The Preparation and Optical Properties of Novel LiLa(MoO4)2:Sm3+,Eu3+ Red Phosphor
Abstract
1. Introduction
2. Experimental
2.1. Sample Preparation
2.2. Characterization of Samples
3. Results and Discussion
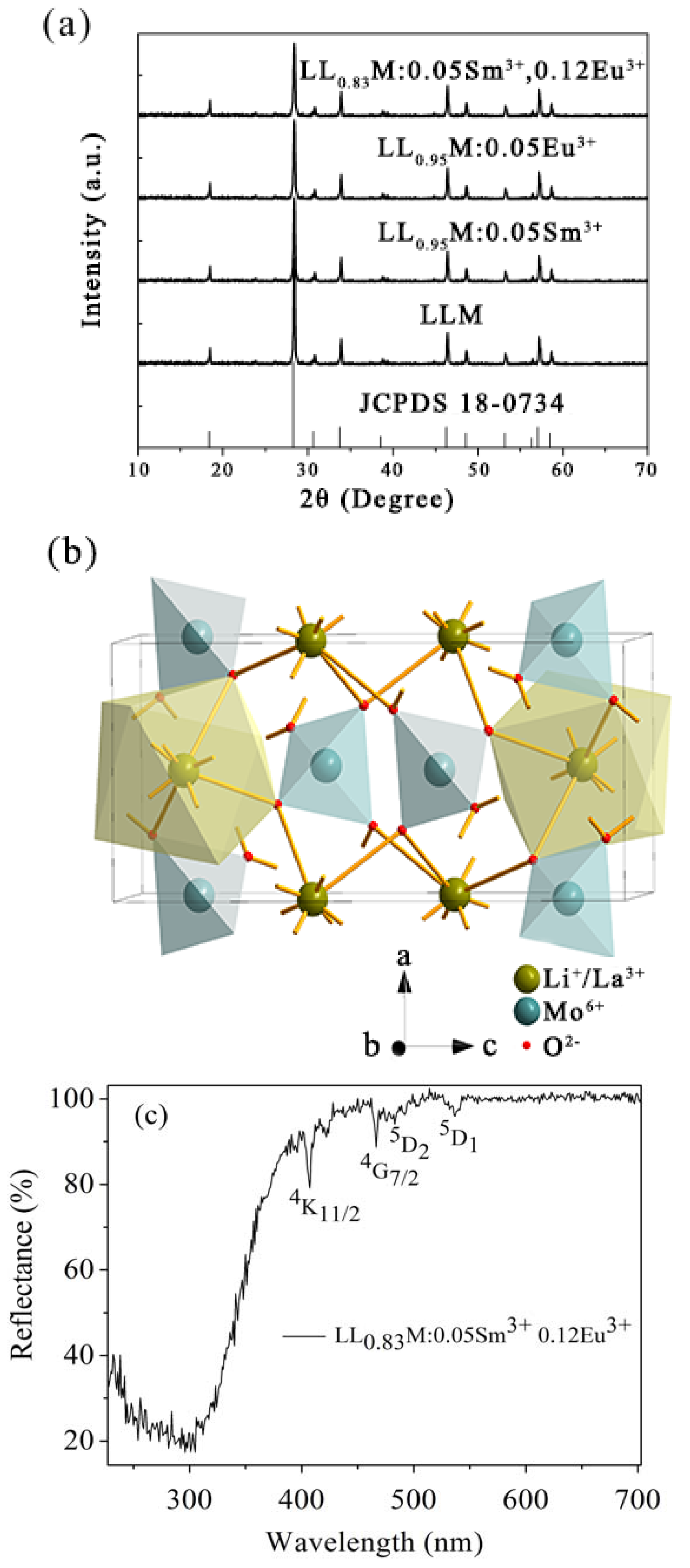
3.1. X-Ray Diffraction Patterns
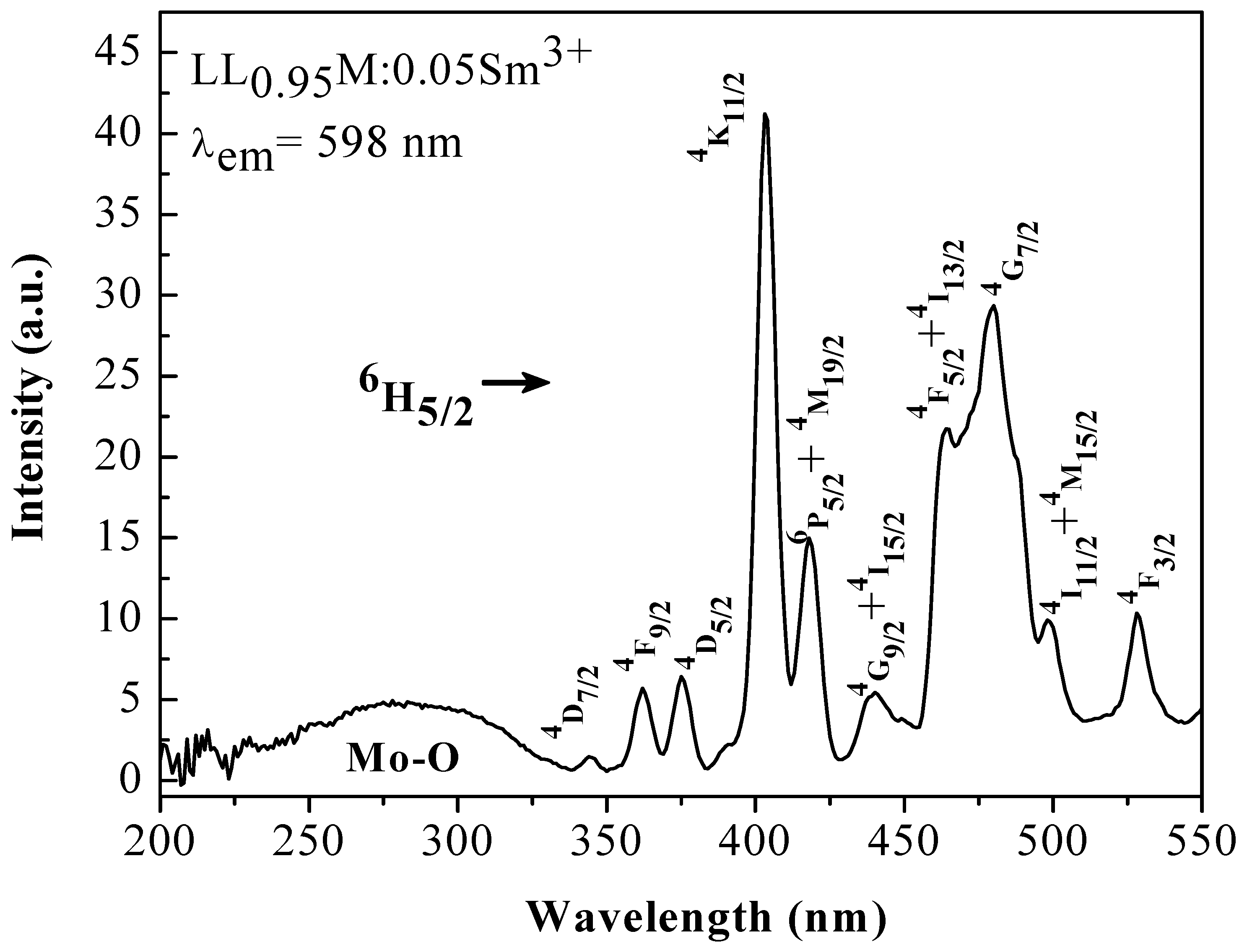
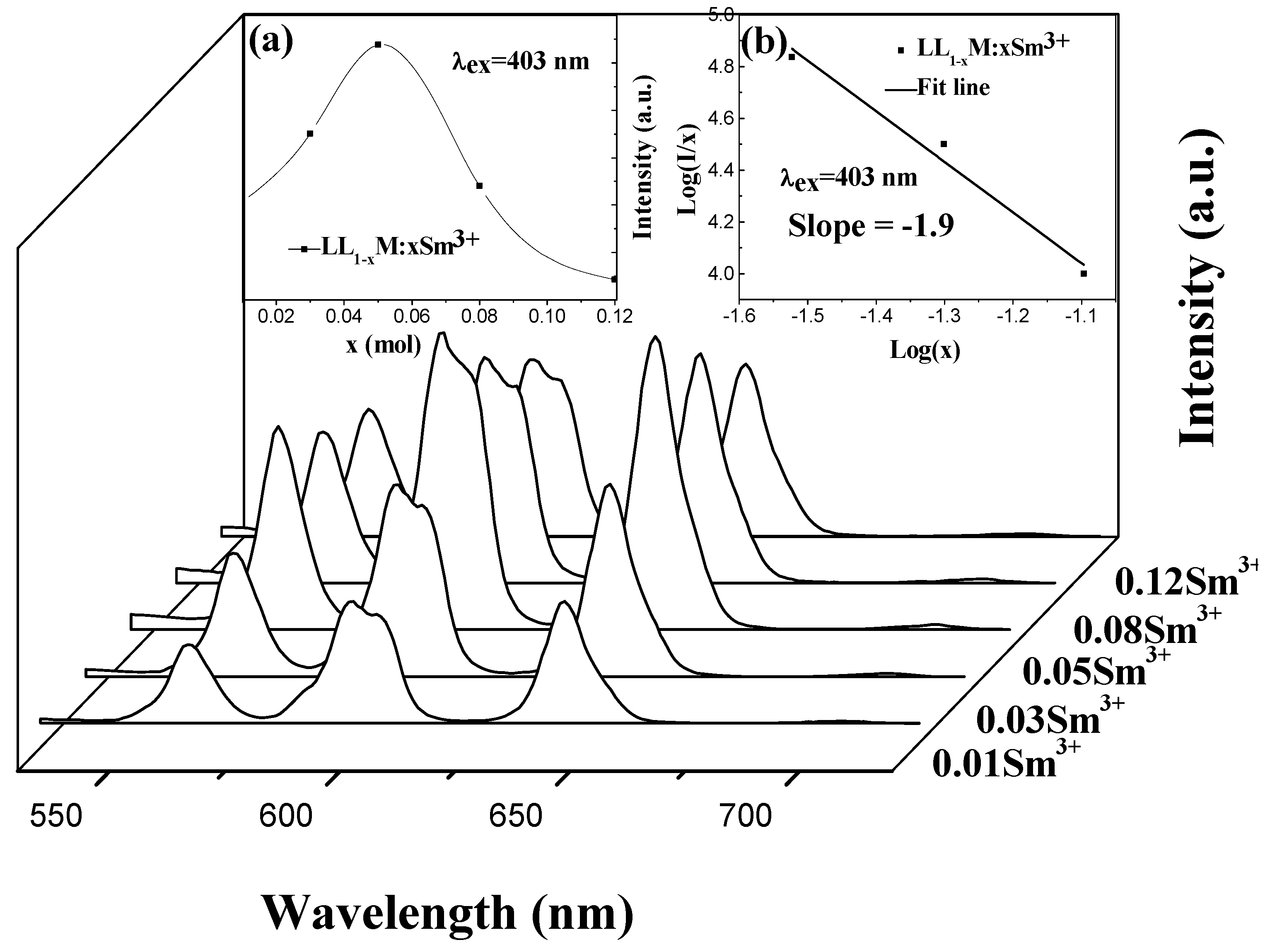
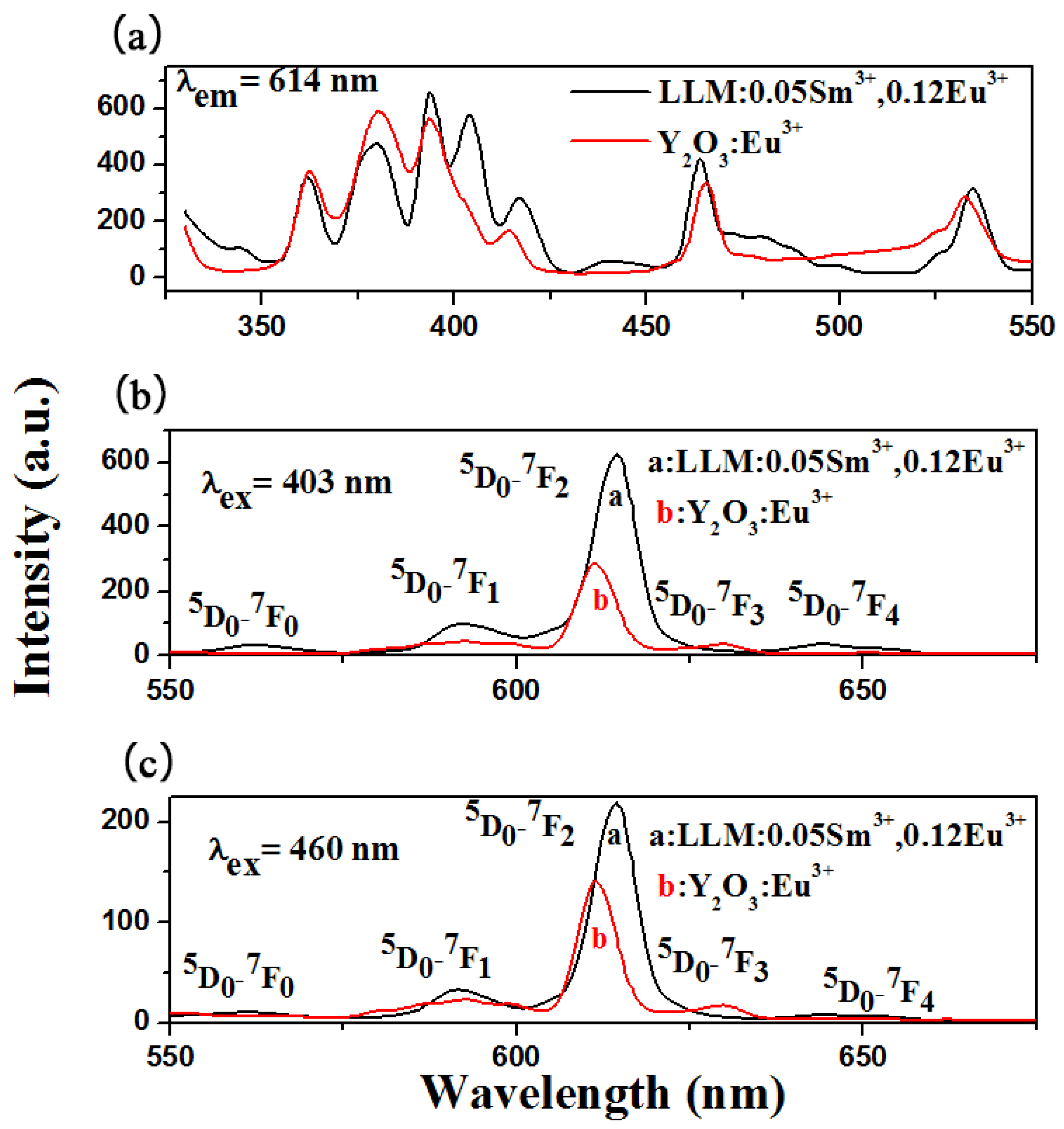
3.2. The Excitation and Emission Spectra of LiLa1−x(MoO4)2 Doped with Sm3+
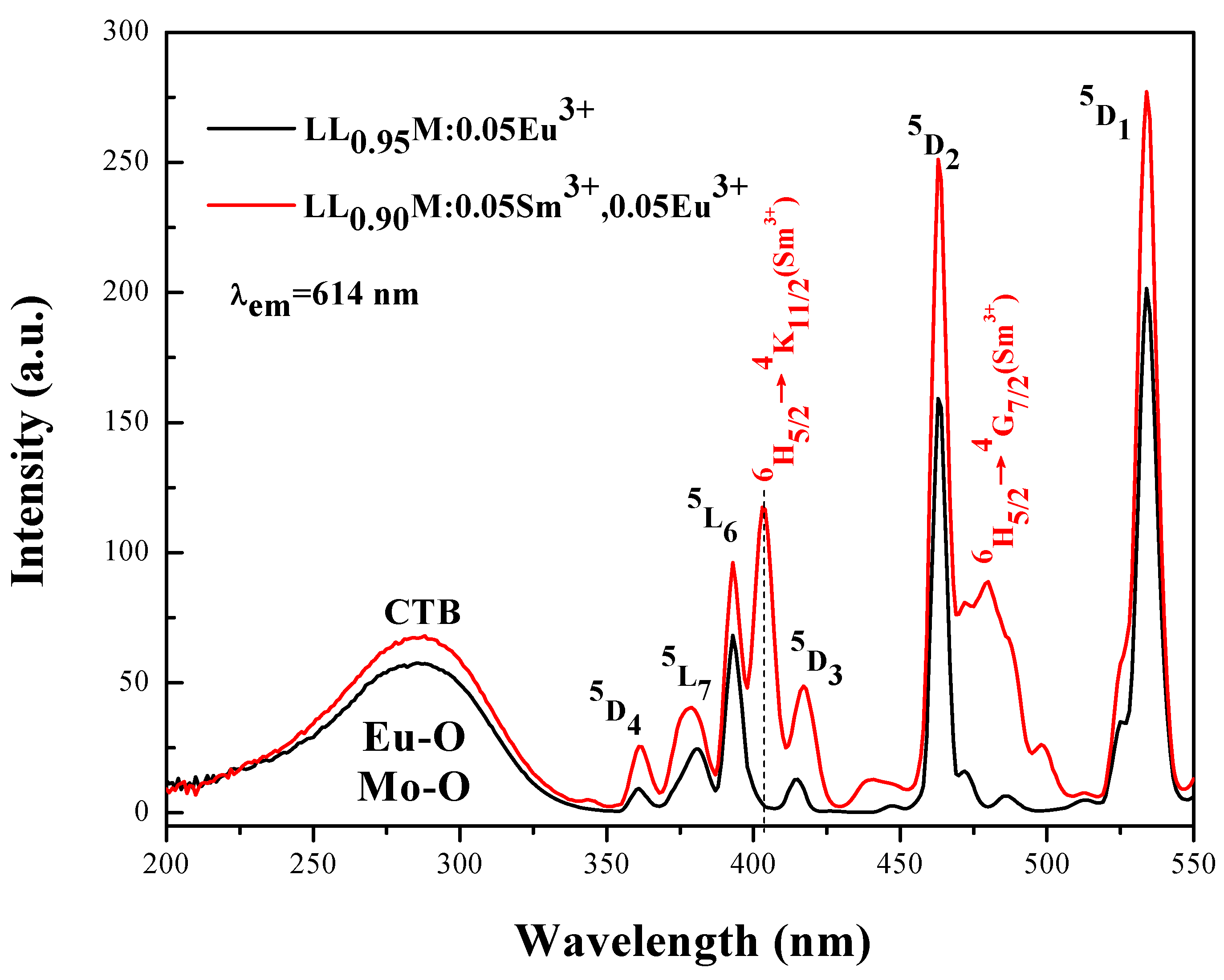
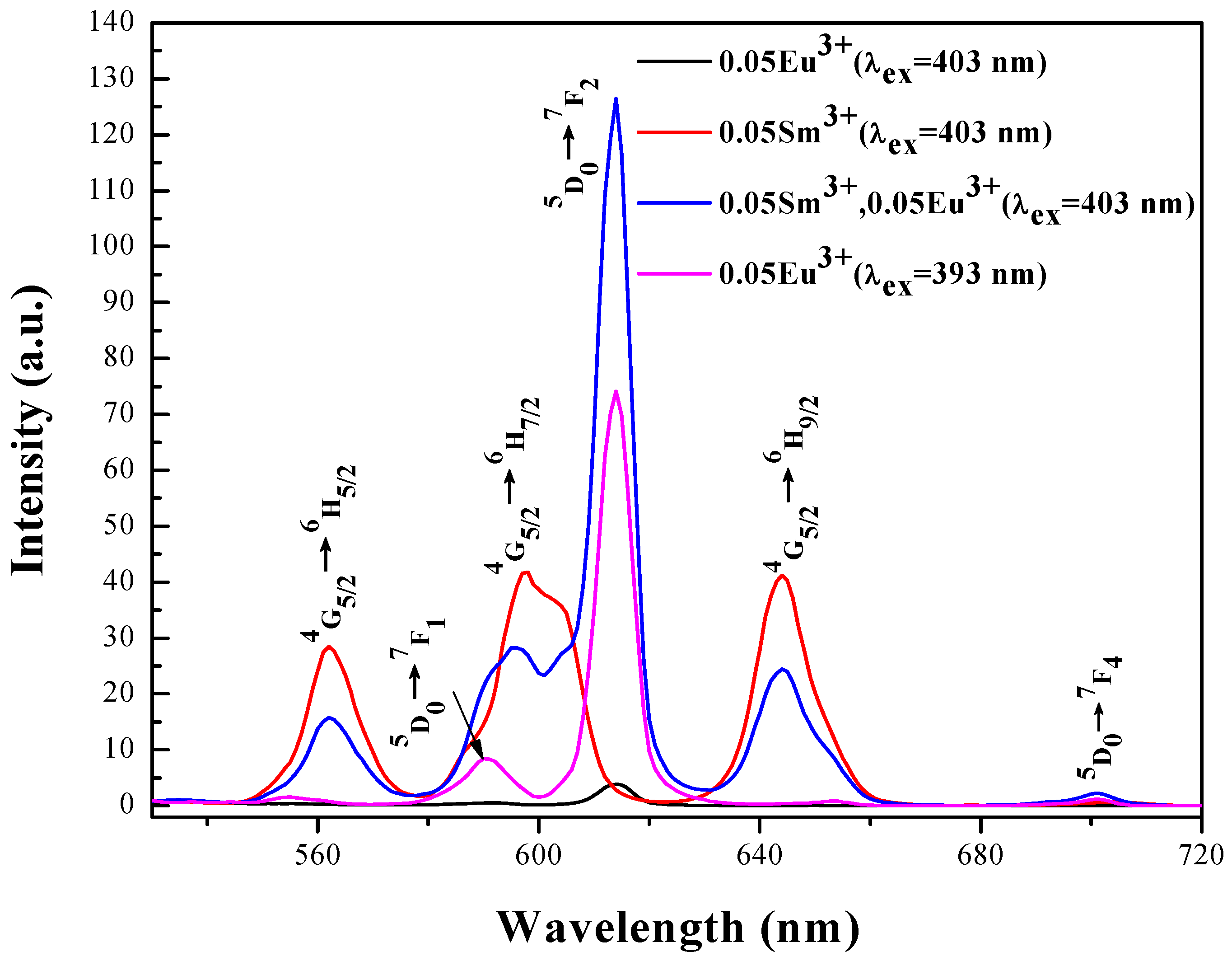
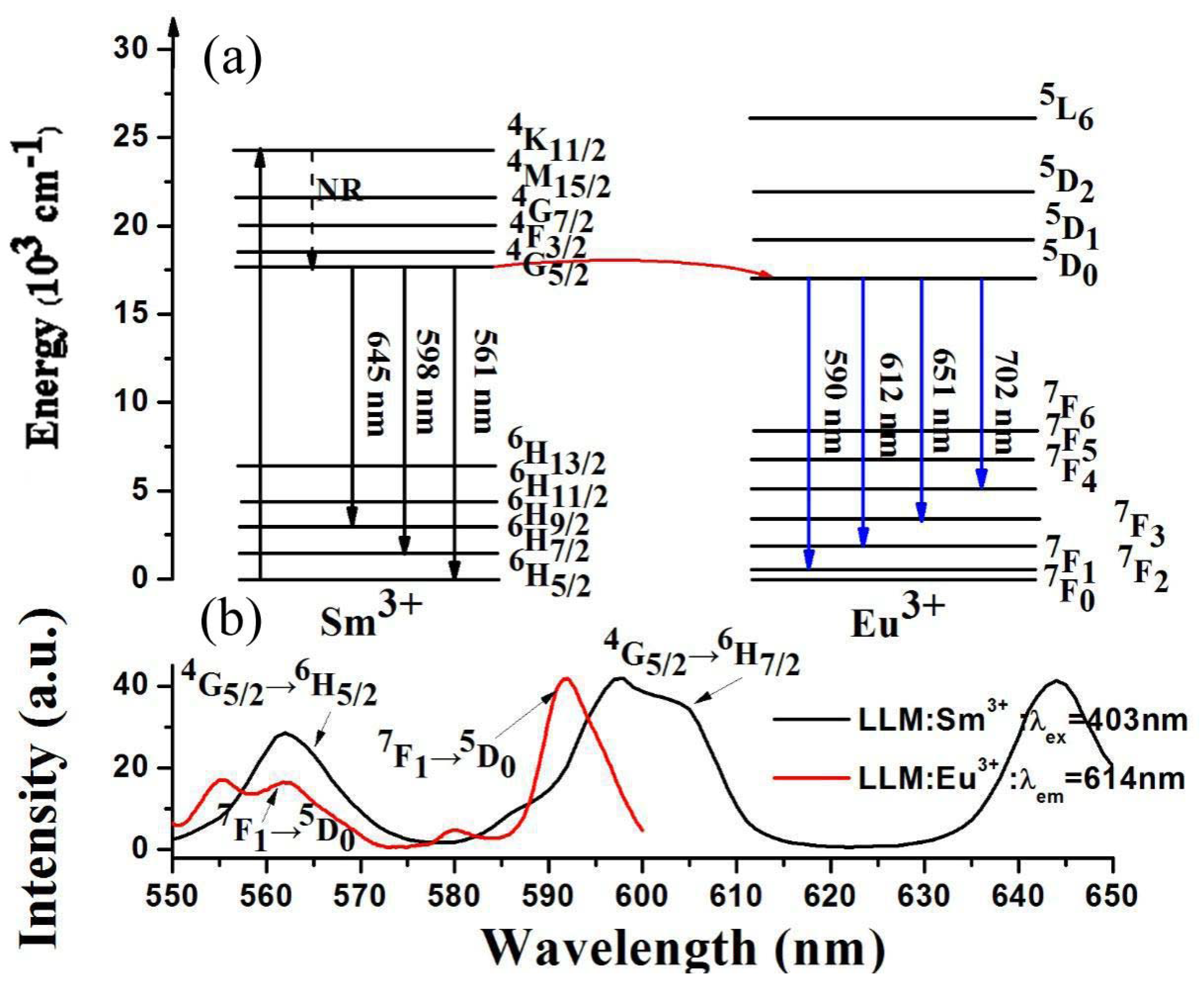
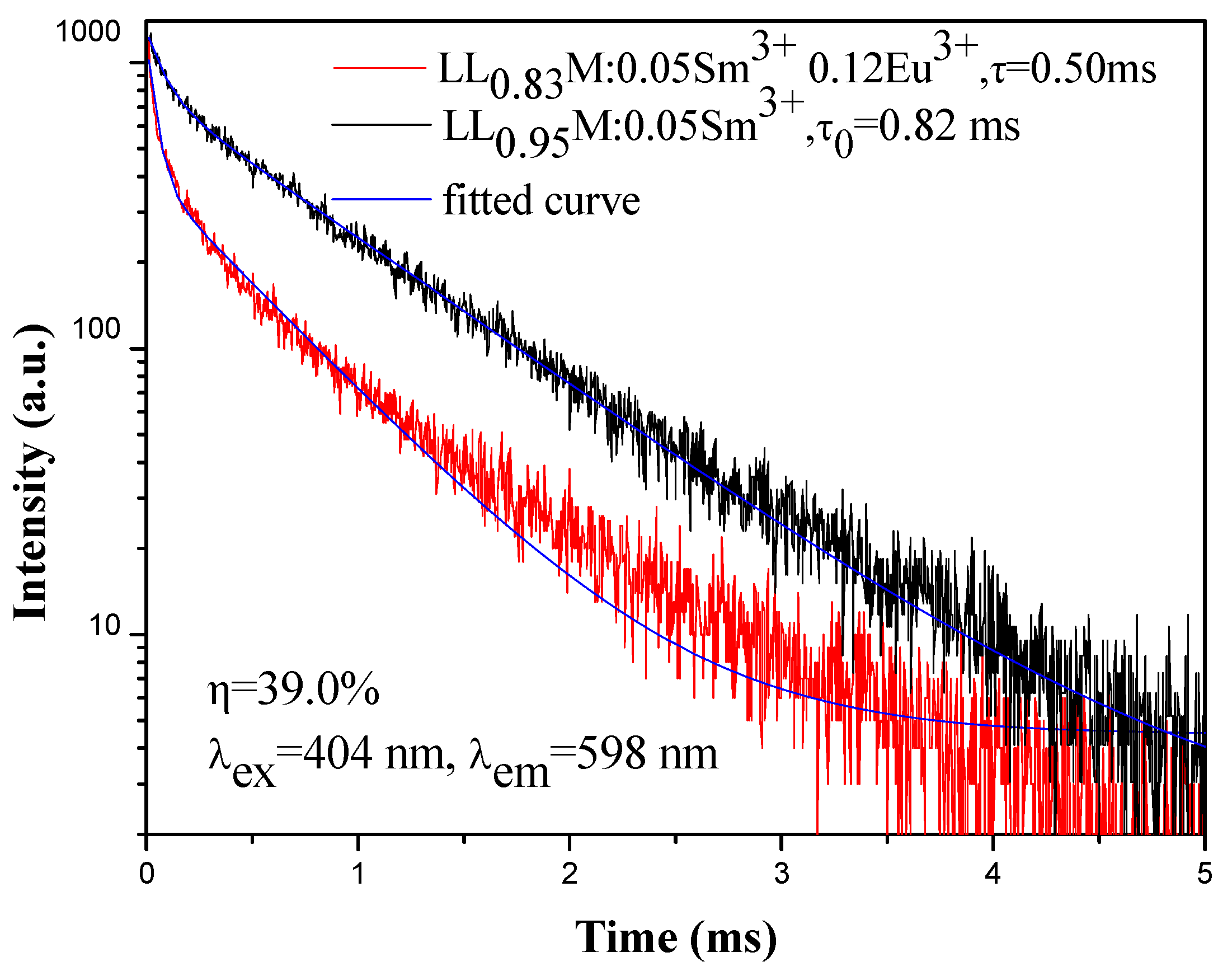
3.3. Energy Transfer from Sm3+ to Eu3+ in LiLa1−x(MoO4)2 Doped with Eu3+ and Sm3+
3.4. Color Coordinates of LL0.95−yM:0.05Sm3+,yEu3+ Phosphors
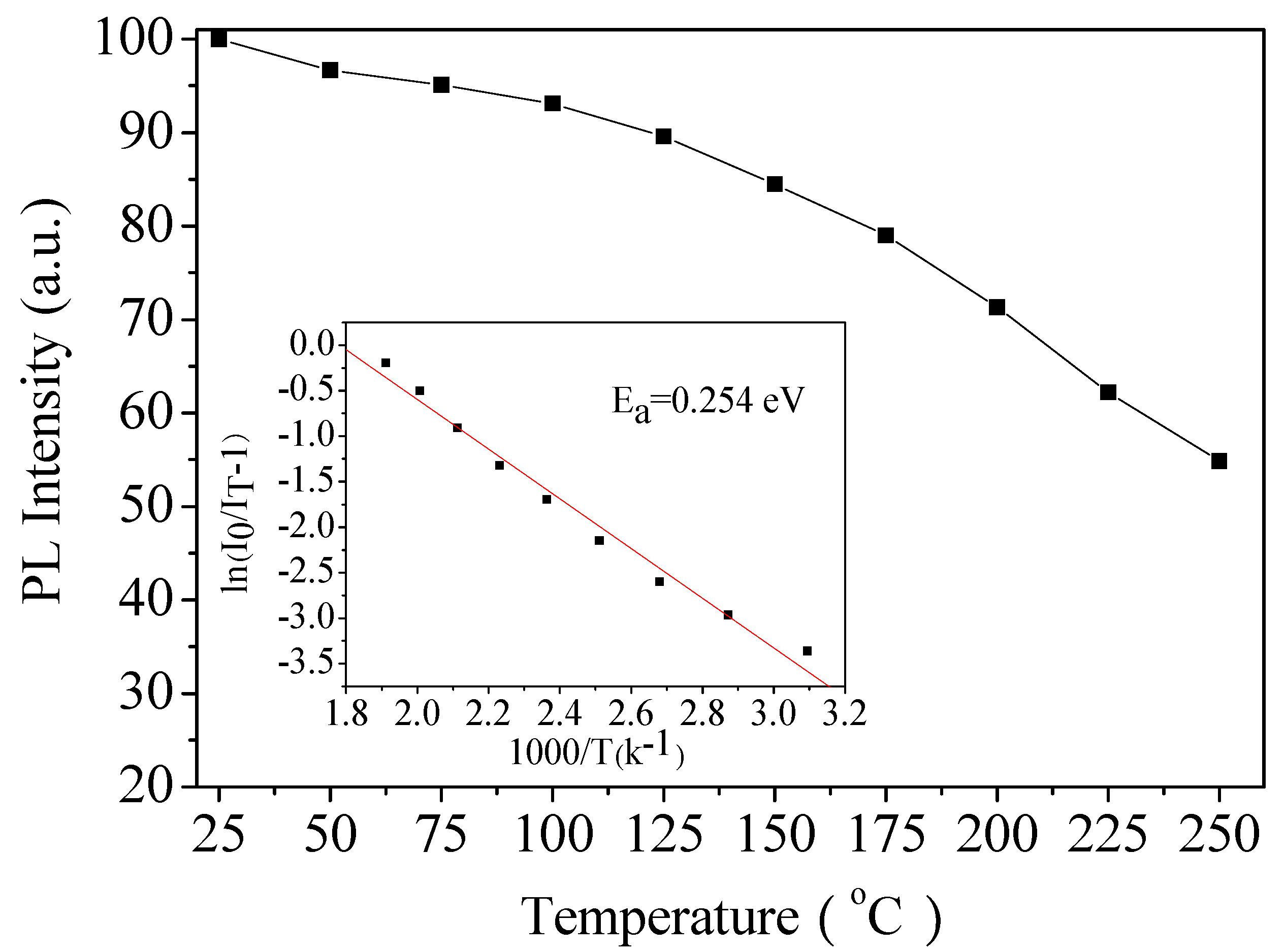
3.5. Thermal Stability Analysis
3.6. The Luminous Efficiency and the Performance for LED Applications
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schubert, E.F.; Kim, J.K. Solid-state light sources getting smart. Science 2005, 308, 1274–1278. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.; Jun, S.; Jang, H.; Lim, J.; Kim, B.; Kim, Y. White-light-emitting diodes with quantum dot color converters for display backlights. Adv. Mater. 2010, 22, 3076–3080. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y.; Taguchi, T. Lighting theory and luminous characteristics of white light-emitting diodes. Opt. Eng. 2005, 44, 16–34. [Google Scholar] [CrossRef]
- Sheu, J.K.; Chang, S.J.; Kuo, C.H.; Su, Y.K.; Wu, L.W.; Lin, Y.C.; Lai, W.C.; Tsai, J.M.; Chi, G.C.; Wu, R.K. White-light emission from near UV InGaN-GaN LED chip precoated with blue/green/red phosphors. IEEE Photonics Technol. Lett. 2003, 15, 18–20. [Google Scholar] [CrossRef]
- Liu, L.; Yuan, X.M.; Xie, A.; Yang, M.; Chen, F. Synthesis and luminescent properties of Eu3+-activated novel borate-based red-emitting phosphors for white LED. Chin. J. Lumin. 2011, 32, 686–692. [Google Scholar] [CrossRef]
