A Series of Robust Copper-Based Triazolyl Isophthalate MOFs: Impact of Linker Functionalization on Gas Sorption and Catalytic Activity †
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and X-ray Crystallography of 1–5
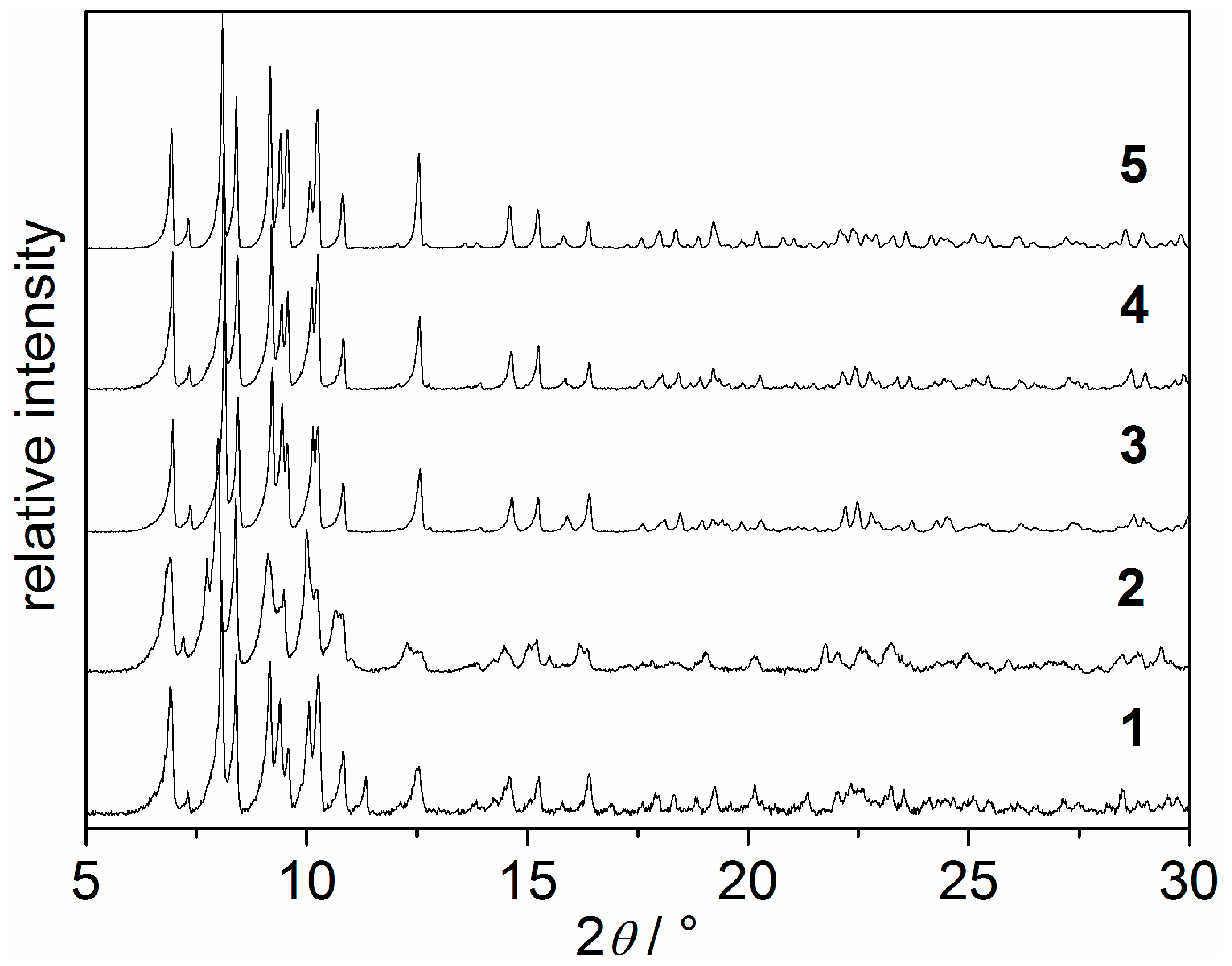
2.2. X-ray Powder Diffraction and Thermal Stability of 1–5
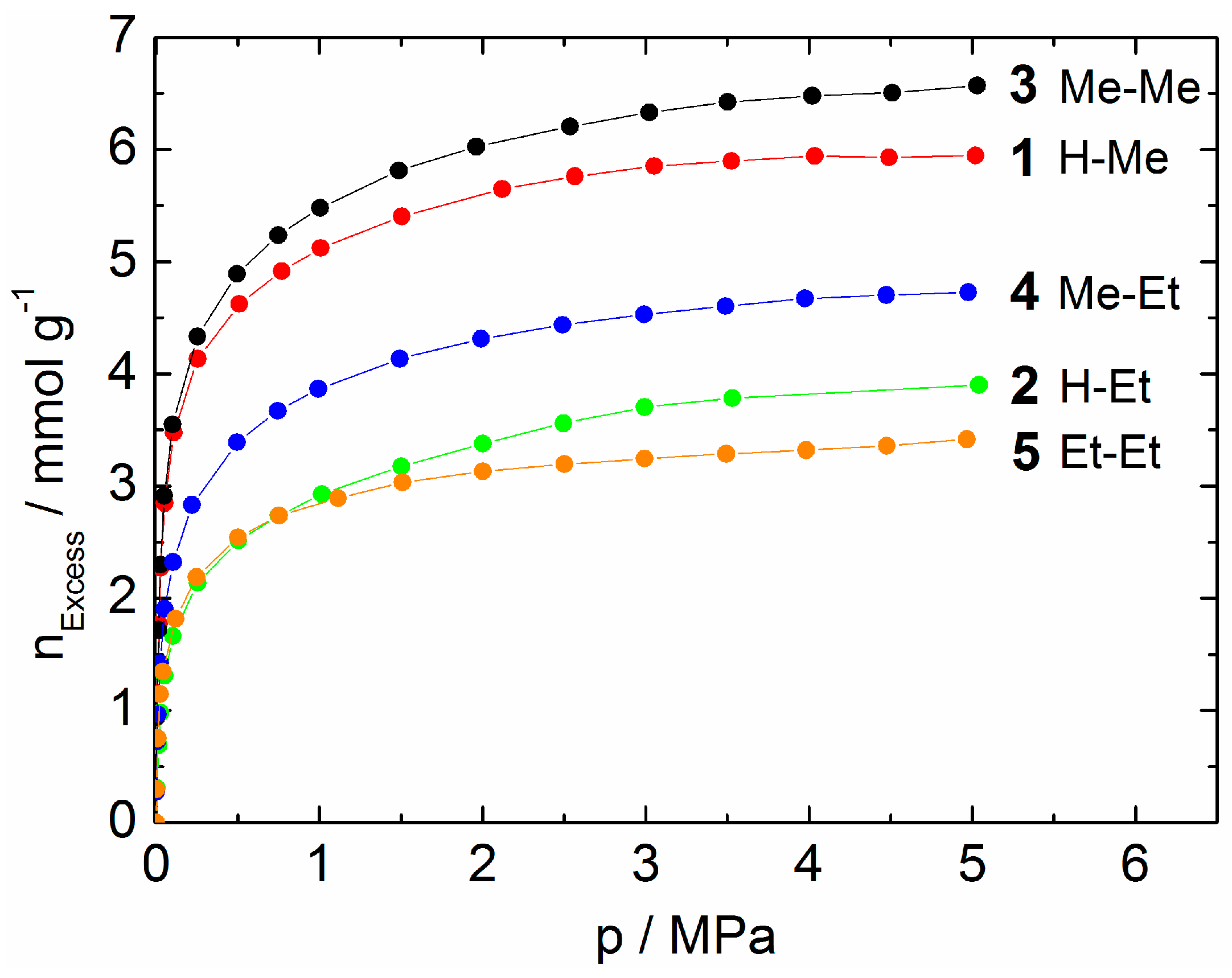
2.3. Adsorption of CO2
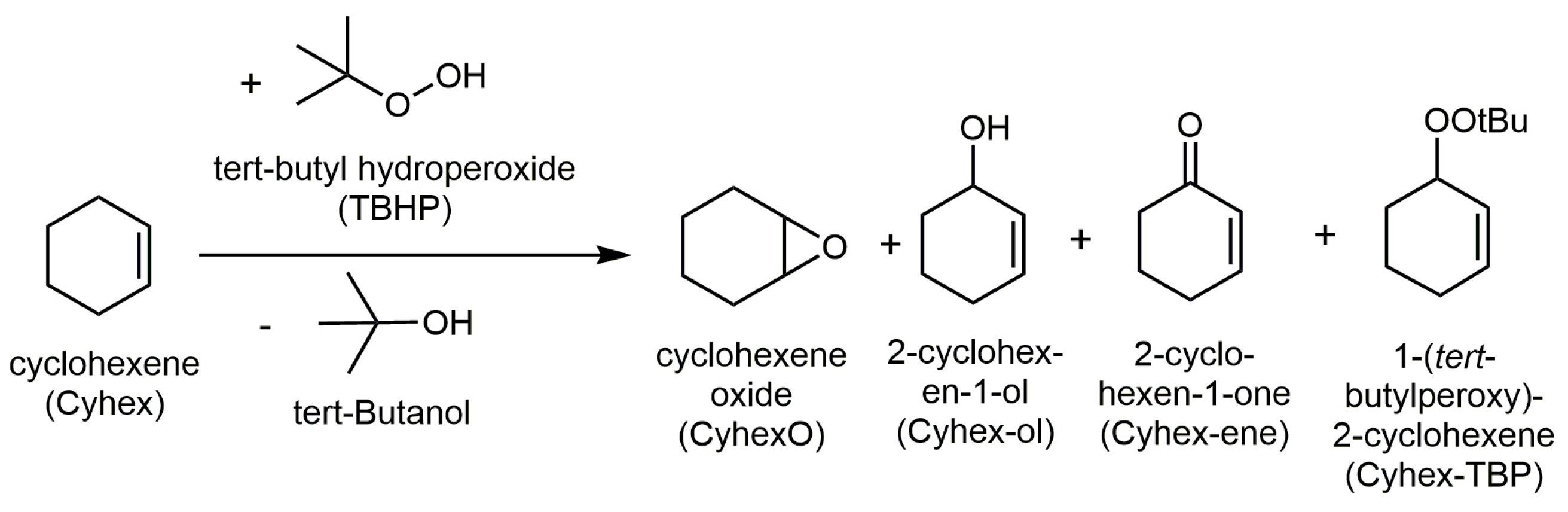
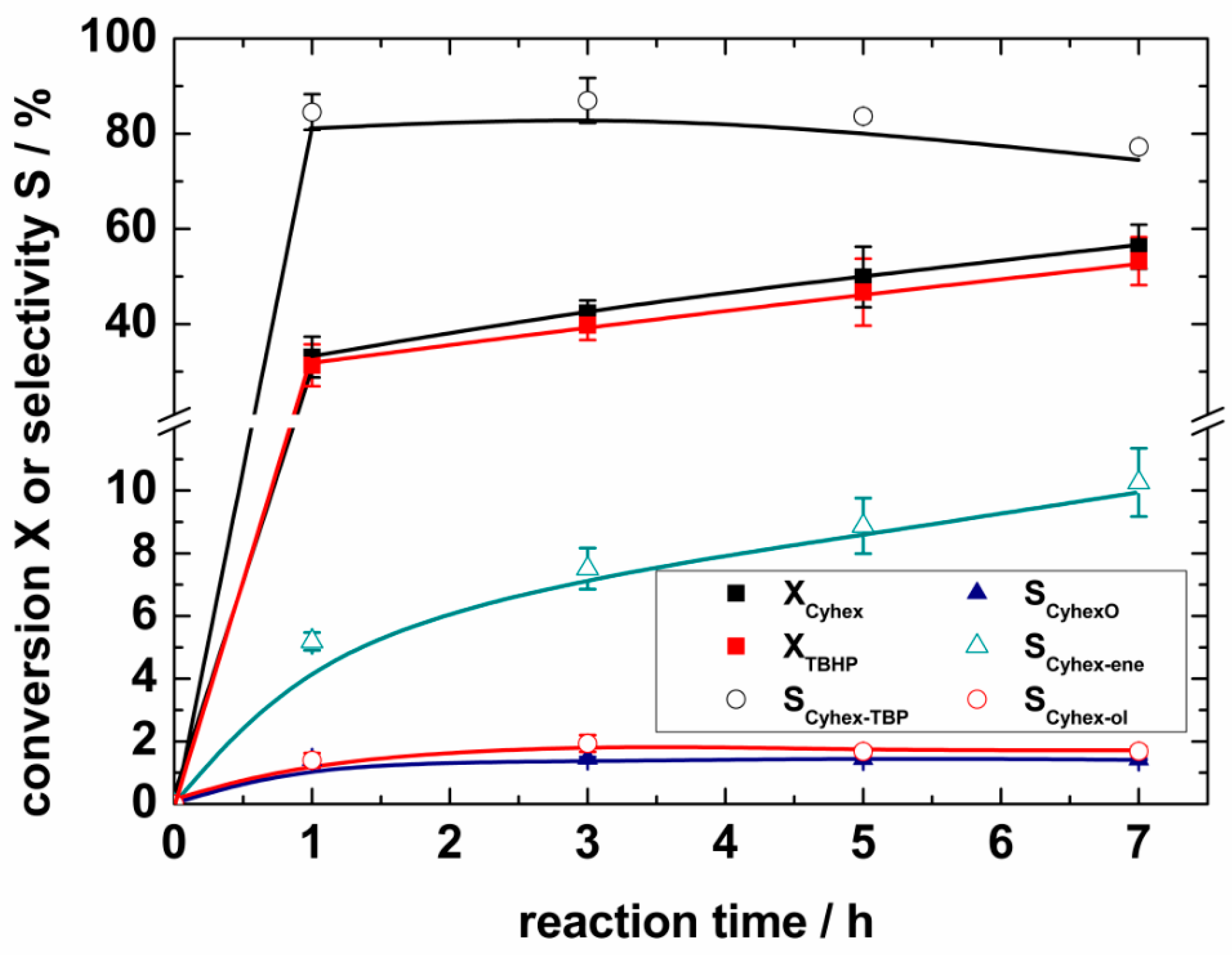
2.4. Catalytic Selective Oxidation of Cyclohexene with TBHP over 1–5
3. Materials and Methods
3.1. Synthesis of [Cu4(μ3-OH)2(R1-R2-trz-ia)3(H2O)x] (R1 = H, Me, Et; R2 = Me, Et; x = 1, 2; 1–5)
3.2. Characterization of 1–5
3.3. Catalytic Selective Oxidation of Cyclohexene
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Murray, L.J.; Dinca, M.; Long, J.R. Hydrogen storage in metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1294–1314. [Google Scholar] [CrossRef] [PubMed]
- Rosi, N.L.; Eckert, J.; Eddaoudi, M.; Vodak, D.T.; Kim, J.; O’Keeffe, M.; Yaghi, O.M. Hydrogen Storage in Microporous Metal-Organic Frameworks. Science 2003, 300, 1127–1130. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Bai, J.; Lu, Z.; Pan, Y.; You, X. Finely tuning MOFs towards high-performance post-combustion CO2 capture materials. Chem. Commun. 2016, 52, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Lässig, D.; Lincke, J.; Moellmer, J.; Reichenbach, C.; Moeller, A.; Gläser, R.; Kalies, G.; Cychosz, K.A.; Thommes, M.; Staudt, R.; et al. A Microporous Copper Metal-Organic Framework with High H2 and CO2 Adsorption Capacity at Ambient Pressure. Angew. Chem. Int. Ed. 2011, 50, 10344–10348. [Google Scholar] [CrossRef] [PubMed]
- Van de Voorde, B.; Bueken, B.; Denayer, J.; de Vos, D. Adsorptive separation on metal-organic frameworks in the liquid phase. Chem. Soc. Rev. 2014, 43, 5766–5788. [Google Scholar] [CrossRef] [PubMed]
- Bourrelly, S.; Llewellyn, P.L.; Serre, C.; Millange, F.; Loiseau, T.; Ferey, G. Different adsorption behaviors of methane and carbon dioxide in the isotypic nanoporous metal terephthalates MIL-53 and MIL-47. J. Am. Chem. Soc. 2005, 127, 13519–13521. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Parker, B.; Huang, X.; Olson, D.H.; Lee, J.; Li, J. Zn(tbip) (H2tbip = 5-tert-Butyl Isophthalic Acid): A Highly Stable Guest-Free Microporous Metal Organic Framework with Unique Gas Separation Capability. J. Am. Chem. Soc. 2006, 128, 4180–4181. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Krungleviciute, V.; Eryazici, I.; Hupp, J.T.; Farha, O.K.; Yildirim, T. Methane storage in metal-organic frameworks: Current records, surprise findings, and challenges. J. Am. Chem. Soc. 2013, 135, 11887–11894. [Google Scholar] [CrossRef] [PubMed]
