Crystallinity and Sub-Band Gap Absorption of Femtosecond-Laser Hyperdoped Silicon Formed in Different N-Containing Gas Mixtures
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
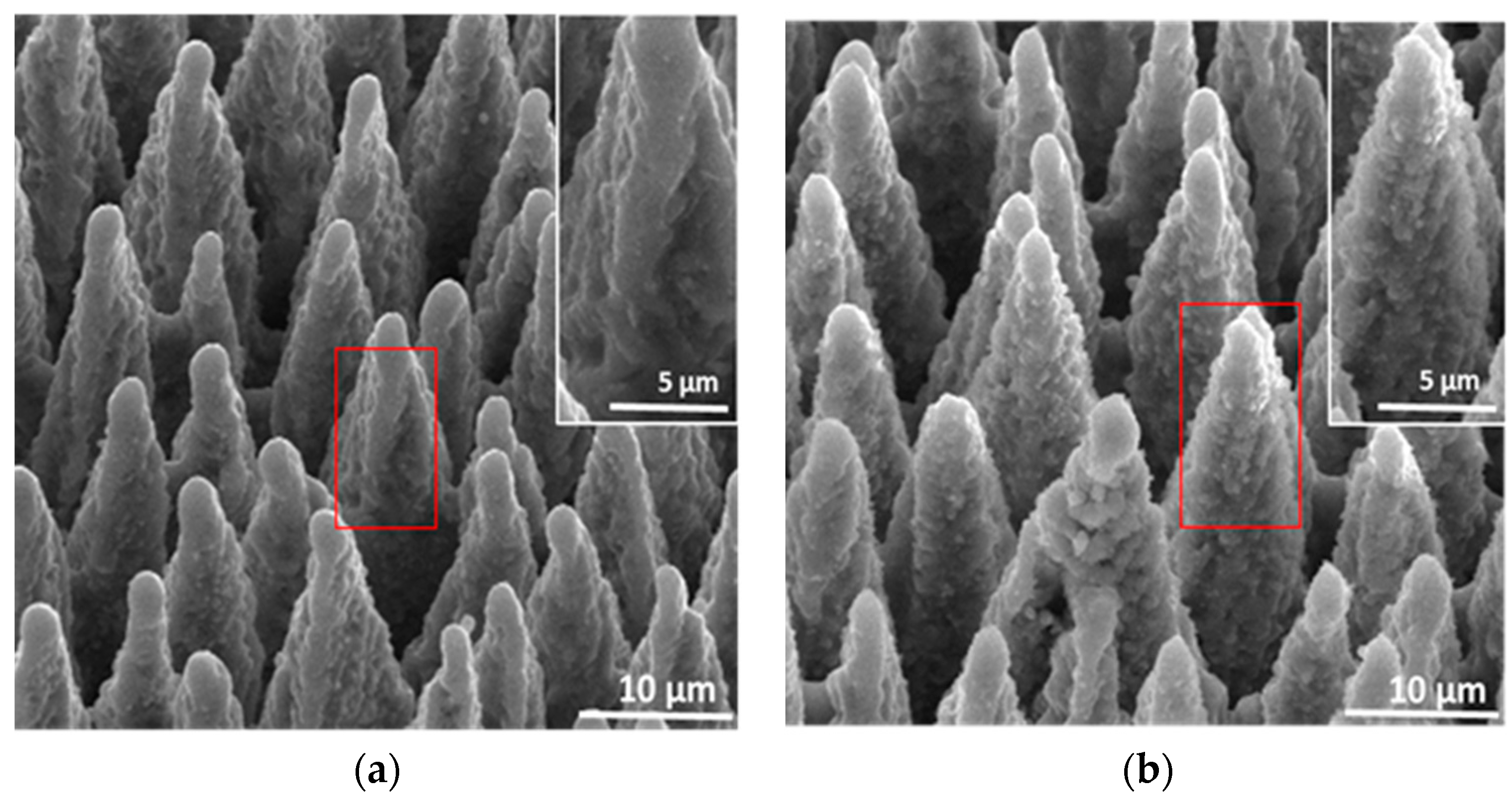
3.1. Surface Morphology
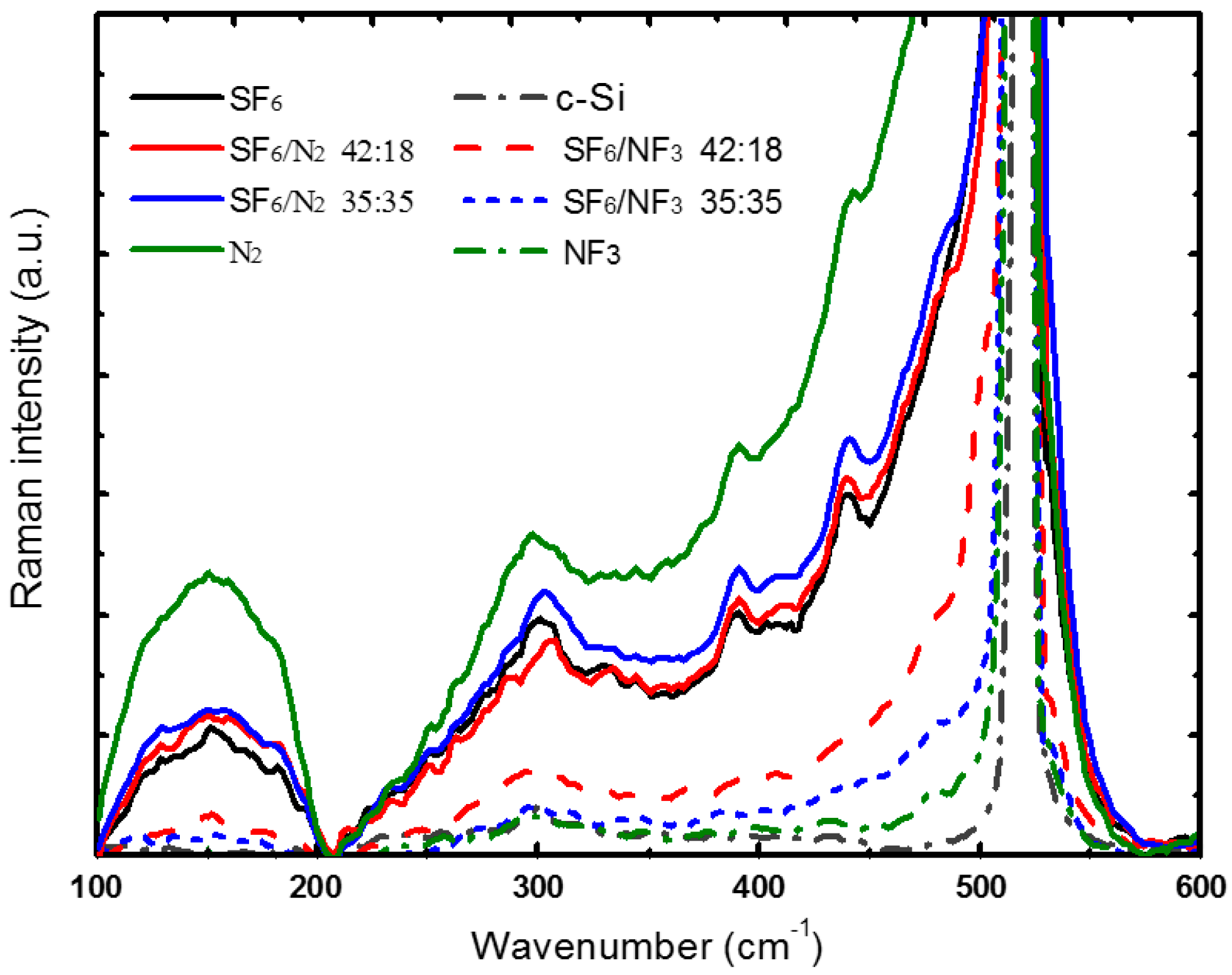
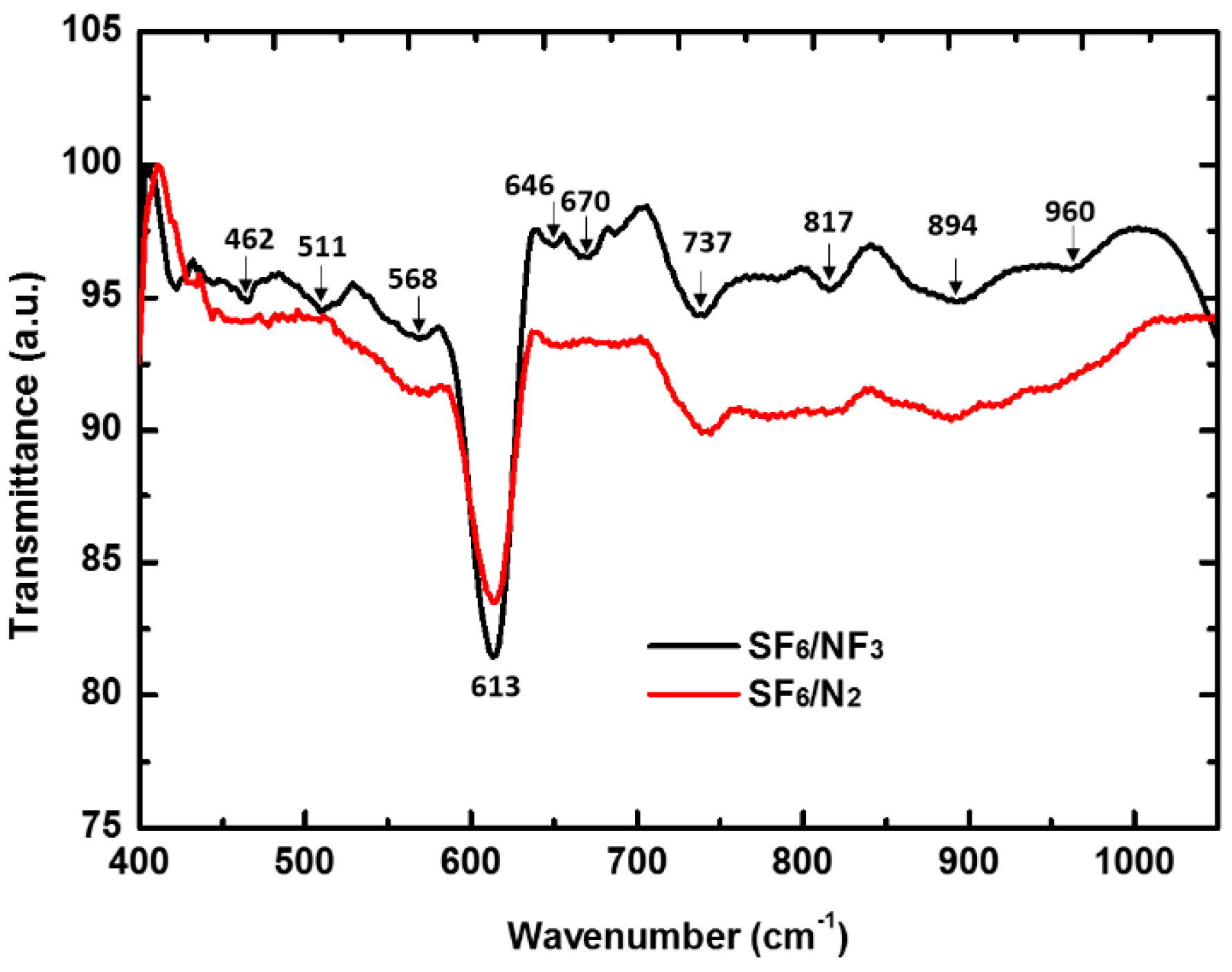
3.2. Nitrogen Co-Doping Characters
3.3. Crystal Properties
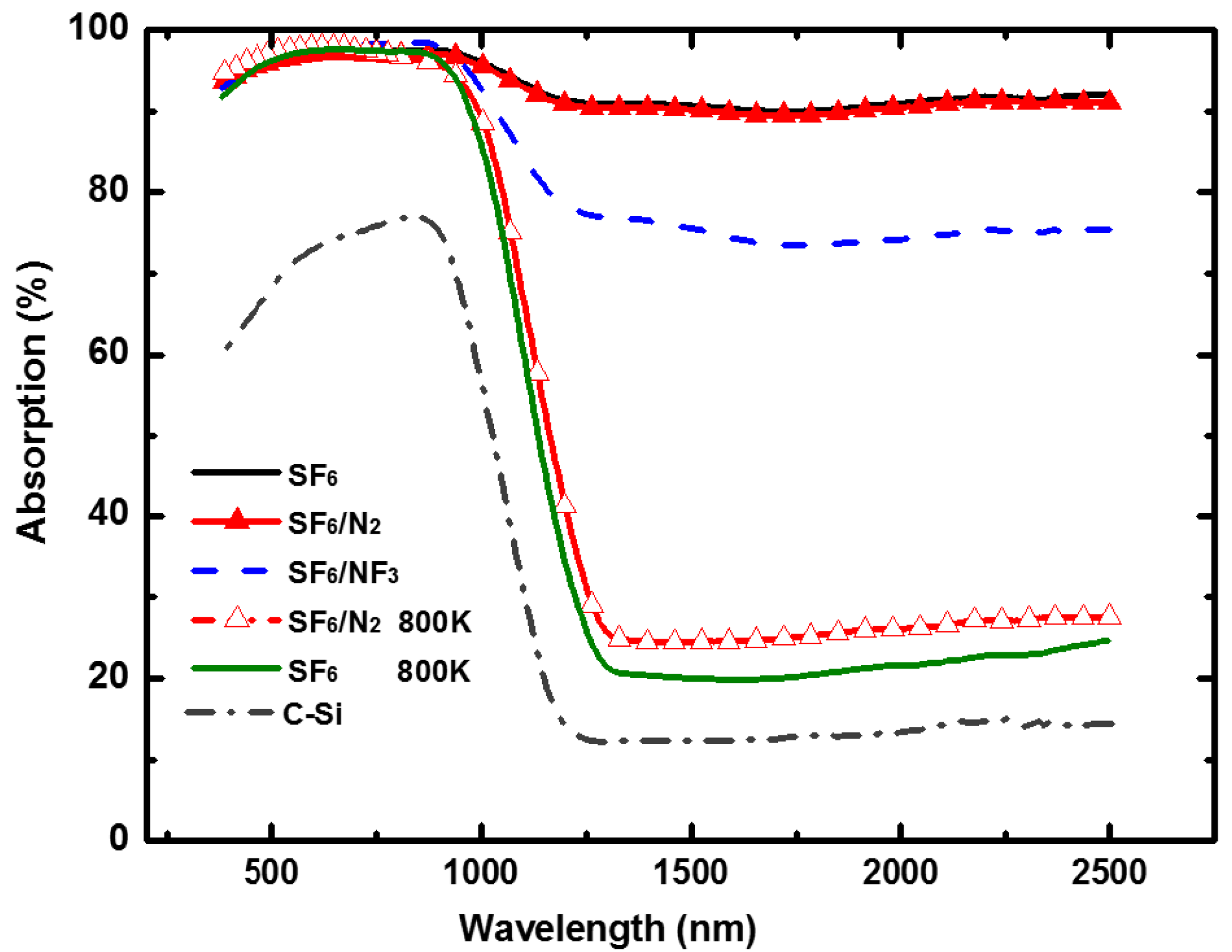
3.4. Optical Light Absorption
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Phillips, K.C.; Gandhi, H.H.; Mazur, E.; Sundranm, S.K. Ultrafast laser processing of materials: A review. Adv. Opt. Photonics 2015, 7, 684–712. [Google Scholar] [CrossRef]
- Zhu, J.T.; Zhao, L.; Li, W.; Wang, Y.; Feng, G.J.; Wang, Z.S. Great enhancement of infrared light absorption of silicon surface-structured by femtosecond laser pulses in N2 ambient. Mater. Lett. 2006, 60, 2187–2189. [Google Scholar] [CrossRef]
- Tull, B.R.; Carey, J.E.; Mazur, E.; McDonald, J.P.; Yalisove, S.M. Silicon Surface Morphologies after Femtosecond Laser Irradiation. MRS Bull. 2006, 3, 626–633. [Google Scholar] [CrossRef]
- Younkin, R.; Carey, J.E.; Mazur, E.; Levinson, J.A.; Friend, C.M. Infrared absorption by conical silicon microstructures made in a variety of background gases using femtosecond-laser pulses. J. Appl. Phys. 2003, 93, 2626–2629. [Google Scholar] [CrossRef]
