Protein-Based Drug-Delivery Materials
Abstract
:1. Introduction
2. Protein Materials
2.1. Keratin
2.2. Collagen
2.3. Elastin
2.4. Silk
2.5. Resilin
2.6. Corn Zein
3. Fabrication Methods of Devices Based on Biopolymers
3.1. Films and Coatings
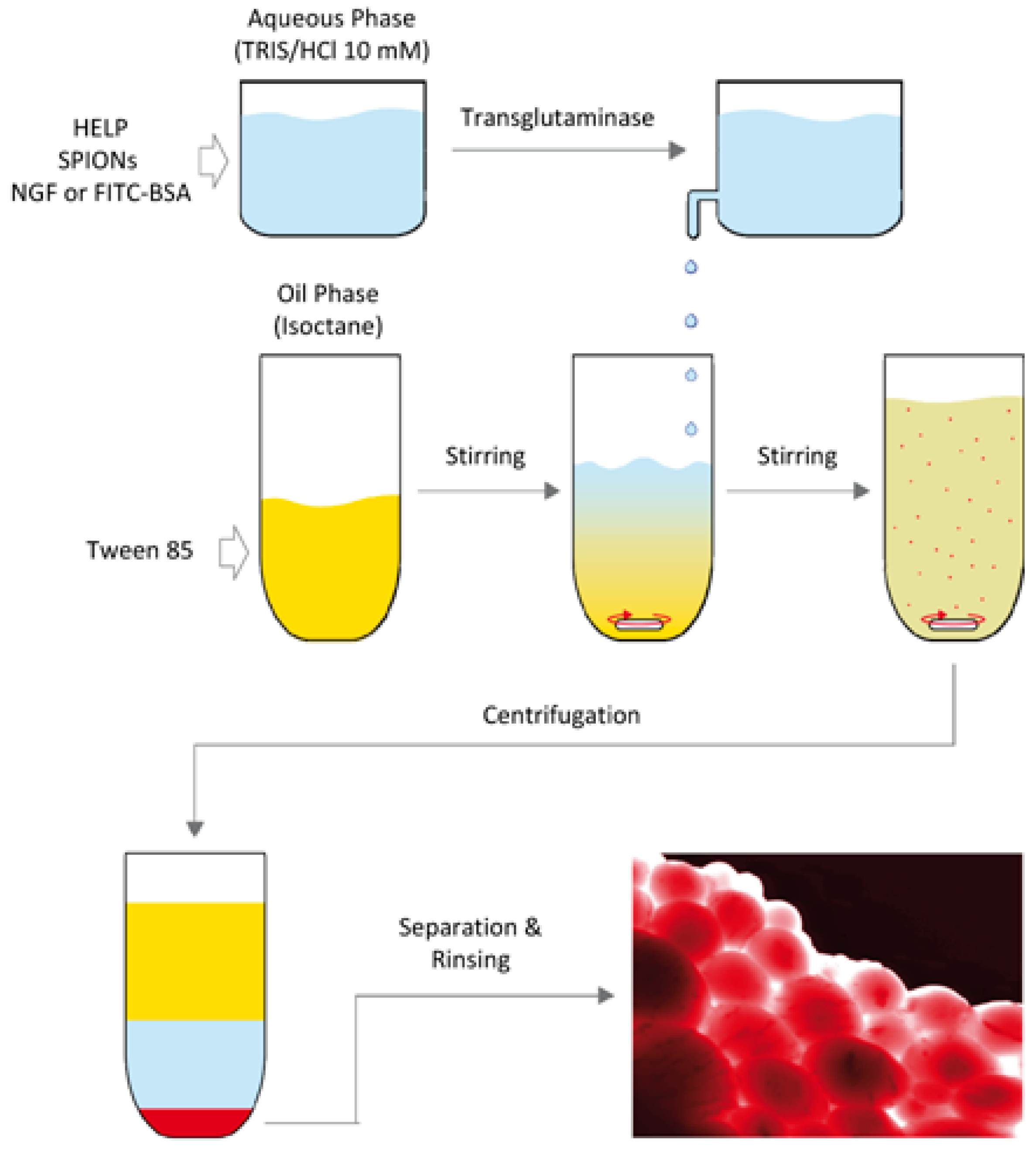
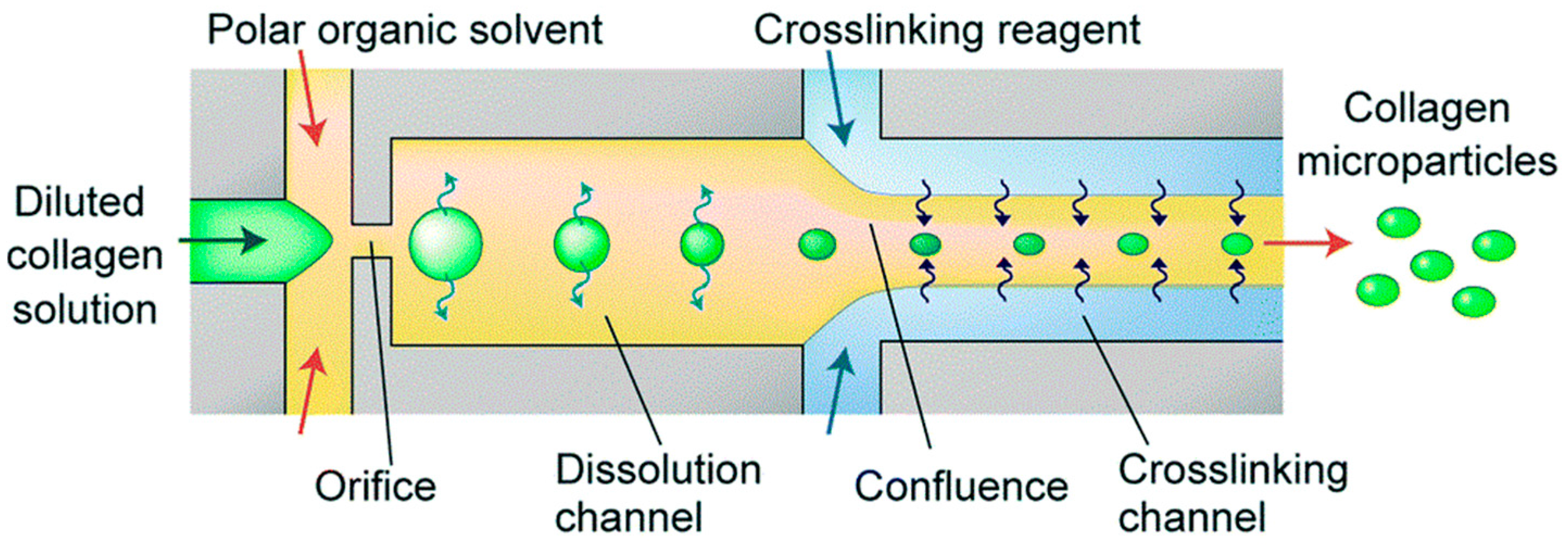
3.2. Particles and Spheres
3.3. Hydrogels
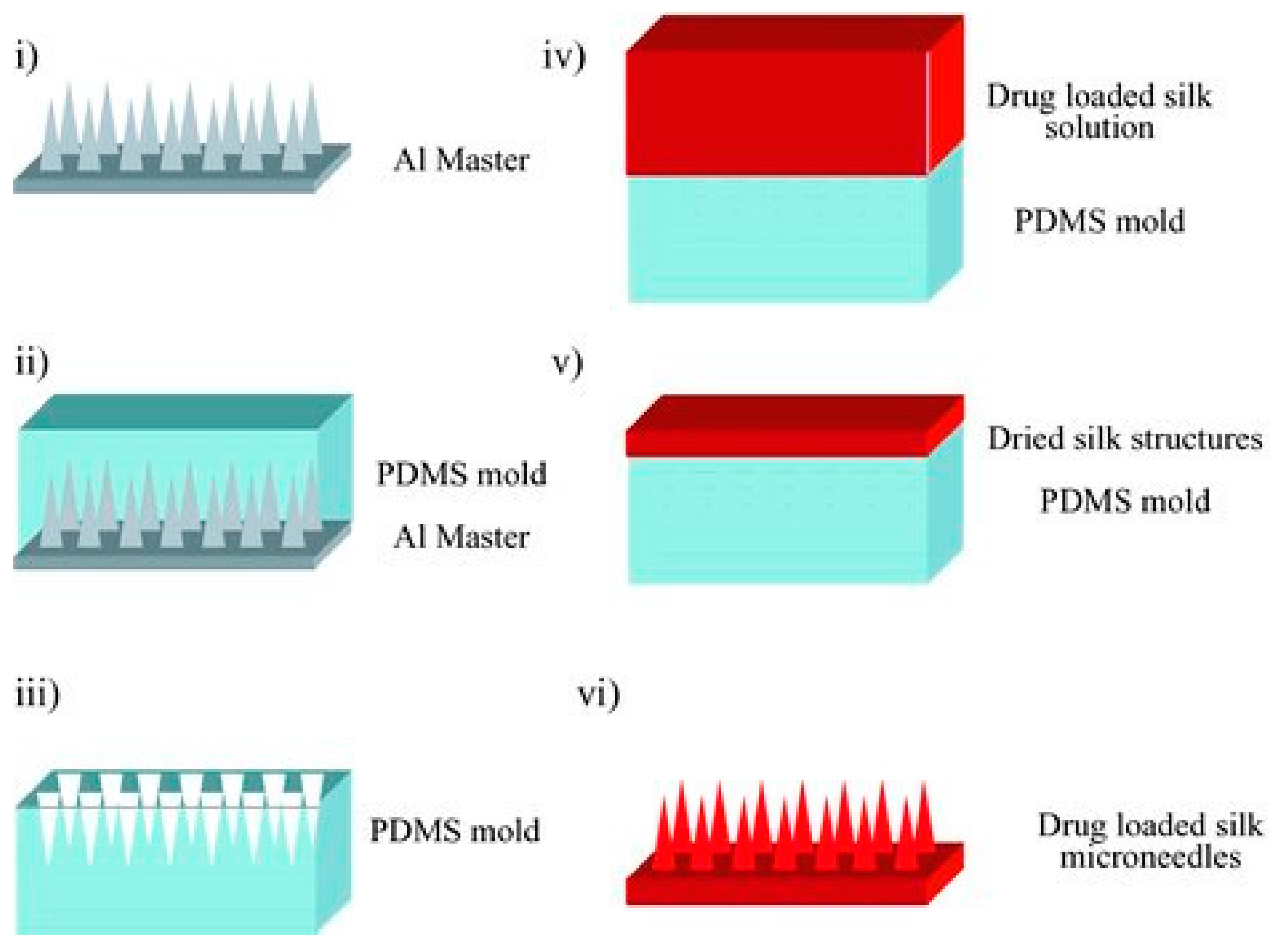
3.4. Microneedles
3.5. Composite Materials
3.5.1. Keratin Composites
3.5.2. Elastin Composites
3.5.3. Collagen Composites
4. Factors to Control Drug-Delivery Efficiency
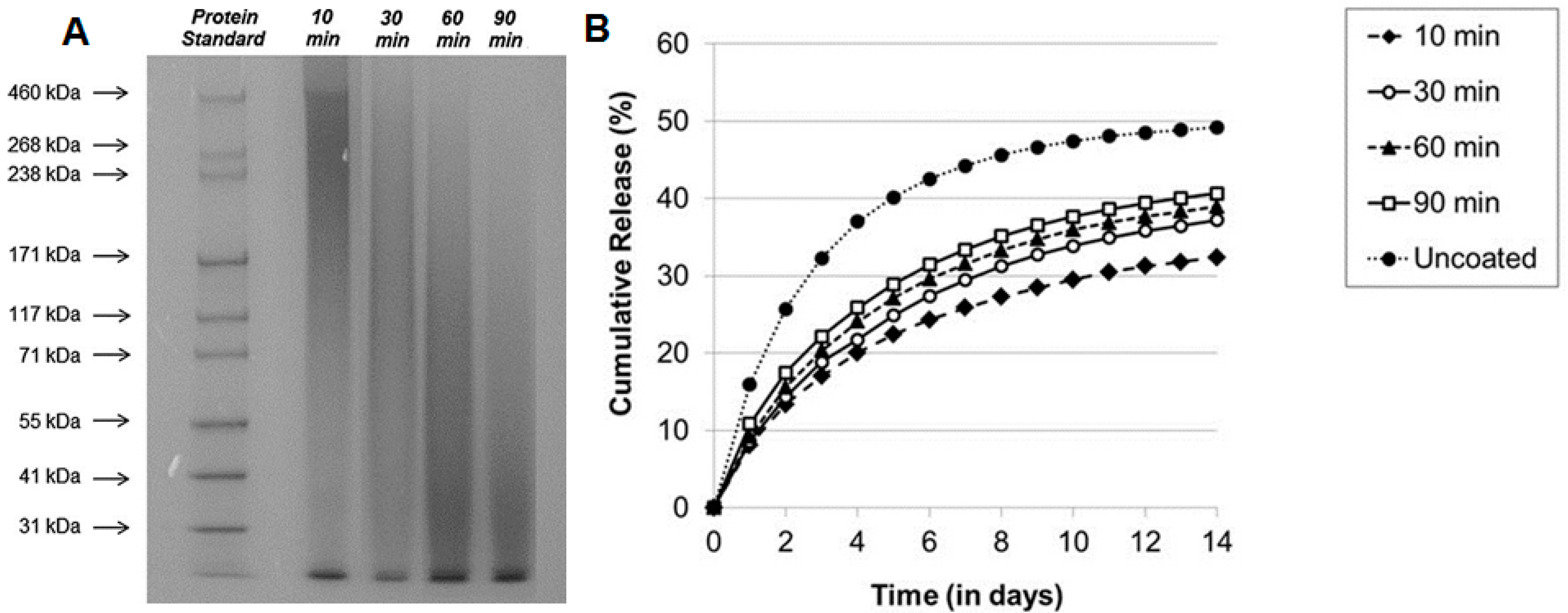
4.1. Molecular Weight
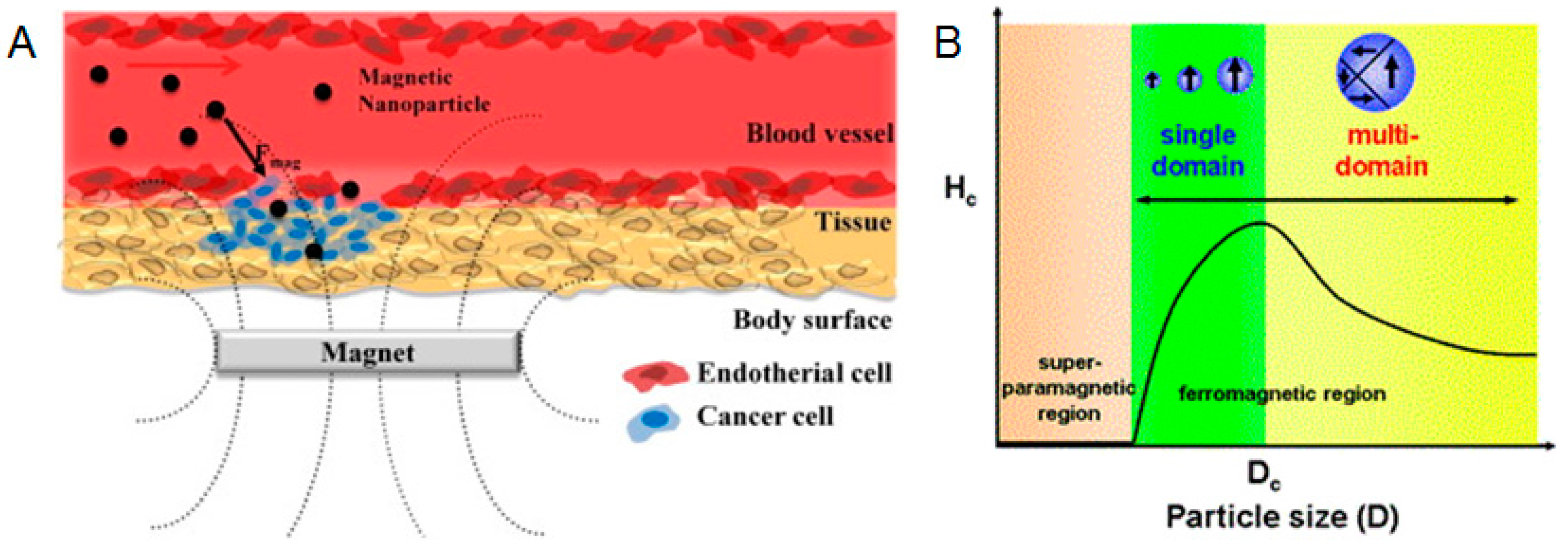
4.2. Nanoparticle Size
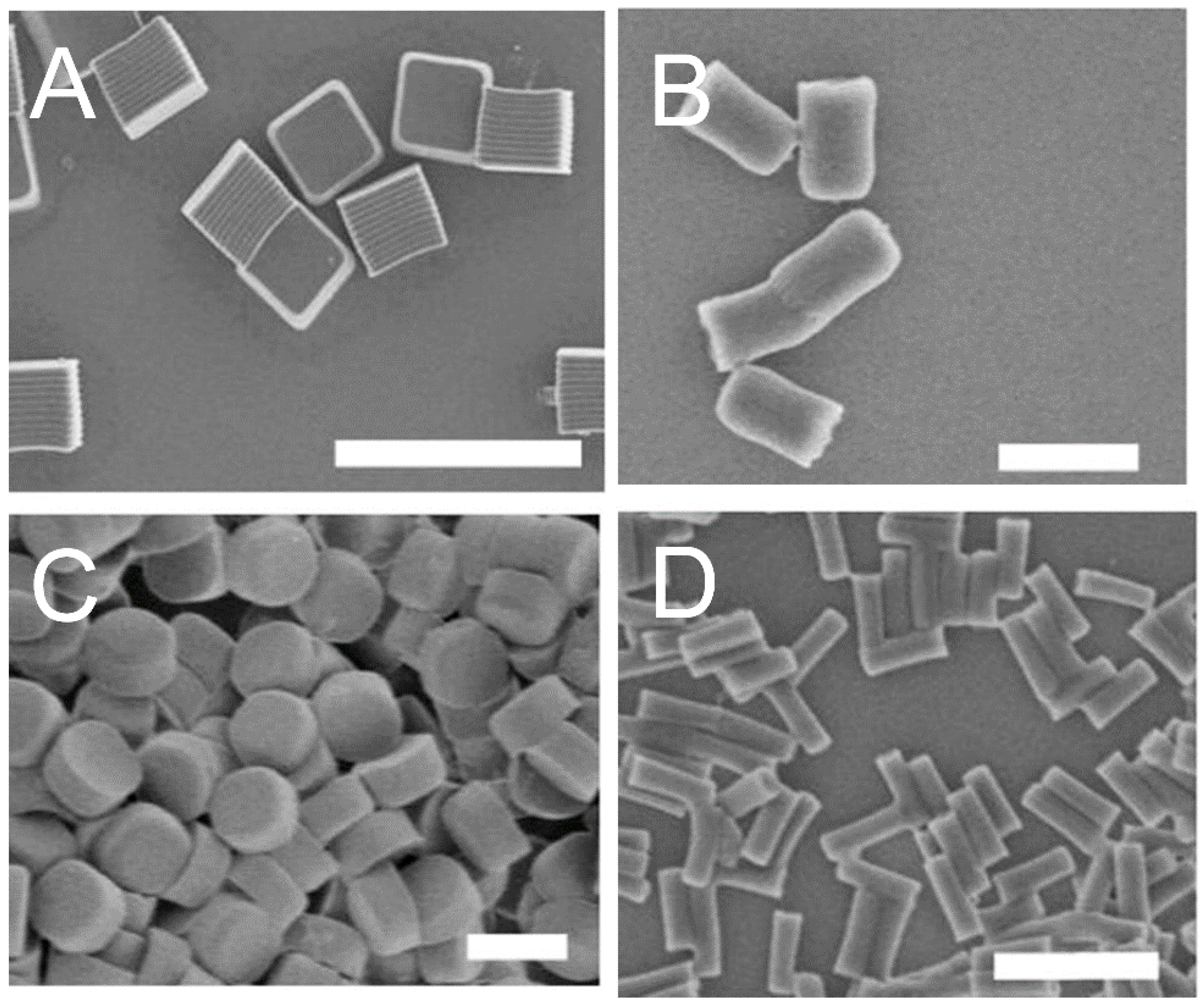
4.3. Morphology and Shape
4.4. Porosity
5. Biomedical Applications
5.1. Bone Healing
5.2. Antibiotic Release
5.3. Diabetes
5.4. Cancer Treatment
5.5. Other Potential Applications
5.5.1. Neuroinflammation
5.5.2. Wound Healing
5.5.3. Corneal Regeneration
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bae, K.H.; Lee, F.; Xu, K.; Keng, C.T.; Tan, S.Y.; Tan, Y.J.; Chen, Q.; Kurisawa, M. Microstructured dextran hydrogels for burst-free sustained release of pegylated protein drugs. Biomaterials 2015, 63, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Li, S.; Han, X.; Al-Youbi, A.O.; Bashammakh, A.S.; El-Shahawi, M.S.; Leblanc, R.M. Determination of the composition, encapsulation efficiency and loading capacity in protein drug delivery systems using circular dichroism spectroscopy. Anal. Chim. Acta 2016, 937, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Hubbell, J.A.; Chilkoti, A. Nanomaterials for drug delivery. Science 2012, 337, 303–305. [Google Scholar] [CrossRef] [PubMed]