- Barea, E.; Turra, F.; Navarro, J.A.R. Separation and Purification of Gases by MOFs. In Metal-Organic Frameworks: Application from Catalysis to Gas Storage; Farrusseng, D., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011; pp. 69–92. [Google Scholar]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; van Duyne, R.P.; Hupp, J.T. Metal-organic framework materials as chemical sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, P.; Gref, R.; Baati, T.; Allan, P.K.; Maurin, G.; Couvreur, P.; Ferey, G.; Morris, R.E.; Serre, C. Metal-organic frameworks in biomedicine. Chem. Rev. 2012, 112, 1232–1268. [Google Scholar] [CrossRef] [PubMed]
- Farrusseng, D.; Aguado, S.; Pinel, C. Metal-Organic Frameworks: Opportunities for Catalysis. Angew. Chem. Int. Ed. 2009, 48, 7502–7513. [Google Scholar] [CrossRef] [PubMed]
- Chughtai, A.H.; Ahmad, N.; Younus, H.A.; Laypkov, A.; Verpoort, F. Metal-organic frameworks: Versatile heterogeneous catalysts for efficient catalytic organic transformations. Chem. Soc. Rev. 2015, 44, 6804–6849. [Google Scholar] [CrossRef] [PubMed]
- Xamena, F.X.L.; Gascon, J.; Spivey, J. Metal Organic Frameworks as Heterogeneous Catalysts; The Royal Society of Chemistry: Cambridge, UK, 2013. [Google Scholar]
- Gascon, J.; Corma, A.; Kapteijn, F.; Xamena, F.X.L. Metal Organic Framework Catalysis: Quo vadis? ACS Catal. 2014, 4, 361–378. [Google Scholar] [CrossRef]
- Kitagawa, S.; Kitaura, R.; Noro, S.-I. Functional porous coordination polymers. Angew. Chem. Int. Ed. 2004, 43, 2334–2375. [Google Scholar] [CrossRef] [PubMed]
- Guillerm, V.; Kim, D.; Eubank, J.F.; Luebke, R.; Liu, X.; Adil, K.; Lah, M.S.; Eddaoudi, M. A supermolecular building approach for the design and construction of metal-organic frameworks. Chem. Soc. Rev. 2014, 43, 6141–6172. [Google Scholar] [CrossRef] [PubMed]
- Henke, S.; Schneemann, A.; Wutscher, A.; Fischer, R.A. Directing the breathing behavior of pillared-layered metal-organic frameworks via a systematic library of functionalized linkers bearing flexible substituents. J. Am. Chem. Soc. 2012, 134, 9464–9474. [Google Scholar] [CrossRef] [PubMed]
- Schwedler, I.; Henke, S.; Wharmby, M.T.; Bajpe, S.R.; Cheetham, A.K.; Fischer, R.A. Mixed-linker solid solutions of functionalized pillared-layer MOFs–adjusting structural flexibility, gas sorption, and thermal responsiveness. Dalton Trans. 2016, 45, 4230–4241. [Google Scholar] [CrossRef] [PubMed]
- Colombo, V.; Montoro, C.; Maspero, A.; Palmisano, G.; Masciocchi, N.; Galli, S.; Barea, E.; Navarro, J.A.R. Tuning the adsorption properties of isoreticular pyrazolate-based metal-organic frameworks through ligand modification. J. Am. Chem. Soc. 2012, 134, 12830–12843. [Google Scholar] [CrossRef] [PubMed]
- Handke, M.; Weber, H.; Lange, M.; Mollmer, J.; Lincke, J.; Gläser, R.; Staudt, R.; Krautscheid, H. Network flexibility: Control of gate opening in an isostructural series of Ag-MOFs by linker substitution. Inorg. Chem. 2014, 53, 7599–7607. [Google Scholar] [CrossRef] [PubMed]
