Poly(Lactic Acid)-Based Nanobiocomposites with Modulated Degradation Rates
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis of Layered Double Hydroxide
2.2.2. Samples Production
2.2.3. Hydrolysis Tests
2.2.4. Gel Permeation Chromatography (GPC Analysis)
2.2.5. Differential Scanning Calorimetry (DSC Analysis)
2.2.6. Rheological Tests (Hydrolysis at High Temperature Analysis)
2.2.7. Mechanical Tests
3. Results and Discussion
3.1. Benchmark LDH Selection
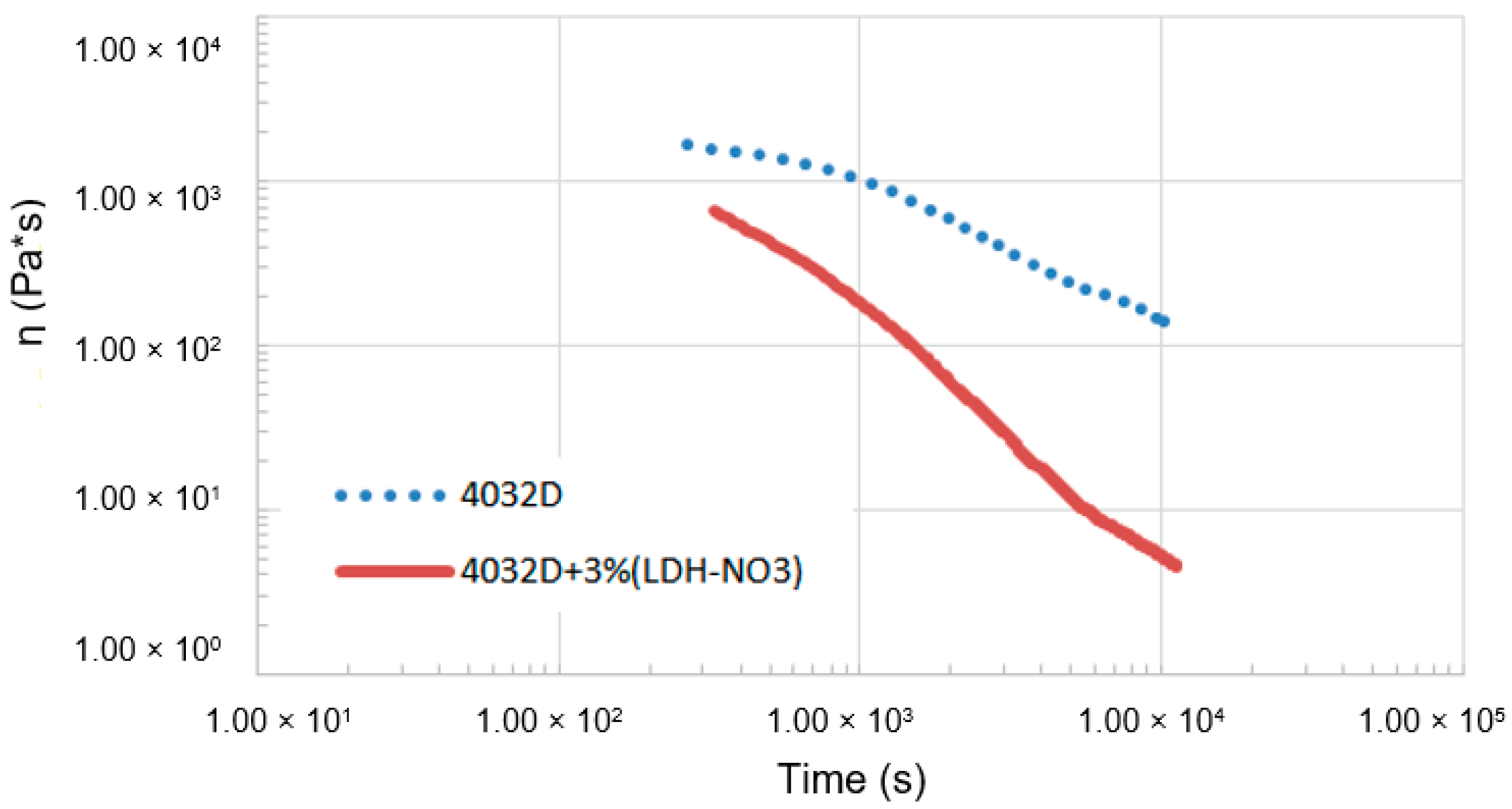
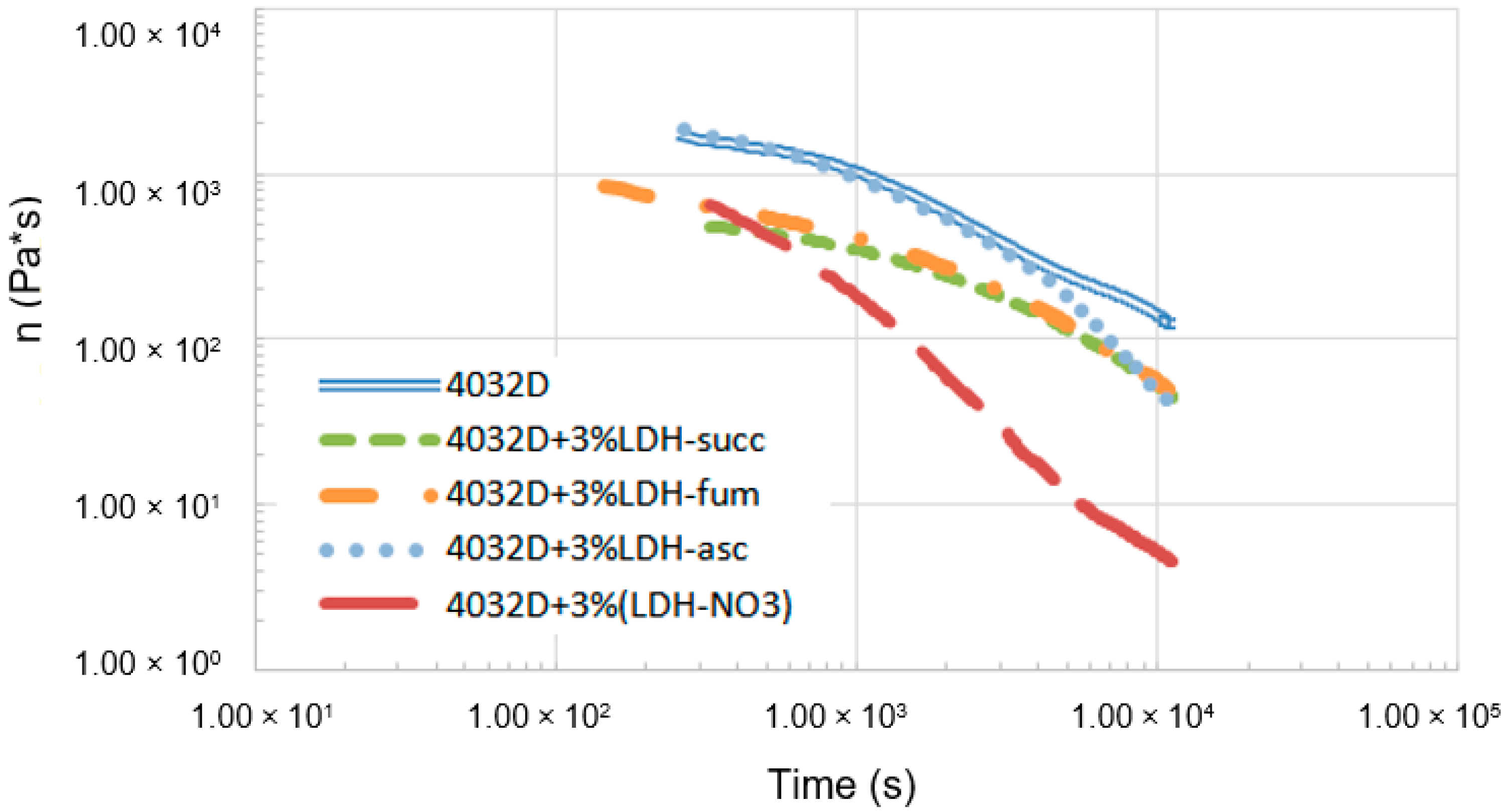
3.1.1. Hydrolysis at High Temperature (Rheological Analysis)
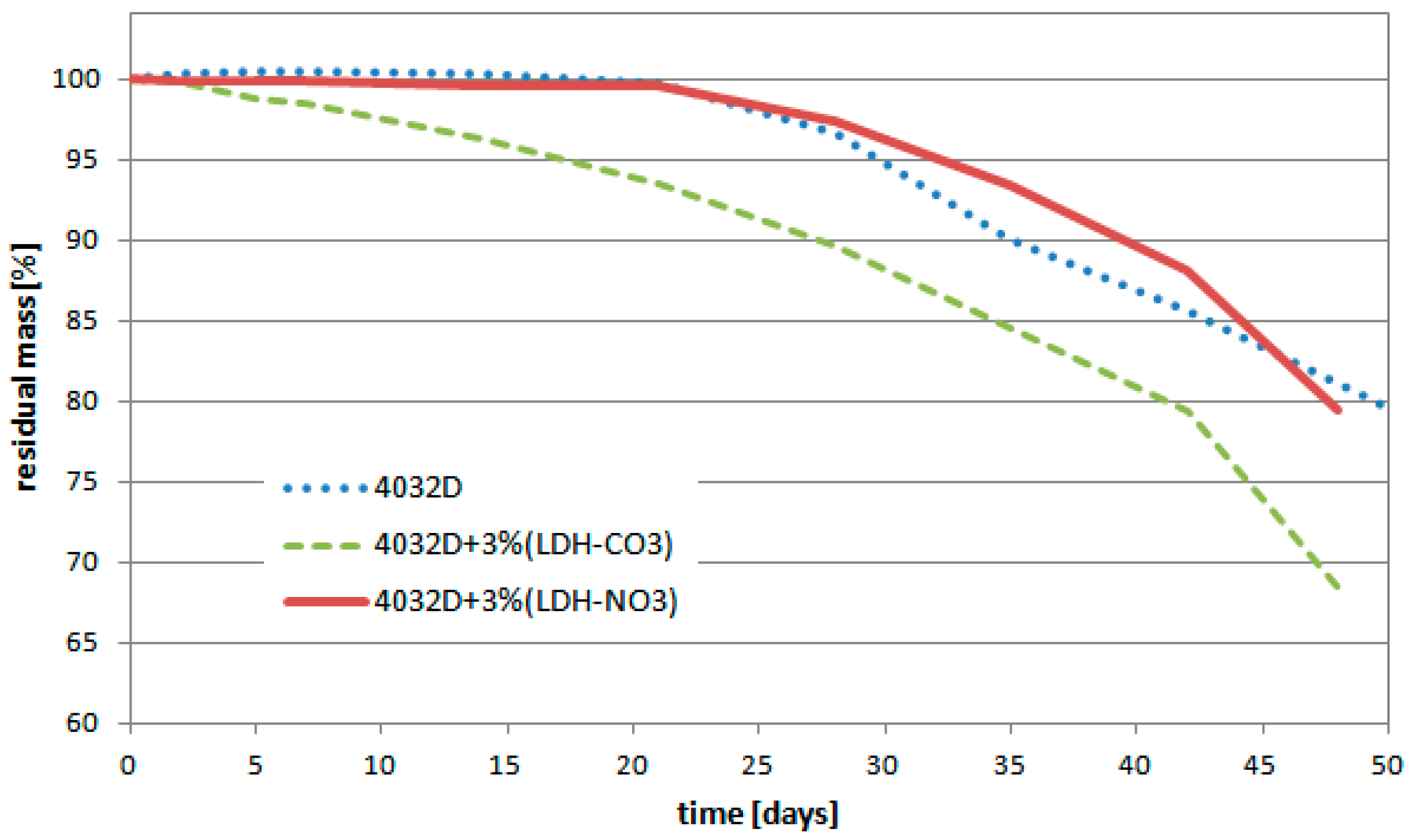
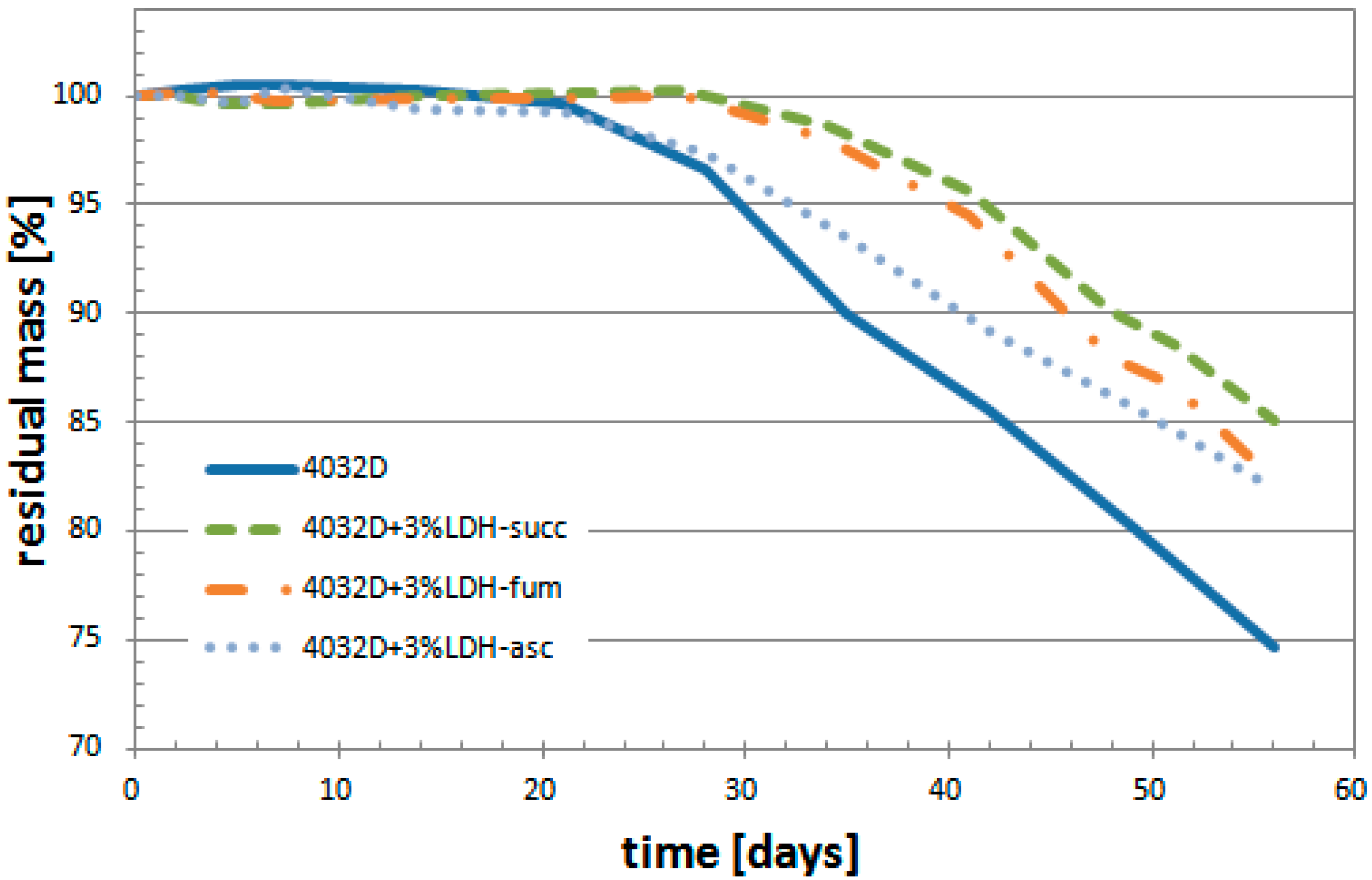
3.1.2. Weight Loss
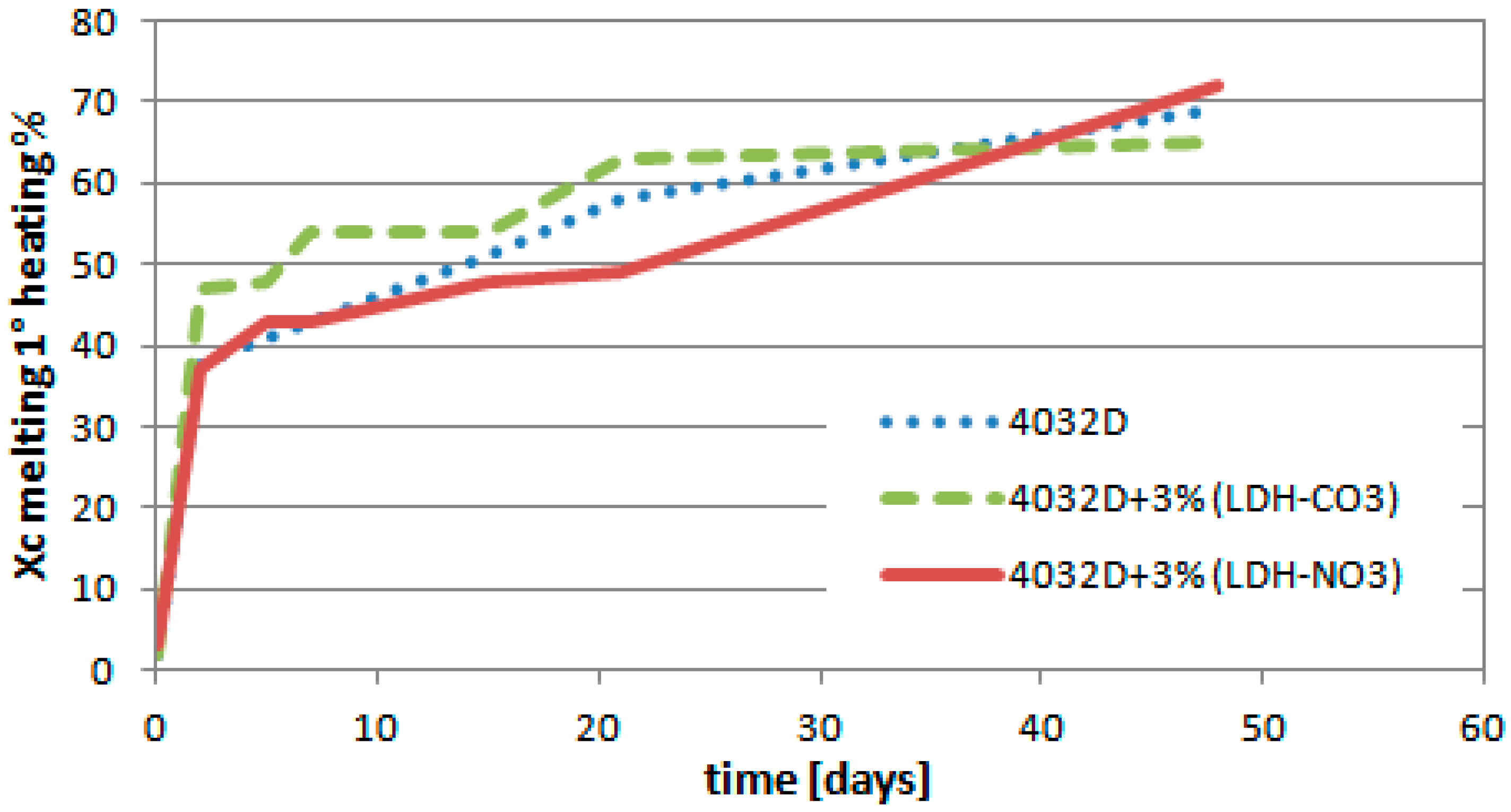
3.1.3. Calorimetric Analysis (DSC)
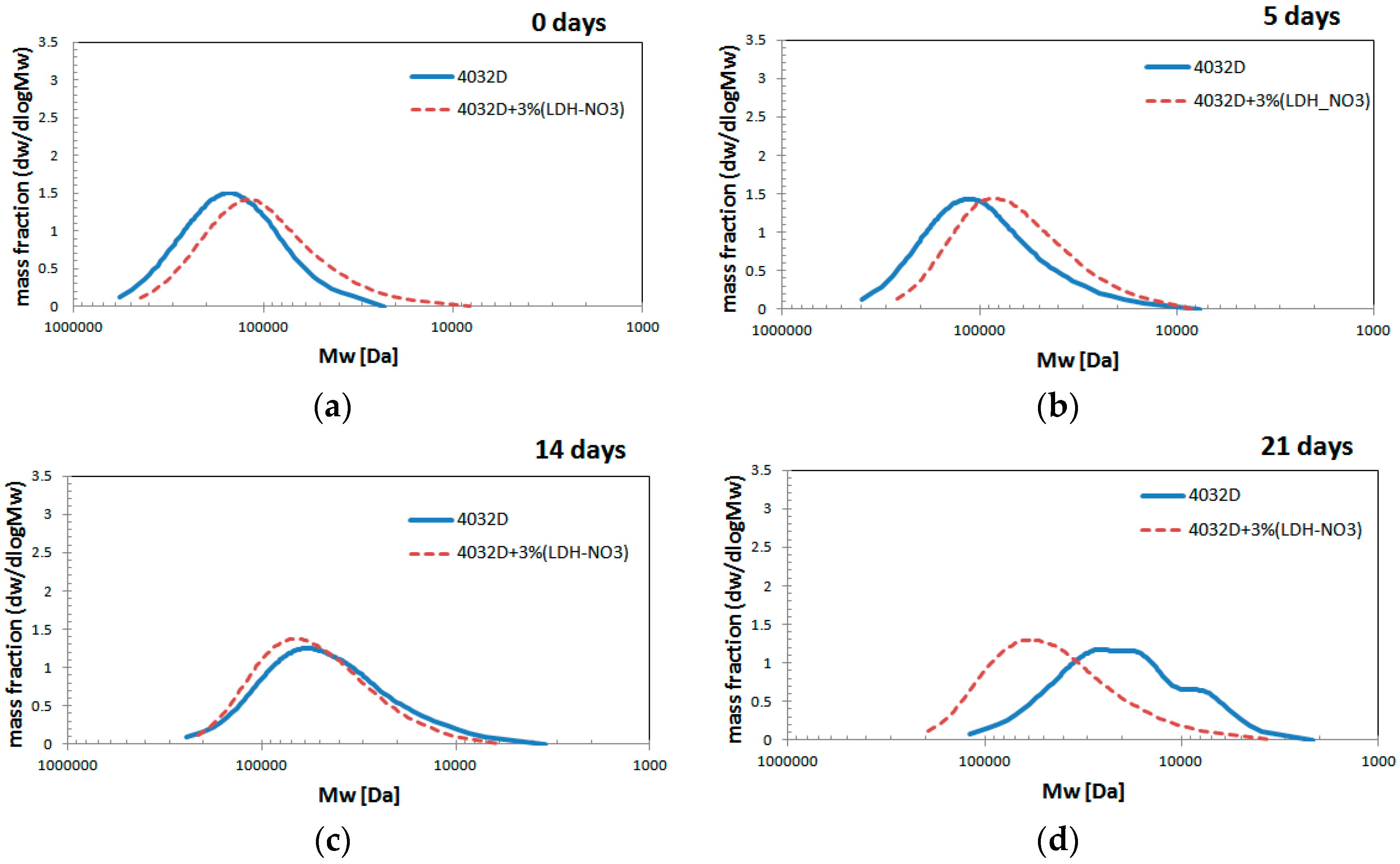
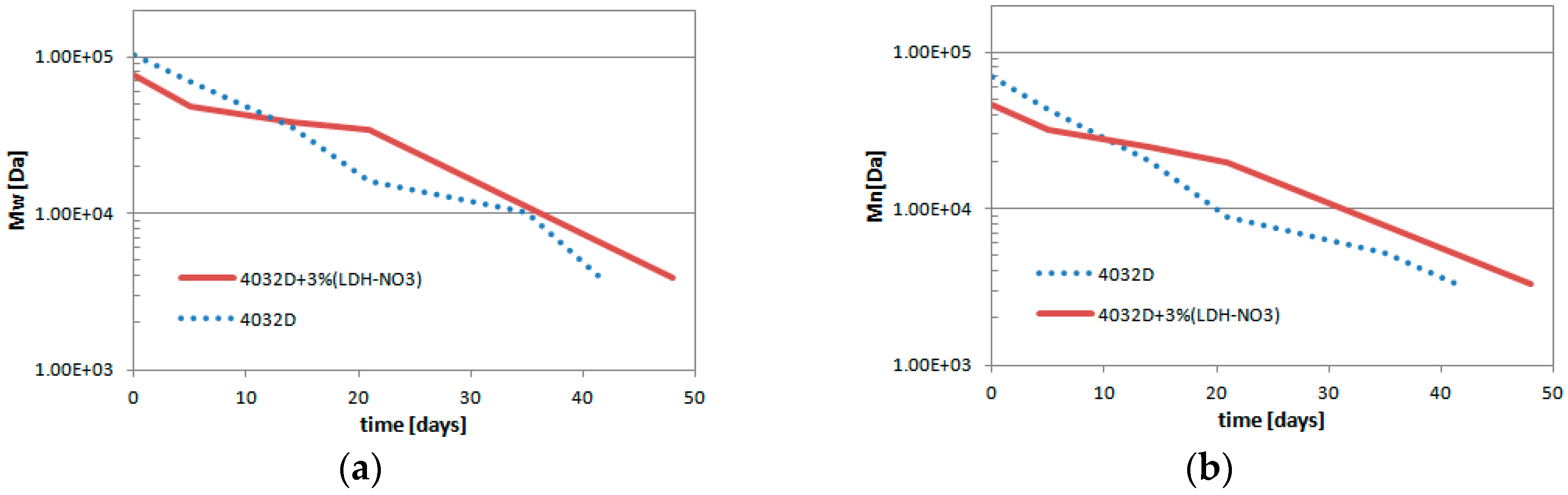
3.1.4. Gel Permeation Chromatography (GPC Analysis)
3.2. Experimental Results: LDH-Organic Acid Selection
3.2.1. Hydrolysis at High Temperature (Rheological Analysis)
3.2.2. Weight Loss
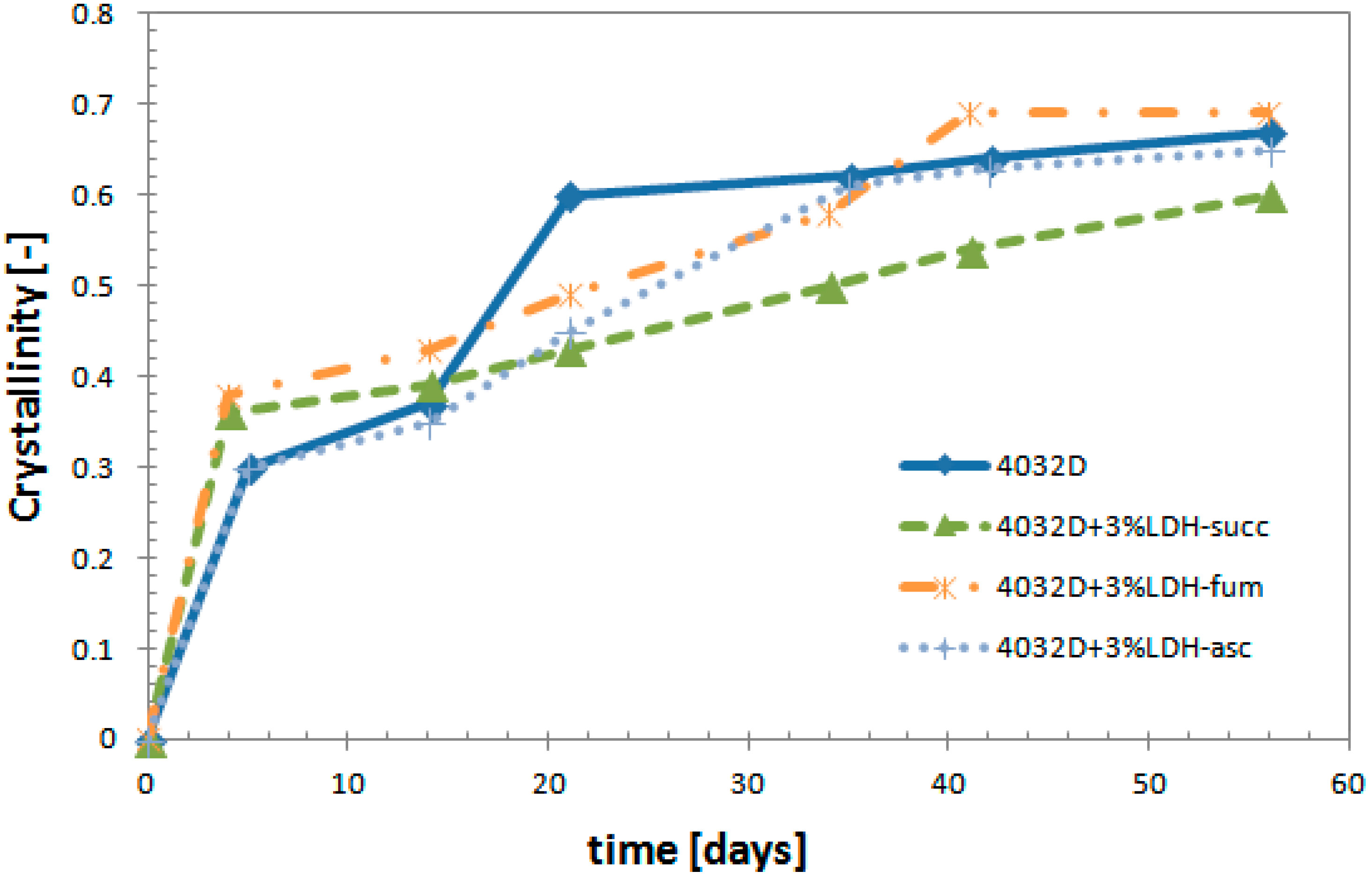
3.2.3. Calorimetric Analysis (DSC)
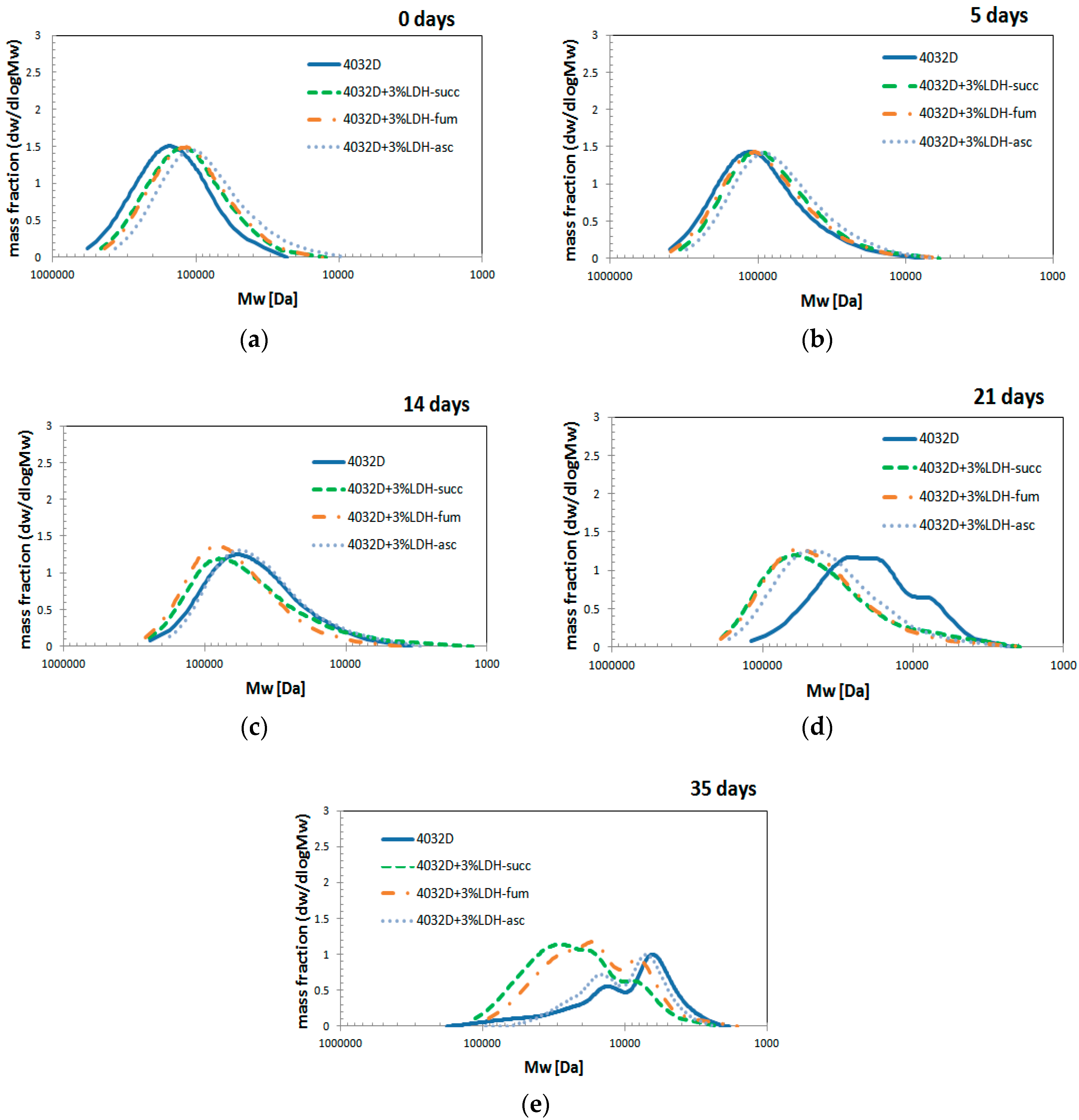
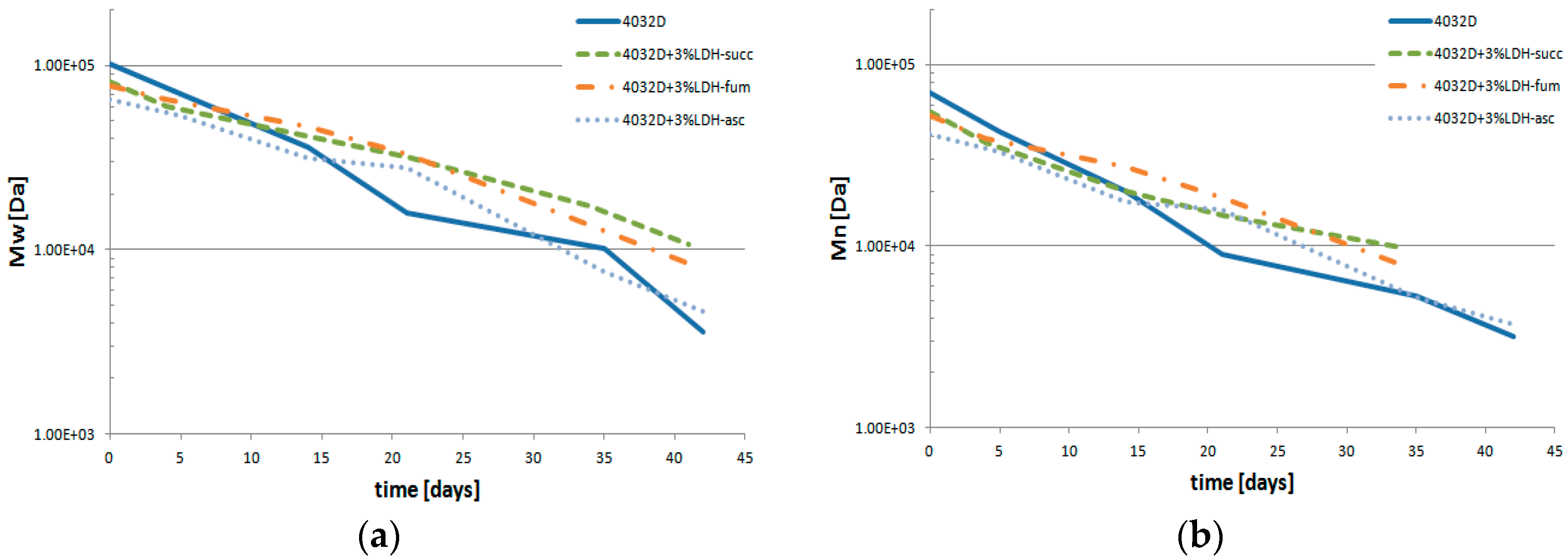
3.2.4. Gel Permeation Chromatography (GPC Analysis)
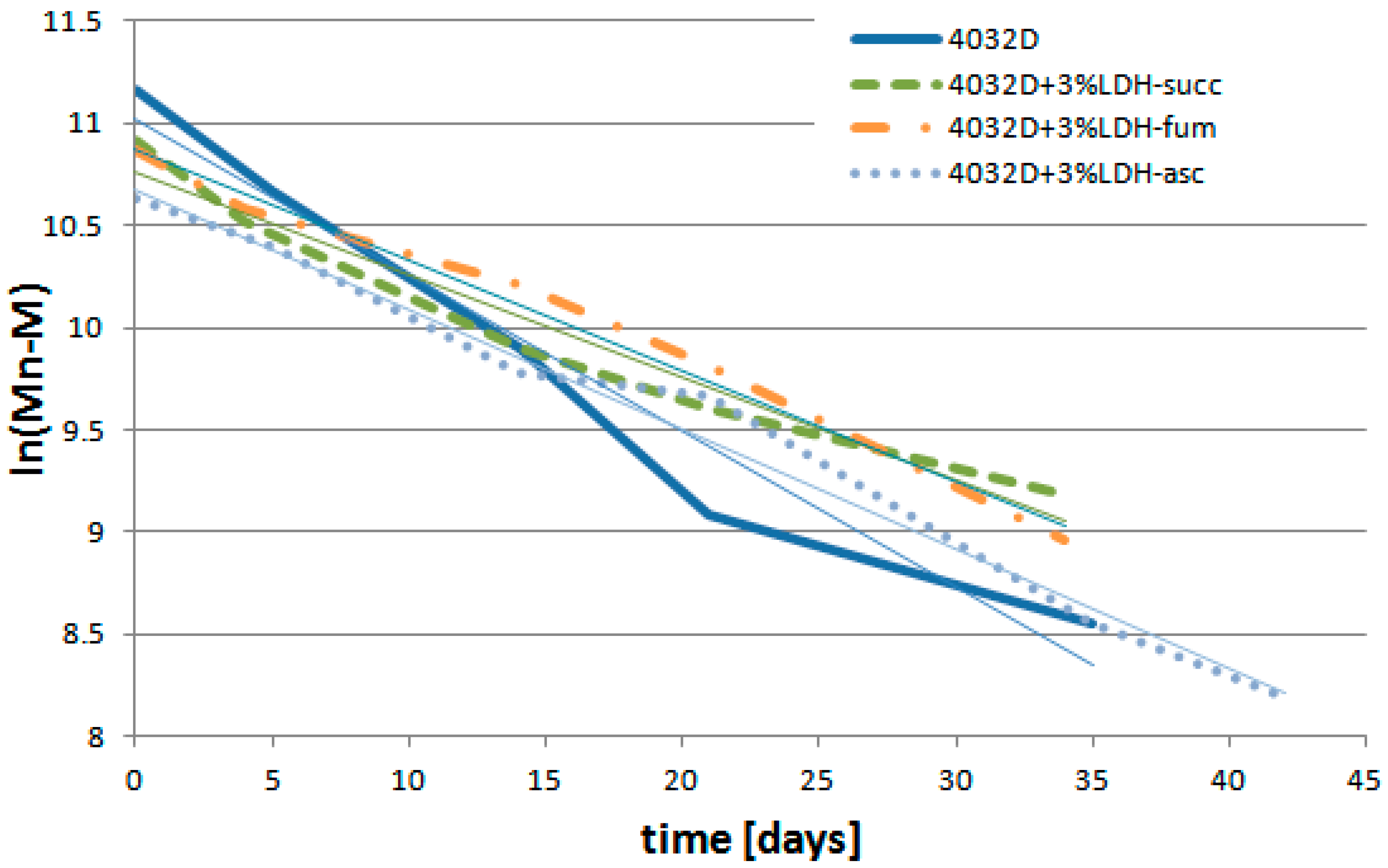
3.2.5. Analysis of PLA Hydrolysis Reaction: Reaction Rates
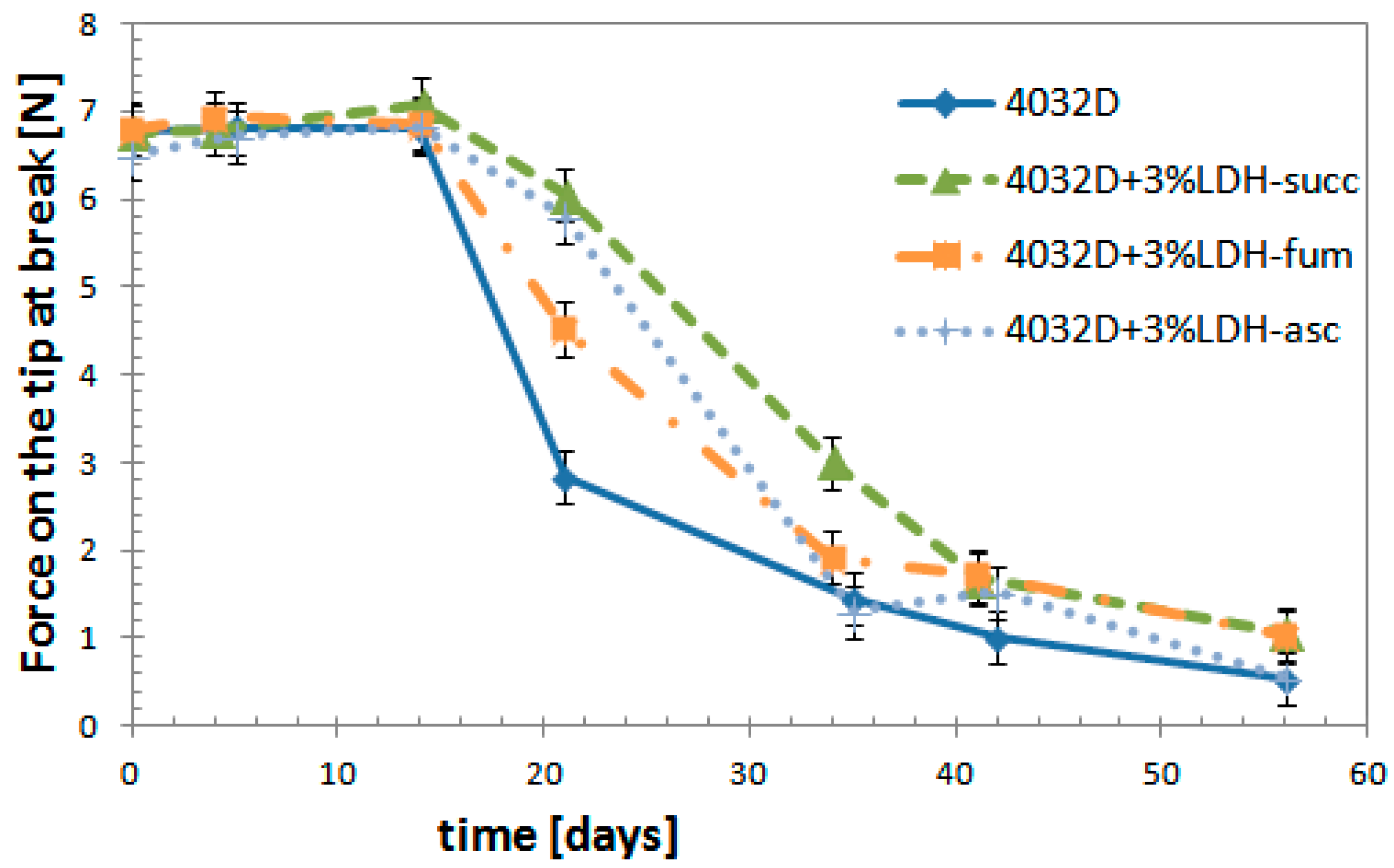
3.2.6. Mechanical Tests
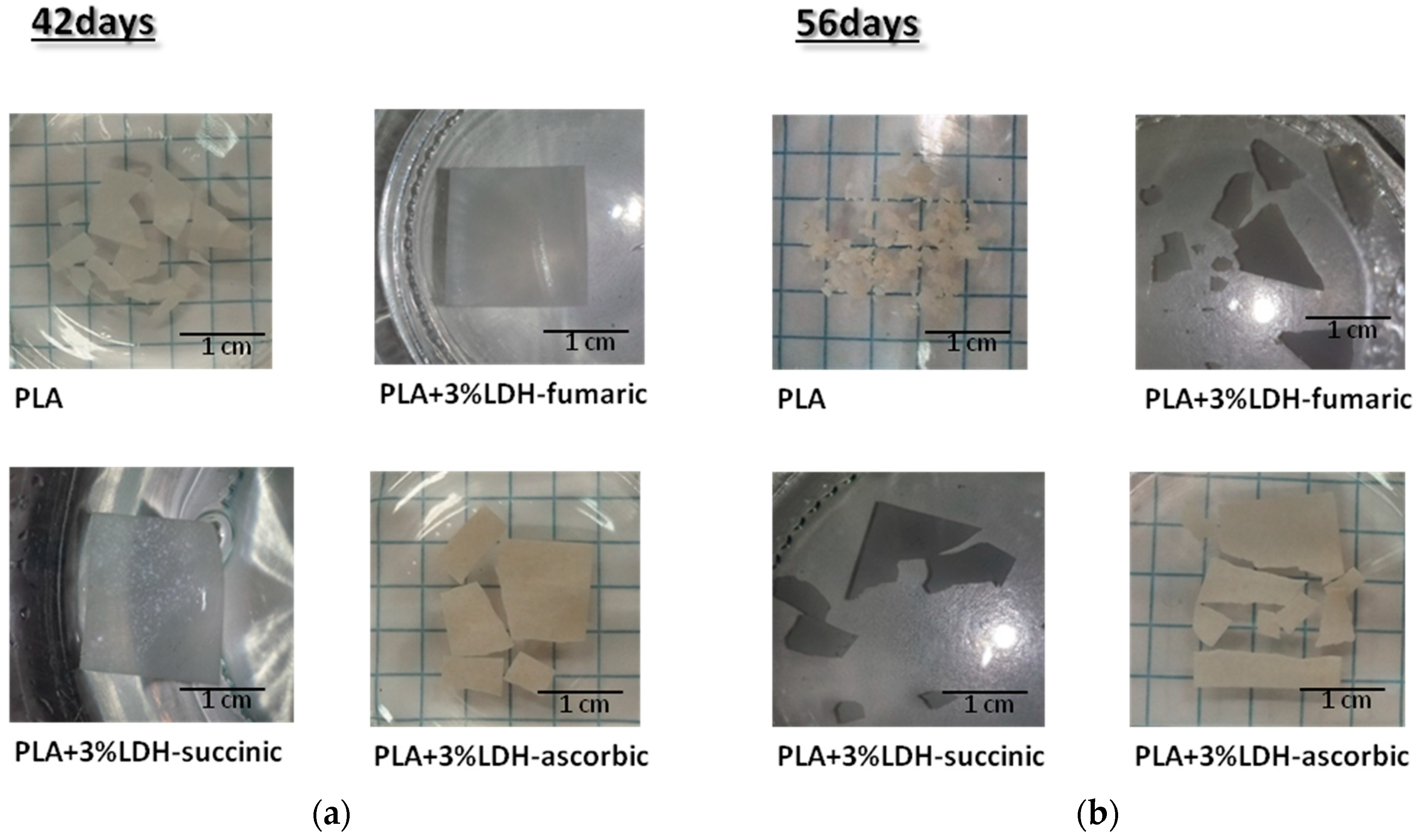
