Genetically Engineered Phage Induced Selective H9c2 Cardiomyocytes Patterning in PDMS Microgrooves
Abstract
:1. Introduction
2. Materials and Methods
2.1. Genetic Modification and Amplification of Phage
- Forward primer: 5′-AGCTTT TGT AGG GGT GAC GGT AGG TGC GGTAC-3′
- Revers primer: 5′-C GCA CCA ACC GTC ACC CCT ACA AA-3′
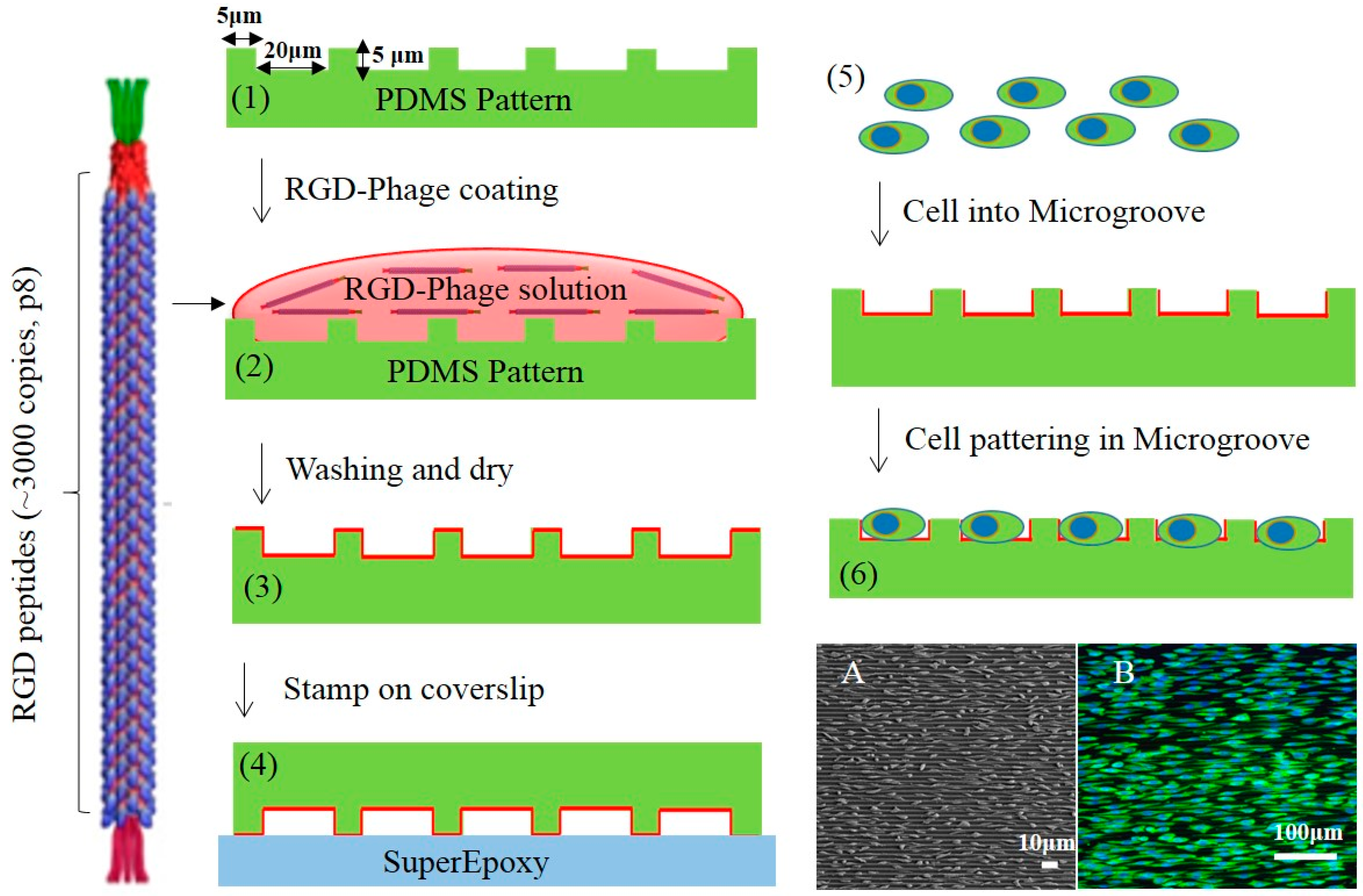
2.2. Micro-Patterned PDMS Substrates Fabrication
2.3. Cell Culture on Micro-Patterned Substrates
2.4. Scanning Electron Microscope (SEM) Imaging
2.5. Statistical Analysis
3. Results and Discussion
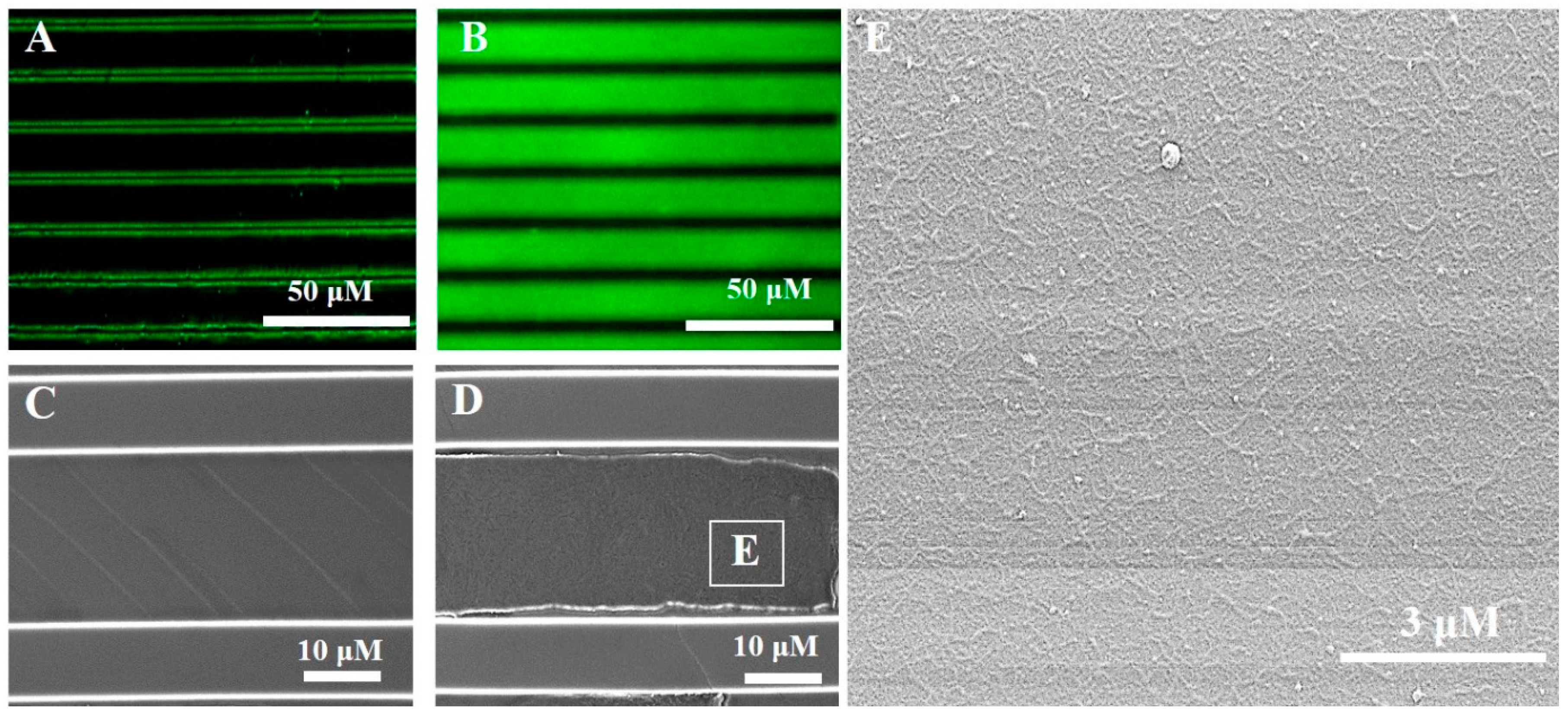
3.1. Immobilization of RGD-Phage on PDMS Micro-Patterned Substrates
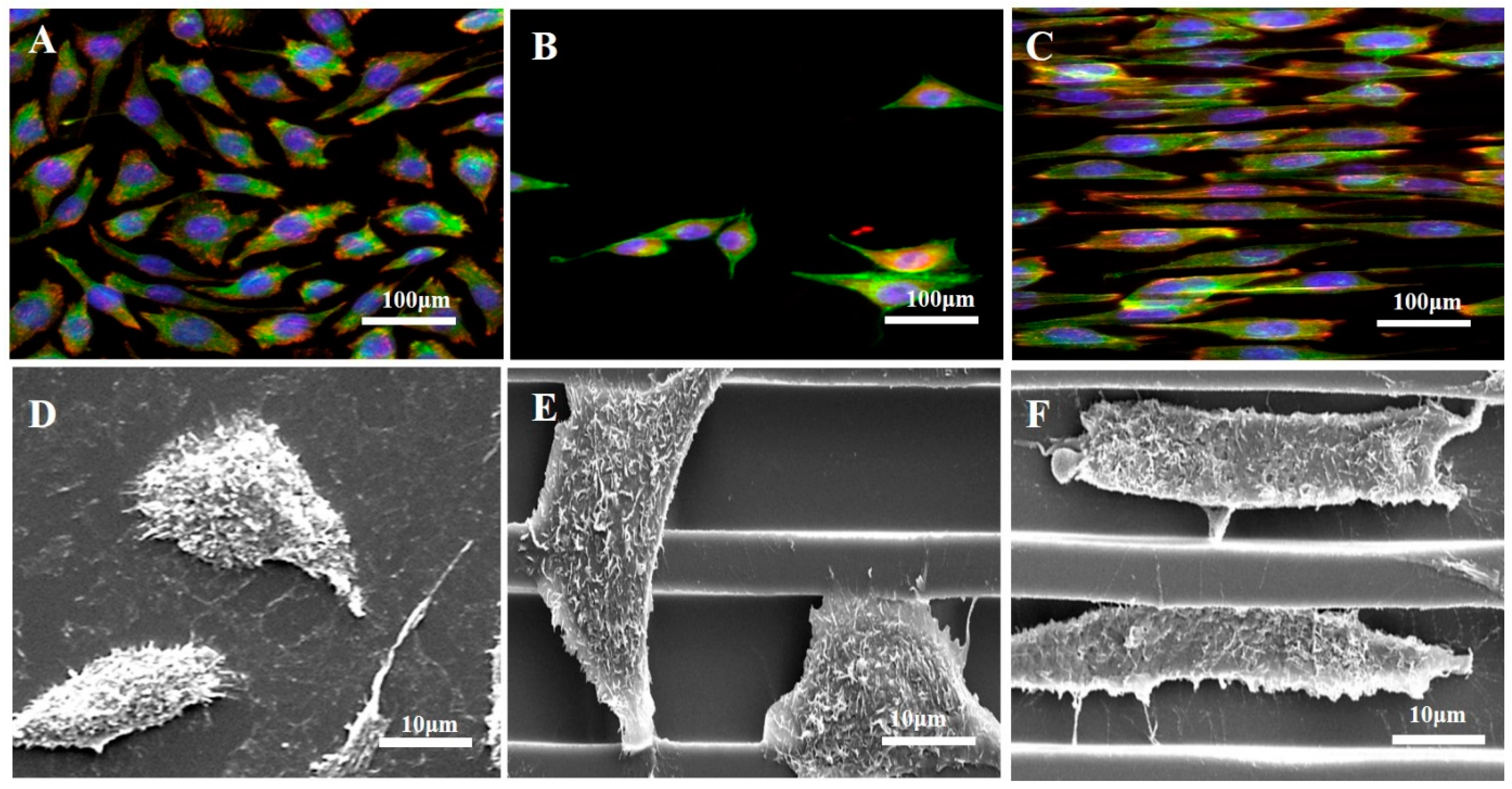
3.2. Cell Adhesion and Morphology Changes on Prepared Surfaces
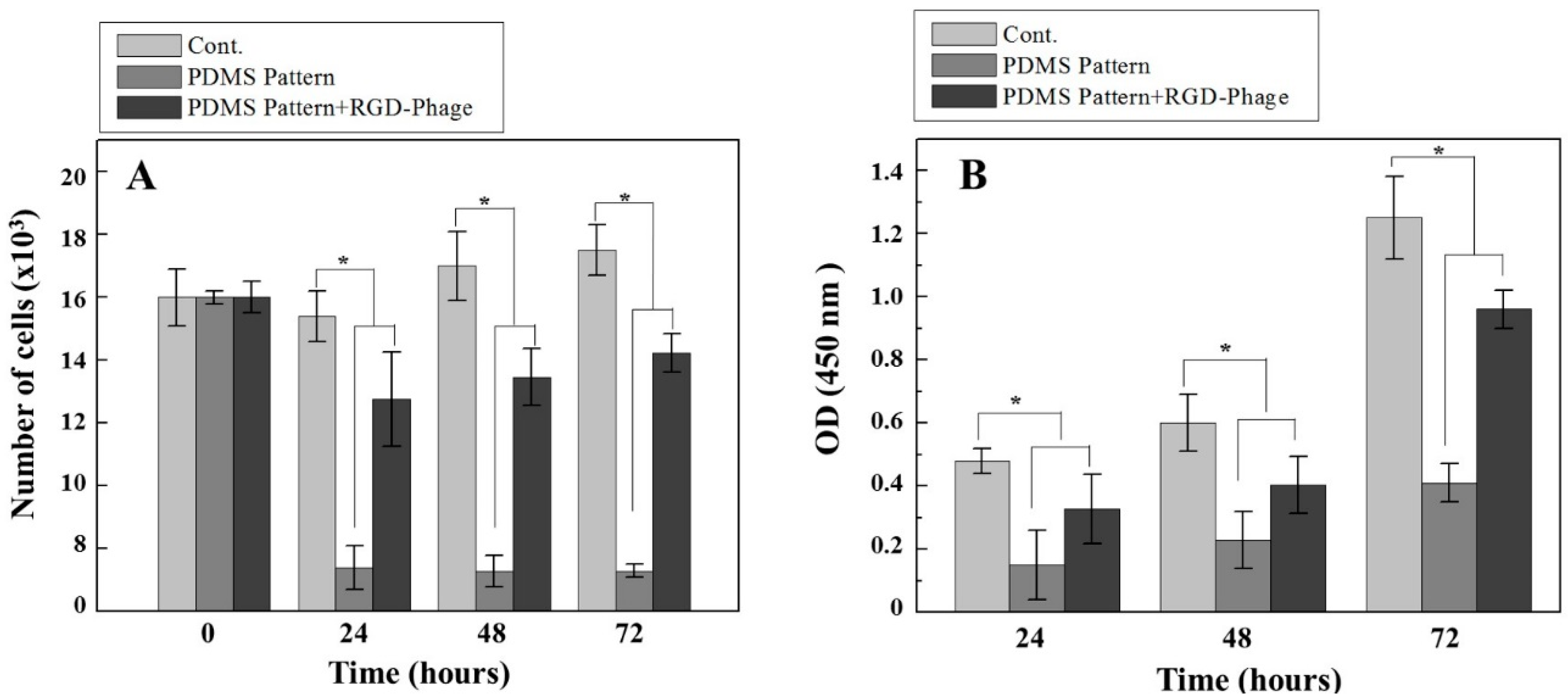
3.3. Cell Proliferation on Micro-Grooved Surfaces
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Martinez, E.; Engel, E.; Planell, J.A.; Samitier, J. Effects of artificial micro- and nano-structured surfaces on cell behaviour. Ann. Anat. Anat. Anz. 2009, 191, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Goto, M.; Tsukahara, T.; Sato, K.; Kitamori, T. Micro- and nanometer-scale patterned surface in a microchannel for cell