- Brozek, C.K.; Dinca, M. Cation exchange at the secondary building units of metal-organic frameworks. Chem. Soc. Rev. 2014, 43, 5456–5467. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.M. Postsynthetic Methods for the Functionalization of Metal-Organic Frameworks. Chem. Rev. 2012, 112, 970–1000. [Google Scholar] [CrossRef] [PubMed]
- Fei, H.; Shin, J.; Meng, Y.S.; Adelhardt, M.; Sutter, J.; Meyer, K.; Cohen, S.M. Reusable oxidation catalysis using metal-monocatecholato species in a robust metal-organic framework. J. Am. Chem. Soc. 2014, 136, 4965–4973. [Google Scholar] [CrossRef] [PubMed]
- Dau, P.V.; Cohen, S.M. Modulating H2 sorption in metal-organic frameworks via ordered functional groups. Chem. Commun. 2014, 50, 12154–12157. [Google Scholar] [CrossRef] [PubMed]
- Farrusseng, D.; Canivet, J.; Quadrelli, A. Design of functional metal-organic frameworks by post-synthetic modification. In Metal-Organic Frameworks: Application from Catalysis to Gas Storage; Farrusseng, D., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011; pp. 23–45. [Google Scholar]
- Deng, H.; Doonan, C.J.; Furukawa, H.; Ferreira, R.B.; Towne, J.; Knobler, C.B.; Wang, B.; Yaghi, O.M. Multiple functional groups of varying ratios in metal-organic frameworks. Science 2010, 327, 846–850. [Google Scholar] [CrossRef] [PubMed]
- Taddei, M.; Costantino, F.; Ienco, A.; Comotti, A.; Dau, V.P.; Cohen, M.S. Synthesis, breathing, and gas sorption study of the first isoreticular mixed-linker phosphonate based metal–organic frameworks. Chem. Commun. 2013, 49, 1315–1317. [Google Scholar] [CrossRef] [PubMed]
- Wee, L.H.; Alaerts, L.; Martens, J.A.; De Vos, D. Metal-organic frameworks as catalysts for organic reactions. In Metal-Organic Frameworks: Application from Catalysis to Gas Storage; Farrusseng, D., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011; pp. 195–208. [Google Scholar]
- Nguyen, J.G.; Cohen, S.M. Moisture-resistant and superhydrophobic metal-organic frameworks obtained via postsynthetic modification. J. Am. Chem. Soc. 2010, 132, 4560–4561. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Li, Y.; Li, Z. Tuning the moisture stability of metal-organic frameworks by incorporating hydrophobic functional groups at different positions of ligands. Chem. Commun. 2011, 47, 7377–7379. [Google Scholar] [CrossRef] [PubMed]
- Dhakshinamoorthy, A.; Alvaro, M.; Garcia, H. Metal-organic frameworks as heterogeneous catalysts for oxidation reactions. Catal. Sci. Technol. 2011, 1, 856–867. [Google Scholar] [CrossRef]
- Luz, I.; Corma, A.; Llabres, I.; Xamena, F.X. Cu-MOFs as active, selective and reusable catalysts for oxidative C-O bond coupling reactions by direct C-H activation of formamides, aldehydes and ethers. Catal. Sci. Technol. 2014, 4, 1829–1836. [Google Scholar]