3.2.7. Visual Analysis of Hydrolyzed Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pantani, R.; Turng, L.S. Manufacturing of advanced biodegradable polymeric components. J. Appl. Polym. Sci. 2015, 132, 42889. [Google Scholar] [CrossRef]
- Soroudi, A.; Jakubowicz, I. Recycling of bioplastics, their blends and biocomposites: A review. Eur. Polym. J. 2013, 49, 2839–2858. [Google Scholar] [CrossRef]
- Garlotta, D. A literature review of poly(lactic acid). J. Polym. Environ. 2001, 9, 63–84. [Google Scholar] [CrossRef]
- Benali, S.; Aouadi, S.; Dechief, A.L.; Murariu, M.; Dubois, P. Key factors for tuning hydrolytic degradation of polylactide/zinc oxide nanocomposites. Nanocomposites 2015, 1, 51–60. [Google Scholar] [CrossRef]
- De Santis, F.; Pantani, R. Melt compounding of poly(Lactic Acid) and talc: assessment of material behavior during processing and resulting crystallization. J. Polym. Res. 2015, 22, 242. [Google Scholar] [CrossRef]
- Speranza, V.; De Meo, A.; Pantani, R. Thermal and hydrolytic degradation kinetics of PLA in the molten state. Polym. Degrad. Stab. 2014, 100, 37–41. [Google Scholar] [CrossRef]
- Harris, A.M.; Lee, E.C. Heat and Humidity Performance of Injection Molded PLA for Durable Applications. J. Appl. Polym. Sci. 2010, 115, 1380–1389. [Google Scholar] [CrossRef]
