The Effect of Surfactant Content over Cu-Ni Coatings Electroplated by the sc-CO2 Technique
Abstract
:1. Introduction
2. Experimental
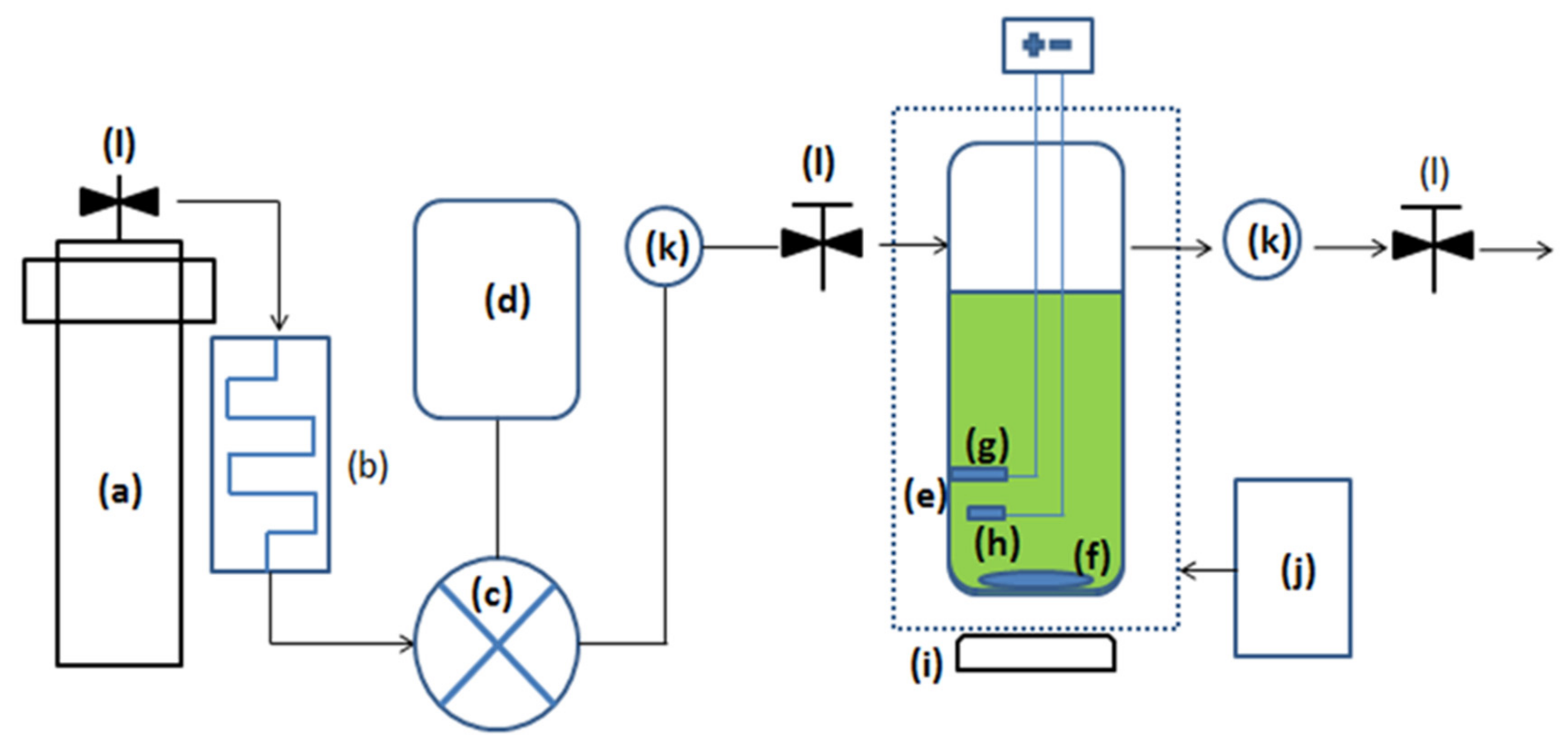
2.1. Materials and System Configuration
2.2. Sample Preparation and Experimental Method
2.3. Current Efficiency Analyses
2.4. Microstructural Analyses
2.5. Internal Stress Analyses
2.6. Electrochemical Analyses
3. Results and Discussion
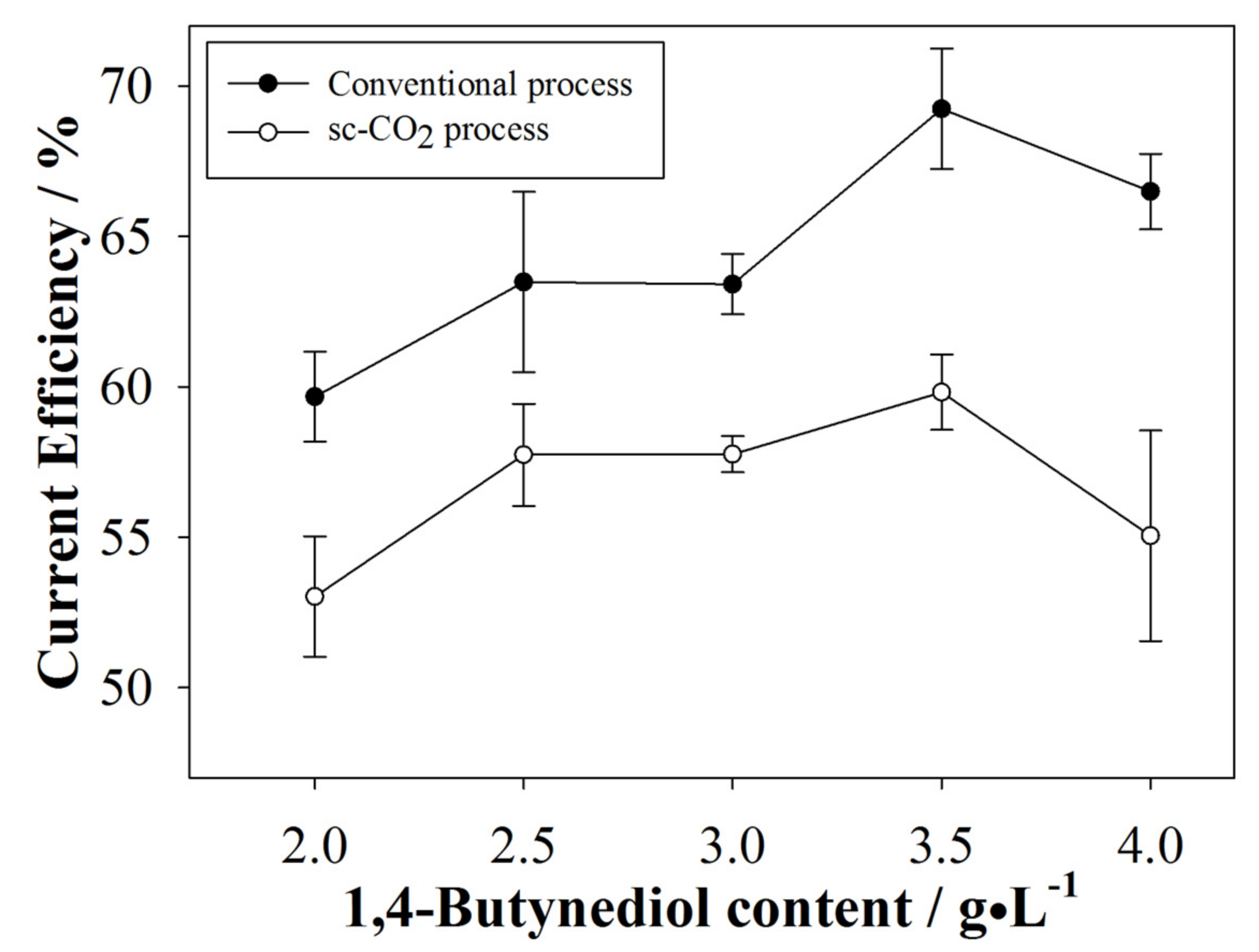
3.1. Current Efficiency (CE) Analyses
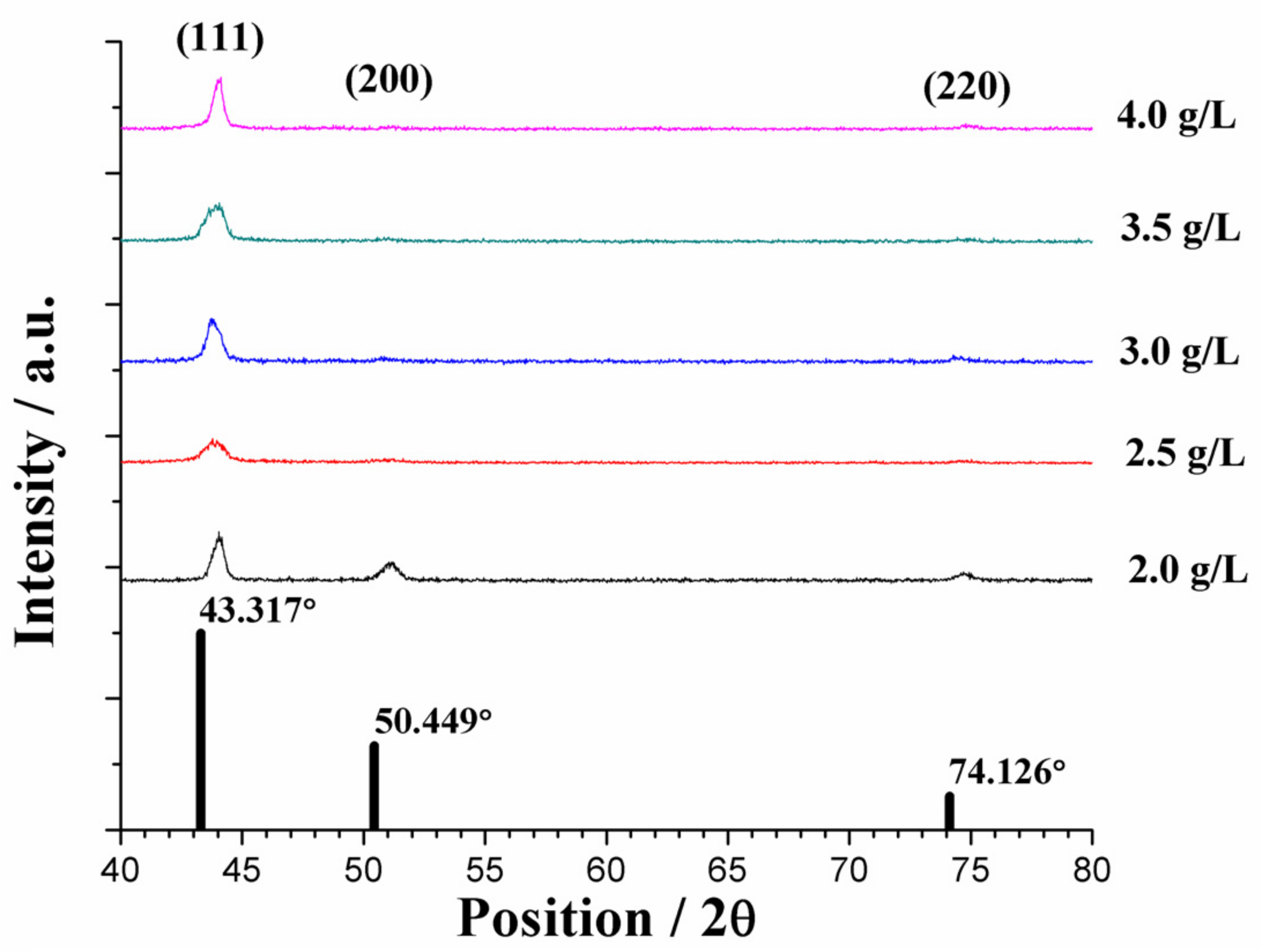
3.2. Microstructural Analyses
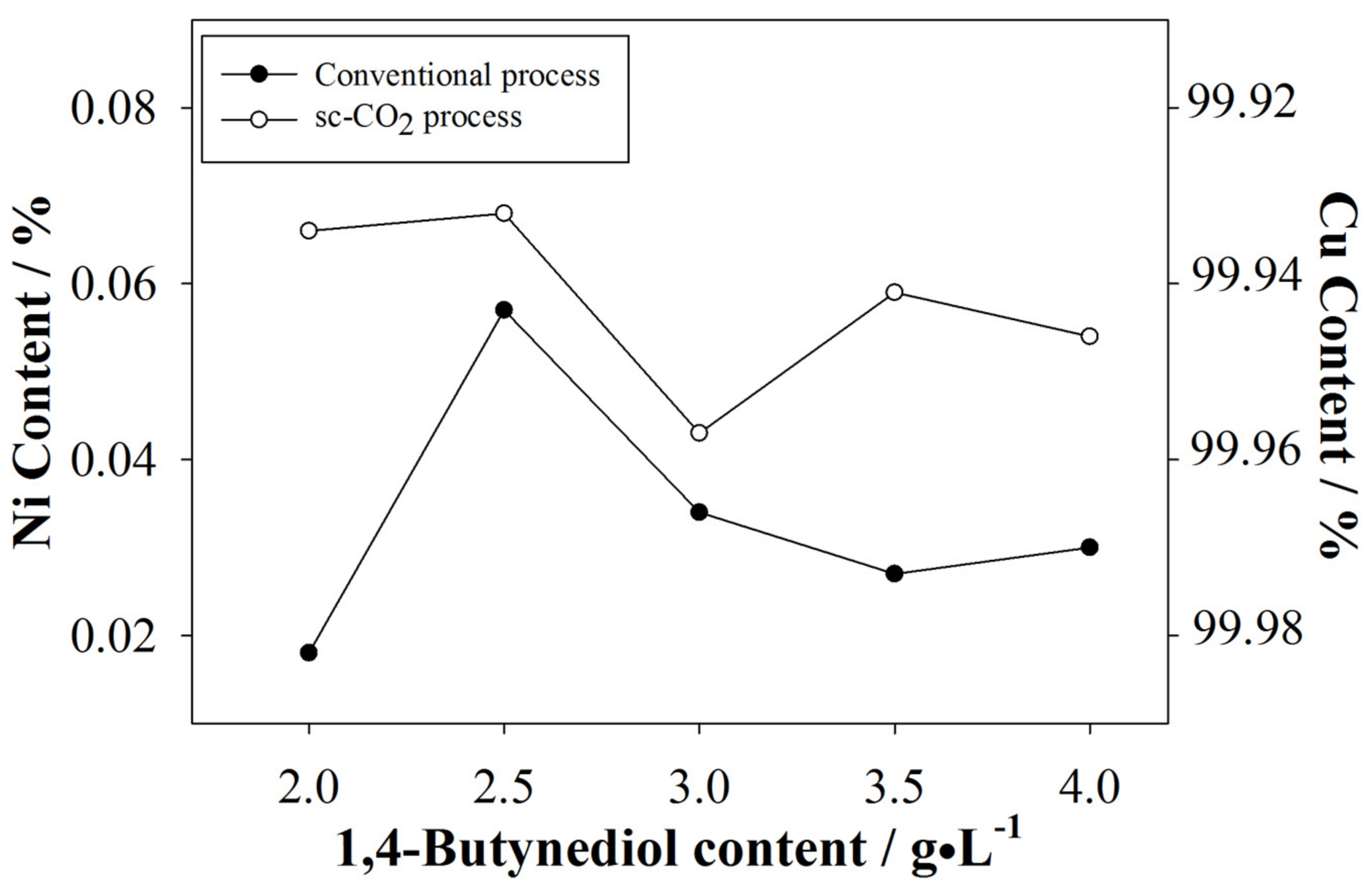
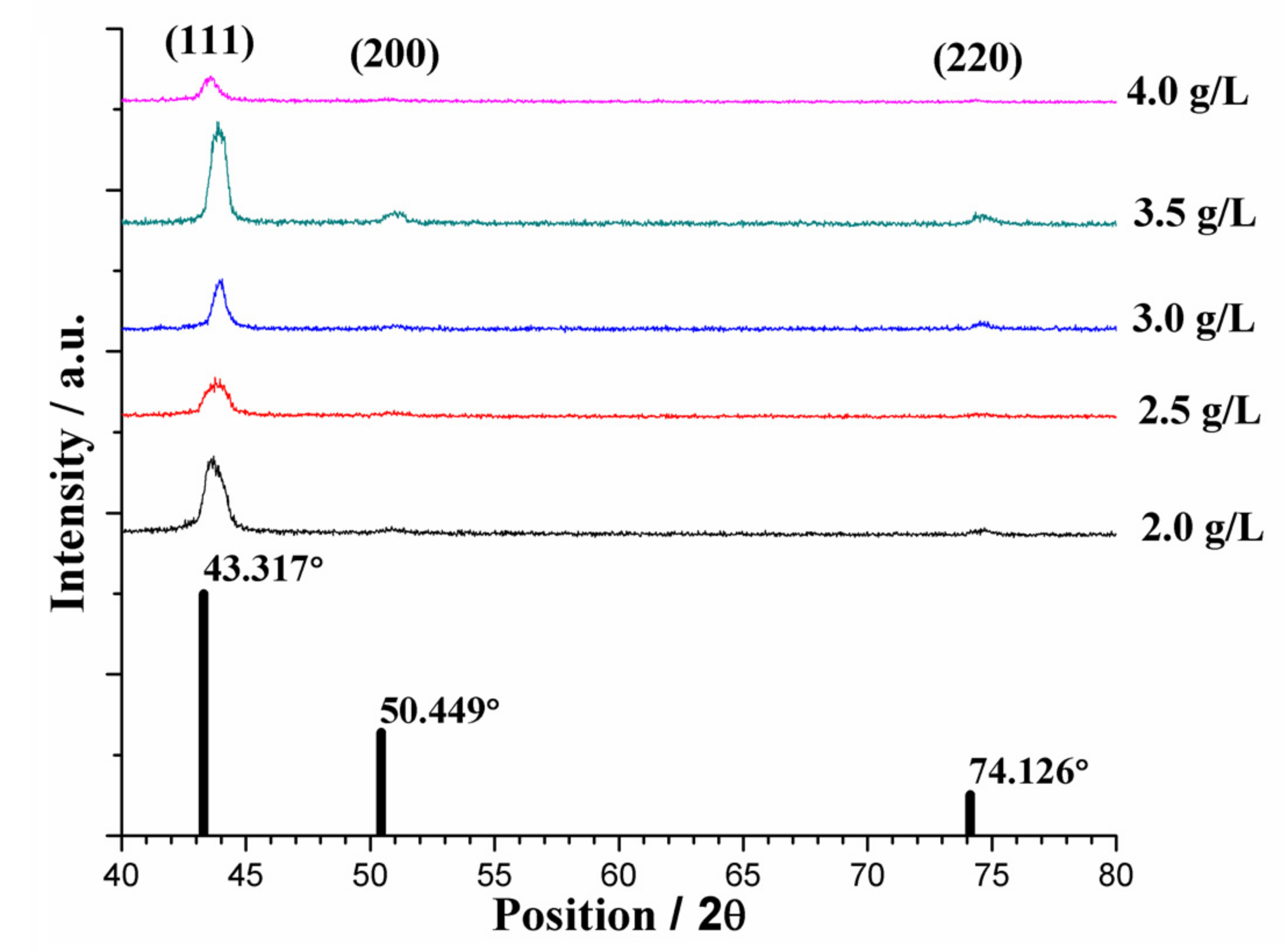
3.2.1. Nickel Content of the Plated Coatings
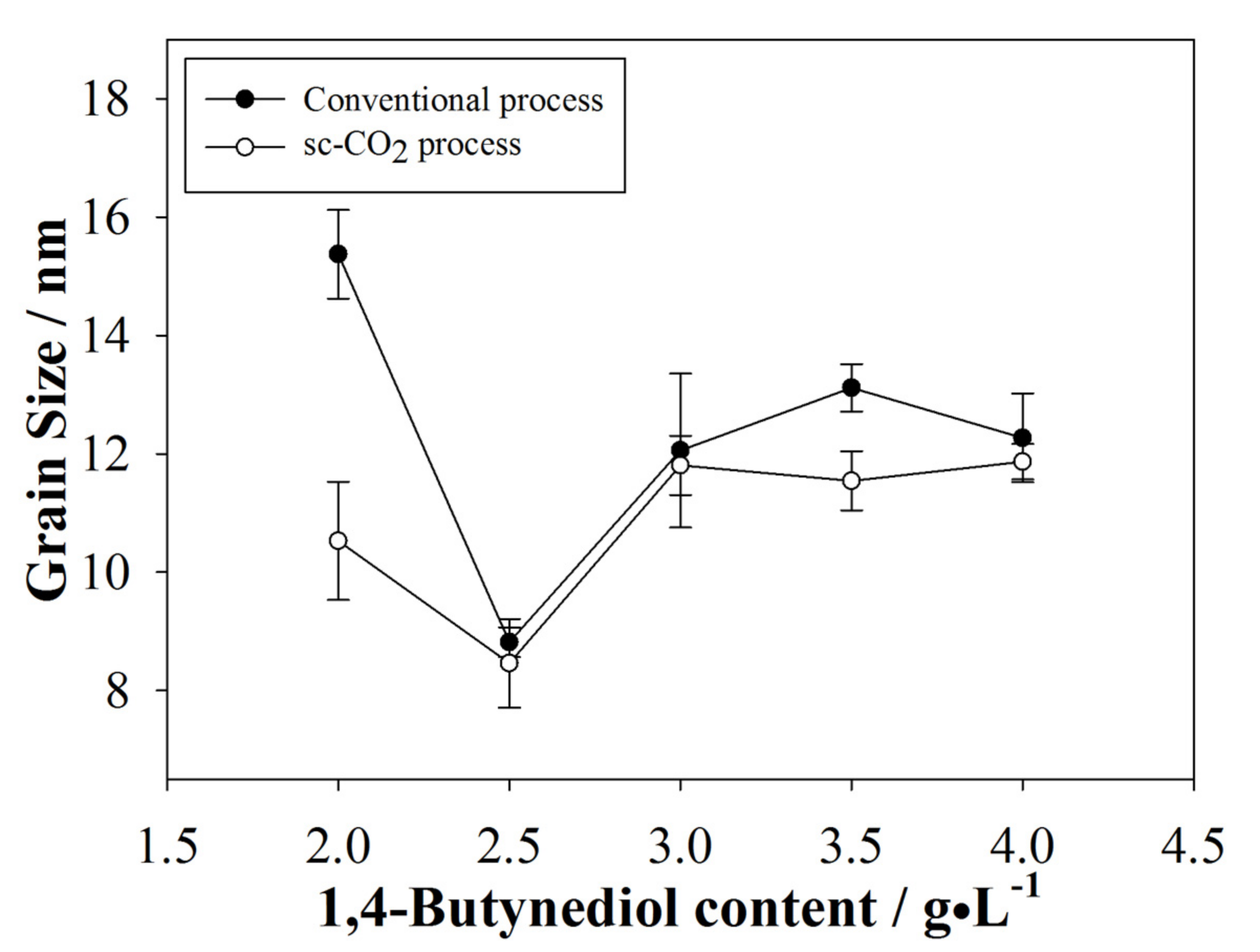
3.2.2. Average Grain Size of the Plated Coatings
3.2.3. Surface Morphology of the Plated Coatings
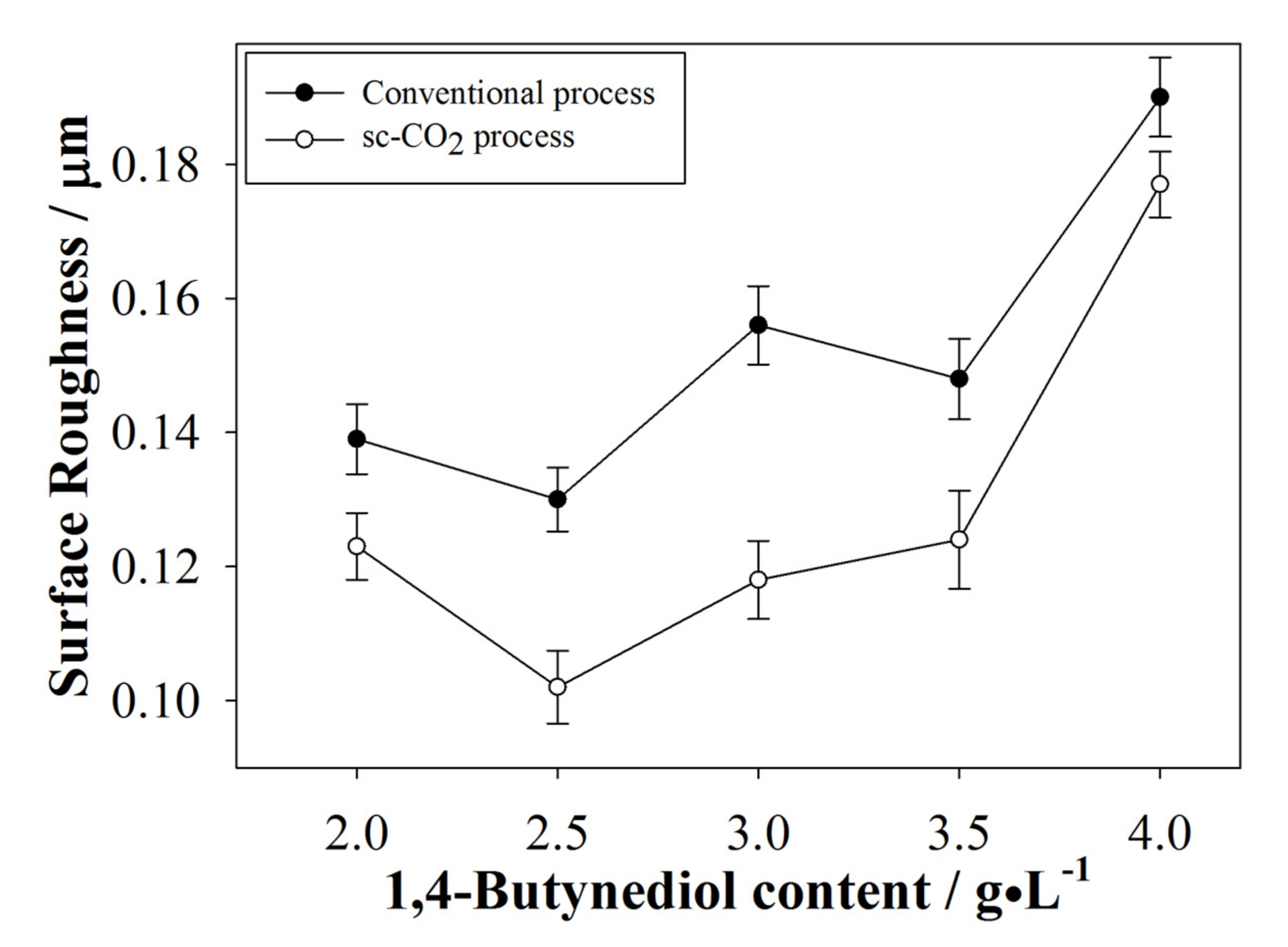
3.2.4. Surface Roughness of the Plated Coatings
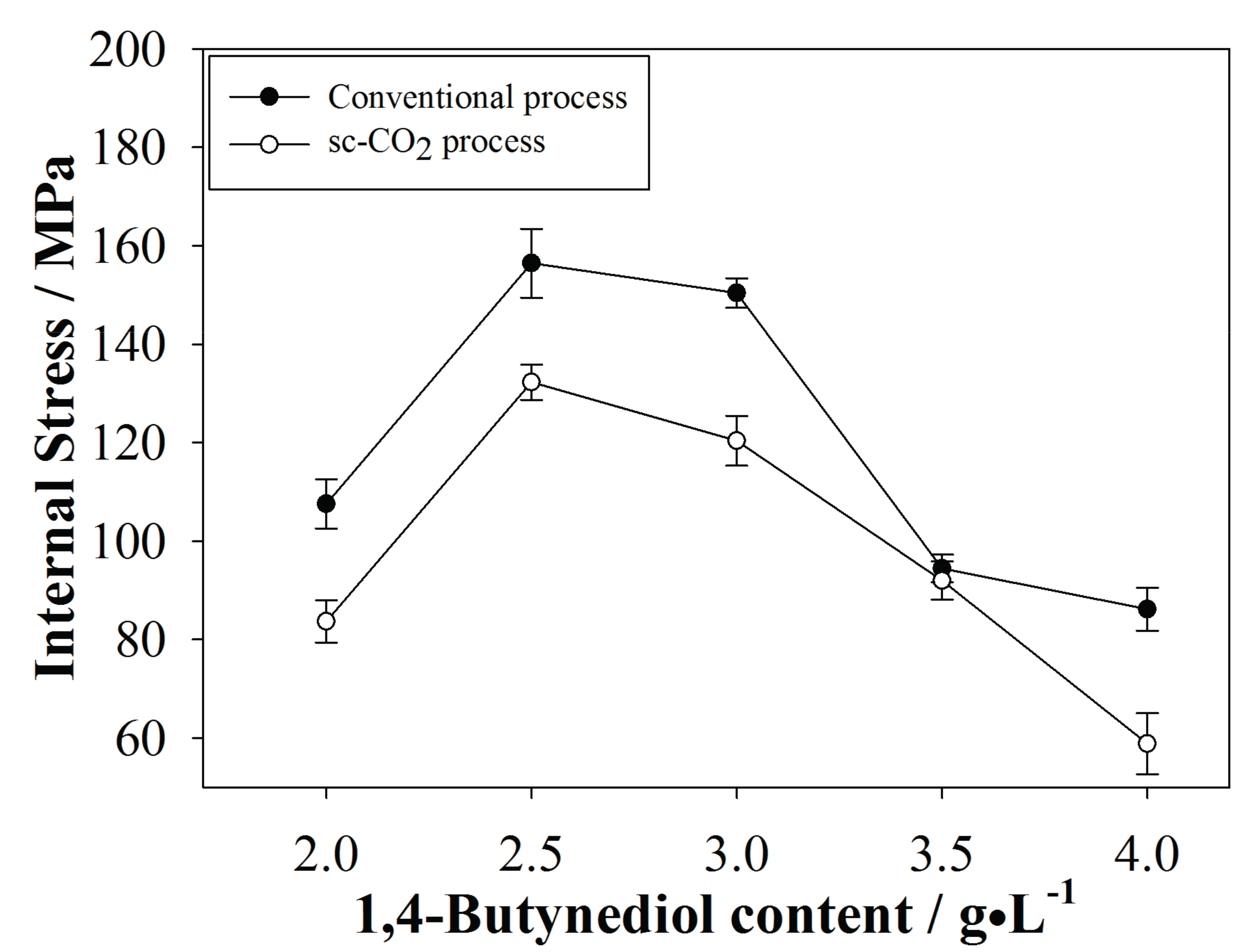
3.3. Internal Stress of the Plated Coatings
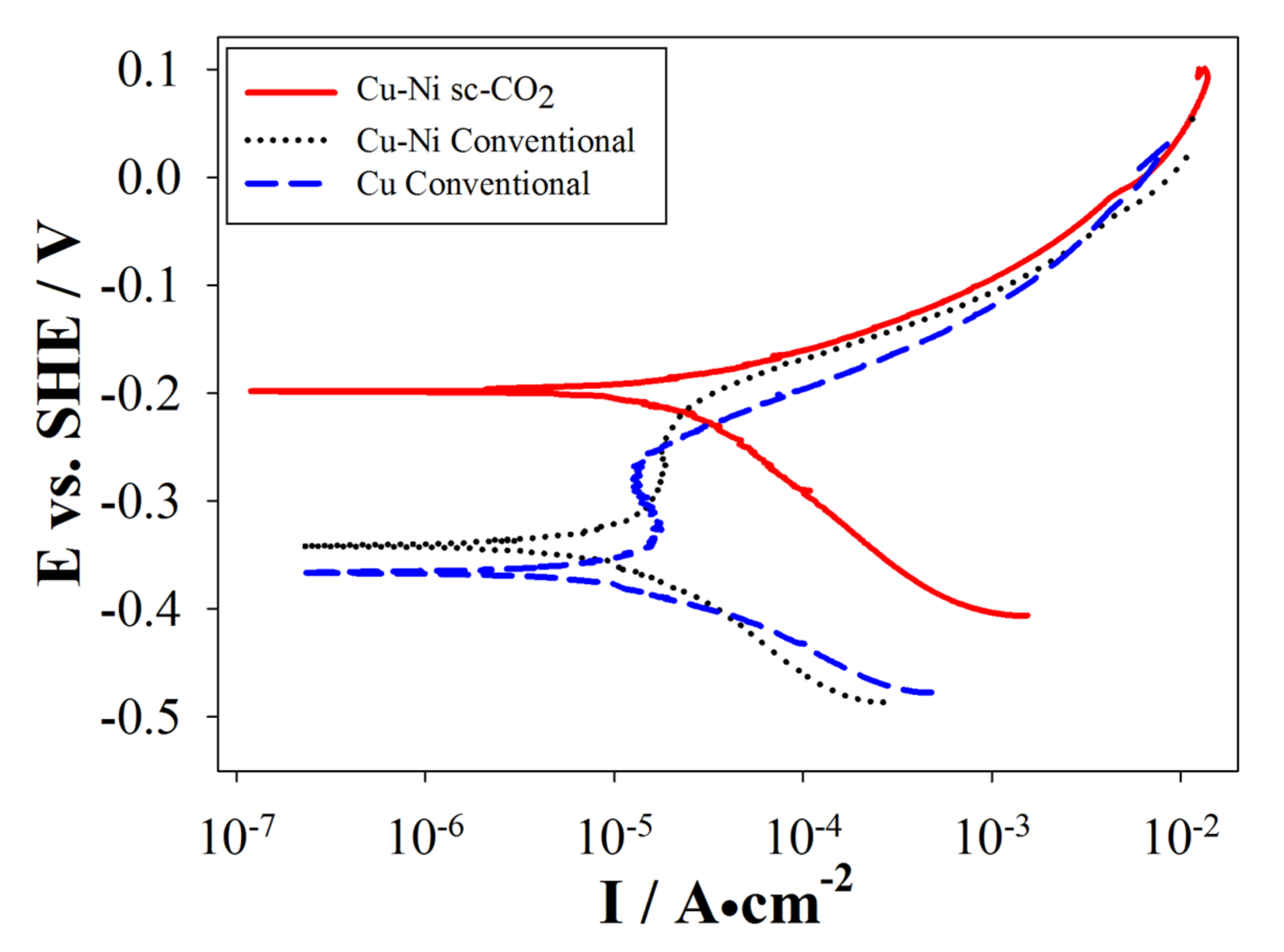
