Study of Perfluorophosphonic Acid Surface Modifications on Zinc Oxide Nanoparticles
Abstract
1. Introduction
2. Results and Discussion
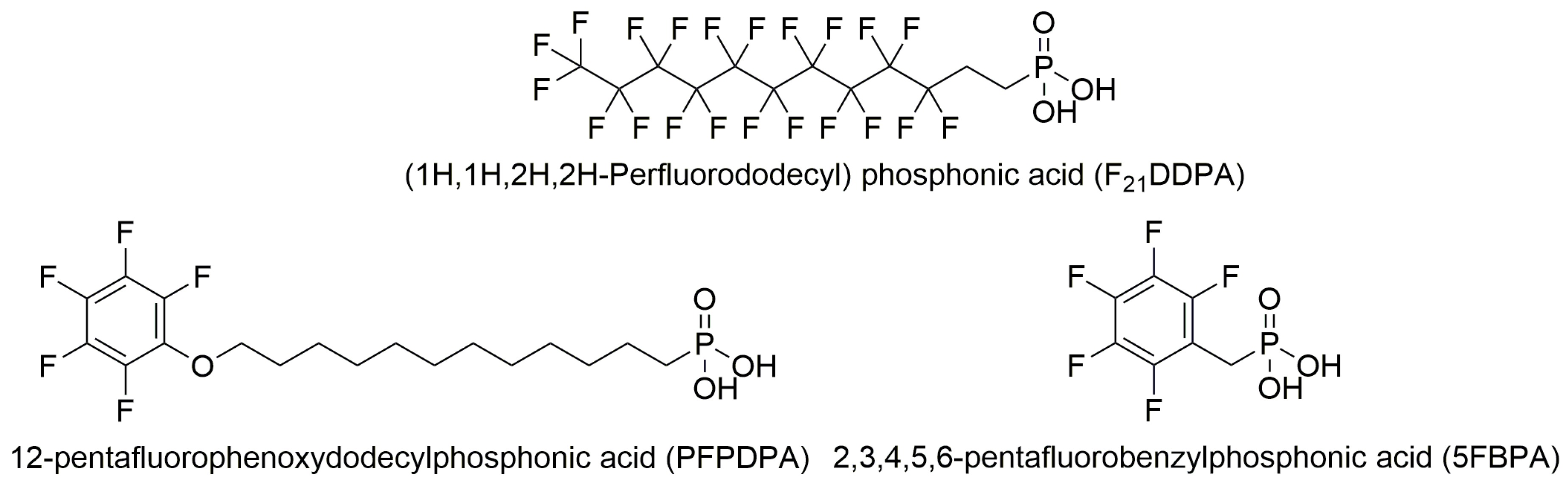
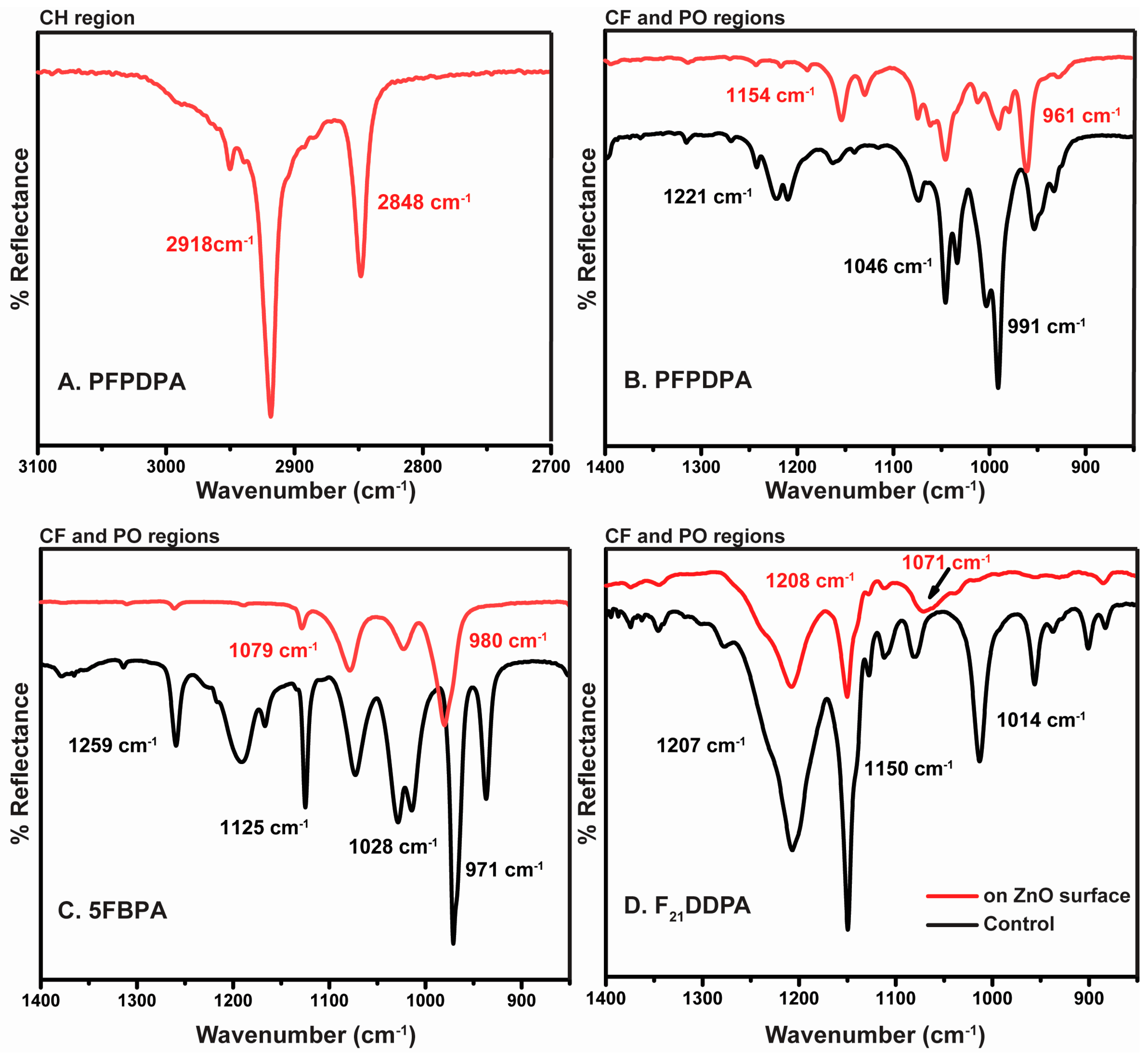
2.1. Attenuated Total Reflectance Infrared Spectroscopy (ATR-IR)
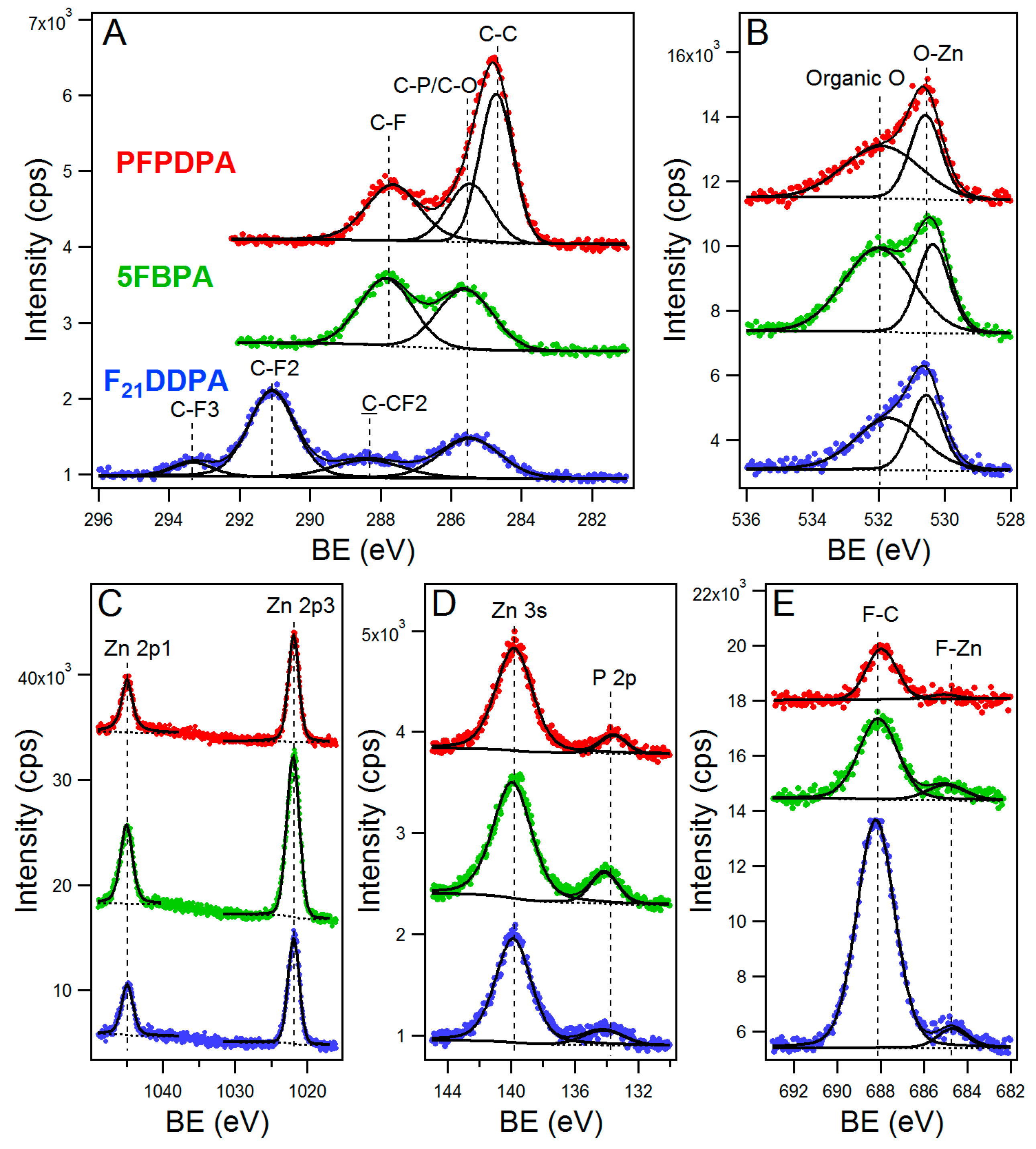
2.2. X-ray Photoelectron Spectroscopy (XPS)
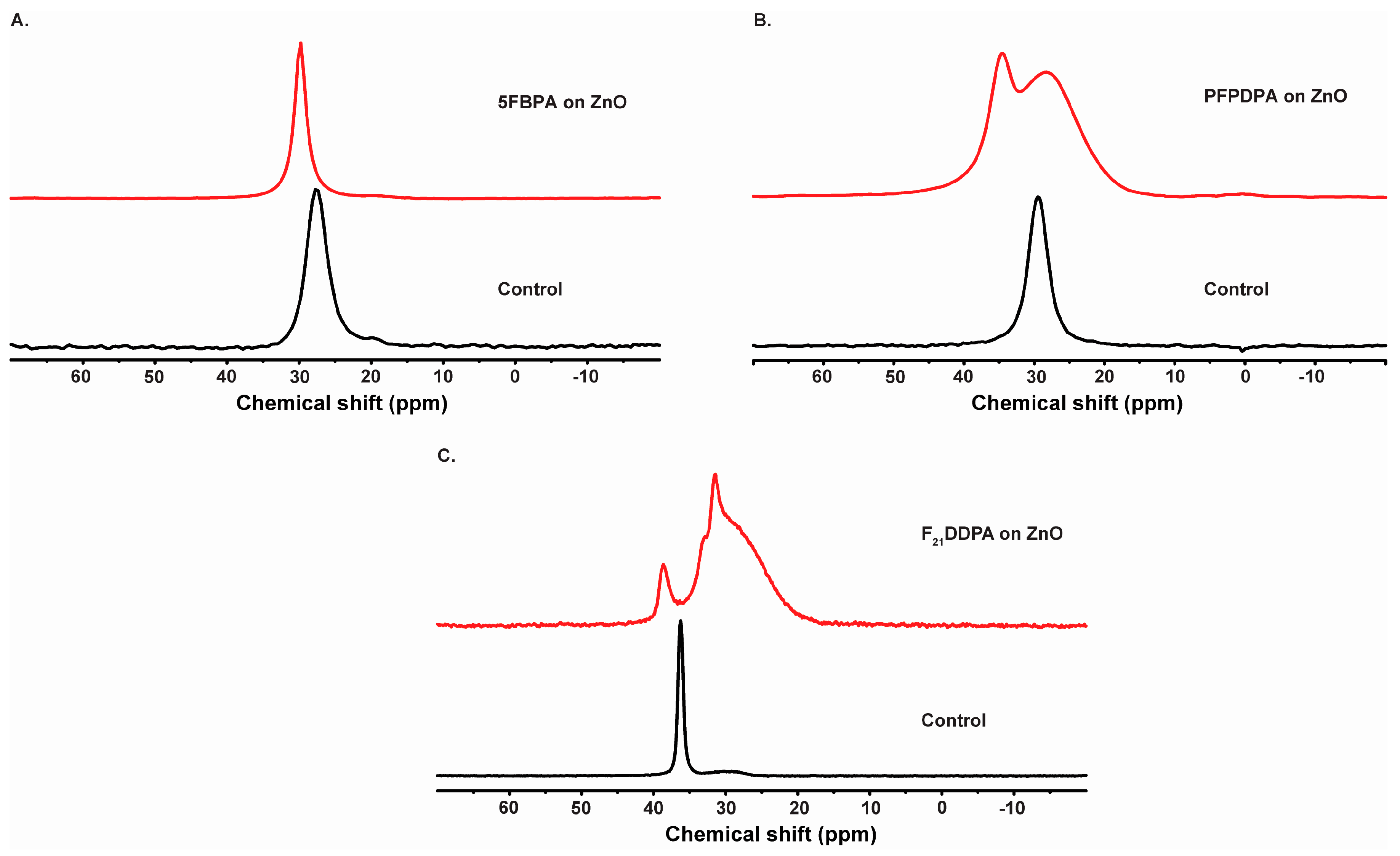
2.3. Solid State Nuclear Magnetic Resonance Spectroscopy (SS-NMR)
2.4. Scanning Electron Microscopy-Energy Dispersive X-ray Spectroscopy (SEM/EDS)
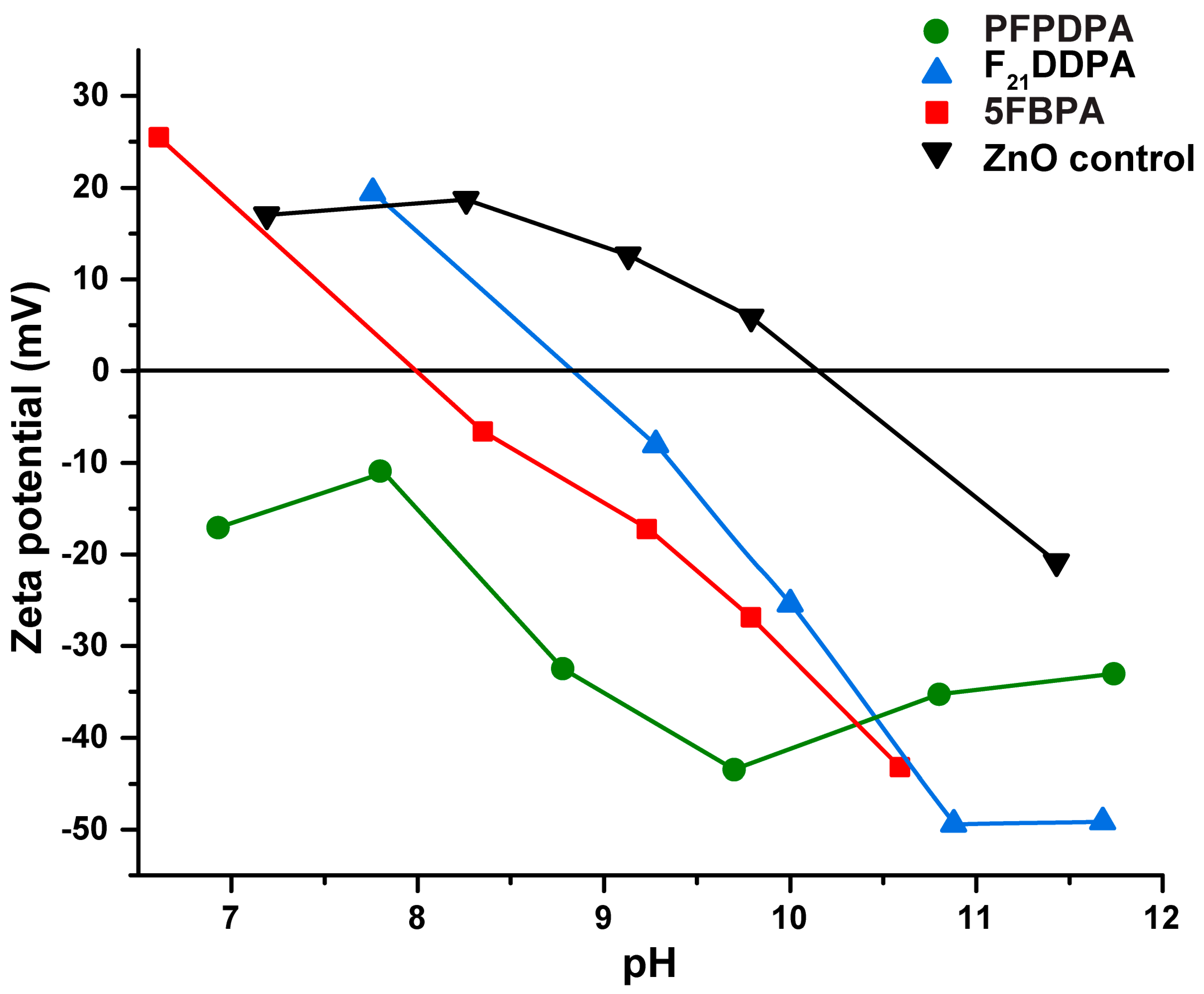
2.5. Dynamic Light Scattering (DLS) and Zeta Potential Measurements
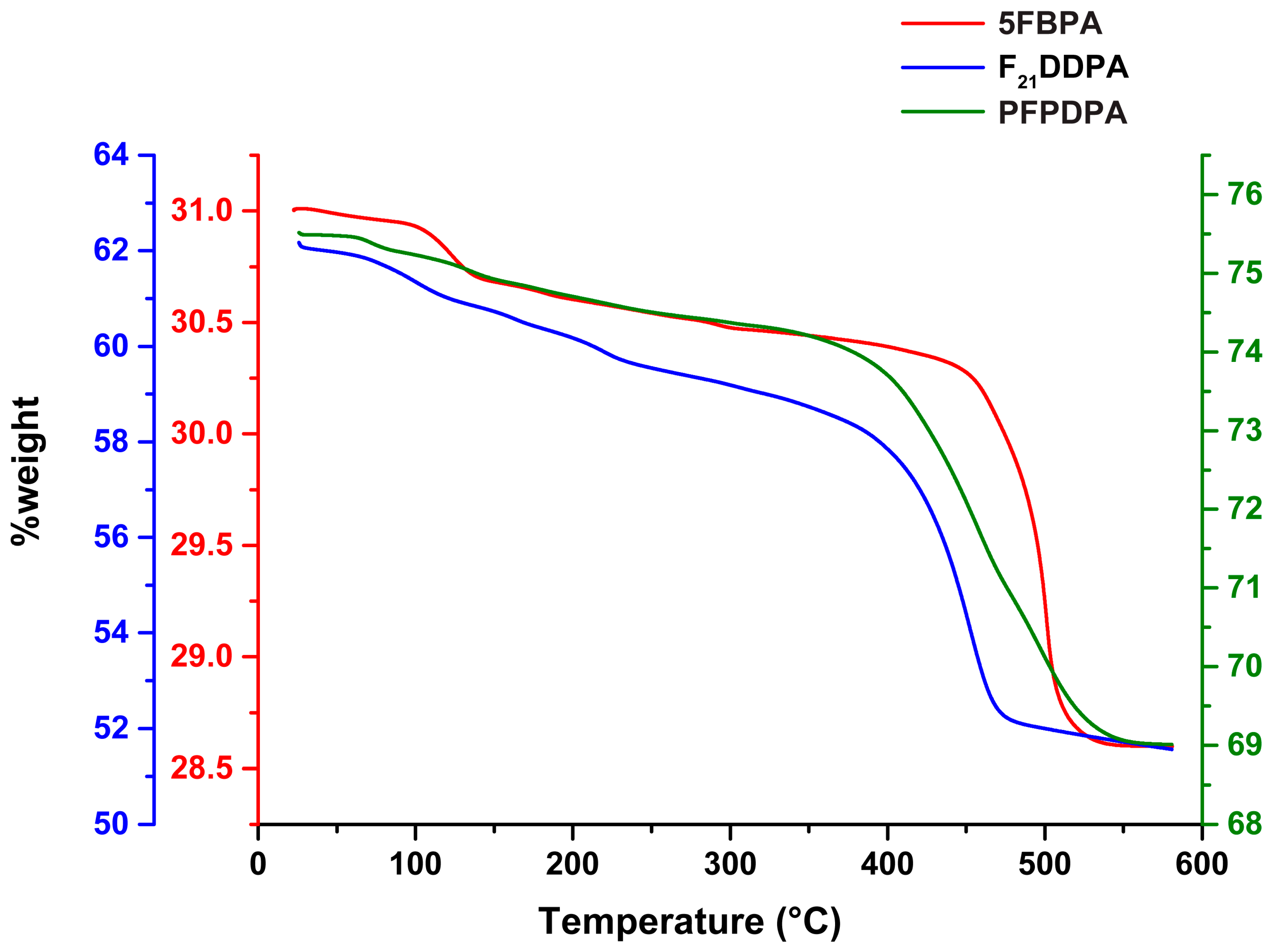
2.6. Thermogravimetric Analysis (TGA)
3. Materials and Methods
3.1. Materials
3.2. Preparation of the Samples
3.3. Characterization of the Films
3.3.1. ATR–IR
3.3.2. XPS
3.3.3. SS-NMR
3.3.4. SEM/EDS
3.3.5. DLS/Zeta Potential
3.3.6. TGA
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Uthirakumar, P.; Hong, C.-H.; Suh, E.-K.; Lee, Y.-S. Novel fluorescent polymer/zinc oxide hybrid particles: Synthesis and application as a luminescence converter for white light-emitting diodes. Chem. Mater. 2006, 18, 4990–4992. [Google Scholar] [CrossRef]
- Fathil, M.; Arshad, M.M.; Ruslinda, A.; Gopinath, S.C.; Adzhri, R.; Hashim, U.; Lam, H. Substrate-gate coupling in ZnO-FET biosensor for cardiac troponin I detection. Sens. Actuators B Chem. 2017, 242, 1142–1154. [Google Scholar] [CrossRef]
- Hau, S.K.; Yip, H.-L.; Baek, N.S.; Zou, J.; O’Malley, K.; Jen, A.K.-Y. Air-stable inverted flexible polymer solar cells using zinc oxide nanoparticles as an electron selective layer. Appl. Phys. Lett. 2008, 92, 225. [Google Scholar] [CrossRef]
- Yip, H.L.; Hau, S.K.; Baek, N.S.; Ma, H.; Jen, A.K.Y. Polymer Solar Cells That Use Self-Assembled-Monolayer-Modified ZnO/Metals as Cathodes. Adv. Mater. 2008, 20, 2376–2382. [Google Scholar] [CrossRef]
- Matsui, M.; Mase, H.; Jin, J.-Y.; Funabiki, K.; Yoshida, T.; Minoura, H. Application of semisquaric acids as sensitizers for zinc oxide solar cell. Dyes Pigment. 2006, 70, 48–53. [Google Scholar] [CrossRef]