- Jia, X.; Jia, T.Q.; Peng, N.N.; Feng, D.H.; Zhang, S.A.; Sun, Z.R. Dynamic of femtosecond lasr-induced periodic surface structures on silicon by high spatical and temporal resolution imaging. J. Appl. Phys. 2014, 14, 143102. [Google Scholar] [CrossRef]
- Crouch, C.H.; Carey, J.E.; Shen, M.; Mazur, E.; Génin, F.Y. Infrared absorption by sulfur-doped silicon formed by femtosecond laser irradiation. Appl. Phys. A 2004, 79, 1635–1641. [Google Scholar] [CrossRef]
- Zhang, S.F.; Li, Y.; Feng, G.J.; Zhu, B.C.; Xiao, S.Y.; Zhou, L.; Zhao, L. Strong infrared absorber: Surface microstructured Au film replicated from black silicon. Opt. Express 2011, 19, 20462–20467. [Google Scholar] [CrossRef] [PubMed]
- Sher, M.J.; Winkler, M.T.; Mazur, E. Pulsed-laser hyperdoping and surface texturing for photovoltaics. MRS Bull. 2011, 36, 439–442. [Google Scholar] [CrossRef]
- Sánchez, K.; Aguilera, I.; Palacios, P.; Wahnón, P. Formation of a reliable intermediate band in Si heavily coimplanted with chalcogens (S, Se, Te) and group III elements (B, Al). Phys. Rev. B 2010, 82, 165201. [Google Scholar] [CrossRef]
- Shao, H.; Li, Y.; Zhang, J.; Ning, B.; Zhang, W.; Ning, X.; Zhao, L.; Zhuang, J. Physical mechanisms for the unique optical properties of chalcogen-hyperdoped silicon. Eur. Phys. Lett. 2012, 99, 46005. [Google Scholar] [CrossRef]
