Hole-Transporting Materials for Printable Perovskite Solar Cells
Abstract
1. Introduction
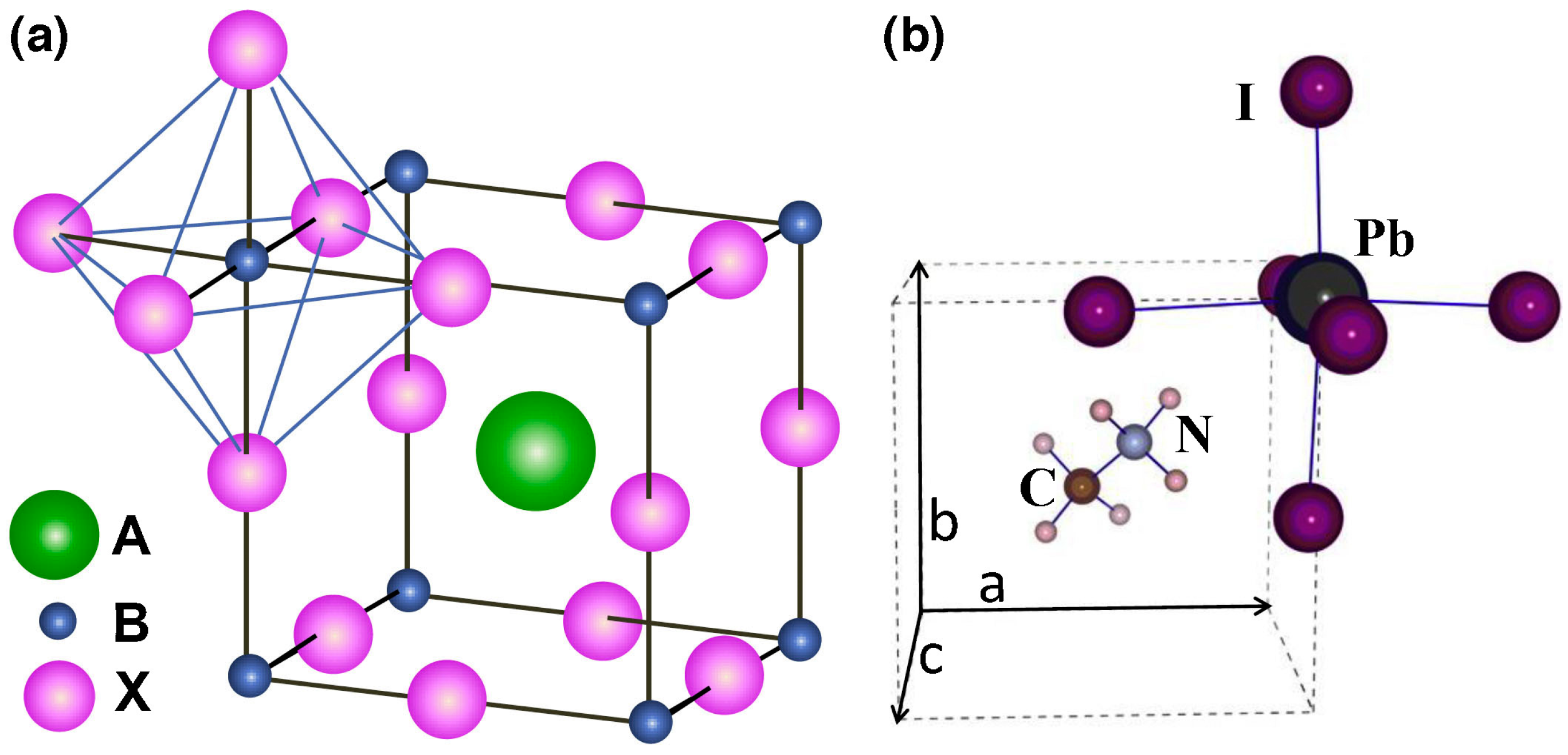
2. Perovskite Solar Cells
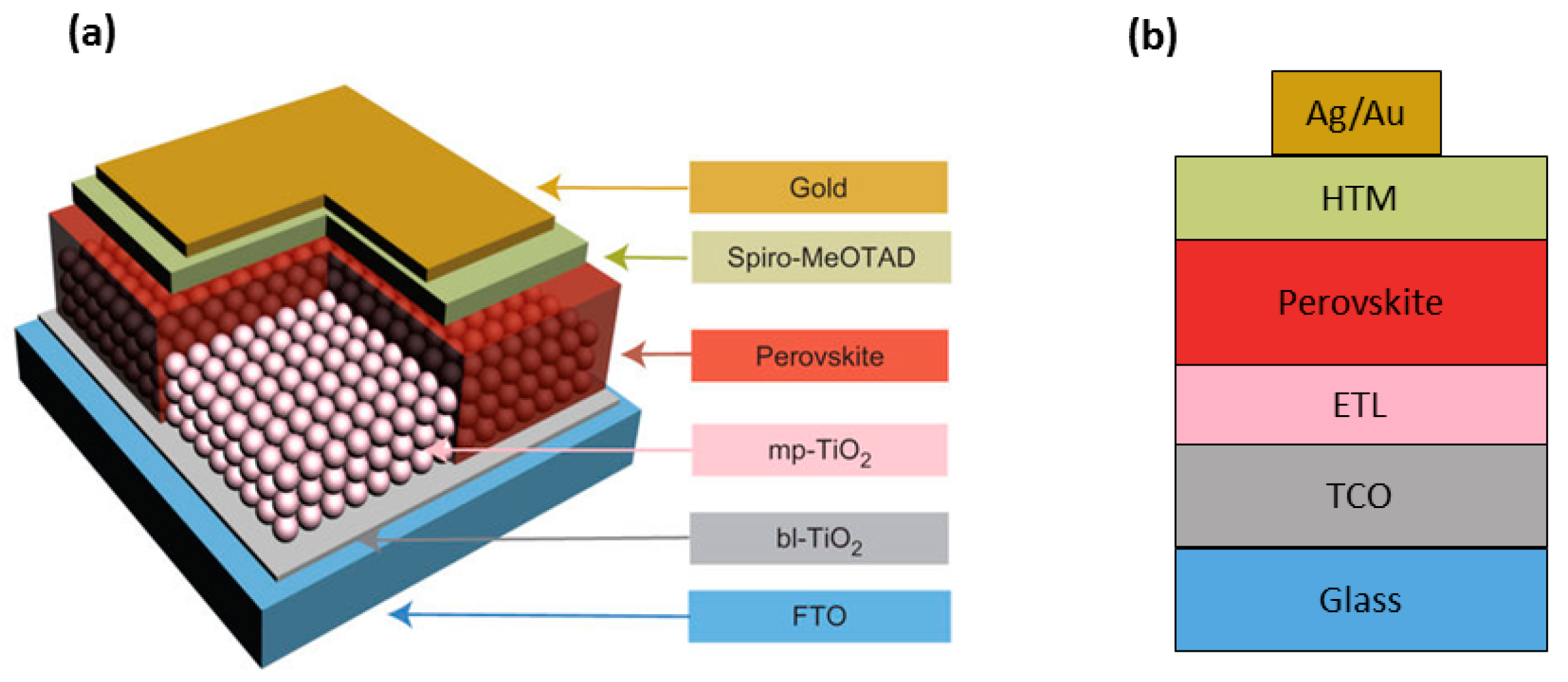
2.1. Mesoscopic and Planar Architectures
2.2. Current Challenges of Perovskite Solar Cells Research
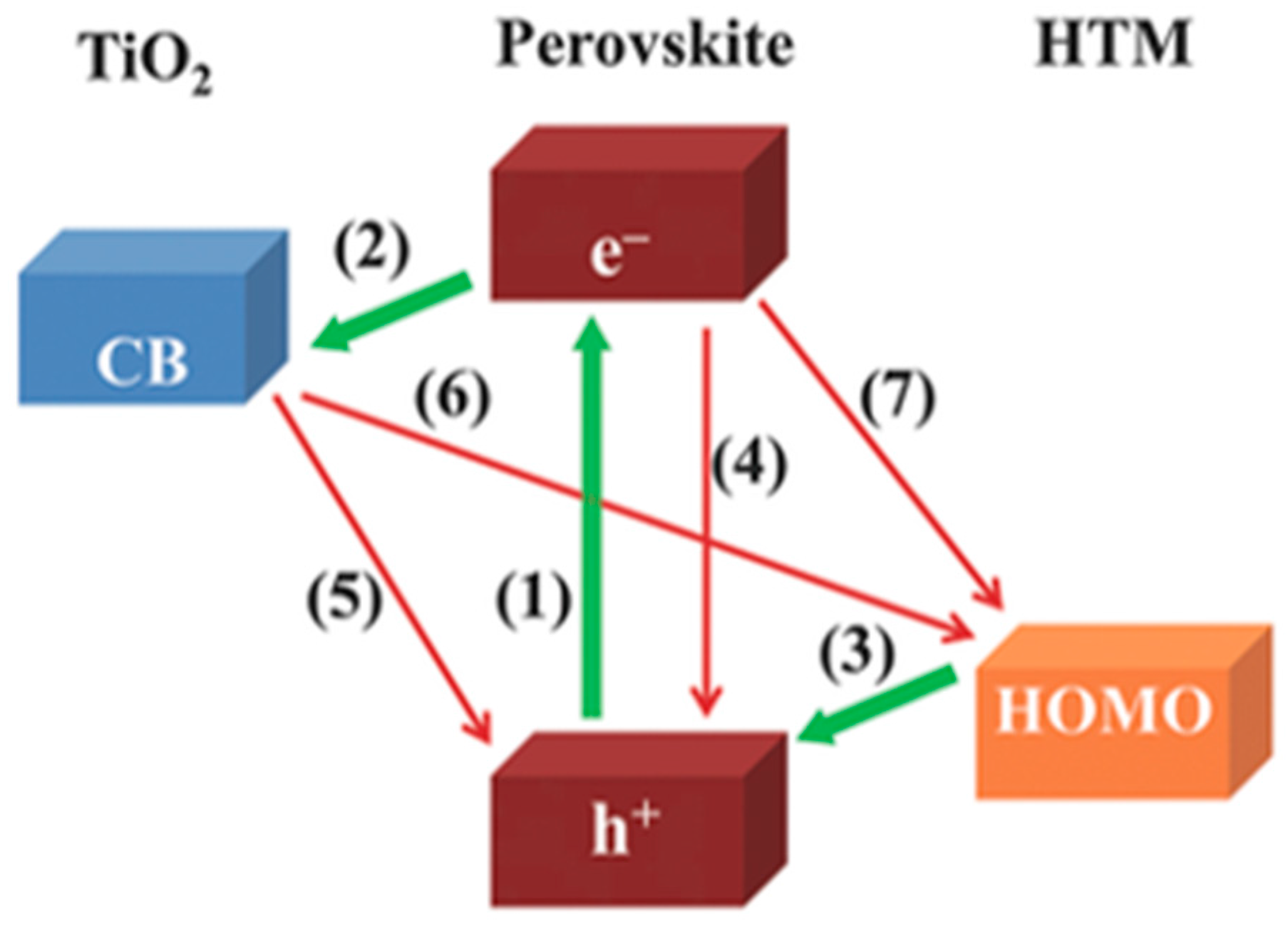
3. Hole-Transporting Materials
- prevent the direct contact between the perovskite and the metal contact, which minimizes charge recombination and avoids degradation at the metal-perovskite interface;
- extract positive charges (holes) from perovskite and transport them to the top-electrode.
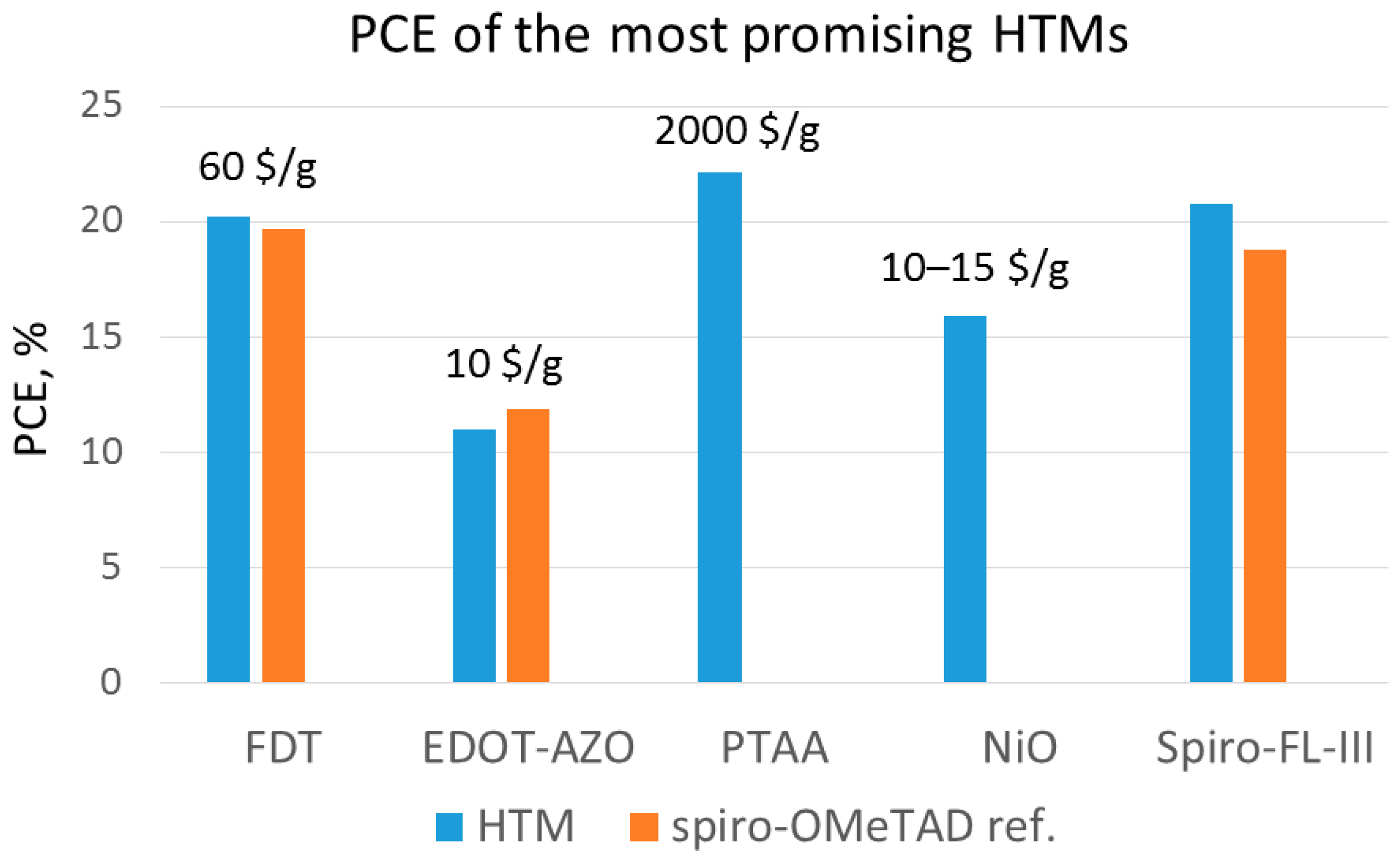
- high costs: spiro-OMeTAD is prohibitively expensive (~500 $/g) [39] because of (a) its onerous multistep synthesis that requires a low temperature (−78 °C); (b) the sensitive (n-butyllithium or Grignard reagents) and aggressive (Br2) reagents involved in the synthetic scheme [40]; (c) the costly sublimation step required for purification [41].
- negative impact on stability: the use of spiro-OMeTAD limits the long-term stability of the devices [39], thus inhibiting the upscaling application in the photovoltaic industry.
- sub-optimal charge transport: when in pristine form, spiro-OMeTAD shows a modest hole-mobility and conductivity (1.67 × 10−5 cm2 V−1 s−1 and 3.54 × 10−7 S cm−1, respectively) [42], thus requiring additives and chemical p-dopants to enhance the hole conductivity by 10-fold and thus the conversion efficiency of PSCs.
3.1. Organic Hole-Transporting Materials
3.1.1. Small-Molecule-Based HTMs
3.1.2. Polymer-Based Hole-Transporters
3.2. Inorganic Hole-Transporting Materials
3.3. Hybrid Hole-Transporters
4. Concluding Remarks and Future Perspectives
- Appropriate ionization potentials are essential to guarantee efficient transfer of the photoexcited holes from the perovskite layer to the HTM.
- Low electron affinities are required to avoid electron recombination at the perovskite|HTM interface.
- HTMs should also be highly transparent, low-cost and present good film-forming properties at the same time, which ensure smooth and pinhole-free coverage.
- High mobility: Doping of the organic HTMs is in most cases necessary to ensure high cells performance, but it is usually responsible for significant degradation processes compared to pristine HTMs. To enhance the stability, it would be ideal to avoid any dopant to the pristine HTM. This requires the HTM to have high mobility. Research efforts in this direction must continue, starting from the promising achievements about the highly order columnar design concept as a strategy to facilitate the mobility of charge-carriers by face-on arrangement. This was the case of material Triazatrux-VII, which demonstrated how to enhance the hole-mobility to achieve exceptional device performance.