- Hoglund, A.; Odelius, K.; Albertsson, A.C. Crucial Differences in the Hydrolytic Degradation between Industrial Polylactide and Laboratory-Scale Poly(l-lactide). ACS Appl. Mater. Interfaces 2012, 4, 2788–2793. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.M.; Lee, E.C. Durability of polylactide-based polymer blends for injection-molded applications. J. Appl. Polym. Sci. 2013, 128, 2136–2144. [Google Scholar] [CrossRef]
- Notta-Cuvier, D.; Odent, J.; Delille, R.; Murariu, M.; Lauro, F.; Raquez, J.M.; Bennani, B.; Dubois, P. Tailoring polylactide (PLA) properties for automotive applications: Effect of addition of designed additives on main mechanical properties. Polym. Test. 2014, 36, 1–9. [Google Scholar] [CrossRef]
- Stloukal, P.; Kalendová, A.; Mattausch, H.; Laske, S.; Holzer, C.; Koutny, M. The influence of a hydrolysis-inhibiting additive on the degradation and biodegradation of PLA and its nanocomposites. Polym. Test. 2015, 41, 124–132. [Google Scholar] [CrossRef]
- Pantani, R.; Sorrentino, A. Influence of crystallinity on the biodegradation rate of injection-moulded poly(lactic acid) samples in controlled composting conditions. Polym. Degrad. Stab. 2013, 98, 1089–1096. [Google Scholar] [CrossRef]
- Drumright, R.E.; Gruber, P.R.; Henton, D.E. Polylactic acid technology. Adv. Mater. 2000, 12, 1841–1846. [Google Scholar] [CrossRef]
- Lostocco, M.R.; Huang, S.J. The hydrolysis of poly(lactic acid) poly(hexamethylene succinate) blends. Polym. Degrad. Stab. 1998, 61, 225–230. [Google Scholar] [CrossRef]
- Hocking, P.J.; Timmins, M.R.; Scherer, T.M.; Fuller, R.C.; Lenz, R.W.; Marchessault, R.H. Enzymatic Degradability of Poly(Beta-Hydroxybutyrate) as a Function of Tacticity. J. Macromol. Sci.-Pure Appl. Chem. 1995, A32, 889–894. [Google Scholar] [CrossRef]