- Garrait, G.; Beyssac, E.; Subirade, M. Development of a novel drug delivery system: Chitosan nanoparticles entrapped in alginate microparticles. J. Microencapsul. 2014, 31, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, R.; Palakurthi, S. Zein in controlled drug delivery and tissue engineering. J. Control. Release 2014, 189, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, V.; Anton, M.; Santé-Lhoutellier, V. The “sisters” α-helices of collagen, elastin and keratin recovered from animal by-products: Functionality, bioactivity and trends of application. Trends Food Sci. Technol. 2016, 51, 65–75. [Google Scholar] [CrossRef]
- Rosenholm, J.M.; Meinander, A.; Peuhu, E.; Niemi, R.; Eriksson, J.E.; Sahlgren, C.; Lindén, M. Targeting of porous hybrid silica nanoparticles to cancer cells. ACS Nano 2008, 3, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Brennan, D.A.; Jao, D.; Siracusa, M.C.; Wilkinson, A.R.; Hu, X.; Beachley, V.Z. Concurrent collection and post-drawing of individual electrospun polymer nanofibers to enhance macromolecular alignment and mechanical properties. Polymer 2016, 103, 243–250. [Google Scholar] [CrossRef]
- Van Herwaarden, S.; Iervolino, E.; Van Herwaarden, F.; Wijffels, T.; Leenaers, A.; Mathot, V. Design, performance and analysis of thermal lag of the ufs1 twin-calorimeter chip for fast scanning calorimetry using the mettler-toledo flash dsc 1. Thermochim. Acta 2011, 522, 46–52. [Google Scholar] [CrossRef]
- Lee, J.W.; Park, J.-H.; Prausnitz, M.R. Dissolving microneedles for transdermal drug delivery. Biomaterials 2008, 29, 2113–2124. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Ahn, S.-H.; Kim, G.H. Three-dimensional collagen/alginate hybrid scaffolds functionalized with a drug delivery system (dds) for bone tissue regeneration. Chem. Mater. 2011, 24, 881–891. [Google Scholar] [CrossRef]