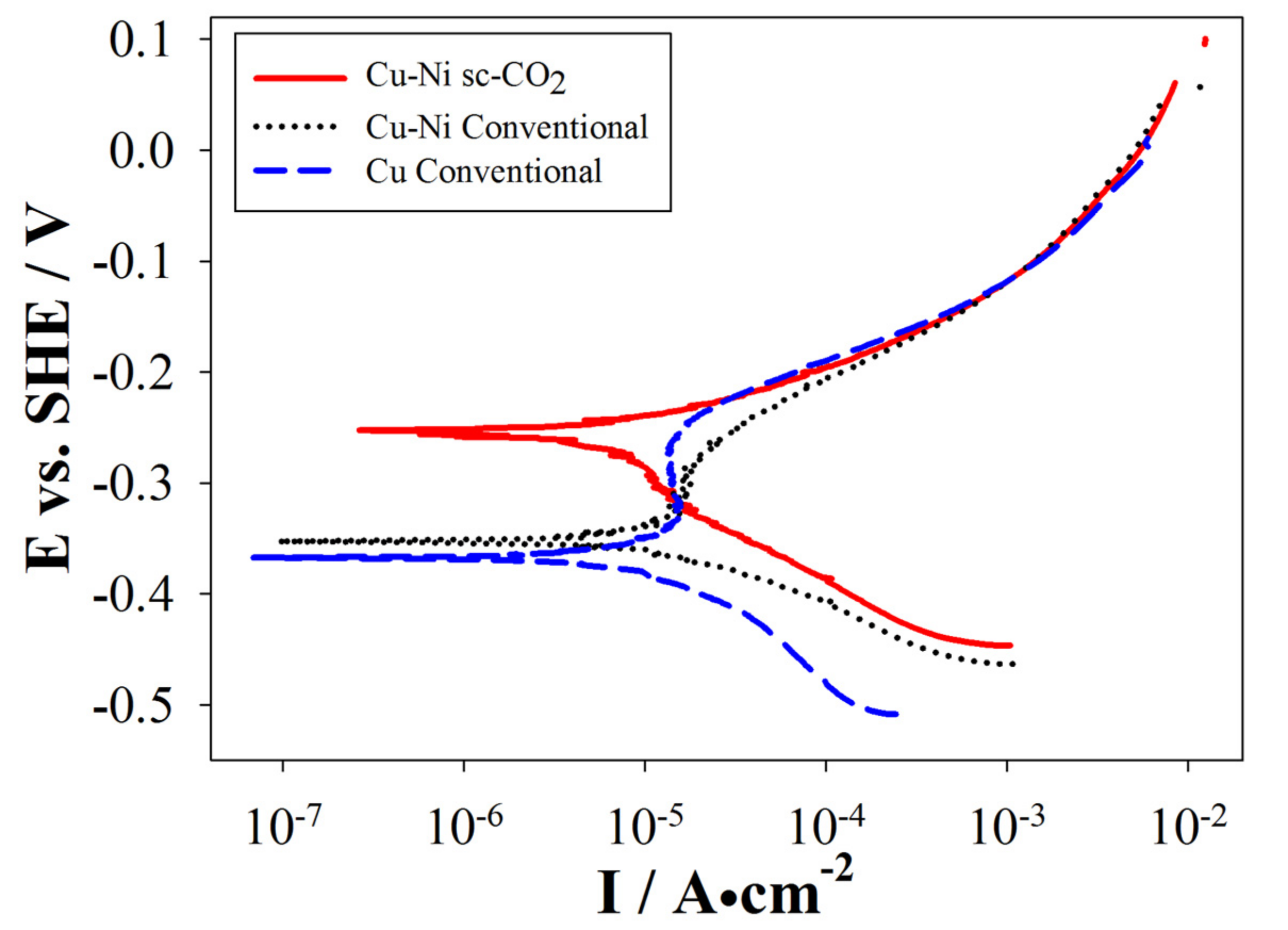
3.4. Electrochemical Properties of the Plated Coatings
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ghosh, S.K.; Grover, A.K.; Dey, G.K.; Suri, A.K. Synthesis of Corrosion-resistant Nanocrystalline Nickel-copper Alloy Coatings by Pulse-plating Technique. Defence Sci. J. 2005, 55, 63–74. [Google Scholar] [CrossRef]
- Wang, S.H.; Guo, X.G.; Yang, H.Y.; Dai, J.C.; Zhu, R.Y.; Gong, J.; Peng, L.M.; Ding, W.J. Electrodeposition mechanism and characterization of Ni-Cu alloy coatings from eutectic-based ionic liquid. Appl. Surf. Sci. 2014, 288, 530–536. [Google Scholar] [CrossRef]
- Kear, G.; Barker, B.D.; Stokes, K.; Walsh, F.C. Electrochemical Corrosion Behavior of 90—10 Cu-Ni Alloy in Chloride-based Electrolytes. J. Appl. Electrochem. 2004, 34, 659–669. [Google Scholar] [CrossRef]
- Baskaran, I.; Sankara Narayanan, T.S.N.; Stephen, A. Pulsed electrodeposition of nanocrystalline Cu–Ni alloy films and evaluation of their characteristic properties. Mater. Lett. 2006, 60, 1990–1995. [Google Scholar] [CrossRef]