- Liu, R.W.; Huang, H.C.; Yeh, W.C.; Tsai, C.J.; Chiu, C.Y.; Yeh, T.Y.; Liu, S.R. A study on the luminescence and energy transfer of single-phase and color-tunable KCaY(PO4)2:Eu2+,Mn2+ phosphor for application in white-light LEDs. Inorg. Chem. 2012, 43, 9636–9641. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, Z.; Hu, G.; Cao, R.; Liang, X.; Xiang, W. A novel deep red phosphor Ca14Zn6Ga10O35:Mn4+ as color converter for warm W-LEDs. J. Alloy. Compd. 2017, 694, 1201–1208. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Beisel, N.F.; Galashov, E.N.; Mandrik, E.M.; Molokeev, M.S.; Yelisseyev, A.P.; Yusuf, A.A.; Xia, Z. Pressure-stimulated synthesis and luminescence properties of microcrystalline (Lu,Y)3Al5O12:Ce3+ garnet phosphors. ACS Appl. Mater. Interfaces 2015, 7, 26235–26243. [Google Scholar] [CrossRef] [PubMed]
- Leaño, J.L., Jr.; Lin, S.; Lazarowska, A.; Mahlik, S.; Grinberg, M.; Liang, C.; Zhou, W.; Molokeev, M.S.; Atuchin, V.V.; Tsai, Y.; et al. Green light-excitable Ce-doped nitridomagnesoaluminate Sr[Mg2Al2N4] phosphor for white light-emitting diodes. Chem. Mater. 2016, 28, 6822–6825. [Google Scholar] [CrossRef]
- Ji, H.; Wang, L.; Molokeev, M.S.; Hirosaki, N.; Xie, R.; Huang, Z.; Xia, Z.; Kate, O.M.; Liu, L.; Atuchin, V.V. Structure evolution and photoluminescence of Lu3(Al, Mg)2(Al, Si)3O12: Ce3+ phosphors: New yellow-color converters for blue LED-driven solid state lighting. J. Mater. Chem. C 2016, 4, 6855–6863. [Google Scholar] [CrossRef]
- Li, Y.T.; Liu, X.H. Photoluminescence properties and energy transfer of KY1−xLnx (MoO4)2 (Ln=Sm3+, Eu3+) red phosphors. J. Lumin. 2014, 151, 52–56. [Google Scholar] [CrossRef]
- Krishna Bharat, L.; Khaja Hussain, S.; Yu, S.J. Near-ultraviolet excitation-based bluish-green emitting K2ZnSiO4:Eu2+ nanophosphors for white light-emitting applications. Dyes Pigment. 2017, 145, 37–42. [Google Scholar] [CrossRef]