culture in microfluidic devices. Anal. Bioanal. Chem. 2008, 390, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, A.W.; Wilkerson, W.R.; Seegert, C.A.; Gibson, A.L.; Hoipkemeier-Wilson, L.; Brennan, A.B. Systematic variation of microtopography, surface chemistry and elastic modulus and the state dependent effect on endothelial cell alignment. J. Biomed. Mater. Res. Part A 2008, 86, 522–534. [Google Scholar] [CrossRef] [PubMed]
- Liliensiek, S.J.; Campbell, S.; Nealey, P.F.; Murphy, C.J. The scale of substratum topographic features modulates proliferation of corneal epithelial cells and corneal fibroblasts. J. Biomed. Mater. Res. Part A 2006, 79, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Christopherson, G.T.; Song, H.; Mao, H.Q. The influence of fiber diameter of electrospun substrates on neural stem cell differentiation and proliferation. Biomaterials 2009, 30, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.E.; Hinds, M.T. Endothelial cell micropatterning: Methods, effects, and applications. Ann. Biomed. Eng. 2011, 39, 2329–2345. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.J.; Clegg, R.E.; Leavesley, D.I.; Pearcy, M.J. Mediation of biomaterial-cell interactions by adsorbed proteins: A review. Tissue Eng. 2005, 11, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Song, S.; Lee, J.; Yoon, J.; Park, J.; Choi, S.; Park, J.K.; Choi, K.; Choi, C. Phenotypic modulation of primary vascular smooth muscle cells by short-term culture on micropatterned substrate. PLoS ONE 2014, 9, e88089. [Google Scholar] [CrossRef] [PubMed]
- Kajzar, A.; Cesa, C.M.; Kirchgessner, N.; Hoffmann, B.; Merkel, R. Toward physiological conditions for cell analyses: Forces of heart muscle cells suspended between elastic micropillars. Biophys. J. 2008, 94, 1854–1866. [Google Scholar] [CrossRef] [PubMed]
- Vernon, R.B.; Gooden, M.D.; Lara, S.L.; Wight, T.N. Microgrooved fibrillar collagen membranes as scaffolds for cell support and alignment. Biomaterials 2005, 26, 3131–3140. [Google Scholar] [CrossRef] [PubMed]
- Kharkar, P.M.; Kiick, K.L.; Kloxin, A.M. Designing degradable hydrogels for orthogonal control of cell microenvironments. Chem. Soc. Rev. 2013, 42, 7335–7372. [Google Scholar] [CrossRef] [PubMed]
- Yakovlev, S.; Gao, Y.; Cao, C.; Chen, L.; Strickland, D.K.; Zhang, L.; Medved, L. Interaction of fibrin with VE-cadherin and anti-inflammatory effect of fibrin-derived fragments. J. Thromb. Haemost. 2011, 9, 1847–1855. [Google Scholar] [CrossRef] [PubMed]
