Luminescent Lanthanoid Calixarene Complexes and Materials
Abstract
1. Introduction
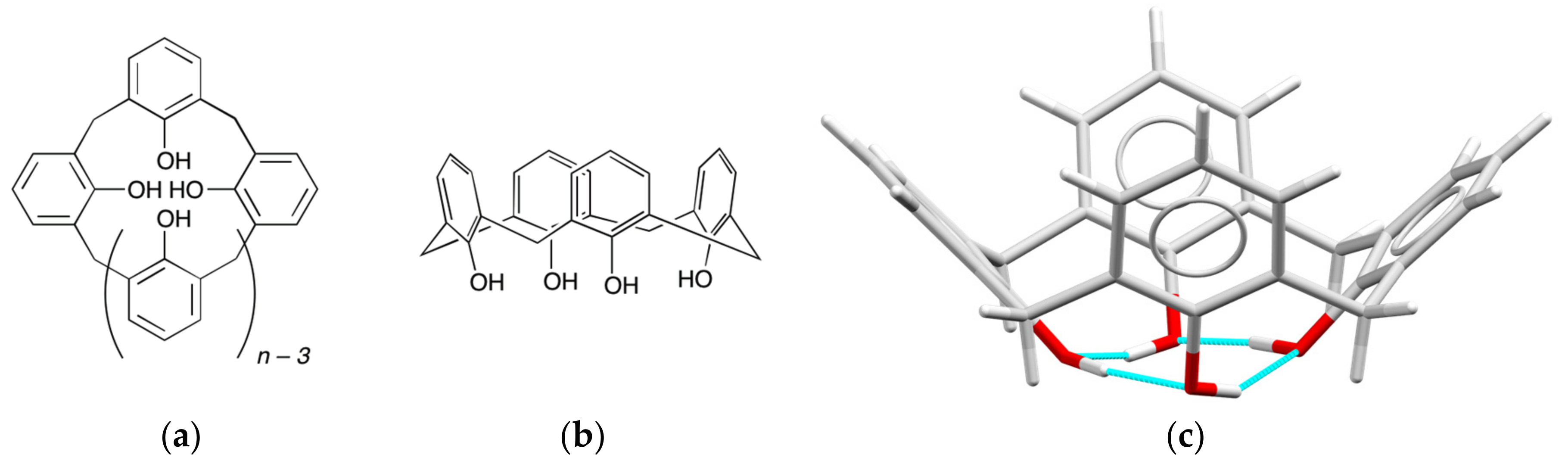
2. Background
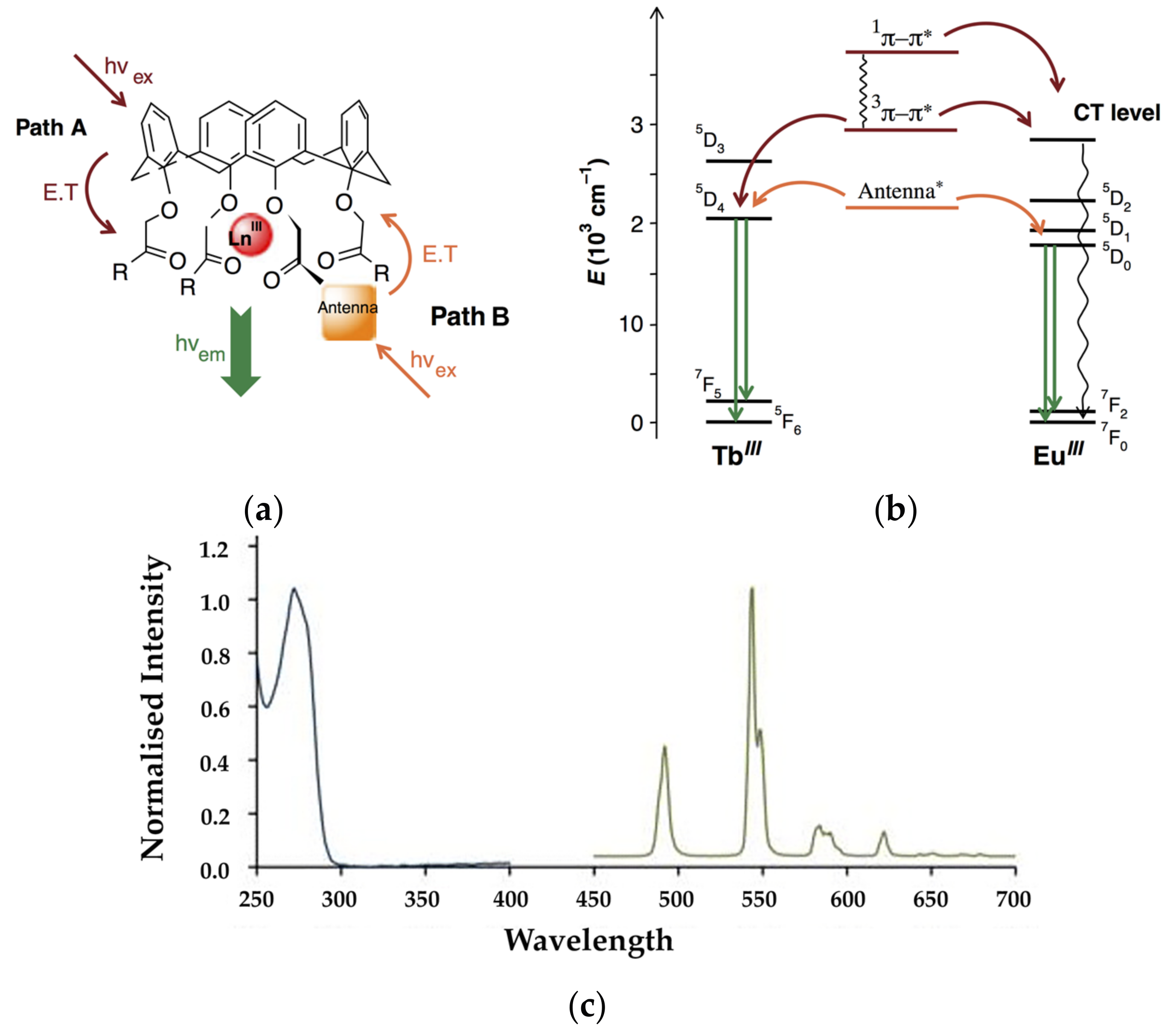
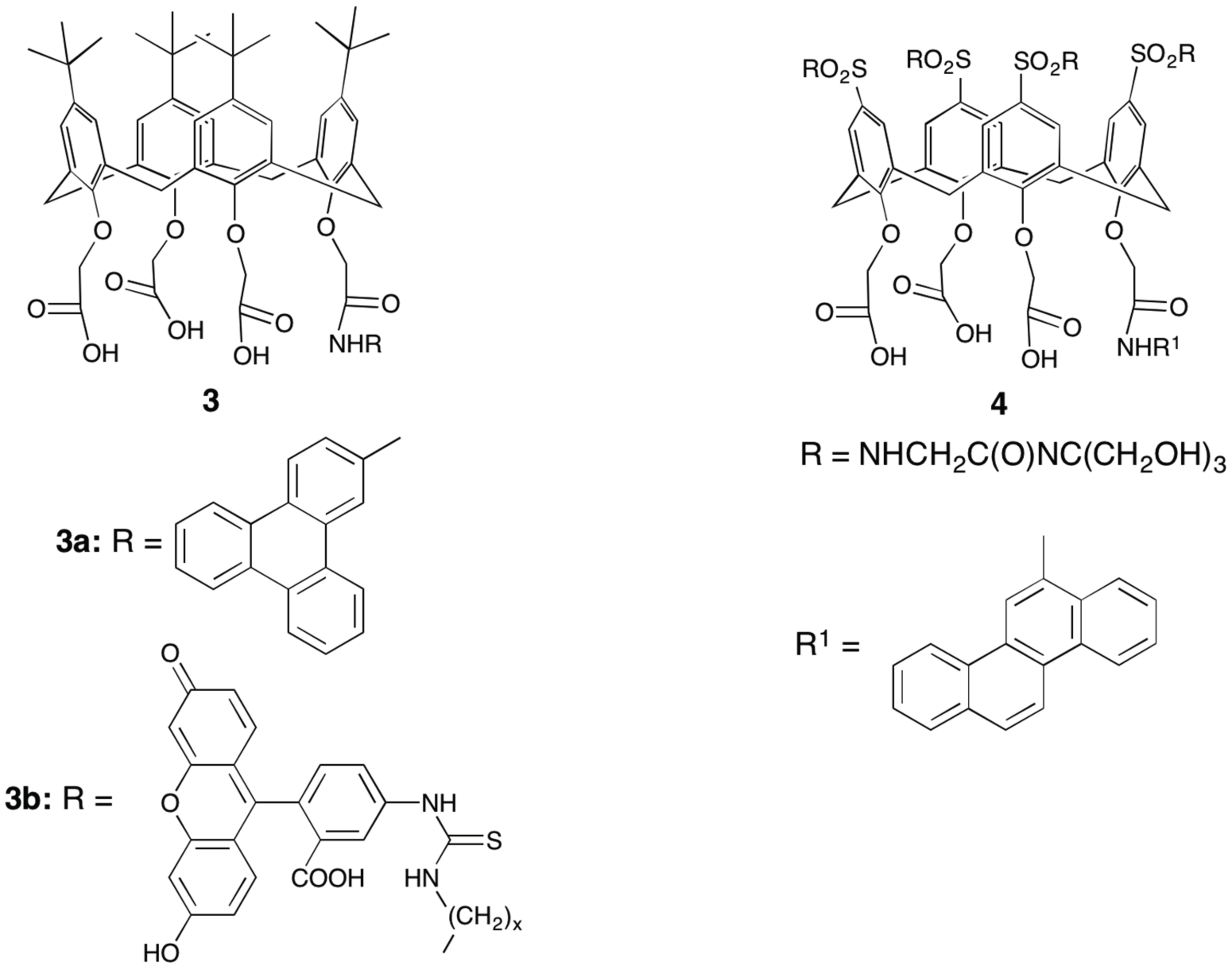
3. Early Work
4. Lanthanoid Calixarene Complexes in Functional Materials
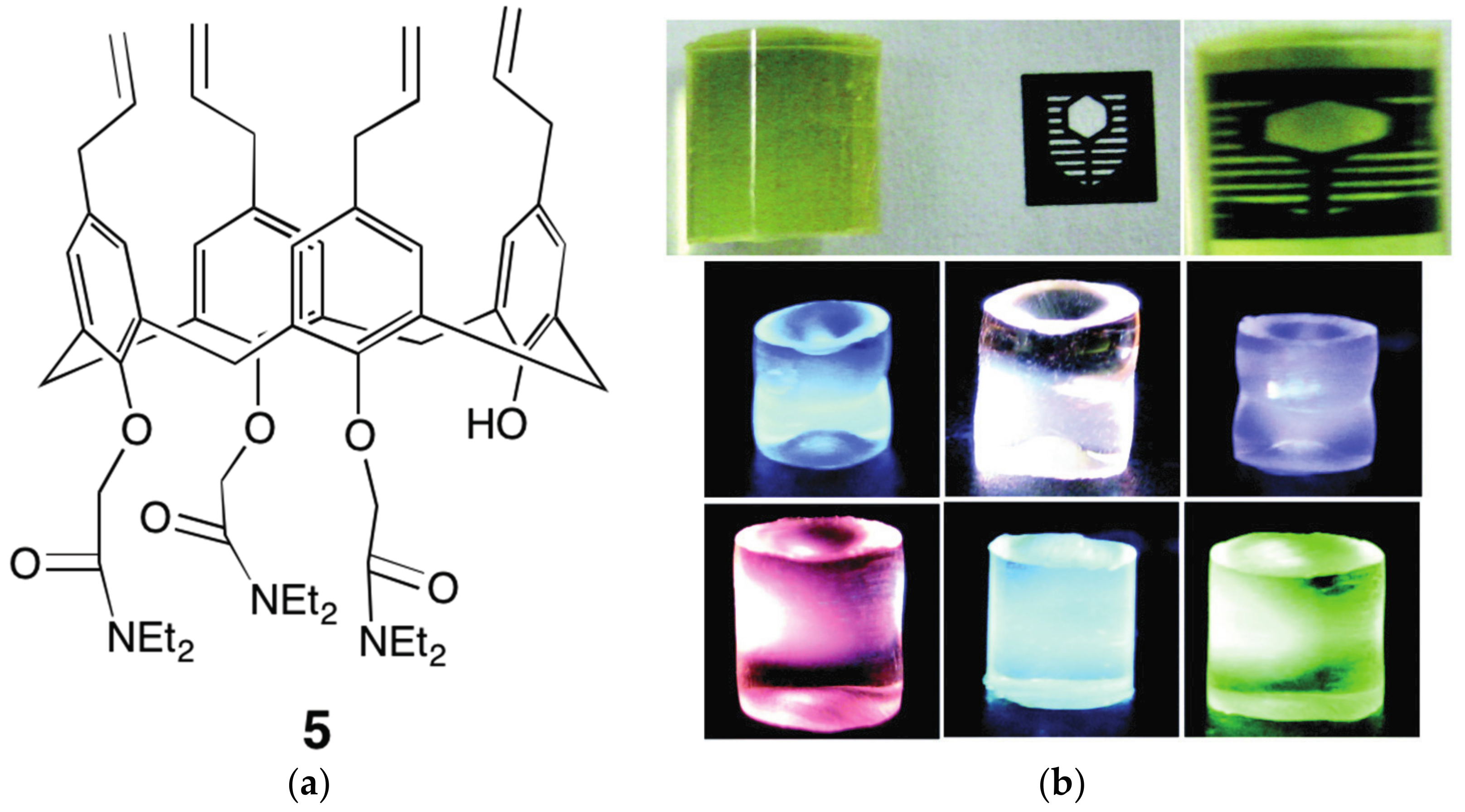
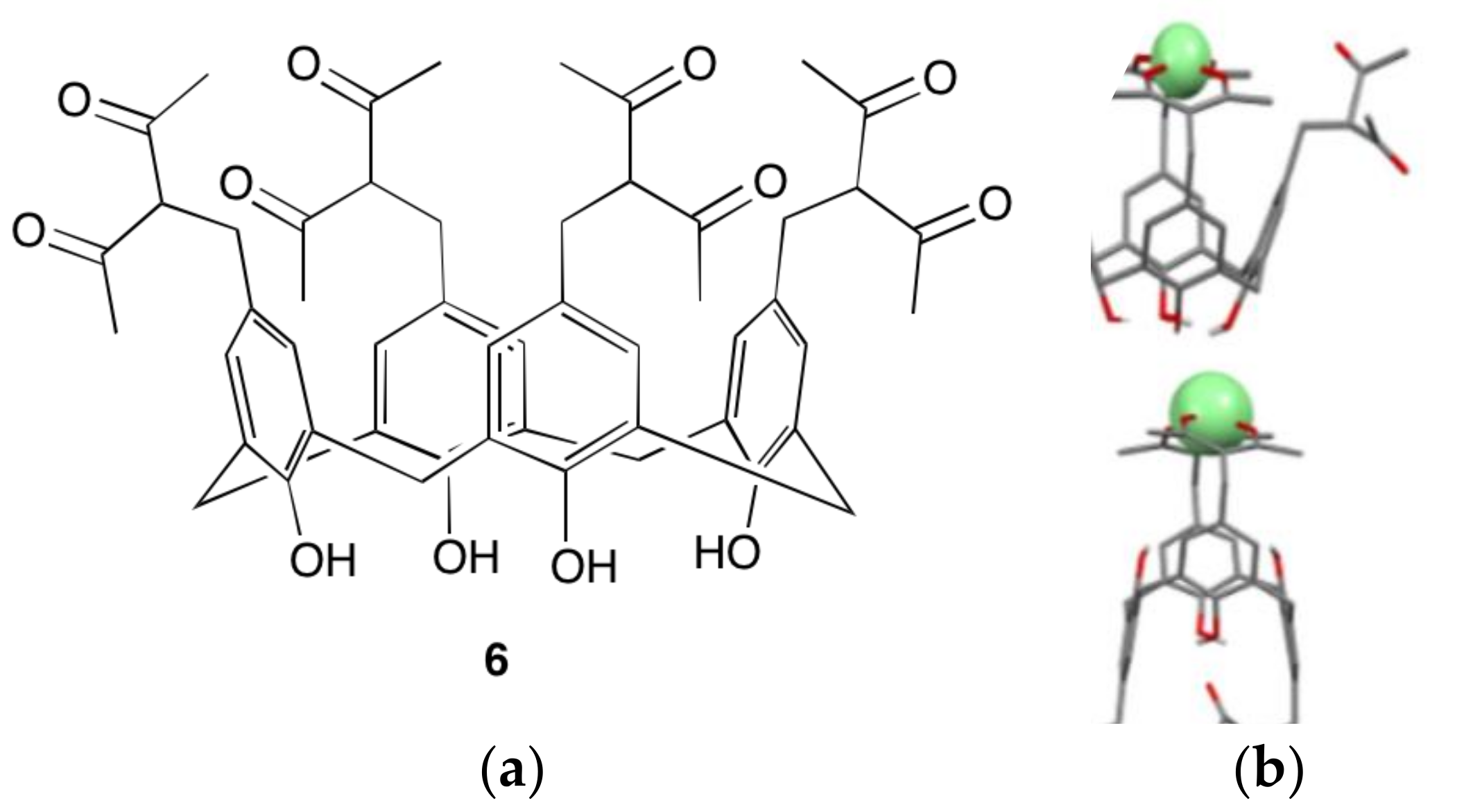
4.1. Polymeric Materials
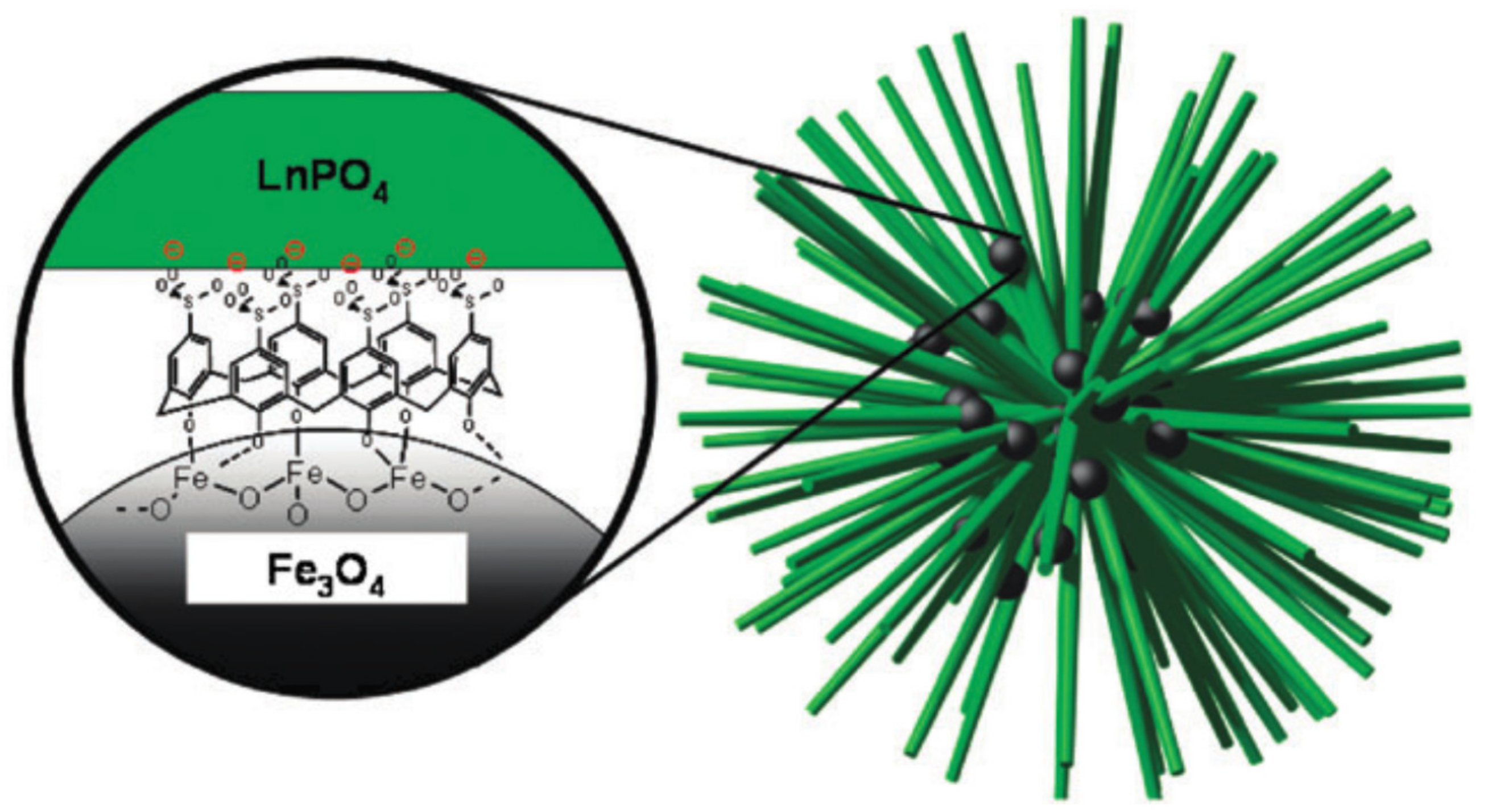
4.2. Nanoparticulate Materials
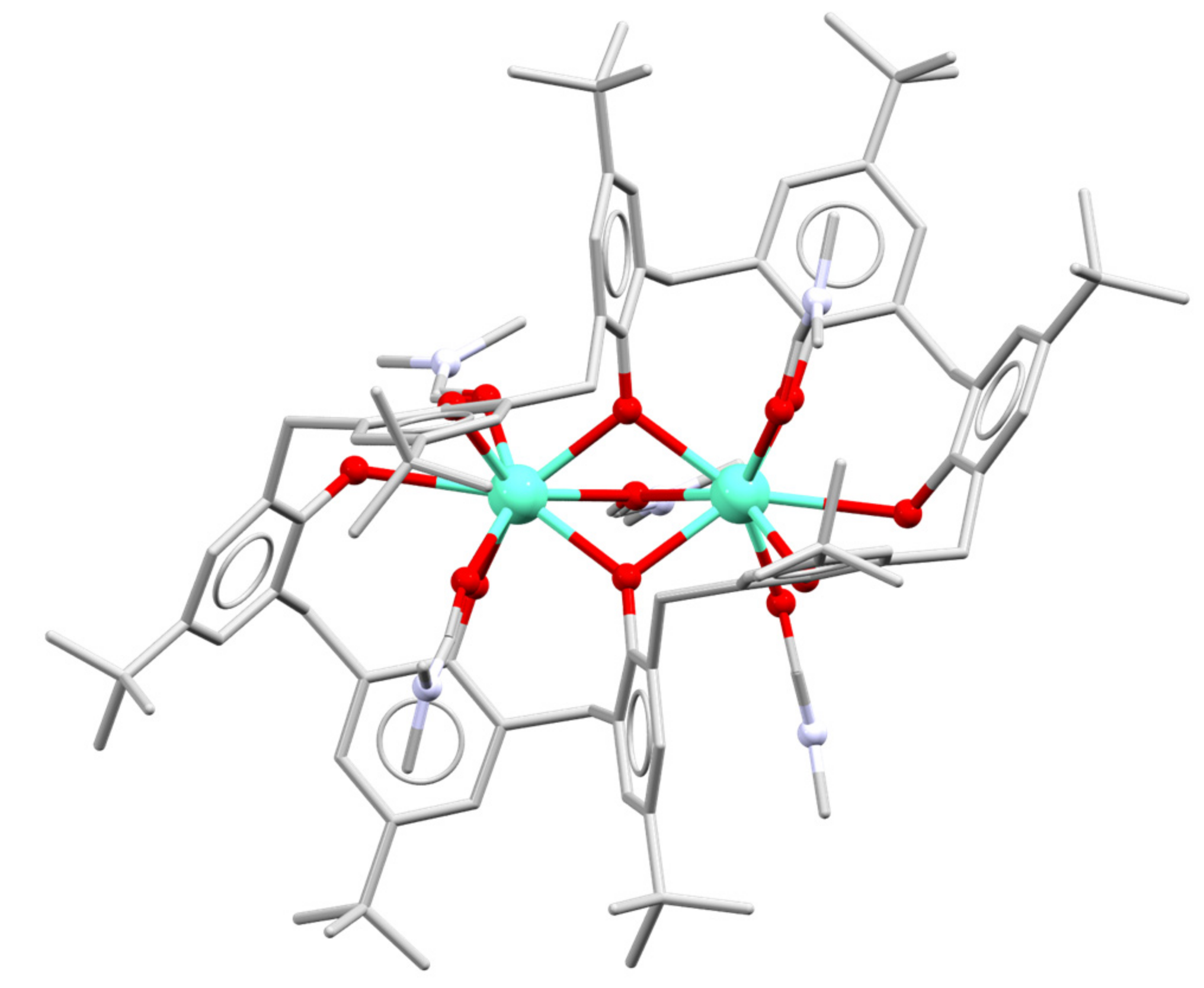
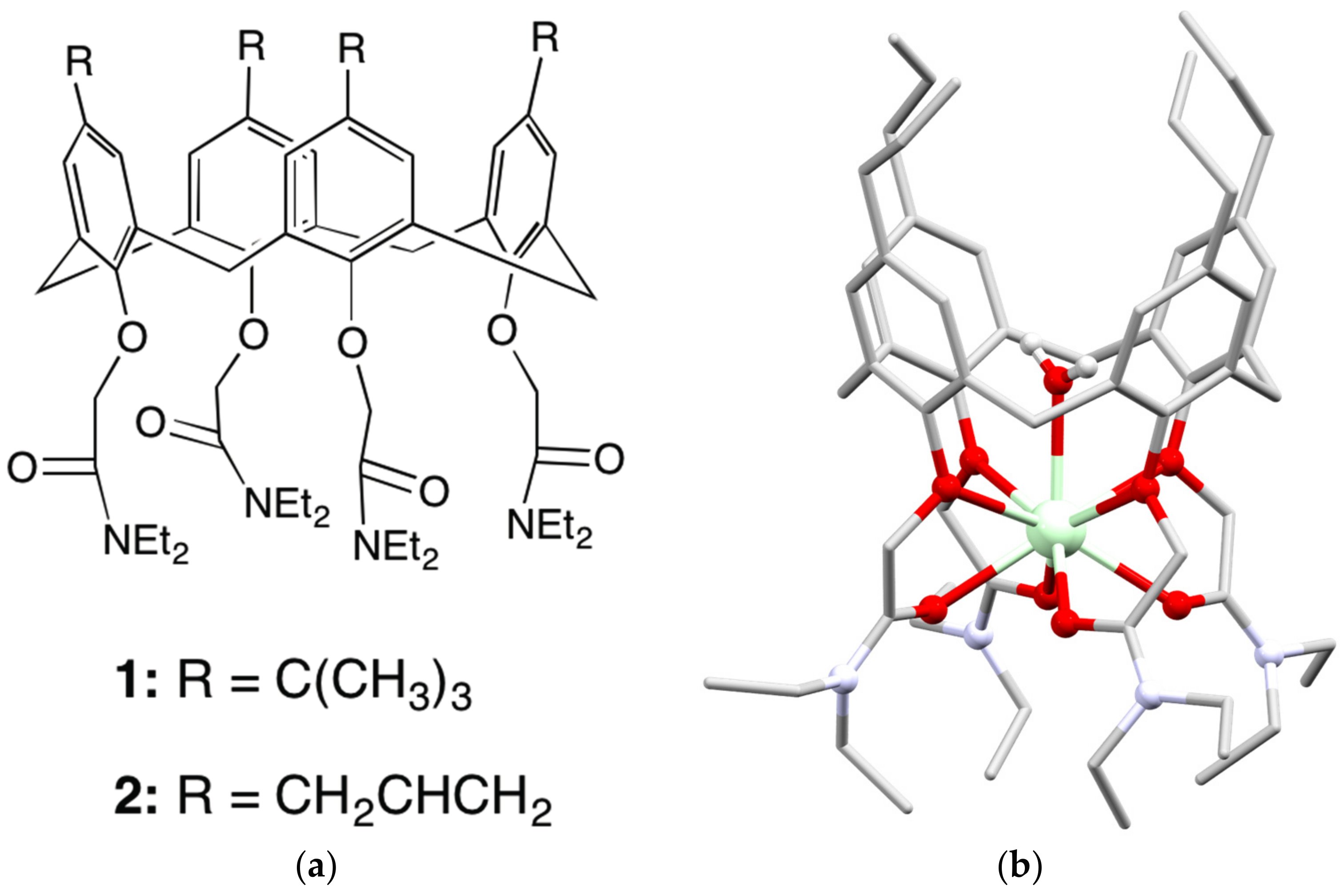
4.3. Lanthanoid Clusters
5. Conclusions
Conflicts of Interest
References
- Gutsche, C.D. Calixarenes: An Introduction, 2nd ed.; RSC Publishing: Cambridge, UK, 2008. [Google Scholar]
- Ogoshi, T.; Yamagishi, T.A.; Nakamoto, Y. Pillar-shaped macrocyclic hosts pillar[n]arenes: New key players for supramolecular chemistry. Chem. Rev. 2016, 116, 7937–8002. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Ko, K.C.; Lee, W.R.; Cho, J.; Moon, J.H.; Moon, D.; Sharma, A.; Lee, J.Y.; Kim, J.S.; Kim, S. Calix[n]triazoles and related conformational studies. Org. Lett. 2017, 19, 5509–5512. [Google Scholar] [CrossRef] [PubMed]
- Chinta, J.P.; Ramanujam, B.; Rao, C.P. Structural aspects of the metal ion complexes of the conjugates of calix[4]arene: Crystal structures and computational models. Coord. Chem. Rev. 2012, 256, 2762–2794. [Google Scholar] [CrossRef]
- Creaven, B.S.; Donlon, D.F.; McGinley, J. Coordination chemistry of calix[4]arene derivatives with lower rim functionalisation and their applications. Coord. Chem. Rev. 2009, 253, 893–962. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L. Application and luminescent properties of the complexes of lanthanide ions based on calixarene derivatives. Prog. Chem. 2010, 22, 427–432. [Google Scholar]
