1. Introduction
Polymer nano-composites are made up of a matrix polymer, which is reinforced by a small quantity of nanoparticles with a high aspect ratio [
1], and the particles or fibres used have a minimum of one dimension in the range of 1 to 100 nm [
2]. Filler materials have been used with different polymers to improve properties such as mechanical strength, heat resistance, impact resistance, and electrical conductivity. Conventional fillers, which are in the macro or micro scale, lack strong interactions with the matrix polymer, and larger fillers are prone to imperfections [
3,
4]. Conventional fibre-reinforced composites with strong filler-matrix interactions show improvements in stiffness and strength while sacrificing toughness. The volume fractions of filler materials also have direct relationships to possible improvements in properties [
2]. The presence of nano fillers in the matrix is more discrete in comparison, and adds to the properties of the base polymer without compromising on qualities such as flexibility. Layered silicates and carbon nanotubes are by far the most investigated nano materials [
5].
The traditional methods used to process nano-composites can be divided into different sub categories. In the mechanical processing of nano materials, severe plastic deformation techniques such as forging, extrusion, drawing, and rolling are employed. The material is subjected to deformation under large strains below the recrystallization temperature [
6,
7,
8]. These conventional techniques have limitations when processing nanoparticles due to load limitations, and it is difficult to achieve uniform microstructures. The formation of impurities and porosity is another problem associated with plastic deformation. Other techniques, such as ball milling, are used for processing, but lack the ability to produce bulk samples [
6,
7,
9]. Shock wave consolidation is also used to process nanomaterials, which can densify powder materials without changing the microstructure or composition, unlike the thermo-mechanical processing methods.
In this context, the additive manufacturing routes, and in particular selective laser sintering (SLS), appear to offer promising solutions. In SLS, the polymer powder is deposited and consolidated layer by layer to produce complex and intricate three-dimensional parts [
10]. The energy required for consolidation is supplied by a focused laser beam directed by a series of mirrors. The laser scanning path is determined by a computer aided design (CAD) model of the part. The sintering process can be characterised by different binding mechanisms used to process various materials. These mechanisms can be classed as solid state sintering, chemically induced binding, and liquid phase sintering. Solid state sintering occurs when the process temperature is from half of the melting temperature to the melting temperature. Diffusion is identified as the most prominent reaction under these conditions. This results in neck formation between adjacent powder particles due to the lowering of free energy. Most materials can be processed by solid state sintering, provided that the temperature is high enough to generate the kinetic energy required for the migration of vacancies. The speed of the process is dependent on the preheating conditions [
11].
SLS has the ability to produce multilayer nano-composites, as it is a layer by layer production method employing localized heating. Studies by Zhang et al. [
12] showed that the toughness of parts increases with smaller particle sizes. The study compares the differences between clay nanoparticle-modified nylon and standard nylon-6. Organic composites containing Carbon or hydrocarbon bonds as the backbone of the composition attained much research attention initially. Paggi et al. [
13] researched a nano-composite comprised of polyamide 12 (PA12) and multi-walled carbon nanotubes (MWCNTs) developed using commercial material components. The polymeric component of the composite is Polyamide Duraform in powder form, and the multi-walled Carbon nanotubes were manufactured by chemical vapour deposition. The materials were mixed using a magnetic stirring technique to achieve homogenization. The specimens produced using SLS are used to identify the process parameters necessary to achieve high mechanical properties suitable for aerospace applications. It has been found that the material’s density and flexural modulus can be improved by adjusting the laser energy density. The study shows that the amount of carbon nanotube which could be mixed in the compound is limited to achieve proper sintering characteristics. The properties of PA12/MWCNTs were further investigated by Gean et al. [
14]. The study revealed an increase in energy absorption in the presence of carbon nanotubes (CNTs) and an increase in tensile strength by 10%, while giving a reduction in elongation by 11–9%. A 12% increase in storage modulus was achieved. Polyamide 12 has also been combined with 3 wt % carbon nanofibers for processing using selective laser sintering [
15]. The microstructural and mechanical characteristics have been evaluated using scanning electron microscopy (SEM) and dynamic mechanical testing. The micrographs have shown that the nanofibers are well-mixed in the polymer matrix of the sintered parts. The results show a 22% increase in the storage modulus from the original material. The composite has the potential for future processing if the powder’s characteristics are sufficiently altered.
Inorganic composites are also used in a range of applications, from producing functionally graded parts to producing biodegradable scaffold structures used in tissue engineering. In a study conducted by Chung et al. [
16], Pure Nylon was blended with silica nanoparticles in the range of 2–6% by volume using a rotary tumbler to determine the effects of different loading and process parameters on the quality of the parts produced. The preferred powders were in the range of 10 to 150 micrometers in diameter, with semi-crystallinity and a low melting point. Previous research conducted on a micro Al
2O
3 particle-filled polyamide showed potential, but the micro ceramics adversely affected the toughness and flexibility of the material. Evidently, nano-fillers are more discrete due to the significant particle size differences, and are likely to contribute to material enhancements without adverse effects. Selective laser sintering is possibly the better way of consolidating the combined powders. The current research is an attempt in this direction, considering Al
2O
3 as the filler material, considering inherent characteristics such as high hardness, thermal stability, insulation, and flame retardancy, while polyamide is the base polymer. Al
2O
3 is proven for its high thermal stability, which allows the particles to absorb laser energy and release it at a slower rate compared to the polymer particles. The slow release in energy improves the solid state sintering of polymer, which is dependent on time and temperature. Nano ceramic particles were chosen instead of micro particles, as their presence in the polymer matrix is negligible.
2. Materials and Methods
Powder preparation is an important aspect in the selective laser sintering process. As mentioned earlier, the mixing of nanoparticles in the matrix polymer is an important and challenging aspect due to the high aspect ratio of nanoparticles. The particles should be uniformly dispersed with a minimum amount of agglomeration to benefit from the composites. The initial tests will be conducted using manually mixed powder specimens due to the lack of immediate availability of resources. One of the other mixing methods identified from the literature will be used for subsequent experimental work. An extrusion process was used by Koo et al. [
17] to produce a polyamide/clay nano-composite. A gravity-fed twin screw extruder was used to achieve the proper dispersion of nanoparticles in the study. A similar melt mixing process was used by Goodridge et al. [
15] to produce a polyamide 12/CNT composite. The melt mixing procedures are followed by a cryogenic blending process to produce the required polymer nano-composite powder. This is conducted by basking the extruded material in liquid Nitrogen for five to ten minutes, followed by blending in a food processor.
Neat Polyamide 11 (PA11) was used as the matrix polymer, as it is a proven material for selective laser sintering and due to availability. Al2O3 powder particles with an approximate diameter of 50 nm were used as the filler material. Powder specimens were produced with Alumina compositions of 1 wt %, 2 wt %, 3 wt %, 5 wt %, and 10 wt % to investigate the effect of Alumina composition on the sintering process. The powder specimens were manually mixed as solid particles.
The specimens were sintered using an experimental Selective laser sintering setup, which allows the sintering conditions and material systems to be varied with minimal restrictions and produce samples using a minimum amount of material. A CO
2 laser is used with a wave length of 10.6 um, which is ideal for processing polymer components. Both polyamide and Alumina have high absorptivity values, 75% and 96% respectively, for a CO
2 laser wave length [
10].