- Dhakshinamoorthy, A.; Alvaro, M.; Garcia, H. Metal organic frameworks as efficient heterogeneous catalysts for the oxidation of benzylic compounds with t-butylhydroperoxide. J. Catal. 2009, 267, 1–4. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Alvaro, M.; Garcia, H. Aerobic Oxidation of Benzylic Alcohols Catalyzed by Metal-Organic Frameworks Assisted by TEMPO. ACS Catal. 2011, 1, 48–53. [Google Scholar] [CrossRef]
- Junghans, U.; Suttkus, C.; Lincke, J.; Lässig, D.; Krautscheid, H.; Gläser, R. Selective oxidation of cyclooctene over copper-containing metal-organic frameworks. Microporous Mesoporous Mater. 2015, 216, 151–160. [Google Scholar] [CrossRef]
- Jiang, D.; Mallat, T.M.; Meier, D.M.; Urakawa, A.; Baiker, A. Copper metal-organic framework: Structure and activity in the allylic oxidation of cyclohexene with molecular oxygen. J. Catal. 2010, 270, 26–33. [Google Scholar] [CrossRef]
- Cancino, P.; Paredes-García, V.; Aguirre, P.; Spodine, E. A reusable CuII based metal–organic framework as a catalyst for the oxidation of olefins. Catal. Sci. Technol. 2014, 4, 2599–2607. [Google Scholar] [CrossRef]
- Aguirre, P.; Brown, K.; Venegas-Yazigi, D.; Paredes-García, V.; Spodine, E. [Cu(H2btec)(bipy)]∞: Reusable Metal Organic Polymer Catalyst for Epoxidation Reactions. Macromol. Symp. 2011, 304, 65–71. [Google Scholar] [CrossRef]
- Brown, K.; Zolezzi, S.; Aguirre, P.; Venegas-Yazigi, D.; Paredes-Garcia, V.; Baggio, R.; Novak, M.A.; Spodine, E. [Cu(H(2)btec)(bipy)](infinity): A novel metal organic framework (MOF) as heterogeneous catalyst for the oxidation of olefins. Dalton Trans. 2009, 1422–1427. [Google Scholar] [CrossRef]
- Pramanik, A.; Abbina, S.; Das, G. Molecular, supramolecular structure and catalytic activity of transition metal complexes of phenoxy acetic acid derivatives. Polyhedron 2007, 26, 5225–5234. [Google Scholar] [CrossRef]
- Fu, Y.; Sun, D.; Qin, M.; Huang, R.; Li, Z. Cu(ii)-and Co(ii)-containing metal–organic frameworks (MOFs) as catalysts for cyclohexene oxidation with oxygen under solvent-free conditions. RSC Adv. 2012, 2, 3309–3314. [Google Scholar] [CrossRef]
- Cancino, P.; Paredes-García, V.; Aliaga, C.; Aguirre, P.; Aravena, D.; Spodine, E. Influence of the lanthanide(iii) ion in {[Cu3Ln2(oda)6(H2O)6 ]·nH2O} n (Ln III: La, Gd, Yb) catalysts on the heterogeneous oxidation of olefins. Catal. Sci. Technol. 2017, 7, 231–242. [Google Scholar] [CrossRef]
- Cancino, P.; Vega, A.; Santiago-Portillo, A.; Navalon, S.; Alvaro, M.; Aguirre, P.; Spodine, E.; García, H. A novel copper(II)–lanthanum(III ) metal organic framework as a selective catalyst for the aerobic oxidation of benzylic hydrocarbons and cycloalkenes. Catal. Sci. Technol. 2016, 6, 3727–3736. [Google Scholar] [CrossRef]
- Lässig, D.; Lincke, J.; Krautscheid, H. Highly functionalised 3,4,5-trisubstituted 1,2,4-triazoles for future use as ligands in coordination polymers. Tetrahedron Lett. 2010, 51, 653–656. [Google Scholar] [CrossRef]