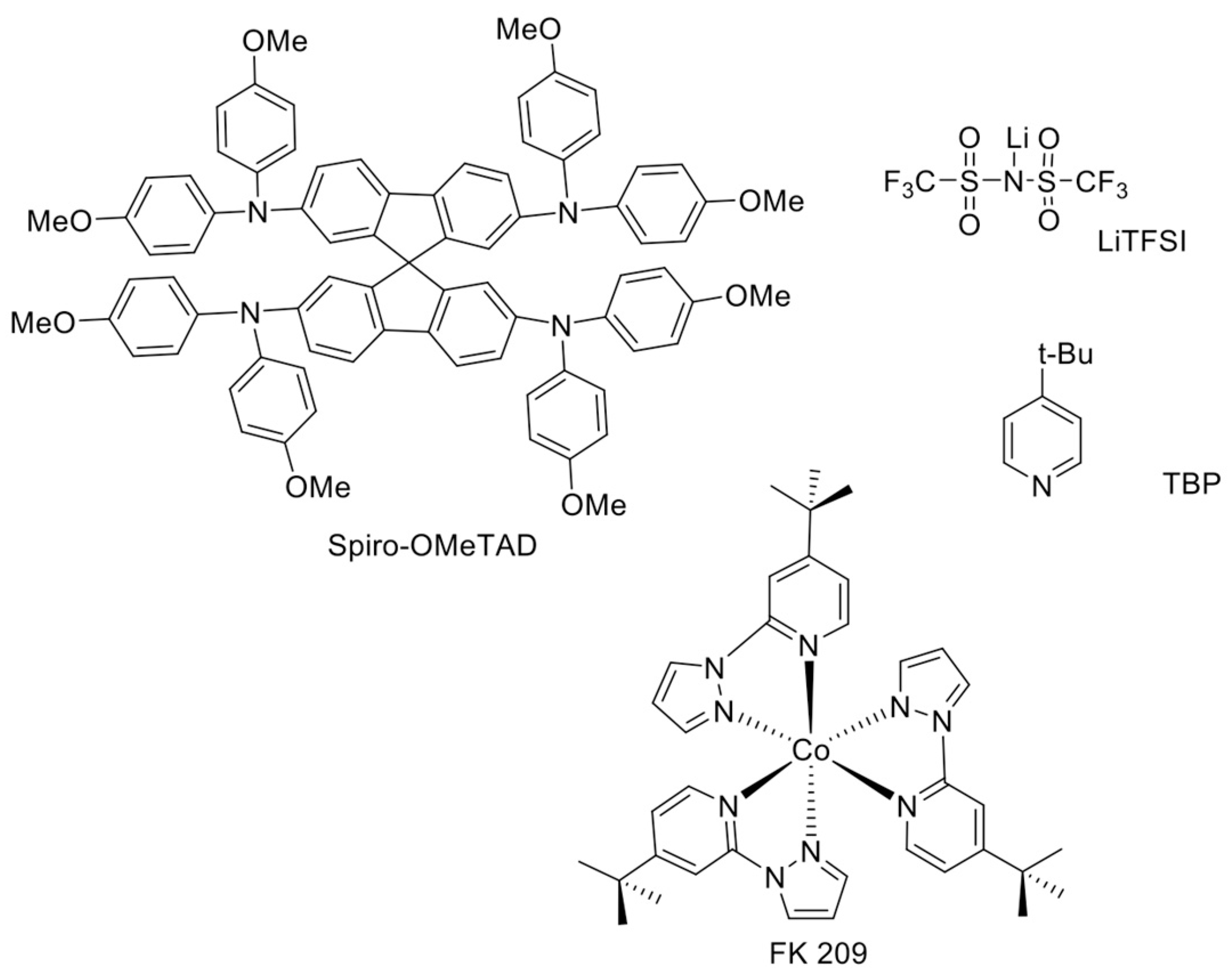
- If the use of dopants cannot be avoided because of the poor hole-mobility of the pristine HTM, hydrophobic dopants or air-stable additives should be chosen, as alternatives to the highly unstable dopants commonly used at present (LiTFSI, TBP, FK 209).
Acknowledgments
Author Contributions
Conflicts of Interest
References
- BP Statistical Review of World Energy 2017. Available online: https://www.bp.com/content/dam/bp/en/corporate/pdf/energy-economics/statistical-review-2017/bp-statistical-review-of-world-energy-2017-full-report.pdf (accessed on 3 August 2017).
- Golušin, M.; Dodić, S.; Popov, S. Sustainable Energy Management; Academmic Press: Cambridge, MA, USA, 2013; ISBN 9780123914279. [Google Scholar]
- Yang, Y.; You, J. Make perovskite solar cells stable. Nature 2017, 544, 155–156. [Google Scholar] [CrossRef] [PubMed]
- Calió, L.; Kazim, S.; Grätzel, M.; Ahmad, S. Hole-Transport Materials for Perovskite Solar Cells. Angew. Chem. Int. Ed. 2016, 55, 14522–14545. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Sun, L. Recent Progress on Hole-Transporting Materials for Emerging Organometal Halide Perovskite Solar Cells. Adv. Energy Mater. 2015, 5. [Google Scholar] [CrossRef]
- Bakr, Z.H.; Wali, Q.; Fakharuddin, A.; Schmidt-Mende, L.; Brown, T.M.; Jose, R. Advances in hole transport materials engineering for stable and efficient perovskite solar cells. Nano Energy 2017, 34, 271–305. [Google Scholar] [CrossRef]
- Krishna, A.; Grimsdale, A.C.; Jang, J.; Nazeeruddin, M.K.; Lessi, M.; Abate, A.; Caramoni, S.; Grätzel, M.; Bellina, F.; Bignozzi, C.A.; et al. Hole transporting materials for mesoscopic perovskite solar cells—Towards a rational design? J. Mater. Chem. A 2017, 9, 1736–1742. [Google Scholar] [CrossRef]
- Gheno, A.; Vedraine, S.; Ratier, B.; Bouclé, J. π-Conjugated Materials as the Hole-Transporting Layer in Perovskite Solar Cells. Metals 2016, 6, 21. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, C.R.; Im, J.H.; Lee, K.B.; Moehl, T.; Marchioro, A.; Moon, S.J.; Humphry-Baker, R.; Yum, J.H.; Moser, J.E.; et al. Lead iodide perovskite sensitized all-solid-state submicron thin film mesoscopic solar cell with efficiency exceeding 9%. Sci. Rep. 2012, 2, 591. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Noh, J.H.; Jeon, N.J.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S., II. High-performance photovoltaic perovskite layers fabricated through intramolecular exchange. Science 2015, 348, 1234–1237. [Google Scholar] [CrossRef] [PubMed]
- Efficiency-Chart NREL. Available online: https://www.nrel.gov/pv/assets/images/efficiency-chart.png (accessed on 7 August 2017).
- Yang, W.S.; Park, B.W.; Jung, E.H.; Jeon, N.J.; Kim, Y.C.; Lee, D.U.; Shin, S.S.; Seo, J.; Kim, E.K.; Noh, J.H.; et al. Iodide management in formamidinium-lead-halide—Based perovskite layers for efficient solar cells. Science 2017, 356, 1376–1379. [Google Scholar] [CrossRef] [PubMed]
- Park, N.G. Perovskite solar cells: An emerging photovoltaic technology. Mater. Today 2015, 18, 65–72. [Google Scholar] [CrossRef]
- Boix, P.P.; Agarwala, S.; Koh, T.M.; Mathews, N.; Mhaisalkar, S.G. Perovskite Solar Cells: Beyond Methylammonium Lead Iodide. J. Phys. Chem. Lett. 2015, 6, 898–907. [Google Scholar] [CrossRef] [PubMed]
- Stranks, S.D.; Eperon, G.E.; Grancini, G.; Menelaou, C.; Alcocer, M.J.P.; Leijtens, T.; Herz, L.M.; Petrozza, A.; Snaith, H.J. Electron-Hole Diffusion Lengths Exceeding 1 Micrometer in an Organometal Trihalide Perovskite Absorber. Science 2013, 342, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Fang, Y.; Shao, Y.; Mulligan, P.; Qiu, J.; Cao, L.; Huang, J. Electron-hole diffusion lengths > 175 μm in solution-grown CH3NH3PbI3 single crystals. Science 2015, 347, 967–970. [Google Scholar] [CrossRef] [PubMed]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- Kazim, S.; Nazeeruddin, M.K.; Grätzel, M.; Ahmad, S. Perovskite as Light Harvester: A Game Changer in Photovoltaics. Angew. Chem. Int. Ed. 2014, 53, 2812–2824. [Google Scholar] [CrossRef] [PubMed]
- Rong, Y.; Liu, L.; Mei, A.; Li, X.; Han, H. Beyond Efficiency: The Challenge of Stability in Mesoscopic Perovskite Solar Cells. Adv. Energy Mater. 2015, 5. [Google Scholar] [CrossRef]
- Correa Baena, J.P.; Steier, L.; Tress, W.; Saliba, M.; Neutzner, S.; Matsui, T.; Giordano, F.; Jacobsson, T.J.; Ram, A.; Kandada, S.; et al. Highly efficient planar perovskite solar cells through band alignment engineering. Energy Environ. Sci. 2015, 8, 2928–2934. [Google Scholar] [CrossRef]
- Yang, D.; Zhou, X.; Yang, R.; Yang, Z.; Yu, W.; Wang, X.; Li, C.; Liu, S.F.; Chang, R.P.H.; Phillips, D.L.; et al. Surface optimization to eliminate hysteresis for record efficiency planar perovskite solar cells. Energy Environ. Sci. 2016, 9, 3071–3078. [Google Scholar] [CrossRef]
- Vivo, P.; Ojanperä, A.; Smått, J.H.; Sandén, S.; Hashmi, S.G.; Kaunisto, K.; Ihalainen, P.; Masood, M.T.; Österbacka, R.; Lund, P.D.; et al. Influence of TiO2 compact layer precursor on the performance of perovskite solar cells. Org. Electron. 2017, 41, 287–293. [Google Scholar] [CrossRef]
- Masood, M.T.; Weinberger, C.; Sarfraz, J.; Rosqvist, E.; Sandén, S.; Sandberg, O.J.; Vivo, P.; Hashmi, G.; Lund, P.D.; Österbacka, R.; et al. Impact of Film Thickness of Ultrathin Dip-Coated Compact TiO2 Layers on the Performance of Mesoscopic Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2017, 9, 17906–17913. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Dar, M.I.; Yi, C.; Luo, J.; Tschumi, M.; Zakeeruddin, S.M.; Nazeeruddin, M.K.; Han, H.; Grätzel, M. Improved performance and stability of perovskite solar cells by crystal crosslinking with alkylphosphonic acid ω-ammonium chlorides. Nat. Chem. 2015, 7, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Burschka, J.; Pellet, N.; Moon, S.J.; Humphry-Baker, R.; Gao, P.; Nazeeruddin, M.K.; Grätzel, M. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature 2013, 499, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; McCleese, C.; Kovalsky, A.; Zhao, Y.; Burda, C. Femtosecond Time-Resolved Transient Absorption Spectroscopy of CH3NH3PbI3 Perovskite Films: Evidence for Passivation Effect of PbI2. J. Am. Chem. Soc. 2014, 136, 12205–12208. [Google Scholar] [CrossRef] [PubMed]
- Srimath Kandada, A.R.; Petrozza, A. Photophysics of Hybrid Lead Halide Perovskites: The Role of Microstructure. Acc. Chem. Res. 2016, 49, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Kanemitsu, Y.; Protesescu, L.; Krieg, F.; Bodnarchuk, M.I.; Nedelcu, G.; Humer, M.; Fiebig, M.; Heiss, W.; Kovalenko, M.V.; Pellaroque, A.; et al. Luminescence spectroscopy of lead-halide perovskites: Materials properties and application as photovoltaic devices. J. Mater. Chem. C 2017, 5, 3427–3437. [Google Scholar] [CrossRef]
- Jeon, N.J.; Noh, J.H.; Kim, Y.C.; Yang, W.S.; Ryu, S.; Seok, S., II. Solvent engineering for high-performance inorganic–organic hybrid perovskite solar cells. Nat. Mater. 2014, 13, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Kang, S.M.; Lee, J.K.; Choi, M. Hysteresis-free low-temperature-processed planar perovskite solar cells with 19.1% efficiency. Energy Environ. Sci. 2016, 9, 2262–2266. [Google Scholar] [CrossRef]
- Pascoe, A.R.; Yang, M.; Kopidakis, N.; Zhu, K.; Reese, M.O.; Rumbles, G.; Fekete, M.; Duffy, N.W.; Cheng, Y.B. Planar versus mesoscopic perovskite microstructures: The influence of CH3NH3PbI3 morphology on charge transport and recombination dynamics. Nano Energy 2016, 22, 439–452. [Google Scholar] [CrossRef]
- Park, N.G. Chapter 1. High Efficiency Mesoscopic Organometal Halide Perovskite Solar Cells. In Unconventional Thin Film Photovoltaics; Royal Society of Chemistry: Cambridge, UK, 2016; pp. 1–31. [Google Scholar]
- Zhu, X. The Perovskite Fever and Beyond. Acc. Chem. Res. 2016, 49, 355–356. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.T.; Rajagopal, A.; Chueh, C.C.; Jen, A.K.Y. Current Challenges and Prospective Research for Upscaling Hybrid Perovskite Photovoltaics. J. Phys. Chem. Lett. 2016, 7, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Asif, A.A.; Singh, R.; Alapatt, G.F. Technical and economic assessment of perovskite solar cells for large scale manufacturing. J. Renew. Sustain. Energy 2015, 7. [Google Scholar] [CrossRef]
- Meloni, S.; Moehl, T.; Tress, W.; Franckevičius, M.; Saliba, M.; Lee, Y.H.; Gao, P.; Nazeeruddin, M.K.; Zakeeruddin, S.M.; Rothlisberger, U.; et al. Ionic polarization-induced current—Voltage hysteresis in CH 3 NH 3 PbX 3 perovskite solar cells. Nat. Commun. 2016, 6. [Google Scholar] [CrossRef]
- Berhe, T.A.; Su, W.N.; Chen, C.H.; Pan, C.J.; Cheng, J.H.; Chen, H.M.; Tsai, M.C.; Chen, L.Y.; Dubale, A.A.; Hwang, B.J.; et al. Organometal halide perovskite solar cells: Degradation and stability. Energy Environ. Sci. 2016, 9, 323–356. [Google Scholar] [CrossRef]
- Grätzel, M.; Bach, U.; Lupo, D.; Comte, P.; Moser, J.E.; Weissörtel, F.; Salbeck, J.; Spreitzer, H. Solid-state dye-sensitized mesoporous TiO2 solar cells with high photon-to-electron conversion efficiencies. Nature 1998, 395, 583–585. [Google Scholar] [CrossRef]
- Saliba, M.; Orlandi, S.; Matsui, T.; Aghazada, S.; Cavazzini, M.; Correa-Baena, J.P.; Gao, P.; Scopelliti, R.; Mosconi, E.; Dahmen, K.H.; et al. A molecularly engineered hole-transporting material for efficient perovskite solar cells. Nat. Energy 2016, 1, 15017. [Google Scholar] [CrossRef]
- Gratia, P.; Magomedov, A.; Malinauskas, T.; Daskeviciene, M.; Abate, A.; Ahmad, S.; Grätzel, M.; Getautis, V.; Nazeeruddin, M.K. A Methoxydiphenylamine-Substituted Carbazole Twin Derivative: An Efficient Hole-Transporting Material for Perovskite Solar Cells. Angew. Chem. Int. Ed. 2015, 54, 11409–11413. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.; Frost, J.M.; Hendon, C.; Molloy, C.D.; Carbery, D.; Walsh, A. Modular design of SPIRO-OMeTAD analogues as hole transport materials in solar cells. Chem. Commun. 2015, 51, 8935–8938. [Google Scholar] [CrossRef] [PubMed]
- Xu, B. Advanced Organic Hole Transport Materials for Solution-Processed Photovoltaic Devices. Ph.D. Thesis, KTH Chemical Science and Engineering, Royal Institute of Technology, Stockholm, Sweden, 2015. [Google Scholar]
- Krüger, J.; Plass, R.; Cevey, L.; Piccirelli, M.; Grätzel, M.; Bach, U. High efficiency solid-state photovoltaic device due to inhibition of interface charge recombination. Appl. Phys. Lett. 2001, 79, 2085–2087. [Google Scholar] [CrossRef]
- Ganesan, P.; Fu, K.; Gao, P.; Raabe, I.; Schenk, K.; Scopelliti, R.; Luo, J.; Wong, L.H.; Grätzel, M.; Nazeeruddin, M.K. A simple spiro-type hole transporting material for efficient perovskite solar cells. Energy Environ. Sci. 2015, 8, 1986–1991. [Google Scholar] [CrossRef]
- Bi, D.; Boschloo, G.; Hagfeldt, A. High-efficient solid-state perovskite solar cell without lithium salt in the hole transport material. Nano 2014, 9. [Google Scholar] [CrossRef]
- Zhao, J.; Zheng, X.; Deng, Y.; Li, T.; Shao, Y.; Gruverman, A.; Shield, J.; Huang, J.; Lee, S.; Lee, K. Is Cu a stable electrode material in hybrid perovskite solar cells for a 30-year lifetime? Energy Environ. Sci. 2016, 9, 3650–3656. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, B.; Dai, J.; Fang, Y.; Bai, Y.; Lin, Y.; Wei, H.; Zeng, X.C.; Huang, J. Defect passivation in hybrid perovskite solar cells using quaternary ammonium halide anions and cations. Nat. Energy 2017, 2. [Google Scholar] [CrossRef]
- Saliba, M.; Matsui, T.; Domanski, K.; Seo, J.Y.; Ummadisingu, A.; Zakeeruddin, S.M.; Correa-Baena, J.P.; Tress, W.R.; Abate, A.; Hagfeldt, A.; et al. Incorporation of rubidium cations into perovskite solar cells improves photovoltaic performance. Science 2016, 354. [Google Scholar] [CrossRef] [PubMed]
- Saliba, M.; Matsui, T.; Seo, J.Y.; Domanski, K.; Correa-Baena, J.P.; Nazeeruddin, M.K.; Zakeeruddin, S.M.; Tress, W.; Abate, A.; Hagfeldt, A.; et al. Cesium-containing Triple Cation Perovskite Solar Cells: Improved Stability, Reproducibility and High Efficiency. Energy Environ. Sci. 2016, 9, 1989–1997. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bi, D.; Yi, C.; Decoppet, J.D.; Luo, J.; Zakeeruddin, S.M.; Hagfeldt, A.; Gratzel, M. A vacuum flash-assisted solution process for high-efficiency large-area perovskite solar cells. Science 2016, 353, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Mei, A.; Li, X.; Liu, L.; Ku, Z.; Liu, T.; Rong, Y.; Xu, M.; Hu, M.; Chen, J.; Yang, Y.; et al. A hole-conductor-free, fully printable mesoscopic perovskite solar cell with high stability. Science 2014, 345, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.V.; Sfyri, G.; Raptis, D.; Stathatos, E.; Lianos, P.; Kahn, A.; Mora-Seró, I.; Tena-Zaera, R.; Liu, Y.; Yang, Y.; et al. Perovskite solar cell with low cost Cu-phthalocyanine as hole transporting material. RSC Adv. 2015, 5, 3786–3791. [Google Scholar] [CrossRef]
- Javier Ramos, F.; Ince, M.; Urbani, M.; Abate, A.; Grätzel, M.; Ahmad, S.; Torres, T.; Nazeeruddin, M.K. Non-aggregated Zn(ii)octa(2,6-diphenylphenoxy) phthalocyanine as a hole transporting material for efficient perovskite solar cells. Dalton Trans. 2015, 44, 10847–10851. [Google Scholar] [CrossRef] [PubMed]
- Shirota, Y.; Kageyama, H. Charge Carrier Transporting Molecular Materials and Their Applications in Devices. Chem. Rev. 2007, 107, 953–1010. [Google Scholar] [CrossRef] [PubMed]
- Jeon, N.J.; Lee, J.; Noh, J.H.; Nazeeruddin, M.K.; Grätzel, M.; Seok, S., II. Efficient Inorganic—Organic Hybrid Perovskite Solar Cells Based on Pyrene Arylamine Derivatives as Hole-Transporting Materials. J. Am. Chem. Soc. 2013, 135, 19087–19090. [Google Scholar] [CrossRef] [PubMed]
- Grisorio, R.; Iacobellis, R.; Listorti, A.; De Marco, L.; Cipolla, M.P.; Manca, M.; Rizzo, A.; Abate, A.; Gigli, G.; Suranna, G.P. Rational Design of Molecular Hole-Transporting Materials for Perovskite Solar Cells: Direct versus Inverted Device Configurations. ACS Appl. Mater. Interfaces 2017, 9, 24778–24787. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Fu, W.; Li, C.Z.; Zhang, Z.; Qiu, W.; Shi, M.; Heremans, P.; Jen, A.K.Y.; Chen, H. Dopant-Free Hole-Transporting Material with a C3h Symmetrical Truxene Core for Highly Efficient Perovskite Solar Cells. J. Am. Chem. Soc. 2016, 138, 2528–2531. [Google Scholar] [CrossRef] [PubMed]