3. Initial Powder Characterization and Laser Sintering Results
3.1. Thermal Characterisation of the Raw Materials
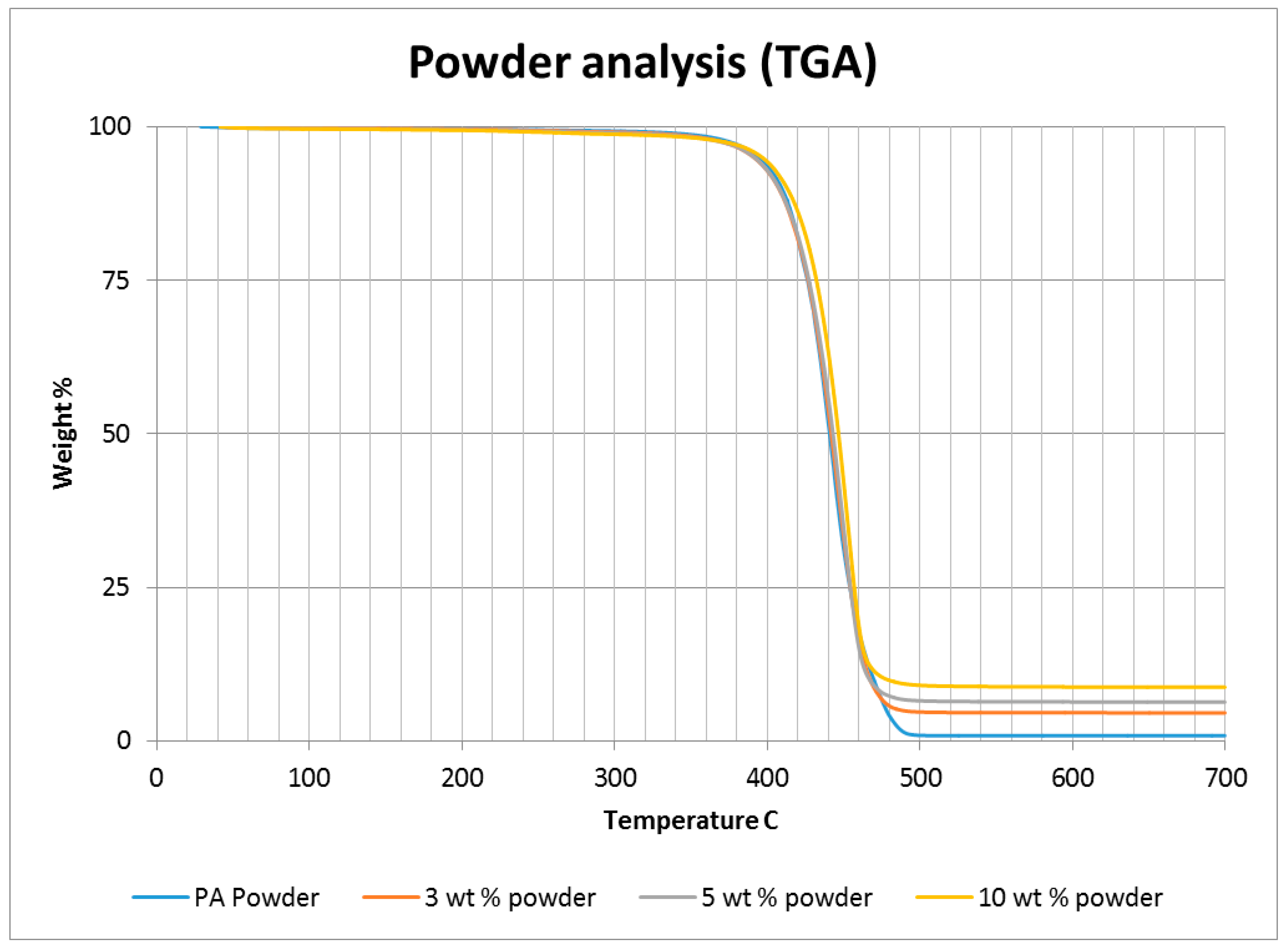
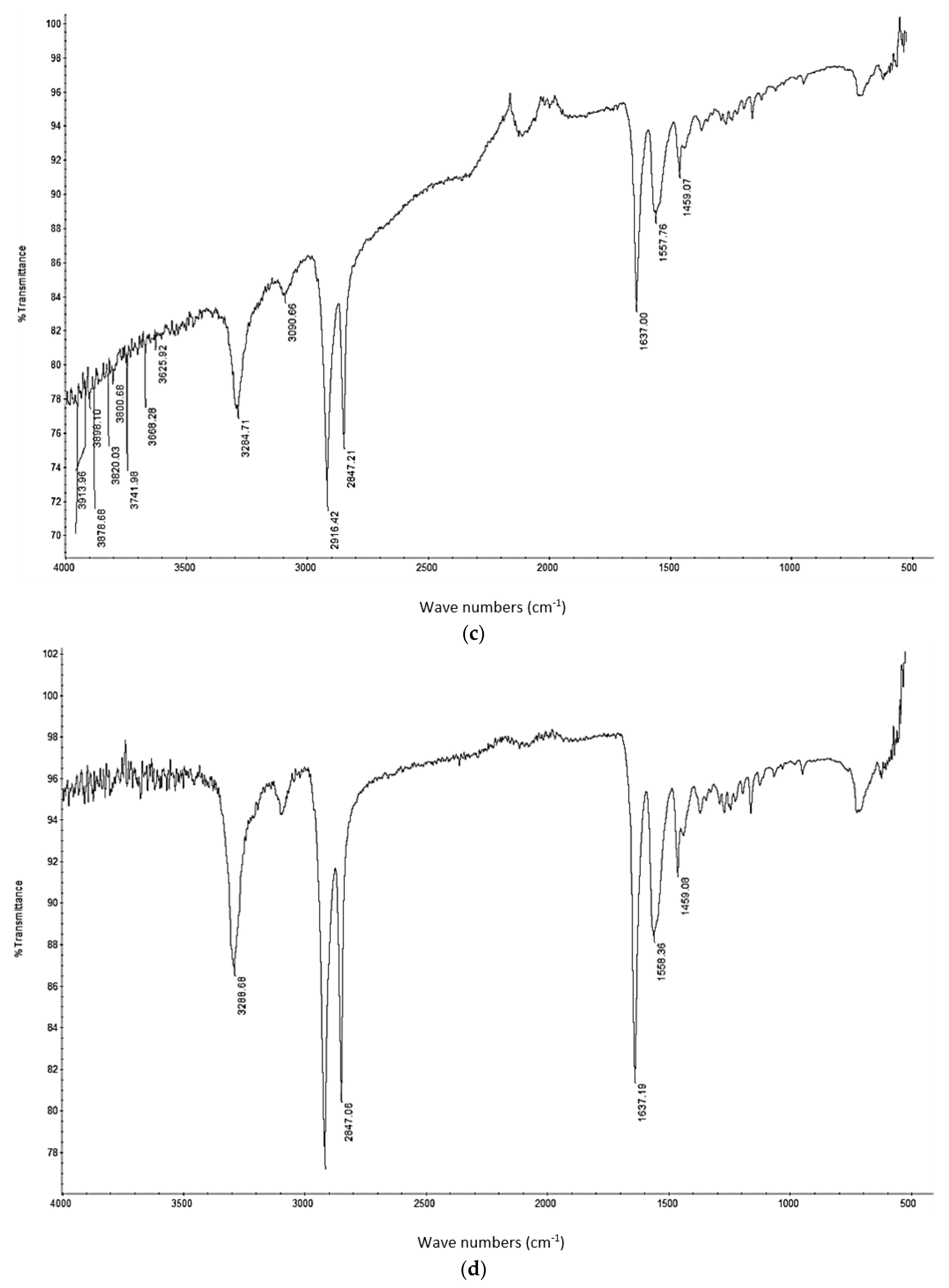
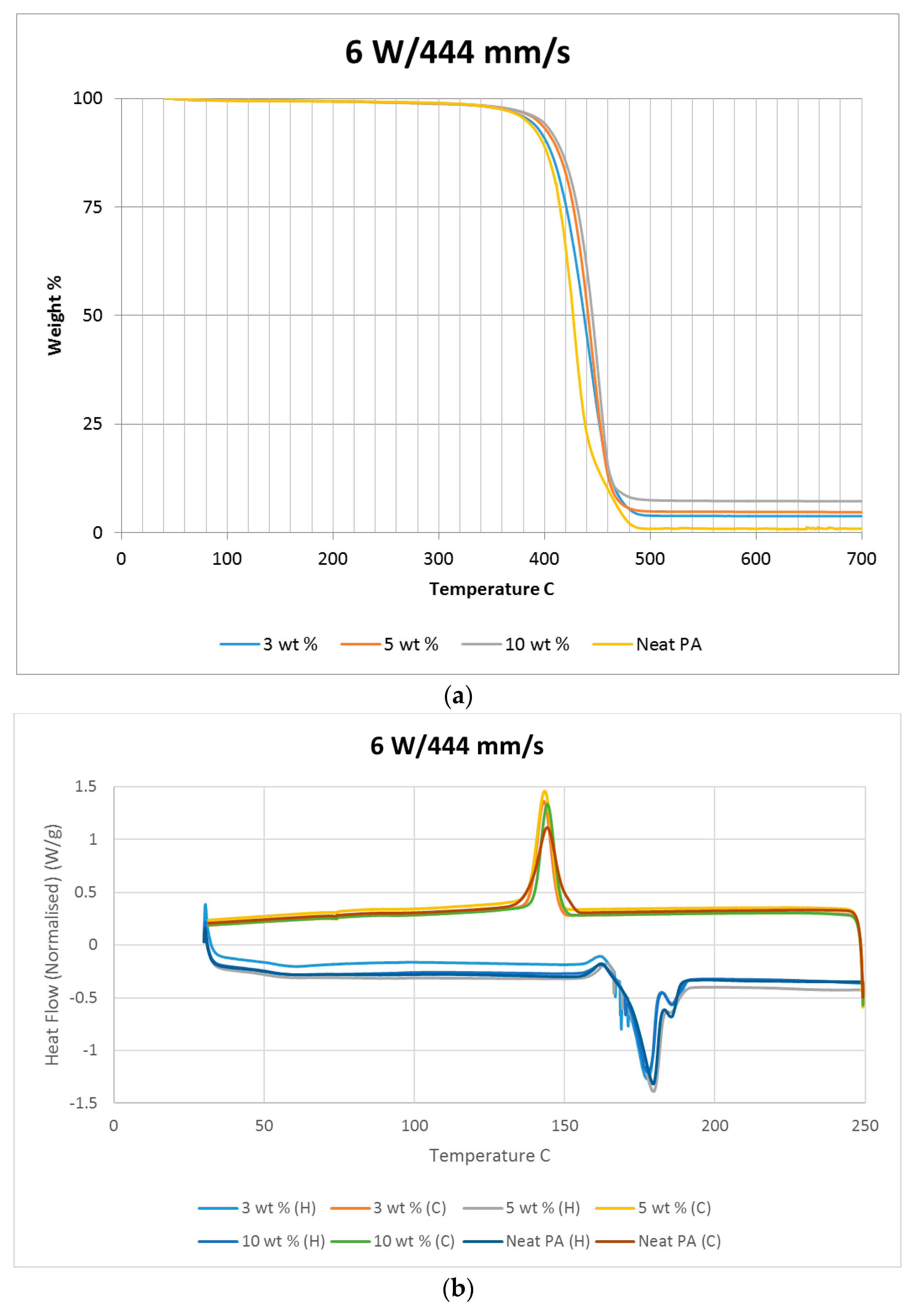
A thermo-gravimetric analysis (TGA) was conducted on the powder and sintered samples to identify the thermal degradation characteristics of the materials. The samples were heated from 30 °C to 700 °C at a rate of 10 °C/min in a TA instruments Discovery TGA system to achieve complete decomposition of PA11. The process was conducted in an air atmosphere and the percentage weight loss was plotted against the temperature and presented in
Figure 1 using powders of varying compositions. The TGA results of the neat polymer presented in
Figure 1 show an initial reduction in weight down to approximately 99.7% when heated from 90 °C to 110 °C, which can be identified as an evaporation of the moisture stored in the PA. The powder produces a graph characteristic of polyamide, as observed from the literature. A