The Influence of the Polymer Amount on the Biological Properties of PCL/ZrO2 Hybrid Materials Synthesized via Sol-Gel Technique
Abstract
:1. Introduction
2. Results and Discussion
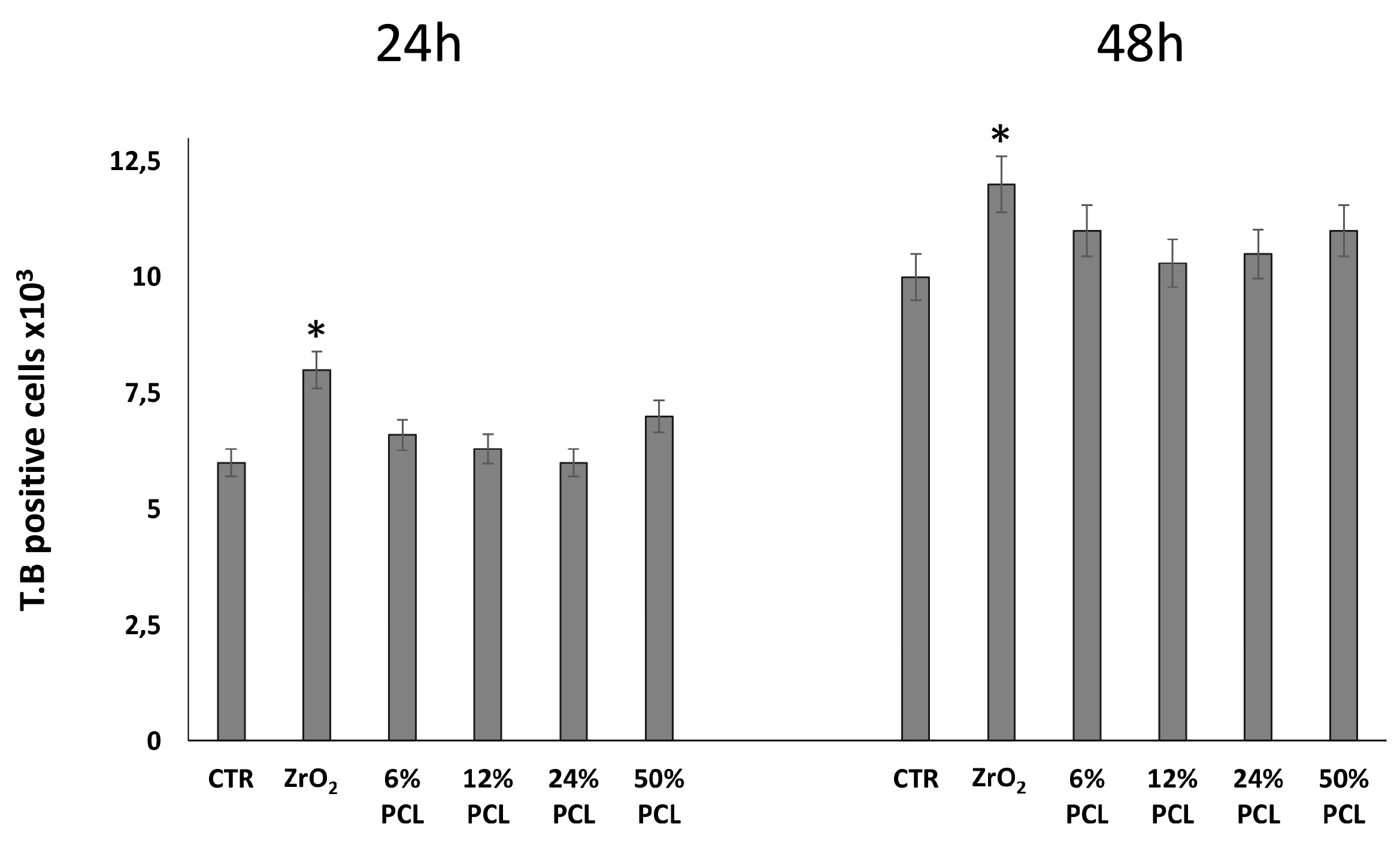
2.1. PCL/ZrO2 Hybrid Materials Do Not Have Cytotoxic Effects
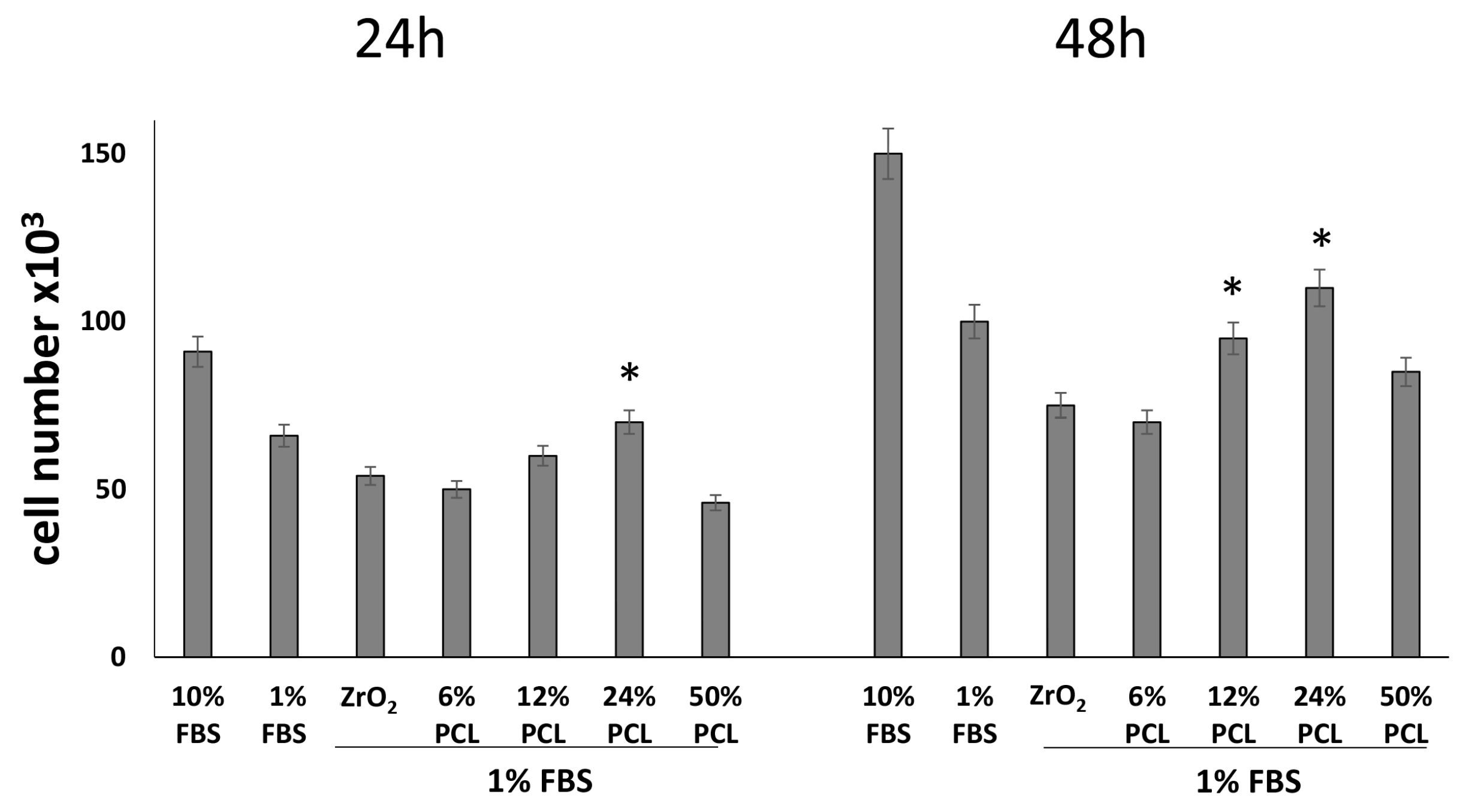
2.2. PCL Content in the PCL/ZrO2 Hybrids Positively Affects the Cell Proliferation
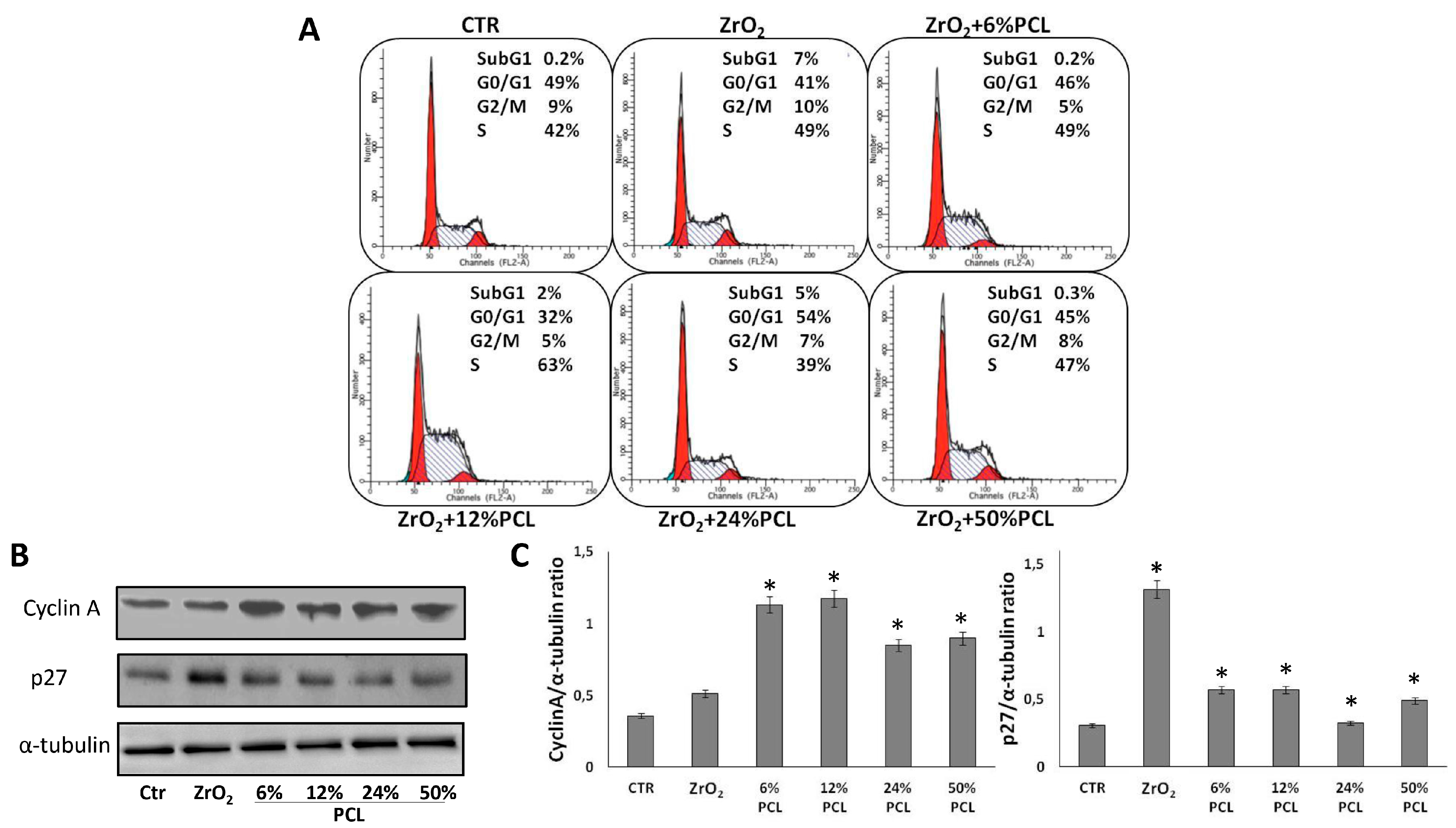
2.3. PCL Content in the PCL/ZrO2 Hybrids Affects the Cell Cycle Progression
3. Materials and Methods
3.1. Sol-Gel Synthesis
3.2. Cell Culture and Treatments
3.3. Trypan Blue Exclusion Test
3.4. Cell Viability Assay
3.5. Evaluation of Cell Cycle Phases by Flow Cytometry
3.6. Preparation of Cell Lysates
3.7. Western Blot Analysis
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Judeinstein, P.; Sanchez, C. Hybrid organic-inorganic materials: A land of multidisciplinarity. J. Mater. Chem. 1996, 6, 511–525. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Klein, L.C. Sol-Gel Technology for Thin Films, Fibers, Preforms, Electronics, and Specialty Shapes; William Andrew Publishing: Norwich, NY, USA, 1988. [Google Scholar]
- Lu, B.; Lin, Y. Sol-gel synthesis and characterization of mesoporous yttria-stabilized zirconia membranes with graded pore structure. J. Mater. Sci. 2011, 46, 7056–7066. [Google Scholar] [CrossRef]
- Catauro, M.; Papale, F.; Sapio, L.; Naviglio, S. Biological influence of Ca/P ratio on calcium phosphate coatings by sol-gel processing. Mater. Sci. Eng. C 2016, 65, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Kim, H.-M.; Kawashita, M. Novel bioactive materials with different mechanical properties. Biomaterials 2003, 24, 2161–2175. [Google Scholar] [CrossRef]
- Gupta, R.; Kumar, A. Bioactive materials for biomedical applications using sol-gel technology. Biomed. Mater. 2008, 3, 034005. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.A.; Yue, S.; Hanna, J.V.; Lee, P.; Newport, R.J.; Smith, M.E.; Jones, J.R. Characterizing the hierarchical structures of bioactive sol–gel silicate glass and hybrid scaffolds for bone regeneration. Phil. Trans. R. Soc. A 2012, 370, 1422–1443. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.; Ribot, F. Design of hybrid organic-inorganic materials synthesized via sol-gel chemistry. New J. Chem. 1994, 18, 1007–1047. [Google Scholar]