- Bekci, D.R.; Karsli, A.; Cakir, A.C.; Sarica, H.; Guloglu, A.; Gunes, S.; Erten-Ela, S. Comparison of ZnO interlayers in inverted bulk heterojunction solar cells. Appl. Energy 2012, 96, 417–421. [Google Scholar] [CrossRef]
- Das, J.; Khushalani, D. Nonhydrolytic Route for Synthesis of ZnO and Its Use as a Recyclable Photocatalyst. J. Phys. Chem. C 2010, 114, 2544–2550. [Google Scholar] [CrossRef]
- Lai, M.-H.; Tubtimtae, A.; Lee, M.-W.; Wang, G.-J. ZnO-Nanorod Dye-Sensitized Solar Cells: New Structure without a Transparent Conducting Oxide Layer. Int. J. Photoenergy 2010, 2010, 497095. [Google Scholar] [CrossRef]
- Hong, R.; Pan, T.; Qian, J.; Li, H. Synthesis and surface modification of ZnO nanoparticles. Chem. Eng. J. 2006, 119, 71–81. [Google Scholar] [CrossRef]
- Wang, X.; Kong, X.; Yu, Y.; Zhang, H. Synthesis and characterization of water-soluble and bifunctional ZnO-Au nanocomposites. J. Phys. Chem. C 2007, 111, 3836–3841. [Google Scholar] [CrossRef]
- Senthilkumar, O.; Yamauchi, K.; Senthilkumar, K.; Yamamae, T.; Fujita, Y.; Nishimoto, N. UV-Blue Light Emission from ZnO Nanoparticles. J. Korean Phys. Soc. 2008, 53, 46–49. [Google Scholar]
- Srikant, V.; Clarke, D.R. On the optical band gap of zinc oxide. J. Appl. Phys. 1998, 83, 5447–5451. [Google Scholar] [CrossRef]
- Lin, H.-F.; Liao, S.-C.; Hung, S.-W. The dc thermal plasma synthesis of ZnO nanoparticles for visible-light photocatalyst. J. Photochem. Photobiol. A Chem. 2005, 174, 82–87. [Google Scholar] [CrossRef]
- Mohamed, R.M.; McKinney, D.; Kadi, M.W.; Mkhalid, I.A.; Sigmund, W. Platinum/zinc oxide nanoparticles: Enhanced photocatalysts degrade malachite green dye under visible light conditions. Ceram. Int. 2016, 42, 9375–9381. [Google Scholar] [CrossRef]
- Maity, S.; Bhunia, C.; Sahu, P. Improvement in optical and structural properties of ZnO thin film through hexagonal nanopillar formation to improve the efficiency of a Si–ZnO heterojunction solar cell. J. Phys. D Appl. Phys. 2016, 49, 205104. [Google Scholar] [CrossRef]
- Brine, H.; Sánchez-Royo, J.F.; Bolink, H.J. Ionic liquid modified zinc oxide injection layer for inverted organic light-emitting diodes. Organ. Electron. 2013, 14, 164–168. [Google Scholar] [CrossRef]
- Quiñones, R.; Rodriguez, K.; Iuliucci, R.J. Investigation of phosphonic acid surface modifications on zinc oxide nanoparticles under ambient conditions. Thin Solid Films 2014, 565, 155–164. [Google Scholar] [CrossRef]