reduction in mass could be identified while heating in the range 400 to 480 °C. The decomposition temperature of the polymer can be identified by producing a derivative graph. This is identified as 443.524 °C at a decomposition rate of 2.55264 (wt %)/(°C). The graph indicates that the polymer powder is completely burned with a residual mass of 0.901276%.
An addition of 3 wt % alumina resulted in minor increases in both the decomposition (445.818 °C) and melting (186.442 °C) temperatures as depicted in
Figure 1. The TGA curve obtained based on the 5 wt % alumina sample presented in
Figure 1 shows the decomposition temperature to increase to 449.373 °C, and a rise in the residual content to 6.37% as a result of the increased alumina content.
Figure 1 indicates a continuation of this trend, with an increased decomposition temperature of 453.222 °C and a residual mass of 8.79% when the alumina content was raised to 10 wt %.
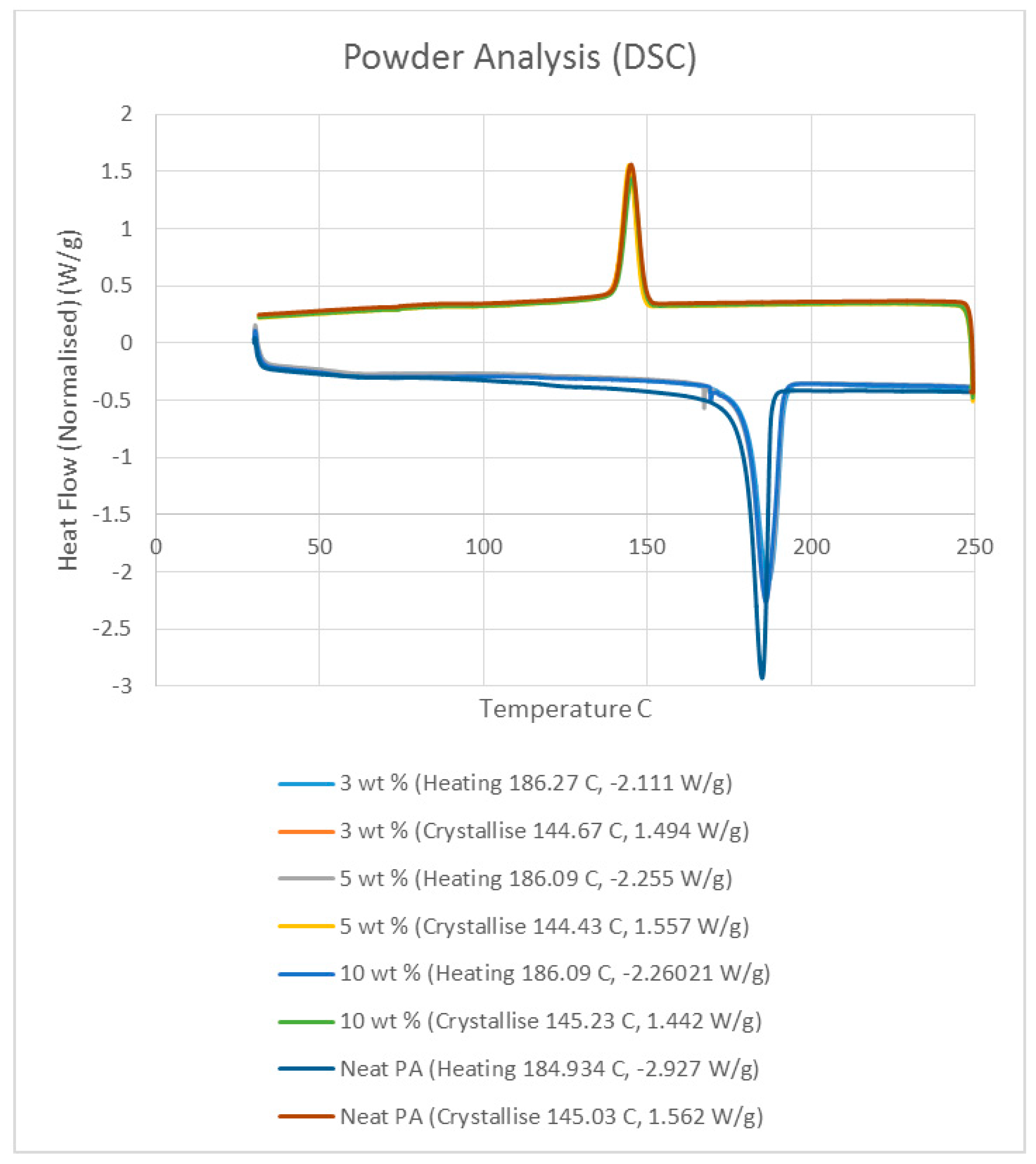
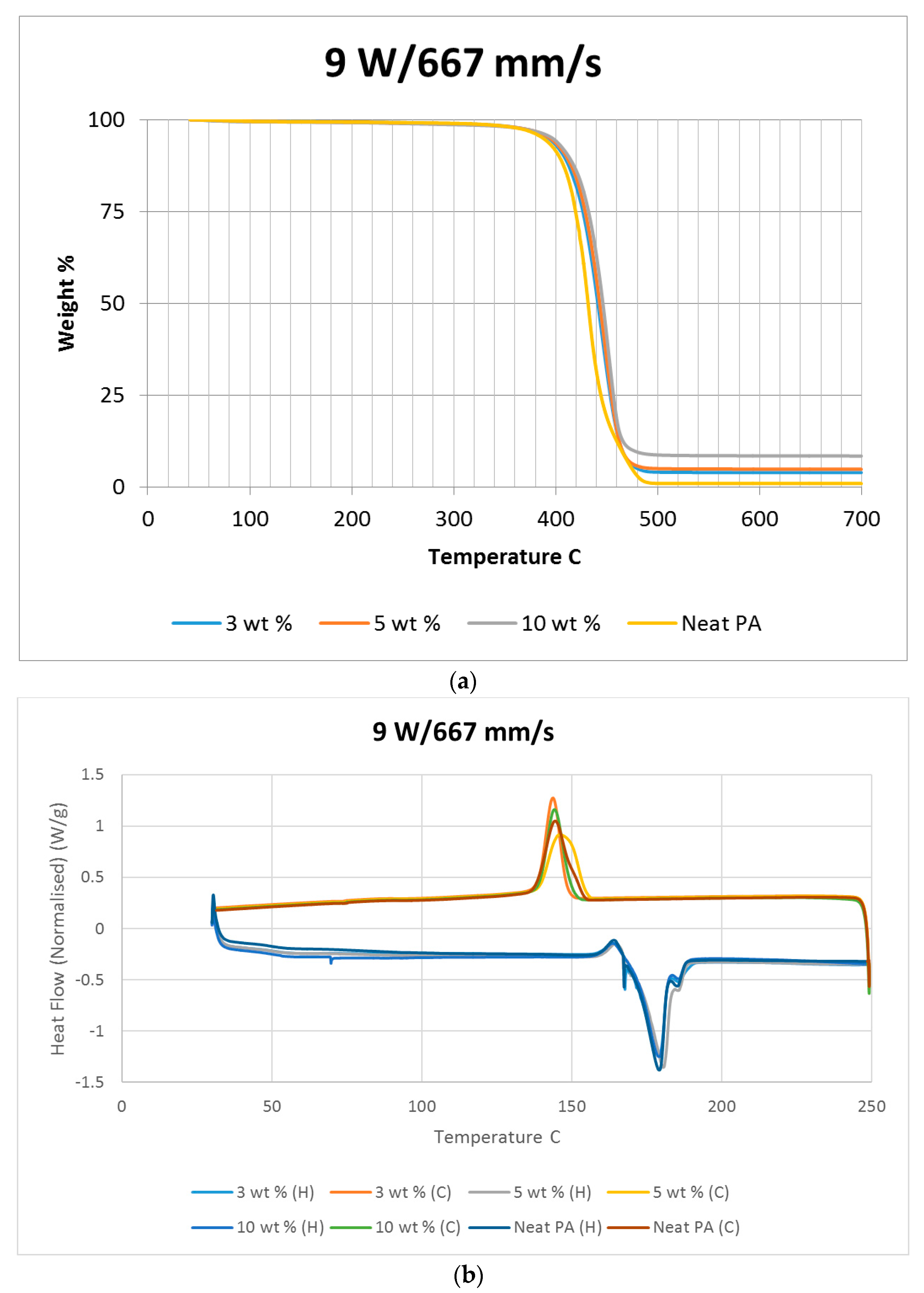