- Alper, M.; Kockar, H.; Safak, M.; Baykul, M.C. Comparison of Ni-Cu alloy films electrodeposited at low and high pH levels. J. Alloys Compd. 2008, 453, 15–19. [Google Scholar] [CrossRef]
- Desislava, G.; Avdeev, G.; Rashkov, R. Electrodeposition and characterization of Ni-Cu alloys. Surf. Coat. Technol. 2014, 240, 204–210. [Google Scholar]
- Nguyen, V.C.; Lee, C.Y.; Chang, L.; Chen, F.J.; Lin, C.S. The relationship between nano crystallite structure and internal stress in Ni coatings electrodeposited by watts bath electrolyte mixed with supercritical CO2. J. Electrochem. Soc. 2012, 159, 393–399. [Google Scholar] [CrossRef]
- Li, Y.W.; Yao, J.H.; Huang, X.X. Effect of Saccharin on the process and properties of nickel electrodeposition from sulfate electrolyte. Int. J. Metall. Mater. Eng. 2016, 2, 123. [Google Scholar]
- Atanassov, N.; Bozhkov, Hr.; Vitkova, St.; Rashkov, St. Morphology and properties of nickel plate deposited in the presence of butynediol. Surf. Technol. 1982, 17, 291–299. [Google Scholar] [CrossRef]