- Rakstys, K.; Abate, A.; Dar, M.I.; Gao, P.; Jankauskas, V.; Jacopin, G.; Kamarauskas, E.; Kazim, S.; Ahmad, S.; Grätzel, M.; et al. Triazatruxene-Based Hole Transporting Materials for Highly Efficient Perovskite Solar Cells. J. Am. Chem. Soc. 2015, 137, 16172–16178. [Google Scholar] [CrossRef] [PubMed]
- Rakstys, K.; Paek, S.; Gao, P.; Gratia, P.; Marszalek, T.; Grancini, G.; Cho, K.T.; Genevicius, K.; Jankauskas, V.; Pisula, W.; et al. Molecular engineering of face-on oriented dopant-free hole transporting material for perovskite solar cells with 19% PCE. J. Mater. Chem. A 2017, 5, 7811–7815. [Google Scholar] [CrossRef]
- Grisorio, R.; Roose, B.; Colella, S.; Listorti, A.; Suranna, G.P.; Abate, A. Molecular Tailoring of Phenothiazine-Based Hole-Transporting Materials for High-Performing Perovskite Solar Cells. ACS Energy Lett. 2017, 2, 1029–1034. [Google Scholar] [CrossRef]
- Cho, A.N.; Chakravarthi, N.; Kranthiraja, K.; Reddy, S.S.; Kim, H.S.; Jin, S.H.; Park, N.G.; Hoogland, S.; Rothenberger, A.; Katsiev, K.; et al. Acridine-based novel hole transporting material for high efficiency perovskite solar cells. J. Mater. Chem. A 2017, 5, 7603–7611. [Google Scholar] [CrossRef]
- Liu, X.; Kong, F.; Ghadari, R.; Jin, S.; Chen, W.; Yu, T.; Hayat, T.; Alsaedi, A.; Guo, F.; Tan, Z.; et al. Thiophene-arylamine hole transporting materials in perovskite solar cells: Substitution position effect. Energy Technol. 2017. [Google Scholar] [CrossRef]
- Pham, H.D.; Wu, Z.; Ono, L.K.; Manzhos, S.; Feron, K.; Motta, N.; Qi, Y.; Sonar, P. Low-Cost Alternative High-Performance Hole-Transport Material for Perovskite Solar Cells and Its Comparative Study with Conventional SPIRO-OMeTAD. Adv. Electron. Mater. 2017, 3. [Google Scholar] [CrossRef]
- Rakstys, K.; Paek, S.; Sohail, M.; Gao, P.; Cho, K.T.; Gratia, P.; Lee, Y.; Dahmen, K.H.; Nazeeruddin, M.K.; Zakeeruddin, S.M.; et al. A highly hindered bithiophene-functionalized dispiro-oxepine derivative as an efficient hole transporting material for perovskite solar cells. J. Mater. Chem. A 2016, 4, 18259–18264. [Google Scholar] [CrossRef]
- Zimmermann, I.; Urieta-Mora, J.; Gratia, P.; Aragó, J.; Grancini, G.; Molina-Ontoria, A.; Ortí, E.; Martín, N.; Nazeeruddin, M.K. High-Efficiency Perovskite Solar Cells Using Molecularly Engineered, Thiophene-Rich, Hole-Transporting Materials: Influence of Alkyl Chain Length on Power Conversion Efficiency. Adv. Energy Mater. 2017, 7. [Google Scholar] [CrossRef]
- Chen, H.; Bryant, D.; Troughton, J.; Kirkus, M.; Neophytou, M.; Miao, X.; Durrant, J.R.; McCulloch, I. One-Step Facile Synthesis of a Simple Hole Transport Material for Efficient Perovskite Solar Cells. Chem. Mater. 2016, 28, 2515–2518. [Google Scholar] [CrossRef]
- Do, K.; Choi, H.; Lim, K.; Jo, H.; Cho, J.W.; Nazeeruddin, M.K.; Ko, J. Star-shaped hole transporting materials with a triazine unit for efficient perovskite solar cells. Chem. Commun. 2014, 50, 10971–10974. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.; Kang, M.S.; Myung, Y.; Seo, J.H.; Banerjee, P.; Marks, T.J.; Ko, J.; Lich, T.; Wang, S.; Luo, Y.; et al. Star-shaped hole transport materials with indeno[1,2-b] thiophene or fluorene on a triazine core for efficient perovskite solar cells. J. Mater. Chem. A 2016, 4, 1186–1190. [Google Scholar] [CrossRef]
- Molina-Ontoria, A.; Zimmermann, I.; Garcia-Benito, I.; Gratia, P.; Roldán-Carmona, C.; Aghazada, S.; Graetzel, M.; Nazeeruddin, M.K.; Martín, N. Benzotrithiophene-Based Hole-Transporting Materials for 18.2% Perovskite Solar Cells. Angew. Chem. Int. Ed. 2016, 55, 6270–6274. [Google Scholar] [CrossRef] [PubMed]
- García-Benito, I.; Zimmermann, I.; Urieta-Mora, J.; Aragó, J.; Molina-Ontoria, A.; Ortí, E.; Martín, N.; Nazeeruddin, M.K.; Bao, Z.; Aspuru-Guzik, A.; et al. Isomerism effect on the photovoltaic properties of benzotrithiophene-based hole-transporting materials. J. Mater. Chem. A 2017, 5, 8317–8324. [Google Scholar] [CrossRef]
- Paek, S.; Rub, M.A.; Choi, H.; Kosa, S.A.; Alamry, K.A.; Cho, J.W.; Gao, P.; Ko, J.; Asiri, A.M.; Nazeeruddin, M.K.; et al. A dual-functional asymmetric squaraine-based low band gap hole transporting material for efficient perovskite solar cells. Nanoscale 2016, 8, 6335–6340. [Google Scholar] [CrossRef] [PubMed]
- Rakstys, K.; Saliba, M.; Gao, P.; Gratia, P.; Kamarauskas, E.; Paek, S.; Jankauskas, V.; Nazeeruddin, M.K. Highly Efficient Perovskite Solar Cells Employing an Easily Attainable Bifluorenylidene-Based Hole-Transporting Material. Angew. Chem. Int. Ed. 2016, 55, 7464–7468. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.K.; Yuan, Z.C.; Shi, G.Z.; Li, Y.X.; Li, Q.; Hui, F.; Sun, B.Q.; Jiang, Z.Q.; Liao, L.S. Dopant-Free Spiro-Triphenylamine/Fluorene as Hole-Transporting Material for Perovskite Solar Cells with Enhanced Efficiency and Stability. Adv. Funct. Mater. 2016, 26, 1375–1381. [Google Scholar] [CrossRef]
- Reddy, S.S.; Gunasekar, K.; Heo, J.H.; Im, S.H.; Kim, C.S.; Kim, D.H.; Moon, J.H.; Lee, J.Y.; Song, M.; Jin, S.H. Highly Efficient Organic Hole Transporting Materials for Perovskite and Organic Solar Cells with Long-Term Stability. Adv. Mater. 2016, 28, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Tiazkis, R.; Paek, S.; Daskeviciene, M.; Malinauskas, T.; Saliba, M.; Nekrasovas, J.; Jankauskas, V.; Ahmad, S.; Getautis, V.; Nazeeruddin, M.K. Methoxydiphenylamine-substituted fluorene derivatives as hole transporting materials: Role of molecular interaction on device photovoltaic performance. Sci. Rep. 2017, 7, 150. [Google Scholar] [CrossRef] [PubMed]
- Bi, D.; Xu, B.; Gao, P.; Sun, L.; Grätzel, M.; Hagfeldt, A. Facile synthesized organic hole transporting material for perovskite solar cell with efficiency of 19.8%. Nano Energy 2016, 23, 138–144. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, J.; Hua, Y.; Liu, P.; Wang, L.; Ruan, C.; Li, Y.; Boschloo, G.; Johansson, E.M.J.; Kloo, L.; et al. Tailor-Making Low-Cost Spiro[fluorene-9,9’-xanthene]-Based 3D Oligomers for Perovskite Solar Cells. Chem 2017, 2, 676–687. [Google Scholar] [CrossRef]
- Xu, B.; Zhu, Z.; Zhang, J.; Liu, H.; Chueh, C.C.; Li, X.; Jen, A.K.Y. 4-Tert-butylpyridine Free Organic Hole Transporting Materials for Stable and Efficient Planar Perovskite Solar Cells. Adv. Energy Mater. 2017. [Google Scholar] [CrossRef]
- Malinauskas, T.; Saliba, M.; Matsui, T.; Daskeviciene, M.; Urnikaite, S.; Gratia, P.; Send, R.; Wonneberger, H.; Bruder, I.; Graetzel, M.; et al. Branched methoxydiphenylamine-substituted fluorene derivatives as hole transporting materials for high-performance perovskite solar cells. Energy Environ. Sci. 2016, 9, 1681–1686. [Google Scholar] [CrossRef]
- Liu, K.; Yao, Y.; Wang, J.; Zhu, L.; Sun, M.; Ren, B.; Xie, L.; Luo, Y.; Meng, Q.; Zhan, X.; et al. Spiro[fluorene-9,9’-xanthene]-based hole transporting materials for efficient perovskite solar cells with enhanced stability. Mater. Chem. Front. 2017, 1, 100–110. [Google Scholar] [CrossRef]
- Wang, H.; Sheikh, A.D.; Feng, Q.; Li, F.; Chen, Y.; Yu, W.; Alarousu, E.; Ma, C.; Haque, M.A.; Shi, D.; et al. Facile Synthesis and High Performance of a New Carbazole-Based Hole-Transporting Material for Hybrid Perovskite Solar Cells. ACS Photonics 2015, 2, 849–855. [Google Scholar] [CrossRef]
- Leijtens, T.; Giovenzana, T.; Habisreutinger, S.N.; Tinkham, J.S.; Noel, N.K.; Kamino, B.A.; Sadoughi, G.; Sellinger, A.; Snaith, H.J. Hydrophobic Organic Hole Transporters for Improved Moisture Resistance in Metal Halide Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2016, 8, 5981–5989. [Google Scholar] [CrossRef] [PubMed]
- Sung, S.D.; Kang, M.S.; Choi, I.T.; Kim, H.M.; Kim, H.; Hong, M.; Kim, H.K.; Lee, W.I.; Docampo, P.; McPherson, I.; et al. 14.8% perovskite solar cells employing carbazole derivatives as hole transporting materials. Chem. Commun. 2014, 50, 14161–14163. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, Y.; Li, F.; Zong, X.; Guo, J.; Sun, Z.; Xue, S. A new carbazole-based hole-transporting material with low dopant content for perovskite solar cells. Electrochim. Acta 2016, 210, 673–680. [Google Scholar] [CrossRef]
- Gabrielsson, E.; Ellis, H.; Feldt, S.; Tian, H.; Boschloo, G.; Hagfeldt, A.; Sun, L. Convergent/Divergent Synthesis of a Linker-Varied Series of Dyes for Dye-Sensitized Solar Cells Based on the D35 Donor. Adv. Energy Mater. 2013, 3, 1647–1656. [Google Scholar] [CrossRef]
- Kang, M.S.; Sung, S.D.; Choi, I.T.; Kim, H.; Hong, M.; Kim, J.; Lee, W.I.; Kim, H.K. Novel Carbazole-Based Hole-Transporting Materials with Star-Shaped Chemical Structures for Perovskite-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2015, 7, 22213–22217. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.D.; Zheng, B.H.; Zhuang, Q.F.; Huang, C.Y.; Cao, H.; Chen, M.D.; Wang, B. Two dimethoxyphenylamine-substituted carbazole derivatives as hole-transporting materials for efficient inorganic-organic hybrid perovskite solar cells. Dyes Pigment. 2017, 146, 589–595. [Google Scholar] [CrossRef]
- Daskeviciene, M.; Paek, S.; Wang, Z.; Malinauskas, T.; Jokubauskaite, G.; Rakstys, K.; Cho, K.T.; Magomedov, A.; Jankauskas, V.; Ahmad, S.; et al. Carbazole-based enamine: Low-cost and efficient hole transporting material for perovskite solar cells. Nano Energy 2017, 32, 551–557. [Google Scholar] [CrossRef]
- Chen, Z.; Li, H.; Zheng, X.; Zhang, Q.; Li, Z.; Hao, Y.; Fang, G. Low-Cost Carbazole-Based Hole-Transport Material for Highly Efficient Perovskite Solar Cells. ChemSusChem 2017, 10, 3111–3117. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zhang, F.; Liu, X.; Sun, M.; Han, J.; You, J.; Wang, S.; Xiao, Y.; Li, X. Dopant-Free Hole-Transport Material with a Tetraphenylethene Core for Efficient Perovskite Solar Cells. Energy Technol. 2017, 5, 1257–1264. [Google Scholar] [CrossRef]
- Zhu, L.; Shan, Y.; Wang, R.; Liu, D.; Zhong, C.; Song, Q.; Wu, F. High-Efficiency Perovskite Solar Cells Based on New TPE Compounds as Hole Transport Materials: The Role of 2,7- and 3,6-Substituted Carbazole Derivatives. Chem. A Eur. J. 2017, 23, 4373–4379. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Sheibani, E.; Liu, P.; Zhang, J.; Tian, H.; Vlachopoulos, N.; Boschloo, G.; Kloo, L.; Hagfeldt, A.; Sun, L. Carbazole-Based Hole-Transport Materials for Efficient Solid-State Dye-Sensitized Solar Cells and Perovskite Solar Cells. Adv. Mater. 2014, 26, 6629–6634. [Google Scholar] [CrossRef] [PubMed]
- Zong, X.; Qiao, W.; Chen, Y.; Sun, Z.; Liang, M.; Xue, S. A new binaphthol based hole-transporting materials for perovskite solar cells. Tetrahedron 2017, 73, 3398–3405. [Google Scholar] [CrossRef]
- Li, X.; Cai, M.; Zhou, Z.; Yun, K.; Xie, F.; Lan, Z.; Hua, J.; Han, L.; Nemnes, G.A.; Iliescu, M.; et al. A comparative study of o,p-dimethoxyphenyl-based hole transport materials by altering π-linker units for highly efficient and stable perovskite solar cells. J. Mater. Chem. A 2017, 5, 10480–10485. [Google Scholar] [CrossRef]
- Nazim, M.; Ameen, S.; Akhtar, M.S.; Khaja Nazeeruddin, M.; Shin, H.S. Tuning electronic structures of thiazolo[5,4-d]thiazole-based hole-transporting materials for efficient perovskite solar cells. Sol. Energy Mater. Sol. Cells 2017, in press. [Google Scholar] [CrossRef]
- Liu, X.; Kong, F.; Cheng, T.; Chen, W.; Tan, Z.; Yu, T.; Guo, F.; Chen, J.; Yao, J.; Dai, S. Tetraphenylmethane-Arylamine Hole-Transporting Materials for Perovskite Solar Cells. ChemSusChem 2017, 10, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Petrus, M.L.; Bein, T.; Dingemans, T.J.; Docampo, P.; Wienk, M.M.; Janssen, R.A.J.; Olivier, Y.; Savenije, T.J.; Siebbeles, L.D.A.; Greenham, N.C.; et al. A low cost azomethine-based hole transporting material for perovskite photovoltaics. J. Mater. Chem. A 2015, 3, 12159–12162. [Google Scholar] [CrossRef]
- Poplavskyy, D.; Nelson, J. Nondispersive hole transport in amorphous films of methoxy-spirofluorene-arylamine organic compound. J. Appl. Phys. 2003, 93, 341–346. [Google Scholar] [CrossRef]
- Shinde, D.B.; Salunke, J.K.; Candeias, N.R.; Tinti, F.; Gazzano, M.; Wadgaonkar, P.P.; Priimagi, A.; Camaioni, N.; Vivo, P. Crystallisation-enhanced bulk hole mobility in phenothiazine-based organic semiconductors. Sci. Rep. 2017, 7, 46268. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Yang, J.; Hua, J.; Tang, J.; Zhang, L.; Long, Y.; Tian, H.; Wang, P.; Grätzel, M.; Miura, H.; et al. Efficient and stable dye-sensitized solar cells based on phenothiazine sensitizers with thiophene units. J. Mater. Chem. 2010, 20, 1772. [Google Scholar] [CrossRef]
- Salunke, J.K.; Wong, F.L.; Feron, K.; Manzhos, S.; Lo, M.F.; Shinde, D.; Patil, A.; Lee, C.S.; Roy, V.A.L.; Sonar, P.; et al. Phenothiazine and carbazole substituted pyrene based electroluminescent organic semiconductors for OLED devices. J. Mater. Chem. C 2016, 4, 1009–1018. [Google Scholar] [CrossRef]
- Venkateswararao, A.; Thomas, K.R.J.; Lee, C.P.; Li, C.T.; Ho, K.C. Organic Dyes Containing Carbazole as Donor and π-Linker: Optical, Electrochemical, and Photovoltaic Properties. ACS Appl. Mater. Interfaces 2014, 6, 2528–2539. [Google Scholar] [CrossRef] [PubMed]
- Grigalevicius, S.; Ma, L.; Grazulevicius, J.V.; Xie, Z.Y. Well defined carbazole-based hole-transporting amorphous molecular materials. Synth. Met. 2006, 156, 46–50. [Google Scholar] [CrossRef]
- Di Giacomo, F.; Razza, S.; Matteocci, F.; D’Epifanio, A.; Licoccia, S.; Brown, T.M.; Di Carlo, A. High efficiency CH3NH3PbI(3 − x)Clx perovskite solar cells with poly(3-hexylthiophene) hole transport layer. J. Power Sources 2014, 251, 152–156. [Google Scholar] [CrossRef]
- Wu, F.; Ji, Y.; Wang, R.; Shan, Y.; Zhu, L. Molecular engineering to enhance perovskite solar cell performance: Incorporation of benzothiadiazole as core unit for low cost hole transport materials. Dyes Pigment. 2017, 143, 356–360. [Google Scholar] [CrossRef]
- Sigma-Aldrich. PTAA-Poly[bis(4-phenyl)(2,4,6-trimethylphenyl)amine]. Available online: http://www.sigmaaldrich.com/catalog/product/aldrich/702471?lang=fi®ion=FI (accessed on 11 August 2017).