- Bain, C.D.; Evall, J.; Whitesides, G.M. Formation of monolayers by the coadsorption of thiols on gold: Variation in the head group, tail group, and solvent. J. Am. Chem. Soc. 1989, 111, 7155–7164. [Google Scholar] [CrossRef]
- Quiñones, R.; Gawalt, E.S. Study of the Formation of Self-Assembled Monolayers on Nitinol. Langmuir 2007, 23, 10123–10130. [Google Scholar] [CrossRef] [PubMed]
- Quiñones, R.; Gawalt, E.S. Polystyrene Formation on Monolayer-Modified Nitinol Effectively Controls Corrosion. Langmuir 2008, 24, 10858–10864. [Google Scholar] [CrossRef] [PubMed]
- Raman, A.; Dubey, M.; Gouzman, I.; Gawalt, E.S. Formation of Self-Assembled Monolayers of Alkylphosphonic Acid on the Native Oxide Surface of SS316L. Langmuir 2006, 22, 6469–6472. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, S.A. Self-assembled monolayers (SAMs) of carboxylic acids: An overview. Cent. Eur. J. Chem. 2011, 9, 369–378. [Google Scholar] [CrossRef]
- Reese, S.; Fox, M.A. Self-Assembled Monolayers on Gold of Thiols Incorporating Conjugated Terminal Groups. J. Phys. Chem. B 1998, 102, 9820–9824. [Google Scholar] [CrossRef]
- Ford, W.E.; Abraham, F.; Scholz, F.; Nelles, G.; Sandford, G.; Wrochem, F.V. Spectroscopic Characterization of Fluorinated Benzylphosphonic Acid Monolayers on AlO x/Al Surfaces. J. Phys. Chem. C 2017, 121, 1690–1703. [Google Scholar] [CrossRef]
- Kim, D.H.; Park, J.-H.; Lee, T.I.; Myoung, J.-M. Superhydrophobic Al-doped ZnO nanorods-based electrically conductive and self-cleanable antireflecting window layer for thin film solar cell. Sol. Energy Mater. Sol. Cells 2016, 150, 65–70. [Google Scholar] [CrossRef]
- Jouet, R.J.; Warren, A.D.; Rosenberg, D.M.; Bellitto, V.J.; Park, K.; Zachariah, M.R. Surface Passivation of Bare Aluminum Nanoparticles Using Perfluoroalkyl Carboxylic Acids. Chem. Mater. 2005, 17, 2987–2996. [Google Scholar] [CrossRef]
- Colvin, V.; Goldstein, A.; Alivisatos, A. Semiconductor nanocrystals covalently bound to metal surfaces with self-assembled monolayers. J. Am. Chem. Soc. 1992, 114, 5221–5230. [Google Scholar] [CrossRef]
