Relationship between Microstructure and Corrosion Behavior of Martensitic High Nitrogen Stainless Steel 30Cr15Mo1N at Different Austenitizing Temperatures
Abstract
:1. Introduction
2. Materials and Methods
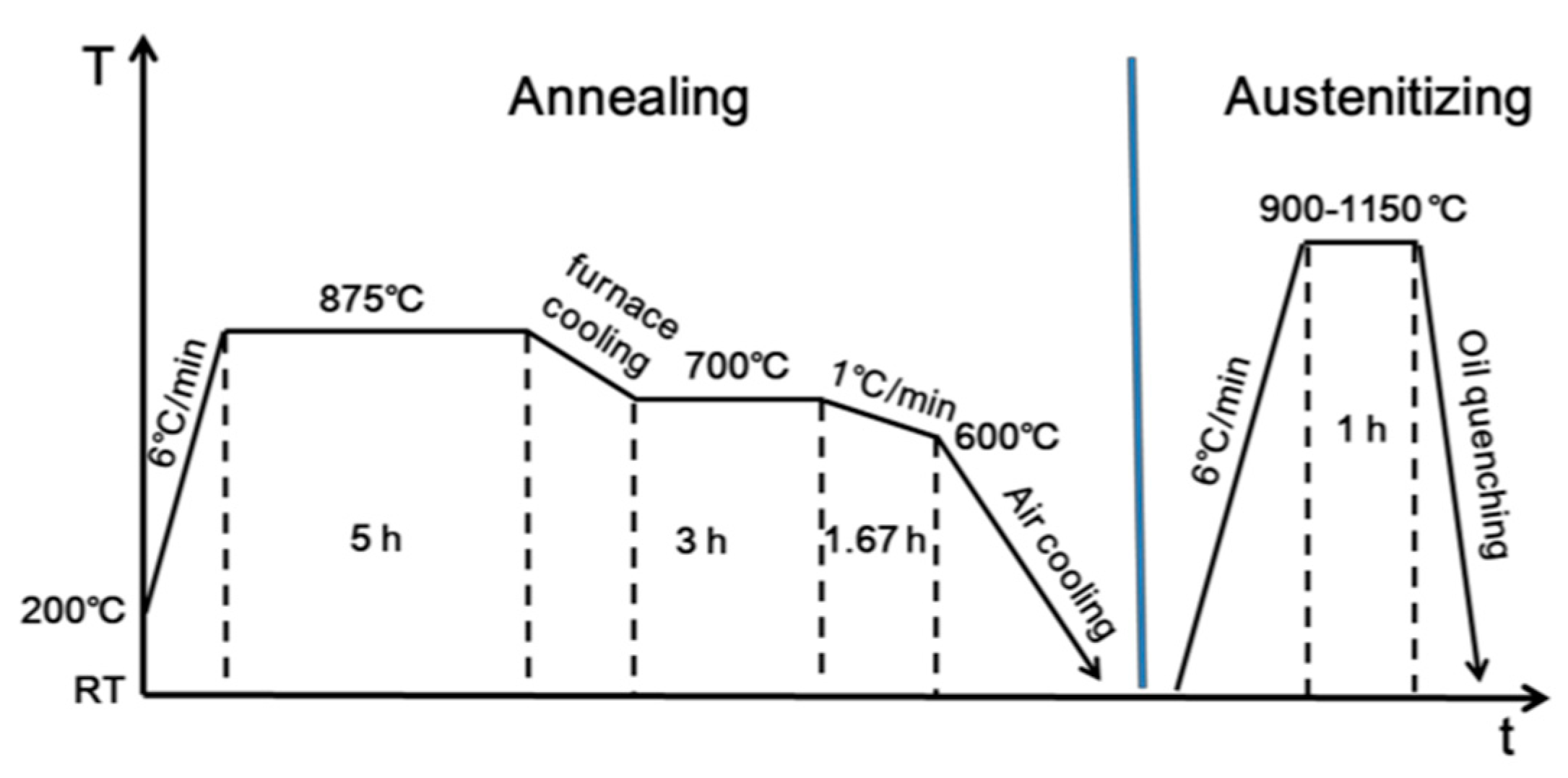
2.1. Material and Heat Treatment
2.2. Thermodynamic Calculations
2.3. Microstructure Characterizations
2.4. Electrochemical Measurement
2.5. Passive Film Analysis
2.6. Immersion Tests
3. Results
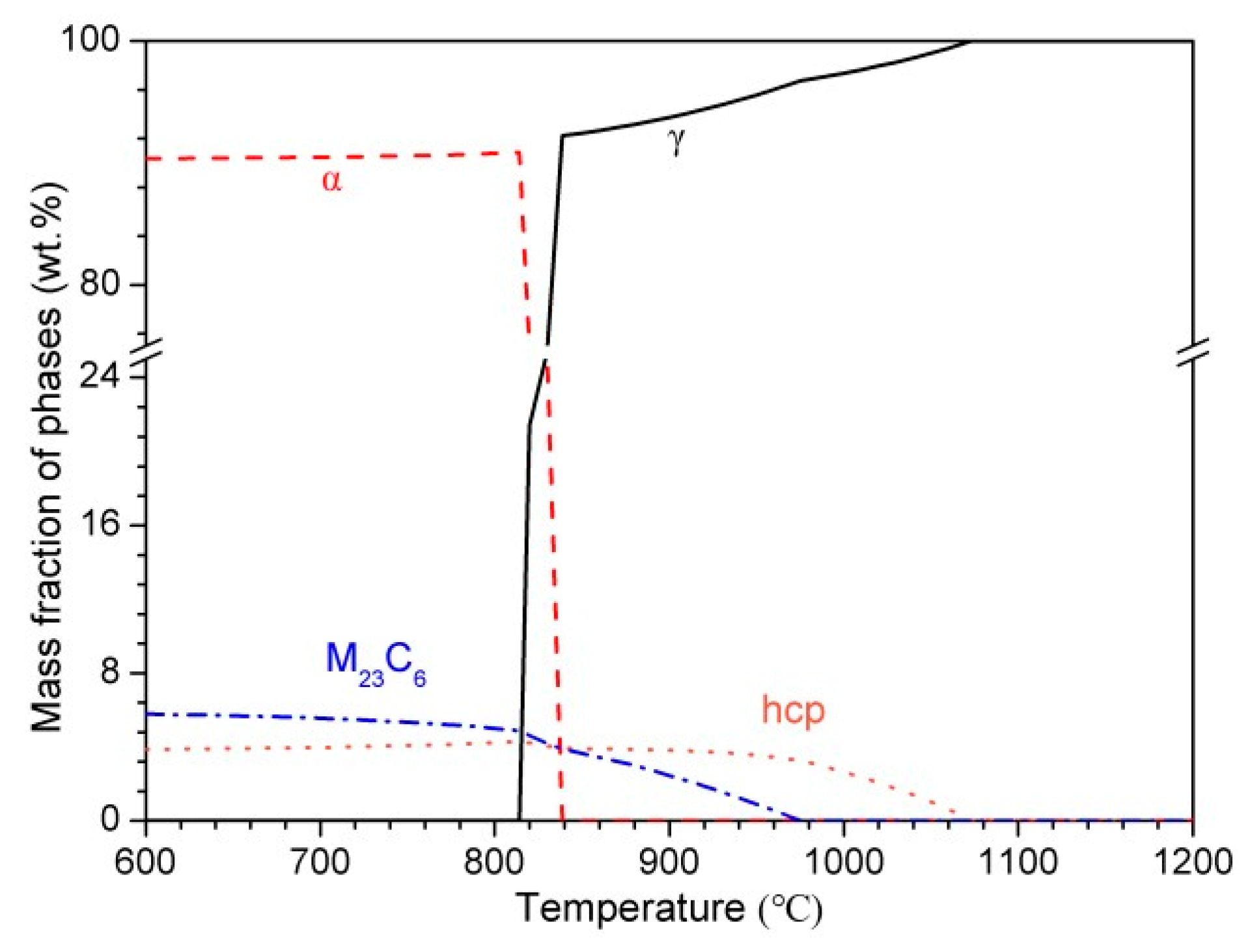