- Ma, W.; Yang, C.; Gong, X.; Lee, K.; Heeger, A.J. Thermally Stable, Efficient Polymer Solar Cells with Nanoscale Control of the Interpenetrating Network Morphology. Adv. Funct. Mater. 2005, 15, 1617–1622. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, C.; Inoue, K.; Harano, K.; Tanaka, H.; Nakamura, E. Enhancement in the efficiency of an organic—Inorganic hybrid solar cell with a doped P3HT hole-transporting layer on a void-free perovskite active layer. J. Mater. Chem. A 2014, 2, 13827–13830. [Google Scholar] [CrossRef]
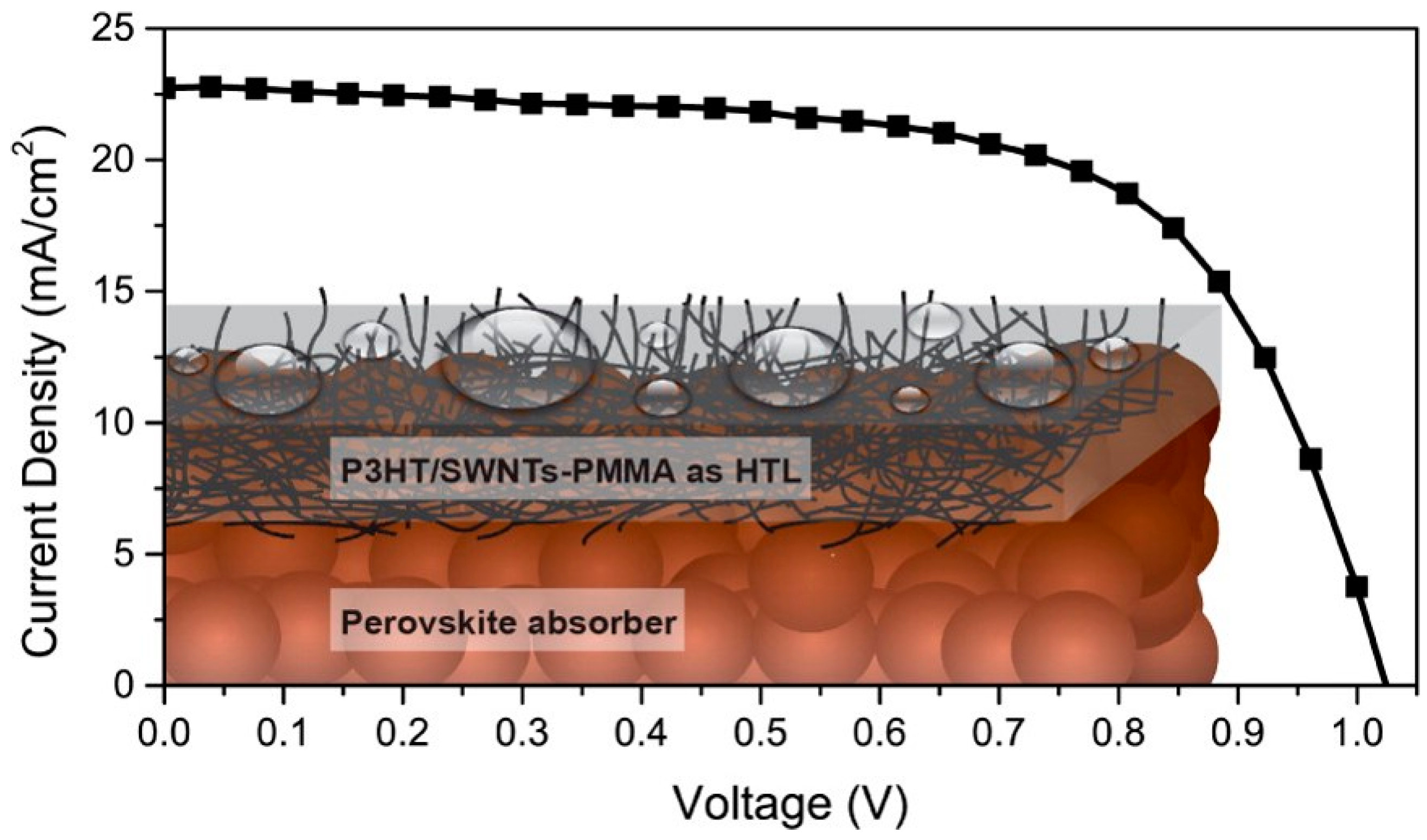
- Cai, M.; Tiong, V.T.; Hreid, T.; Bell, J.; Wang, H.; Bradforth, S.E.; Thompson, M.E.; Salamo, G.J.; Viswanathan, T.; Biris, A.R.; et al. An efficient hole transport material composite based on poly(3-hexylthiophene) and bamboo-structured carbon nanotubes for high performance perovskite solar cells. J. Mater. Chem. A 2015, 3, 2784–2793. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, H.; Wei, Z.; Yang, Y.; Lin, H.; Yang, S. High-performance, stable and low-cost mesoscopic perovskite (CH3NH3PbI3) solar cells based on poly(3-hexylthiophene)-modified carbon nanotube cathodes. Front. Optoelectron. 2016, 9, 71–80. [Google Scholar] [CrossRef]
- You, J.; Yang, Y.(M.); Hong, Z.; Song, T.B.; Meng, L.; Liu, Y.; Jiang, C.; Zhou, H.; Chang, W.H.; Li, G.; et al. Moisture assisted perovskite film growth for high performance solar cells. Appl. Phys. Lett. 2014, 105, 183902. [Google Scholar] [CrossRef]
- Dubey, A.; Adhikari, N.; Venkatesan, S.; Gu, S.; Khatiwada, D.; Wang, Q.; Mohammad, L.; Kumar, M.; Qiao, Q. Solution processed pristine PDPP3T polymer as hole transport layer for efficient perovskite solar cells with slower degradation. Sol. Energy Mater. Sol. Cells 2016, 145, 193–199. [Google Scholar] [CrossRef]
- Wong-Stringer, M.; Bishop, J.E.; Smith, J.A.; Mohamad, D.K.; Parnell, A.J.; Kumar, V.; Rodenburg, C.; Lidzey, D.G.; Burn, P.L.; Gentle, I.R.; et al. Efficient perovskite photovoltaic devices using chemically doped PCDTBT as a hole-transport material. J. Mater. Chem. A 2017, 5, 15714–15723. [Google Scholar] [CrossRef]
- Heo, J.; Im, S.; Noh, J.; Mandal, T.; Lim, C. Efficient inorganic-organic hybrid heterojunction solar cells containing perovskite compound and polymeric hole conductors. Nat. Photonics 2013, 7, 486–491. [Google Scholar] [CrossRef]
- Yu, Z.; Zhang, Y.; Jiang, X.; Li, X.; Lai, J.; Hu, M.; Elawad, M.; Gurzadyan, G.G.; Yang, X.; Sun, L.; et al. High-efficiency perovskite solar cells employing a conjugated donor—Acceptor co-polymer as a hole-transporting material. RSC Adv. 2017, 7, 27189–27197. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhao, Y.; Zhang, C.; Zou, D.; Chen, Y.; Lin, Z.; Zhen, H.; Ling, Q.; He, Y.; Maculan, G.; et al. A facile one-pot synthesis of hyper-branched carbazole-based polymer as a hole-transporting material for perovskite solar cells. J. Mater. Chem. A 2017, 5, 6613–6621. [Google Scholar] [CrossRef]
- Liu, J.; Ge, Q.; Zhang, W.; Ma, J.; Ding, J.; Yu, G.; Hu, J. Highly π-extended copolymer as additive-free hole-transport material for perovskite solar cells. Nano Res. 2017, 1–10. [Google Scholar] [CrossRef]
- Xu, Y.; Bu, T.; Li, M.; Qin, T.; Yin, C.; Wang, N.; Li, R.; Zhong, J.; Li, H.; Peng, Y.; et al. Non-Conjugated Polymer as an Efficient Dopant-Free Hole-Transporting Material for Perovskite Solar Cells. ChemSusChem 2017, 10, 2578–2584. [Google Scholar] [CrossRef] [PubMed]
- Gaml, E.A.; Dubey, A.; Reza, K.M.; Hasan, M.N.; Adhikari, N.; Elbohy, H.; Bahrami, B.; Zeyada, H.; Yang, S.; Qiao, Q. Alternative benzodithiophene (BDT) based polymeric hole transport layer for efficient perovskite solar cells. Sol. Energy Mater. Sol. Cells 2017, 168, 8–13. [Google Scholar] [CrossRef]
- Park, N.G.; Grätzel, M.; Miyasaka, T. Organic-Inorganic Halide Perovskite Photovoltaics: From Fundamentals to Device Architectures; Springer: Cham, Switzerland, 2016; ISBN 9783319351148. [Google Scholar]