- Du, H.Y.; Sun, J.F.; Xia, Z.G. Luminescence Properties of Ba2Mg (BO3) 2: Eu2 + red phosphors synthesized by a microwave-assisted Sol-Gel route sensors and displays: principles, materials, and processing. J. Electrochem. Soc. 2009, 156, 361–366. [Google Scholar] [CrossRef]
- Xie, R.J.; Hirosaki, N. Silicon-based oxynitride and nitride phosphors for white LEDs—A review. Sci. Technol. Adv. Mater. 2007, 8, 588–600. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, B.; Dong, F.; Wang, S.; Su, W.S. A first-principles study of the electronic structure and mechanical and optical properties of CaAlSiN3. Phys. Chem. Chem. Phys. 2015, 17, 15065–15070. [Google Scholar] [CrossRef] [PubMed]
- Pust, P.; Weiler, V.; Hecht, C.; Tücks, A.; Wochnik, A.S.; Henß, A.; Wiechert, D.; Scheu, C.; Schmidt, P.J.; Schnick, W. Narrow-band red-emitting Sr[LiAl3N4]:Eu2+ as a next-generation LED-phosphor material. Nat. Mater. 2014, 13, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Wagatha, P.; Pust, P.; Weiler, V.; Wochnik, S.A.; Schmidt, J.P.; Scheu, C.; Schnick, W. Ca18.75Li10.5[Al39N55]:Eu2+-supertetrahedron phosphor for solid-state lighting. Chem. Mater. 2016, 28, 1220–1226. [Google Scholar] [CrossRef]
- Ji, H.O.; Heejoon, K.; Yun, J.E.; Hoo, K.P.; Young, R.D. Synthesis of narrow-band red-emitting K2SiF6:Mn4+ phosphors for a deep red monochromatic LED and ultrahigh color quality warm-white LEDs. J. Mater. Chem. C 2015, 3, 607–615. [Google Scholar] [CrossRef]
- Sijbom, F.H.; Joos, J.J.; Martin, I.L.; van den Eeckhout, K.; Poelman, D.; Smet, F.P. Luminescent behavior of the K2SiF6:Mn4+ red phosphor at high fluxes and at the microscopic level. ECS J. Solid. State Sci. Technol. 2016, 5, R3040–R3048. [Google Scholar] [CrossRef]
- Ji, H.O.; Yun, J.E.; Hee, C.Y.; Young-Duk, H.; Young, R.D. Evaluation of new color metrics: Guidelines for developing narrow-band red phosphors for WLEDs. J. Mater. Chem. C 2016, 4, 8315–8584. [Google Scholar] [CrossRef]