- Guo, X.G.; Li, X.M.; Zheng, Y.H.; Lai, C.; Li, W.L.; Luo, B.B.; Zhang, D.X. Effects of surfactants on high regularity of 3D porous nickel for Zn2+ adsorption application. J. Nanomater. 2014. [Google Scholar] [CrossRef]
- Tsai, W.L.; Hsu, P.C.; Hwu, Y.; Chen, C.H.; Chang, L.W.; Je, J.H.; Lin, H.M.; Groso, A.; Margaritondo, G. Building on bubbles in metal electrodeposition. Nature 2002, 417, 139. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Sone, M.; Mizushima, A.; Abe, K.; Tao, X.T.; Ichihara, S.; Miyata, S. Electroplating of Nanostructured Nickel in Emulsion of Supercritical Carbon Dioxide in Electrolyte Solution. Chem. Lett. 2002, 11, 1086–1087. [Google Scholar] [CrossRef]
- Yoshida, H.; Sone, M.; Mizushima, A.; Yan, H.; Wakabayashi, H.; Abe, K.; Tao, X.T.; Ichihara, S.; Miyata, S. Application of Emulsion of Dense Carbon Dioxide in Electroplating Solution with Nonionic Surfactants for Nickel Electroplating. Surf. Coat. Technol. 2003, 173, 285–292. [Google Scholar] [CrossRef]