- Li, M.H.; Shen, P.S.; Wang, K.C.; Guo, T.F.; Chen, P. Inorganic p-type contact materials for perovskite-based solar cells. J. Mater. Chem. A 2015, 3, 9011–9019. [Google Scholar] [CrossRef]
- Zhang, P.P.; Zhou, Z.J.; Kou, D.X.; Wu, S.X. Perovskite Thin Film Solar Cells Based on Inorganic Hole Conducting Materials. Int. J. Photoenergy 2017, 2017, 1–10. [Google Scholar] [CrossRef]
- Wang, K.C.; Jeng, J.Y.; Shen, P.S.; Chang, Y.C.; Diau, E.W.G.; Tsai, C.H.; Chao, T.Y.; Hsu, H.C.; Lin, P.Y.; Chen, P.; et al. P-type Mesoscopic Nickel Oxide/Organometallic Perovskite Heterojunction Solar Cells. Sci. Rep. 2014, 4, 4756. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.C.; Shen, P.S.; Li, M.H.; Chen, S.; Lin, M.W.; Chen, P.; Guo, T.F. Low-Temperature Sputtered Nickel Oxide Compact Thin Film as Effective Electron Blocking Layer for Mesoscopic NiO/CH3NH3PbI3 Perovskite Heterojunction Solar Cells. ACS Appl. Mater. Interfaces 2014, 6, 11851–11858. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Seo, J.; Park, S.; Shin, S.S.; Kim, Y.C.; Jeon, N.J.; Shin, H.W.; Ahn, T.K.; Noh, J.H.; Yoon, S.C.; et al. Efficient CH3NH3PbI3 Perovskite Solar Cells Employing Nanostructured p-Type NiO Electrode Formed by a Pulsed Laser Deposition. Adv. Mater. 2015, 27, 4013–4019. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wu, Y.; Liu, J.; Qin, C.; Yang, X.; Islam, A.; Cheng, Y.B.; Han, L. Hybrid interfacial layer leads to solid performance improvement of inverted perovskite solar cells. Energy Environ. Sci. 2015, 8, 629. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, M.; Xu, X.; Bu, L.; Zhang, W.; Li, W.; Zhao, Z.; Wang, M.; Cheng, Y.B.; He, H. Dalton Transactions: An international journal of inorganic chemistry p-Type mesoscopic NiO as an active interfacial layer for carbon counter electrode based perovskite solar cells. Dalton. Trans. 2015, 44, 3879–4390. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, M.; Xu, X.; Cai, F.; Yuan, H.; Bu, L.; Li, W.; Zhu, A.; Zhao, Z.; Wang, M.; et al. NiO nanosheets as efficient top hole transporters for carbon counter electrode based perovskite solar cells. J. Mater. Chem. A 2015, 3, 24121–24127. [Google Scholar] [CrossRef]
- Xu, X.; Liu, Z.; Zuo, Z.; Zhang, M.; Zhao, Z.; Shen, Y.; Zhou, H.; Chen, Q.; Yang, Y.; Wang, M. Hole Selective NiO Contact for Efficient Perovskite Solar Cells with Carbon Electrode. Nano Lett. 2015, 15, 2402–2408. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.; Zuo, Z.; Cui, J.; Shen, Y.; Moehl, T.; Zakeeruddin, S.M.; Grätzel, M.; Wang, M. Efficient screen printed perovskite solar cells based on mesoscopic TiO2 /Al2O3 /NiO/carbon architecture. Nano Energy 2015, 17, 171–179. [Google Scholar] [CrossRef]
- You, J.; Meng, L.; Song, T.B.; Guo, T.F.; Yang, Y.; Chang, W.H.; Hong, Z.; Chen, H.; Zhou, H.; Chen, Q.; et al. Improved air stability of perovskite solar cells via solution-processed metal oxide transport layers. Nat. Nanotechnol. 2016, 11, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wu, Y.; Yue, Y.; Liu, J.; Zhang, W.; Yang, X.; Chen, H.; Bi, E.; Ashraful, I.; Grätzel, M.; et al. Efficient and stable large-area perovskite solar cells with inorganic charge extraction layers. Science 2015, 350, 944–948. [Google Scholar] [CrossRef] [PubMed]
- Jeng, J.Y.; Chen, K.C.; Chiang, T.Y.; Lin, P.Y.; Tsai, T.D.; Chang, Y.C.; Guo, T.F.; Chen, P.; Wen, T.C.; Hsu, Y.J. Nickel Oxide Electrode Interlayer in CH3NH3PbI3 Perovskite/PCBM Planar-Heterojunction Hybrid Solar Cells. Adv. Mater. 2014, 26, 4107–4113. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Bai, Y.; Zhang, T.; Liu, Z.; Long, X.; Wei, Z.; Wang, Z.; Zhang, L.; Wang, J.; Yan, F.; et al. High-Performance Hole-Extraction Layer of Sol-Gel-Processed NiO Nanocrystals for Inverted Planar Perovskite Solar Cells. Angew. Chem. Int. Ed. 2014, 53, 12571–12575. [Google Scholar] [CrossRef]