- Morke, C.; Rebl, H.; Finke, B.; Dubs, M.; Nestler, P.; Airoudj, A.; Roucoules, V.; Schnabelrauch, M.; Körtge, A.; Anselme, K.; et al. Abrogated Cell Contact Guidance on Amino-Functionalized Microgrooves. ACS Appl. Mater. Interfaces 2017, 9, 10461–10471. [Google Scholar] [CrossRef] [PubMed]
- Laughlin, T.D.; Miles, J.R.; Wright-Johnson, E.C.; Rempel, L.A.; Lents, C.A.; Pannier, A.K. Development of pre-implantation porcine blastocysts cultured within alginate hydrogel systems either supplemented with secreted phosphoprotein 1 or conjugated with Arg-Gly-Asp Peptide. Reprod. Fertil. Dev. 2017. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.; Naskar, D.; Sapru, S.; Bhattacharjee, P.; Dey, T.; Ghosh, A.K.; Mandal, M.; Kundu, S.C. Hydroxyapatite reinforced inherent RGD containing silk fibroin composite scaffolds: Promising platform for bone tissue engineering. Nanomedicine 2017, 13, 1745–1759. [Google Scholar] [CrossRef] [PubMed]
- Singhvi, R.; Stephanopoulos, G.; Wang, D.I. Effects of substratum morphology on cell physiology. Biotechnol. Bioeng. 1994, 43, 764–771. [Google Scholar] [CrossRef] [PubMed]