- Bergmann, J.; Stein, K.; Kobalz, M.; Handke, M.; Lange, M.; Möllmer, J.; Heinke, F.; Oeckler, O.; Gläser, R.; Staudt, R.; et al. A series of isomorphous Metal-Organic Frameworks with rtl topology–Metal distribution and tunable sorption capacity via substitution of metal ions. Microporous Mesoporous Mater. 2015, 216, 56–63. [Google Scholar] [CrossRef]
- Lincke, J.; Lässig, D.; Kobalz, M.; Bergmann, J.; Handke, M.; Möllmer, J.; Lange, M.; Roth, C.; Möller, A.; Staudt, R.; et al. An Isomorphous Series of Cubic, Copper-Based Triazolyl Isophthalate MOFs: Linker Substitution and Adsorption Properties. Inorg. Chem. 2012, 51, 7579–7586. [Google Scholar] [CrossRef] [PubMed]
- Kobalz, M.; Lincke, J.; Kobalz, K.; Erhart, O.; Bergmann, J.; Lässig, D.; Lange, M.; Möllmer, J.; Gläser, R.; Staudt, R.; et al. Paddle Wheel Based Triazolyl Isophthalate MOFs: Impact of Linker Modification on Crystal Structure and Gas Sorption Properties. Inorg. Chem. 2016, 55, 3030–3039. [Google Scholar] [CrossRef] [PubMed]
- Lincke, J.; Lässig, D.; Moellmer, J.; Reichenbach, C.; Puls, A.; Moeller, A.; Gläser, R.; Kalies, G.; Staudt, R.; Krautscheid, H. A novel copper-based MOF material: Synthesis, characterization and adsorption studies. Microporous Mesoporous Mater. 2011, 142, 62–69. [Google Scholar] [CrossRef]
- Kobalz, K.; Kobalz, M.; Möllmer, J.; Junghans, U.; Lange, M.; Bergmann, J.; Dietrich, S.; Wecks, M.; Gläser, R.; Krautscheid, H. Bis(carboxyphenyl)-1,2,4-triazole Based Metal-Organic Frameworks: Impact of Metal Ion Substitution on Adsorption Performance. Inorg. Chem. 2016, 55, 6938–6948. [Google Scholar] [CrossRef] [PubMed]
- Worch, C.; Kettner, F.; Lässig, D.; Lincke, J.; Krautscheid, H.; Gläser, R. Tuning the catalytic activity of the heteronuclear coordination polymers [CoxZn1−x(tdc)(bipy)] and [CoxZn1−x(Me2trz–pba)2] in the epoxidation of cyclooctene via isomorphous substitution. Catal. Commun. 2014, 44, 46–49. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Asiri, A.M.; Garcia, H. Metal-Organic Frameworks as Catalysts for Oxidation Reactions. Chem. Eur. J. 2016, 22, 8012–8024. [Google Scholar] [CrossRef] [PubMed]
- Dhakshinamoorthy, A.; Asiri, A.M.; Garcia, H. Mixed-metal or mixed-linker metal organic frameworks as heterogeneous catalysts. Catal. Sci. Technol. 2016, 6, 5238–5261. [Google Scholar] [CrossRef]
- Leus, K.; Liu, Y.-Y.; van der Voort, P. Metal-Organic Frameworks as Selective or Chiral Oxidation Catalysts. Catal. Rev. Sci. Eng. 2014, 56, 1–56. [Google Scholar] [CrossRef]
- Blatov, V.A.; Shevchenko, A.P.; Serezhkin, V.N. TOPOS 3.2: A new version of the program package for multipurpose crystal-chemical analysis. J. Appl. Crystallogr. 2000, 33, 1193. [Google Scholar] [CrossRef]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef]
- Computing Pore Size Distribution—PSDsolv. Available online: http://supriyob.wordpress.com/about/research/ (accessed on 21 August 2016).