- Li, S.M.; Garreau, H.; Vert, M. Structure Property Relationships in the Case of the Degradation of Massive Aliphatic Poly-(Alpha-Hydroxy Acids) in Aqueous-Media. 1. Poly(dl-Lactic Acid). J. Mater. Sci. Mater. Med. 1990, 1, 123–130. [Google Scholar] [CrossRef]
- De Jong, S.J.; Arias, E.R.; Rijkers, D.T.S.; Van Nostrum, C.F.; Kettenes-Van den Bosch, J.J.; Hennink, W.E. New insights into the hydrolytic degradation of poly(lactic acid): participation of the alcohol terminus. Polymer 2001, 42, 2795–2802. [Google Scholar] [CrossRef]
- Shih, C. A graphical method for the determination of the mode of hydrolysis of biodegradable polymers. Pharm. Res. 1995, 12, 2036–2040. [Google Scholar] [CrossRef] [PubMed]
- Belbella, A.; Vauthier, C.; Fessi, H.; Devissaguet, J.P.; Puisieux, F. In vitro degradation of nanospheres from poly(d,l-lactides) of different molecular weights and polydispersities. Int. J. Pharmaceut. 1996, 129, 95–102. [Google Scholar] [CrossRef]
- Gleadall, A.; Pan, J.; Kruft, M.A.; Kellomäki, M. Degradation mechanisms of bioresorbable polyesters. Part 1. Effects of random scission, end scission and autocatalysis. Acta Biomater. 2014, 10, 2223–2232. [Google Scholar] [CrossRef] [PubMed]
- Gorrasi, G.; Pantani, R. Effect of PLA grades and morphologies on hydrolytic degradation at composting temperature: Assessment of structural modification and kinetic parameters. Polym. Degrad. Stab. 2013, 98, 1006–1014. [Google Scholar] [CrossRef]