- Eldsäter, C.; Erlandsson, B.; Renstad, R.; Albertsson, A.-C.; Karlsson, S. The biodegradation of amorphous and crystalline regions in film-blown Poly (ϵ-caprolactone). Polymer 2000, 41, 1297–1304. [Google Scholar] [CrossRef]
- Choi, E.J.; Kim, C.H.; Park, J.K. Synthesis and characterization of starch-g-polycaprolactone copolymer. Macromolecules 1999, 32, 7402–7408. [Google Scholar] [CrossRef]
- Causa, F.; Battista, E.; Della Moglie, R.; Guarnieri, D.; Iannone, M.; Netti, P.A. Surface investigation on biomimetic materials to control cell adhesion: The case of rgd conjugation on PCL. Langmuir 2010, 26, 9875–9884. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Sun, X.S. Properties of soy protein isolate/polycaprolactone blends compatibilized by methylene diphenyl diisocyanate. Polymer 2001, 42, 6961–6969. [Google Scholar] [CrossRef]
- De Santis, R.; Gloria, A.; Russo, T.; D’Amora, U.; Zeppetelli, S.; Dionigi, C.; Sytcheva, A.; Herrmannsdörfer, T.; Dediu, V.; Ambrosio, L. A basic approach toward the development of nanocomposite magnetic scaffolds for advanced bone tissue engineering. J. Appl. Polym. Sci. 2011, 122, 3599–3605. [Google Scholar] [CrossRef]
- Hutmacher, D.W.; Schantz, T.; Zein, I.; Ng, K.W.; Teoh, S.H.; Tan, K.C. Mechanical properties and cell cultural response of polycaprolactone scaffolds designed and fabricated via fused deposition modeling. J. Biomed. Mater. Res. A 2001, 55, 203–216. [Google Scholar] [CrossRef]
- Kyriakidou, K.; Lucarini, G.; Zizzi, A.; Salvolini, E.; Mattioli Belmonte, M.; Mollica, F.; Gloria, A.; Ambrosio, L. Dynamic co-seeding of osteoblast and endothelial cells on 3d polycaprolactone scaffolds for enhanced bone tissue engineering. J. Bioact. Compat. Polym. 2008, 23, 227–243. [Google Scholar] [CrossRef]
- Bañobre-López, M.; Pineiro-Redondo, Y.; De Santis, R.; Gloria, A.; Ambrosio, L.; Tampieri, A.; Dediu, V.; Rivas, J. Poly (caprolactone) based magnetic scaffolds for bone tissue engineering. J. Appl. Phys. 2011, 109, 07B313. [Google Scholar] [CrossRef]