- Gelb, L.D.; Gubbins, K.E. Pore Size Distributions in Porous Glasses: A Computer Simulation Study. Langmuir 1999, 15, 305–308. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Gubbins, K.E. Fast method for computing pore size distributions of model materials. Langmuir 2006, 22, 7726–7731. [Google Scholar] [CrossRef] [PubMed]
- Horike, S.; Shimomura, S.; Kitagawa, S. Soft porous crystals. Nat. Chem. 2009, 1, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, S.; Kondo, M. Functional Micropore Chemistry of Crystalline Metal Complex-Assembled Compounds. Bull. Chem. Soc. Jpn. 1998, 71, 1739–1753. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Gurvich, L. Physico-chemical attractive force. II. J. Phys. Chem. Soc. Russ 1915, 47, 805–827. [Google Scholar]
- Lowell, S.; Shields, J.; Thomas, M.A.; Thommes, M. Characterization of Porous Solids and Powders: Surface Area, Pore Size and Density; Springer: Dordrecht, The Netherlands, 2010. [Google Scholar]
- Tonigold, M.; Lu, Y.; Mavrandonakis, A.; Puls, A.; Staudt, R.; Möllmer, J.; Sauer, J.; Volkmer, D. Pyrazolate-based cobalt(II)-containing metal-organic frameworks in heterogeneous catalytic oxidation reactions: Elucidating the role of entatic states for biomimetic oxidation processes. Chem. Eur. J. 2011, 17, 8671–8695. [Google Scholar] [CrossRef] [PubMed]
- Calero, S.; Gomez-Alvarez, P. Insights into the Adsorption of Water and Small Alcohols on the Open-Metal Sites of Cu-BTC via Molecular Simulation. J. Phys. Chem. C 2015, 119, 467–472. [Google Scholar] [CrossRef]
- Harvey, S.D.; Eckberg, A.D.; Thallapally, P.K. Evaluation of copper-1,3,5-benzenetricarboxylate metal-organic framework (Cu-MOF) as a selective sorbent for Lewis-base analytes. J. Sep. Sci. 2011, 34, 2418–2426. [Google Scholar] [CrossRef] [PubMed]
- Van Assche, T.R.C.; Duerinck, T.; Gutierrez Sevillano, J.J.; Calero, S.; Baron, G.V.; Denayer, J.F.M. High Adsorption Capacities and Two-Step Adsorption of Polar Adsorbates on Copper-Benzene-1,3,5-tricarboxylate Metal-Organic Framework. J. Phys. Chem. C 2013, 117, 18100–18111. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Alvaro, M.; Garcia, H. Commercial metal-organic frameworks as heterogeneous catalysts. Chem. Commun. 2012, 48, 11275–11288. [Google Scholar] [CrossRef] [PubMed]
- Schlichte, K.; Kratzke, T.; Kaskel, S. Improved synthesis, thermal stability and catalytic properties of the metal-organic framework compound CU3(BTC)(2). Microporous Mesoporous Mater. 2004, 73, 81–88. [Google Scholar] [CrossRef]
- Breck, D.W. Zeolite Molecular Sieves; Wiley-VCH Verlag GmbH & Co. KGaA: New York, NY, USA, 1974. [Google Scholar]
