the hydrophilicity and water resistance effect of styrene-acrylonitrile copolymer treated by CF4 and O2 plasmas. Plasma Process. Polym. 2015, 12, 1075–1084. [Google Scholar] [CrossRef]
- Lopez-Garcia, J.; Lehocky, M.; Humpolicek, P.; Novak, I. On the correlation of surface charge and energy in non-thermal plasma-treated polyethylene. Surf. Interface Anal. 2014, 46, 625–629. [Google Scholar] [CrossRef]
- Kolska, Z.; Makajova, Z.; Kolarova, K.; Kasalkova, N.; Trostova, S.; Reznickova, A.; Siegel, J.; Svorcik, V. Electrokinetic Potential and Other Surface Properties of Polymer Foils and Their Modifications, 1st ed.; Yilmaz, F., Ed.; IntechOpen: London, UK, 23 January 2013; Chapter 8. [Google Scholar] [CrossRef]
- Lopez-Garcia, J.; Bilek, F.; Lehocky, M.; Junkar, I.; Mozetic, M.; Sowe, M. Enhanced printability of polyethylene through air plasma treatment. Vacuum 2013, 95, 43–49. [Google Scholar] [CrossRef]
- Mozetic, M.; Ostrikov, K.; Ruzic, D.N.; Curreli, D.; Cvelbar, U.; Vesel, A.; Primc, G.; Leisch, M.; Jousten, K.; Malyshev, O.B.; et al. Recent advances in vacuum sciences and applications. J. Phys. D Appl. Phys. 2014, 47, 153001. [Google Scholar] [CrossRef]

| Sample | Contact Angle (°) After DC | Contact Angle (°) After RF | |||||
|---|---|---|---|---|---|---|---|
| Plasma Duration (min) | Power Input (W) | θw | θe | θd | θw | θe | θd |
| 0 | 0 | 108.9 | 90.7 | 75.2 | 108.9 | 90.7 | 75.2 |
| 1 | 10 | 85.0 | 69.7 | 68.2 | 83.1 | 70.4 | 57.4 |
| 1 | 20 | 81.9 | 68.3 | 62.6 | 88.1 | 74.2 | 70.3 |
| 1 | 50 | 77.6 | 62.6 | 60.6 | 75.2 | 69.0 | 51.8 |
| 2 | 10 | 82.0 | 70.4 | 68.9 | 83.1 | 76.1 | 63.5 |
| 2 | 20 | 78.7 | 69.7 | 67.2 | 81.7 | 74.6 | 62.6 |
| 2 | 50 | 81.4 | 66.8 | 62.1 | 82.5 | 67.3 | 65.1 |
| 5 | 10 | 74.2 | 66.8 | 66.9 | 86.9 | 78.6 | 65.7 |
| 5 | 20 | 81.9 | 69.1 | 61.8 | 83.0 | 62.7 | 66.4 |
| 5 | 50 | 80.0 | 63.1 | 59.8 | 78.7 | 68.1 | 63.7 |
| 10 | 10 | 81.5 | 61.4 | 66.8 | 87.6 | 78.4 | 61.9 |
| 10 | 20 | 81.4 | 71.8 | 65.8 | 81.0 | 67.3 | 67.7 |
| 10 | 50 | 88.6 | 70.1 | 70.0 | 75.7 | 67.2 | 63.3 |
| 20 | 10 | 80.9 | 63.4 | 70.5 | 83.6 | 73.6 | 62.2 |
| 20 | 20 | 77.6 | 69.3 | 67.1 | 78.8 | 67.1 | 63.3 |
| 20 | 50 | 78.5 | 73.5 | 67.2 | 81.8 | 59.8 | 59.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-García, J.; Cupessala, F.; Humpolíček, P.; Lehocký, M. Physical and Morphological Changes of Poly(tetrafluoroethylene) after Using Non-Thermal Plasma-Treatments. Materials 2018, 11, 2013. https://doi.org/10.3390/ma11102013
López-García J, Cupessala F, Humpolíček P, Lehocký M. Physical and Morphological Changes of Poly(tetrafluoroethylene) after Using Non-Thermal Plasma-Treatments. Materials. 2018; 11(10):2013. https://doi.org/10.3390/ma11102013
Chicago/Turabian StyleLópez-García, Jorge, Florence Cupessala, Petr Humpolíček, and Marian Lehocký. 2018. "Physical and Morphological Changes of Poly(tetrafluoroethylene) after Using Non-Thermal Plasma-Treatments" Materials 11, no. 10: 2013. https://doi.org/10.3390/ma11102013
APA StyleLópez-García, J., Cupessala, F., Humpolíček, P., & Lehocký, M. (2018). Physical and Morphological Changes of Poly(tetrafluoroethylene) after Using Non-Thermal Plasma-Treatments. Materials, 11(10), 2013. https://doi.org/10.3390/ma11102013