- Cilurzo, F.; Selmin, F.; Aluigi, A.; Bellosta, S. Regenerated keratin proteins as potential biomaterial for drug delivery. Polym. Adv. Technol. 2013, 24, 1025–1028. [Google Scholar] [CrossRef]
- Srinivasan, B.; Kumar, R.; Shanmugam, K.; Sivagnam, U.T.; Reddy, N.P.; Sehgal, P.K. Porous keratin scaffold–promising biomaterial for tissue engineering and drug delivery. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 92, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J. Mesoporous bioglass/silk fibroin scaffolds as a drug delivery system: Fabrication, drug loading and release in vitro and repair calvarial defects in vivo. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2014, 29, 401–406. [Google Scholar] [CrossRef]
- Friess, W. Collagen–biomaterial for drug delivery. Eur. J. Pharm. Biopharm. 1998, 45, 113–136. [Google Scholar] [CrossRef]
- Rafat, M.; Li, F.; Fagerholm, P.; Lagali, N.S.; Watsky, M.A.; Munger, R.; Matsuura, T.; Griffith, M. Peg-stabilized carbodiimide crosslinked collagen–chitosan hydrogels for corneal tissue engineering. Biomaterials 2008, 29, 3960–3972. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, C.E.; Marelli, B.; Omenetto, F.G.; Funderburgh, J.L.; Kaplan, D.L. 3D functional corneal stromal tissue equivalent based on corneal stromal stem cells and multi-layered silk film architecture. PLoS ONE 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Song, W.; Liu, S.; Ren, L. Corneal regeneration by utilizing collagen based materials. Sci. China Chem. 2016, 59, 1548–1553. [Google Scholar] [CrossRef]
- Almine, J.F.; Bax, D.V.; Mithieux, S.M.; Nivison-Smith, L.; Rnjak, J.; Waterhouse, A.; Wise, S.G.; Weiss, A.S. Elastin-based materials. Chem. Soc. Rev. 2010, 39, 3371–3379. [Google Scholar] [CrossRef] [PubMed]
- Barenghi, R.; Beke, S.; Romano, I.; Gavazzo, P.; Farkas, B.; Vassalli, M.; Brandi, F.; Scaglione, S. Elastin-coated biodegradable photopolymer scaffolds for tissue engineering applications. BioMed Res. Int. 2014. [Google Scholar] [CrossRef] [PubMed]