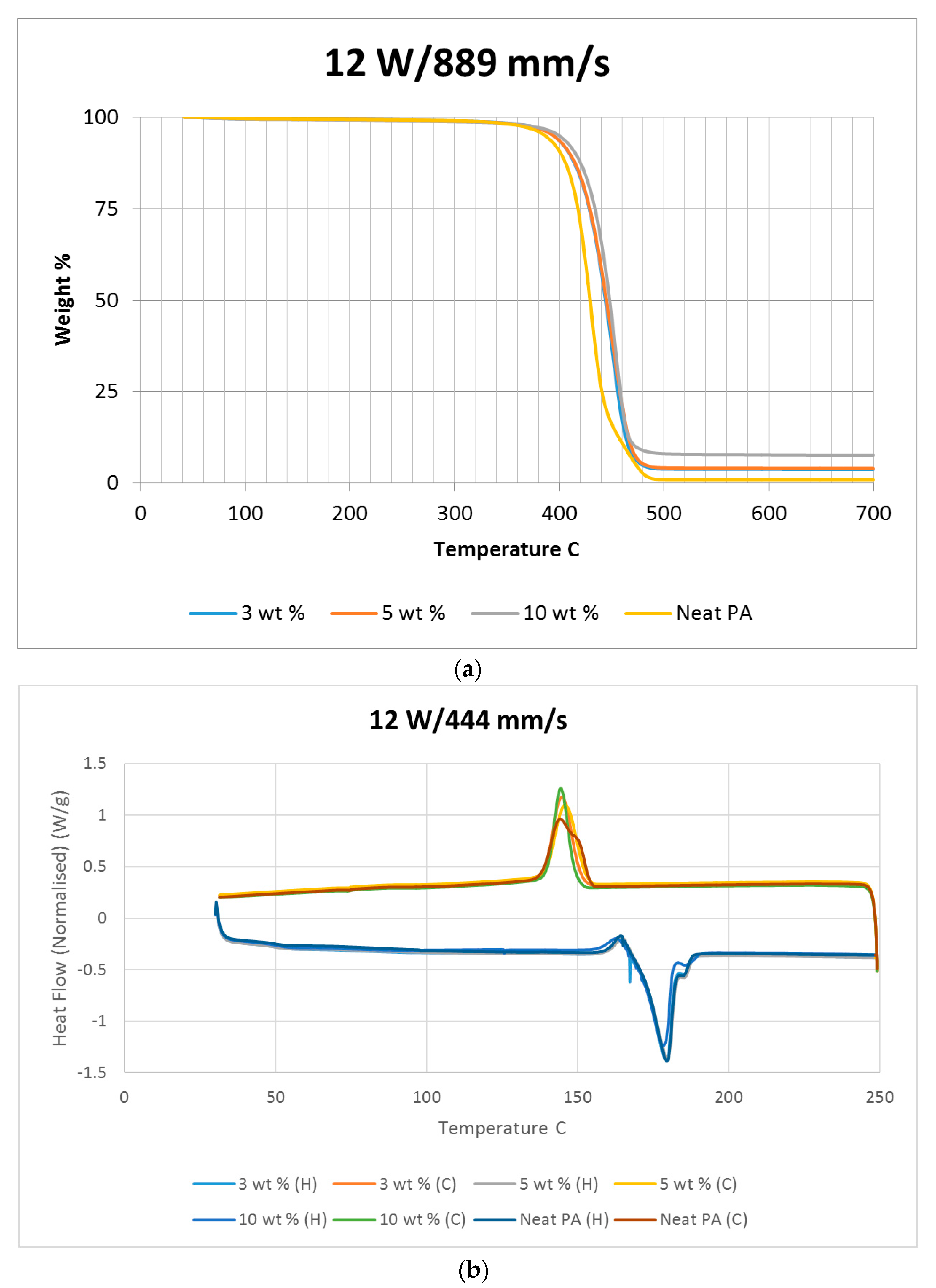
Differential scanning calorimetry (DSC) was done on all of the specimens using the temperature range identified prior to polymer decomposition. The samples were subjected to two heating cycles and a cooling cycle in the range of 30 °C to 250 °C at a rate of 10 °C/min in a nitrogen environment with a feed rate of 10 L/min. The results of the DSC tests are presented in
Figure 2. As it may be observed from
Figure 2, for the case of the neat polyamide, the melting temperature was noted to be 184.918 °C from the initial heating cycle of DSC, while the crystallization temperature was 145.025 °C. The secondary heating cycle yielded a lower melting temperature at 179.525 °C, indicating a deterioration of the polymer from the previous heating cycle. The addition of a nano alumina content of 3 wt % increased the melting temperature to 186.442 °C, as seen in
Figure 2. The crystallization temperature was reduced to 144.675 °C, showing no major variation in thermal properties after the addition of the nano filler.