- Mobasseri, R.; Tian, L.; Soleimani, M.; Ramakrishna, S.; Naderi-Manesh, H. Bio-active molecules modified surfaces enhanced mesenchymal stem cell adhesion and proliferation. Biochem. Biophys. Res. Commun. 2017, 483, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Jin, Y.H.; Salieb-Beugelaar, G.B.; Nam, C.H.; Stieglitz, T. Genetically engineered bacteriophage delivers a tumor necrosis factor alpha antagonist coating on neural electrodes. Biomed. Mater. 2014, 9, 015009. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jeon, H.; Haidar, A.; Abdul-Khaliq, H.; Veith, M.; Aktas, C.; Kim, Y.J. Recombinant Phage Coated 1D Al2O3 Nanostructures for Controlling the Adhesion and Proliferation of Endothelial Cells. BioMed Res. Int. 2015, 2015, 909807. [Google Scholar] [PubMed]
- Hertveldt, K.; Belien, T.; Volckaert, G. General M13 phage display: M13 phage display in identification and characterization of protein-protein interactions. Methods Mol. Biol. 2009, 502, 321–339. [Google Scholar] [PubMed]
- Huang, N.F.; Patel, S.; Thakar, R.G.; Wu, J.; Hsiao, B.S.; Chu, B.; Lee, R.J.; Li, S. Myotube assembly on nanofibrous and micropatterned polymers. Nano Lett. 2006, 6, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Andalib, M.N.; Dzenis, Y.; Donahue, H.J.; Lim, J.Y. Biomimetic substrate control of cellular mechanotransduction. Biomater. Res. 2016, 20, 11. [Google Scholar] [CrossRef] [PubMed]