- Cui, J.; Meng, F.; Zhang, H.; Cao, K.; Yuan, H.; Cheng, Y.; Huang, F.; Wang, M. CH3NH3PbI3-Based Planar Solar Cells with Magnetron-Sputtered Nickel Oxide. ACS Appl. Mater. Interfaces 2014, 6, 22862–22870. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Peng, J.; Wang, W.; Xia, Z.; Yuan, J.; Lu, J.; Huang, X.; Ma, W.; Song, H.; Chen, W.; et al. Sequential Deposition of CH3NH3PbI3 on Planar NiO Film for Efficient Planar Perovskite Solar Cells. ACS Photonics 2014, 1, 547–553. [Google Scholar] [CrossRef]
- Jiang, F.; Choy, W.C.H.; Li, X.; Zhang, D.; Cheng, J. Post-treatment-Free Solution-Processed Non-stoichiometric NiOx Nanoparticles for Efficient Hole-Transport Layers of Organic Optoelectronic Devices. Adv. Mater. 2015, 27, 2930–2937. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Chen, P.; Que, M.; Xing, Y.; Que, W.; Niu, C.; Shao, J. Highly Efficient Flexible Perovskite Solar Cells Using Solution-Derived NiOx Hole Contacts. ACS Nano 2016, 10, 3630–3636. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cheng, J.; Lin, F.; He, H.; Mao, J.; Wong, K.S.; Jen, A.K.Y.; Choy, W.C.H. Pinhole-Free and Surface-Nanostructured NiOx Film by Room-Temperature Solution Process for High-Performance Flexible Perovskite Solar Cells with Good Stability and Reproducibility. ACS Nano 2016, 10, 1503–1511. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.W.; Chueh, C.C.; Jen, A.K.Y. A Low-Temperature, Solution-Processable, Cu-Doped Nickel Oxide Hole-Transporting Layer via the Combustion Method for High-Performance Thin-Film Perovskite Solar Cells. Adv. Mater. 2015, 27, 7874–7880. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Liang, P.W.; Williams, S.T.; Cho, N.; Chueh, C.C.; Glaz, M.S.; Ginger, D.S.; Jen, A.K.Y. High-Performance and Environmentally Stable Planar Heterojunction Perovskite Solar Cells Based on a Solution-Processed Copper-Doped Nickel Oxide Hole-Transporting Layer. Adv. Mater. 2015, 27, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.; Park, I.J.; Kim, M.; Lee, S.; Bae, C.; Jung, H.S.; Park, N.G.; Kim, J.Y.; Shin, H.; Yu, H.; et al. An ultra-thin, un-doped NiO hole transporting layer of highly efficient (16.4%) organic—Inorganic hybrid perovskite solar cells. Nanoscale 2016, 8, 11403–11412. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhu, A.; Cai, F.; Tao, L.; Zhou, Y.; Zhao, Z.; Chen, Q.; Cheng, Y.B.; Zhou, H.; Spiccia, L.; et al. Nickel oxide nanoparticles for efficient hole transport in p-i-n and n-i-p perovskite solar cells. J. Mater. Chem. A 2017, 5, 6597–6605. [Google Scholar] [CrossRef]
- Christians, J.A.; Fung, R.C.M.; Kamat, P.V. An Inorganic Hole Conductor for Organo-Lead Halide Perovskite Solar Cells. Improved Hole Conductivity with Copper Iodide. J. Am. Chem. Soc. 2014, 136, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Nejand, B.A.; Ahmadi, V.; Gharibzadeh, S.; Shahverdi, H.R. Cuprous Oxide as a Potential Low-Cost Hole-Transport Material for Stable Perovskite Solar Cells. ChemSusChem 2016, 9, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xia, Z.; Liang, J.; Wang, X.; Liu, Y.; Liu, C.; Zhang, S.; Zhou, H. Towards printed perovskite solar cells with cuprous oxide hole transporting layers: A theoretical design. Semicond. Sci. Technol. 2015, 30, 054004. [Google Scholar] [CrossRef]
- Qin, P.; Tanaka, S.; Ito, S.; Tetreault, N.; Manabe, K.; Nishino, H.; Nazeeruddin, M.K.; Grätzel, M. Inorganic hole conductor-based lead halide perovskite solar cells with 12.4% conversion efficiency. Nat. Commun. 2014, 5, 3834. [Google Scholar] [CrossRef] [PubMed]
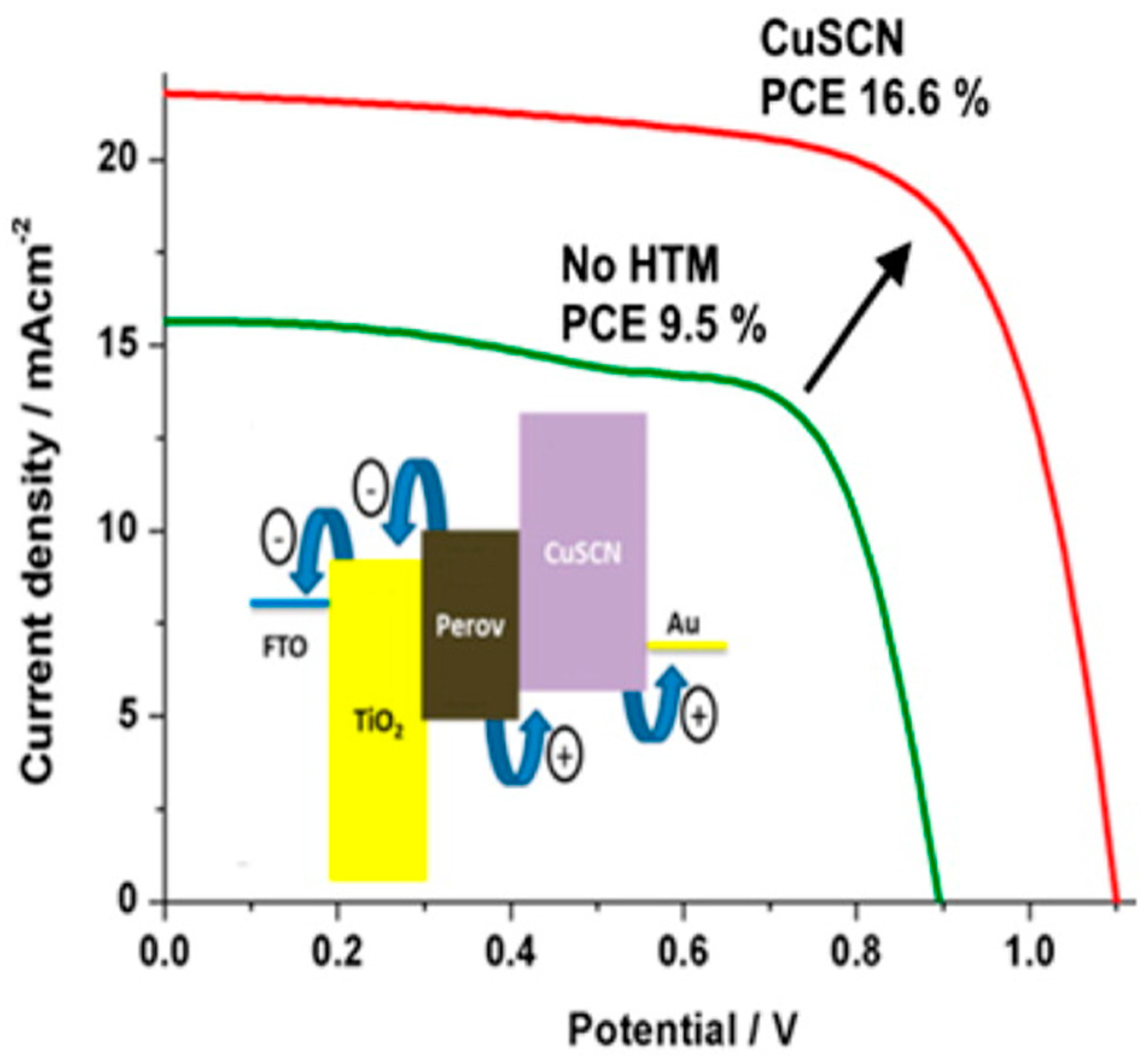
- Madhavan, V.E.; Zimmermann, I.; Roldán-Carmona, C.; Grancini, G.; Buffiere, M.; Belaidi, A.; Nazeeruddin, M.K. Copper Thiocyanate Inorganic Hole-Transporting Material for High-Efficiency Perovskite Solar Cells. ACS Energy Lett. 2016, 1, 1112–1117. [Google Scholar] [CrossRef]