The characteristic curves showed a similar trend, indicating that the chemical structure of the matrix polymer remains unaltered during thermal processing when mixed with a given filler. The enthalpy for the melting process is reduced by the addition of the nano particles, indicating a lesser heat requirement to achieve melting. The trend continues with the melting temperature increasing to 186.095 °C, indicating an increase in heat absorption due to the higher alumina content at 5%. Overall, the temperature variations are minor with varying compositions, but a significant variation in the crystallization enthalpy may be noted between the neat polymer and the composites. The enthalpy of crystallization first increased by almost 50% when 3% nanoparticles are added to the neat polymer. However, with further increase in the nano alumina to 5% and 10%, the enthalpy of crystallization decreased below that of the neat polyamide. The enthalpy for the crystallization process is also known as the latent heat of crystallization, which is the amount of energy released to transform from a liquid state to a solid state.
While cooling neat polyamide of a given mass, a certain energy is released at the point of crystallization, corresponding to the peak shown on the cooling curve of
Figure 2. With increased amounts of Al
2O
3 content, the relative amounts of latent heat should have been lesser. However, the nano Al
2O
3 particles, being more laser absorptive, gather relatively higher heat energy while heating. This heat is also not immediately conducted due to the presence of polyamide particles. Some of this heat is released during the cooling cycles of the polyamide-Al
2O
3 composite powders, compensating for some of the reduction in the energy released from the polyamide content. This is probably the reason why the peaks at crystallization are almost at the same level for samples of varying compositions.
3.2. Initial Laser Sintering Trials
The results of the DSC and TGA tests were used to evaluate the potential for laser sintering where the polymer and polymer composites are thermally stable for the laser sintering process. The clear melting and crystallization peaks, and the narrow but not overlapping range between the peaks indicated by the DSC graphs, suggests that the polymer and composite samples are suitable for laser sintering. The energy requirement for producing preliminary samples was estimated using temperature values obtained by DSC. The scan lines were simplified as cylindrical paths, while the density of the nanoparticles was neglected for a simplified initial