- Tonigold, M.; Lu, Y.; Bredenkötter, B.; Rieger, B.; Bahnmüller, S.; Hitzbleck, J.; Langstein, G.; Volkmer, D. Heterogeneous Catalytic Oxidation by MFU-1: A Cobalt(II)-Containing Metal-Organic Framework. Angew. Chem. Int. Ed. 2009, 48, 7546–7550. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Tonigold, M.; Bredenkotter, B.; Volkmer, D.; Hitzbleck, J.; Langstein, G. A Cobalt(II)-containing Metal-Organic Framework Showing Catalytic Activity in Oxidation Reactions. Z. Anorg. Allg. Chem. 2008, 634, 2411–2417. [Google Scholar] [CrossRef]
- Stoe & Cie GmbH. X-Area; Stoe & Cie GmbH: Darmstadt, Germany, 2006. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A: Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, K. Diamond 3.2f; Crystal Impact GbR: Bonn, Germany, 2010. [Google Scholar]
- Stoe & Cie GmbH. WinXPow 3.0.2.7; Stoe & Cie GmbH: Darmstadt, Germany, 2012. [Google Scholar]
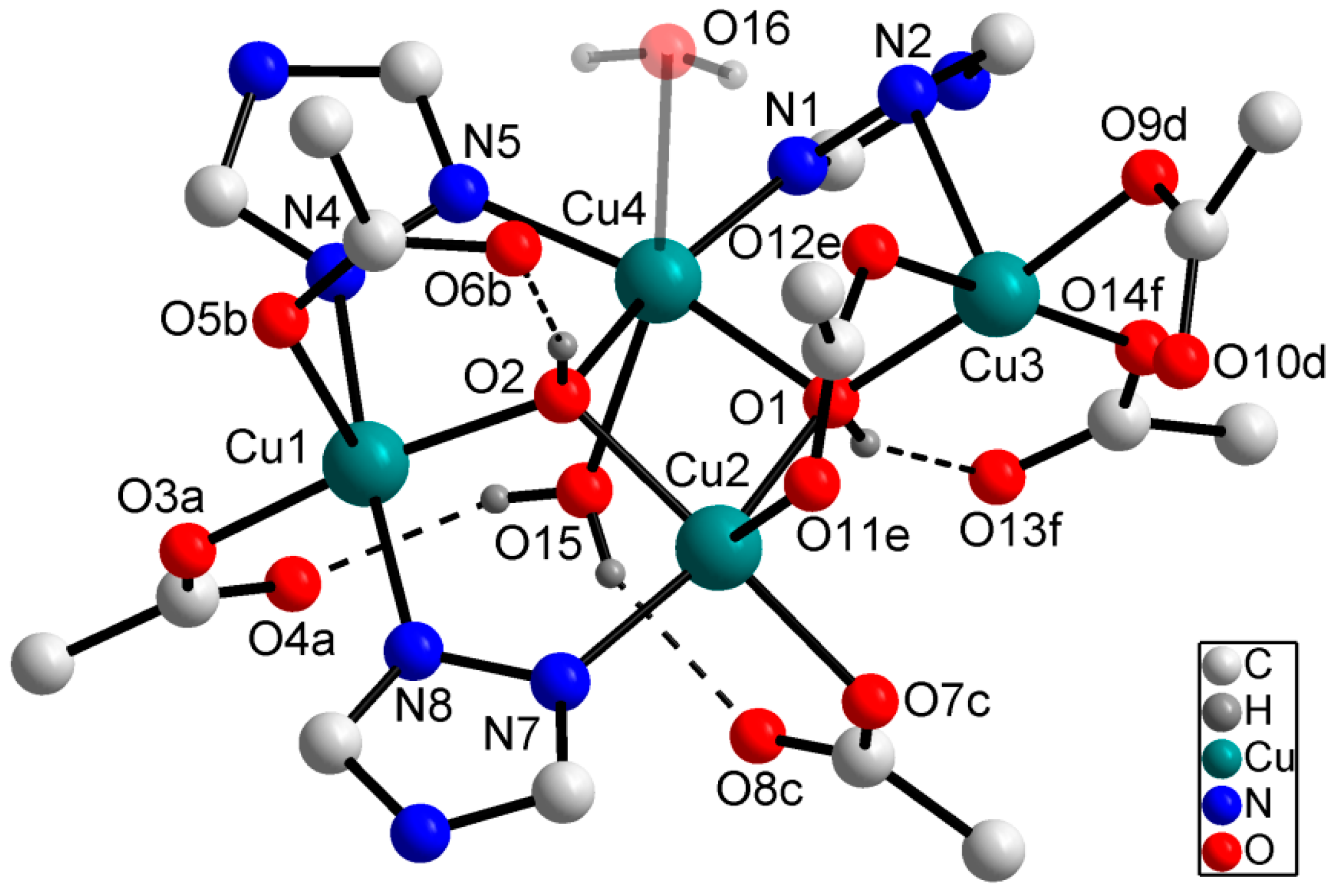
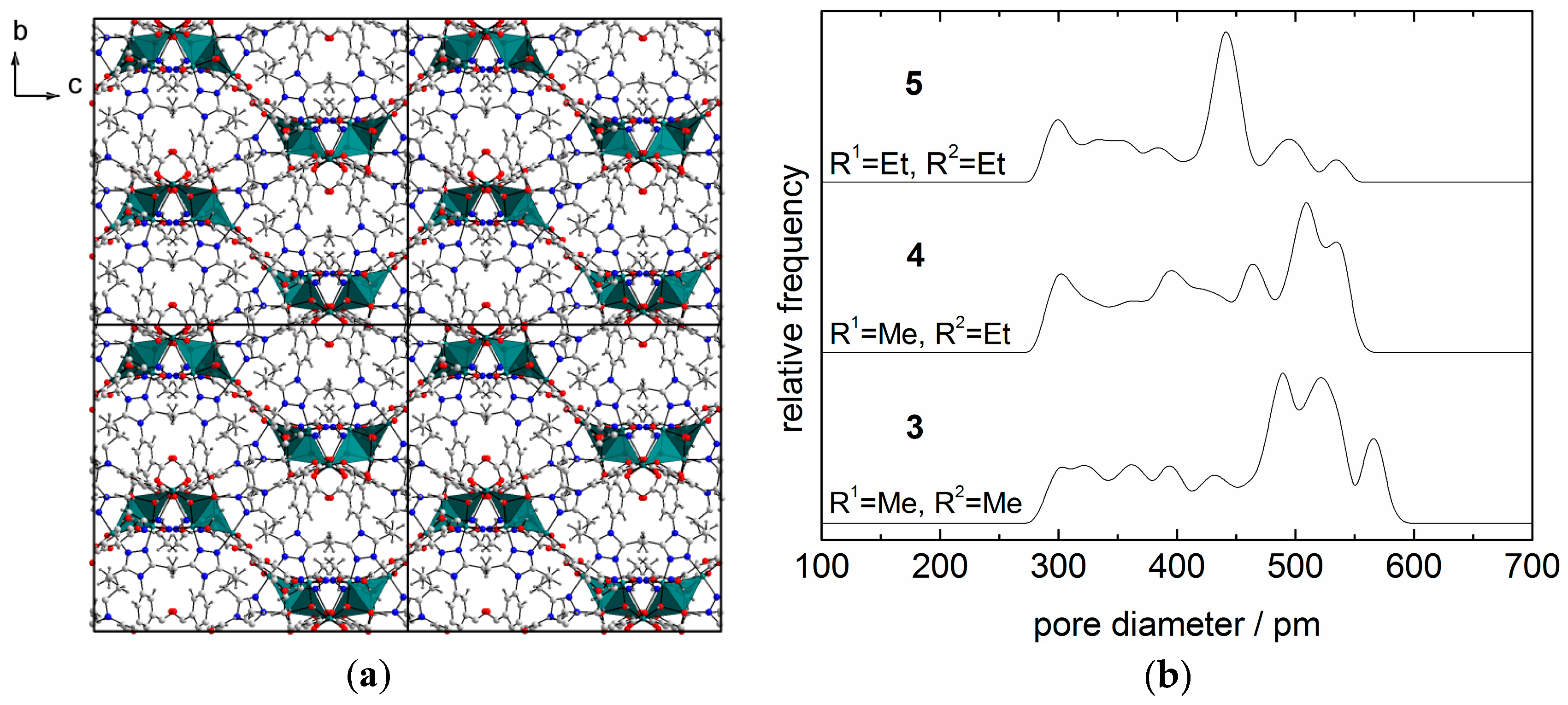

| MOF | Ligand | R1 | R2 | 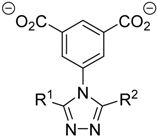 |
| 1 | (H-Me-trz-ia)2− | H | Me | |
| 2 | (H-Et-trz-ia)2− | H | Et | |
| 3 | (Me2-trz-ia)2− | Me | Me | |
| 4 | (Me-Et-trz-ia)2− | Me | Et | |
| 5 | (Et2-trz-ia)2− | Et | Et |
| MOF | 3 (R1 = R2 = Me) | 4 (R1 = Me, R2 = Et) | 5 (R1 = R2 = Et) |
| space group | (No. 61) | (No. 61) | (No. 61) |
| a/pm | 1875.00(8) | 1872.71(3) | 1854.69(5) |
| b/pm | 2428.78(6) | 2461.21(5) | 2420.12(7) |
| c/pm | 2540.75(7) | 2525.07(6) | 2547.32(7) |
| V/106 pm3 | 11,570.6(7) | 11,638.4(4) | 11,433.8(6) |
| Z | 8 | 8 | 8 |
| MOF | 3 | 4 | 5 | MOF | 3 | 4 | 5 |
|---|---|---|---|---|---|---|---|
| bond length/pm | angle/° | ||||||
| Cu1-O2 | 193.2(4) | 192.3(2) | 192.7(2) | Cu3-O1 | 196.8(3) | 194.7(3) | 195.5(2) |
| Cu1-N4 | 202.1(4) | 202.0(3) | 201.5(2) | Cu3-O12e | 195.6(4) | 195.8(3) | 195.1(2) |
| Cu1-O3a | 194.1(4) | 192.7(3) | 194.7(2) | Cu3-O9d | 196.4(4) | 194.7(3) | 194.3(2) |
| Cu1-N8 | 200.3(4) | 202.6(3) | 201.8(2) | Cu3-O14f | 197.5(4) | 196.1(3) | 196.6(2) |
| Cu1⋯O5b | 243.2(4) | 256.6(4) | 240.2(3) | Cu3⋯N2 | 246.9(5) | 268.3(5) | 256.2(3) |
| Cu2-O1 | 196.9(3) | 195.9(2) | 198.3(2) | Cu4-O1 | 197.6(3) | 197.0(3) | 198.0(2) |
| Cu2-O2 | 198.0(3) | 197.1(3) | 196.2(2) | Cu4-O2 | 196.8(4) | 196.3(2) | 195.7(2) |
| Cu2-O7c | 195.0(4) | 197.4(3) | 195.1(2) | Cu4-N1 | 198.4(5) | 198.5(3) | 198.8(3) |
| Cu2-N7 | 201.4(5) | 199.0(3) | 201.8(3) | Cu4-N5 | 203.6(4) | 201.0(3) | 202.5(2) |
| Cu2⋯O11e | 224.4(4) | 221.9(3) | 219.6(3) | Cu4⋯O15 | 230.0(4) | 243.0(4) | 225.4(3) |
