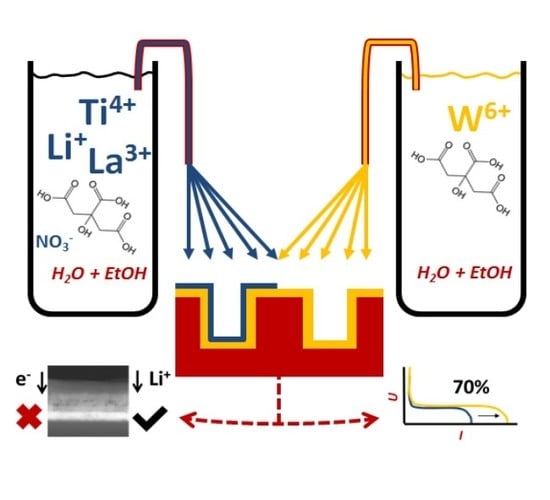
Wet-Chemical Synthesis of 3D Stacked Thin Film Metal-Oxides for All-Solid-State Li-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of the Tungsten Precursor (W-Precursor)
2.2. Synthesis of the Lithium Lanthum Titanium Precursor (Li-La-Ti Precursor)
2.3. Substrates
2.4. Thin Film Synthesis
2.5. Characterization
3. Results and Discussion
3.1. Planar Tungsten Oxide and Lithium Lanthanum Titanium Oxide Stacks
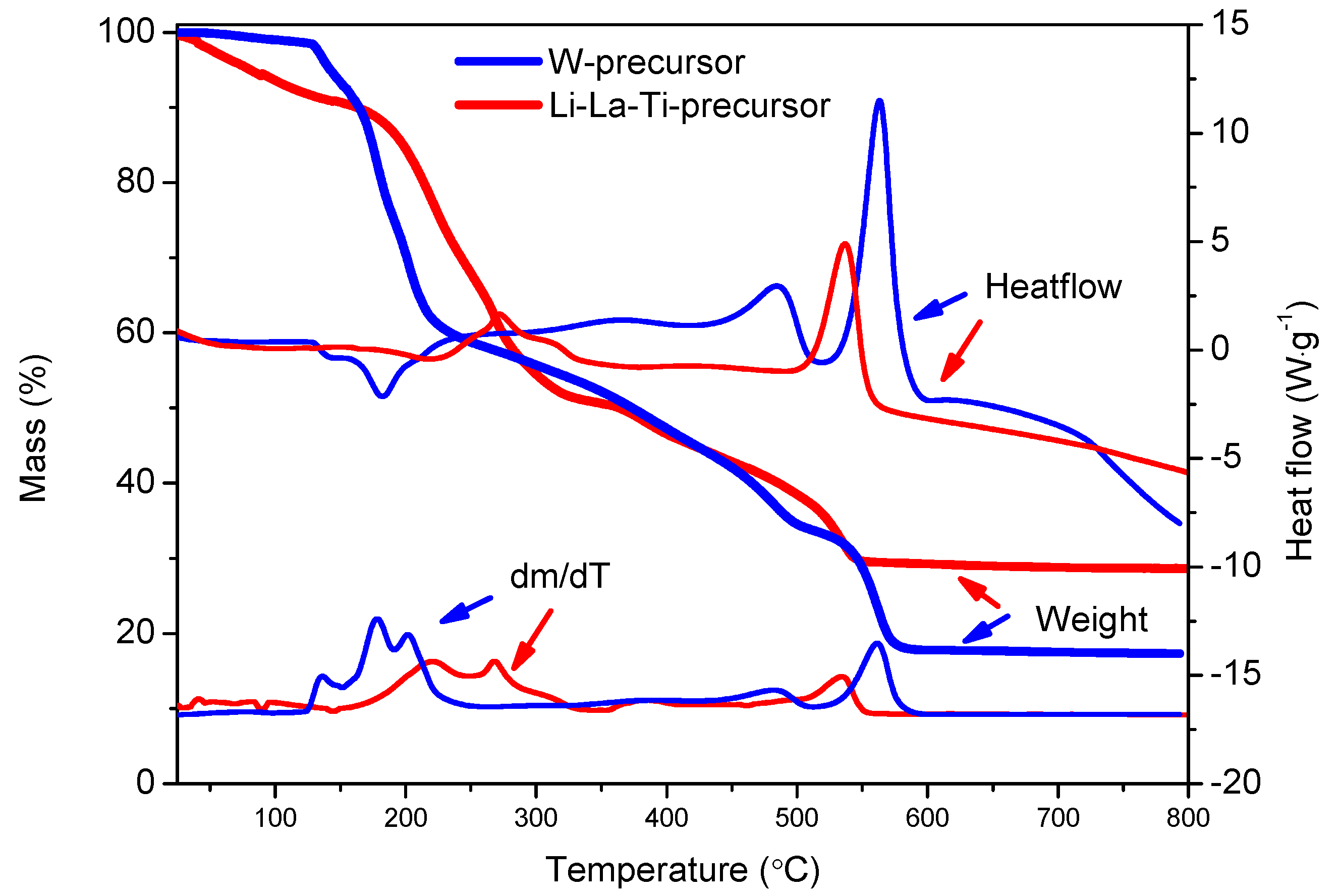
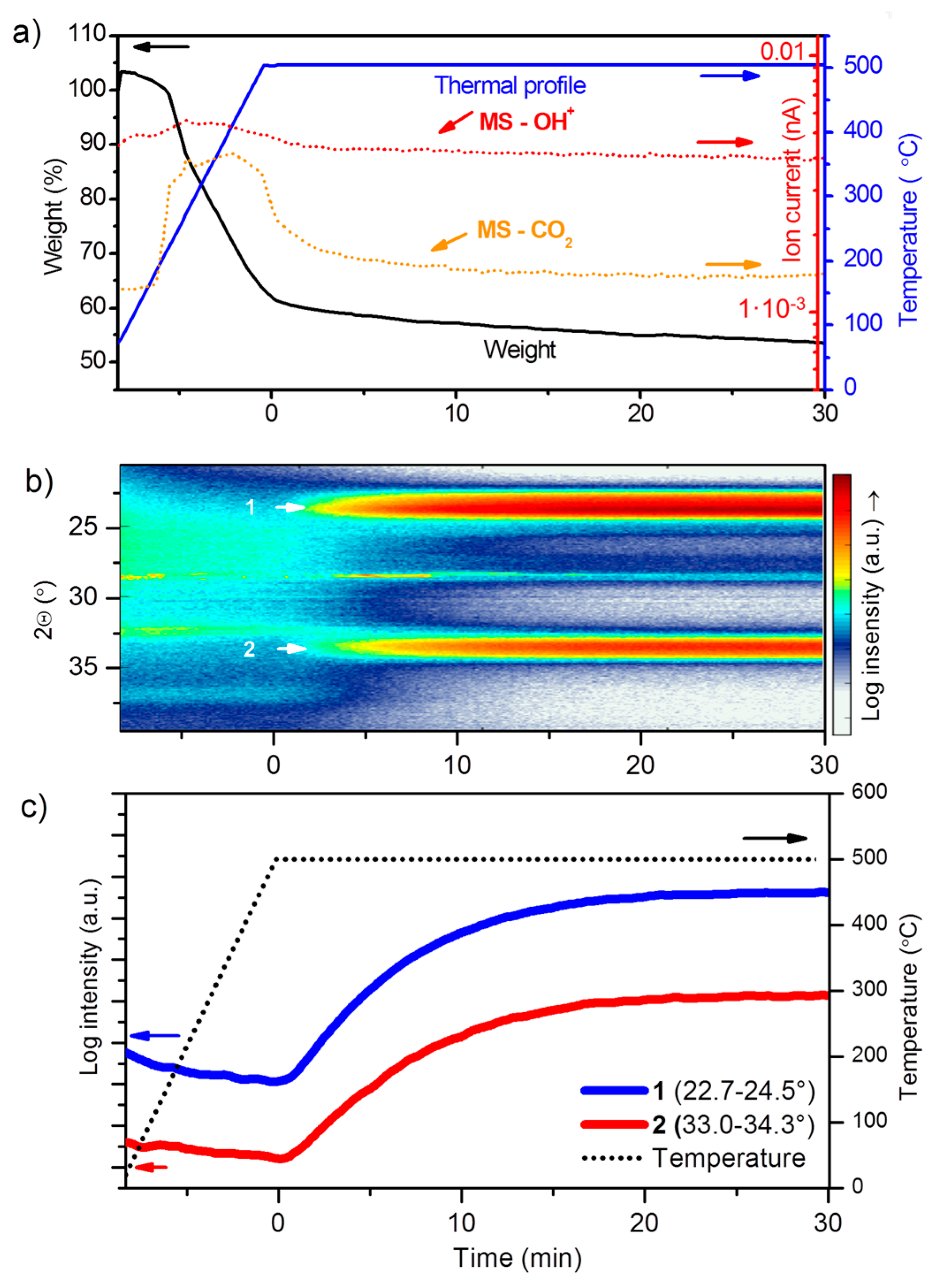
3.1.1. Thermal Decomposition of the Precursor
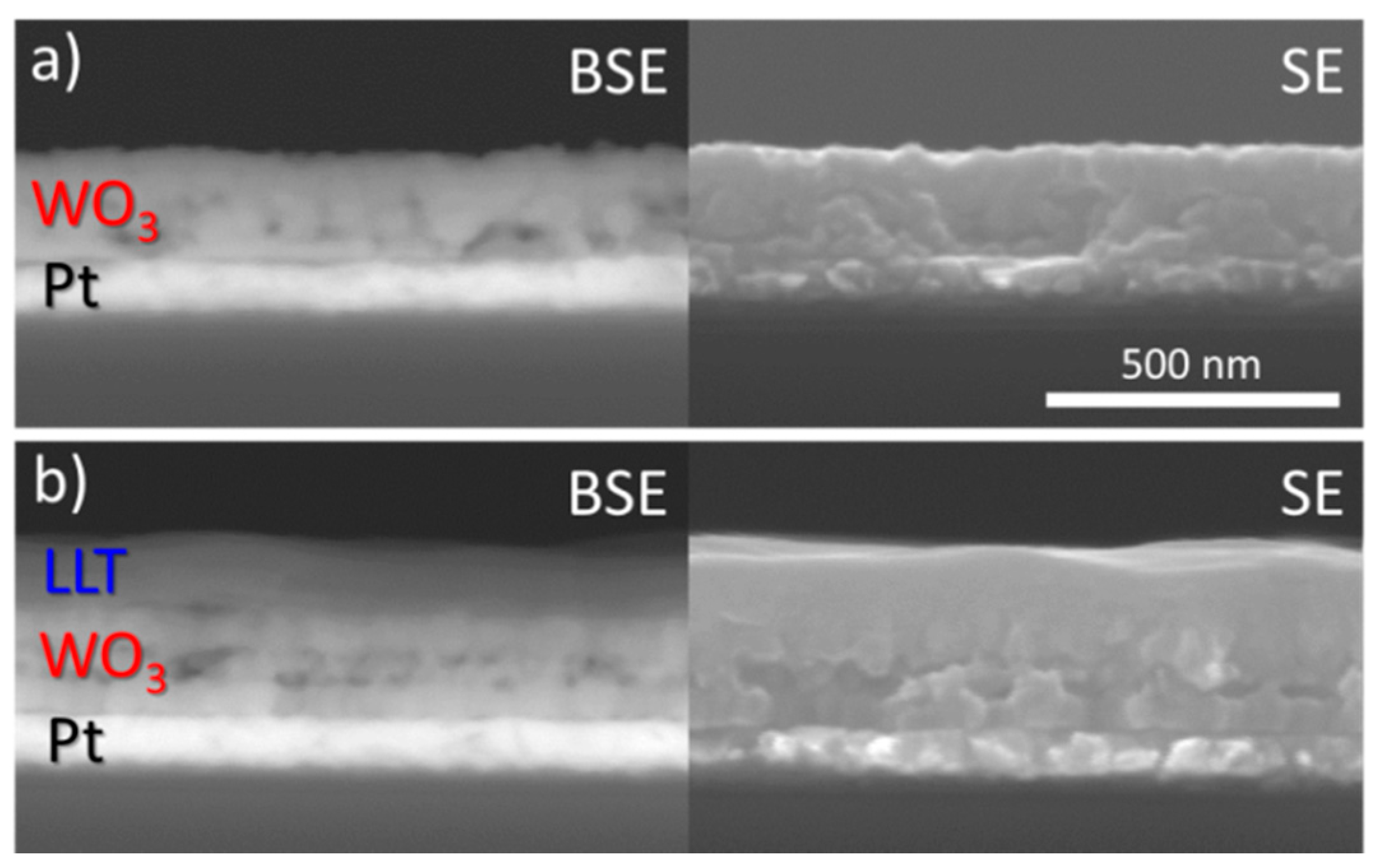