- Bartolo, P.; Domingos, M.; Gloria, A.; Ciurana, J. Biocell printing: Integrated automated assembly system for tissue engineering constructs. CIRP Ann.-Manuf. Technol. 2011, 60, 271–274. [Google Scholar] [CrossRef]
- Catauro, M.; Bollino, F.; Papale, F.; Piccolella, S.; Pacifico, S. Sol-gel synthesis and characterization of SiO2/PCL hybrid materials containing quercetin as new materials for antioxidant implants. Mater. Sci. Eng. C 2016, 58, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Domingos, M.; Chiellini, F.; Gloria, A.; Ambrosio, L.; Bartolo, P.; Chiellini, E. Effect of process parameters on the morphological and mechanical properties of 3d bioextruded poly (ϵ-caprolactone) scaffolds. Rapid Prototyp. J. 2012, 18, 56–67. [Google Scholar] [CrossRef]
- Catauro, M.; Bollino, F. Anti-inflammatory entrapment in polycaprolactone/silica hybrid material prepared by sol-gel route, characterization, bioactivity and in vitro release behavior. J. Appl. Biomater. Funct. Mater. 2013, 11, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, F.; Moradi, L.; Shadmehr, M.B.; Bonakdar, S.; Droodinia, A.; Safshekan, F. In-vivo characterization of a 3d hybrid scaffold based on PCL/decellularized aorta for tracheal tissue engineering. Mater. Sci. Eng. C 2017, 81, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Perrin, D.E.; English, J.P. Polycaprolactone. In Handbook of Biodegradable Polymers; Domb, A.J., Kost, J.K., Wiseman, D.M., Eds.; CRC Press: Amsterdam, The Netherlands, 1997; Volume 7, pp. 63–77. [Google Scholar]
- Lee, S.J.; Liu, J.; Oh, S.H.; Soker, S.; Atala, A.; Yoo, J.J. Development of a composite vascular scaffolding system that withstands physiological vascular conditions. Biomaterials 2008, 29, 2891–2898. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.-J.; Meredith, C.; Johnson, C.; Galis, Z.S. The effect of scaffold degradation rate on three-dimensional cell growth and angiogenesis. Biomaterials 2004, 25, 5735–5742. [Google Scholar] [CrossRef] [PubMed]
- Catauro, M.; Bollino, F.; Papale, F. Biocompatibility improvement of titanium implants by coating with hybrid materials synthesized by sol-gel technique. J. Biomed. Mater. Res. A 2014, 102, 4473–4479. [Google Scholar] [CrossRef] [PubMed]