- Bradberry, S.J.; Savyasachi, A.J.; Martinez-Calvo, M.; Gunnlaugsson, T. Development of responsive visibly and nir luminescent and supramolecular coordination self-assemblies using lanthanide ion directed synthesis. Coord. Chem. Rev. 2014, 273, 226–241. [Google Scholar] [CrossRef]
- Binnemans, K. Lanthanide-based luminescent hybrid materials. Chem. Rev. 2009, 109, 4283–4374. [Google Scholar] [CrossRef] [PubMed]
- Bunzli, J.C.G. Lanthanide luminescence for biomedical analyses and imaging. Chem. Rev. 2010, 110, 2729–2755. [Google Scholar] [CrossRef] [PubMed]
- Atwood, J.L.; Barbour, L.J.; Jerga, A. Storage of methane and freon by interstitial van der waals confinement. Science 2002, 296, 2367–2369. [Google Scholar] [CrossRef] [PubMed]
- Bunzli, J.C.G. On the design of highly luminescent lanthanide complexes. Coord. Chem. Rev. 2015, 293, 19–47. [Google Scholar] [CrossRef]
- Eliseeva, S.V.; Bunzli, J.C.G. Lanthanide luminescence for functional materials and bio-sciences. Chem. Soc. Rev. 2010, 39, 189–227. [Google Scholar] [CrossRef] [PubMed]
- Eliseeva, S.V.; Bunzli, J.C.G. Rare earths: Jewels for functional materials of the future. New J. Chem. 2011, 35, 1165–1176. [Google Scholar] [CrossRef]
- Lincheneau, C.; Quinlan, E.; Kitchen, J.A.; McCabe, T.; Matthews, S.E.; Gunnlaugsson, T. Delayed lanthanide luminescent Tb(III) complexes formed from lower rim amide functionalised calix[4]arenes. Supramol. Chem. 2013, 25, 869–880. [Google Scholar] [CrossRef]
- Bunzli, J.C.G.; Froidevaux, P.; Harrowfield, J.M. Photophysical properties of lanthanide dinuclear complexes with p-tert-butylcalix[8]arene. Inorg. Chem. 1993, 32, 3306–3311. [Google Scholar] [CrossRef]
- Sato, N.; Shinkai, S. Energy-transfer luminescence of lanthanide ions encapsulated in sensitizer-modified calix[4]arenes. J. Chem. Soc. Perk. Trans. 2 1993, 621–624. [Google Scholar] [CrossRef]