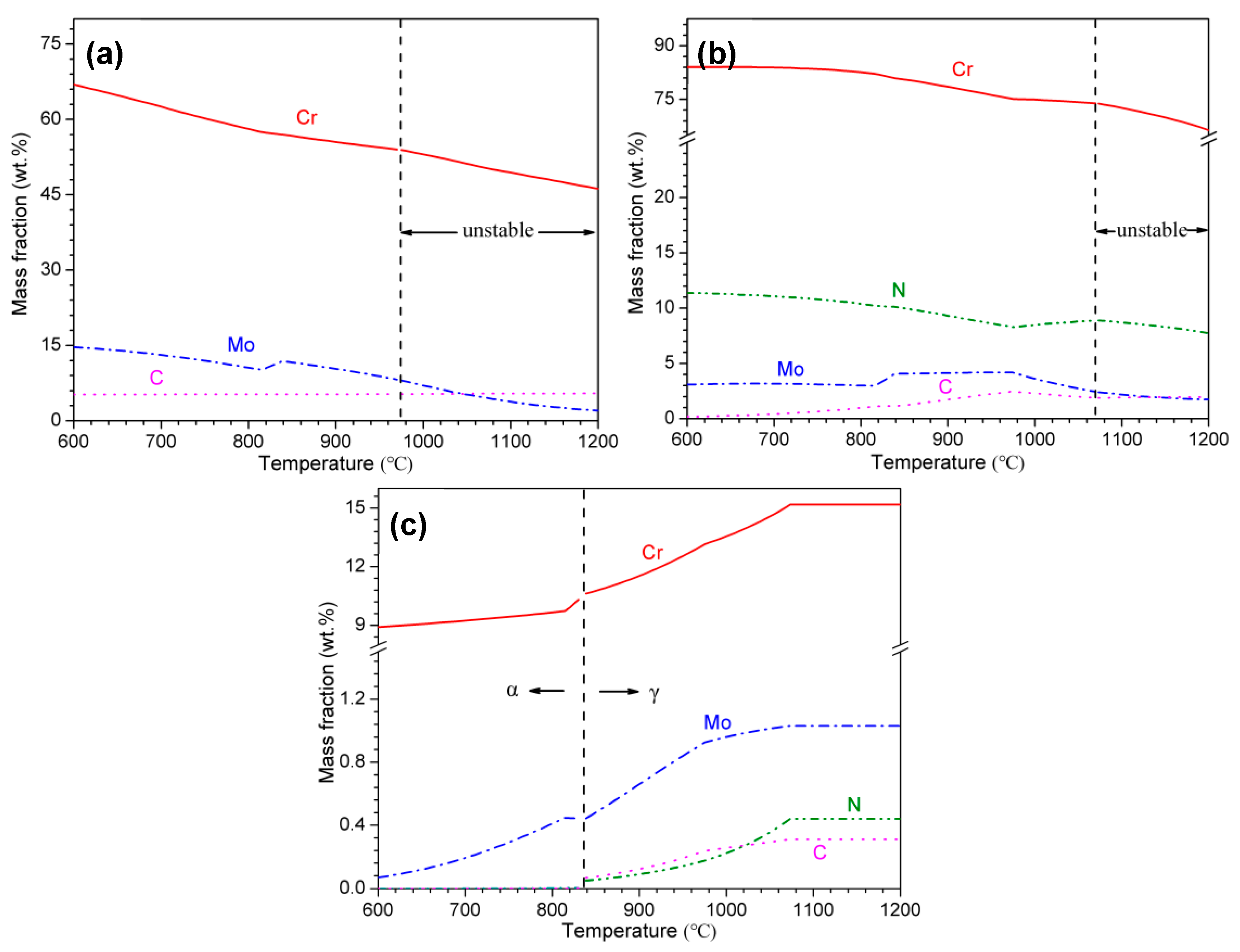
3.1. Thermodynamic Calculations
3.2. Microstructure Characterizations
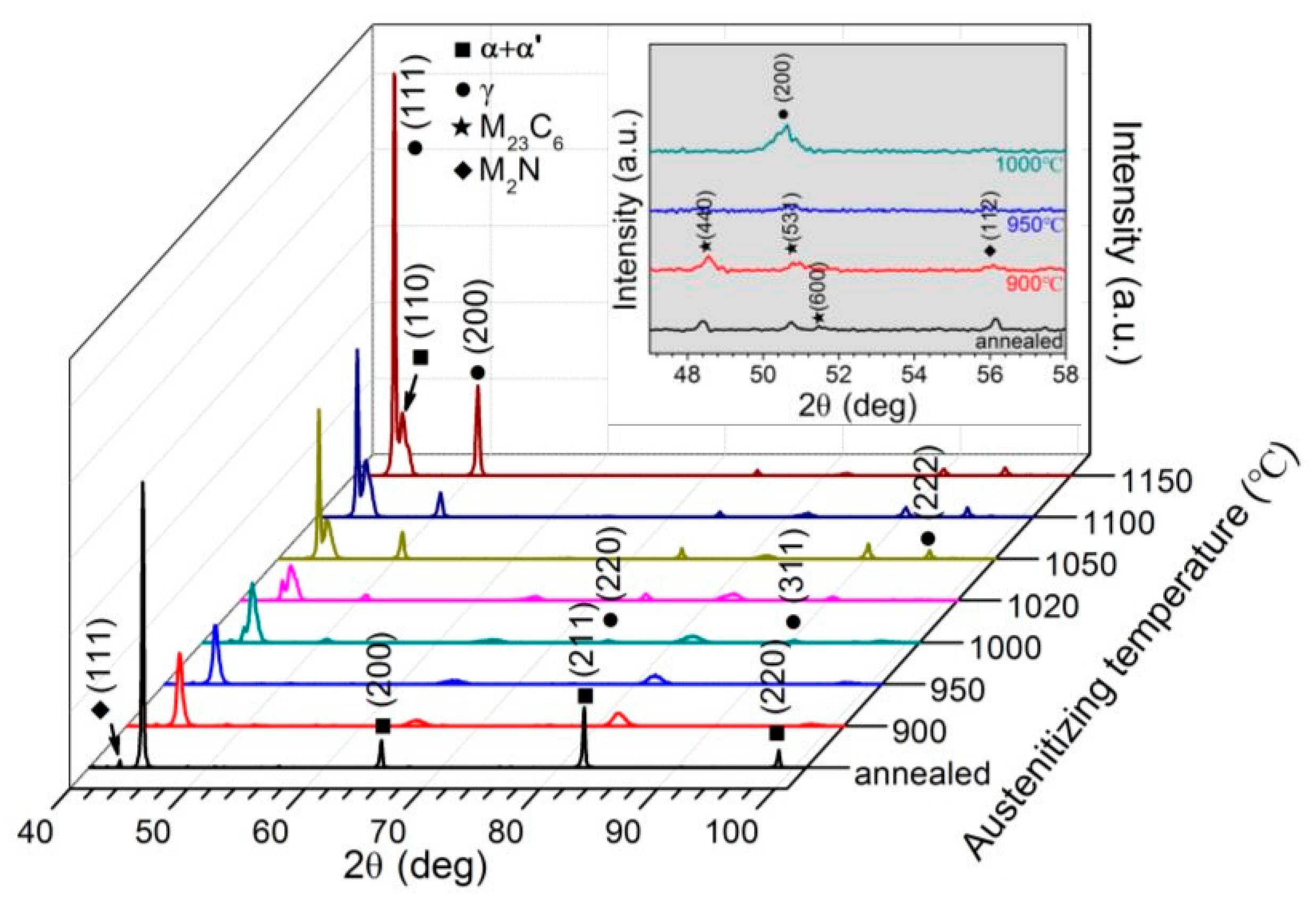
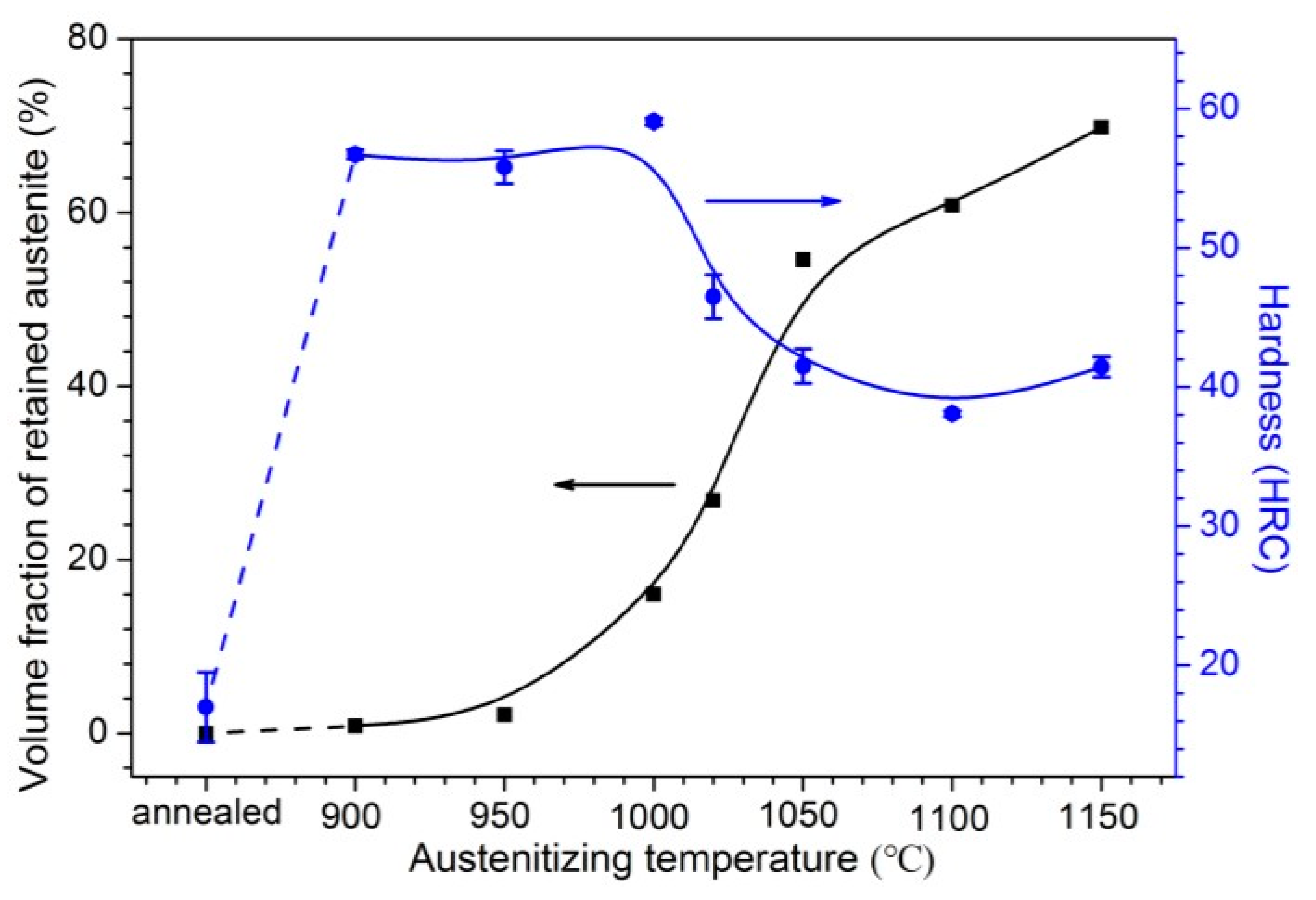
3.2.1. X-ray Diffraction and Hardness Tests
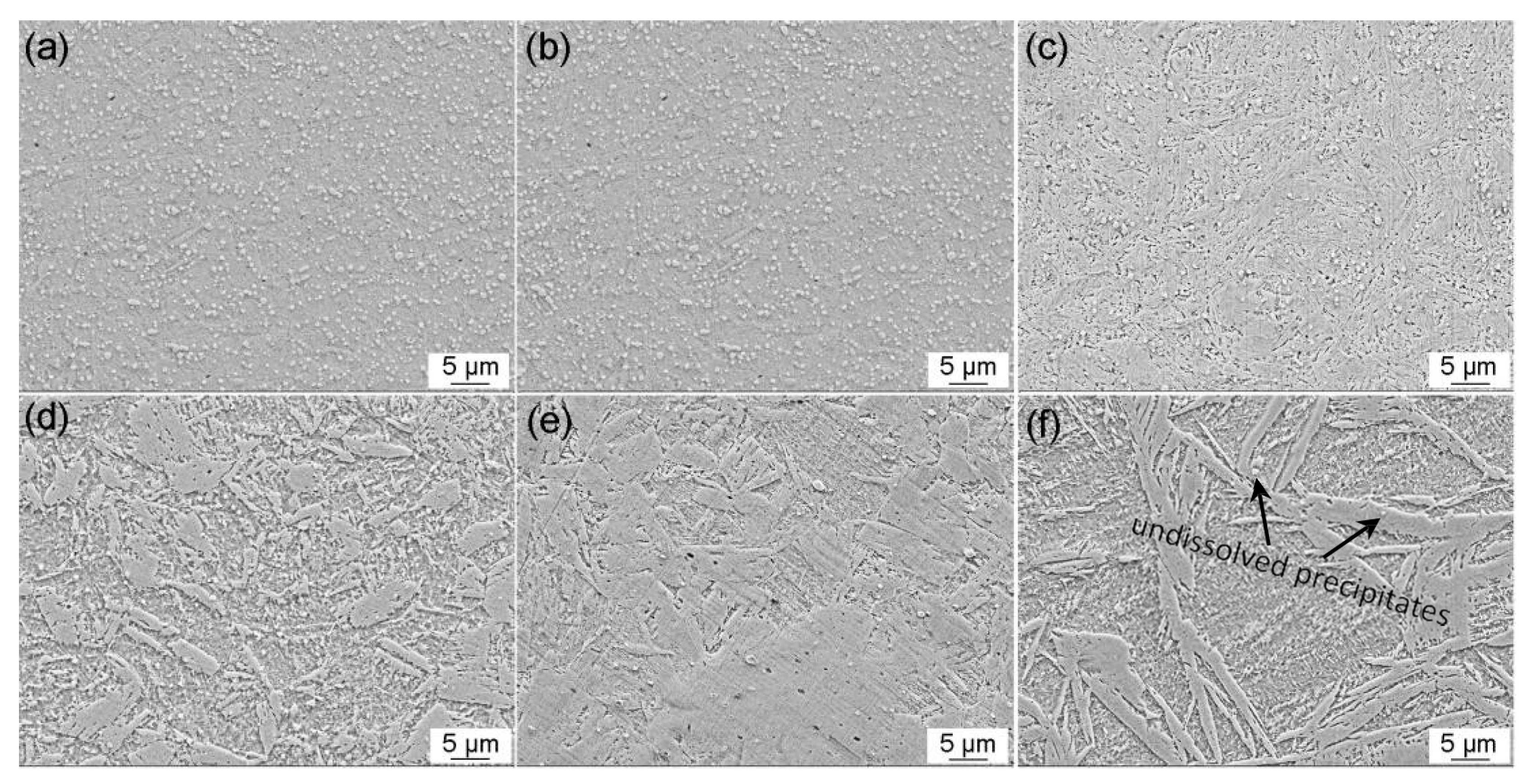
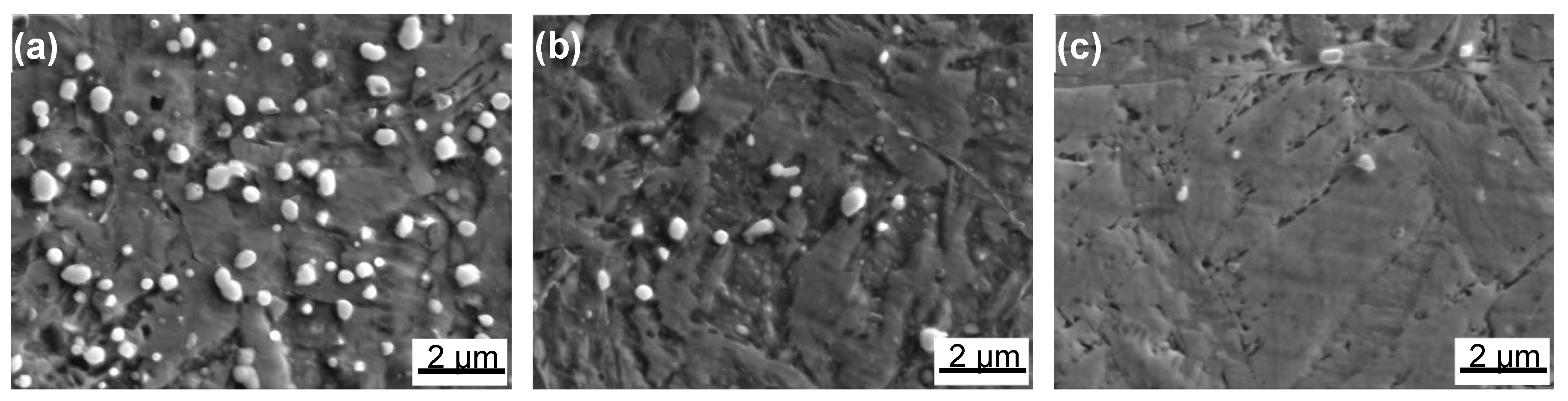
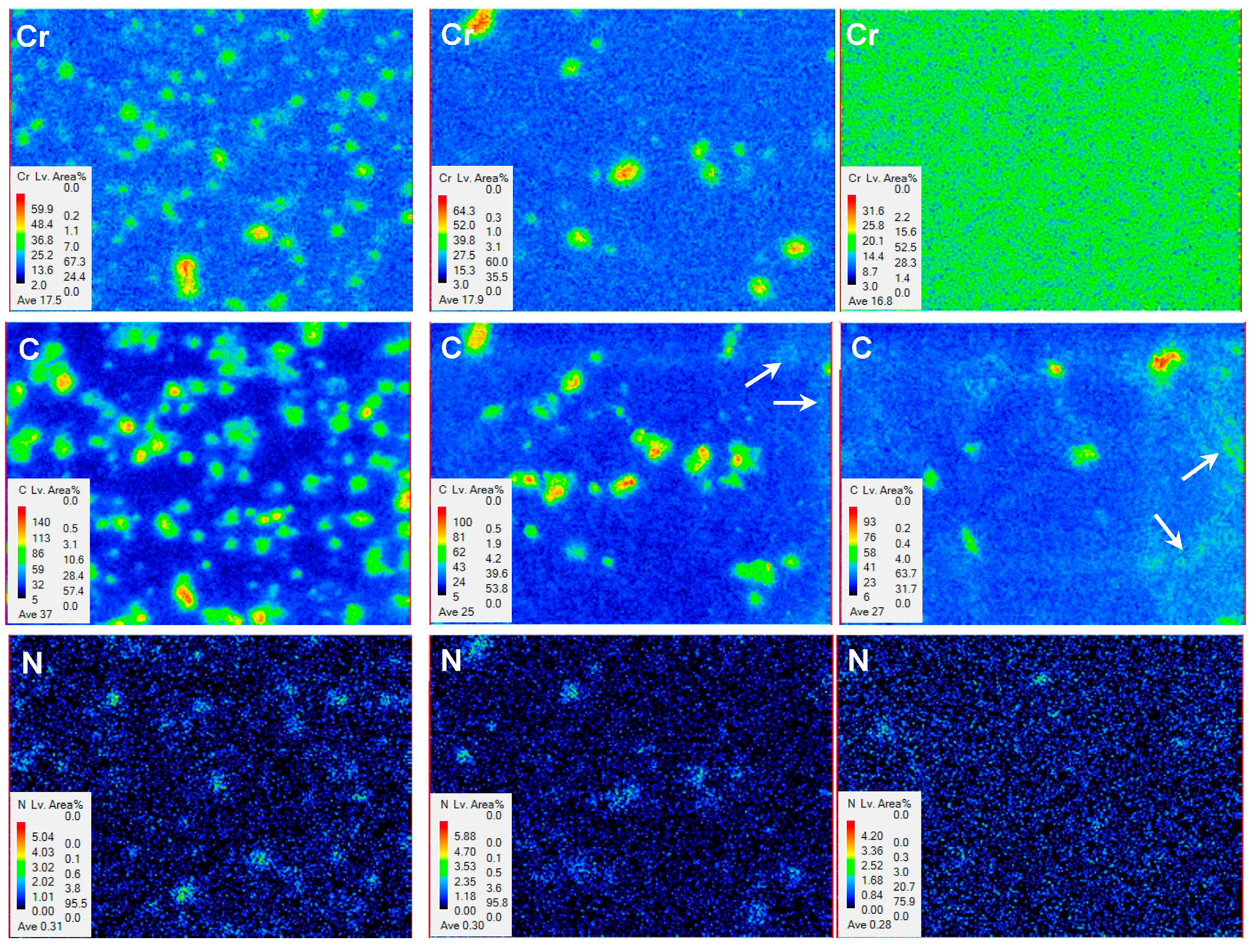
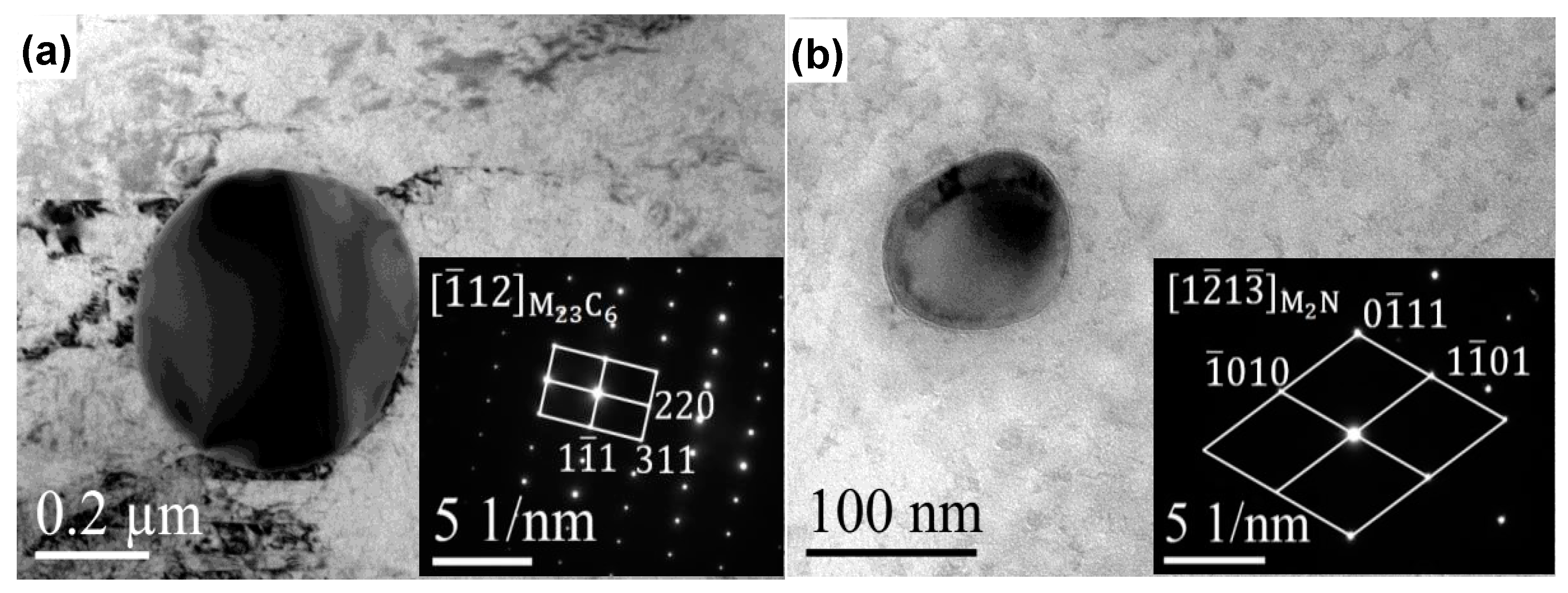
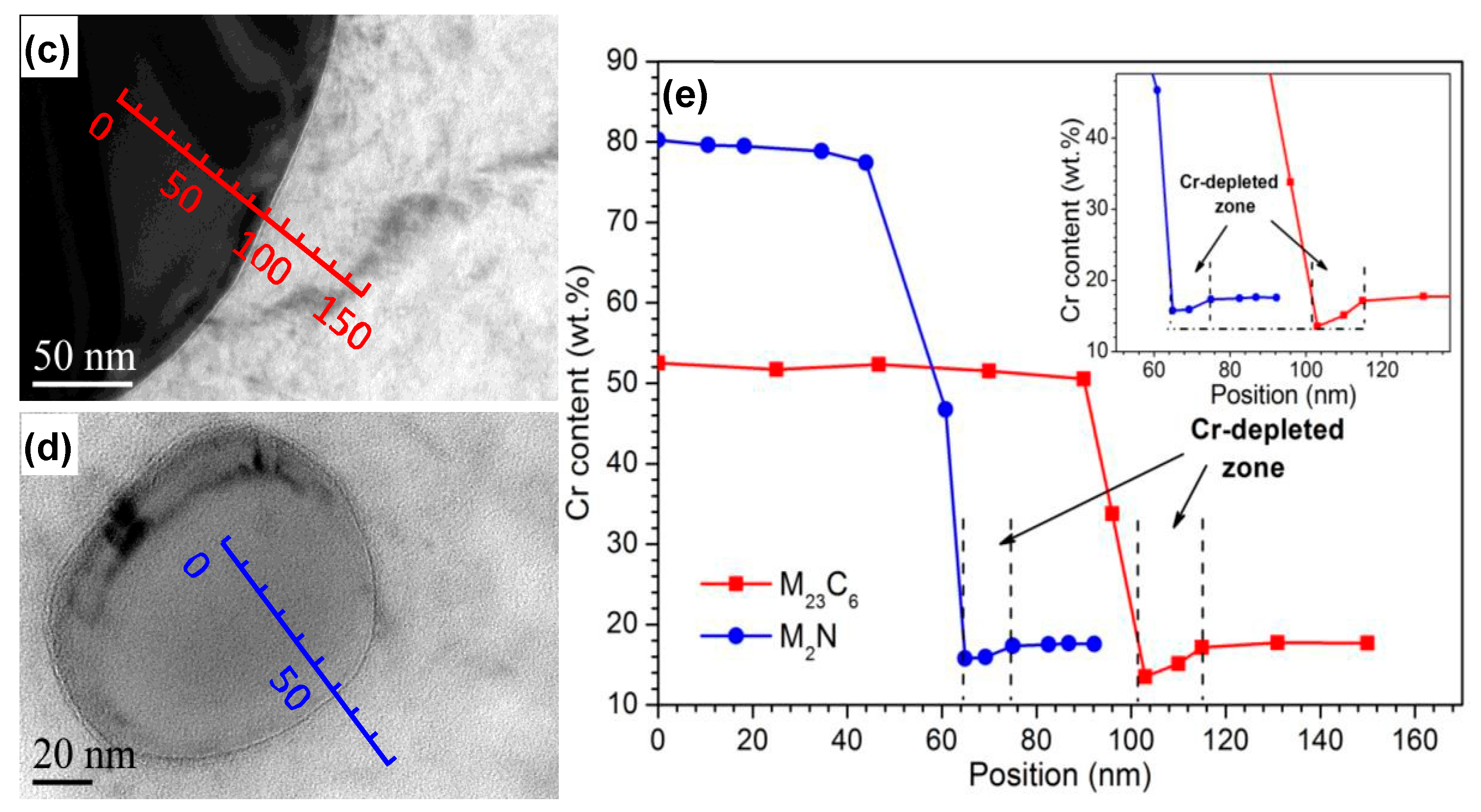