- Shi, P.L.; Xia, Z.G.; Molokeev, M.S.; Atuchin, V.V. Crystal chemistry and luminescence properties of red-emitting CsGd1-xEux(MoO4)2 solid-solution phosphors. Dalton Trans. 2014, 43, 9669–9676. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Lin, F.; Wu, X.; Hai, O.; Wei, T.; Jiao, Y.; Li, H. Synthesis and luminescent properties of KGd(MoO4)2Sm3+ red phosphor for white light emitting diodes. Mater. Res. Bull. 2017, 90, 66–72. [Google Scholar] [CrossRef]
- Zhiping, Y.A.N.G.; Hongyan, D.O.N.G.; Pengfei, L.I.U.; Chuncai, H.O.U.; Liang, X.; Can, W.A.N.G.; Fachun, L.U. Photoluminescence properties of Sm3+-doped LiY(MoO4)2 red phosphors. J. Rare Earths 2014, 32, 404–408. [Google Scholar] [CrossRef]
- Liao, J.S.; You, H.Y.; Zhou, D.; Wen, H.R.; Hong, R.J. Sol-gel preparation and photoluminescence properties of LiLa(MoO4)2:Eu3+ phosphors. Opt. Mater. 2012, 34, 1468–1472. [Google Scholar] [CrossRef]
- Liu, J.; Lian, H.Z.; Shi, C.S. Improved optical photoluminescence by charge compensation in the phosphor system CaMoO4:Eu3+. Opt. Mater. 2007, 29, 1591–1594. [Google Scholar] [CrossRef]
- Hu, Y.; Zhuang, W.; Ye, H.; Wang, D.; Zhang, S.; Huang, X. A novel red phosphor for white light emitting diodes. J. Alloy. Compd. 2005, 390, 226–229. [Google Scholar] [CrossRef]
- Lin, H.Y.; Fang, Y.C.; Chu, S.Y. Energy transfer Sm3+→Eu3+ in potential red phosphor (Ca, Ba)3(VO4)2:Sm3+, Eu3+ for use in organic solar cells and white light-emitting diodes. J. Am. Ceram. Soc. 2010, 93, 3850–3856. [Google Scholar] [CrossRef]
- Wang, Z.; Liang, H.; Gong, M.; Su, Q. Novel red phosphor of Bi3+, Sm3+ co-activated NaEu(MoO4)2. Opt. Mater. 2007, 29, 896–900. [Google Scholar] [CrossRef]
- Wang, X.X.; Xian, Y.L.; Wang, G.; Shi, J.X.; Su, Q.; Gong, M.L. Luminescence investigation of Eu–Sm co-doped GdxyEuxSmy(MoO) phosphors as red phosphors for UV InGaN-based light-emitting diode. Opt. Mater. 2008, 30, 521–526. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, J.; Lü, S.; Zhao, H.; Zhang, X.; Wang, X.J. Fabrication of Eu3+ and Sm3+ codoped micro/nanosized MMoO4 (M = Ca, Ba, and Sr) via facile hydrothermal method and their Photoluminescence properties through energy transfer. J. Phys. Chem. C 2008, 112, 5860–5864. [Google Scholar] [CrossRef]