- Limaye, M.V.; Chen, S.C.; Lee, C.Y.; Chen, L.Y.; Singh, S.B.; Shao, Y.C.; Wang, Y.F.; Hsieh, S.H.; Hsueh, H.C.; Chiou, J.W.; et al. Understanding of sub-band absorption of femtosecond-laser sulfur hyperdoped silicon using synchrotron-based techiniques. Sci. Rep. 2015, 5, 11466. [Google Scholar] [CrossRef] [PubMed]
- Sher, M.J.; Mazur, E. Intermediate band conduction in femtosecond-laser hyperdoped silicon. Appl. Phys. Lett. 2014, 105, 032103. [Google Scholar] [CrossRef]
- Myers, R.A.; Farrell, R.; Karger, A.M.; Carey, J.E.; Mazur, E. Enhancing near-infrared avalanche photodiode performance by femtosecond laser microstructuring. Appl. Opt. 2006, 35, 8825–8831. [Google Scholar] [CrossRef]
- Otto, M.; Algsinger, M.; Branz, H.; Geseman, B.; Gimpl, T.; Fuchsel, K.; Kasebier, T.; Kontermann, S.; Koynov, S.; Li, X.P.; et al. Black silicon photovoltaics. Adv. Opt. Mater. 2014, 2, 147–164. [Google Scholar] [CrossRef]
- Carey, J.E.; Crouch, C.H.; Shen, M.; Mazur, E. Visible and near-infrared responsivity of femtosecond-laser microstructured silicon photodiodes. Opt. Lett. 2005, 30, 1773–1775. [Google Scholar] [CrossRef] [PubMed]
- Casalino, M.; Coppola, G.; Iodice, M.; Rendina, I.; Sirleto, L. Near-infrared sub-bandgap all-silicon photodetectors: State of the art and perspectives. Sensors 2010, 10, 10571–10600. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.H.; Carey, J.E.; Liu, M.G.; Guo, X.Y.; Mazur, E.; Campbell, J.C. Microstructured silicon photodetector. Appl. Phys. Lett. 2006, 89, 033506. [Google Scholar] [CrossRef]
