Pitting Initiation and Propagation of X70 Pipeline Steel Exposed to Chloride-Containing Environments
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Materials and Samples Preparation
2.2. Microscopy Observations
2.3. Corrosion Tests
2.4. Electrochemical Measurements
3. Results and Discussion
3.1. Corrosion Tests
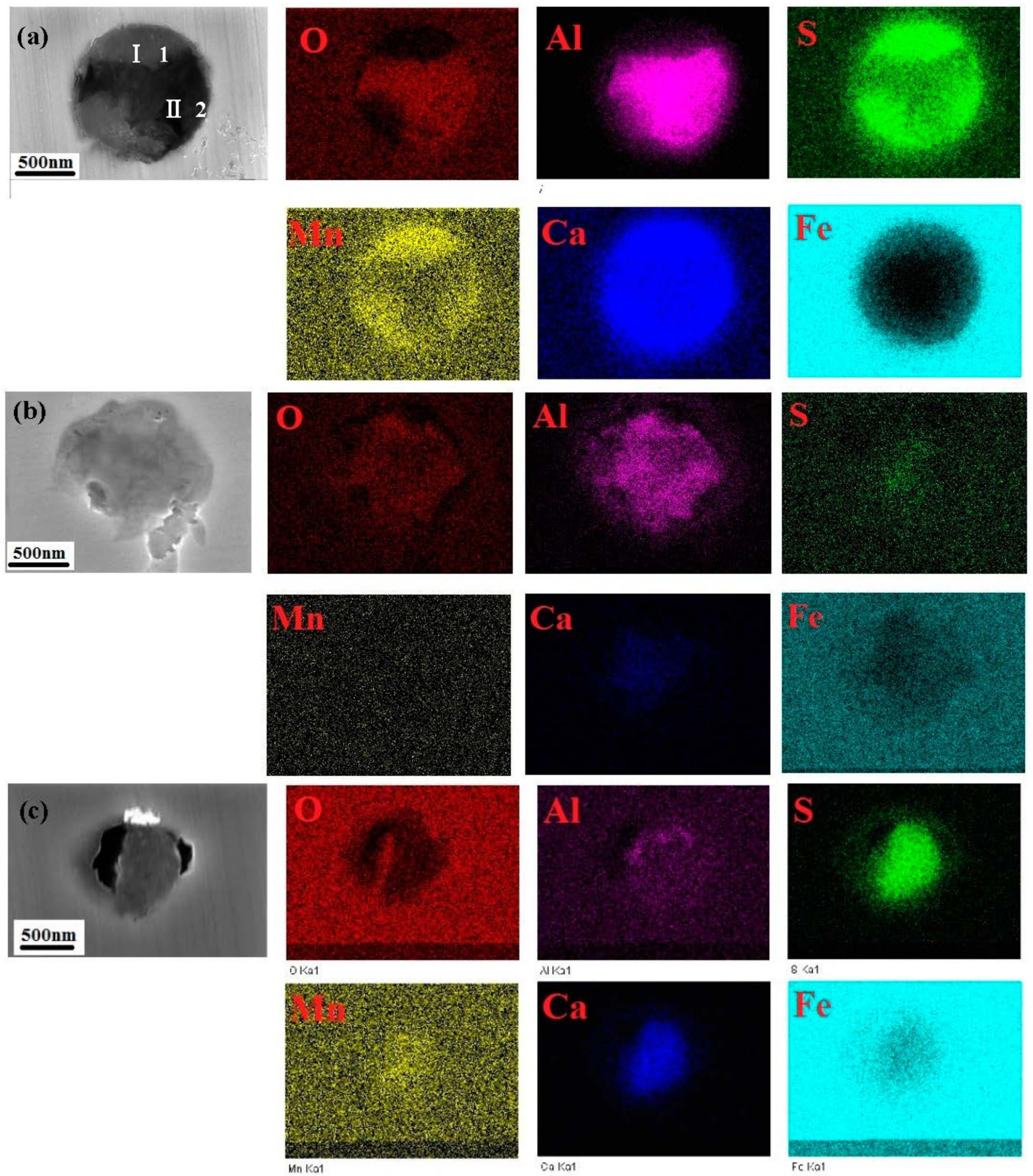
3.2. SEM Inclusions Observations
3.3. Potentiodynamic Polarization Tests
3.4. Characterization of the Corrosion Attack
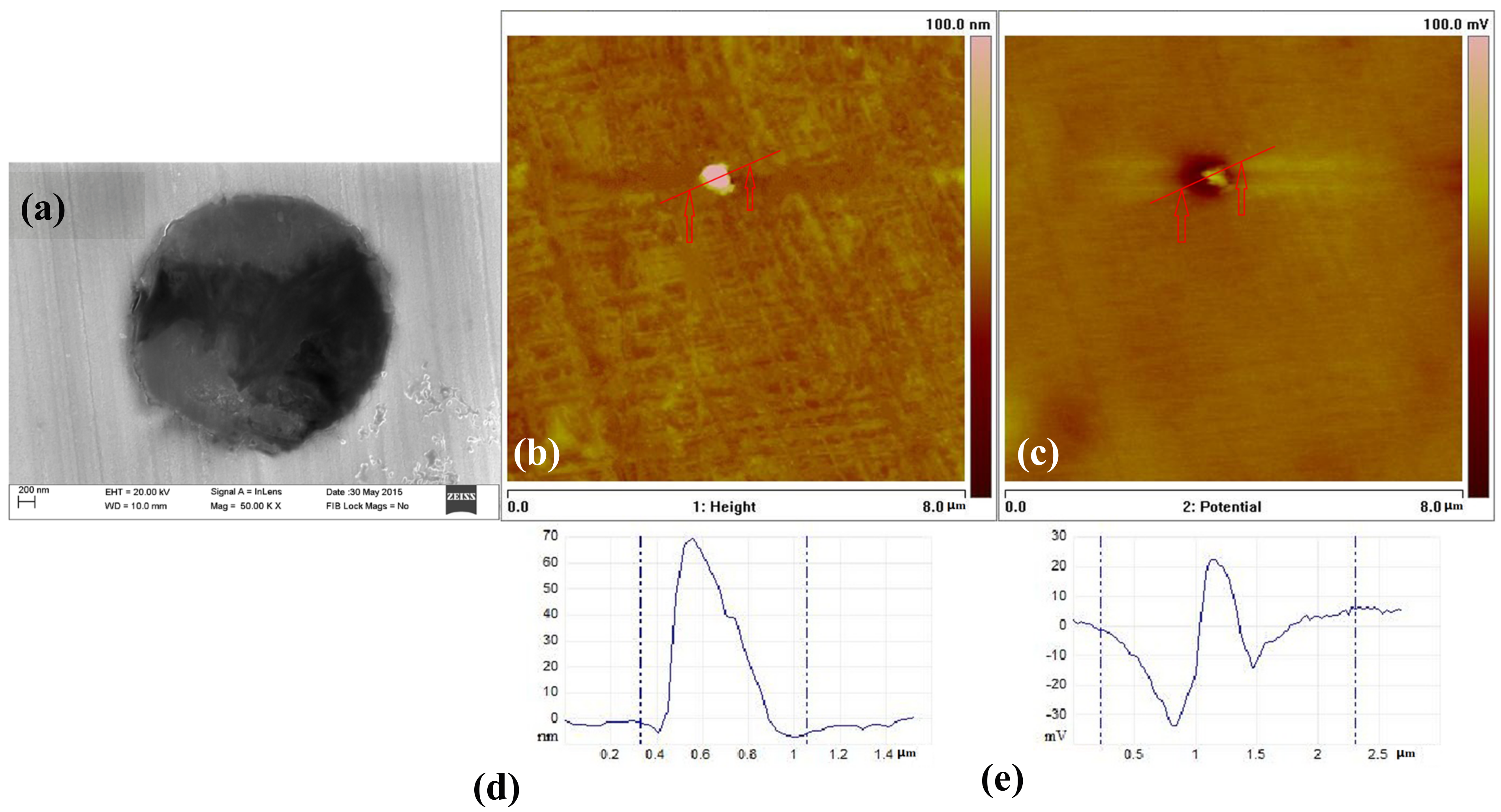
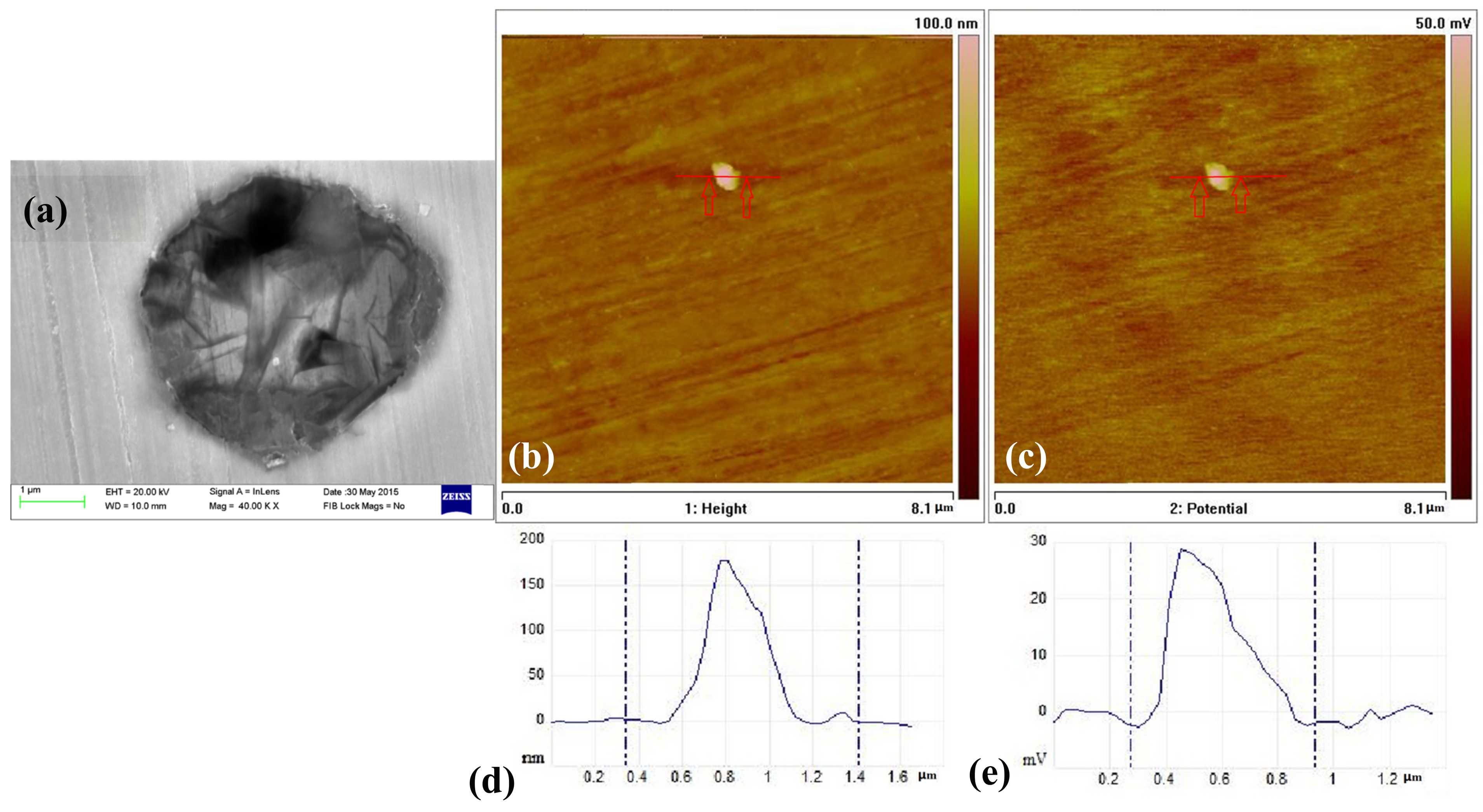
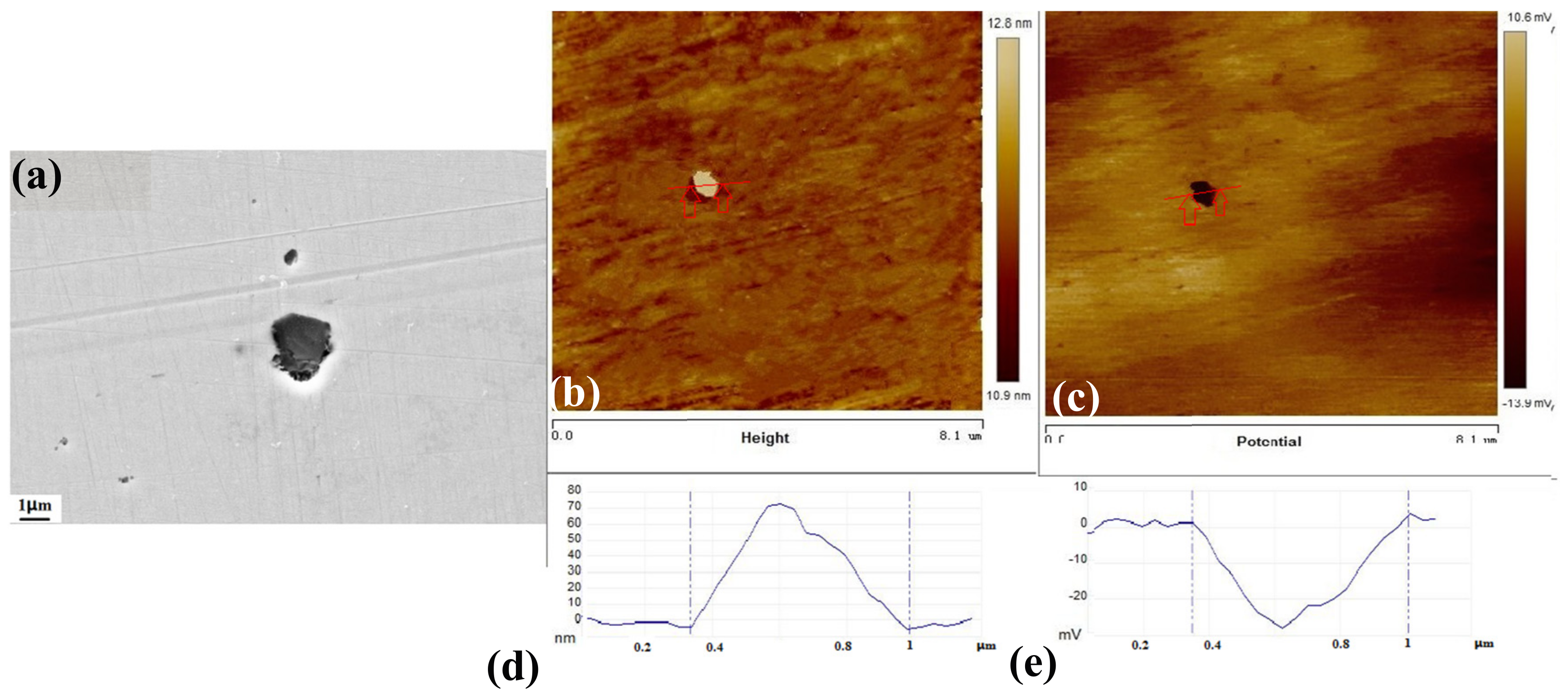
3.5. The Inclusions’ Potential Obtained by SKPFM