3.1.2. Morphology and Thickness of Deposited Films
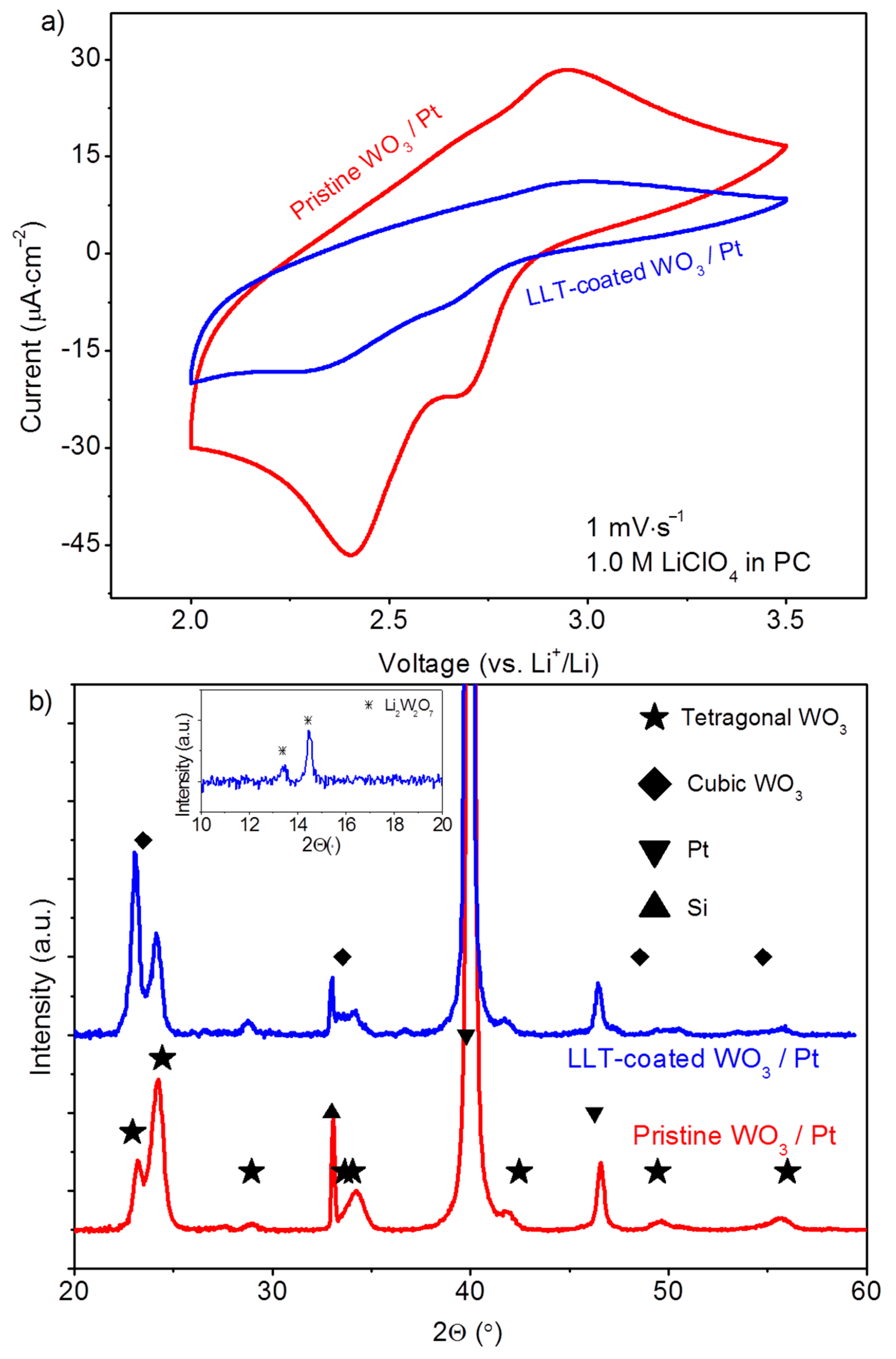
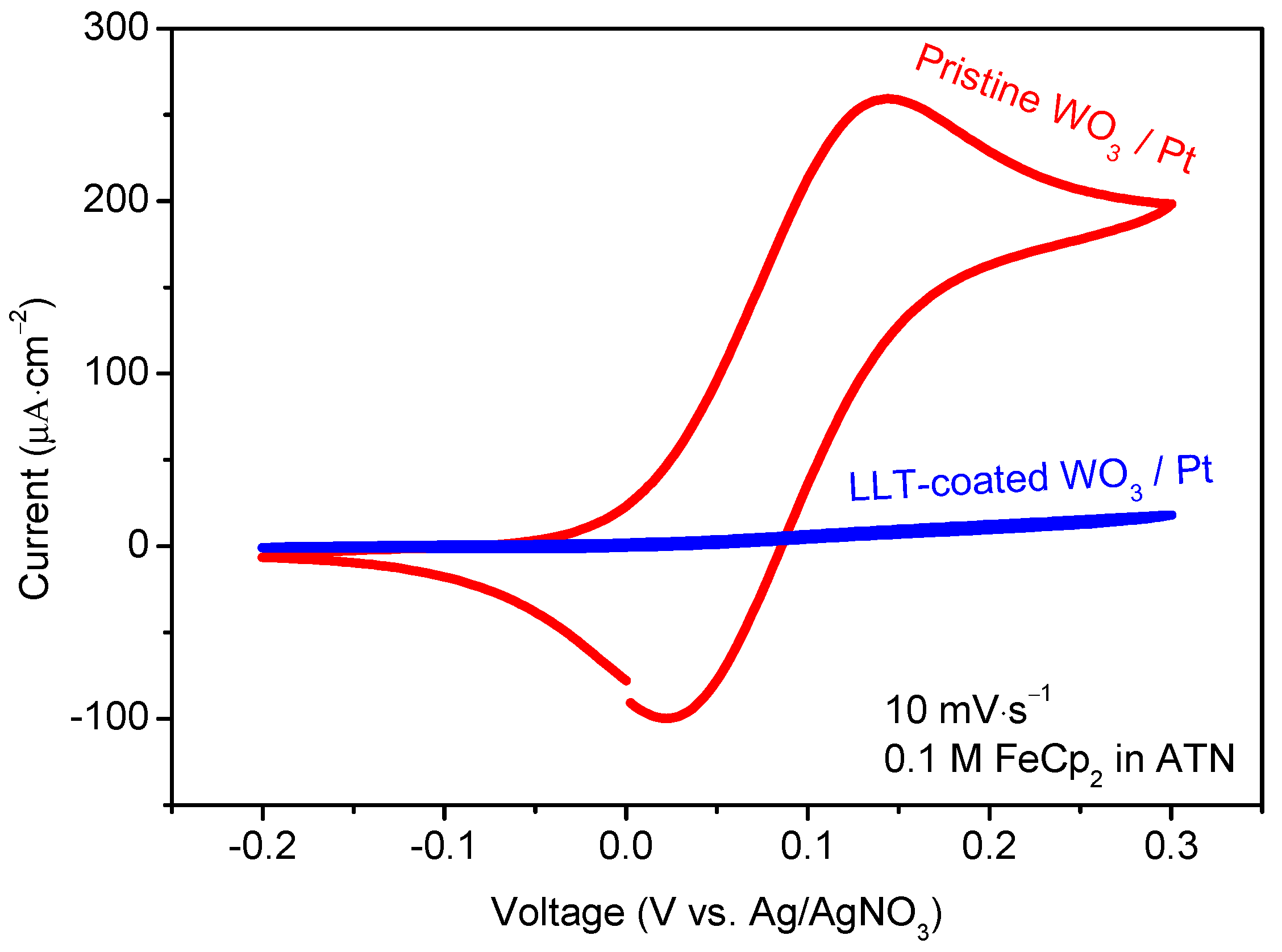
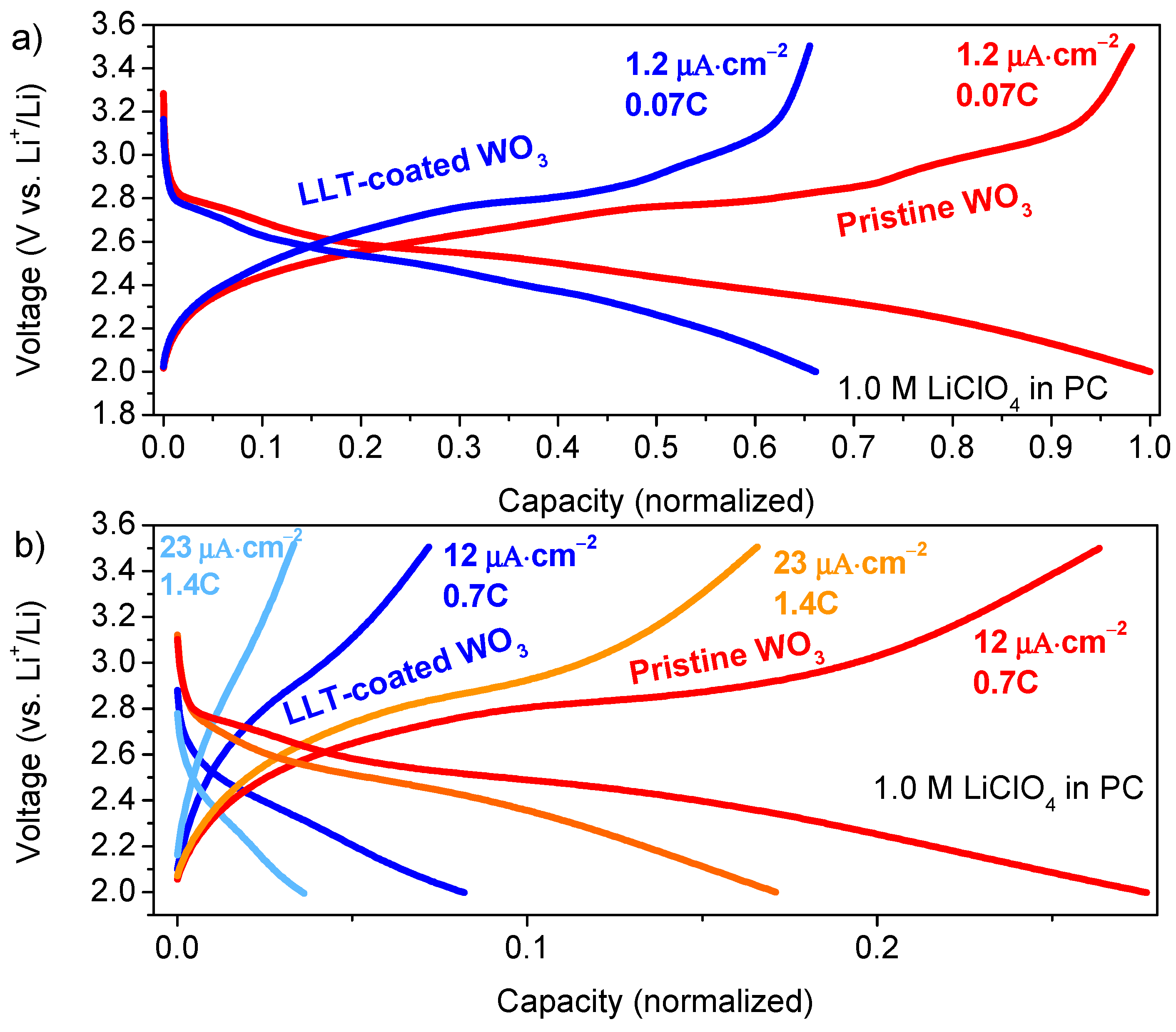
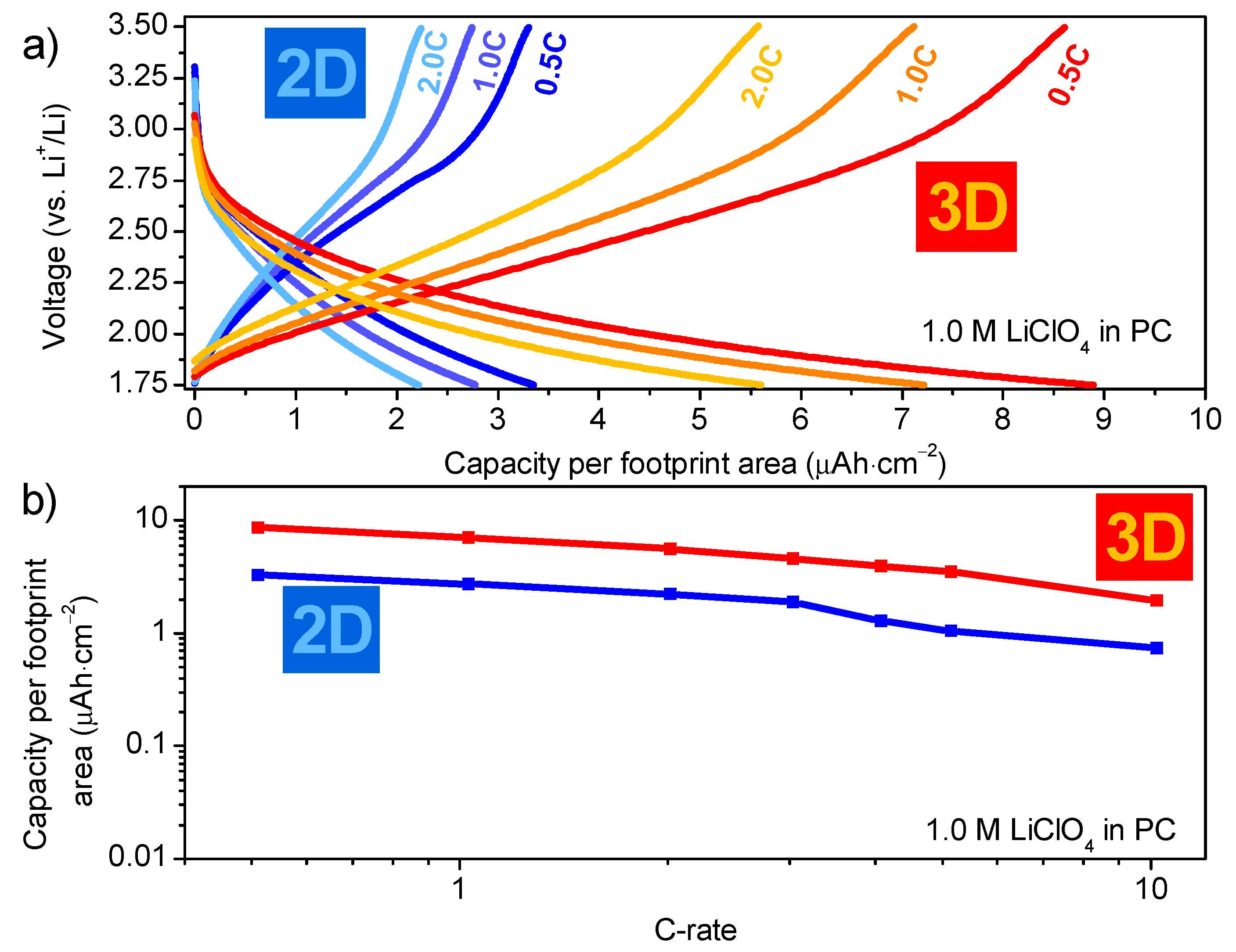
3.1.3. Functional Properties of Planar Films—Electrochemical Activity
3.2. Tungsten Oxide and Lithium Lanthanum Titanium Oxide Stacks 3D Deposition
- (i)
- The probability of a droplet reaching the bottom of the trenches scales with the area of the trench opening (top). As the width of the trench reduces, less material is able to reach and spread at the bottom of the trench.