- Mailoa, J.P.; Akey, A.J.; Simmons, C.B.; Hutchinson, D.; Mathews, J.; Sullivan, J.T.; Recht, D.; Winkler, M.T.; Williams, J.S.; Warrender, J.M.; et al. Room-temperature sub-band gap optoelectronic response of hyperdoped silicon. Nat. Commun. 2014, 5, 3011. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.C.; Zheng, H.Y.; Tan, C.W.; Wang, F.; Yu, H.Y.; Pey, K.L. Femtosecond laser induced surface nanostructuring and simultaneous crystallization of amorphous thin silicon film. Opt. Express 2010, 18, 19379–19385. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.J.; Lin, Y.T.; Sher, M.J.; Winkler, M.T.; Mazur, E.; Grádecak, S. Pressure-induced phase transformations during femtosecond-laser doping of silicon. J. Appl. Phys. 2011, 110, 053524. [Google Scholar] [CrossRef]
- Smith, M.J.; Sher, M.J.; Franta, B.J.; Lin, Y.T.; Mazur, E.; Gradečak, S. The origins of pressure-induced phase transformations during the surface texturing of silicon using femtosecond laser irradiation. J. Appl. Phys. 2012, 112, 083518. [Google Scholar] [CrossRef]
- Newman, B.K.; Sher, M.J.; Mazur, E.; Buonassisi, T. Reactivation of sub-bandgap absorption in chalcogen-hyperdoped silicon. Appl. Phys. Lett. 2011, 98, 251905. [Google Scholar] [CrossRef]