- Chilkoti, A.; Christensen, T.; MacKay, J.A. Stimulus responsive elastin biopolymers: Applications in medicine and biotechnology. Curr. Opin. Chem. Biol. 2006, 10, 652–657. [Google Scholar] [CrossRef] [PubMed]
- MacEwan, S.R.; Chilkoti, A. Applications of elastin-like polypeptides in drug delivery. J. Control. Release 2014, 190, 314–330. [Google Scholar] [CrossRef] [PubMed]
- Annabi, N.; Mithieux, S.M.; Camci-Unal, G.; Dokmeci, M.R.; Weiss, A.S.; Khademhosseini, A. Elastomeric recombinant protein-based biomaterials. Biochem. Eng. J. 2013, 77, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Shmidov, Y.; Matson, J.B.; Bitton, R. Multi-scale characterization of thermoresponsive dendritic elastin-like peptides. Colloids Surf. B Biointerfaces 2017, 153, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Mobini, S.; Solati-Hashjin, M.; Peirovi, H.; Osman, N.A.A.; Gholipourmalekabadi, M.; Barati, M.; Samadikuchaksaraei, A. Bioactivity and biocompatibility studies on silk-based scaffold for bone tissue engineering. J. Med. Biol. Eng. 2013, 33, 207–214. [Google Scholar] [CrossRef]
- Vollrath, F.; Porter, D. Spider silk as archetypal protein elastomer. Soft Matter 2006, 2, 377–385. [Google Scholar] [CrossRef]
- Pritchard, E.M.; Hu, X.; Finley, V.; Kuo, C.K.; Kaplan, D.L. Effect of silk protein processing on drug delivery from silk films. Macromol. Biosci. 2013, 13, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Seib, F.P.; Kaplan, D.L. Silk for drug delivery applications: Opportunities and challenges. Isr. J. Chem. 2013, 53, 756–766. [Google Scholar] [CrossRef]
- Kaplan, D.L.; Meinel, L. Silk-Based Drug Delivery System. Available online: https://www.google.com/patents/US20130195831 (accessed on 8 May 2017).