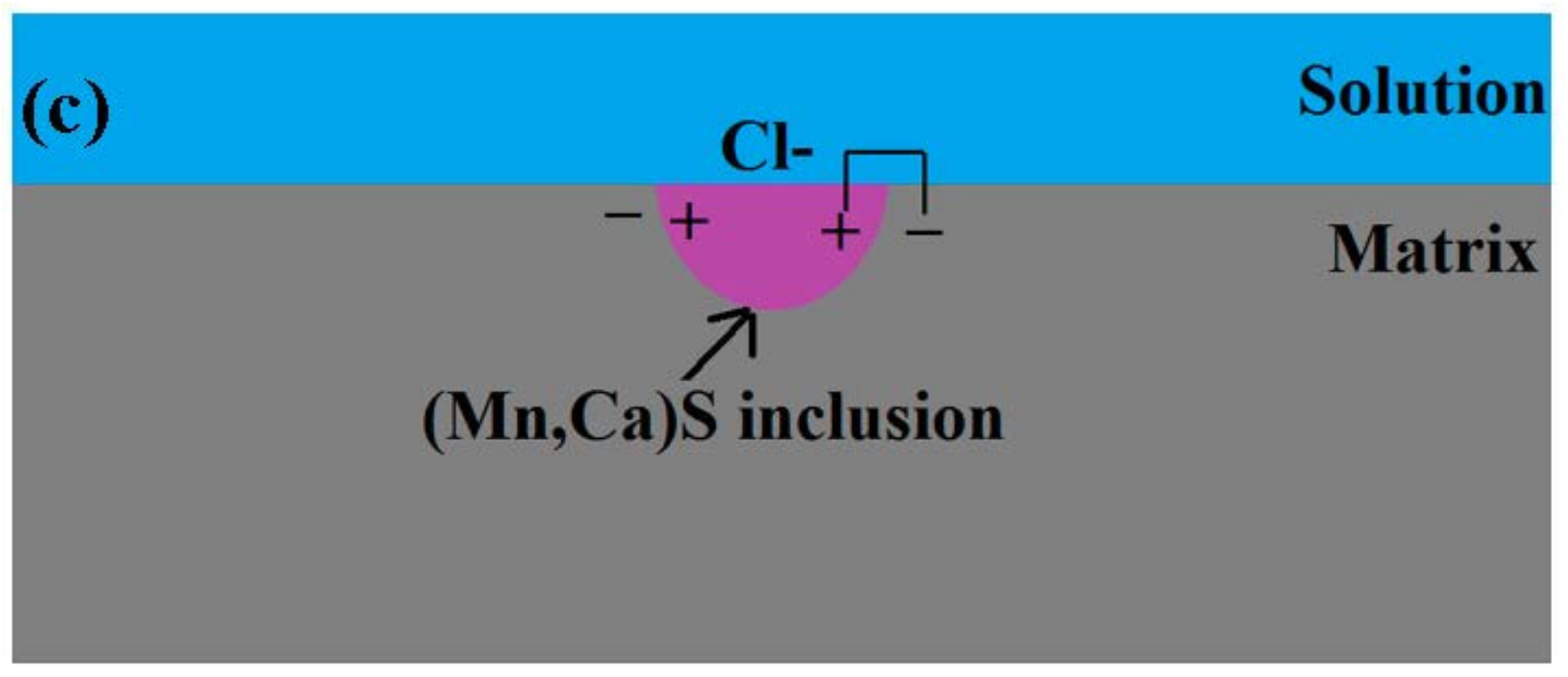
3.6. Pitting Initiation Mechanism
4. Conclusions
- (1)
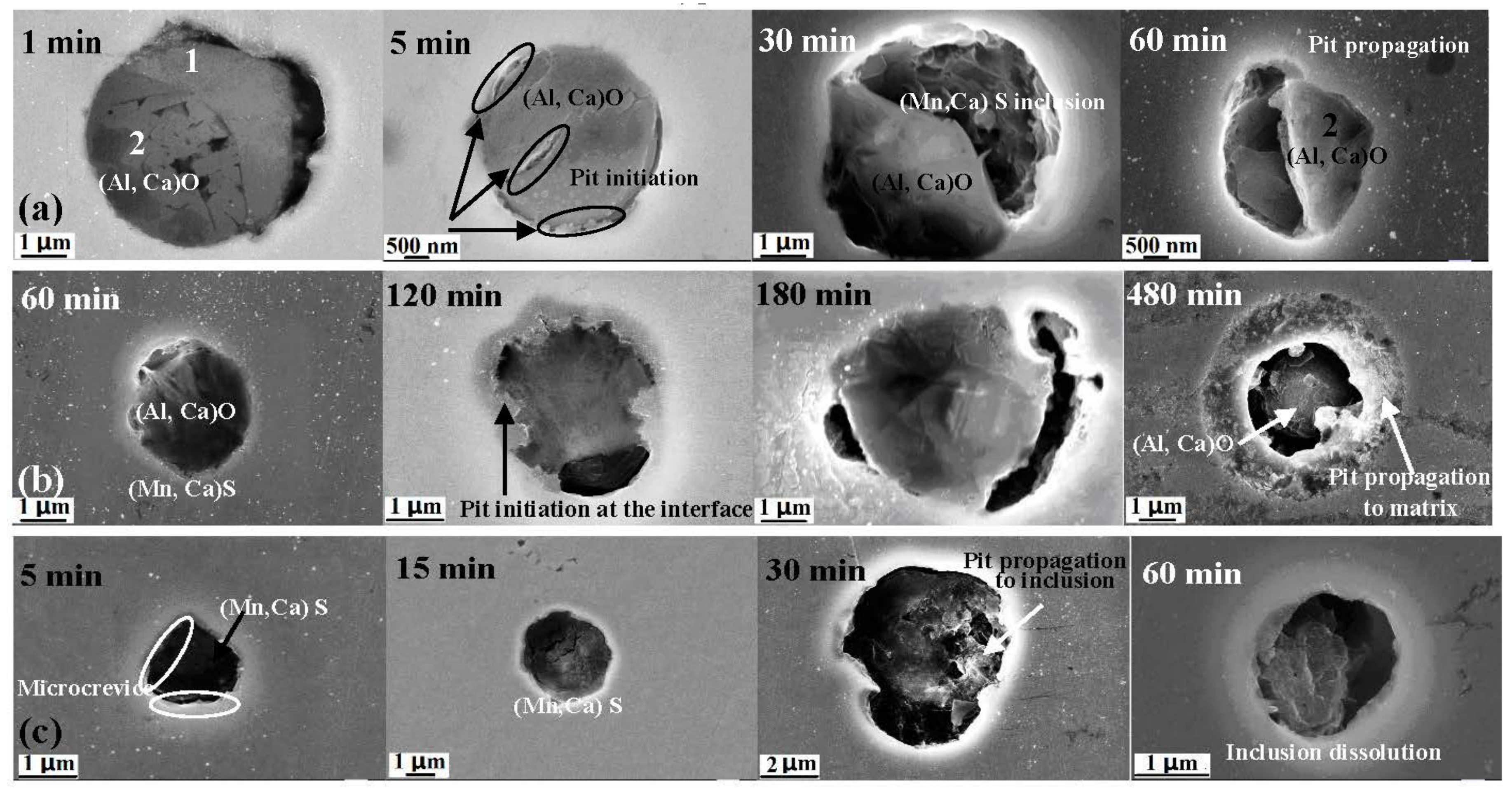
- Three types of inclusions were observed in the X70 steel. Type A is a complex inclusion, one part is rich in (Mn, Ca)S and another part is rich in (Al, Ca)O. Type B is rich in (Al, Ca)O and has smaller amounts of (Mn, Ca)S. Type C is rich in (Mn, Ca)S and has smaller amounts of (Al, Ca)O.
- (2)
- Three types of inclusions can induce pitting corrosion, but the pitting corrosion resistance is different: type A inclusion < type C inclusion < type B inclusion, and (Mn, Ca)S < matrix < (Al, Ca)O. The type A inclusion exhibited both lower and higher potentials than the matrix, while the type B inclusion exhibited higher potential than the matrix. Corrosion and AFM potential test results are consistent. Pitting corrosion is more likely to occur at the interface between the inclusion and the matrix because of its lower surface potential.