- Franta, B.J.; Pastor, D.; Gandhi, H.H.; Rekemeyer, P.H.; Gradecák, S.; Aziz, M.J.; Mazur, E. Simultaneous high crystallinity and sub-bandgap optical absorptance in hyperdoped black silicon using nanosecond laser annealing. J. Appl. Phys. 2015, 118, 225303. [Google Scholar] [CrossRef]
- Kim, T.G.; Warrender, J.M.; Aziz, M.J. Strong sub-band-gap infrared absorption in silicon supersaturated with sulfur. Appl. Phys. Lett. 2006, 88, 241902. [Google Scholar] [CrossRef]
- Sun, H.; Liang, C.; Feng, G.; Zhu, Z.; Zhuang, J.; Zhao, L. Improving crystallinity of femtosecond-laser hyperdoped silicon via co-doping with nitrogen. Opt. Mater. Express 2016, 6, 1321–1328. [Google Scholar] [CrossRef]
- Dong, X.; Li, N.; Zhu, Z.; Shao, H.; Rong, X.; Liang, C.; Sun, H.; Feng, G.; Zhao, L.; Zhuang, J. A nitrogen-hyperdoped silicon material formed by femtosecond laser irradiation. Appl. Phys. Lett. 2014, 104, 091907. [Google Scholar] [CrossRef]
- Dong, X.; Li, N.; Liang, C.; Sun, H.; Feng, G.; Zhu, Z.; Shao, H.; Rong, X.; Zhao, L.; Zhuang, J. Strong mid-Infrared absorption and high crystallinity of microstructured silicon formed by femtosecond laser irradiation in NF3 atmosphere. Appl. Phys. Express 2013, 6, 081301. [Google Scholar] [CrossRef]
- Taguchi, A.; Kageshima, H.; Wada, K. First-principles investigations of notrigen-doping effects on defects aggregation process in Czochralski Si. J. Appl. Phys. 2005, 5, 053514. [Google Scholar] [CrossRef]