- Furphy, B.M.; Harrowfield, J.M.; Kepert, D.L.; Skelton, B.W.; White, A.H.; Wilner, F.R. Bimetallic lanthanide complexes of the calixarenes—Europium(III) and tert-butylcalix[8]arene. Inorg. Chem. 1987, 26, 4231–4236. [Google Scholar] [CrossRef]
- Harrowfield, J.M.; Ogden, M.I.; White, A.H. Lanthanide ion complexes of calixarenes. VIII. Bimetallic lanthanide complexes of p-t-Butylcalix[8]arene from dimethylformamide solutions. Aust. J. Chem. 1991, 44, 1249–1262. [Google Scholar] [CrossRef]
- Harrowfield, J.M.; Ogden, M.I.; White, A.H. Lanthanide ion complexes of calixarenes. VII. Bimetallic lanthanide complexes of para-tert-butylcalix[8]arene from Dimethylsulfoxide solutions. Aust. J. Chem. 1991, 44, 1237–1247. [Google Scholar] [CrossRef]
- Froidevaux, P.; Bunzli, J.C.G. Complexes of lanthanoid salts with macrocyclic ligands. 42. Energy-transfer processes in lanthanide dinuclear complexes with p-tert-butylcalix[8]arene—An example of dipole-dipolar mechanism. J. Phys. Chem. 1994, 98, 532–536. [Google Scholar] [CrossRef]
- Latva, M.; Takalo, H.; Mukkala, V.M.; Matachescu, C.; RodriguezUbis, J.C.; Kankare, J. Correlation between the lowest triplet state energy level of the ligand and lanthanide(III) luminescence quantum yield. J. Lumin. 1997, 75, 149–169. [Google Scholar] [CrossRef]
- Sabbatini, N.; Guardigli, M.; Mecati, A.; Balzani, V.; Ungaro, R.; Ghidini, E.; Casnati, A.; Pochini, A. Encapsulation of lanthanide ions in calixarene receptors. A strongly luminescent terbium(3+) complex. J. Chem. Soc. Chem. Commun. 1990, 878–879. [Google Scholar] [CrossRef]
- Horrocks, W.D.; Sudnick, D.R. Lanthanide ion luminescence probes of the structure of biological macromolecules. Acc. Chem. Res. 1981, 14, 384–392. [Google Scholar] [CrossRef]