- (3)
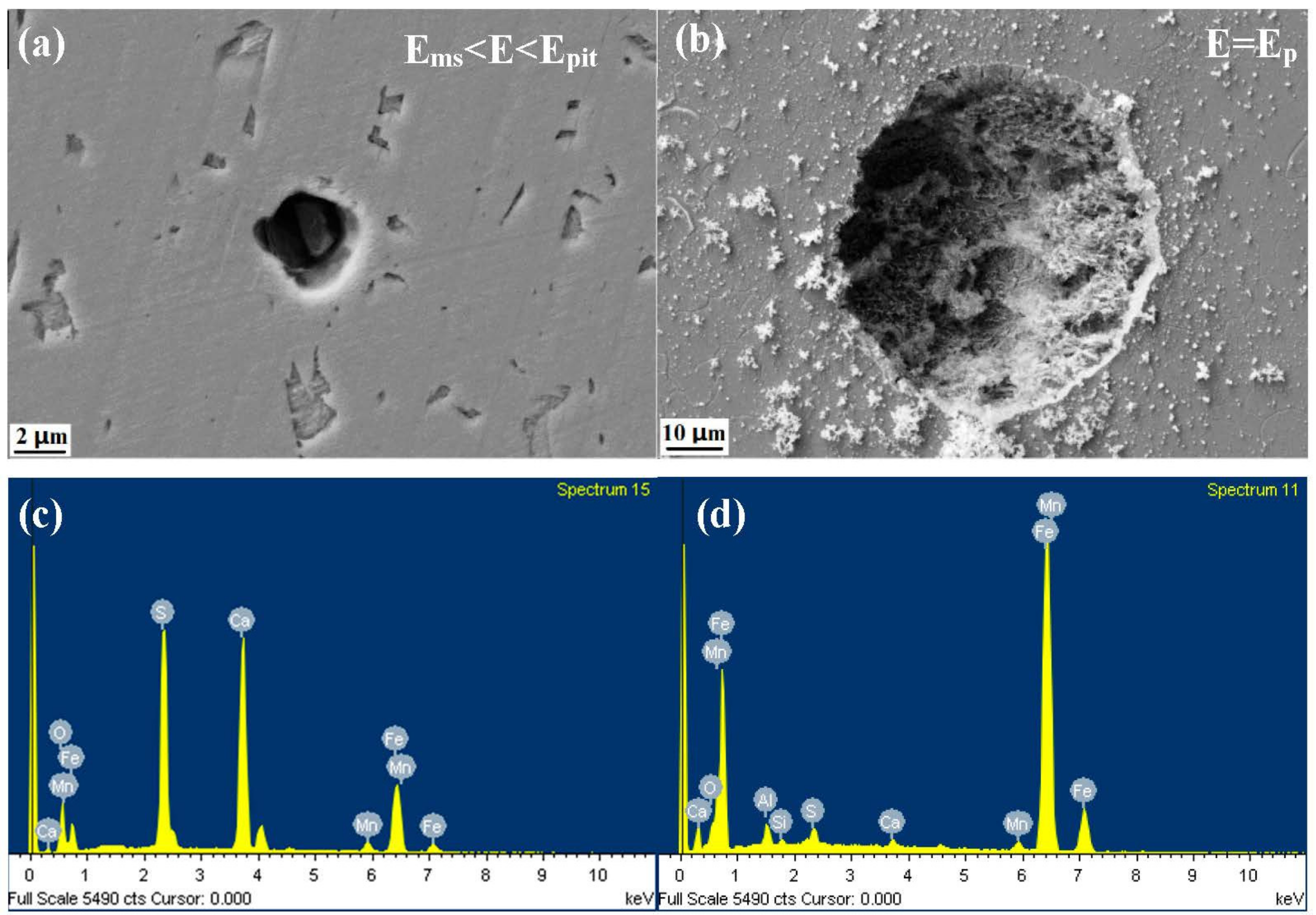
- The inclusions’ morphology was observed at different potentials. The passive film of the type A inclusion was broken at the potential Ems < E < Ep, while the type A inclusion can induce metastable pitting continuously. The passive film of the type B inclusion is inactive at the potential Ems < E < Ep, while the type B inclusion cannot induce metastable pitting continuously. The passive film of the type C inclusion was broken at the potential Ems <E < Ep, while the type C inclusion can lead to continuous metastable pitting corrosion. Thus, the types A and C are active inclusions and the type B is an inactive inclusion.