- Yoshida, H.; Sone, M.; Mizushima, A.; Abe, K.; Tao, X.T.; Ichihara, S.; Miyata, S. The effects of dense carbon dioxide on nickel plating using emulsion of carbon dioxide in electroplating solution. Surf. Coat. Technol. 2004, 182, 329–334. [Google Scholar]
- Chiu, S.Y.; Chung, S.T.; Lin, C.Y.; Tsai, W.T. Electrodeposition of Ni-Al2O3 composite coatings employing supercritical CO2 baths. Surf. Coat. Technol. 2014, 247, 68–73. [Google Scholar] [CrossRef]
- Berche, B.; Henkel, M.; Kenna, R. Critical phenomena: 150 years since Cagnaird de la Tour. Rev. Bras. Ens. Fis. 2009, 31. [Google Scholar] [CrossRef]
- Chuang, H.C.; Hong, G.Y.; Sanchez, J. Fabrication of high aspect ratio copper nanowires using supercritical CO2 fluids electroplating technique in AAO template. Mater. Sci. Semicond. Process. 2016, 45, 17–26. [Google Scholar] [CrossRef]
- Chuang, H.C.; Lai, W.H. Fabrication of through-silicon vias by supercritical CO2 emulsion-enabled nickel electroplating. Mater. Sci. Semicond. Process. 2014, 23, 27–33. [Google Scholar] [CrossRef]