- Lange, I.; Reiter, S.; Pätzel, M.; Zykov, A.; Nefedov, A.; Hildebrandt, J.; Hecht, S.; Kowarik, S.; Wöll, C.; Heimel, G.; et al. Tuning the Work Function of Polar Zinc Oxide Surfaces using Modified Phosphonic Acid Self-Assembled Monolayers. Adv. Funct. Mater. 2014, 24, 7014–7024. [Google Scholar] [CrossRef]
- Boardman, L.D.; Pellerite, M.J. Fluorinated Phosphonic Acids. Google Patents US6824882 B2, 30 November 2004. [Google Scholar]
- Kraft, U.; Zschieschang, U.; Ante, F.; Kalblein, D.; Kamella, C.; Amsharov, K.; Jansen, M.; Kern, K.; Weber, E.; Klauk, H. Fluoroalkylphosphonic acid self-assembled monolayer gate dielectrics for threshold-voltage control in low-voltage organic thin-film transistors. J. Mater. Chem. 2010, 20, 6416–6418. [Google Scholar] [CrossRef]
- Xu, T.; Yu, L. How to design low bandgap polymers for highly efficient organic solar cells. Mater. Today 2014, 17, 11–15. [Google Scholar] [CrossRef]
- Timpel, M.; Nardi, M.V.; Krause, S.; Ligorio, G.; Christodoulou, C.; Pasquali, L.; Giglia, A.; Frisch, J.; Wegner, B.; Moras, P. Surface Modification of ZnO (0001)-Zn with Phosphonate-Based Self-Assembled Monolayers: Binding Modes, Orientation, and Work Function. Chem. Mater. 2014, 26, 5042–5050. [Google Scholar] [CrossRef]
- Raman, A.; Quiñones, R.; Barriger, L.; Eastman, R.; Parsi, A.; Gawalt, E.S. Understanding Organic Film Behavior on Alloy and Metal Oxides. Langmuir 2010, 26, 1747–1754. [Google Scholar] [CrossRef] [PubMed]
- Quiñones, R.; Raman, A.; Gawalt, E.S. An approach to differentiating between multi- and monolayers using MALDI-TOF MS. Surf. Interface Anal. 2007, 39, 593–600. [Google Scholar] [CrossRef]
- Whitesides, G.M.; Laibinis, P.E. Wet chemical approaches to the characterization of organic surfaces: Self-assembled monolayers, wetting, and the physical-organic chemistry of the solid-liquid interface. Langmuir 1990, 6, 87–96. [Google Scholar] [CrossRef]
- Snyder, R.G.; Struss, H.L.; Elliger, C.A. Carbon-hydrogon atretching modes and structure of n-alkyl chains. 1. Long, disordered Chains. J. Phys. Chem. 1982, 86, 5145–5150. [Google Scholar] [CrossRef]
- Wood, C.; Li, H.; Winget, P.; Brédas, J.-L. Binding Modes of Fluorinated Benzylphosphonic Acids on the Polar ZnO Surface and Impact on Work Function. J. Phys. Chem. C 2012, 116, 19125–19133. [Google Scholar] [CrossRef]