- Ha, J.U.; Xanthos, M. Novel modifiers for layered double hydroxides and their effects on the properties of polylactic acid composites. Appl. Clay Sci. 2010, 47, 303–310. [Google Scholar] [CrossRef]
- Arias, V.; Höglund, A.; Odelius, K.; Albertsson, A.C. Tuning the Degradation Profiles of Poly(l-lactide)-Based Materials through Miscibility. Biomacromolecules 2014, 15, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Balaguer, M.P.; Aliaga, C.; Fito, C.; Hortal, M. Compostability assessment of nano-reinforced poly(lactic acid) films. Waste Manag. 2016, 48, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Gorrasi, G.; Sorrentino, A.; Pantani, R. Modulation of Biodegradation Rate of Poly(lactic acid) by Silver Nanoparticles. J. Polym. Environ. 2015, 23, 316–320. [Google Scholar] [CrossRef]
- Hakim, R.H.; Cailloux, J.; Santana, O.O.; Bou, J.; Sánchez-Soto, M.; Odent, J.; Raquez, J.M.; Dubois, P.; Carrasco, F.; Maspoch, M.L. PLA/SiO2 composites: Influence of the filler modifications on the morphology, crystallization behavior, and mechanical properties. J. Appl. Polym. Sci. 2017, 134, 45367. [Google Scholar] [CrossRef]
- Bayer, I.S. Thermomechanical Properties of Polylactic Acid-Graphene Composites: A State-of-the-Art Review for Biomedical Applications. Materials 2017, 10, 748. [Google Scholar] [CrossRef]
- Wu, T.M.; Wu, C.Y. Biodegradable poly(lactic acid)/chitosan-modified montmorillonite nanocomposites: Preparation and characterization. Polym. Degrad. Stab. 2006, 91, 2198–2204. [Google Scholar] [CrossRef]