- (ii)
- Thermophoretic forces, i.e., the repelling force caused by a huge pressure gradient near to the hot surface [58], are larger in the case of small trenches. Carrier gas diffusion into a small trench is more difficult than in to a broad trench, leading to an increased thermophoretic force for smaller trench. Therefore, layer deposition into deeper parts of the trenches is becoming more difficult.
- (iii)
- A necking mechanism is observed, especially for the smallest type of trench measured. The surface tension of the gel leads to necking (contacting similar material) instead of wetting the alien (Si) material down the trench.
3.3. Towards Functional WO3 3D Coatings
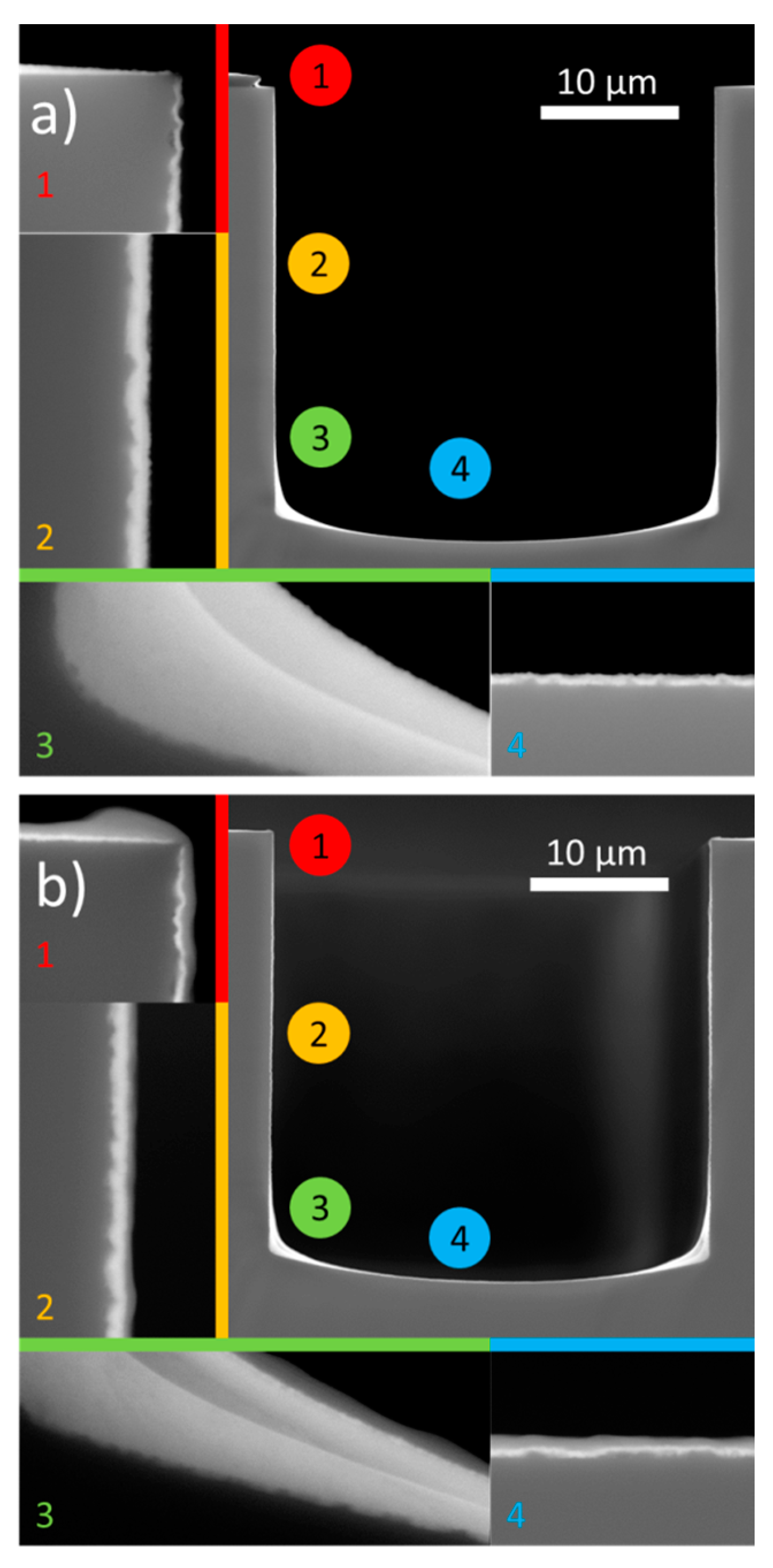
3.3.1. WO3 Deposition on High Aspect Ratio Substrates
3.3.2. Thermal Decomposition and Crystallization
3.3.3. Functional Measurements of 3D WO3 Coatings
- (i)
- The deposition of W-citrate on the trench structures (Figure 6a) illustrates that layer thickness cannot be controlled perfectly, which leads to local differences in capacity of the layer, therefore lowering the capacity enhancement.
- (ii)
- Although the coating is present on the micro-cylinders, certain defects can be observed (Figure S4).
- (iii)
- The current collector may remain an issue; upon cycling, the voltage drop at the start of the measurement is significantly larger for the 3D samples than for the 2D samples (Figure 8a). This illustrates that higher resistance is presence in these 3D samples, which is primarily attributed to the partial oxidation of the TiN current collector (20 nm). As the TiN current collector is much thicker for the 2D samples (80 nm), and the applied currents are much lower, this leads to lower IR-drop for planar films, compared to the 3D counterparts.