estimate. The calculations were conducted for a laser beam diameter of 0.54 mm, polymer density of 1.04 × 103 kg/m3, and a specific heat value of 1700 JK−1 kg−1. A bench mark scan speed of 500 mm/s was used for the calculations, which is a medium speed governed by the SLS system. Using the DSC results together with the basic heat transfer principles, the energy requirement for sufficient sintering was evaluated to be 9.5 W.
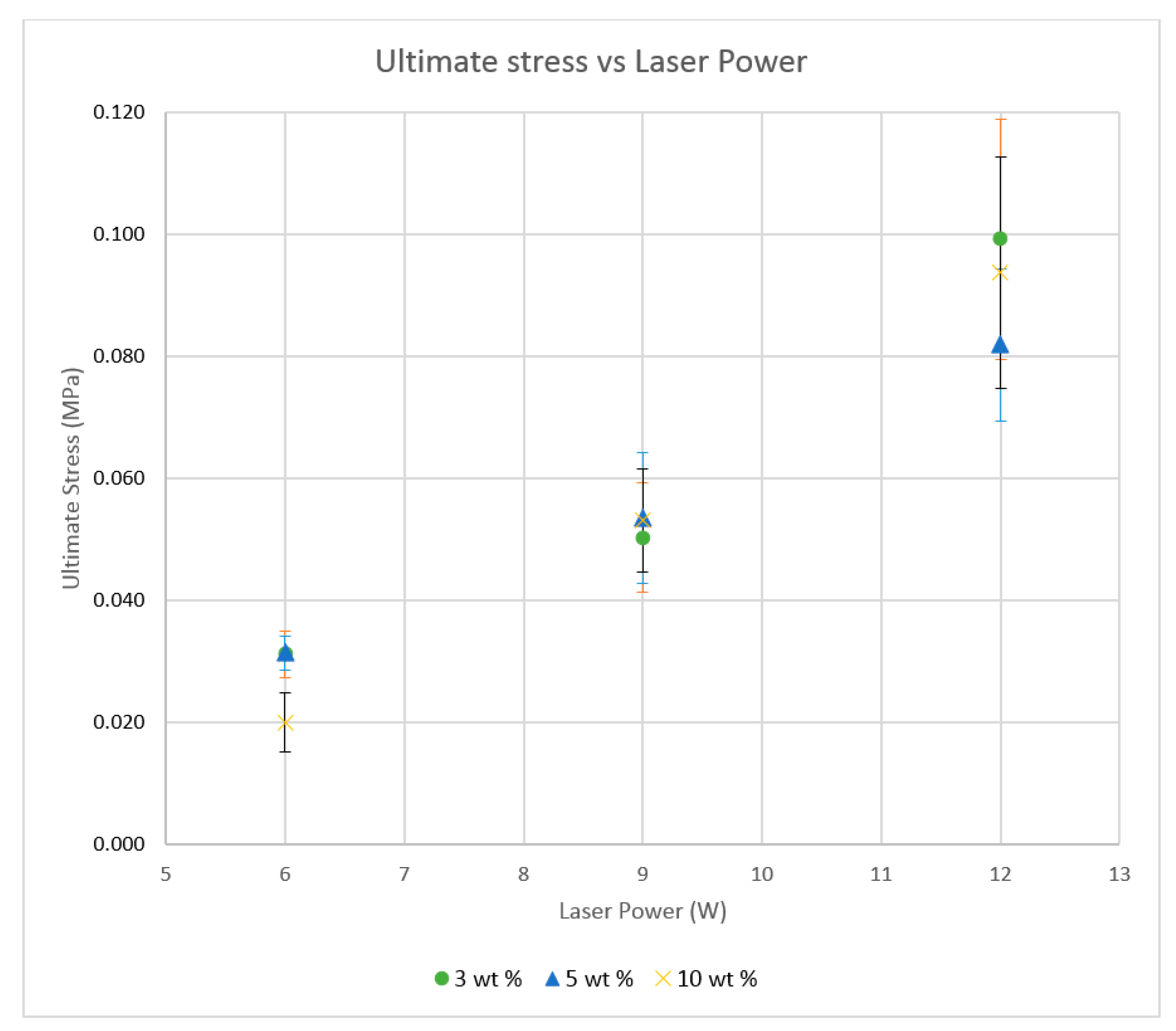
Single-layer samples were produced according to the American Society for testing Materials (ASTM) standards using predicted energy densities, while varying the laser power and scan speeds. The samples were subjected to thin film tensile testing to evaluate the mechanical properties of the composite material. Further testing was conducted using a range of energy densities to achieve better tensile properties [
13]. The dispersion of the nanoparticles was analyzed by SEM and EBSD methods. The crystallinity and the rate of crystallinity of the composite can be evaluated by differential scanning calorimetry as shown by Kim et al. [
18]. The thermal stability of the composite can be evaluated using thermo-gravimetric testing [
19]. The thermal stability is a deciding factor for the repeatability of the process. Small rectangular specimens were produced using a range of laser power outputs and scan speeds within the successful energy density levels to determine preliminary sintering characteristics in the multi-layer samples [
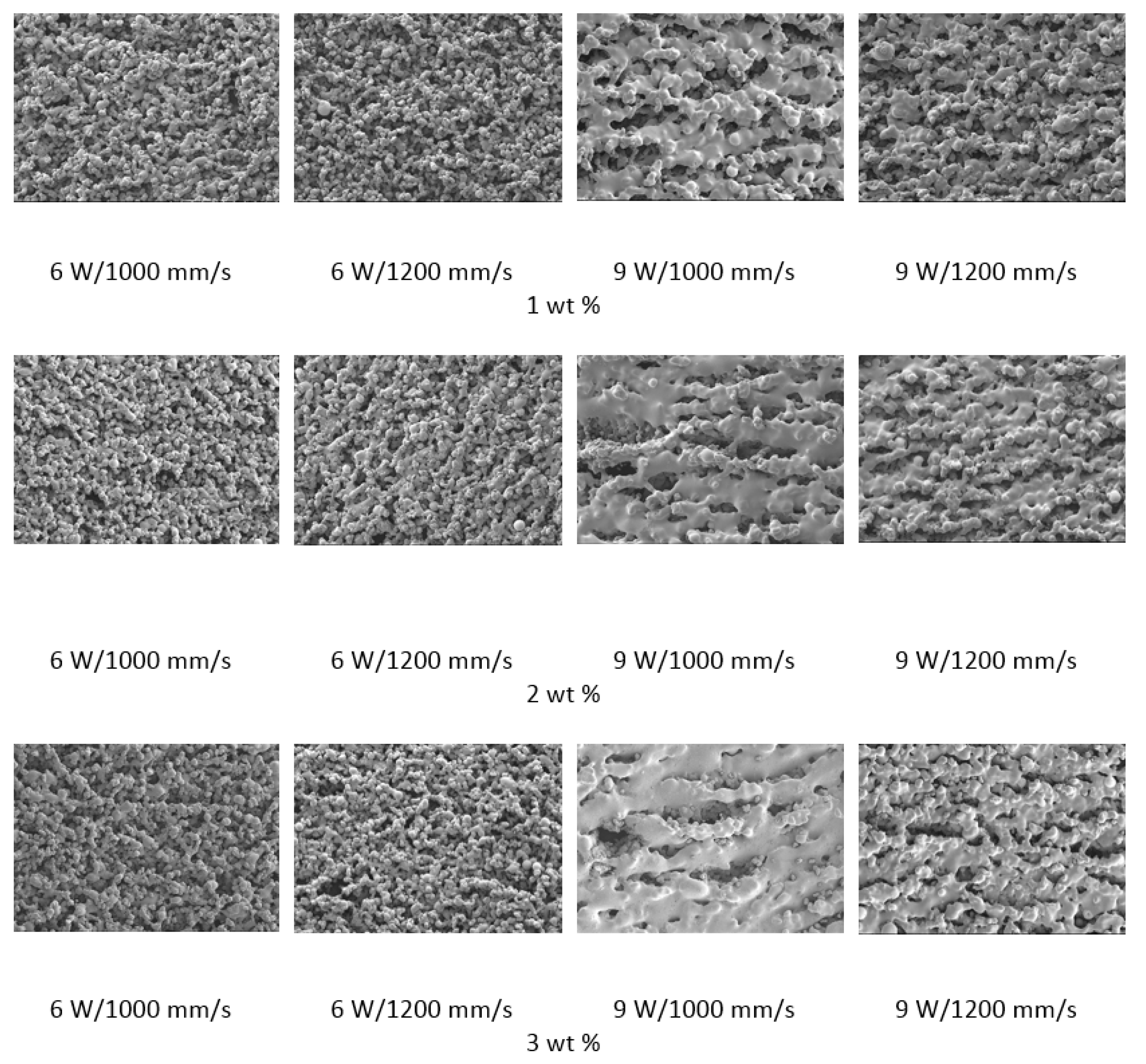
15]. The samples were then evaluated with SEM to determine the consolidation of material and predict the energy density requirements.
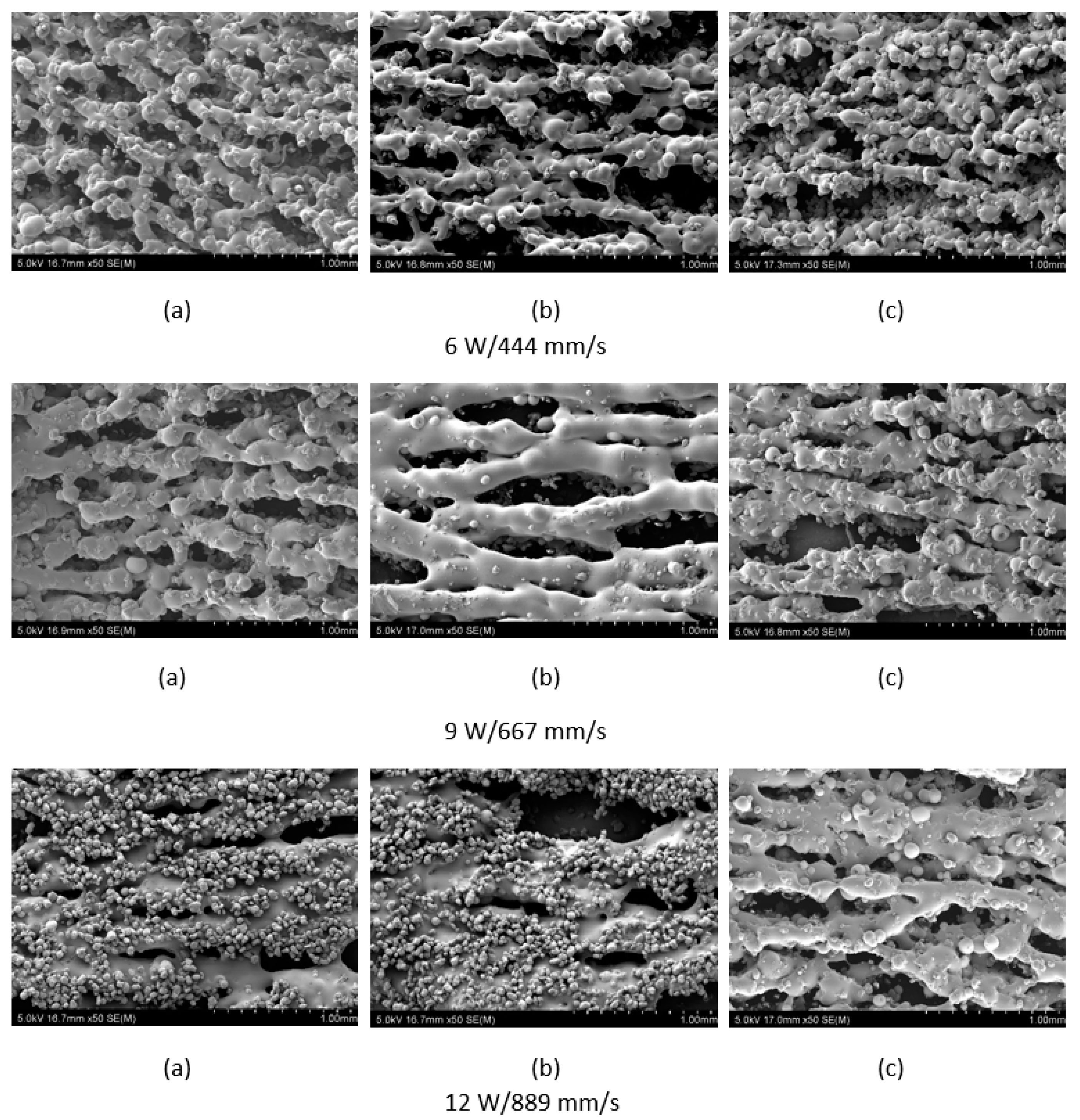
The polymer powder particles were in a non-melted state when sintered at 6 W with an Alumina content of 1 wt %. Signs of solid state diffusion could be identified due to neck formation among adjacent particles. The melt flow of polymer increased with increasing Alumina content under the same conditions. This can be seen by the developed scan lines in the sintering direction. A small amount of inter scan line coalescence can be seen at the 3 wt % loading. The interscan line melt flow decreased when the scan speed was increased while maintaining the same laser power. The melt flow of the polymer was significantly increased when the laser power was increased to 9 W. Clearly formed scan lines can be observed and the fusion between scan lines is improved at 9 W/1000 mm/s with a nanoparticle content of 3 wt %. The sintering behaviour is clearly improved with increasing nanoparticle content.