- Driscoll, C.R.; Skelton, B.W.; Massi, M.; Ogden, M.I. Structural characterisation and photophysical properties of lanthanoid complexes of a tetra-amide functionalised calix[4]arene. Supramol. Chem. 2016, 28, 567–574. [Google Scholar] [CrossRef]
- Sabbatini, N.; Guardigli, M.; Manet, I.; Ungaro, R.; Casnati, A.; Fischer, C.; Ziessel, R.; Ulrich, G. Synthesis and luminescence of Eu3+ and Tb3+ complexes with novel calix[4]arene ligands carrying 2,2’-bipyridine subunits. New J. Chem. 1995, 19, 137–140. [Google Scholar]
- Casnati, A.; Fischer, C.; Guardigli, M.; Isernia, A.; Manet, I.; Sabbatini, N.; Ungaro, R. Synthesis of calix[4]arene receptors incorporating (2,2′-bipyridin-6-yl)methyl and (9-methyl-1,10-phenanthrolin-2-yl)methyl chromophores and luminescence of their Eu3+ and Tb3+ complexes. J. Chem. Soc., Perk. Trans. 2 1996, 395–399. [Google Scholar] [CrossRef]
- Ulrich, G.; Ziessel, R.; Manet, I.; Guardigli, M.; Sabbatini, N.; Fraternali, F.; Wipff, G. Calix[4]arene podands and barrelands incorporating 2,2′-bipyridine moieties and their lanthanide complexes: Luminescence properties. Chem. Eur. J. 1997, 3, 1815–1822. [Google Scholar] [CrossRef]
- Rudkevich, D.M.; Verboom, W.; Vandertol, E.; Vanstaveren, C.J.; Kaspersen, F.M.; Verhoeven, J.W.; Reinhoudt, D.N. Calix[4]aarene-triacids as receptors for lanthanides—Synthesis and luminescence of neutral Eu3+ and Tb3+ complexes. J. Chem. Soc. Perk. Trans. 2 1995, 131–134. [Google Scholar] [CrossRef]
- Steemers, F.J.; Verboom, W.; Reinhoudt, D.N.; Vandertol, E.B.; Verhoeven, J.W. New sensitizer-modified calix[4]arenes enabling near-uv excitation of complexed luminescent lanthanide ions. J. Am. Chem. Soc. 1995, 117, 9408–9414. [Google Scholar] [CrossRef]
- Wolbers, M.P.O.; van Veggel, F.; Peters, F.G.A.; van Beelen, E.S.E.; Hofstraat, J.W.; Geurts, F.A.J.; Reinhoudt, D.N. Sensitised near-infrared emission from Nd3+ and Er3+ complexes of fluorescein-bearing calix[4]arene cages. Chem. Eur. J. 1998, 4, 772–780. [Google Scholar] [CrossRef]