- Alpass, C.R.; Murphy, J.D.; Falster, R.J.; Wilshaw, P.R. Nitrogen diffusion and interaction with dislocations in single-crystal silicon. J. Appl. Phys. 2009, 105, 013519. [Google Scholar] [CrossRef]
- Zhang, H.; Stavola, M.; Seacrist, M. Nitrogen-containing point defects in multi-crystalline Si solar-cell materials. J. Appl. Phys. 2013, 114, 093707. [Google Scholar] [CrossRef]
- Sawada, H.; Kawakami, K. First-principles calculation of the interaction between nitrogen atoms and vacancies in silicon. Phys. Rev. B 2000, 62, 1851–1858. [Google Scholar] [CrossRef]
- Goss, J.P.; Hahn, I.; Jones, R.; Briddon, P.R.; Oberg, S. Vibrational modes and electronic properties of nitrogen defects in silicon. Phys. Rev. B 2003, 67, 045206. [Google Scholar] [CrossRef]
- Zhu, Z.; Shao, H.; Dong, X.; Li, N.; Ning, B.; Ning, X.; Zhao, L.; Zhuang, J. Electronic band structure and sub-band-gap absorption of nitrogen hyperdoped silicon. Sci. Rep. 2015, 5, 10513. [Google Scholar] [CrossRef] [PubMed]
- Aben, T. Generation and annihilation of point defects by doping impurities during FZ silicon crystal growth. J. Cryst. Growth 2011, 334, 4–15. [Google Scholar]
- Kaku, M.; Masuda, K.; Miyazaki, K. Observation of revival structure in femtosecond-laser-induced alignment of N2 with high-order harmonic generation. Jpn. J. Appl. Phys. 2004, 43, 591–593. [Google Scholar] [CrossRef]
- Hishikawa, A.; Iwamae, A.; Hoshina, K.; Kono, M.; Yamanouchi, K. Coulomb explosion dynamics of N2 in intense laser field by mass-resolved momentum imaging. Chem. Phys. 1998, 231, 315–329. [Google Scholar] [CrossRef]