- Jung, M.; Kim, Y.C.; Jeon, N.J.; Yang, W.S.; Seo, J.; Noh, J.H.; Seok, S., II. Thermal Stability of CuSCN Hole Conductor-Based Perovskite Solar Cells. ChemSusChem 2016, 9, 2592–2596. [Google Scholar] [CrossRef] [PubMed]
- Palma, A.L.; Cinà, L.; Pescetelli, S.; Agresti, A.; Raggio, M.; Paolesse, R.; Bonaccorso, F.; Di Carlo, A. Reduced graphene oxide as efficient and stable hole transporting material in mesoscopic perovskite solar cells. Nano Energy 2016, 22, 349–360. [Google Scholar] [CrossRef]
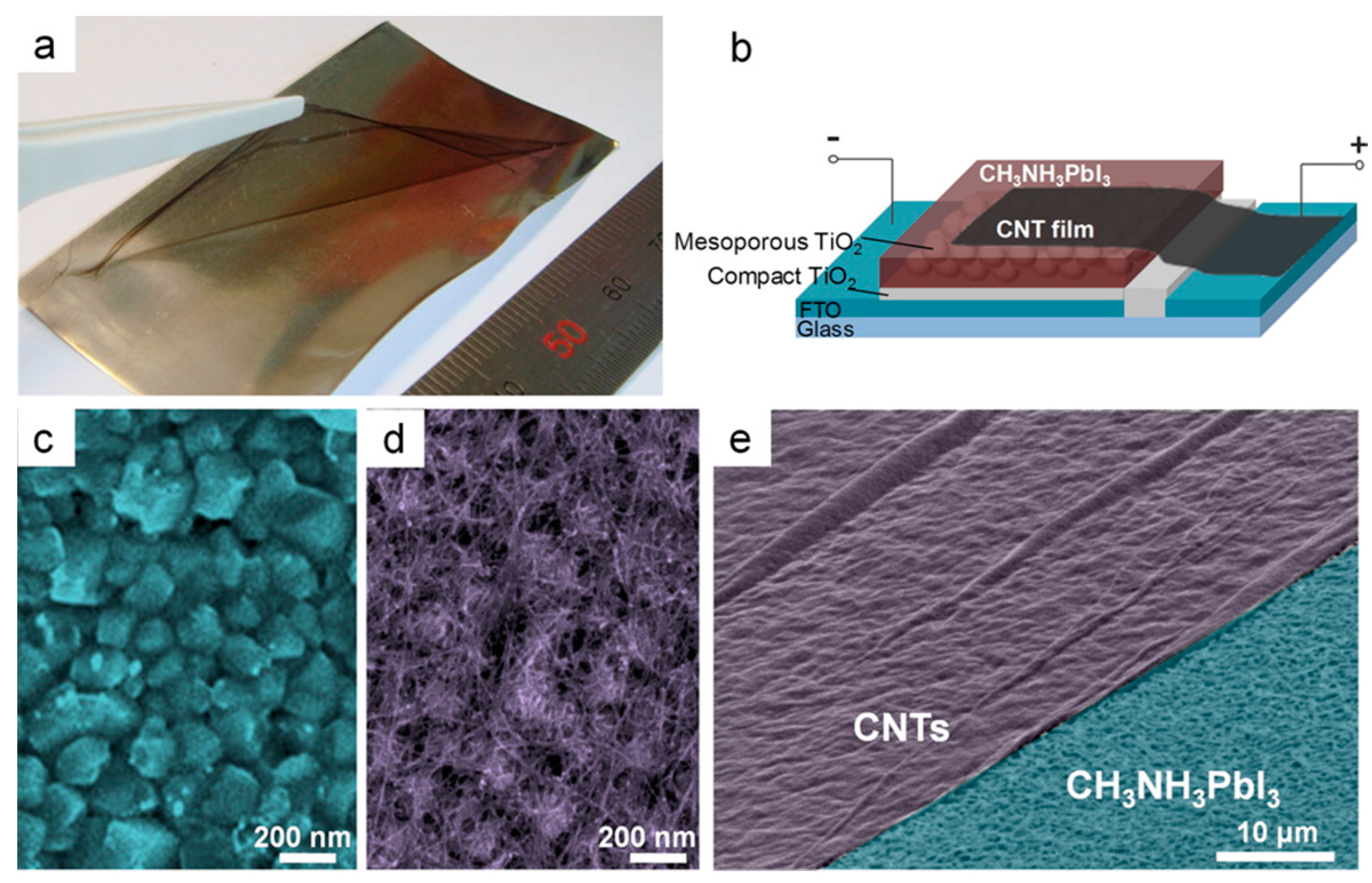
- Li, Z.; Kulkarni, S.A.; Boix, P.P.; Shi, E.; Cao, A.; Fu, K.; Batabyal, S.K.; Zhang, J.; Xiong, Q.; Wong, L.H.; et al. Laminated Carbon Nanotube Networks for Metal Electrode-Free Efficient Perovskite Solar Cells. ACS Nano 2014, 8, 6797–6804. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Menamparambath, M.M.; Hwang, J.Y.; Baik, S. Hierarchically Structured Hole Transport Layers of Spiro-OMeTAD and Multiwalled Carbon Nanotubes for Perovskite Solar Cells. ChemSusChem 2015, 8, 2358–2362. [Google Scholar] [CrossRef] [PubMed]
- Habisreutinger, S.N.; Nicholas, R.J.; Snaith, H.J. Carbon Nanotubes in Perovskite Solar Cells. Adv. Energy Mater. 2017, 7, 1601839. [Google Scholar] [CrossRef]
- Aitola, K.; Sveinbjörnsson, K.; Correa-Baena, J.P.; Kaskela, A.; Abate, A.; Tian, Y.; Johansson, E.M.J.; Grätzel, M.; Kauppinen, E.I.; Hagfeldt, A.; et al. Carbon nanotube-based hybrid hole-transporting material and selective contact for high efficiency perovskite solar cells. Energy Environ. Sci. 2016, 9, 461–466. [Google Scholar] [CrossRef]
- Habisreutinger, S.N.; Leijtens, T.; Eperon, G.E.; Stranks, S.D.; Nicholas, R.J.; Snaith, H.J. Carbon Nanotube/Polymer Composites as a Highly Stable Hole Collection Layer in Perovskite Solar Cells. Nano Lett. 2014, 14, 5561–5568. [Google Scholar] [CrossRef] [PubMed]
- Aitola, K.; Domanski, K.; Correa-Baena, J.P.; Sveinbjörnsson, K.; Saliba, M.; Abate, A.; Grätzel, M.; Kauppinen, E.; Johansson, E.M.J.; Tress, W.; et al. High Temperature-Stable Perovskite Solar Cell Based on Low-Cost Carbon Nanotube Hole Contact. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]

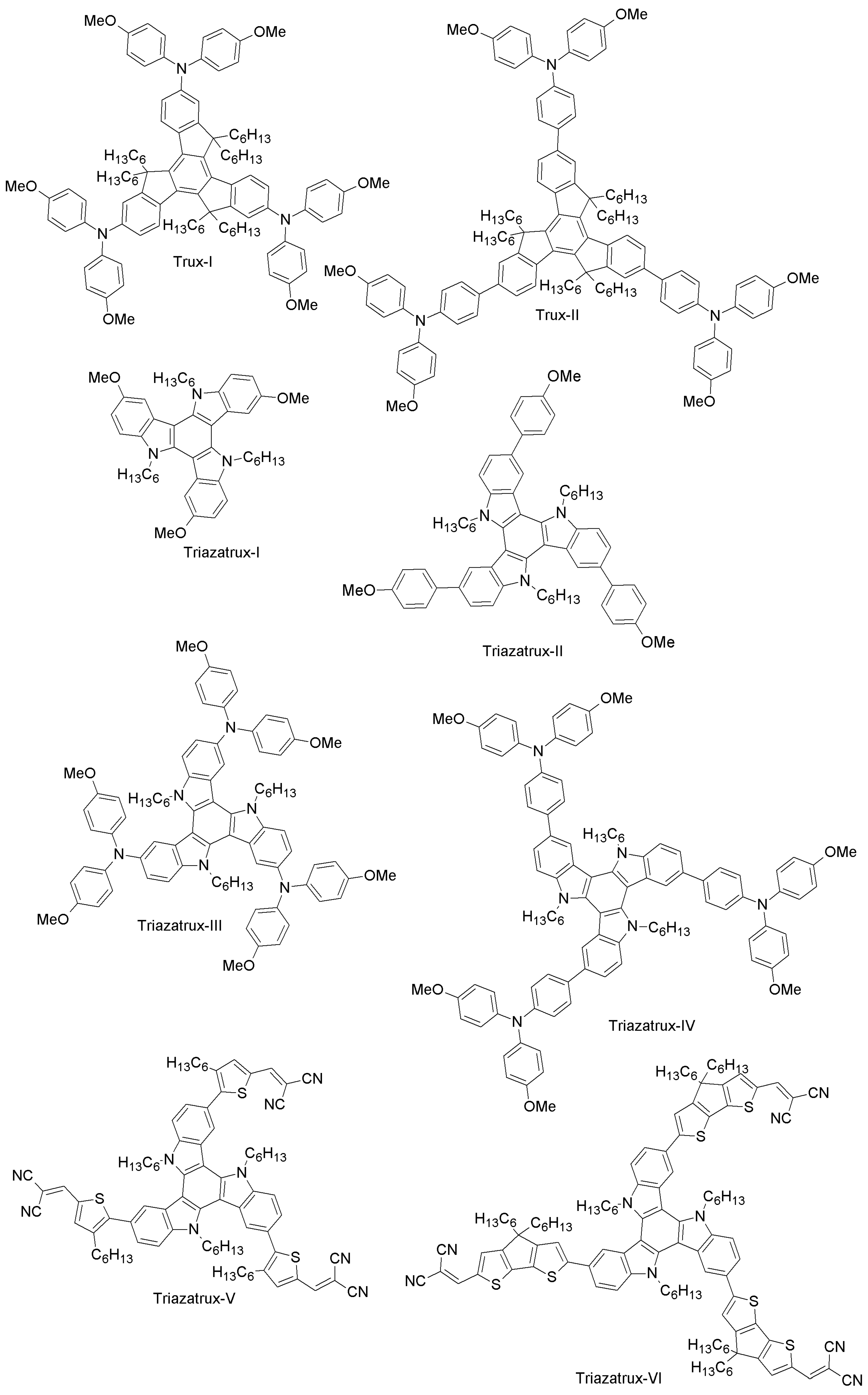



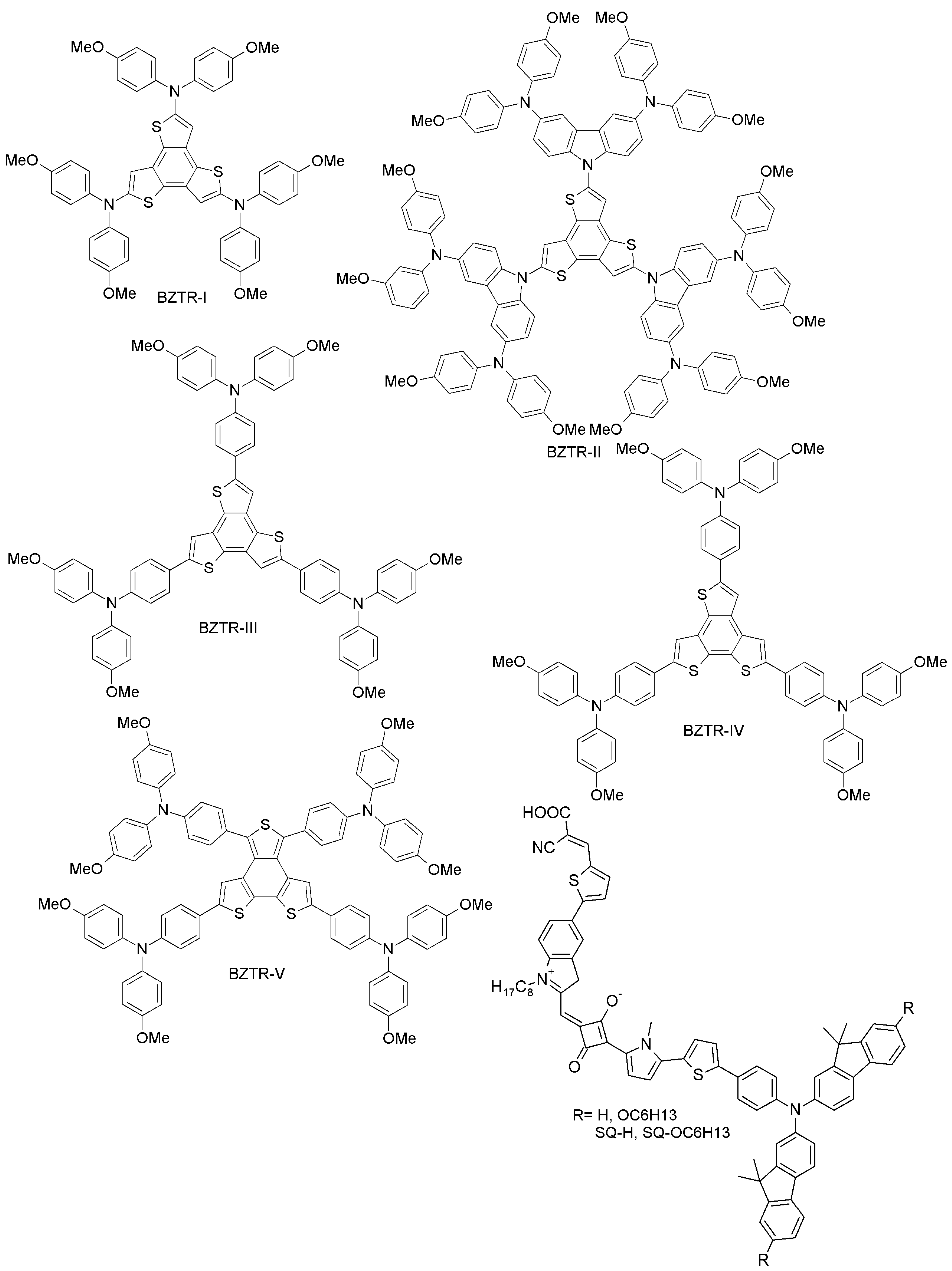
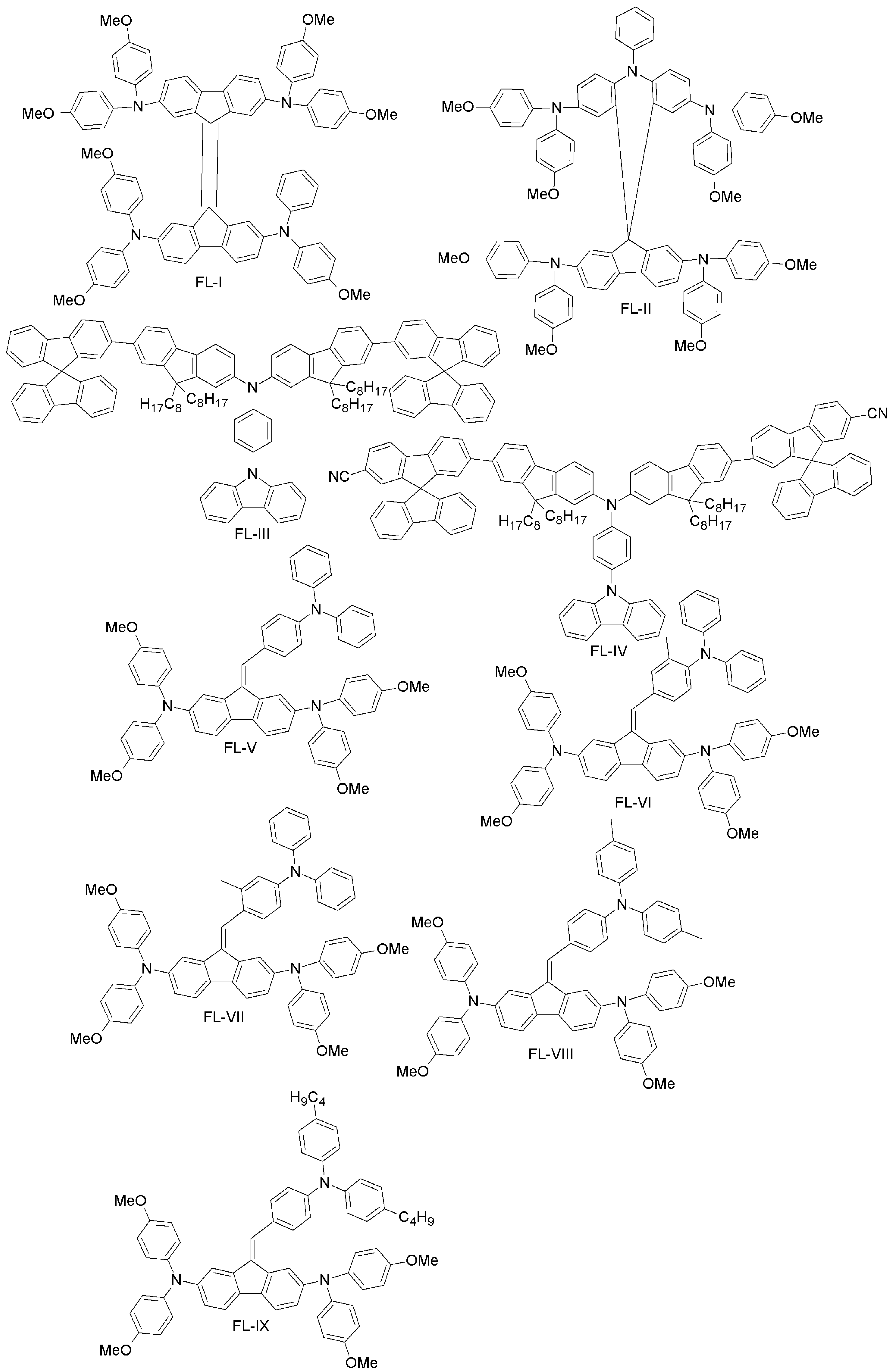
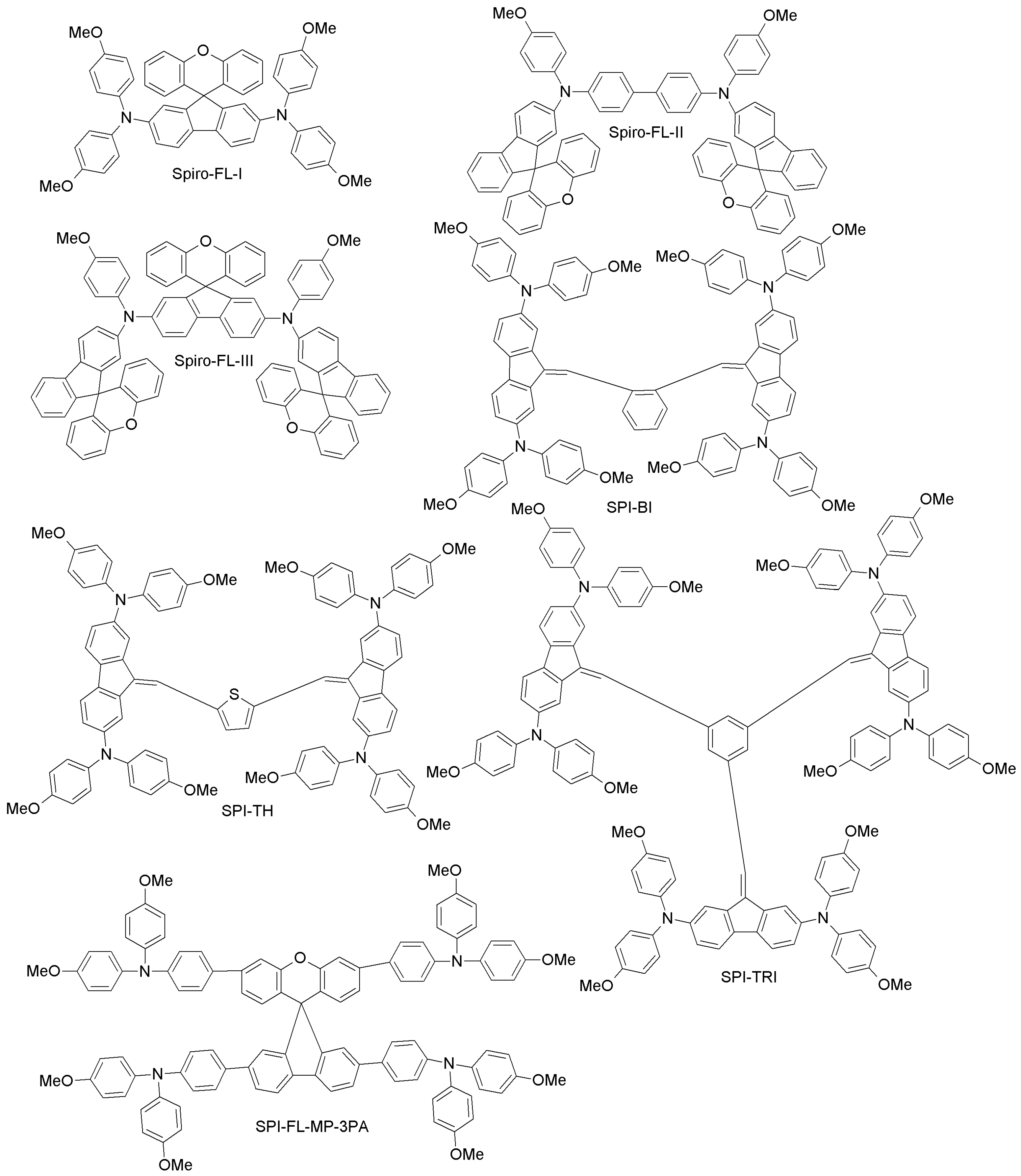


| Category | HTM | PCE (%), HTM | PCE (%), Spiro-OMeTAD | Reference |
|---|---|---|---|---|
| Pyrene-based | PY-1 | 3.3 | 12.70 | [55] |
| PY-2 | 12.3 | 12.70 | [55] | |
| PY-3 | 12.4 | 12.70 | [55] | |
| Truxene-core | Trux-I | 18.6 (10.2 [56]) | 16.0 (9.5 [56]) | [57] |
| Trux-II | 13.4 | 9.50 | [56] | |
| Triazatrux-I | 8.9 | 17.10 | [58] | |
| Triazatrux-II | 17.7 | 17.10 | [58] | |
| Triazatrux-III | 15.8 | 17.10 | [58] | |
| Triazatrux-IV | 11.5 | 17.10 | [58] | |
| Triazatrux-V | 8.88 | 19.01 | [59] | |
| Triazatrux-VI | 14.87 | 19.01 | [59] | |
| Triazatrux-VII | 19.03 | 19.01 | [59] | |
| Phenothiazine-based | PH-I | 2.10 | 17.70 | [60] |
| PH-II | 17.60 | 17.70 | [60] | |
| Acridine-, thiophene-, biphenyl-, bithiophene-, tetrathiophene-, difluorobenzene, and phenyl-based | AC-I | 16.42 | 16.26 | [61] |
| Thio-I | 9.05 | 8.83 (15.63) 1 | [62] | |
| Thio-II | 15.13 | 8.83 (15.63) 1 | [62] | |
| BPH-I | 13.27 | 16.81 | [63] | |
| BPH-II | 16.42 | 16.81 | [63] | |
| BTHIO | 19.40 | 18.80 | [64] | |
| TETRATH-I | 18.13 | 17.80 | [65] | |
| TETRATH-II | 17.3 | 17.80 | [65] | |
| TETRATH-III | 15.7 | 17.80 | [65] | |
| TETRATH-IV | 9.7 | 17.80 | [65] | |
| DFTAB | 10.4 | 15 | [66] | |
| Triazine-based | TRIAZ-I | 12.5 | 13.45 | [67] |
| TRIAZ-II | 10.9 | 13.45 | [67] | |
| TRIAZ-III | 13.2 | 13.8 | [68] | |
| TRIAZ-IV | 12.6 | 13.8 | [68] | |
| Benzotrithiophene- and squaraine-based | BZTR-I | 16 | 18.1 | [69] |
| BZTR-II | 17 | 18.1 | [69] | |
| BZTR-III | 18.2 | 18.1 | [69] | |
| BZTR-IV | 19 | 18.9 | [70] | |
| BZTR-VHYX | 18.2 | 18.9 | [70] | |
| SQ-H | 14.74 | 15.33 | [71] | |
| SQ-OC6H13 | 14.73 | 15.33 | [71] | |