- Teulé, F.; Miao, Y.-G.; Sohn, B.-H.; Kim, Y.-S.; Hull, J.J.; Fraser, M.J.; Lewis, R.V.; Jarvis, D.L. Silkworms transformed with chimeric silkworm/spider silk genes spin composite silk fibers with improved mechanical properties. Proc. Natl. Acad. Sci. USA 2012, 109, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.-X.; Qian, Z.-G.; Ki, C.S.; Park, Y.H.; Kaplan, D.L.; Lee, S.Y. Native-sized recombinant spider silk protein produced in metabolically engineered escherichia coli results in a strong fiber. Proc. Natl. Acad. Sci. USA 2010, 107, 14059–14063. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.; Hu, X.; Cebe, P.; Kaplan, D.L. Mechanism of resilin elasticity. Nat. Commun. 2012. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Tong, Z.; Jia, X.; Kiick, K.L. Resilin-like polypeptide hydrogels engineered for versatile biological function. Soft Matter 2013, 9, 665–673. [Google Scholar] [CrossRef] [PubMed]
- McGann, C.L.; Levenson, E.A.; Kiick, K.L. Resilin-based hybrid hydrogels for cardiovascular tissue engineering. Macromol. Chem. Phys. 2013, 214, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Sousa, F.; Luzardo-Álvarez, A.; Blanco-Méndez, J.; Martín-Pastor, M. Nmr techniques in drug delivery: Application to zein protein complexes. Int. J. Pharm. 2012, 439, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Bouman, J.; Belton, P.; Venema, P.; van der Linden, E.; de Vries, R.; Qi, S. Controlled release from zein matrices: Interplay of drug hydrophobicity and ph. Pharm. Res. 2016, 33, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Nonthanum, P.; Lee, Y.; Padua, G.W. Effect of γ-zein on the rheological behavior of concentrated zein solutions. J. Agric. Food Chem. 2012, 60, 1742–1747. [Google Scholar] [CrossRef] [PubMed]
- Ramshaw, J.A.; Werkmeister, J.A.; Glattauer, V. Collagen-based biomaterials. Biotechnol. Genet. Eng. Rev. 1996, 13, 335–382. [Google Scholar] [CrossRef] [PubMed]
- Lickorish, D.; Ramshaw, J.A.; Werkmeister, J.A.; Glattauer, V.; Howlett, C.R. Collagen–hydroxyapatite composite prepared by biomimetic process. J. Biomed. Mater. Res. Part A 2004, 68, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Sionkowska, A.; Kozłowska, J. Properties and modification of porous 3-d collagen/hydroxyapatite composites. Int. J. Biol. Macromol. 2013, 52, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Sell, S.A.; McClure, M.J.; Garg, K.; Wolfe, P.S.; Bowlin, G.L. Electrospinning of collagen/biopolymers for regenerative medicine and cardiovascular tissue engineering. Adv. Drug Deliv. Rev. 2009, 61, 1007–1019. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Macosko, C.W.; Urry, D.W. Swelling behavior of γ-irradiation cross-linked elastomeric polypentapeptide-based hydrogels. Macromolecules 2001, 34, 4114–4123. [Google Scholar] [CrossRef]
- Yan, H.-B.; Zhang, Y.-Q.; Ma, Y.-L.; Zhou, L.-X. Biosynthesis of insulin-silk fibroin nanoparticles conjugates and in vitro evaluation of a drug delivery system. J. Nanoparticle Res. 2009, 11, 1937–1946. [Google Scholar] [CrossRef]
- Srokowski, E.M.; Woodhouse, K.A. Development and characterisation of novel cross-linked bio-elastomeric materials. J. Biomater. Sci. Polym. Ed. 2008, 19, 785–799. [Google Scholar] [CrossRef] [PubMed]
- Cappello, J.; Textor, G.; Bauerle, B. Bioresorption of implanted protein polymer films controlled by adjustment of their silk/elastin block lengths. In Industrial Biotechnological Polymers; Technomic Publishing Company: Lancaster, UK, 1995; pp. 249–256. [Google Scholar]
- Nowatzki, P.J.; Tirrell, D.A. Physical properties of artificial extracellular matrix protein films prepared by isocyanate crosslinking. Biomaterials 2004, 25, 1261–1267. [Google Scholar] [CrossRef]
- Huang, L.; McMillan, R.A.; Apkarian, R.P.; Pourdeyhimi, B.; Conticello, V.P.; Chaikof, E.L. Generation of synthetic elastin-mimetic small diameter fibers and fiber networks. Macromolecules 2000, 33, 2989–2997. [Google Scholar] [CrossRef]
- Lee, J.; Macosko, C.W.; Urry, D.W. Elastomeric polypentapeptides cross-linked into matrixes and fibers. Biomacromolecules 2001, 2, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, A.; Kaneko, S.; Tanabe, T.; Yamauchi, K. Rapid fabrication of keratin–hydroxyapatite hybrid sponges toward osteoblast cultivation and differentiation. Biomaterials 2005, 26, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Sierpinski, P.; Garrett, J.; Ma, J.; Apel, P.; Klorig, D.; Smith, T.; Koman, L.A.; Atala, A.; Van Dyke, M. The use of keratin biomaterials derived from human hair for the promotion of rapid regeneration of peripheral nerves. Biomaterials 2008, 29, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Apel, P.J.; Garrett, J.P.; Sierpinski, P.; Ma, J.; Atala, A.; Smith, T.L.; Koman, L.A.; Van Dyke, M.E. Peripheral nerve regeneration using a keratin-based scaffold: Long-term functional and histological outcomes in a mouse model. J. Hand Surg. 2008, 33, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
- Aboushwareb, T.; Eberli, D.; Ward, C.; Broda, C.; Holcomb, J.; Atala, A.; Van Dyke, M. A keratin biomaterial gel hemostat derived from human hair: Evaluation in a rabbit model of lethal liver injury. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 90, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, T.; Okitsu, N.; Tachibana, A.; Yamauchi, K. Preparation and characterization of keratin–chitosan composite film. Biomaterials 2002, 23, 817–825. [Google Scholar] [CrossRef]