| Pressure Ratio of Gas Mixture | N (1019 Atoms/cm3) | S (1019 Atoms/cm3) |
|---|---|---|
| SF6/NF3 (35:35 kPa) | 4.50298 | 4.43861 |
| SF6/N2 (35:35 kPa) | 1.31992 | 4.56782 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, H.; Xiao, J.; Zhu, S.; Hu, Y.; Feng, G.; Zhuang, J.; Zhao, L. Crystallinity and Sub-Band Gap Absorption of Femtosecond-Laser Hyperdoped Silicon Formed in Different N-Containing Gas Mixtures. Materials 2017, 10, 351. https://doi.org/10.3390/ma10040351
Sun H, Xiao J, Zhu S, Hu Y, Feng G, Zhuang J, Zhao L. Crystallinity and Sub-Band Gap Absorption of Femtosecond-Laser Hyperdoped Silicon Formed in Different N-Containing Gas Mixtures. Materials. 2017; 10(4):351. https://doi.org/10.3390/ma10040351
Chicago/Turabian StyleSun, Haibin, Jiamin Xiao, Suwan Zhu, Yue Hu, Guojin Feng, Jun Zhuang, and Li Zhao. 2017. "Crystallinity and Sub-Band Gap Absorption of Femtosecond-Laser Hyperdoped Silicon Formed in Different N-Containing Gas Mixtures" Materials 10, no. 4: 351. https://doi.org/10.3390/ma10040351
APA StyleSun, H., Xiao, J., Zhu, S., Hu, Y., Feng, G., Zhuang, J., & Zhao, L. (2017). Crystallinity and Sub-Band Gap Absorption of Femtosecond-Laser Hyperdoped Silicon Formed in Different N-Containing Gas Mixtures. Materials, 10(4), 351. https://doi.org/10.3390/ma10040351