- Won, Y.H.; Ho, Z.; Jang, S.; Jeon, D.Y. Red-emitting LiLa2O2BO3:Sm 3+, Eu 3+ phosphor for near-ultraviolet light-emitting diodes-based solid-state lighting. J. Electrochem. Soc. 2008, 155, J226–J229. [Google Scholar] [CrossRef]
- Yan, X.; Li, W.; Sun, K. A novel red emitting phosphor CaIn2O4:Eu3+, Sm3+ with a broadened near-ultraviolet absorption band for solid-state lighting. Mater. Res. Bull. 2010, 46, 87–91. [Google Scholar] [CrossRef]
- Xue, Y.N.; Xiao, F.; Zhang, Q.Y. Enhanced red light emission from LaBSiO5:Eu3+, R3+ (R = Bi or Sm) phosphors. Spectrochim. Acta A 2011, 78, 607–611. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.C.; Chu, S.Y.; Kao, P.C.; Chuang, Y.M.; Zeng, Z.L. Energy transfer and thermal quenching behaviors of CaLa2(MoO4)4:Sm3+,Eu3+ red phosphors. J. Electrochem. Soc. 2011, 158, J1–J5. [Google Scholar] [CrossRef]
- Li, P.; Wang, Z.; Yang, Z.; Guo, Q. SrIn2O4:Eu3+, Sm3+: A red emitting phosphor with a broadened near-ultraviolet absorption band for solid-state lighting. J. Electrochem. Soc. 2011, 158, H1201. [Google Scholar] [CrossRef]
- Kang, D.; Yoo, H.S.; Sang, H.J.; Kim, H.; Jeon, D.Y. Synthesis and photoluminescence properties of a novel red-emitting Na2Y2Ti3O10:Eu3+,Sm3+ phosphor for white-light-emitting diodes. J. Phys. Chem. C 2011, 115, 24334–24340. [Google Scholar] [CrossRef]
- Li, P.; Xu, Z.; Zhao, S.; Zhang, F.; Wang, Y. Luminescent characteristics and energy transfer of Ca2BO3Cl:Sm3+,Eu3+ red phosphor. Mater. Res. Bull. 2012, 47, 3825–3829. [Google Scholar] [CrossRef]
- Min, X.; Huang, Z.; Fang, M.; Liu, Y.G.; Tang, C.; Wu, X. Energy transfer from Sm3+ to Eu3+ in red-emitting phosphor LaMgAl11O19:Sm3+, Eu3+ for solar cells and near-ultraviolet white light-emitting diodes. Inorg. Chem. 2014, 53, 6060–6065. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Hu, Y.H.; Chen, L.; Wang, X.J. Luminescence properties and energy transfer of a red-emitting Ca3(PO4)2:Sm3+,Eu3+ phosphor. J. Mater. Sci. Mater. Electron. 2015, 26, 5360–5367. [Google Scholar] [CrossRef]