- (4)
- Three kinds of possible mechanisms of inclusions inducing pitting corrosion were established for the X70 steel.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Frankel, G.S. Pitting corrosion of metals-a review of the critical factors. J. Electrochem. Soc. 1998, 145, 2186–2198. [Google Scholar] [CrossRef]
- Newman, R.C. 2001 W.R. Whitney award lecture: Understanding the corrosion stainless steel. Corrosion 2001, 57, 1030–1041. [Google Scholar] [CrossRef]
- Ryan, M.P.; Williams, D.E.; Chater, R.J. Why stainless steel corrodes. Nature 2002, 415, 770–774. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Chen, C.; Shao, Y.W.; Meng, G.; Wang, F.H. Corrosion of pure magnesium under thin electrolyte layers. Electrochim. Acta. 2008, 53, 7921–7931. [Google Scholar] [CrossRef]
- Park, I1.-J.; Lee, S.-M.; Kang, M. Pitting corrosion behavior in advanced high strength steels. J. Alloys Compd. 2015, 619, 205–210. [Google Scholar]
- Zheng, S.Q.; Li, C.Y.; Qi, Y.M. Mechanism of (Mg,Al,Ca)-oxide inclusion-induced pitting corrosion in 316L stainless steel exposed to sulphur environments containing chloride ion. Corros. Sci. 2013, 67, 20–31. [Google Scholar] [CrossRef]
- Wranglen, G. Pitting and sulphide inclusions in steel. Corros. Sci. 1974, 14, 331–349. [Google Scholar] [CrossRef]
- Williams, D.E.; Zhu, Y.Y. Explanation for initiation of pitting corrosion of stainless steels at sulfide inclusions. J. Electrochem. Soc. 2000, 147, 1763–1766. [Google Scholar] [CrossRef]
- Williams, D.E.; Stewart, J.; Balkwill, P.H. The nucleation, growth and stability of micropits in stainless steel. Corros. Sci. 1994, 36, 1213–1235. [Google Scholar] [CrossRef]
- Wranglen, G. Pitting and sulphide inclusions in steel. In Proceedings of the International Congress on Localized Corrosion, Willamburg, VA, USA, 6–10 December 1971. [Google Scholar]
- Zheng, S.J.; Wang, Y.J.; Zhang, B. Identification of MnCr2O4 nano-octahedron in catalyzing pitting corrosion of austenitic stainless steels. Acta Mater. 2010, 58, 5070–5085. [Google Scholar] [CrossRef]
- Eklund, G.S. Initiation of pitting at sulfide inclusions in stainless steel. J. Electrochem. Soc. 1974, 121, 467–473. [Google Scholar] [CrossRef]
- Punckt, C.; Bölscher, M.; Rotermund, H.H.; Mikhailov, A.S. Sudden Onset of Pitting Corrosion on Stainless Steel as a Critical Phenomenon. Science 2004, 305, 1133–1136. [Google Scholar] [CrossRef] [PubMed]
- Gholami, M.; Hoseinpoor, M.; Moayed, M.H. A statistical study on the effect of annealing temperature on pitting corrosion resistance of 2205 duplex stainless steel. Corros. Sci. 2015, 94, 156–164. [Google Scholar] [CrossRef]
- Abbasi Aghuy, A.; Zakeri, M.; Moayed, M.H.; Mazinani, M. Effect of grain size on pitting corrosion of 304L austenitic stainless steel. Corros. Sci. 2015, 94, 368–376. [Google Scholar] [CrossRef]
- Lin, B.; Hu, R.G.; Ye, C.Q. A study on the initiation of pitting corrosion in carbon steel in chloride-containing media using scanning electrochemical probes. Electrochim. Acta 2010, 55, 6542–6545. [Google Scholar] [CrossRef]
- Westcott, C.; Williams, D.E. Modeling of the initiation and growth of pits at constant potential. J. Electrochem. Soc. 1985, 132, 1796–1804. [Google Scholar]
- Tang, Y.M.; Zuo, Y.; Zhao, X.H. The metastable pitting behaviors of mild steel in bicarbonate and nitrite solutions containing Cl−. Corros. Sci. 2008, 50, 989–994. [Google Scholar] [CrossRef]
- Wang, H.; Xie, J.; Yan, K.P.; Duan, M.; Zuo, Y. The nucleation and growth of metastable pitting on pure iron. Corros. Sci. 2009, 51, 181–185. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, T.; Meng, G.; Shao, Y.; Wang, F. Effect of pitting nucleation on critical pitting temperature of 316L stainless steel by nitric acid passivation. Corros. Sci. 2015, 91, 232–244. [Google Scholar] [CrossRef]
- Soltis, J. Passivity breakdown, pit initiation and propagation of pits in metallic materials—Review. Corros. Sci. 2015, 90, 5–22. [Google Scholar] [CrossRef]
- Tian, W.M.; Du, N.; Li, S.M. Metastable pitting corrosion of 304 stainless steel in 3.5% NaCl solution. Corros. Sci. 2014, 85, 372–379. [Google Scholar] [CrossRef]