- Steemers, F.J.; Meuris, H.G.; Verboom, W.; Reinhoudt, D.N.; vanderTol, E.B.; Verhoeven, J.W. Water-soluble neutral calix[4]arene-lanthanide complexes: Synthesis and luminescence properties. J. Org. Chem. 1997, 62, 4229–4235. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zhang, H.J. Hybrid materials based on lanthanide organic complexes: A review. Chem. Soc. Rev. 2013, 42, 387–410. [Google Scholar] [CrossRef] [PubMed]
- Stanley, J.M.; Holliday, B.J. Luminescent lanthanide-containing metallopolymers. Coord. Chem. Rev. 2012, 256, 1520–1530. [Google Scholar] [CrossRef]
- Driscoll, C.R.; Reid, B.L.; McIldowie, M.J.; Muzzioli, S.; Nealon, G.L.; Skelton, B.W.; Stagni, S.; Brown, D.H.; Massi, M.; Ogden, M.I. A “plug-and-play” approach to the preparation of transparent luminescent hybrid materials based on poly(methyl methacrylate), a calix[4]arene cross-linking agent, and terbium ions. Chem. Commun. 2011, 47, 3876–3878. [Google Scholar] [CrossRef] [PubMed]
- Ennis, B.W.; Muzzioli, S.; Reid, B.L.; D’Alessio, D.M.; Stagni, S.; Brown, D.H.; Ogden, M.I.; Massi, M. Recyclable calix[4]arene-lanthanoid luminescent hybrid materials with color-tuning and color-switching properties. Dalton Trans. 2013, 42, 6894–6901. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.F.; Zhang, H.Y.; Yan, B. Photoactive binary and ternary lanthanide (Eu3+, Tb3+, Nd3+) hybrids with p-tert-butylcalix[4]arene derived Si–O linkages and polymers. Dalton Trans. 2010, 39, 8882–8892. [Google Scholar] [CrossRef] [PubMed]
- Ovsyannikov, A.; Solovieva, S.; Antipin, I.; Ferlay, S. Coordination polymers based on calixarene derivatives: Structures and properties. Coord. Chem. Rev. 2017, 352, 151–186. [Google Scholar] [CrossRef]