| Fluorene- and spiro-fluorene-based | FL-I | 17.8 | 18.4 | [72] |
| FL-II | 16.73 | 14.84 | [73] | |
| FL-III | 17.25 | 16.67 | [74] | |
| FL-IV | 15.90 | 16.67 | [74] | |
| FL-V | 14.52 | 17.88 | [75] | |
| FL-VI | 15.09 | 17.88 | [75] | |
| FL-VII | 9.15 | 17.88 | [75] | |
| FL-VIII | 16.79 | 17.88 | [75] | |
| FL-IX | 16.45 | 17.88 | [75] | |
| Spiro-FL-I | 19.8 | 20.8 | [76] | |
| Spiro-FL-II | 13.6 | 18.8 | [77] | |
| Spiro-FL-III | 20.8 | 18.8 | [77] | |
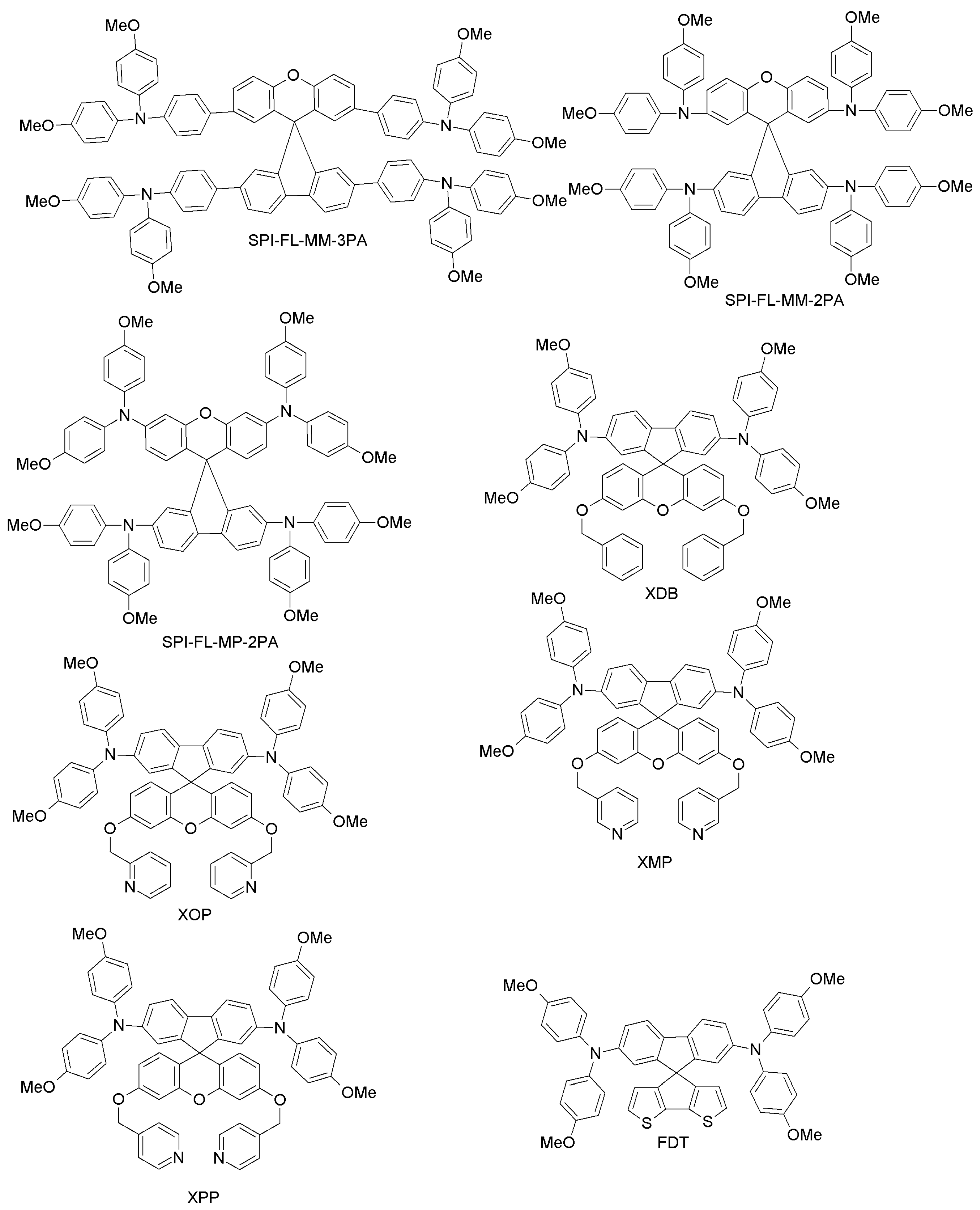
| XDB | 5.4 | 5.5 | [78] | |
| XOP | 15.0 | 5.5 | [78] | |
| XMP | 16.5 | 5.5 | [78] | |
| XPP | 17.2 | 5.5 | [78] | |
| FDT | 20.2 | 19.7 | [39] | |
| SPI-BI | 17.77 | 18.25 | [79] | |
| SPI-TH | 19.96 | 18.25 | [79] | |
| SPI-TRI | 19.47 | 18.25 | [79] | |
| SPI-FL-MM-3PA | 13.46 | 14.98 | [80] | |
| SPI-FL-MP-3PA | 15.59 | 14.98 | [80] | |
| SPI-FL-MM-2PA | 12.56 | 14.98 | [80] | |
| SPI-FL-MP-2PA | 13.43 | 14.98 | [80] | |
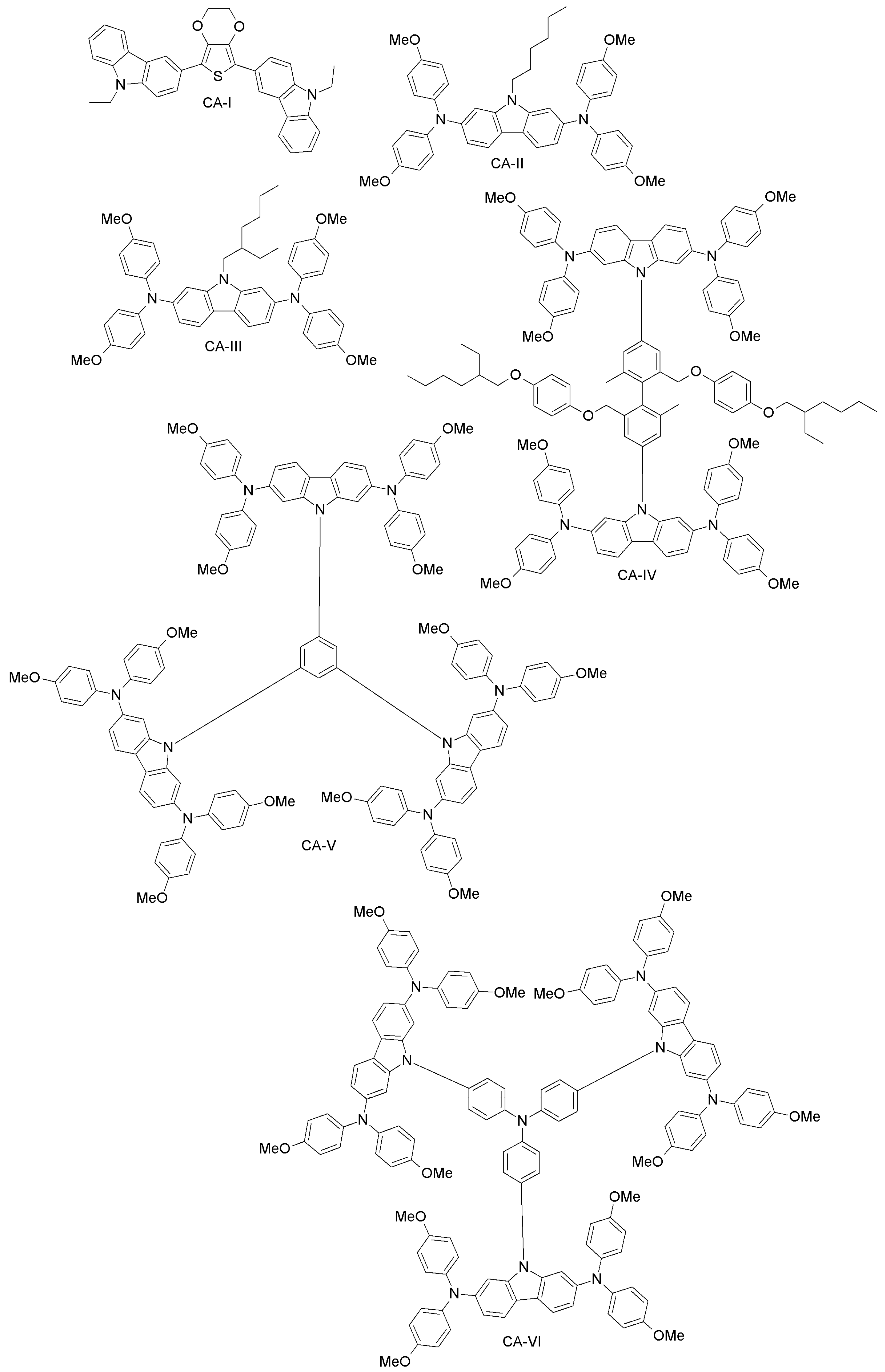
| Carbazole-based | CA-I | 12.3 | 12.17 | [81] |
| CA-II | 8.5 | 10.2 | [82] | |
| CA-III | 10.2 | 10.2 | [82] | |
| CA-IV | 13.28 | 15.23 | [83] | |
| CA-V | 14.79 | 15.23 | [83] | |
| CA-VI | 13.86 | 15.23 | [83] | |
| CA-VII | 4.53 | 5.10 | [84] | |
| CA-VIII | 0.19 | 5.10 | [84] | |
| CA-IX | 7.6 | 10.2 | [85] | |
| CA-X | 9.8 | 10.2 | [85] | |
| CA-XI | 10.96 | 13.76 | [86] | |
| CA-XII | 12.61 | 13.76 | [86] | |
| CA-XIII | 13.0 | 13.76 | [86] | |
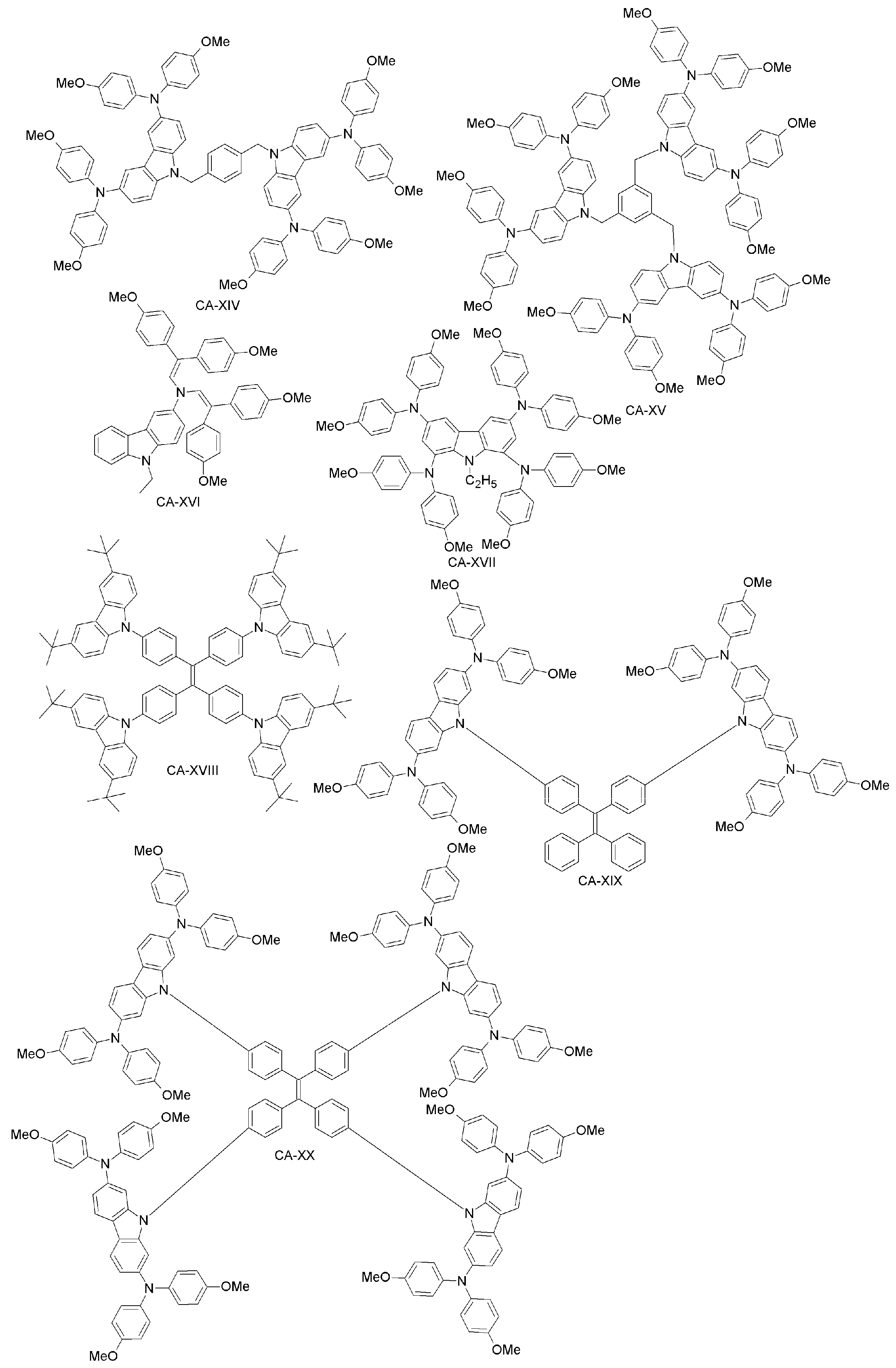
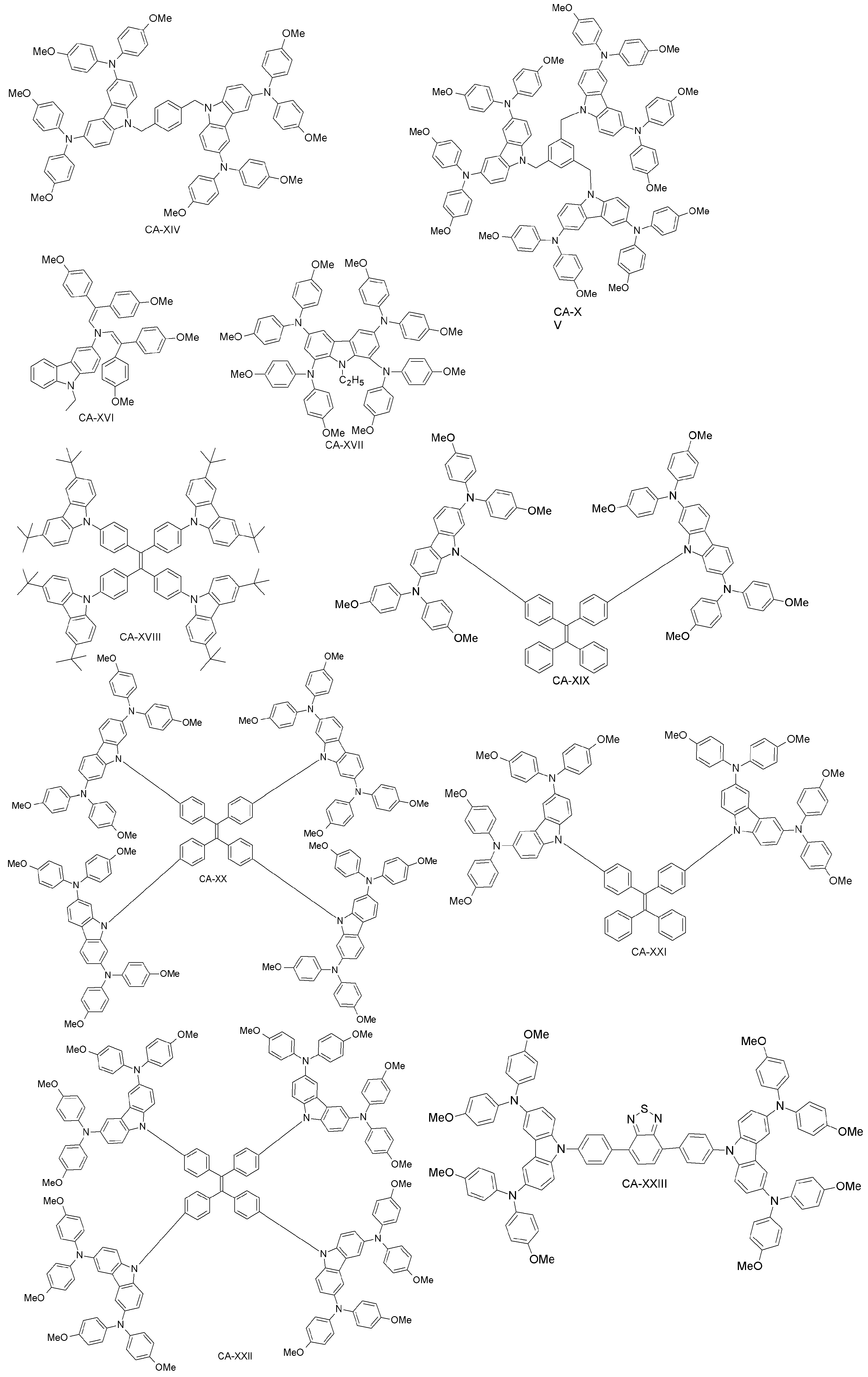
| CA-XIV | 11.4 | 12.0 | [87] | |
| CA-XV | 13.1 | 12.0 | [87] | |
| CA-XVI | 17.8 | 18.6 | [88] | |
| CA-XVII | 17.81 | 18.59 | [89] | |
| CA-XVIII | 12.42 | 14.32 | [90] | |
| CA-XIX | 14.92 | 15.01 | [91] | |
| CA-XX | 16.74 | 15.01 | [91] | |
| CA-XXI | 0 | 15.01 | [91] | |
| CA-XXII | 13.30 | 15.01 | [91] | |
| CA-XXIII | 16.87 | 15.53 | [92] | |
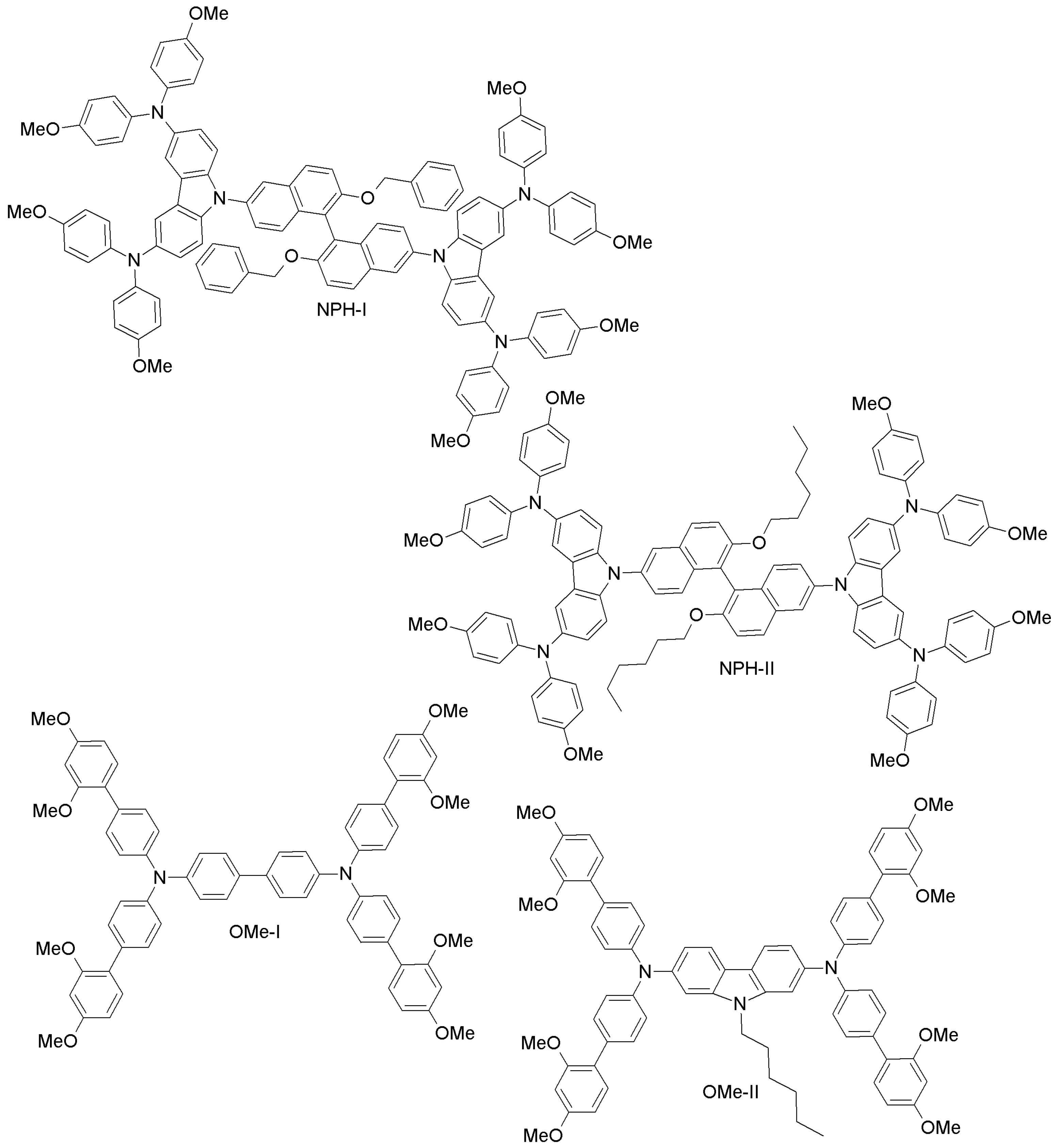
| Other small molecules (Naphthalene (NPH), di- and tetra-phenylmethane (DPA-TPM, TPA-TPM), and ethylene dioxythiophene (EDOT)) | NPH-I | 10.05 | 10.06 | [93] |
| NPH-II | 8.66 | 10.06 | [93] | |
| OMe-I | 18.34 | - | [94] | |
| OMe-II | 16.14 | - | [94] | |
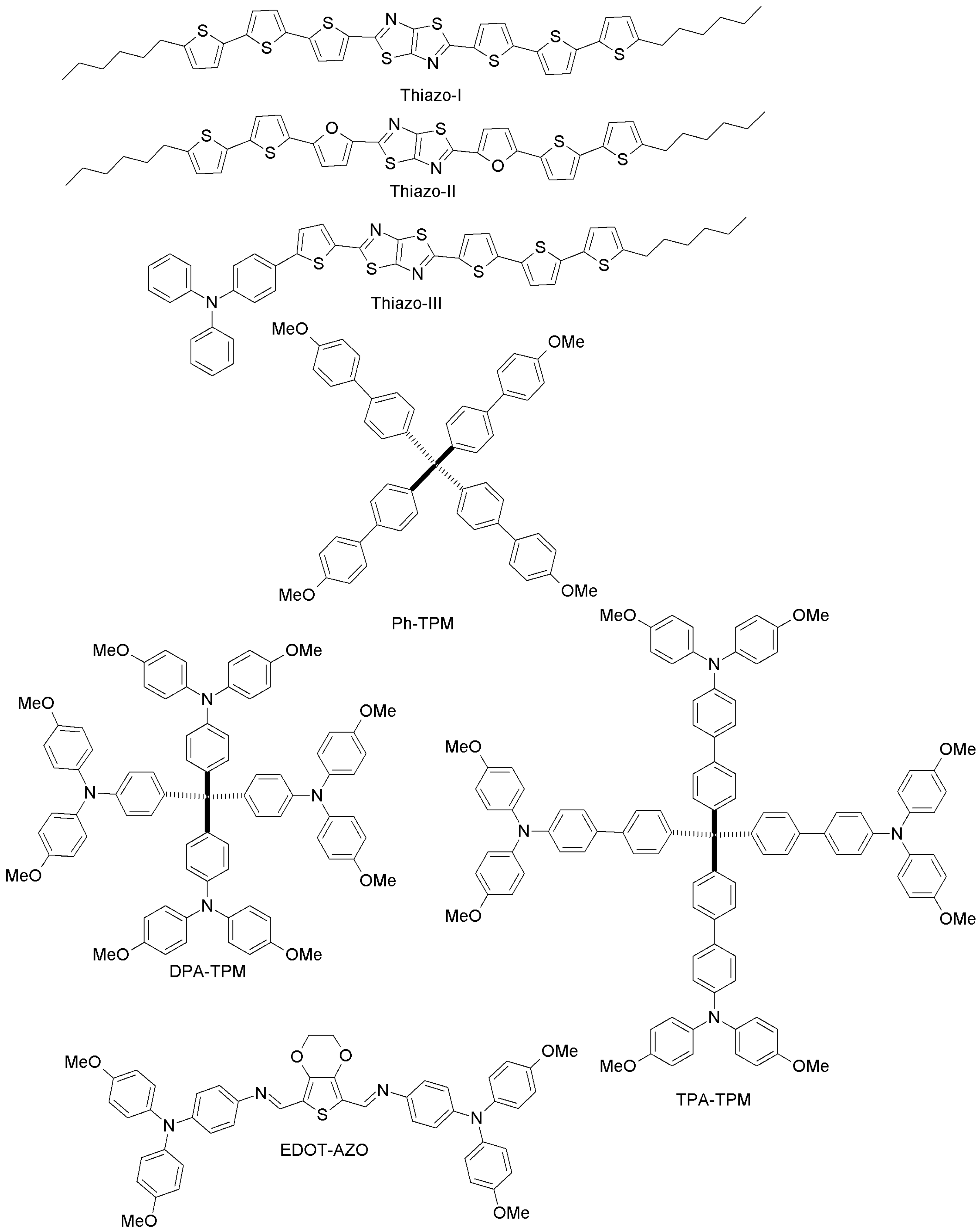
| Thiazo-I | 10.60 | - | [95] | |
| Thiazo-II | 4.37 | - | [95] | |
| Thiazo-III | 8.63 | - | [95] | |
| Ph-TPM | 4.62 | 15.49 | [96] | |
| DPA-TPM | 9.33 | 15.49 | [96] | |
| TPA-TPM | 15.06 | 15.49 | [96] | |
| EDOT-AZO | 11.0 | 11.9 | [97] |
| HTM | PCE (%), HTM | PCE (%), Reference | Reference |
|---|---|---|---|
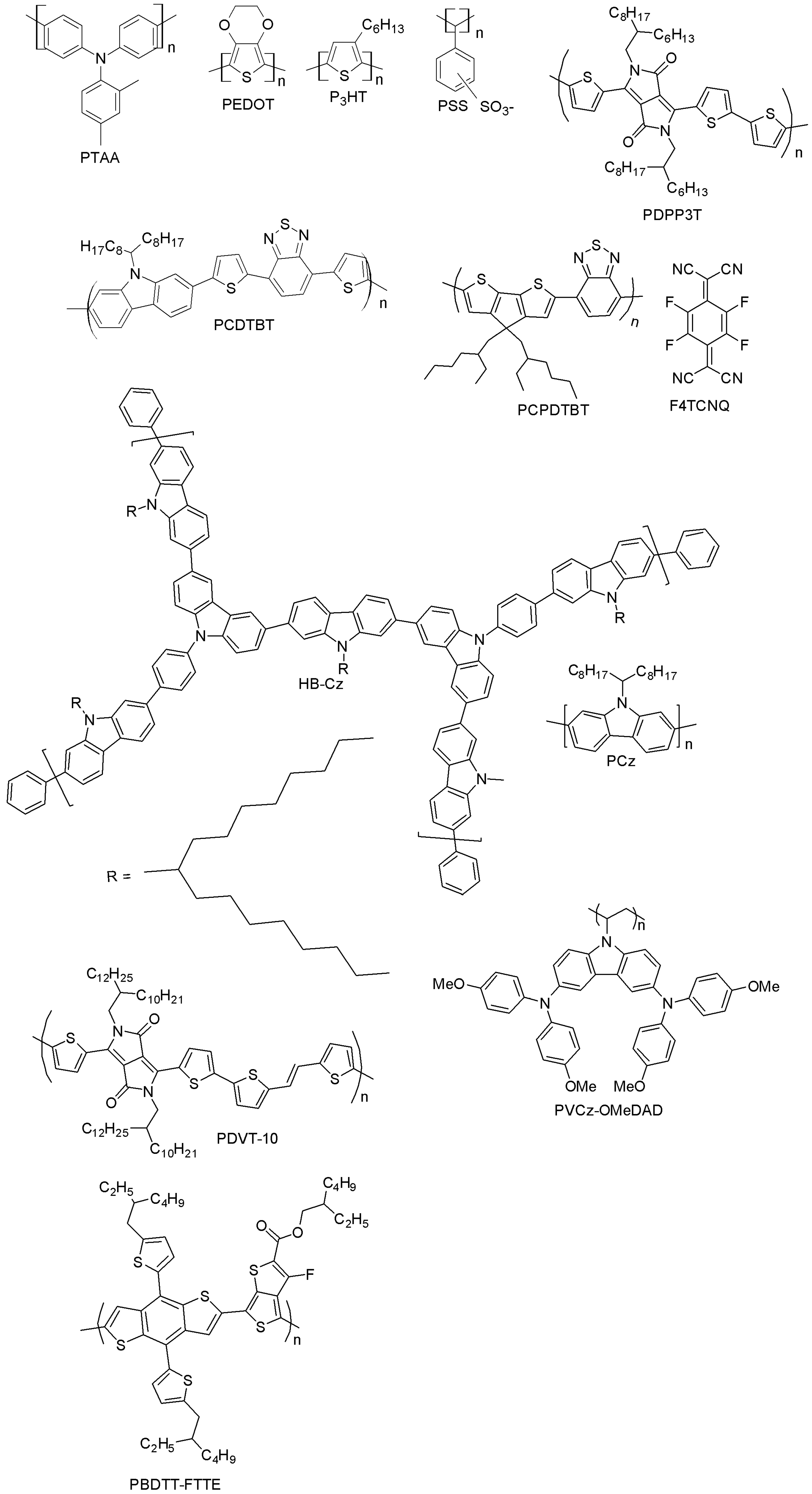
| PTAA | 22.1 | - | [12] |
| P3HT | 13.0 | [108] | |
| PEDOT:PSS | 18.1 | [30] | |
| PDPP3T | 12.32 | 12.34 | [112] |
| PCDTBT | 15.9 | 16.6 | [113] |
| PCPDTBT | 15.1 | - | [115] |
| HB-CZ | 14.07 | 9.05 (P3HT), 6.60 (PCz) | [116] |
| PDVT-10 | 13.4 | 11.28 (PTAA) | [117] |
| PVCz-OMeDAD | 16.09 | 9.62 | [118] |
| PBDTT-FTTE | 11.6 | 10.3 | [119] |
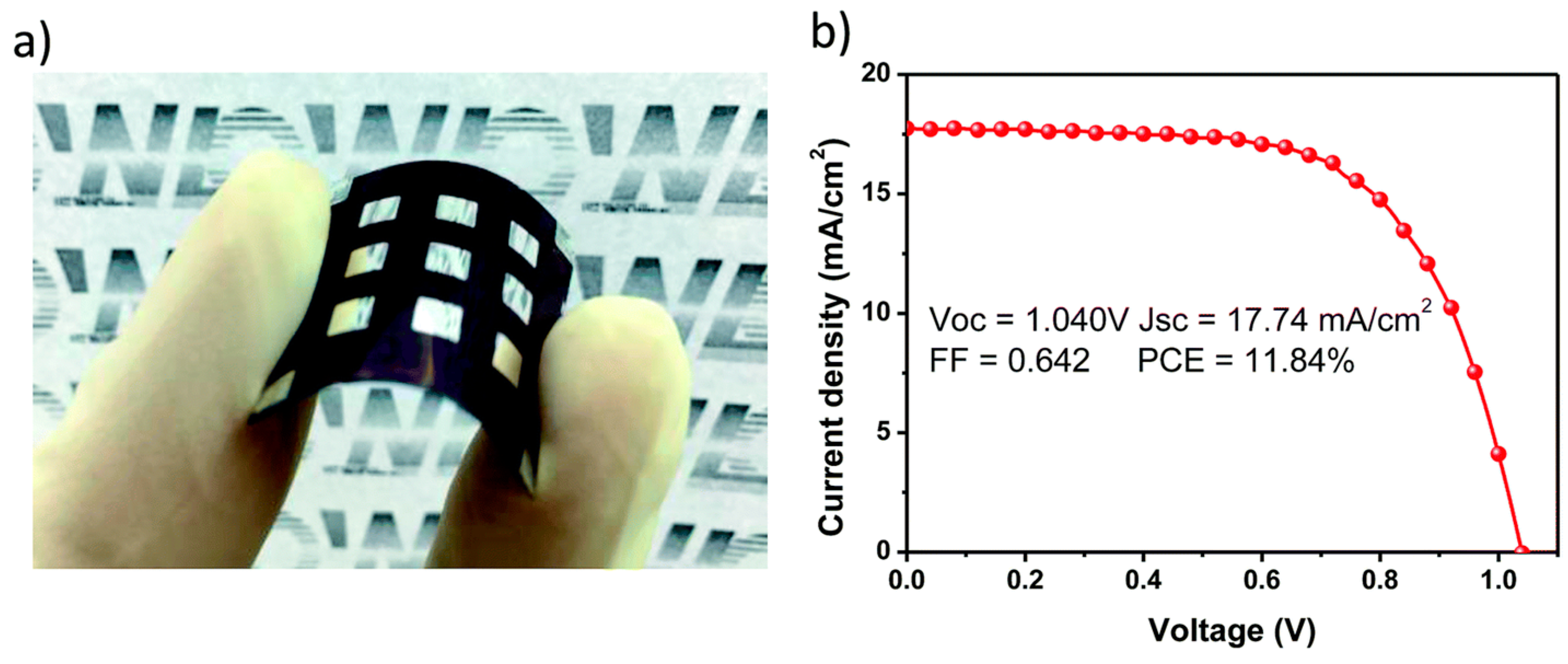
| Scan Direction | VOC (V) | JSC (mA/cm2) | FF (%) | PCE (%) |
|---|---|---|---|---|
| Forward | 1.03 | 20.58 | 74.7 | 15.89 |
| Reverse | 1.03 | 20.66 | 74.2 | 15.90 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vivo, P.; Salunke, J.K.; Priimagi, A. Hole-Transporting Materials for Printable Perovskite Solar Cells. Materials 2017, 10, 1087. https://doi.org/10.3390/ma10091087
Vivo P, Salunke JK, Priimagi A. Hole-Transporting Materials for Printable Perovskite Solar Cells. Materials. 2017; 10(9):1087. https://doi.org/10.3390/ma10091087
Chicago/Turabian StyleVivo, Paola, Jagadish K. Salunke, and Arri Priimagi. 2017. "Hole-Transporting Materials for Printable Perovskite Solar Cells" Materials 10, no. 9: 1087. https://doi.org/10.3390/ma10091087
APA StyleVivo, P., Salunke, J. K., & Priimagi, A. (2017). Hole-Transporting Materials for Printable Perovskite Solar Cells. Materials, 10(9), 1087. https://doi.org/10.3390/ma10091087