- Zoccola, M.; Aluigi, A.; Vineis, C.; Tonin, C.; Ferrero, F.; Piacentino, M.G. Study on cast membranes and electrospun nanofibers made from keratin/fibroin blends. Biomacromolecules 2008, 9, 2819–2825. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Teller, S.; Clifton, R.J.; Jia, X.; Kiick, K.L. Tunable mechanical stability and deformation response of a resilin-based elastomer. Biomacromolecules 2011, 12, 2302. [Google Scholar] [CrossRef] [PubMed]
- Renner, J.N.; Cherry, K.M.; Su, R.S.-C.; Liu, J.C. Characterization of resilin-based materials for tissue engineering applications. Biomacromolecules 2012, 13, 3678–3685. [Google Scholar] [CrossRef] [PubMed]
- Straley, K.S.; Heilshorn, S.C. Independent tuning of multiple biomaterial properties using protein engineering. Soft Matter 2009, 5, 114–124. [Google Scholar] [CrossRef]
- Koria, P.; Yagi, H.; Kitagawa, Y.; Megeed, Z.; Nahmias, Y.; Sheridan, R.; Yarmush, M.L. Self-assembling elastin-like peptides growth factor chimeric nanoparticles for the treatment of chronic wounds. Proc. Natl. Acad. Sci. USA 2011, 108, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- Gellynck, K.; Verdonk, P.C.; Van Nimmen, E.; Almqvist, K.F.; Gheysens, T.; Schoukens, G.; Van Langenhove, L.; Kiekens, P.; Mertens, J.; Verbruggen, G. Silkworm and spider silk scaffolds for chondrocyte support. J. Mater. Sci. Mater. Med. 2008, 19, 3399–3409. [Google Scholar] [CrossRef] [PubMed]
- Huemmerich, D.; Slotta, U.; Scheibel, T. Processing and modification of films made from recombinant spider silk proteins. Appl. Phys. A Mater. Sci. Proc. 2006, 82, 219–222. [Google Scholar] [CrossRef]
- Hermanson, K.D.; Huemmerich, D.; Scheibel, T.; Bausch, A.R. Engineered microcapsules fabricated from reconstituted spider silk. Adv. Mater. 2007, 19, 1810–1815. [Google Scholar] [CrossRef]
- Lammel, A.; Schwab, M.; Hofer, M.; Winter, G.; Scheibel, T. Recombinant spider silk particles as drug delivery vehicles. Biomaterials 2011, 32, 2233–2240. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.-M.; Padua, G.W. Properties and microstructure of plasticized zein films. Cereal Chem. 1997, 74, 771–775. [Google Scholar] [CrossRef]
- Liu, X.; Sun, Q.; Wang, H.; Zhang, L.; Wang, J.-Y. Microspheres of corn protein, zein, for an ivermectin drug delivery system. Biomaterials 2005, 26, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Li, Y.; Wu, F. Zein nanofibrous membranes as templates for biomineralization of hydroxyapatite crystallites. Polym. Compos. 2013, 34, 1163–1171. [Google Scholar] [CrossRef]
- Li, Y.; Yao, C. Mineralization of hydroxyapatite crystallites on zein microspheres. Polym. Compos. 2012, 33, 961–966. [Google Scholar] [CrossRef]
- Wang, X.; Yucel, T.; Lu, Q.; Hu, X.; Kaplan, D.L. Silk nanospheres and microspheres from silk/pva blend films for drug delivery. Biomaterials 2010, 31, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- Gil, C.S.; Gil, V.S.; Carvalho, S.M.; Silva, G.R.; Magalhães, J.T.; Oréfice, R.L.; Mansur, A.; Mansur, H.S.; Patricio, P.S.; Oliveira, L.C. Recycled collagen films as biomaterials for controlled drug delivery. New J. Chem. 2016, 40, 8502–8510. [Google Scholar] [CrossRef]
- Sahiner, M.; Alpaslan, D.; Bitlisli, B.O. Collagen-based hydrogel films as drug-delivery devices with antimicrobial properties. Polym. Bull. 2014, 71, 3017–3033. [Google Scholar] [CrossRef]
- Mwangi, T.K.; Bowles, R.D.; Tainter, D.M.; Bell, R.D.; Kaplan, D.L.; Setton, L.A. Synthesis and characterization of silk fibroin microparticles for intra-articular drug delivery. Int. J. Pharm. 2015, 485, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Li, Y.; Xie, M.-B. Silk fibroin-based nanoparticles for drug delivery. Int. J. Mol. Sci. 2015, 16, 4880–4903. [Google Scholar] [CrossRef] [PubMed]
- Ciofani, G.; Genchi, G.G.; Guardia, P.; Mazzolai, B.; Mattoli, V.; Bandiera, A. Recombinant human elastin-like magnetic microparticles for drug delivery and targeting. Macromol. Biosci. 2014, 14, 632–642. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Hori, A.; Sugaya, S.; Yajima, Y.; Utoh, R.; Yamato, M.; Seki, M. Cell-sized condensed collagen microparticles for preparing microengineered composite spheroids of primary hepatocytes. Lab Chip 2015, 15, 3941–3951. [Google Scholar] [CrossRef] [PubMed]
- Joscelyne, S.M.; Trägårdh, G. Membrane emulsification—A literature review. J. Membr. Sci. 2000, 169, 107–117. [Google Scholar] [CrossRef]
- Numata, K.; Yamazaki, S.; Naga, N. Biocompatible and biodegradable dual-drug release system based on silk hydrogel containing silk nanoparticles. Biomacromolecules 2012, 13, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Ita, K. Transdermal delivery of drugs with microneedles—Potential and challenges. Pharmaceutics 2015, 7, 90–105. [Google Scholar] [CrossRef] [PubMed]
- Sivamani, R.K.; Stoeber, B.; Wu, G.C.; Zhai, H.; Liepmann, D.; Maibach, H. Clinical microneedle injection of methyl nicotinate: Stratum corneum penetration. Skin Res. Technol. 2005, 11, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Tsioris, K.; Raja, W.K.; Pritchard, E.M.; Panilaitis, B.; Kaplan, D.L.; Omenetto, F.G. Fabrication of silk microneedles for controlled-release drug delivery. Adv. Funct. Mater. 2012, 22, 330–335. [Google Scholar] [CrossRef]