- Gándara, F.; Gutiérrez-Puebla, E.; Iglesias, M.; Snejko, N.; Monge, M.Á. Isolated hexanuclear hydroxo lanthanide secondary building units in a rare-earth polymeric framework based on p-sulfonatocalix[4]arene. Cryst. Growth Des. 2010, 10, 128–134. [Google Scholar] [CrossRef]
- Barros, B.S.; de Oliveira, C.A.F.; Kulesza, J.; Alves, S.; Bochenska, M. Synthesis, characterization and luminescent properties of new coordination polymers based on p-tert-butylcalix[4]arene-tetracarboxylic acid and lanthanide cations. In Adaptive, Active and Multifunctional Smart Materials Systems; Vincenzini, P., Hahn, Y.B., Iannotta, S., Lendlein, A., Palermo, V., Paul, S., Sibilia, C., Silva, S.R.P., Srinivasan, G., Eds.; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2013; Volume 77, pp. 132–137. [Google Scholar]
- Lee, J.Y.; Kim, H.J.; Jung, J.H.; Sim, W.; Lee, S.S. Networking of calixcrowns: From heteronuclear endo/exocyclic coordination polymers to a photoluminescence switch. J. Am. Chem. Soc. 2008, 130, 13838–13839. [Google Scholar] [CrossRef] [PubMed]
- Zairov, R.; Mustafina, A.; Shamsutdinova, N.; Nizameev, I.; Moreira, B.; Sudakova, S.; Podyachev, S.; Fattakhova, A.; Safina, G.; Lundstrom, I.; et al. High performance magneto-fluorescent nanoparticles assembled from terbium and gadolinium 1,3-diketones. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Zairov, R.; Shamsutdinova, N.; Podyachev, S.; Sudakova, S.; Gimazetdinova, G.; Rizvanov, I.; Syakaev, V.; Babaev, V.; Amirov, R.; Mustafina, A. Structure impact in antenna effect of novel upper rim substituted tetra-1,3-diketone calix[4]arenes on Tb(III) green and Yb(III) NIR-luminescence. Tetrahedron 2016, 72, 2447–2455. [Google Scholar] [CrossRef]