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Radzir, N.N.M.; Hanifah, S.A.; Ahmad, A.; Hassan, N.H.; Bella, F. Effect of lithium bis(trifluoromethylsulfonyl)imide salt-doped UV-cured glycidyl methacrylate. J. Solid State Electrochem. 2015, 19, 3079–3085. [Google Scholar] [CrossRef]
- Nair, J.R.; Destro, M.; Bella, F.; Appetecchi, G.B.; Gerbaldi, C. Thermally cured semi-interpenetrating electrolyte networks (s-IPN) for safe and aging-resistant secondary lithium polymer batteries. J. Power Sources 2016, 306, 258–267. [Google Scholar] [CrossRef]
- Knauth, P. Inorganic Solid Li Ion Conductors: An Overview. Solid State Ion. 2009, 180, 911–916. [Google Scholar] [CrossRef]
- Mahmoud, M.M.; Cui, Y.; Rohde, M.; Ziebert, C.; Link, G.; Seifert, H.J. Microwave Crystallization of Lithium Aluminum Germanium Phosphate Solid-State Electrolyte. Materials 2016, 9, 506. [Google Scholar] [CrossRef] [PubMed]
- Oudenhoven, J.F.M.; Baggetto, L.; Notten, P.H.L. All-Solid-State Lithium-Ion Microbatteries: A Review of Various Three-Dimensional Concepts. Adv. Energy Mater. 2011, 1, 10–33. [Google Scholar] [CrossRef]
- Long, J.W.; Dunn, B.; Rolison, D.R.; White, H.S. Three-Dimensional Battery Architectures. Chem. Rev. 2004, 104, 4463–4492. [Google Scholar] [CrossRef] [PubMed]
- Notten, P.H.L.; Roozeboom, F.; Niessen, R.A.H.; Baggetto, L. 3-D Integrated All-Solid-State Rechargeable Batteries. Adv. Mater. 2007, 19, 4564–4567. [Google Scholar] [CrossRef]
- Xie, J.; Peter-Paul, R.M.L.H.; Li, D.; Raijmakers, L.H.J.; Notten, P.H.L. Planar and 3D Deposition of Li4Ti5O12 Thin Film Electrodes by MOCVD. Solid State Ion. 2016, 287, 83–88. [Google Scholar] [CrossRef]
- Donders, M.E.; Oudenhoven, J.F.M.; Baggetto, L.; Knoops, H.C.M.; van de Sanden, M.C.M.; Kessels, W.M.M.; Notten, P.H.L. All-Solid-State Batteries: A Challenging Route Towards 3D Integration. ECS Trans. 2010, 33, 213–222. [Google Scholar] [CrossRef]
- Donders, M.E.; Arnoldbik, W.M.; Knoops, H.C.M.; Kessels, W.M.M.; Notten, P.H.L. Atomic Layer Deposition of LiCoO2 Thin-Film Electrodes for All-Solid-State Li-Ion Micro-Batteries. J. Electrochem. Soc. 2013, 160, A3066–A3071. [Google Scholar] [CrossRef]
- Dobbelaere, T.; Mattelaer, F.; Dendooven, J.; Vereecken, P.; Detavernier, C. Plasma-Enhanced Atomic Layer Deposition of Iron Phosphate as a Positive Electrode for 3D Lithium-Ion Microbatteries. Chem. Mater. 2016, 28, 3435–3445. [Google Scholar] [CrossRef]
- Nisula, M.; Shindo, Y.; Koga, H.; Karppinen, M. Atomic Layer Deposition of Lithium Phosphorus Oxynitride. Chem. Mater. 2015, 27, 6987–6993. [Google Scholar] [CrossRef]
- Xie, J.; Oudenhoven, J.F.M.; Peter-Paul, R.M.L.H.; Li, D.; Notten, P.H.L. Chemical Vapor Deposition of Lithium Phosphate Thin-Films for 3D All-Solid-State Li-Ion Batteries. J. Electrochem. Soc. 2014, 162, A249–A254. [Google Scholar] [CrossRef]
- Lin, C.-F.; Noked, M.; Kozen, A.C.; Liu, C.; Zhao, O.; Gregorczyk, K.; Hu, L.; Lee, S.B.; Rubloff, G.W. Solid Electrolyte Lithium Phosphous Oxynitride as a Protective Nanocladding Layer for 3D High-Capacity Conversion Electrodes. ACS Nano 2016, 10, 2693–2701. [Google Scholar] [CrossRef] [PubMed]
- Kozen, A.C.; Pearse, A.J.; Lin, C.-F.; Noked, M.; Rubloff, G.W. Atomic Layer Deposition of the Solid Electrolyte LiPON. Chem. Mater. 2015, 27, 5324–5331. [Google Scholar] [CrossRef]
- Perng, Y.-C.; Cho, J.; Sun, S.Y.; Membreno, D.; Cirigliano, N.; Dunn, B.; Chang, J.P. Synthesis of Ion Conducting LixAlySizO Thin Films by Atomic Layer Deposition. J. Mater. Chem. A 2014, 2, 9566–9573. [Google Scholar] [CrossRef]
- Li, X.; Liu, J.; Banis, M.N.; Lushington, A.; Li, R.; Cai, M.; Sun, X. Atomic Layer Deposition of Solid-State Electrolyte Coated Cathode Materials with Superior High-Voltage Cycling Behavior for Lithium Ion Battery Application. Energy Environ. Sci. 2014, 7, 768–778. [Google Scholar] [CrossRef]
- Pearse, A.J.; Schmitt, T.E.; Fuller, E.J.; El-Gabaly, F.; Lin, C.F.; Gerasopoulos, K.; Kozen, A.C.; Talin, A.A.; Rubloff, G.; Gregorczyk, K.E. Nanoscale Solid State Batteries Enabled by Thermal Atomic Layer Deposition of a Lithium Polyphospazene Solid State Electrolyte. Chem. Mater. 2017, 29, 3740–3753. [Google Scholar] [CrossRef]
- Létiche, M.; Eustache, E.; Frexias, J.; Demortière, A.; De Andrade, V.; Morgenroth, L.; Tilmant, P.; Vaurette, F.; Troadec, D.; Roussel, P.; et al. Atomic Layer Deposition of Functional Layers for on Chip 3D Li-ion All Solid State Microbattery. Adv. Energy Mater. 2017, 7, 161402. [Google Scholar] [CrossRef]
- Gielis, S.; Hardy, A.; Van Bael, M.K. Conformal Coating on Three-Dimenstional Substrates. Eur. Pat. Appl. 2947178, 2015. [Google Scholar]
- Van den Ham, E.J.; Gielis, S.; Van Bael, M.K.; Hardy, A. Ultrasonic Spray Deposition of Metal Oxide Films on High Aspect Ratio Microstructures for 3D All-Solid-State Li-Ion Batteries. ACS Energy Lett. 2016, 1, 1184–1188. [Google Scholar] [CrossRef]
- Yu, A.; Kumagai, N.; Liu, Z.; Lee, J.Y. Electrochemical Lithium Intercalation into WO3 and Lithium Tungstates Li X WO3 + X/2 of Various Structures. J. Solid State Electrochem. 1998, 2, 394–400. [Google Scholar] [CrossRef]