- Rechmann, J.; Sarfraz, A.; Götzinger, A.C.; Dirksen, E.; Müller, T.J.J.; Erbe, A. Surface Functionalization of Oxide-Covered Zinc and Iron with Phosphonated Phenylethynyl Phenothiazine. Langmuir 2015, 31, 7306–7316. [Google Scholar] [CrossRef] [PubMed]
- Di Mauro, A.; Smecca, E.; D’Urso, A.; Condorelli, G.G.; Fragalà, M.E. Tetra-anionic porphyrin loading onto ZnO nanoneedles: A hybrid covalent/non covalent approach. Mater. Chem. Phys. 2014, 143, 977–982. [Google Scholar] [CrossRef]
- Ishizaki, T.; Teshima, K.; Masuda, Y.; Sakamoto, M. Liquid phase formation of alkyl- and perfluoro-phosphonic acid derived monolayers on magnesium alloy AZ31 and their chemical properties. J. Colloid Interface Sci. 2011, 360, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Tajima, K.; Tong, Y.; Ye, S.; Hashimoto, K. Surface-Segregated Monolayers: A New Type of Ordered Monolayer for Surface Modification of Organic Semiconductors. J. Am. Chem. Soc. 2009, 131, 17597–17604. [Google Scholar] [CrossRef] [PubMed]
- Davidowski, S.K.; Holland, G.P. Solid-State NMR Characterization of Mixed Phosphonic Acid Ligand Binding and Organization on Silica Nanoparticles. Langmuir 2016, 32, 3253–3261. [Google Scholar] [CrossRef] [PubMed]
- Holland, G.P.; Sharma, R.; Agola, J.O.; Amin, S.; Solomon, V.C.; Singh, P.; Buttry, D.A.; Yarger, J.L. NMR Characterization of Phosphonic Acid Capped SnO2 Nanoparticles. Chem. Mater. 2007, 19, 2519–2526. [Google Scholar] [CrossRef]
- Yah, W.O.; Takahara, A.; Lvov, Y.M. Selective Modification of Halloysite Lumen with Octadecylphosphonic Acid: New Inorganic Tubular Micelle. J. Am. Chem. Soc. 2011, 134, 1853–1859. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Lee, H.; Lynch, V.M.; Mallouk, T.E. Synthesis and structural characterization of a homologous series of divalent-metal phosphonates, MII(O3PR).cntdot.H2O and MII(HO3PR)2. Inorg. Chem. 1988, 27, 2781–2785. [Google Scholar] [CrossRef]
- Hotchkiss, P.J.; Malicki, M.; Giordano, A.J.; Armstrong, N.R.; Marder, S.R. Characterization of phosphonic acid binding to zinc oxide. J. Mater. Chem. 2011, 21, 3107–3112. [Google Scholar] [CrossRef]