- Catauro, M.; Bollino, F.; Papale, F.; Mozzati, M.C.; Ferrara, C.; Mustarelli, P. ZrO2/PEG hybrid nanocomposites synthesized via sol-gel: Characterization and evaluation of the magnetic properties. J. Non-Cryst. Solids 2015, 413, 1–7. [Google Scholar] [CrossRef]
- Catauro, M.; Bollino, F.; Papale, F.; Mozetic, P.; Rainer, A.; Trombetta, M. Biological response of human mesenchymal stromal cells to titanium grade 4 implants coated with PCL/ZrO2 hybrid materials synthesized by sol-gel route: In vitro evaluation. Mater. Sci. Eng. C 2014, 45, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Catauro, M.; Mozzati, M.C.; Bollino, F. Sol–gel hybrid materials for aerospace applications: Chemical characterization and comparative investigation of the magnetic properties. Acta Astronaut. 2015, 117, 153–162. [Google Scholar] [CrossRef]
- Catauro, M.; Raucci, M.; Ausanio, G. Sol-gel processing of drug delivery zirconia/polycaprolactone hybrid materials. J. Mater. Sci.-Mater. M 2008, 19, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Catauro, M.; Bollino, F.; Papale, F. Preparation, characterization, and biological properties of organic-inorganic nanocomposite coatings on titanium substrates prepared by sol-gel. J. Biomed. Mater. Res. A 2014, 102, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Catauro, M.; Bollino, F.; Papale, F.; Pacifico, S.; Galasso, S.; Ferrara, C.; Mustarelli, P. Synthesis of zirconia/polyethylene glycol hybrid materials by sol-gel processing and connections between structure and release kinetic of indomethacin. Drug Deliv. 2014, 21, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Naviglio, S.; Spina, A.; Chiosi, E.; Fusco, A.; Illiano, F.; Pagano, M.; Romano, M.; Senatore, G.; Sorrentino, A.; Sorvillo, L. Inorganic phosphate inhibits growth of human osteosarcoma U2OS cells via adenylate cyclase/camp pathway. J. Cell. Biochem. 2006, 98, 1584–1596. [Google Scholar] [CrossRef] [PubMed]
- Catauro, M.; Papale, F.; Bollino, F.; Gallicchio, M.; Pacifico, S. Biological evaluation of zirconia/peg hybrid materials synthesized via sol-gel technique. Mater. Sci. Eng. C 2014, 40, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, G.; Singaravelu, S.; Muthukumar, T.; Thyagarajan, S.; Perumal, P.T.; Sivagnanam, U.T. Design and characterization of 3d hybrid collagen matrixes as a