- Singaravelu, S.; Ramanathan, G.; Raja, M.; Nagiah, N.; Padmapriya, P.; Kaveri, K.; Sivagnanam, U.T. Biomimetic interconnected porous keratin–fibrin–gelatin 3d sponge for tissue engineering application. Int. J. Biol. Macromol. 2016, 86, 810–819. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhu, L.; Liu, R.; Huang, D.; Jin, X.; Che, N.; Li, Z.; Qu, X.; Kang, H.; Huang, Y. Biological stimuli responsive drug carriers based on keratin for triggerable drug delivery. J. Mater. Chem. 2012, 22, 19964–19973. [Google Scholar] [CrossRef]
- Han, S.; Ham, T.R.; Haque, S.; Sparks, J.L.; Saul, J.M. Alkylation of human hair keratin for tunable hydrogel erosion and drug delivery in tissue engineering applications. Acta Biomater. 2015, 23, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Youn, P.; Furgeson, D. Thermo-targeted drug delivery of geldanamycin to hyperthermic tumor margins with diblock elastin-based biopolymers. J. Control. Release 2011, 155, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Dash, B.C.; Mahor, S.; Carroll, O.; Mathew, A.; Wang, W.; Woodhouse, K.A.; Pandit, A. Tunable elastin-like polypeptide hollow sphere as a high payload and controlled delivery gene depot. J. Control. Release 2011, 152, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Lee, D.-W.; Park, K.; Park, S.-J.; Choi, E.-J.; Park, E.S.; Kim, H.R. Temperature-triggered tumor-specific delivery of anticancer agents by crgd-conjugated thermosensitive liposomes. Colloids Surf. B: Biointerfaces 2014, 116, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Barbaresso, R.C.; Rău, I.; Zgârian, R.G.; Meghea, A.; Ghica, M.V. Niflumic acid-collagen delivery systems used as anti-inflammatory drugs and analgesics in dentistry. C. R. Chim. 2014, 17, 12–17. [Google Scholar] [CrossRef]
- Wu, H.; Liu, S.; Xiao, L.; Dong, X.; Lu, Q.; Kaplan, D.L. Injectable and ph-responsive silk nanofiber hydrogels for sustained anticancer drug delivery. ACS Appl. Mater. Interfaces 2016, 8, 17118–17126. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, E.M.; Kaplan, D.L. Silk fibroin biomaterials for controlled release drug delivery. Expert Opin. Drug Deliv. 2011, 8, 797–811. [Google Scholar] [CrossRef] [PubMed]
- Wilz, A.; Pritchard, E.M.; Li, T.; Lan, J.-Q.; Kaplan, D.L.; Boison, D. Silk polymer-based adenosine release: Therapeutic potential for epilepsy. Biomaterials 2008, 29, 3609–3616. [Google Scholar] [CrossRef] [PubMed]
- Pandit, M.; Sagar, A.J.; Rao, M.N. Studies on silk fibroin. I. Molecular weight, sedimentation coefficient, viscosity and optical rotation of silk fibroin from carbonate-extracted silk fiber. Archiv. Biochem. Biophys. 1972, 149, 259–268. [Google Scholar] [CrossRef]
- Yamada, H.; Nakao, H.; Takasu, Y.; Tsubouchi, K. Preparation of undegraded native molecular fibroin solution from silkworm cocoons. Mater. Sci. Eng. C 2001, 14, 41–46. [Google Scholar] [CrossRef]
- Fang, J.-Y.; Chen, J.-P.; Leu, Y.-L.; Wang, H.-Y. Characterization and evaluation of silk protein hydrogels for drug delivery. Chem. Pharm. Bull. 2006, 54, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Li, L.; Chen, D. Mesoporous silica nanoparticles: Synthesis, biocompatibility and drug delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef] [PubMed]
- Brannon-Peppas, L.; Blanchette, J.O. Nanoparticle and targeted systems for cancer therapy. Adv. Drug Deliv. Rev. 2004, 56, 1649–1659. [Google Scholar] [CrossRef] [PubMed]
- Widder, K.J.; Senyei, A.E.; Scarpelli, D.G. Magnetic microspheres: A model system for site specific drug delivery in vivo. Exp. Biol. Med. 1978, 158, 141–146. [Google Scholar] [CrossRef]
- Alexiou, C.; Arnold, W.; Klein, R.J.; Parak, F.G.; Hulin, P.; Bergemann, C.; Erhardt, W.; Wagenpfeil, S.; Luebbe, A.S. Locoregional cancer treatment with magnetic drug targeting. Cancer Res. 2000, 60, 6641–6648. [Google Scholar] [PubMed]
- Mody, V.V.; Cox, A.; Shah, S.; Singh, A.; Bevins, W.; Parihar, H. Magnetic nanoparticle drug delivery systems for targeting tumor. Appl. Nanosci. 2014, 4, 385–392. [Google Scholar] [CrossRef]
- Lu, F.; Wu, S.H.; Hung, Y.; Mou, C.Y. Size effect on cell uptake in well-suspended, uniform mesoporous silica nanoparticles. Small 2009, 5, 1408–1413. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Saravanakumar, G.; Kim, K.; Kwon, I.C. Targeted delivery of low molecular drugs using chitosan and its derivatives. Adv. Drug Deliv. Rev. 2010, 62, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Jun, Y.-W.; Choi, J.-S.; Cheon, J. Heterostructured magnetic nanoparticles: Their versatility and high performance capabilities. Chem. Commun. 2007, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Foo, C.W.P.; Patwardhan, S.V.; Belton, D.J.; Kitchel, B.; Anastasiades, D.; Huang, J.; Naik, R.R.; Perry, C.C.; Kaplan, D.L. Novel nanocomposites from spider silk–silica fusion (chimeric) proteins. Proc. Natl. Acad. Sci. USA 2006, 103, 9428–9433. [Google Scholar]
- Mieszawska, A.J.; Fourligas, N.; Georgakoudi, I.; Ouhib, N.M.; Belton, D.J.; Perry, C.C.; Kaplan, D.L. Osteoinductive silk–silica composite biomaterials for bone regeneration. Biomaterials 2010, 31, 8902–8910. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Luo, Z.-S.; Pang, W.-Q.; Fan, Y.-W.; Chen, X.-H.; Cui, F.-Z. Dilute solution routes to various controllable morphologies of mcm-41 silica with a basic medium. Chem. Mater. 2001, 13, 258–263. [Google Scholar] [CrossRef]
- Gratton, S.E.; Ropp, P.A.; Pohlhaus, P.D.; Luft, J.C.; Madden, V.J.; Napier, M.E.; DeSimone, J.M. The effect of particle design on cellular internalization pathways. Proc. Natl. Acad. Sci. USA 2008, 105, 11613–11618. [Google Scholar] [CrossRef] [PubMed]