- Shervedani, R.K.; Hatefi-Mehrjardi, A.; Babadi, M.K. Comparative electrochemical study of self-assembled monolayers of 2-mercaptobenzoxazole, 2-mercaptobenzothiazole, and 2-mercaptobenzimidazole formed on polycrystalline gold electrode. Electrochim. Acta 2007, 52, 7051–7060. [Google Scholar] [CrossRef]
- Feichtenschlager, B.; Pabisch, S.; Peterlik, H.; Kickelbick, G. Nanoparticle Assemblies as Probes for Self-Assembled Monolayer Characterization: Correlation between Surface Functionalization and Agglomeration Behavior. Langmuir 2012, 28, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Quiñones, R.; Garretson, S.; Behnke, G.; Fagan, J.W.; Mueller, K.T.; Agarwal, S.; Gupta, R.K. Fabrication of phosphonic acid films on nitinol nanoparticles by dynamic covalent assembly. Thin Solid Films 2017, 642, 195–206. [Google Scholar] [CrossRef]
- Anuradha, K.; Bangal, P.; Madhavendra, S.S. Macromolecular Arabinogalactan Polysaccharide Mediated Synthesis of Silver Nanoparticles, Characterization and Evaluation. Macromol. Res. 2015, 24, 152–162. [Google Scholar] [CrossRef]
- Ling, X.Y.; Reinhoudt, D.N.; Huskens, J. Ferrocenyl-Functionalized Silica Nanoparticles: Preparation, Characterization, and Molecular Recognition at Interfaces. Langmuir 2006, 22, 8777–8783. [Google Scholar] [CrossRef] [PubMed]
- Sizovs, A.; Song, X.; Waxham, M.N.; Jia, Y.; Feng, F.; Chen, J.; Wicker, A.C.; Xu, J.; Yu, Y.; Wang, J. Precisely Tunable Engineering of Sub-30 nm Monodisperse Oligonucleotide Nanoparticles. J. Am. Chem. Soc. 2014, 136, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Schmitt Pauly, C.; Genix, A.-C.; Alauzun, J.G.; Guerrero, G.; Appavou, M.-S.; Pérez, J.; Oberdisse, J.; Mutin, P.H. Simultaneous Phase Transfer and Surface Modification of TiO2 Nanoparticles Using Alkylphosphonic Acids: Optimization and Structure of the Organosols. Langmuir 2015, 31, 10966–10974. [Google Scholar] [CrossRef] [PubMed]
- Oueiny, C.; Berlioz, S.; Patout, L.; Perrin, F.X. Aqueous dispersion of multiwall carbon nanotubes with phosphonic acid derivatives. Colloids Surf. A 2016, 493, 41–51. [Google Scholar] [CrossRef]
- Mikolajczyk, A.; Gajewicz, A.; Rasulev, B.; Schaeublin, N.; Maurer-Gardner, E.; Hussain, S.; Leszczynski, J.; Puzyn, T. Zeta Potential for Metal Oxide Nanoparticles: A Predictive Model Developed by a Nano-Quantitative Structure–Property Relationship Approach. Chem. Mater. 2015, 27, 2400–2407. [Google Scholar] [CrossRef]