- Chuang, H.C.; Lai, W.H.; Sanchez, J. An investigation of supercritical-CO2 copper electroplating parameters for application in TSV chips. J. Micromech. Microeng. 2014, 25, 015004. [Google Scholar] [CrossRef]
- Weil, R. Origins of Stress in Electrodeposits Pt. 2: Review of the Literature Dealing with Stress in Electrodeposited Metals. Plating 1971, 58, 50–56. [Google Scholar]
- Nguyen, V.C.; Lee, C.Y.; Chen, F.J.; Lin, C.S.; Liu, T.Y. Study on the internal stress of nickel coating electrodeposited in an electrolyte mixed with supercritical carbon dioxide. Surf. Coat. Technol. 2012, 206, 3201–3207. [Google Scholar] [CrossRef]
- Chuang, H.C.; Teng, Y.C.; Sanchez, J. Study on the effects of pressure and material characterization in thin film and TSV fabricated by supercritical carbon dioxide electrolyte. Mater. Sci. Semicond. Process. 2016, 56, 5–13. [Google Scholar] [CrossRef]
- Badea, G.E.; Caraban, A.; Sebesan, M.; Dzitac, S.; Cret, P.; Setel, A. Polarisation measurements used for corrosion rates determination. J. Sustain. Energy 2010, 1, 83–87. [Google Scholar]