- Chun, A.L. Nanoparticles: Shape also matters. Nat. Nanotechnol. 2008. [Google Scholar] [CrossRef]
- Almería, B.; Deng, W.; Fahmy, T.M.; Gomez, A. Controlling the morphology of electrospray-generated plga microparticles for drug delivery. J. Colloid Interface Sci. 2010, 343, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Champion, J.A.; Katare, Y.K.; Mitragotri, S. Particle shape: A new design parameter for micro- and nanoscale drug delivery carriers. J. Control. Release 2007, 121, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Itokazu, M.; Yang, W.; Aoki, T.; Ohara, A.; Kato, N. Synthesis of antibiotic-loaded interporous hydroxyapatite blocks by vacuum method and in vitro drug release testing. Biomaterials 1998, 19, 817–819. [Google Scholar] [CrossRef]
- Kundu, B.; Soundrapandian, C.; Nandi, S.K.; Mukherjee, P.; Dandapat, N.; Roy, S.; Datta, B.K.; Mandal, T.K.; Basu, D.; Bhattacharya, R.N. Development of new localized drug delivery system based on ceftriaxone-sulbactam composite drug impregnated porous hydroxyapatite: A systematic approach for in vitro and in vivo animal trial. Pharm. Res. 2010, 27, 1659–1676. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, P.; Serre, C.; Vallet-Regí, M.; Sebban, M.; Taulelle, F.; Férey, G. Metal–organic frameworks as efficient materials for drug delivery. Angew. Chem. 2006, 118, 6120–6124. [Google Scholar] [CrossRef]
- Athanasou, N.A. Pathology of bone injury. Diagn. Histopathol. 2009, 15, 437–443. [Google Scholar] [CrossRef]
- Zugravu, M.V.; Smith, R.A.; Reves, B.T.; Jennings, J.A.; Cooper, J.O.; Haggard, W.O.; Bumgardner, J.D. Physical properties and in vitro evaluation of collagen–chitosan–calcium phosphate microparticle-based scaffolds for bone tissue regeneration. J. Biomater. Appl. 2012. [Google Scholar] [CrossRef] [PubMed]
- Wenk, E.; Merkle, H.P.; Meinel, L. Silk fibroin as a vehicle for drug delivery applications. J. Control. Release 2011, 150, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Bano, S.; Ghosh, A.S.; Mandal, M.; Kim, H.-W.; Dey, T.; Kundu, S.C. Silk fibroin nanoparticles support in vitro sustained antibiotic release and osteogenesis on titanium surface. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1193–1204. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Thouas, G.A. Metallic implant biomaterials. Mater. Sci. Eng. R: Rep. 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Donath, M.Y.; Shoelson, S.E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011, 11, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.P.; Simionescu, D.T.; Warner, H.; Wang, B.; Patnaik, S.S.; Liao, J.; Simionescu, A. Mitigation of diabetes-related complications in implanted collagen and elastin scaffolds using matrix-binding polyphenol. Biomaterials 2013, 34, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Alam, A.M.; Shubhra, Q.T. Surface modified thin film from silk and gelatin for sustained drug release to heal wound. J. Mater. Chem. B 2015, 3, 6473–6479. [Google Scholar] [CrossRef]
- Salehi, S.; Fathi, M.; Javanmard, S.H.; Bahners, T.; Gutmann, J.S.; Ergün, S.; Steuhl, K.P.; Fuchsluger, T.A. Generation of pgs/pcl blend nanofibrous scaffolds mimicking corneal stroma structure. Macromol. Mater. Eng. 2014, 299, 455–469. [Google Scholar] [CrossRef]
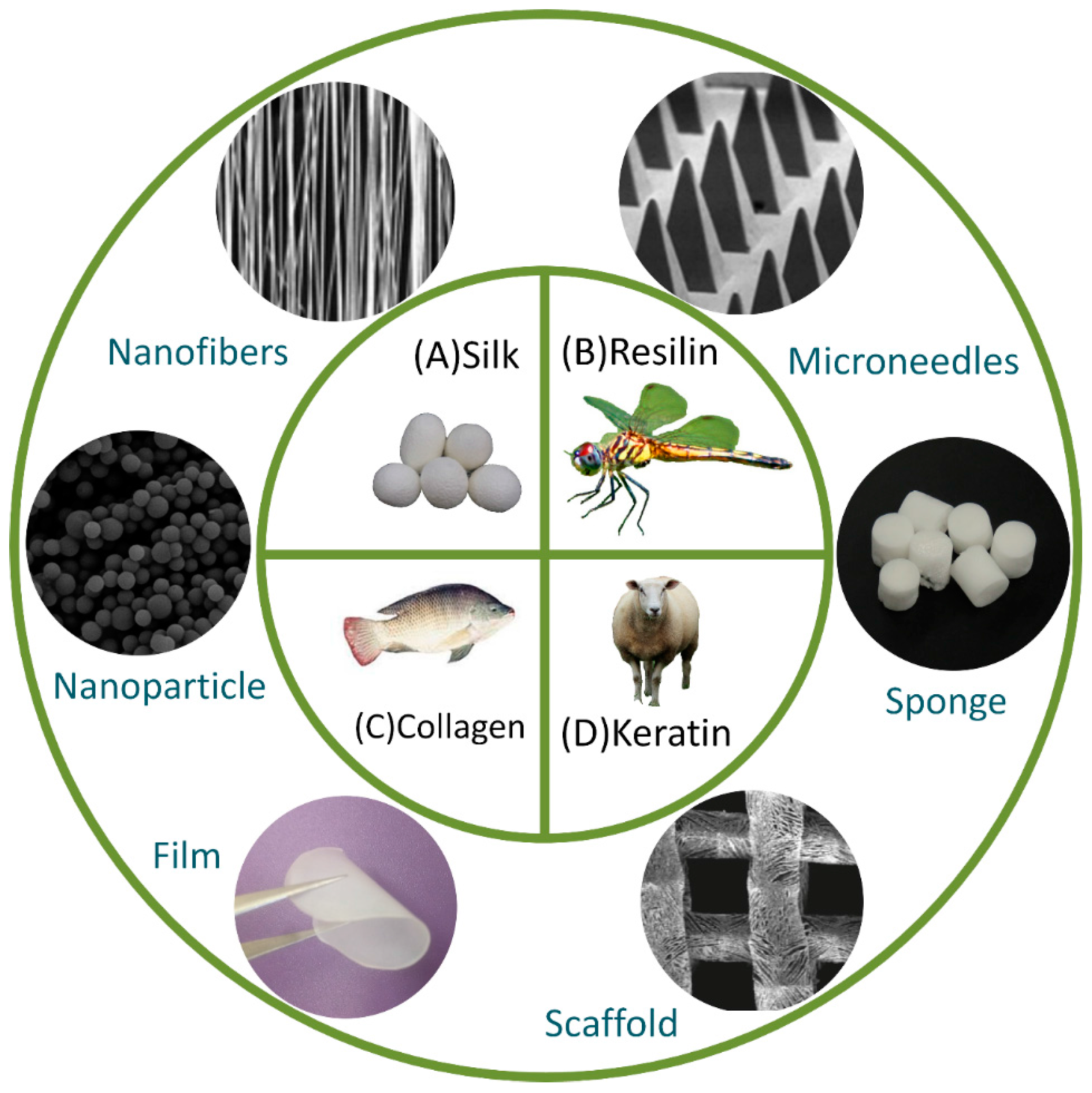
| Material | Applications | Structural Design |
|---|---|---|
| Collagen | Engineering of cartilage, corneal, nerve, ocular, skin, and tendon/ligament tissues, surgical conduits, wound repair, integrated in a variety of composite materials to enhance favorable drug-delivery properties | Hydrogels [15,38,39,40], Films [17], Fibers [41] |
| Elastin | Controlled drug delivery, engineering of cartilage, liver, ocular, and vascular graft tissue, highly tunable thermoresponsive intracellular functionalized peptide drugs, wound healing applications | Hydrogels [42,43,44], Films [45,46], Fibers [47,48] |
| Keratin | Antibacterial, drug delivery, tissue engineering, trauma and medical devices, wound healing | Hydrogels [49,50,51,52], Films, [53], Fibers [54] |
| Resilin | Engineering of native vocal fold, cardiovascular, human cartilage tissues, protein-engineered bioactive materials to promote cell adhesion, degradation, growth factor delivery, and cell differentiation | Hydrogels [34,55,56,57], Nanoparticles [58] |
| Silk | Adhesive fillers, engineering of cartilage or load bearing tissues, wound dressing, enzyme immobilization, drug delivery | Hydrogels [59], Films [60], Microcapsules [61], Microparticles [62] |
| Zein | Biomineralization, controlled drug release, enhanced mechanical strength, microbial resistance, positive cell attachment and osteoblast growth | Films [63], Microspheres [64], Nanofibers [65], Nanoparticles [66] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jao, D.; Xue, Y.; Medina, J.; Hu, X. Protein-Based Drug-Delivery Materials. Materials 2017, 10, 517. https://doi.org/10.3390/ma10050517
Jao D, Xue Y, Medina J, Hu X. Protein-Based Drug-Delivery Materials. Materials. 2017; 10(5):517. https://doi.org/10.3390/ma10050517
Chicago/Turabian StyleJao, Dave, Ye Xue, Jethro Medina, and Xiao Hu. 2017. "Protein-Based Drug-Delivery Materials" Materials 10, no. 5: 517. https://doi.org/10.3390/ma10050517
APA StyleJao, D., Xue, Y., Medina, J., & Hu, X. (2017). Protein-Based Drug-Delivery Materials. Materials, 10(5), 517. https://doi.org/10.3390/ma10050517