- Shamsutdinova, N.A.; Podyachev, S.N.; Sudakova, S.N.; Mustafina, A.R.; Zairov, R.R.; Burilov, V.A.; Nizameev, I.R.; Rizvanov, I.K.; Syakaev, V.V.; Gabidullin, B.M.; et al. A facile synthetic route to convert Tb(III) complexes of novel tetra-1,3-diketone calix[4]resorcinarene into hydrophilic luminescent colloids. New J. Chem. 2014, 38, 4130–4140. [Google Scholar] [CrossRef]
- Fang, J.; Saunders, M.; Guo, Y.L.; Lu, G.Z.; Raston, C.L.; Iyer, K.S. Green light-emitting Lapo4: Ce3+:Tb3+ koosh nanoballs assembled by p-sulfonato-calix[6]arene coated superparamagnetic Fe3O4. Chem. Commun. 2010, 46, 3074–3076. [Google Scholar] [CrossRef] [PubMed]
- Harrowfield, J.; Koutsantonis, G. Calixarenes as cluster keepers. In Calixarenes in the Nanoworld; Vicens, J., Harrowfield, J., Baklouti, L., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 197–212. [Google Scholar]
- Furphy, B.M.; Harrowfield, J.M.; Ogden, M.I.; Skelton, B.W.; White, A.H.; Wilner, F.R. Lanthanide ion complexes of the calixarenes. Part 4. Double inclusion by p-t-butylcalix[4]arene (H4L). Crystal structures of [Eu2(HL)2(dmf)4].7dmf (dmf = dimethylformamide) and H4L.dmso (dmso = dimethyl sulphoxide). J. Chem. Soc. Dalton Trans. 1989, 2217–2221. [Google Scholar] [CrossRef]
- Sanz, S.; McIntosh, R.D.; Beavers, C.M.; Teat, S.J.; Evangelisti, M.; Brechin, E.K.; Dalgarno, S.J. Calix[4]arene-supported rare earth octahedra. Chem. Commun. 2012, 48, 1449–1451. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, D.N.; Winpenny, R.E.P.; Layfield, R.A. Lanthanide single-molecule magnets. Chem. Rev. 2013, 113, 5110–5148. [Google Scholar] [CrossRef] [PubMed]
- Bilyk, A.; Hall, A.K.; Harrowfield, J.M.; Hosseini, M.W.; Skelton, B.W.; White, A.H. A unique rare-earth cluster within a calixarene sandwich: Parallels in the chemistry of cyclosiloxanes and calixarenes. Aust. J. Chem. 2001, 53, 895–898. [Google Scholar] [CrossRef]
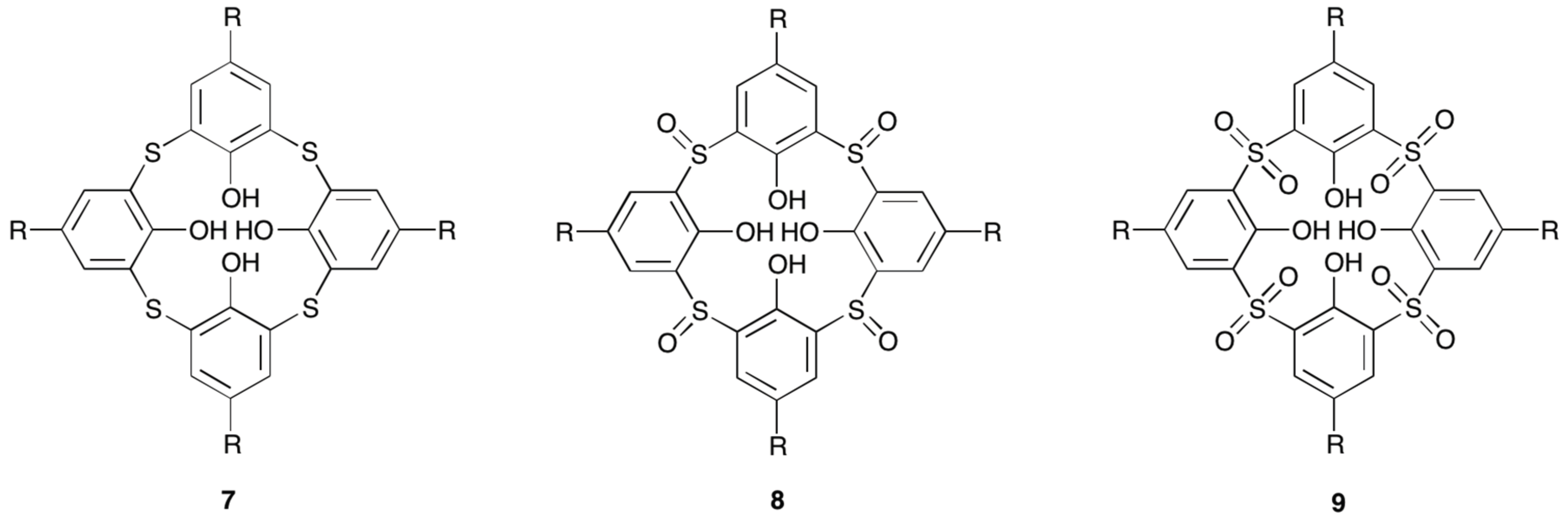