| Cu4⋯O16 | - | 266.0(5) | - | ||||
| O2-Cu1⋯O5b | 91.6(1) | 84.9(1) | 91.89(8) | O1-Cu3⋯N2 | 82.8(2) | 79.7(1) | 81.24(9) |
| O2-Cu1-N4 | 87.5(2) | 88.1(1) | 86.77(9) | O1-Cu3-O14f | 95.7(2) | 95.1(1) | 96.01(9) |
| O2-Cu2⋯O11e | 97.3(1) | 99.0(1) | 98.08(8) | O1-Cu4⋯O15 | 88.5(1) | 86.8(1) | 90.22(9) |
| O2-Cu2-O1 | 80.7(1) | 81.2(1) | 81.08(8) | O1-Cu4-O2 | 80.8(1) | 81.1(1) | 81.28(8) |
| MOF | Pore Fraction/% [56] | ρ/g cm−3 | Vpore (cal.)/cm3 g−1 | Vpore (CO2)/cm3 g−1 | Vpore (N2)/cm3 g−1 | SBET/m2 g−1 |
|---|---|---|---|---|---|---|
| 1 | - a | - a | - a | 0.33 | 0.26 | 580 |
| 2 | - a | - a | - a | 0.22 | 0.16 | 345 |
| 3 | 39 | 1.244 | 0.31 | 0.36 | 0.26 | 648 |
| 4 | 34 | 1.306 | 0.26 | 0.26 | 0.25 | 680 |
| 5 | 28 | 1.357 | 0.21 | 0.19 | 0.14 | 319 |
| Catalyst | Cu Content a/wt % | TOF/h−1 |
|---|---|---|
| 1 | 21.9 | 2.8 |
| 2 | 21.2 | 3.1 |
| 3 | 22.5 | 2.6 |
| 4 | 21.9 | 3.0 |
| 5 | 18.7 | 4.4 |
| Cu3(BTC)2 | 29.6 | 3.0 |
| Cu(NO3)2 | 28.7 | 3.9 |
| Catalyst | t/h | XCyhex/% | SCyhex-TBP/% | TOF/h−1 | Reference |
|---|---|---|---|---|---|
| 5 | 7 | 56 | 77 | 4.4 | this work |
| MFU-1 a | 11 | 25 | 64 | 3.8 | [73] |
| [CoII(BPD)]·3DMF b | 12 | 62 | 83 | 3.7 | [74] |
| [Cu2L2] c | 7 | 82 | 56 | 6.6 | [50] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Junghans, U.; Kobalz, M.; Erhart, O.; Preißler, H.; Lincke, J.; Möllmer, J.; Krautscheid, H.; Gläser, R. A Series of Robust Copper-Based Triazolyl Isophthalate MOFs: Impact of Linker Functionalization on Gas Sorption and Catalytic Activity †. Materials 2017, 10, 338. https://doi.org/10.3390/ma10040338
Junghans U, Kobalz M, Erhart O, Preißler H, Lincke J, Möllmer J, Krautscheid H, Gläser R. A Series of Robust Copper-Based Triazolyl Isophthalate MOFs: Impact of Linker Functionalization on Gas Sorption and Catalytic Activity †. Materials. 2017; 10(4):338. https://doi.org/10.3390/ma10040338
Chicago/Turabian StyleJunghans, Ulrike, Merten Kobalz, Oliver Erhart, Hannes Preißler, Jörg Lincke, Jens Möllmer, Harald Krautscheid, and Roger Gläser. 2017. "A Series of Robust Copper-Based Triazolyl Isophthalate MOFs: Impact of Linker Functionalization on Gas Sorption and Catalytic Activity †" Materials 10, no. 4: 338. https://doi.org/10.3390/ma10040338
APA StyleJunghans, U., Kobalz, M., Erhart, O., Preißler, H., Lincke, J., Möllmer, J., Krautscheid, H., & Gläser, R. (2017). A Series of Robust Copper-Based Triazolyl Isophthalate MOFs: Impact of Linker Functionalization on Gas Sorption and Catalytic Activity †. Materials, 10(4), 338. https://doi.org/10.3390/ma10040338