3.2.2. Microscopy Observation
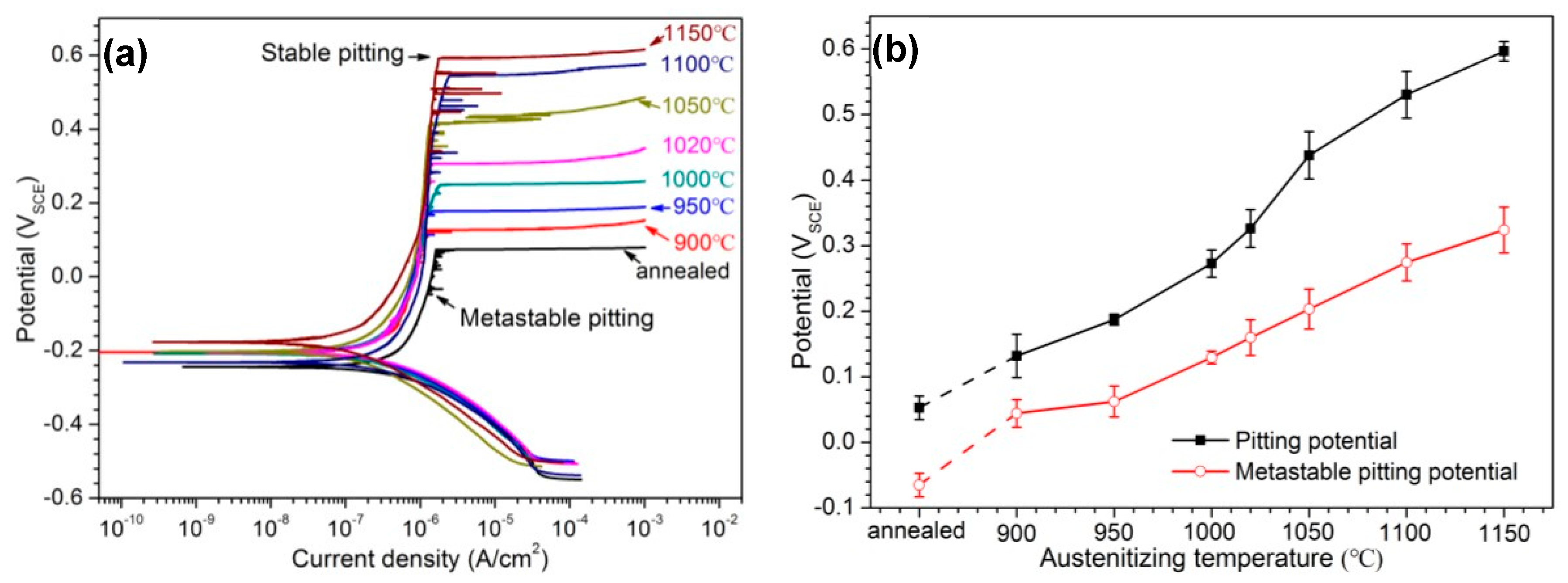
3.3. Electrochemical Measurement
3.4. XPS Results
3.5. Immersion Tests
4. Discussion
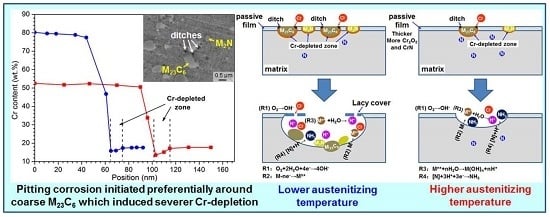
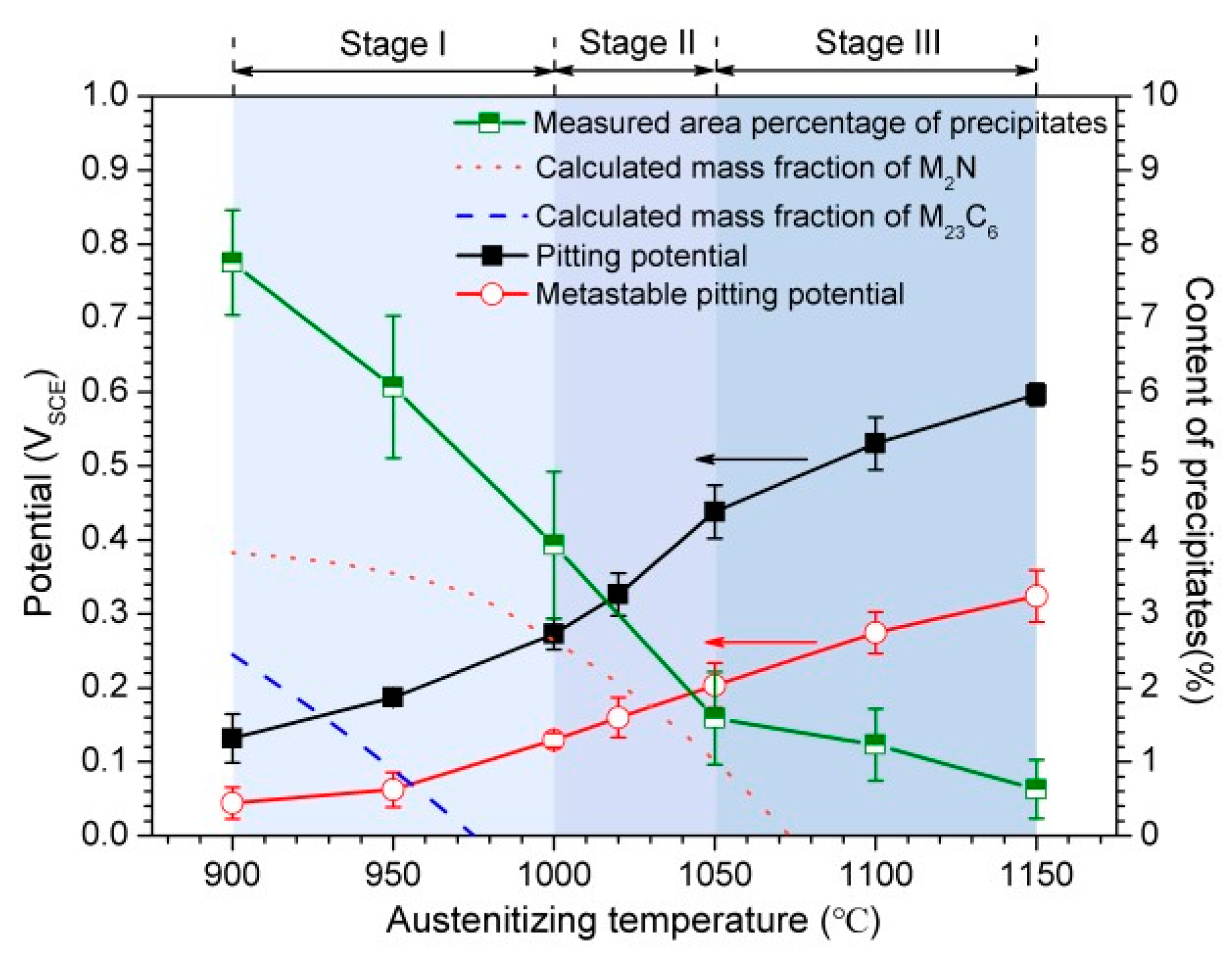
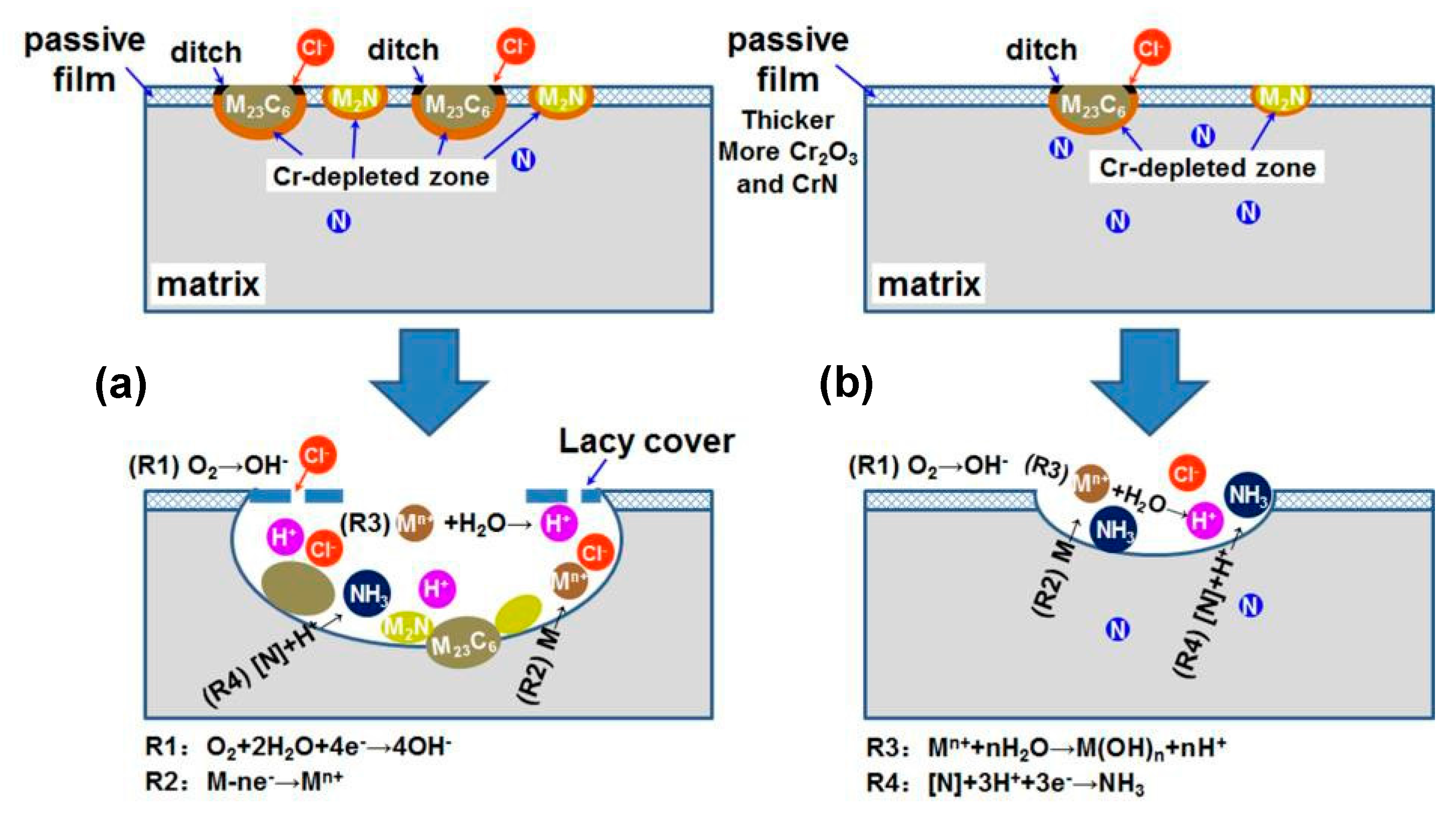
4.1. Explanation of Preferential Metastable Pit Initiation Sites
4.2. Influence of Austenitizing Temperature on Pit Initiation
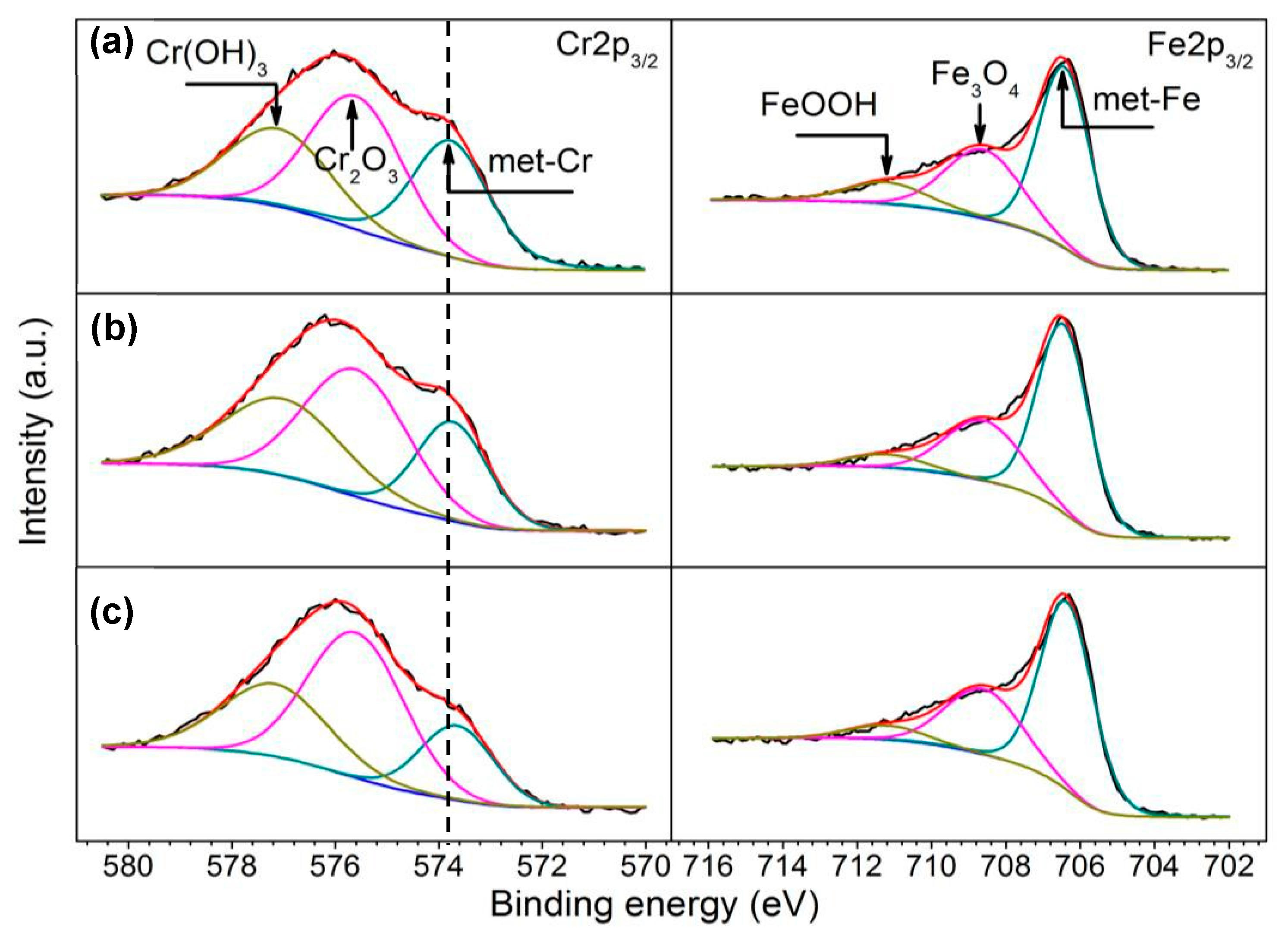
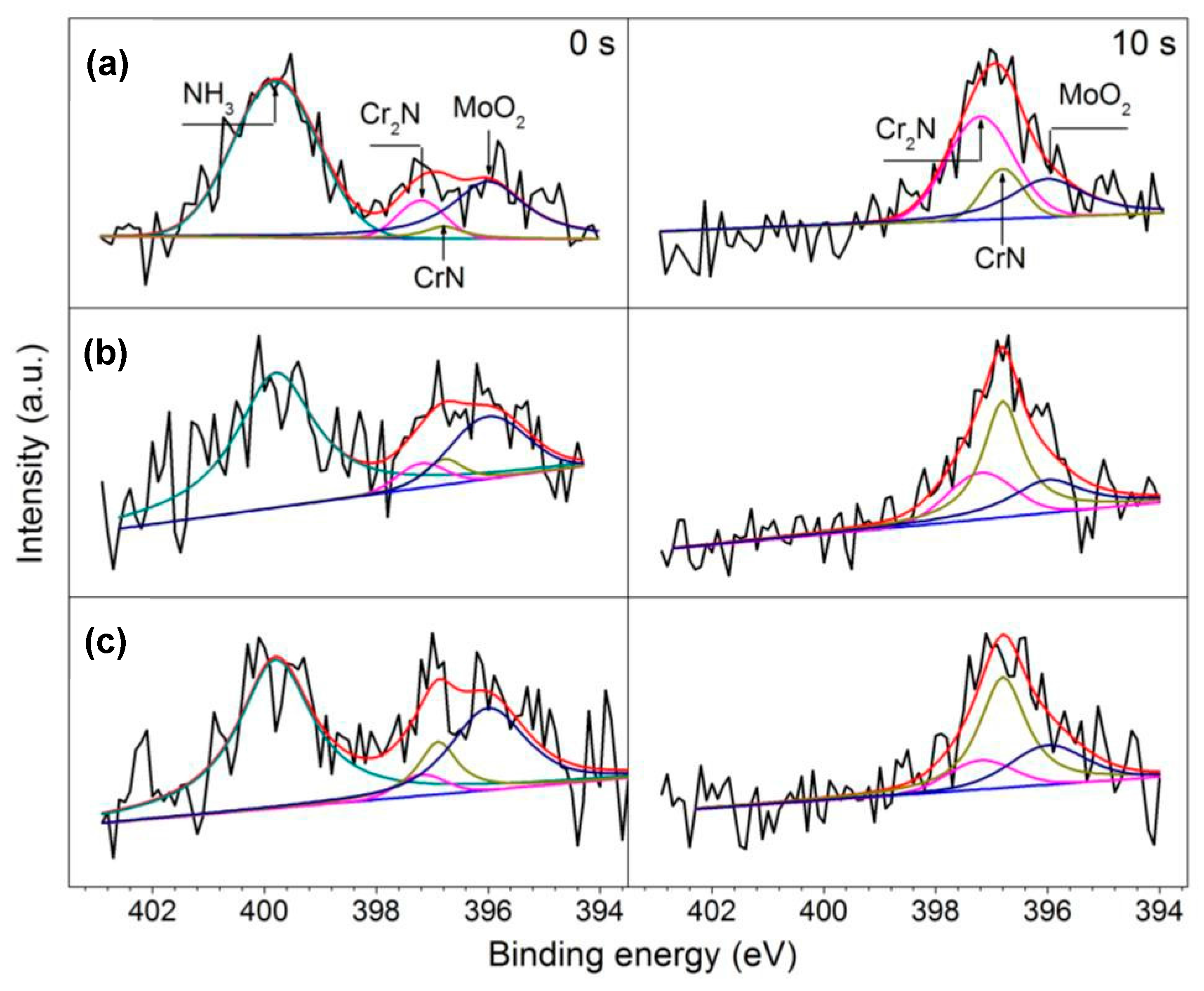