- Nalawade, P.; Aware, B.; Kadam, V.J.; Hirlekar, R.S. Layered double hydroxides: A review. J. Sci. Ind. Res. 2009, 68, 267–272. [Google Scholar]
- Sisti, L.; Totaro, G.; Fiorini, M.; Celli, A.; Coelho, C.; Hennous, M.; Verney, V.; Leroux, F. Poly(butylene succinate)/layered double hydroxide bionanocomposites: Relationships between chemical structure of LDH anion, delamination strategy, and final properties. J. Appl. Polym. Sci. 2013, 130, 1931–1940. [Google Scholar] [CrossRef]
- Zhou, Q.; Xanthos, M. Nanoclay and crystallinity effects on the hydrolytic degradation of polylactides. Polym. Degrad. Stab. 2008, 93, 1450–1459. [Google Scholar] [CrossRef]
- Eili, M.; Shameli, K.; Ibrahim, N.A.; Wan Yunus, W.M.Z. Degradability Enhancement of Poly(Lactic Acid) by Stearate-Zn3Al LDH Nanolayers. Int. J. Mol. Sci. 2012, 13, 7938–7951. [Google Scholar] [CrossRef] [PubMed]
- Oyarzabal, A.; Mugica, A.; Müller, A.J.; Zubitur, M. Hydrolytic degradation of nanocomposites based on poly(l-lactic acid) and layered double hydroxides modified with a model drug. J. Appl. Polym. Sci. 2016, 133, 43648. [Google Scholar] [CrossRef]
- Lu, Z.; Pan, Y.; Liu, X.; Zheng, G.; Schubert, D.W.; Liu, C. Molar mass and temperature dependence of rheological properties of polymethylmethacrylate melt. Mater. Lett. 2018, 221, 62–65. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, X.; Kaschta, J.; Hao, X.; Liu, C.; Schubert, D.W. Viscoelastic and electrical behavior of poly(methyl methacrylate)/carbon black composites prior to and after annealing. Polymer 2017, 113, 34–38. [Google Scholar] [CrossRef]