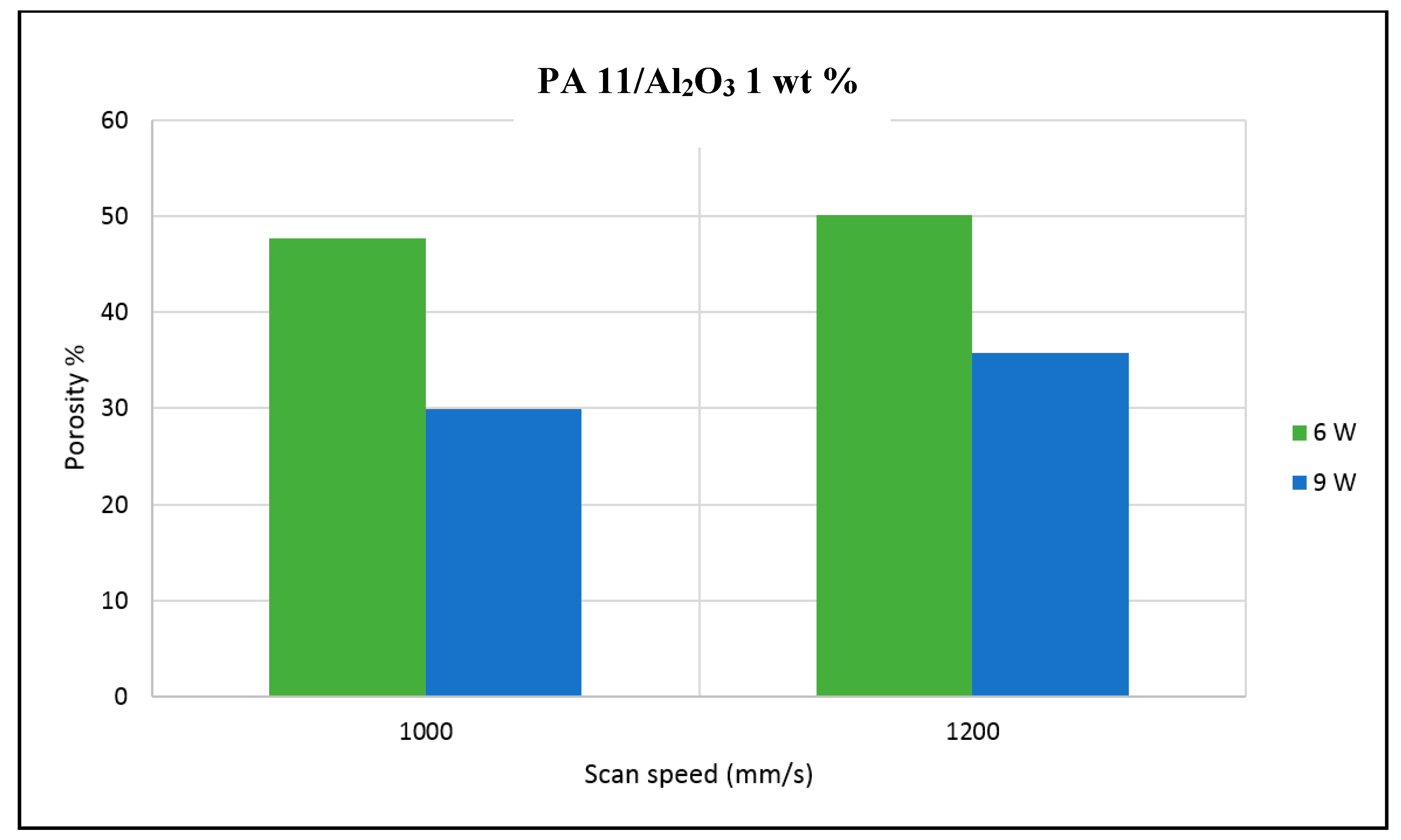
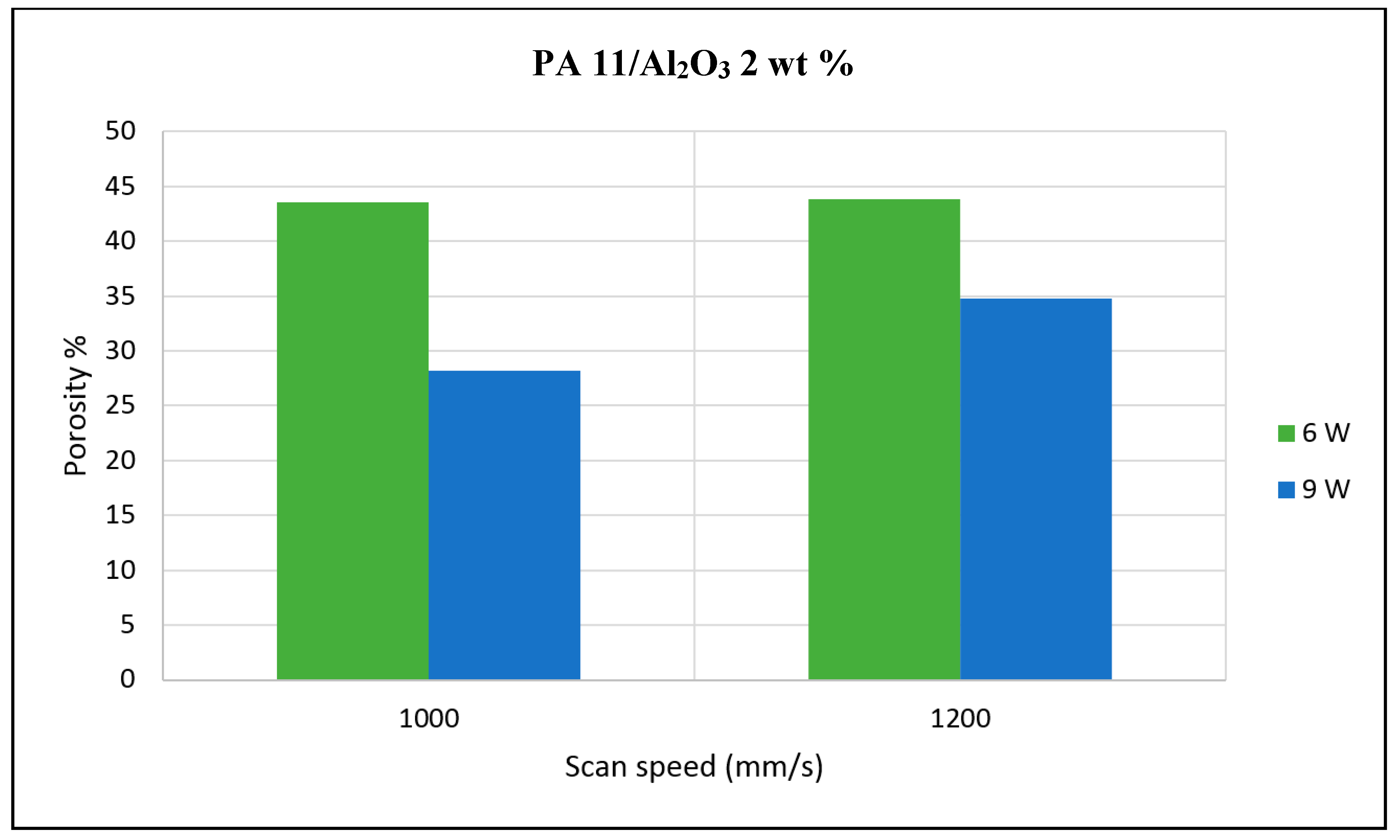
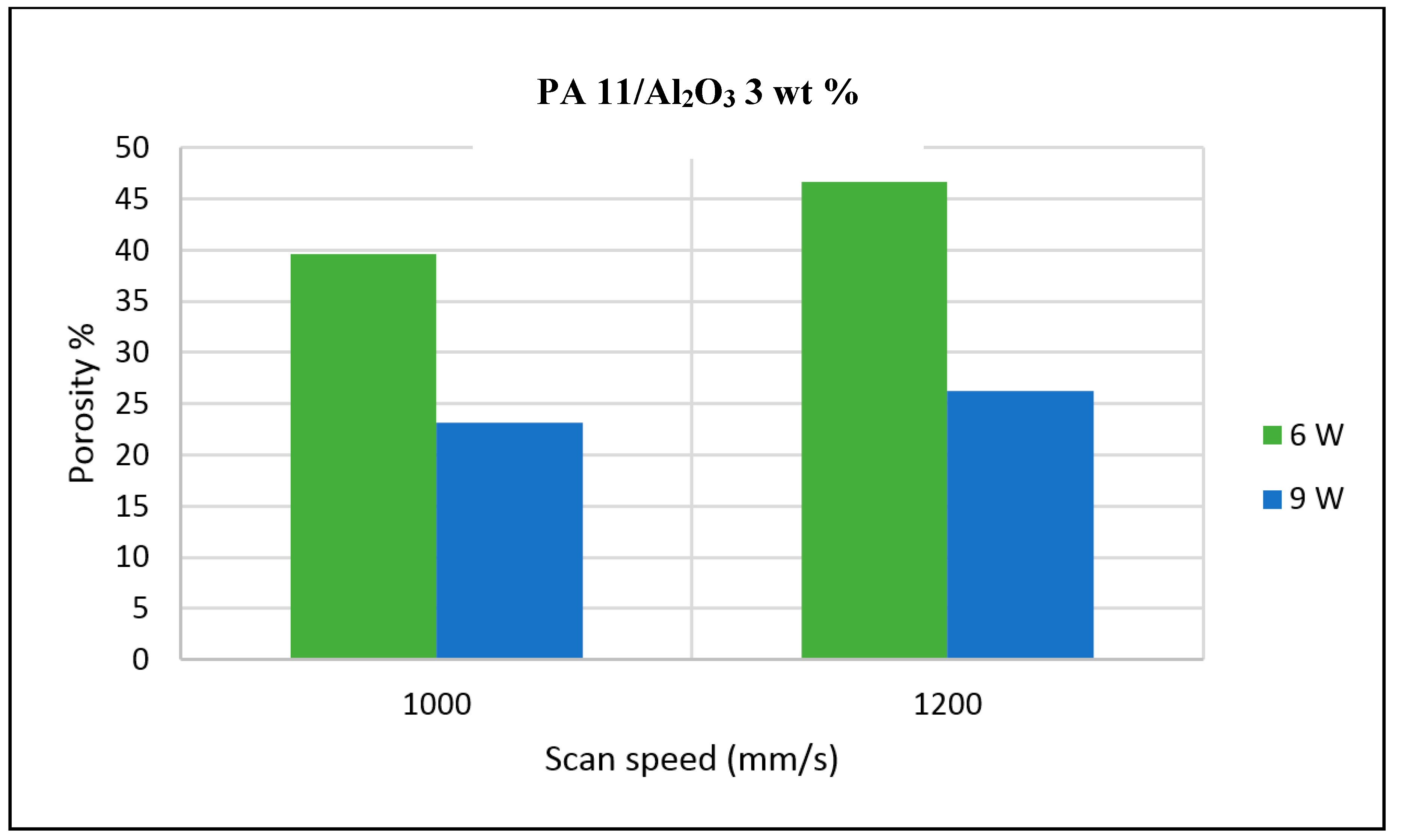
The porosity of the sintered parts were measured using an image analysis software, which identifies the voids in SEM images of single-layer samples. The porosity results support the observations made above, as the highest porosity is seen at the smallest loading condition as seen in
Figure 3 and
Figure 4, and the lowest porosity is achieved at the highest loading condition under high laser power and low scan speed. The porosity reduction at a higher loading condition is potentially due to the higher laser absorptivity of alumina compared to the matrix polymer, which increases the efficiency of the sintering process. The energy absorbed from the nanoparticles is also released to the surrounding polymer