- Shibata, T.; Zhu, Y. A stochastic analysis of flow velocity effects on the pit generation process on anodized titanium. Corros. Sci. 1995, 37, 853. [Google Scholar] [CrossRef]
- Shibata, T.; Zhu, Y. The effect of film formation temperature on the stochastic processes of pit generation on anodized titanium. Corros. Sci. 1994, 36, 1735. [Google Scholar] [CrossRef]
- Amin, M.A. Metastable and stable pitting events on Al induced by chlorate and perchlorate anions—Polarization, XPS and SEM studies. Electrochim. Acta 2009, 54, 1857–1863. [Google Scholar] [CrossRef]
- Jeon, S.-H.; Kim, S.-T.; Choi, M.-S. Effects of cerium on the compositional variations in and around inclusions and the initiation and propagation of pitting corrosion in hyperduplex stainless steels. Corros. Sci. 2013, 75, 367–375. [Google Scholar] [CrossRef]
- Jeon, S.-H.; Kim, S.-T.; Lee, I.-S. Effects of sulfur addition on pitting corrosion and machinability behavior of super duplex stainless steel containing rare earth metals: Part 2. Corros. Sci. 2010, 52, 3537–3547. [Google Scholar] [CrossRef]
- Jeon, S.-H.; Kim, S.-T.; Lee, I.-S. Effects of copper addition on the formation of inclusions and the resistance to pitting corrosion of high performance duplex stainless steels. Corros. Sci. 2011, 53, 1408–1416. [Google Scholar] [CrossRef]
- Zhang, F. Localized corrosion behaviour of reinforcement steel in simulated concrete pore solution. Corros. Sci. 2009, 51, 2130–2138. [Google Scholar] [CrossRef]
- Suter, T.; Bohni, H. Microelectrodes for corrosion studies in Microsystems. Electrochim. Acta 2001, 47, 191–199. [Google Scholar] [CrossRef]
- Suter, T. A new microelectrochemical method to study pit initiation on stainless steels. Electrochim. Acta 1997, 42, 3275–3280. [Google Scholar] [CrossRef]





| C | Si | Mn | P | S | Cr | Ni | Ca | Cu | Al | Ti | Other | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.066 | 0.29 | 1.39 | 0.008 | 0.002 | 0.032 | 0.201 | 0.051 | 0.17 | 0.034 | 0.015 | 0.300 | Bal. |
| Type of Inclusion | Chemical Composition (wt %) | |||||
|---|---|---|---|---|---|---|
| O | Al | Mn | S | Ca | Fe | |
| b in Figure 1b | 33.9 | 31.1 | 8.1 | 4.5 | 3.1 | 21.4 |
| c in Figure 1c | 31.8 | 36.7 | 0.6 | 0.5 | 0.4 | 30.1 |
| Type of Inclusion | Chemical Composition (wt %) | |||||
|---|---|---|---|---|---|---|
| O | Al | Mn | S | Ca | Fe | |
| Type A in Figure 2a-1 | 28.62 | 8.44 | 12.01 | 11.48 | 8.64 | 30.80 |
| Type A in Figure 2a-2 | 40.38 | 30.32 | 0.56 | 0.48 | 2.55 | 22.46 |
| Type B in Figure 2b | 29.8 | 18.59 | 1.88 | 4.46 | 4.88 | 37.96 |
| Type C in Figure 2c | 14.73 | 4.7 | 13.59 | 16.35 | 10.97 | 41.05 |
| Type of Inclusion | Chemical composition (wt %) | |||||
|---|---|---|---|---|---|---|
| O | Al | Mn | S | Ca | Fe | |
| Figure 7-a1-1 min | 18.6 | 6.4 | 15.0 | 20.6 | 8.6 | 30.8 |
| Figure 7-a1-60 min | 0.2 | 0.3 | 1.6 | 0.2 | 0.2 | 97.5 |
| Figure 7-a2-1 min | 42.4 | 31.3 | 0.7 | 0.8 | 2.4 | 22.4 |
| Figure 7-a2-60 min | 40.4 | 31.4 | 0.6 | 0.5 | 1.5 | 25.6 |
| Figure 7-b-60 min | 29.8 | 18.6 | 1.9 | 4.5 | 4.8 | 37.9 |
| Figure 7-b-480 min | 31.8 | 20.9 | 0.9 | 1.5 | 0.8 | 44.1 |
| Figure 7-c-5 min | 11.6 | 4.5 | 16.6 | 19.4 | 11.9 | 36.0 |
| Figure 7-c-60 min | 5.7 | 1.7 | 1.6 | 2.4 | 1.9 | 86.7 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Kan, B.; Li, J.; Su, Y.; Qiao, L.; Volinsky, A.A. Pitting Initiation and Propagation of X70 Pipeline Steel Exposed to Chloride-Containing Environments. Materials 2017, 10, 1076. https://doi.org/10.3390/ma10091076
Yang Z, Kan B, Li J, Su Y, Qiao L, Volinsky AA. Pitting Initiation and Propagation of X70 Pipeline Steel Exposed to Chloride-Containing Environments. Materials. 2017; 10(9):1076. https://doi.org/10.3390/ma10091076
Chicago/Turabian StyleYang, Zixuan, Bo Kan, Jinxu Li, Yanjing Su, Lijie Qiao, and Alex A. Volinsky. 2017. "Pitting Initiation and Propagation of X70 Pipeline Steel Exposed to Chloride-Containing Environments" Materials 10, no. 9: 1076. https://doi.org/10.3390/ma10091076
APA StyleYang, Z., Kan, B., Li, J., Su, Y., Qiao, L., & Volinsky, A. A. (2017). Pitting Initiation and Propagation of X70 Pipeline Steel Exposed to Chloride-Containing Environments. Materials, 10(9), 1076. https://doi.org/10.3390/ma10091076