- Siparsky, G.L.; Voorhees, K.J.; Miao, F.D. Hydrolysis of polylactic acid (PLA) and polycaprolactone (PCL) in aqueous acetonitrile solutions: Autocatalysis. J. Environ. Polym. Degrad. 1998, 6, 31–41. [Google Scholar] [CrossRef]
- Partini, M.; Argenio, O.; Coccorullo, I.; Pantani, R. Degradation kinetics and rheology of biodegradable polymers. J. Therm. Anal. Calorim. 2009, 98, 645–653. [Google Scholar] [CrossRef]
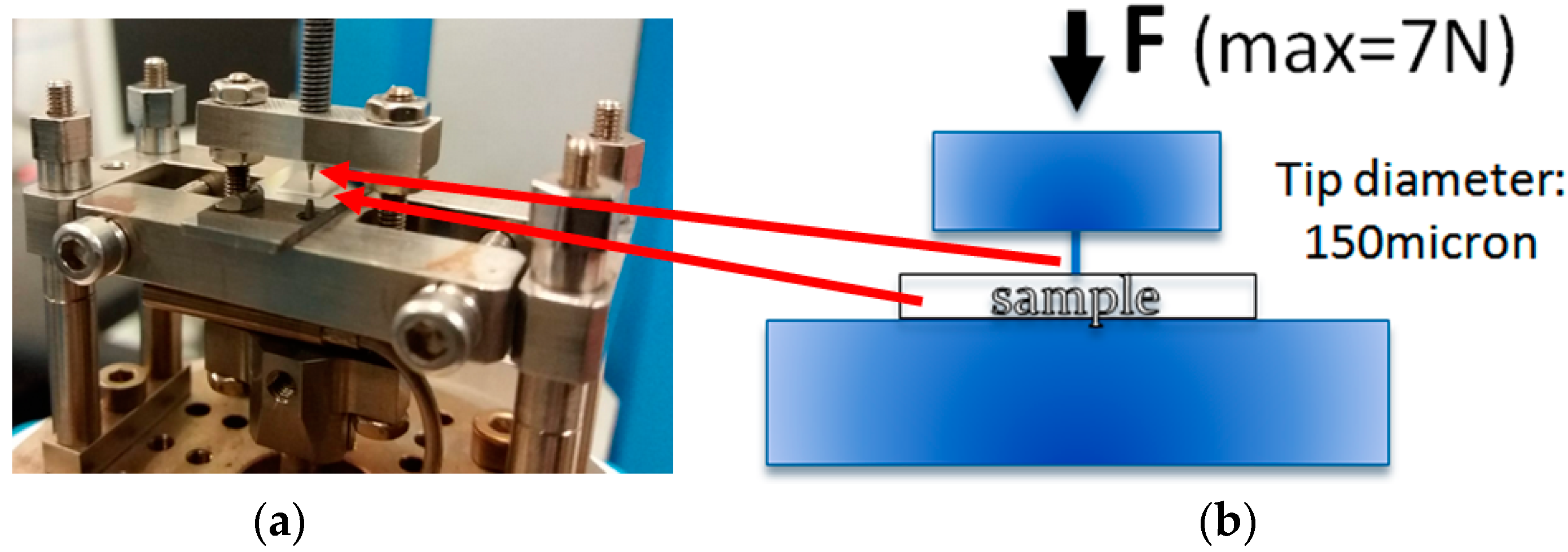
| Groups | Extruded Materials (Filler 3 w/w) |
|---|---|
| Pure PLA | 4032D |
| PLA + LDH | 4032D + (LDH-CO3) |
| 4032D + (LDH-NO3) | |
| PLA + (LDH-organic acids) | 4032D + LDH-succinic acid |
| 4032D + LDH-fumaric acid | |
| 4032D + LDH-ascorbic acid |
| Sample | Days of Hydrolysis | |||||
|---|---|---|---|---|---|---|
| 0 | 5 | 14 | 21 | 48 | ||
| 4032D | Tg (°C) | 63.8 | 63.7 | 61.4 | 61.4 | 51 |
| Tm (°C) | 169.9 | 168.4 | 167.8 | 164.9 | 154.3 | |
| 4032D + 3% (LDH-CO3) | Tg | 61.8 | / | / | / | 50.2 |
| Tm | 166.4 | 164.8 | 162.9 | 158.6 | 150.3 | |
| 4032D + 3% (LDH-NO3) | Tg | 63.3 | 62.9 | 62.2 | 62.5 | 56.9 |
| Tm | 168.2 | 167.5 | 166.8 | 166 | 155.3 |
| Sample | Days of Hydrolysis | |||||
|---|---|---|---|---|---|---|
| 0 | 5 | 14 | 21 | 48 | ||
| 4032D | Tg (°C) | 63.8 | 63.7 | 61.4 | 61.4 | 51 |