dermal substitute in skin tissue engineering. Mater. Sci. Eng. C 2017, 72, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lee, S.J.; Chung, S.; Lee, J.H.; Kim, W.D.; Lee, J.Y.; Park, S.A. Cell-laden 3d bioprinting hydrogel matrix depending on different compositions for soft tissue engineering: Characterization and evaluation. Mater. Sci. Eng. C 2017, 71, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, S.I.; Chakarov, S.; Momchilova, A.; Pankov, R. Live-cell biosensor for assessment of adhesion qualities of biomaterials. Mater. Sci. Eng. C 2017, 78, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Navarro, L.; Mogosanu, D.-E.; Ceaglio, N.; Luna, J.; Dubruel, P.; Rintoul, I. Novel poly (diol sebacate) s as additives to modify paclitaxel release from poly (lactic-co-glycolic acid) thin films. J. Pharm. Sci. 2017, 106, 2106–2114. [Google Scholar] [CrossRef] [PubMed]
- Morgenstern, S.; Flor, R.; Kessler, G.; Klein, B. Automated determination of nad-coupled enzymes. Determination of lactic dehydrogenase. Anal. Biochem. 1965, 13, 149–161. [Google Scholar] [CrossRef]
- Ferri, F.F. Ferri’s Best Test E-Book: A Practical Guide to Laboratory Medicine and Diagnostic Imaging, 3rd ed.; Elsevier Health Sciences: Philadelphia, PA, USA, 2014. [Google Scholar]
- Spina, A.; Sorvillo, L.; Di Maiolo, F.; Esposito, A.; D’Auria, R.; Di Gesto, D.; Chiosi, E.; Naviglio, S. Inorganic phosphate enhances sensitivity of human osteosarcoma U2OS cells to doxorubicin via a p53-dependent pathway. J. Cell. Physiol. 2013, 228, 198–206. [Google Scholar] [CrossRef] [PubMed]
- McKeehan, W.L. Glutaminolysis in animal cells. In Carbohydrate Metabolism in Cultured Cells; Morgan, M.J., Ed.; Springer: Boston, MA, USA, 1986; pp. 111–150. [Google Scholar]
- Sekiya, S.; Suzuki, A. Direct conversion of mouse fibroblasts to hepatocyte-like cells by defined factors. Nature 2011, 475, 390–393. [Google Scholar] [CrossRef] [PubMed]
- Messer, R.L.; Lockwood, P.E.; Wataha, J.C.; Lewis, J.B.; Norris, S.; Bouillaguet, S. In vitro cytotoxicity of traditional versus contemporary dental ceramics. J. Prosthet. Dent. 2003, 90, 452–458. [Google Scholar] [CrossRef]