- Falconnet, D.; Csucs, G.; Grandin, H.M.; Textor, M. Surface engineering approaches to micropattern surfaces for cell-based assays. Biomaterials 2006, 27, 3044–3063. [Google Scholar] [CrossRef] [PubMed]
- Mateo, C.; Grazu, V.; Palomo, J.M.; Lopez-Gallego, F.; Fernandez-Lafuente, R.; Guisan, J.M. Immobilization of enzymes on heterofunctional epoxy supports. Nat. Protoc. 2007, 2, 1022–1033. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Kwon, C.; Jeon, H. Genetically Engineered Phage Induced Selective H9c2 Cardiomyocytes Patterning in PDMS Microgrooves. Materials 2017, 10, 973. https://doi.org/10.3390/ma10080973
Kim Y, Kwon C, Jeon H. Genetically Engineered Phage Induced Selective H9c2 Cardiomyocytes Patterning in PDMS Microgrooves. Materials. 2017; 10(8):973. https://doi.org/10.3390/ma10080973
Chicago/Turabian StyleKim, Youngjun, Chunga Kwon, and Hojeong Jeon. 2017. "Genetically Engineered Phage Induced Selective H9c2 Cardiomyocytes Patterning in PDMS Microgrooves" Materials 10, no. 8: 973. https://doi.org/10.3390/ma10080973
APA StyleKim, Y., Kwon, C., & Jeon, H. (2017). Genetically Engineered Phage Induced Selective H9c2 Cardiomyocytes Patterning in PDMS Microgrooves. Materials, 10(8), 973. https://doi.org/10.3390/ma10080973