- Niklasson, G.A.; Granqvist, C.G. Electrochromics for Smart Windows: Thin Films of Tungsten Oxide and Nickel Oxide, and Devices Based on These. J. Mater. Chem. 2007, 17, 127–156. [Google Scholar] [CrossRef]
- Regragui, M.; Addou, M.; Outzourhit, A.; Bernede, J.C.; El Idrissi, E.; Benseddik, E.; Kachouane, A. Preparation and Characterization of Pyrolytic Spray Deposited Electrochromic Tungsten Trioxide Fims. Thin Solid Films 2000, 358, 40–45. [Google Scholar] [CrossRef]
- Garciacanadas, J. Charging and Diffusional Aspects of Li+ Insertion in Electrochromic a-WO3. Solid State Ion. 2004, 175, 521–525. [Google Scholar] [CrossRef]
- Nishio, K.; Sei, T.; Tsuchiya, T. Preparation of Electrochromic Thin Film by Sol-Gel Process. J. Ceram. Soc. Jpn. 1999, 107, 199–203. [Google Scholar] [CrossRef]
- Guo, C.; Yin, S.; Yan, M.; Kobayashi, M.; Kakihana, M.; Sato, T. Morphology-Controlled Synthesis of W18O49 Nanostructures and Their Near-Infrared Absorption Properties. Inorg. Chem. 2012, 51, 4763–4771. [Google Scholar] [CrossRef] [PubMed]
- Denayer, J.; Aubry, P.; Bister, G.; Spronck, G.; Colson, P.; Vertruyen, B.; Lardot, V.; Cambier, F.; Henrist, C.; Cloots, R. Improved Coloration Contrast and Electrochromic Efficiency of Tungsten Oxide Films Thanks to a Surfactant-Assisted Ultrasonic Spray Pyrolysis Process. Sol. Energy Mater. Sol. Cells 2014, 130, 623–628. [Google Scholar] [CrossRef]
- Ji, R.; Zheng, D.; Zhou, C.; Cheng, J.; Yu, J.; Li, L. Low-Temperature Preparation of Tungsten Oxide Anode Buffer Layer via Ultrasonic Spray Pyrolysis Method for Large-Area Organic Solar Cells. Materials 2017, 10, 820. [Google Scholar] [CrossRef] [PubMed]
- Li, W.J.; Fu, Z.W. Nanostructured WO3 Thin Film as a New Anode Material for Lithium-Ion Batteries. Appl. Surf. Sci. 2010, 256, 2447–2452. [Google Scholar] [CrossRef]
- Perereira-Ramos, J.P.; Baddour-Hadjean, R.; Kumagai, N.; Tanno, K. Improvement of the Electrochemical Behaviour of WO3 as Reversible Cathodic Material for Lithium Batteries. Electrochim. Acta 1993, 38, 431–436. [Google Scholar] [CrossRef]
- Huang, K.; Zhang, Q. Rechargeable Lithium Battery Based on a Single Hexagonal Tungsten Trioxide Nanowire. Nano Energy 2012, 1, 172–175. [Google Scholar] [CrossRef]
- Van den Ham, E.J.; Elen, K.; Kokal, I.; Yagci, M.B.; Peys, N.; Bonneux, G.; Ulu, F.; Marchal, W.; Van Bael, M.; Hardy, A. From Liquid to Thin Film: Colloidal Suspensions for Tungsten Oxide as an Electrode Material for Li-Ion Batteries. RSC Adv. 2016, 6, 51747–51756. [Google Scholar] [CrossRef]
- Li, W.; Sasaki, A.; Oozu, H.; Aoki, K.; Kakushima, K.; Kataoka, Y.; Nishiyama, A.; Sugii, N.; Wakabayashi, H.; Tsutsui, K.; et al. Improvement of Charge/discharge Performance for Lithium Ion Batteries with Tungsten Trioxide Electrodes. Microelectron. Reliab. 2015, 55, 402–406. [Google Scholar] [CrossRef]
- Zhong, Q.; Dahn, J.R.; Colbow, K. Lithium Intercalation into WO3 and the Phase Diagram of LixWO3. Phys. Rev. B 1992, 46, 2554–2560. [Google Scholar] [CrossRef]
- Nishio, K.; Tsuchiya, T. Electrochromic Thin Films Prepared by Sol-Gel Process. Sol. Energy Mater. Sol. Cells 2001, 68, 279–293. [Google Scholar] [CrossRef]
- Delmas, C.; Nadiri, A.; Soubeyroux, J.L. The Nasicon-Type Titanium Phosphates ATi2(PO4)3 (A = Li, Na) as Electrode Materials. Solid State Ion. 1988, 28–30, 419–423. [Google Scholar] [CrossRef]
- Takada, K.; Tansho, M.; Yanase, I.; Inada, T.; Kajiyama, A.; Kouguchi, M.; Kondo, S.; Watanabe, M. Lithium Ion Conduction in LiTi(PO4)3. Solid State Ion. 2001, 139, 241–247. [Google Scholar] [CrossRef]
- Kim, S.; Hirayama, M.; Taminato, S.; Kanno, R. Epitaxial Growth and Lithium Ion Conductivity of Lithium-Oxide Garnet for an All Solid-State Battery Electrolyte. Dalton Trans. 2013, 42, 13112–13117. [Google Scholar] [CrossRef] [PubMed]
- Reinacher, J.; Berendts, S.; Janek, J. Preparation and Electrical Properties of Garnet-Type Li6BaLa2Ta2O12 Lithium Solid Electrolyte Thin Films Prepared by Pulsed Laser Deposition. Solid State Ion. 2014, 258, 1–7. [Google Scholar] [CrossRef]
- Tadanaga, K.; Egawa, H.; Hayashi, A.; Tatsumisago, M.; Mosa, J.; Aparicio, M.; Duran, A. Preparation of Lithium Ion Conductive Al-Doped Li7La3Zr2O12 Thin Films by a Sol–gel Process. J. Power Sources 2015, 273, 844–847. [Google Scholar] [CrossRef]
- Aaltonen, T.; Alnes, M.; Nilsen, O.; Costelle, L.; Fjellvåg, H. Lanthanum Titanate and Lithium Lanthanum Titanate Thin Films Grown by Atomic Layer Deposition. J. Mater. Chem. 2010, 20, 2877–2881. [Google Scholar] [CrossRef]
- Van den Ham, E.J.; Peys, N.; De Dobbelaere, C.; D’Haen, J.; Mattelaer, F.; Detavernier, C.; Notten, P.H.L.; Hardy, A.; Van Bael, M.K. Amorphous and Perovskite Li3xLa(2/3)−xTiO3 (thin) Films via Chemical Solution Deposition: Solid Electrolytes for All-Solid-State Li-Ion Batteries. J. Sol-Gel Sci. Technol. 2015, 73, 536–543. [Google Scholar] [CrossRef]
- Knaepen, W.; Detavernier, C.; Van Meirhaeghe, R.L.; Jordan Sweet, J.; Lavoie, C. In-Situ X-Ray Diffraction Study of Metal Induced Crystallization of Amorphous Silicon. Thin Solid Films 2008, 516, 4946–4952. [Google Scholar] [CrossRef]