| Tm (°C) | 169.9 | 168.4 | 167.8 | 164.9 | 154.3 | |
| 4032D + 3% LDH-succ | Tg | 62.8 | 62.3 | 61.8 | 61.7 | 56.6 |
| Tm | 169.8 | 167 | 166.7 | 166.5 | 162.5 | |
| 4032D + 3% LDH-fum | Tg | 62.2 | 61.5 | 61.1 | 60.9 | 56.1 |
| Tm | 169.2 | 166.9 | 166.7 | 166.5 | 161 | |
| 4032D + 3% LDH-asc | Tg | 61.2 | 61.1 | 59.1 | 58.6 | 55.8 |
| Tm | 169.6 | 168 | 167.4 | 167.3 | 158.2 |
| k′ (Days−1) | R2 | |
|---|---|---|
| 4032D | 0.0765 | 0.9628 |
| 4032D + 3%LDH-succ | 0.0503 | 0.9591 |
| 4032D + 3%LDH-fum | 0.0543 | 0.9871 |
| 4032D + 3%LDH-asc | 0.0587 | 0.9868 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valentina, I.; Haroutioun, A.; Fabrice, L.; Vincent, V.; Roberto, P. Poly(Lactic Acid)-Based Nanobiocomposites with Modulated Degradation Rates. Materials 2018, 11, 1943. https://doi.org/10.3390/ma11101943
Valentina I, Haroutioun A, Fabrice L, Vincent V, Roberto P. Poly(Lactic Acid)-Based Nanobiocomposites with Modulated Degradation Rates. Materials. 2018; 11(10):1943. https://doi.org/10.3390/ma11101943
Chicago/Turabian StyleValentina, Iozzino, Askanian Haroutioun, Leroux Fabrice, Verney Vincent, and Pantani Roberto. 2018. "Poly(Lactic Acid)-Based Nanobiocomposites with Modulated Degradation Rates" Materials 11, no. 10: 1943. https://doi.org/10.3390/ma11101943
APA StyleValentina, I., Haroutioun, A., Fabrice, L., Vincent, V., & Roberto, P. (2018). Poly(Lactic Acid)-Based Nanobiocomposites with Modulated Degradation Rates. Materials, 11(10), 1943. https://doi.org/10.3390/ma11101943