- Li, G.; Wei, Y.; Long, W.; Xu, G. Photoluminescence properties, energy transfer and thermal stability of the novel red-emitting CaGd2(WO4)4:Eu3+, Sm3+ phosphors. Mater. Res. Bull. 2017, 95, 86–94. [Google Scholar] [CrossRef]
- Zhang, L.C.; Zhang, C.L.; Horng, J.H.; Chen, Z.C. Luminescence properties and energy transfer of Sm3+-Eu3+ Co-doped Gd2(MoO4)3 red phosphors. Adv. Mater. 2012, 591–593, 931–934. [Google Scholar] [CrossRef]
- Huang, X.Y.; Lin, Z.B.; Zhang, L.Z.; Wang, G.F. Growth and spectral characteristics of Yb3+-doped LiLa(MoO4)2 crystal. J. Alloys. Compd. 2007, 306, 208–211. [Google Scholar] [CrossRef]
- Zhao, W.; Zhou, W.W.; Wei, B. Spectroscopic assessment Dy3+:LiLa(MoO4)2 crystal as an active medium for all-solid-state direct yellow-emitting lasers. J. Alloy. Compd. 2012, 538, 136–143. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Chimitova, O.D.; Gavrilova, T.A.; Molokeev, M.S.; Kim, S.; Surovtsev, N.V.; Bazarov, B.G. Synthesis, structural and vibrational properties of microcrystalline RbNd (MoO4)2. J. Cryst. Growth. 2011, 318, 683–686. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Grossman, V.G.; Adichtchev, S.V.; Surovtsev, N.V.; Gavrilova, T.A.; Bazarov, B.G. Structural and vibrational properties of microcrystalline TlM (MoO4)2 (M = Nd, Pr) molybdates. Opt. Mater. 2012, 34, 812–816. [Google Scholar] [CrossRef]
- Chimitova, O.D.; Atuchin, V.V.; Bazarov, B.G.; Molokeev, M.S. The formation and structural parameters of new double molybdates RbLn (MoO4)2 (Ln = Pr, Nd, Sm, Eu). Proc. SPIE. 2013. [Google Scholar] [CrossRef]
- Li, L.L.; Liu, Y.L.; Li, R.Q.; Leng, Z.H.; Gan, S.C. Tunable luminescence properties of the novel Tm3+- and Dy3+-codoped LiLa(MoO4)x(WO4)2−x phosphors for white light-emitting diodes. RSC Adv. 2015, 5, 7049–7057. [Google Scholar] [CrossRef]