4.3. Influence of Austenitizing Temperature on Passive Film
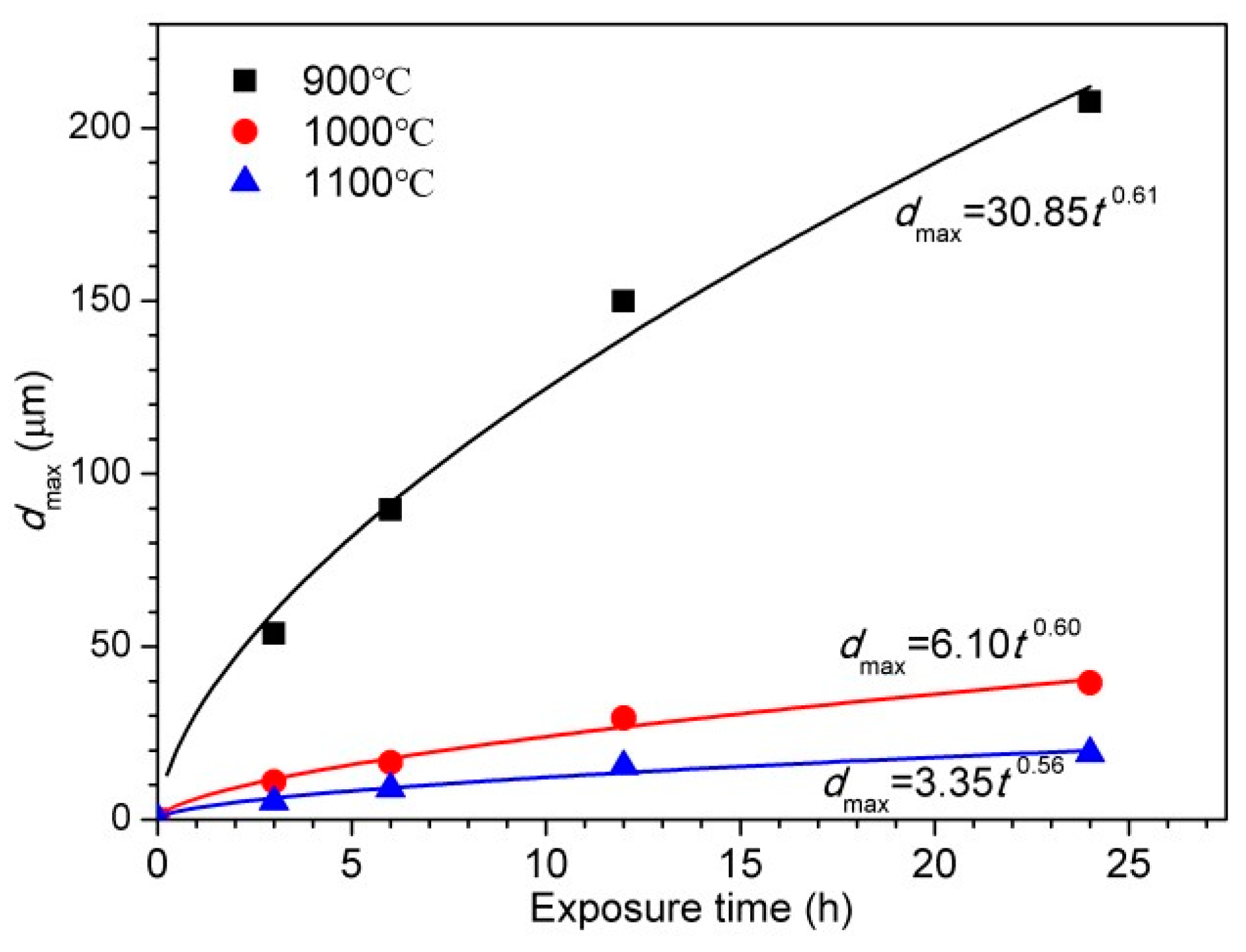
4.4. Effect of Austenitizing Temperature on Pit Growth Kinetics
5. Conclusions
- (1)
- With increasing austenitizing temperature, the fraction of precipitates decreased and retained austenite increased, resulting in more homogeneous distribution and higher contents of Cr, Mo, C and N in the matrix. The precipitates were identified as Cr-rich M23C6 and M2N, and M2N was finer and distributed more homogeneously than M23C6. The Cr-depleted zone around M23C6 was wider and its minimum Cr concentration was lower than M2N.
- (2)
- The metastable pits initiated preferentially around coarse M23C6 which induced severer Cr-depletion, and the pit growth followed the power law. The dissolution of M23C6 and M2N at higher austenitizing temperature reduced the pit initiation sites. As austenitizing temperature increased, the metastable and stable pitting potentials increased and the pit growth rate decreased, revealing less susceptible metastable pit initiation, larger repassivation tendency and higher corrosion resistance. The determining factor of pitting potential could be divided into three stages: dissolution of M23C6 (below 1000 °C), dissolution of M2N (from 1000 to 1050 °C) and existence of a few undissolved precipitates and non-metallic inclusions (above 1050 °C).