- Fedele, L.; Colla, L.; Bobbo, S. Viscosity and thermal conductivity measurements of water-based nanofluids containing titanium oxide nanoparticles. Int. J. Refrig. 2012, 35, 1359–1366. [Google Scholar] [CrossRef]
- Borlaf, M.; Colomer, M.T.; Cabello, F.; Serna, R.; Moreno, R. Electrophoretic Deposition of TiO2/Er3+ Nanoparticulate Sols. J. Phys. Chem. B 2013, 117, 1556–1562. [Google Scholar] [CrossRef] [PubMed]
- Raman, A.; Gawalt, E.S. Self-Assembled Monolayers of Alkanoic Acids on the Native Oxide Surface of SS316L by Solution Deposition. Langmuir 2007, 23, 2284–2288. [Google Scholar] [CrossRef] [PubMed]
- Parks, G.A. The Isoelectric Points of Solid Oxides, Solid Hydroxides, and Aqueous Hydroxo Complex Systems. Chem. Rev. 1965, 65, 177–198. [Google Scholar] [CrossRef]
- Lopes, K.P.; Cavalcante, L.S.; Simões, A.Z.; Gonçalves, R.F.; Escote, M.T.; Varela, J.A.; Longo, E.; Leite, E.R. NiTiO3 nanoparticles encapsulated with SiO2 prepared by sol–gel method. J. Sol-Gel Sci. Technol. 2008, 45, 151–155. [Google Scholar] [CrossRef]
- Marsalek, R. Particle size and zeta potential of ZnO. APCBEE Procedia 2014, 9, 13–17. [Google Scholar] [CrossRef]
- Clement, S.; Deng, W.; Drozdowicz-Tomsia, K.; Liu, D.; Zachreson, C.; Goldys, E.M. Bright, water-soluble CeF3 photo-, cathodo-, and X-ray luminescent nanoparticles. J. Nanopart. Res. 2015, 17, 7. [Google Scholar] [CrossRef]
- Ivanov, M.R.; Haes, A.J. Anionic Functionalized Gold Nanoparticle Continuous Full Filling Separations: Importance of Sample Concentration. Anal. Chem. 2012, 84, 1320–1326. [Google Scholar] [CrossRef] [PubMed]
- Helmy, R.; Fadeev, A.Y. Self-Assembled Monolayers supported on TiO2: Comparison of C18H37SiX3 (X=H, Cl, OCH3), C18H37Si(CH3)2Cl and C18H37PO(OH)2. Langmuir 2002, 18, 8924–8928. [Google Scholar] [CrossRef]
- Ye, H.-J.; Shao, W.-Z.; Zhen, L. Tetradecylphosphonic acid modified BaTiO3 nanoparticles and its nanocomposite. Colloids Surf. A 2013, 427 (Suppl. C), 19–25. [Google Scholar] [CrossRef]
- Ruiterkamp, G.J.; Hempenius, M.A.; Wormeester, H.; Vancso, G.J. Surface functionalization of titanium dioxide nanoparticles with alkanephosphonic acids for transparent nanocomposites. J. Nanopart. Res. 2011, 13, 2779–2790. [Google Scholar] [CrossRef]