- Tsai, W.T.; Chuang, Y.C. Effect of surfactant on the electrodeposition of Ni–P coating in emulsified supercritical CO2 baths. Thin Solid Films 2013, 529, 322–326. [Google Scholar]
- Kriaa, K.; Serin, J.P.; Contamine, F.; Cézac, P.; Mercadier, J. 2-Butyne-1, 4-diol hydrogenation in supercritical CO2: Effect of hydrogen concentration. J. Supercrit. Fluids 2009, 49, 227–232. [Google Scholar] [CrossRef]
- European Chemical Agency. But-2-Yne-1,4-Diol Summary Risk Assessment Report. Available online: https://echa.europa.eu/documents/10162/d25f048d-21b9-4949-a7c6-7d9ae3a1aa3a (accessed on 11 April 2017).

| Parameter | Conventional Electroplating | sc-CO2 Electroplating |
|---|---|---|
| Pressure (MPa) | 0.1 | 15 |
| Temperature (°C) | 50 | 50 |
| Agitation speed (rpm) | 500 | 500 |
| Current density (A/dm2) | 2.5 | 2.5 |
| Surfactant content (g/L) | 2.0, 2.5, 3.0, 3.5, 4.0 | 2.0, 2.5, 3.0, 3.5, 4.0 |
| Plating time (h) | 1 | 1 |
| Parameter | Setting |
|---|---|
| Exposed area of WE | 1 cm2 |
| Plated thickness | 50 µm |
| OCP stabilization time | 30 min |
| Scanning potential | Vs. reference SCE |