particles, as the operating temperatures are not sufficient to melt the alumina particles. This helps maintain the polymer particles at an elevated temperature for an extended duration, resulting in better sintering. The SEM morphology results and the porosity results from
Figure 3,
Figure 4,
Figure 5 and
Figure 6 suggest that material consolidation is higher at a laser power of 9 W and a scan speed of 1000 mm/s. The energy density for this configuration was calculated as 16,666 J/m
2 according to Equation (1):
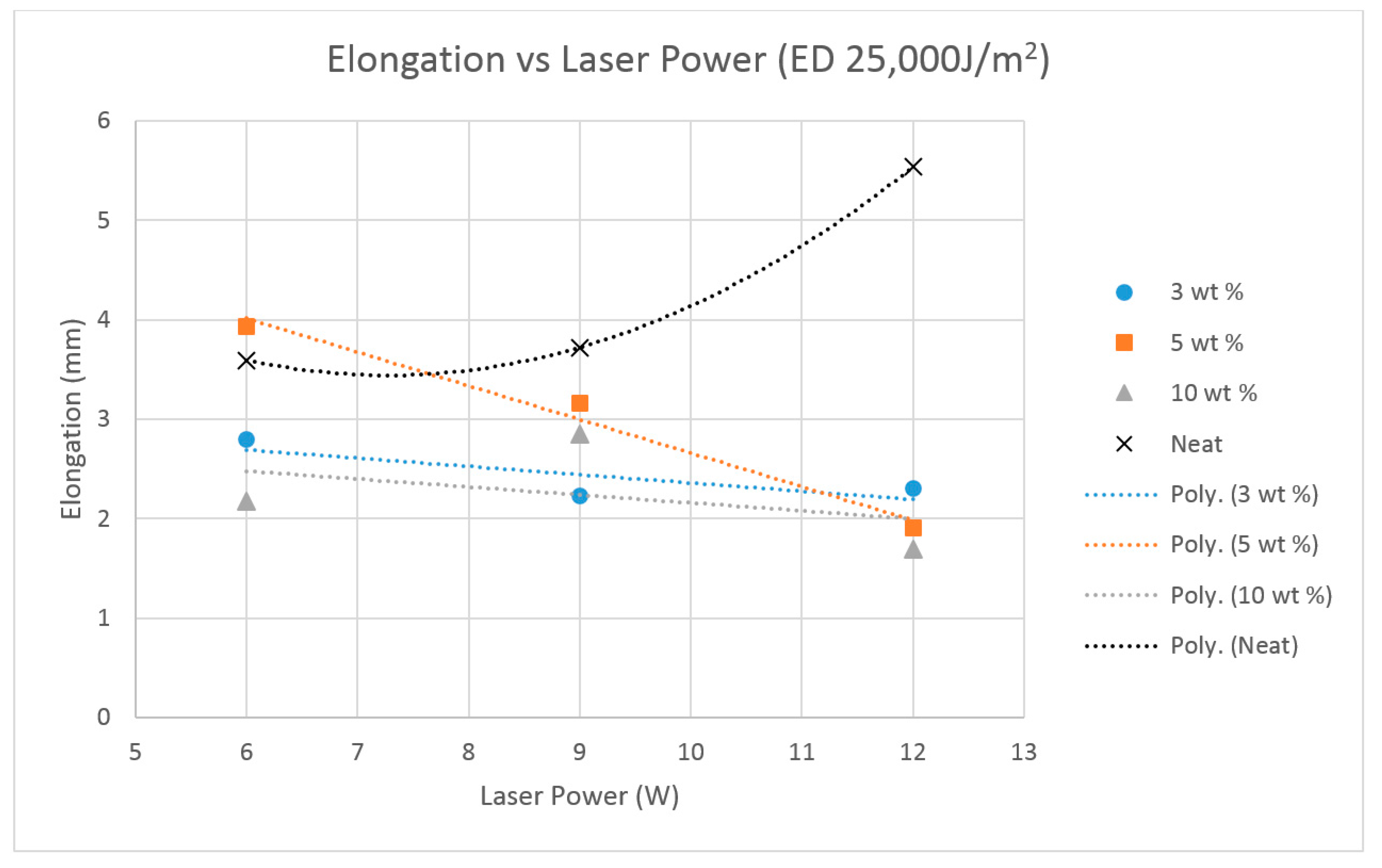
The previous literature on Polyamide (PA) suggests that the elongation properties of sintered PA specimens significantly improve when the laser energy density is increased to 25,000 J/m
2 [
19]. Hence, the higher energy density was used for producing the Al
2O
3 samples.