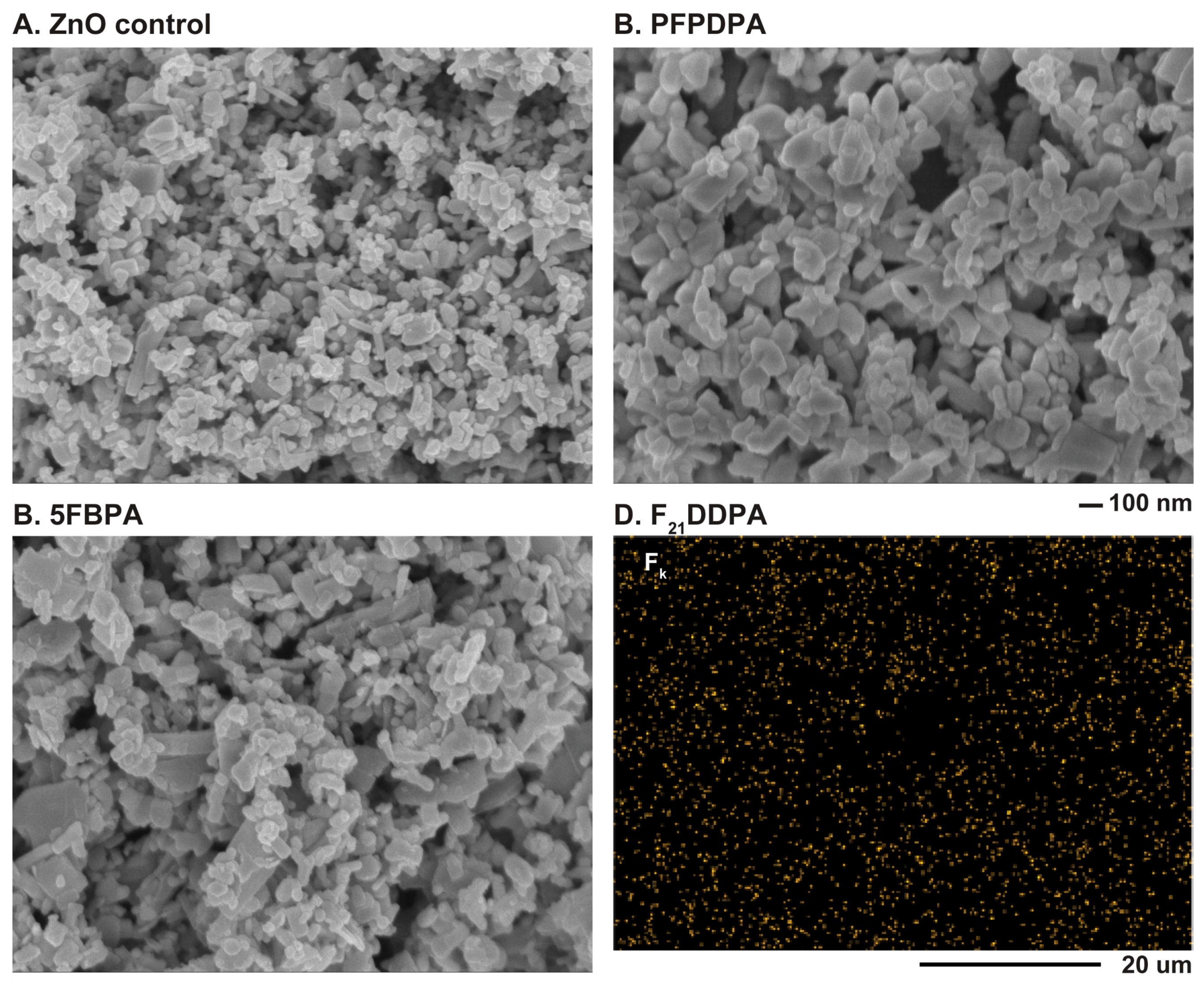
| Sample | Zn2p % | O1s % | C1s % | F1s % | P2p % | C/P | F/P |
|---|---|---|---|---|---|---|---|
| PFPDPA | 14.9 | 28.3 | 44.9 | 10.1 | 1.8 | 24.8 (18) | 5.5 (5) |
| 5FBPA | 25.0 | 31.2 | 23.5 | 17.6 | 2.7 | 8.7 (7) | 6.5 (5) |
| F21DDPA | 13.5 | 19.5 | 25.9 | 39.1 | 2.1 | 12.4 (12) | 18.7 (21) |
| ZnO Control | 47.5 | 52.5 | - | - | - | - | - |
| Modifications | Average Particle Size (nm) | Particle Distribution (±nm) |
|---|---|---|
| ZnO | 139.8 | 18.6 |
| 5FBPA | 167.0 | 27.9 |
| PFPDPA | 194.1 | 40.8 |
| F21DDPA | 166.0 | 32.7 |
| Modification | Water | THF | ||
|---|---|---|---|---|
| Particle Size (nm) | Zeta Potential (mV) | Particle Size (nm) | Zeta Potential (mV) | |
| ZnO | 497.2 ± 12.2 | −11.09 ± 0.42 | 413.2 ± 18.0 | −11.48 ± 4.40 |
| 5FBPA | 247.1 ± 8.9 | −20.31 ± 0.66 | 279.1 ± 3.5 | −48.04 ± 2.68 |
| PFPDPA | 275.3 ± 9.5 | −22.53 ± 0.39 | 243.5 ± 0.7 | −52.29 ± 3.02 |
| F21DDPA | 217.5 ± 8.4 | −18.64 ± 0.56 | 244.1 ± 2.2 | −89.12 ± 1.96 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quiñones, R.; Shoup, D.; Behnke, G.; Peck, C.; Agarwal, S.; Gupta, R.K.; Fagan, J.W.; Mueller, K.T.; Iuliucci, R.J.; Wang, Q. Study of Perfluorophosphonic Acid Surface Modifications on Zinc Oxide Nanoparticles. Materials 2017, 10, 1363. https://doi.org/10.3390/ma10121363
Quiñones R, Shoup D, Behnke G, Peck C, Agarwal S, Gupta RK, Fagan JW, Mueller KT, Iuliucci RJ, Wang Q. Study of Perfluorophosphonic Acid Surface Modifications on Zinc Oxide Nanoparticles. Materials. 2017; 10(12):1363. https://doi.org/10.3390/ma10121363
Chicago/Turabian StyleQuiñones, Rosalynn, Deben Shoup, Grayce Behnke, Cynthia Peck, Sushant Agarwal, Rakesh K. Gupta, Jonathan W. Fagan, Karl T. Mueller, Robbie J. Iuliucci, and Qiang Wang. 2017. "Study of Perfluorophosphonic Acid Surface Modifications on Zinc Oxide Nanoparticles" Materials 10, no. 12: 1363. https://doi.org/10.3390/ma10121363
APA StyleQuiñones, R., Shoup, D., Behnke, G., Peck, C., Agarwal, S., Gupta, R. K., Fagan, J. W., Mueller, K. T., Iuliucci, R. J., & Wang, Q. (2017). Study of Perfluorophosphonic Acid Surface Modifications on Zinc Oxide Nanoparticles. Materials, 10(12), 1363. https://doi.org/10.3390/ma10121363