- (3)
- The increasing of austenitizing temperature promoted the thickening of passive film; enrichment of Cr2O3, Cr3+ and CrN; and higher nitrogen content in solid solution, thereby enhancing the stability of passive film and repassivation ability of pits.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Franz-Josef, E. An overview of performance characteristics, experiences and trends of aerospace engine bearings technologies. Chin. J. Aeronaut. 2007, 20, 378–384. [Google Scholar]
- Berns, H.; Ebert, F.-J.; Zoch, H.-W. The new low nitrogen steel LNS—A material for advanced aircraft engine and aerospace bearing applications. In Bearing Steels: Into the 21st Century; Hoo, J.J.C., Green, W.B., Eds.; ASTM International: West Conshohocken, PA, USA, 1998; pp. 354–373. [Google Scholar]
- Li, H.B.; Zhou, E.Z.; Ren, Y.B.; Zhang, D.W.; Xu, D.K.; Yang, C.G.; Feng, H.; Jiang, Z.H.; Li, X.G.; Gu, T.Y.; et al. Investigation of microbiologically influenced corrosion of high nitrogen nickel-free stainless steel by Pseudomonas aeruginosa. Corros. Sci. 2016, 111, 811–821. [Google Scholar] [CrossRef]
- Li, H.B.; Jiang, Z.H.; Yang, Y.; Cao, Y.; Zhang, Z.R. Pitting corrosion and crevice corrosion behaviors of high nitrogen austenitic stainless steels. Int. J. Miner. Met. Mater. 2009, 16, 517–524. [Google Scholar] [CrossRef]
- Ha, H.-Y.; Lee, C.-H.; Lee, T.-H.; Kim, S. Effects of nitrogen and tensile direction on stress corrosion cracking susceptibility of Ni-free FeCrMnC-based duplex stainless steels. Materials 2017, 10, 294. [Google Scholar] [CrossRef]
- Li, H.-B.; Jiang, Z.-H.; Shen, M.-H.; You, X.-M. High nitrogen austenitic stainless steels manufactured by nitrogen gas alloying and adding nitrided ferroalloys. J. Iron Steel Res. Int. 2007, 14, 64–69. [Google Scholar] [CrossRef]
- Levey, P.R.; Vanbennekom, A. A mechanistic study of the effects of nitrogen on the corrosion properties of stainless steels. Corrosion 1995, 51, 911–921. [Google Scholar] [CrossRef]
- Lu, Y.; Bandy, R.; Clayton, C.; Newman, R. Surface enrichment of nitrogen during passivation of a highly resistant stainless steel. J. Electrochem. Soc. 1983, 130, 1774–1776. [Google Scholar] [CrossRef]
- Clayton, C.R.; Martin, K.G. Evidence of anodic segregation of nitrogen in high nitrogen stainless steels and its influence on passivity. In Proceedings of the High Nitrogen Steels—HNS 88, Lille, France, 18–20 May 1988. [Google Scholar]
- Clayton, C.R.; Halada, G.P.; Kearns, J.R. Passivity of high nitrogen stainless alloys—The role of metal oxyanions and salt films. Mater. Sci. Eng. A 1995, 198, 135–144. [Google Scholar] [CrossRef]
- Valentin, G.; Gavriljuk, H.B. High Nitrogen Steels: Structure, Properties, Manufacture, Applications; Springer: Berlin/Heidelberg, Germany, 1999. [Google Scholar]
- Li, H.-B.; Jiao, W.-C.; Feng, H.; Jiang, Z.-H.; Ren, C.-D. Influence of austenitizing temperature on the microstructure and corrosion resistance of 55Cr18Mo1VN high-nitrogen plastic mould steel. Acta Metall. Sin. 2016, 29, 1148–1160. [Google Scholar] [CrossRef]
- El Mehtedi, M.; Ricci, P.; Drudi, L.; El Mohtadi, S.; Cabibbo, M.; Spigarelli, S. Analysis of the effect of deep cryogenic treatment on the hardness and microstructure of X30 CrMoN 15 1 steel. Mater. Des. 2012, 33, 136–144. [Google Scholar] [CrossRef]
- Speidel, M.O. Properties and applications of high nitrogen steels. In Proceedings of the High Nitrogen Steels—HNS 88, Lille, France, 18–20 May 1988. [Google Scholar]
- Kaluba, W.J.; Kaluba, T.; Taillard, R. The austenitizing behaviour of high-nitrogen martensitic stainless steels. Scr. Mater. 1999, 41, 1289–1293. [Google Scholar] [CrossRef]
- Gavriljuk, V.G.; Berns, H. Precipitates in tempered stainless martensitic steels alloyed with nitrogen, carbon or both. Mater. Sci. Forum 1999, 318, 71–80. [Google Scholar] [CrossRef]
- Shimada, T.; Yamamoto, A.; Abe, S. The effect of nitrogen content on material properties of high carbon stainless steel SUS440A. Tetsue-to-Hagane 1996, 82, 309–314. [Google Scholar] [CrossRef]
- Trojahn, W.; Streit, E.; Chin, H.; Ehlert, D. Progress in bearing performance of advanced nitrogen alloyed stainless steel, Cronidur 30. Mater. Sci. Eng. Technol. 1999, 30, 605–611. [Google Scholar] [CrossRef]
- Isfahany, A.N.; Saghafian, H.; Borhani, G. The effect of heat treatment on mechanical properties and corrosion behavior of AISI420 martensitic stainless steel. J. Alloys Compd. 2011, 509, 3931–3936. [Google Scholar] [CrossRef]
- Lu, S.-Y.; Yao, K.-F.; Chen, Y.-B.; Wang, M.-H.; Shao, Y.; Ge, X.-Y. Effects of austenitizing temperature on the microstructure and electrochemical behavior of a martensitic stainless steel. J. Appl. Electrochem. 2015, 45, 375–383. [Google Scholar] [CrossRef]
- Choi, Y.-S.; Kim, J.-G.; Park, Y.-S.; Park, J.-Y. Austenitizing treatment influence on the electrochemical corrosion behavior of 0.3C-14Cr-3Mo martensitic stainless steel. Mater. Lett. 2007, 61, 244–247. [Google Scholar] [CrossRef]
- Park, J.; Park, Y. Effects of austenitizing treatment on the corrosion resistance of 14Cr-3Mo martensitic stainless steel. Corrosion 2006, 62, 541–547. [Google Scholar] [CrossRef]
- Lu, S.-Y.; Yao, K.-F.; Chen, Y.-B.; Wang, M.-H.; Ge, X.-Y. Influence of heat treatment on the microstructure and corrosion resistance of 13 wt pct Cr-type martensitic stainless steel. Metall. Mater. Trans. A 2015, 46, 6090–6102. [Google Scholar] [CrossRef]
- Geng, X.; Feng, H.; Jiang, Z.; Li, H.; Zhang, B.; Zhang, S.; Wang, Q.; Li, J. Microstructure, mechanical and corrosion properties of friction stir welding high nitrogen martensitic stainless steel 30Cr15Mo1N. Metals 2016, 6, 301. [Google Scholar] [CrossRef]
- Leem, D.-S.; Lee, Y.-D.; Jun, J.-H.; Choi, C.-S. Amount of retained austenite at room temperature after reverse transformation of martensite to austenite in a Fe-13%Cr-7%Ni-3%Si martensitic stainless steel. Scr. Mater. 2001, 45, 767–772. [Google Scholar] [CrossRef]
- Database for Surface Spectroscopies as XPS, AES and UPS. Available online: http://www.lasurface.com (accessed on 11 November 2016).
- ASTM. ASTM G48–11. Standard Test Methods for Pitting and Crevice Corrosion Resistance of Stainless Steels and Related Alloys by Use of Ferric Chloride Solution; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- ISO. ISO 8407: 2009(E). Corrosion of Metals and Alloys—Removal of Corrosion Products from Corrosion Test Specimens; International Standards Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Schlüter, K.; Shi, Z.; Zamponi, C.; Cao, F.; Quandt, E.; Atrens, A. Corrosion performance and mechanical properties of sputter-deposited MgY and MgGd alloys. Corros. Sci. 2014, 78, 43–54. [Google Scholar] [CrossRef]