- Sims-Mourtada, J.; Niamat, R.A.; Samuel, S.; Eskridge, C.; Kmiec, E.B. Enrichment of breast cancer stem-like cells by growth on electrospun polycaprolactone-chitosan nanofiber scaffolds. Int. J. Nanomed. 2014, 9, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Allo, B.A.; Rizkalla, A.S.; Mequanint, K. Hydroxyapatite formation on sol-gel derived poly (ε-caprolactone)/bioactive glass hybrid biomaterials. ACS Appl. Mater. Interfaces 2012, 4, 3148–3156. [Google Scholar] [CrossRef] [PubMed]
- Catauro, M.; Bollino, F.; Papale, F. Surface modifications of titanium implants by coating with bioactive and biocompatible poly (ε-caprolactone)/sio 2 hybrids synthesized via sol-gel. Arab. J. Chem. 2015. [Google Scholar] [CrossRef]
- Teng, S.-H.; Wang, P.; Dong, J.-Q. Bioactive hybrid coatings of poly (ε-caprolactone)–silica xerogel on titanium for biomedical applications. Mater. Lett. 2014, 129, 209–212. [Google Scholar] [CrossRef]
- Ciprioti, S.V.; Bollino, F.; Tranquillo, E.; Catauro, M. Synthesis, thermal behavior and physicochemical characterization of ZrO2/PEG inorganic/organic hybrid materials via sol-gel technique. J. Therm. Anal. Calorim. 2017, 130, 535–540. [Google Scholar] [CrossRef]
- Naviglio, S.; Matteucci, C.; Matoskova, B.; Nagase, T.; Nomura, N.; Di Fiore, P.P.; Draetta, G.F. Ubpy: A growth-regulated human ubiquitin isopeptidase. EMBO J. 1998, 17, 3241–3250. [Google Scholar] [CrossRef] [PubMed]
- Moraleva, A.; Magoulas, C.; Polzikov, M.; Hacot, S.; Mertani, H.C.; Diaz, J.J.; Zatsepina, O. Involvement of the specific nucleolar protein surf6 in regulation of proliferation and ribosome biogenesis in mouse nih/3t3 fibroblasts. Cell Cycle 2017, 16, 1979–1991. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Yamamoto, M.; Okada, H.; Yashiki, T.; Shimamoto, T. A new technique to efficiently entrap leuprolide acetate into microcapsules of polylactic acid or copoly (lactic/glycolic) acid. Chem. Pharm. Bull. 1988, 36, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- Tokiwa, Y.; Suzuki, T. Hydrolysis of polyesters by lipases. Nature 1977, 270, 76–78. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, V.; Campo, M.S. Bpv-4 E8 transforms NIH 3T3 cells, up-regulates cyclin a and cyclin a-associated kinase activity and de-regulates expression of the cdk inhibitor p27 Kip1. Oncogene 1998, 17, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Guardavaccaro, D.; Pagano, M. Stabilizers and destabilizers controlling cell cycle oscillators. Mol. Cell 2006, 22, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Sapio, L.; Sorvillo, L.; Illiano, M.; Chiosi, E.; Spina, A.; Naviglio, S. Inorganic phosphate prevents Erk1/2 and Stat3 activation and improves sensitivity to doxorubicin of mda-mb-231 breast cancer cells. Molecules 2015, 20, 15910–15928. [Google Scholar] [CrossRef] [PubMed]



| Sample Labels | Total Proteins g/dL | AST U/L | ALT U/L | LDH U/L |
|---|---|---|---|---|
| Blank | 1.0 ± 0.05 | 3 ± 0.1 | 6 ± 0.2 | 54 ± 1 |
| Control | 1.1 ± 0.04 | 5 ± 0.1 | 6 ± 0.1 | 58 ± 2 |
| ZrO2 | 1.1 ± 0.03 | 6 ± 0.3 | 6 ± 0.2 | 61 ± 3 |
| 6 wt % PCL | 1.0 ± 0.03 | 5 ± 0.2 | 6 ± 0.1 | 64 ± 2 * |
| 12 wt % PCL | 1.1 ± 0.02 | 5 ± 0.3 | 6 ± 0.3 | 63 ± 3 |
| 24 wt % PCL | 1.1 ± 0.05 | 6 ± 0.2 | 6 ± 0.4 | 62 ± 3 |
| 50 wt % PCL | 1.0 ± 0.02 | 5 ± 0.1 | 6 ± 0.1 | 64 ± 2 * |
| DOXO | 1.1 ± 0.05 | 23 ± 1 * | 6 ± 0.2 | 130 ± 5 * |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catauro, M.; Tranquillo, E.; Illiano, M.; Sapio, L.; Spina, A.; Naviglio, S. The Influence of the Polymer Amount on the Biological Properties of PCL/ZrO2 Hybrid Materials Synthesized via Sol-Gel Technique. Materials 2017, 10, 1186. https://doi.org/10.3390/ma10101186
Catauro M, Tranquillo E, Illiano M, Sapio L, Spina A, Naviglio S. The Influence of the Polymer Amount on the Biological Properties of PCL/ZrO2 Hybrid Materials Synthesized via Sol-Gel Technique. Materials. 2017; 10(10):1186. https://doi.org/10.3390/ma10101186
Chicago/Turabian StyleCatauro, Michelina, Elisabetta Tranquillo, Michela Illiano, Luigi Sapio, Annamaria Spina, and Silvio Naviglio. 2017. "The Influence of the Polymer Amount on the Biological Properties of PCL/ZrO2 Hybrid Materials Synthesized via Sol-Gel Technique" Materials 10, no. 10: 1186. https://doi.org/10.3390/ma10101186
APA StyleCatauro, M., Tranquillo, E., Illiano, M., Sapio, L., Spina, A., & Naviglio, S. (2017). The Influence of the Polymer Amount on the Biological Properties of PCL/ZrO2 Hybrid Materials Synthesized via Sol-Gel Technique. Materials, 10(10), 1186. https://doi.org/10.3390/ma10101186