- Bi, W.B.; Meng, Q.Y.; Sun, W.J. Luminescent properties and energy transfer mechanism of NaGd(MoO4)2:Sm3+,Eu3+ phosphors. Ceram. Int. 2016, 42, 14086–14093. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Aleksandrovsky, A.S.; Chimitova, O.D.; Diao, C.; Gavrilova, T.A.; Kesler, V.G.; Molokeev, M.S.; Krylov, A.S.; Bazarov, B.G.; Bazarova, J.G.; et al. Electronic structure of β-RbSm(MoO4)2 and chemical bonding in molybdates. Dalton Trans. 2014, 44, 1805–1815. [Google Scholar] [CrossRef] [PubMed]
- Atuchin, V.V.; Aleksandrovsky, A.S.; Molokeev, M.S.; Krylov, A.S.; Oreshonkov, A.S.; Zhou, D. Structural and spectroscopic properties of self-activated monoclinic molybdate BaSm2(MoO4)4. J. Alloys. Compd. 2017, 729, 843–849. [Google Scholar] [CrossRef]
- Vanuitert, L.G. Characterization of Energy Transfer Interactions between Rare Earth Ions. J. Electrochem. Soc. 1967, 114, 1048–1053. [Google Scholar] [CrossRef]
- Xu, Q.G.; Sun, J.Y.; Cui, D.P.; Di, Q.M.; Zeng, J.H. Synthesis and luminescence properties of novel Sr3Gd(PO4)3:Dy3+ phosphor. J. Lumin. 2015, 158, 301–305. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Aleksandrovsky, A.S.; Chimitova, O.D.; Gavrilova, T.A.; Krylov, A.S.; Molokeev, M.S.; Oreshonkov, A.S.; Bazarov, B.G.; Bazarova, J.G. Synthesis and spectroscopic properties of monoclinic α-Eu2 (MoO4)3. J. Phys. Chem. C 2014, 118, 15404–15411. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Subanakov, A.K.; Aleksandrovsky, A.S.; Bazarov, B.G.; Bazarova, J.G.; Gavrilova, T.A.; Krylov, A.S.; Molokeev, M.S.; Oreshonkov, A.S.; Stefanovich, S.Y. Structural and spectroscopic properties of new noncentrosymmetric self-activated borate Rb3EuB6O12 with B5O10 units. Mater. Design. 2018, 140, 488–494. [Google Scholar] [CrossRef]
- Das, S.; Reddy, A.A.; Babu, S.S.; Prakash, G.V. Tunable visible upconversion emission in Er3+/Yb3+-codoped KCaBO3 phosphors by introducing Ho3+ ions. Mater. Lett. 2014, 120, 232–235. [Google Scholar] [CrossRef]