- Candelaria, A.; Pinedo, C. Influence of the heat treatment on the corrosion resistance of the martensitic stainless steel type AISI 420. J. Mater. Sci. Lett. 2003, 22, 1151–1153. [Google Scholar] [CrossRef]
- Bai, G.; Lu, S.; Li, D.; Li, Y. Influences of niobium and solution treatment temperature on pitting corrosion behaviour of stabilised austenitic stainless steels. Corros. Sci. 2016, 108, 111–124. [Google Scholar] [CrossRef]
- Tang, Y.; Zuo, Y.; Wang, J.; Zhao, X.; Niu, B.; Lin, B. The metastable pitting potential and its relation to the pitting potential for four materials in chloride solutions. Corros. Sci. 2014, 80, 111–119. [Google Scholar] [CrossRef]
- Fu, Y.; Wu, X.; Han, E.-H.; Ke, W.; Yang, K.; Jiang, Z. Effects of nitrogen on the passivation of nickel-free high nitrogen and manganese stainless steels in acidic chloride solutions. Electrochim. Acta 2009, 54, 4005–4014. [Google Scholar] [CrossRef]
- Muto, I.; Sato, E.; Ito, S. Pitting corrosion behavior of stainless steels in a marine environment and its estimation method. Zairyo-to-Kankyo 1993, 42, 714–720. [Google Scholar] [CrossRef]
- Woldemedhin, M.; Shedd, M.; Kelly, R. Evaluation of the maximum pit size model on stainless steels under thin film electrolyte conditions. J. Electrochem. Soc. 2014, 161, E3216–E3224. [Google Scholar] [CrossRef]
- Berns, H.; Ehrhardt, R. Carbon or nitrogen alloyed quenched and tempered stainless steels—A comparative study. Steel Res. Int. 1996, 67, 343–349. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, T.; Meng, G.; Shao, Y.; Wang, F. Effect of pitting nucleation on critical pitting temperature of 316L stainless steel by nitric acid passivation. Corros. Sci. 2015, 91, 232–244. [Google Scholar] [CrossRef]
- Frankel, G. Pitting corrosion of metals—A review of the critical factors. J. Electrochem. Soc. 1998, 145, 2186–2198. [Google Scholar] [CrossRef]
- Della Rovere, C.; Aquino, J.; Ribeiro, C.; Silva, R.; Alcântara, N.; Kuri, S. Corrosion behavior of radial friction welded supermartensitic stainless steel pipes. Mater. Des. 2015, 65, 318–327. [Google Scholar] [CrossRef]
- Zhang, S.; Jiang, Z.; Li, H.; Feng, H.; Zhang, B. Detection of susceptibility to intergranular corrosion of aged super austenitic stainless steel S32654 by a modified electrochemical potentiokinetic reactivation method. J. Alloys Compd. 2017, 695, 3083–3093. [Google Scholar] [CrossRef]
- Chan, K.W.; Tjong, S.C. Effect of secondary phase precipitation on the corrosion behavior of duplex stainless steels. Materials 2014, 7, 5268–5304. [Google Scholar] [CrossRef]
- Moteshakker, A.; Danaee, I. Microstructure and corrosion resistance of dissimilar weld-joints between duplex stainless steel 2205 and austenitic stainless steel 316L. J. Mater. Sci. Technol. 2016, 32, 282–290. [Google Scholar] [CrossRef]
- Habibzadeh, S.; Li, L.; Shum-Tim, D.; Davis, E.C.; Omanovic, S. Electrochemical polishing as a 316L stainless steel surface treatment method: Towards the improvement of biocompatibility. Corros. Sci. 2014, 87, 89–100. [Google Scholar] [CrossRef]
- Lv, J.; Liang, T.; Wang, C. Surface enriched molybdenum enhancing the corrosion resistance of 316L stainless steel. Mater. Lett. 2016, 171, 38–41. [Google Scholar]
- Fuertes, N.; Pettersson, R. Passive film properties and electrochemical response of different phases in a Cu-alloyed stainless steel after long term heat treatment. J. Electrochem. Soc. 2016, 163, C377–C385. [Google Scholar] [CrossRef]
- Willenbruch, R.; Clayton, C.; Oversluizen, M.; Kim, D.; Lu, Y. An XPS and electrochemical study of the influence of molybdenum and nitrogen on the passivity of austenitic stainless steel. Corros. Sci. 1990, 31, 179–190. [Google Scholar] [CrossRef]
- Lu, Y.; Ives, M.; Clayton, C. Synergism of alloying elements and pitting corrosion resistance of stainless steels. Corros. Sci. 1993, 35, 89–96. [Google Scholar] [CrossRef]
- Cavanaugh, M.; Buchheit, R.; Birbilis, N. Modeling the environmental dependence of pit growth using neural network approaches. Corros. Sci. 2010, 52, 3070–3077. [Google Scholar] [CrossRef]
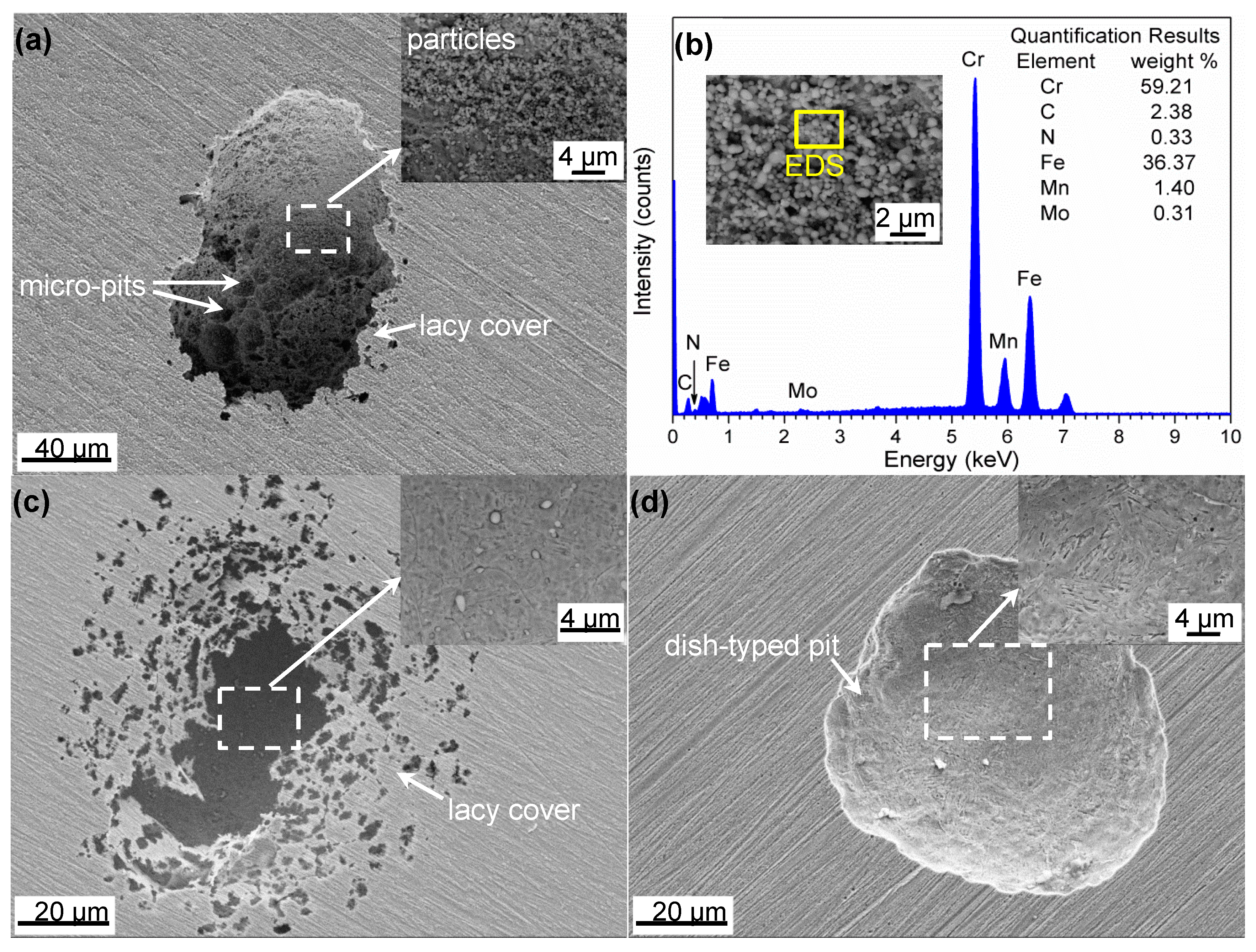
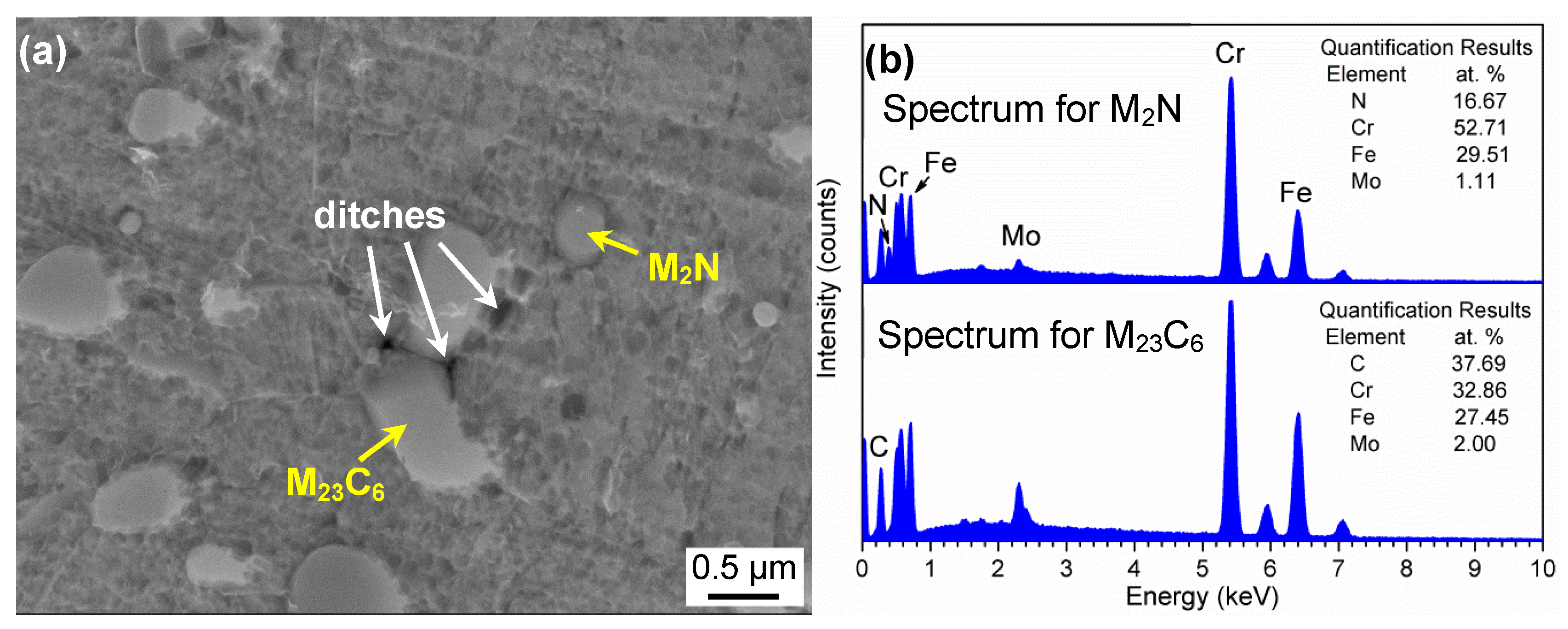
| C | Cr | Mo | N | Mn | Si | Ni | S | P | O |
|---|---|---|---|---|---|---|---|---|---|
| 0.31 | 15.17 | 1.03 | 0.44 | 0.44 | 0.52 | 0.07 | 0.002 | 0.011 | 0.0015 |
| Temperature (°C) | 900 | 950 | 1000 | 1050 | 1100 | 1150 |
|---|---|---|---|---|---|---|
| Area percentage of precipitates (%) | 7.75 ± 0.71 | 6.07 ± 0.96 | 3.94 ± 0.99 | 1.59 ± 0.63 | 1.23 ± 0.48 | 0.63 ± 0.40 |
| Specimens | Component of Passive Films (at %) | Cr3+/(Fe2+ + Fe3+) | |||
|---|---|---|---|---|---|
| Fe3O4 | FeOOH | Cr2O3 | Cr(OH)3 | ||
| 900 °C | 12.51 | 4.68 | 6.97 | 4.52 | 0.24 |
| 1000 °C | 11.39 | 2.49 | 8.47 | 6.34 | 0.29 |
| 1100 °C | 11.92 | 2.56 | 9.04 | 5.33 | 0.31 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Z.; Feng, H.; Li, H.; Zhu, H.; Zhang, S.; Zhang, B.; Han, Y.; Zhang, T.; Xu, D. Relationship between Microstructure and Corrosion Behavior of Martensitic High Nitrogen Stainless Steel 30Cr15Mo1N at Different Austenitizing Temperatures. Materials 2017, 10, 861. https://doi.org/10.3390/ma10080861
Jiang Z, Feng H, Li H, Zhu H, Zhang S, Zhang B, Han Y, Zhang T, Xu D. Relationship between Microstructure and Corrosion Behavior of Martensitic High Nitrogen Stainless Steel 30Cr15Mo1N at Different Austenitizing Temperatures. Materials. 2017; 10(8):861. https://doi.org/10.3390/ma10080861
Chicago/Turabian StyleJiang, Zhouhua, Hao Feng, Huabing Li, Hongchun Zhu, Shucai Zhang, Binbin Zhang, Yu Han, Tao Zhang, and Dake Xu. 2017. "Relationship between Microstructure and Corrosion Behavior of Martensitic High Nitrogen Stainless Steel 30Cr15Mo1N at Different Austenitizing Temperatures" Materials 10, no. 8: 861. https://doi.org/10.3390/ma10080861
APA StyleJiang, Z., Feng, H., Li, H., Zhu, H., Zhang, S., Zhang, B., Han, Y., Zhang, T., & Xu, D. (2017). Relationship between Microstructure and Corrosion Behavior of Martensitic High Nitrogen Stainless Steel 30Cr15Mo1N at Different Austenitizing Temperatures. Materials, 10(8), 861. https://doi.org/10.3390/ma10080861