- Cruywagen, J.J.; Krüger, L.; Rohwer, E.A. Complexation of tungsten(VI) with citrate. J. Chem. Soc. Dalton Trans. 1991. [Google Scholar] [CrossRef]
- Rajendran, M.; Subba Rao, M. Formation of BaTiO3 from Citrate Precursor. J. Solid State Chem. 1994, 113, 239–247. [Google Scholar] [CrossRef]
- Peys, N.; Ling, Y.; Dewulf, D.; Gielis, S.; De Dobbelaere, C.; Cuypers, D.; Adriaensens, P.; Van Doorslaer, S.; De Gendt, S.; Hardy, A.; et al. V6O13 Films by Control of the Oxidation State from Aqueous Precursor to Crystalline Phase. Dalton Trans. 2013, 42, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Hardy, A.; Mondelaers, D.; Van Bael, M.K.; Mullens, J.; Van Poucke, L.C.; Vanhoyland, G.; D’Haen, J. Synthesis of (Bi,La)4Ti3O12 by a New Aqueous Solution-Gel Route. J. Eur. Ceram. Soc. 2004, 24, 905–909. [Google Scholar] [CrossRef]
- Truijen, I.; Hardy, A.; Van Bael, M.K.; Van den Rul, H.; Mullens, J. Study of the Decomposition of Aqueous Citratoperoxo-Ti(IV)-Gel Precursors for Titania by Means of TGA-MS and FTIR. Thermochim. Acta 2007, 456, 38–47. [Google Scholar] [CrossRef]
- Jain, S.R.; Adiga, K.C.; Verneker, V.R.P. A New Approach to Thermochemical Calculations of Condensed Fuel-Oxidizer Mixtures. Combust. Flame 1981, 40, 71–79. [Google Scholar] [CrossRef]
- Fortal’nova, E.A.; Mosunov, A.V.; Safronenko, M.G.; Venskovskii, N.U.; Politova, E.D. Electrical Properties of (La1/2Li1/3 + x)TiO3 Solid Solutions. Inorg. Mater. 2006, 42, 393–398. [Google Scholar] [CrossRef]
- Darmawi, S.; Burkhardt, S.; Leichtweiss, T.; Weber, D.A.; Wenzel, S.; Janek, J.; Elm, M.T.; Klar, P.J. Correlation of Electrochromic Properties and Oxidation States in Nanocrystalline Tungsten Trioxide. Phys. Chem. Chem. Phys. 2015, 17, 15903–15911. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.H.; Whittingham, M.S. Lithium Incorporation in Tungsten Oxides. Solid State Ion. Commun. 1980, 1, 151–161. [Google Scholar] [CrossRef]
- Pralong, V.; Venkatesh, G.; Malo, S.; Caignaert, V.; Baies, R.; Raveau, B. Electrochemical Synthesis of a Lithium-Rich Rock-Salt-Type Oxide. Inorg. Chem. 2014, 53, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Raistrick, I.D.; Mark, A.J.; Huggins, R.A. Thermodynamics of Electrochemical Insertion of Lithium into Tungsten Bronzes. Solid State Ion. 1981, 5, 351–354. [Google Scholar] [CrossRef]
- Dong, M.; Chatzus, I. The Imbibition and Flow of a Wetting Liquid along the Corners of a Square Capillary Tube. J. Colloid Interface Sci. 1995, 172, 278–288. [Google Scholar] [CrossRef]
- Ten Dam, J.; Badloe, D.; Ramanathan, A.; Djanashvili, K.; Kapteijn, F.; Hanefeld, U. Synthesis, Characterisation and Catalytic Performance of a Mesoporous Tungsten Silicate: W-TUD-1. Appl. Catal. A Gen. 2013, 468, 150–159. [Google Scholar] [CrossRef]
- Perednis, D.; Gauckler, L.J. Thin Film Deposition Using Spray Pyrolysis. J. Electroceram. 2005, 14, 103–111. [Google Scholar] [CrossRef]
- Baggetto, L.; Oudenhoven, J.F.M.; Van Dongen, T.; Klootwijk, J.H.; Mulder, M.; Niessen, R.A.H.; De Croon, M.; Notten, P.H.L. On the Electrochemistry of an Anode Stack for All-Solid-State 3D-Integrated Batteries. J. Power Sources 2009, 189, 402–410. [Google Scholar] [CrossRef]
- Saha, N.C.; Tompkins, H.G. Titanium Nitride Oxidation Chemistry: An X-Ray Photoelectron Spectroscopy Study. J. Appl. Phys. 1992, 72, 3072–3079. [Google Scholar] [CrossRef]
- Marchal, W.; De Dobbelaere, C.; Kesters, J.; Bonneux, G.; Vandenbergh, J.; Damm, H.; Junkers, T.; Maes, W.; D’Haen, J.; Van Bael, M.K.; et al. Combustion Deposition of MoO3 Films: From Fundamentals to OPV Applications. RSC Adv. 2015, 5, 91349–91362. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van den Ham, E.J.; Maino, G.; Bonneux, G.; Marchal, W.; Elen, K.; Gielis, S.; Mattelaer, F.; Detavernier, C.; Notten, P.H.L.; Van Bael, M.K.; et al. Wet-Chemical Synthesis of 3D Stacked Thin Film Metal-Oxides for All-Solid-State Li-Ion Batteries. Materials 2017, 10, 1072. https://doi.org/10.3390/ma10091072
Van den Ham EJ, Maino G, Bonneux G, Marchal W, Elen K, Gielis S, Mattelaer F, Detavernier C, Notten PHL, Van Bael MK, et al. Wet-Chemical Synthesis of 3D Stacked Thin Film Metal-Oxides for All-Solid-State Li-Ion Batteries. Materials. 2017; 10(9):1072. https://doi.org/10.3390/ma10091072
Chicago/Turabian StyleVan den Ham, Evert Jonathan, Giulia Maino, Gilles Bonneux, Wouter Marchal, Ken Elen, Sven Gielis, Felix Mattelaer, Christophe Detavernier, Peter H. L. Notten, Marlies K. Van Bael, and et al. 2017. "Wet-Chemical Synthesis of 3D Stacked Thin Film Metal-Oxides for All-Solid-State Li-Ion Batteries" Materials 10, no. 9: 1072. https://doi.org/10.3390/ma10091072
APA StyleVan den Ham, E. J., Maino, G., Bonneux, G., Marchal, W., Elen, K., Gielis, S., Mattelaer, F., Detavernier, C., Notten, P. H. L., Van Bael, M. K., & Hardy, A. (2017). Wet-Chemical Synthesis of 3D Stacked Thin Film Metal-Oxides for All-Solid-State Li-Ion Batteries. Materials, 10(9), 1072. https://doi.org/10.3390/ma10091072