- Iki, N.; Tanaka, T.; Hiro-oka, S.; Shinoda, K. Self-assembly of a trilanthanide(III) core sandwiched between two thiacalix[4]arene ligands. Eur. J. Inorg. Chem. 2016, 5020–5027. [Google Scholar] [CrossRef]
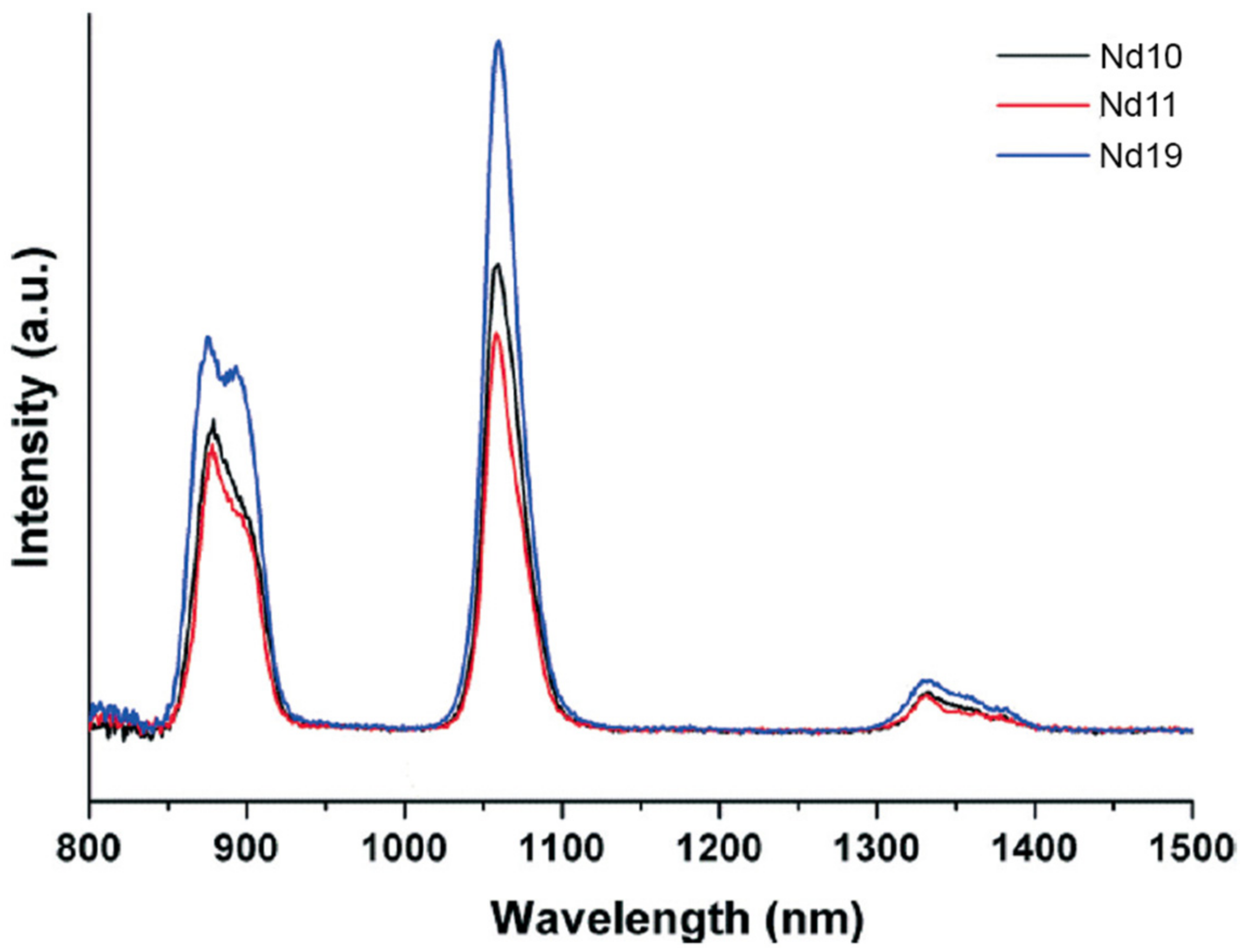
- Su, K.Z.; Jiang, F.L.; Wu, M.Y.; Qian, J.J.; Pang, J.D.; Yuan, D.Q.; Hong, M.C. Syntheses, structures, luminescence and magnetic properties of three high-nuclearity neodymium compounds based on mixed sulfonylcalix[4]arene-phosphonate ligands. CrystEngComm 2016, 18, 4921–4928. [Google Scholar] [CrossRef]
- Shangguan, L.Q.; Xing, H.; Mondal, J.H.; Shi, B.B. Novel rare earth fluorescent supramolecular polymeric assemblies constructed by orthogonal pillar[5]arene-based molecular recognition, Eu(III)-coordination and π–π donor–acceptor interactions. Chem. Commun. 2017, 53, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Shamsutdinova, N.; Zairov, R.; Mustafina, A.; Podyachev, S.; Sudakova, S.; Nizameev, I.; Kadirov, M.; Amirov, R. Interfacial interactions of hard polyelectrolyte-stabilized luminescent colloids with substrates. Colloids Surf. A 2015, 482, 231–240. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Massi, M.; Ogden, M.I. Luminescent Lanthanoid Calixarene Complexes and Materials. Materials 2017, 10, 1369. https://doi.org/10.3390/ma10121369
Massi M, Ogden MI. Luminescent Lanthanoid Calixarene Complexes and Materials. Materials. 2017; 10(12):1369. https://doi.org/10.3390/ma10121369
Chicago/Turabian StyleMassi, Massimiliano, and Mark I. Ogden. 2017. "Luminescent Lanthanoid Calixarene Complexes and Materials" Materials 10, no. 12: 1369. https://doi.org/10.3390/ma10121369
APA StyleMassi, M., & Ogden, M. I. (2017). Luminescent Lanthanoid Calixarene Complexes and Materials. Materials, 10(12), 1369. https://doi.org/10.3390/ma10121369