| PPS speed | 1 mV/s |
| PPS range | Dependent on OCP: ±250 mV (Total scan range: 500 mV) |
| Corrosive solution | 3.5% NaCl |
| Temperature | 20 °C ± 2 °C |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuang, H.-C.; Sánchez, J.; Cheng, H.-Y. The Effect of Surfactant Content over Cu-Ni Coatings Electroplated by the sc-CO2 Technique. Materials 2017, 10, 428. https://doi.org/10.3390/ma10040428
Chuang H-C, Sánchez J, Cheng H-Y. The Effect of Surfactant Content over Cu-Ni Coatings Electroplated by the sc-CO2 Technique. Materials. 2017; 10(4):428. https://doi.org/10.3390/ma10040428
Chicago/Turabian StyleChuang, Ho-Chiao, Jorge Sánchez, and Hsiang-Yun Cheng. 2017. "The Effect of Surfactant Content over Cu-Ni Coatings Electroplated by the sc-CO2 Technique" Materials 10, no. 4: 428. https://doi.org/10.3390/ma10040428
APA StyleChuang, H.-C., Sánchez, J., & Cheng, H.-Y. (2017). The Effect of Surfactant Content over Cu-Ni Coatings Electroplated by the sc-CO2 Technique. Materials, 10(4), 428. https://doi.org/10.3390/ma10040428