- Som, S.; Das, S.; Dutta, S.; Visser, H.G.; Pandey, M.K.; Kumar, P.; Dubey, R.K.; Sharma, S.K. Synthesis of strong red emitting Y2O3:Eu3+ phosphor by potential chemical routes: Comparative investigations on the structural evolutions, photometric properties and Judd–Ofelt analysis. RSC Adv. 2015, 5, 70887–70898. [Google Scholar] [CrossRef]

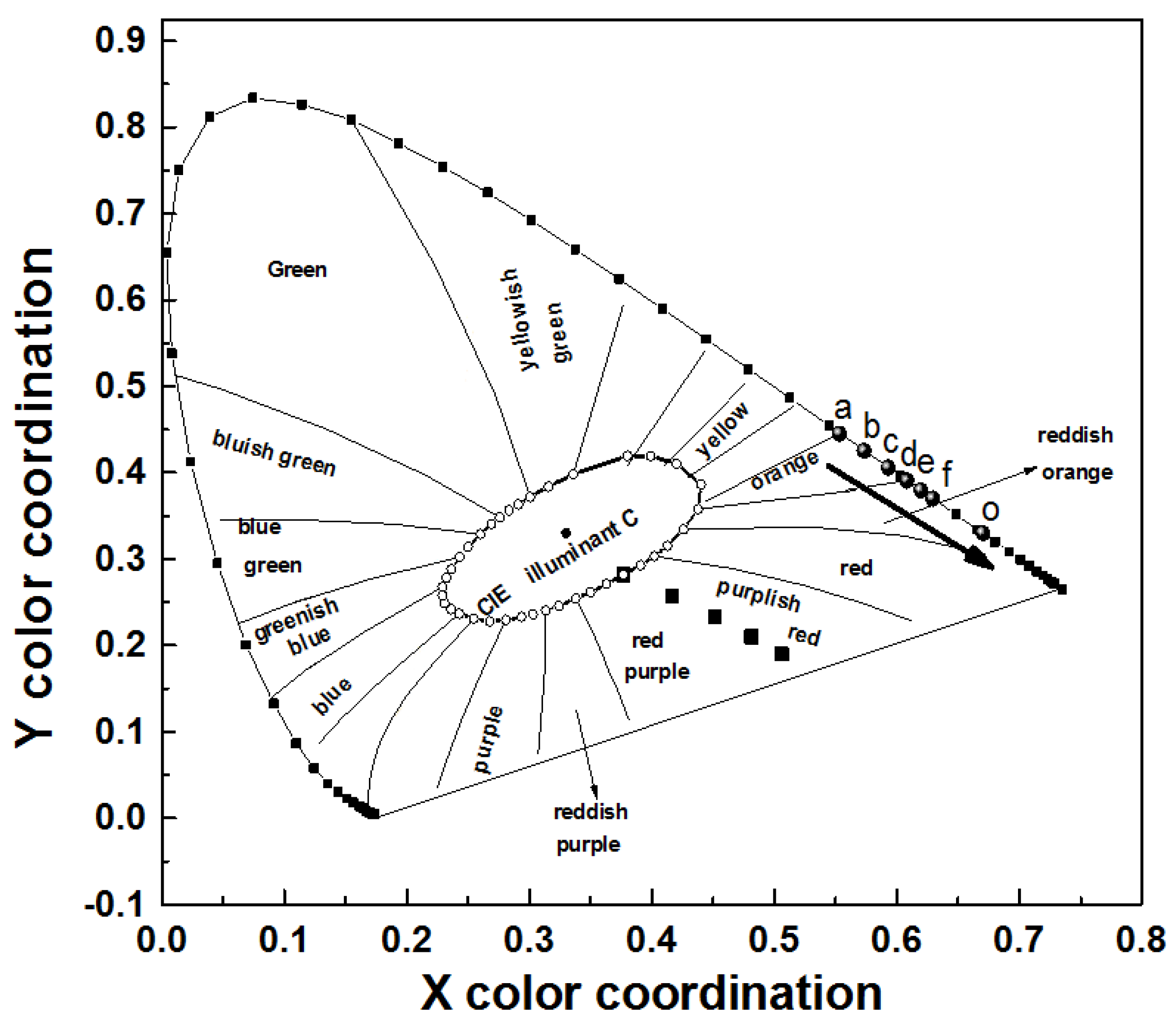
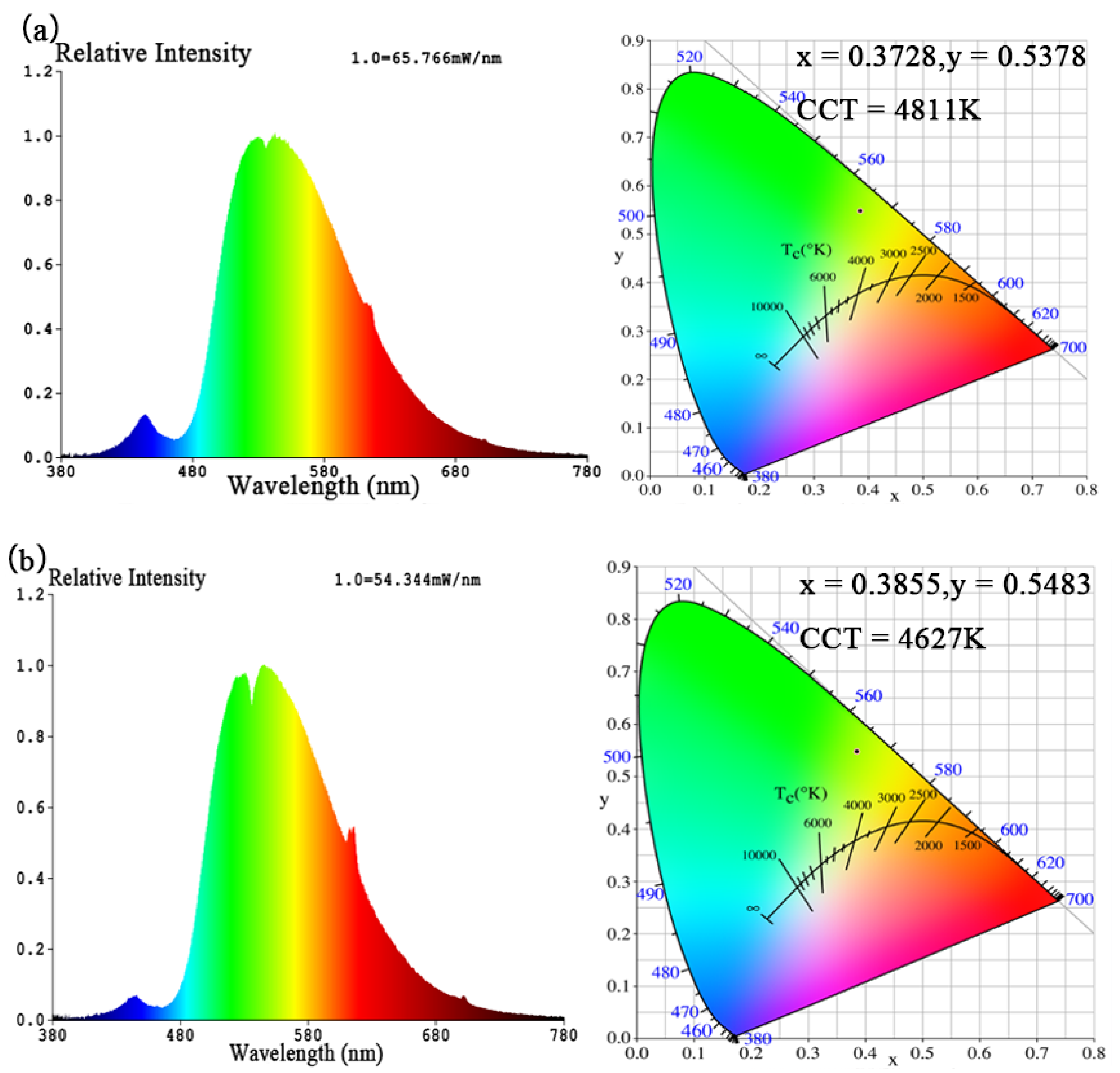
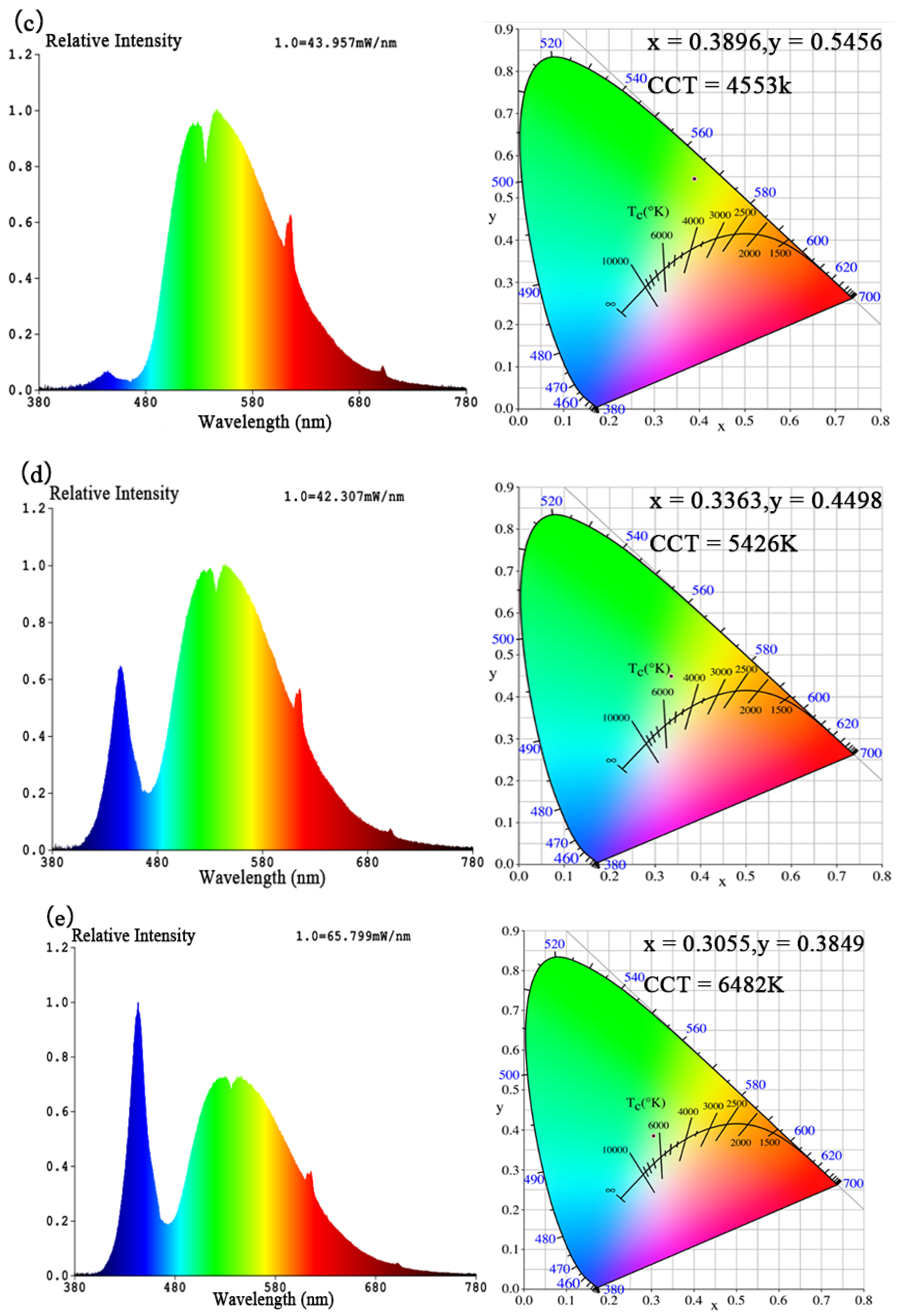
| Figure 12 | ENA6550 A:B:Yellow Phosphor:LLM(Eu,Sm) | CCT (K) | Color Purity (%) | CRI | CIE (x, y) | Luminous Efficacy (lm/w) | Voltage (V) | Current (A) | Power (W) | |
|---|---|---|---|---|---|---|---|---|---|---|
| x | y | |||||||||
| (a) | 1.2:0.4:0.2:0.2 | 4811 | 73.6 | 50.1 | 0.3728 | 0.5378 | 158.15 | 37.6 | 0.6 | 22.6 |
| (b) | 1.2:0.4:0.2:0.5 | 4627 | 80.6 | 45.6 | 0.3855 | 0.5483 | 133.43 | 36.8 | 0.6 | 22.1 |
| (c) | 1.2:0.4:0.2:0.8 | 4553 | 81.0 | 46.4 | 0.3896 | 0.5456 | 107.20 | 36.8 | 0.6 | 22.1 |
| (d) | 1.2:0.4:0.1:0.52 | 5426 | 36.2 | 62.6 | 0.3363 | 0.4498 | 107.08 | 36.6 | 0.6 | 22.0 |
| (e) | 1.2:0.4:0.1:0.52 | 6482 | 10.4 | 65.0 | 0.3055 | 0.3849 | 121.36 | 36.8 | 0.6 | 22.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Luo, L.; Huang, B.; He, J.; Zhang, W.; Zhao, W.; Wang, J. The Preparation and Optical Properties of Novel LiLa(MoO4)2:Sm3+,Eu3+ Red Phosphor. Materials 2018, 11, 297. https://doi.org/10.3390/ma11020297
Wang J, Luo L, Huang B, He J, Zhang W, Zhao W, Wang J. The Preparation and Optical Properties of Novel LiLa(MoO4)2:Sm3+,Eu3+ Red Phosphor. Materials. 2018; 11(2):297. https://doi.org/10.3390/ma11020297
Chicago/Turabian StyleWang, Jiaxi, Li Luo, Baoyu Huang, Jingqi He, Wei Zhang, Weiren Zhao, and Jianqing Wang. 2018. "The Preparation and Optical Properties of Novel LiLa(MoO4)2:Sm3+,Eu3+ Red Phosphor" Materials 11, no. 2: 297. https://doi.org/10.3390/ma11020297
APA StyleWang, J., Luo, L., Huang, B., He, J., Zhang, W., Zhao, W., & Wang, J. (2018). The Preparation and Optical Properties of Novel LiLa(MoO4)2:Sm3+,Eu3+ Red Phosphor. Materials, 11(2), 297. https://doi.org/10.3390/ma11020297





