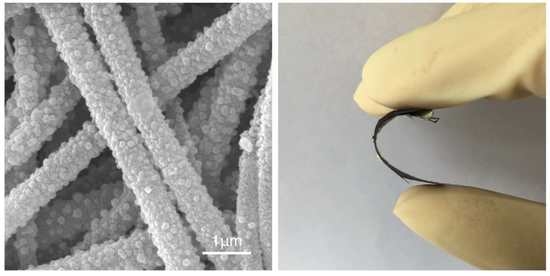
Preparation of Palladium/Silver-Coated Polyimide Nanotubes: Flexible, Electrically Conductive Fibers
Abstract
:1. Introduction
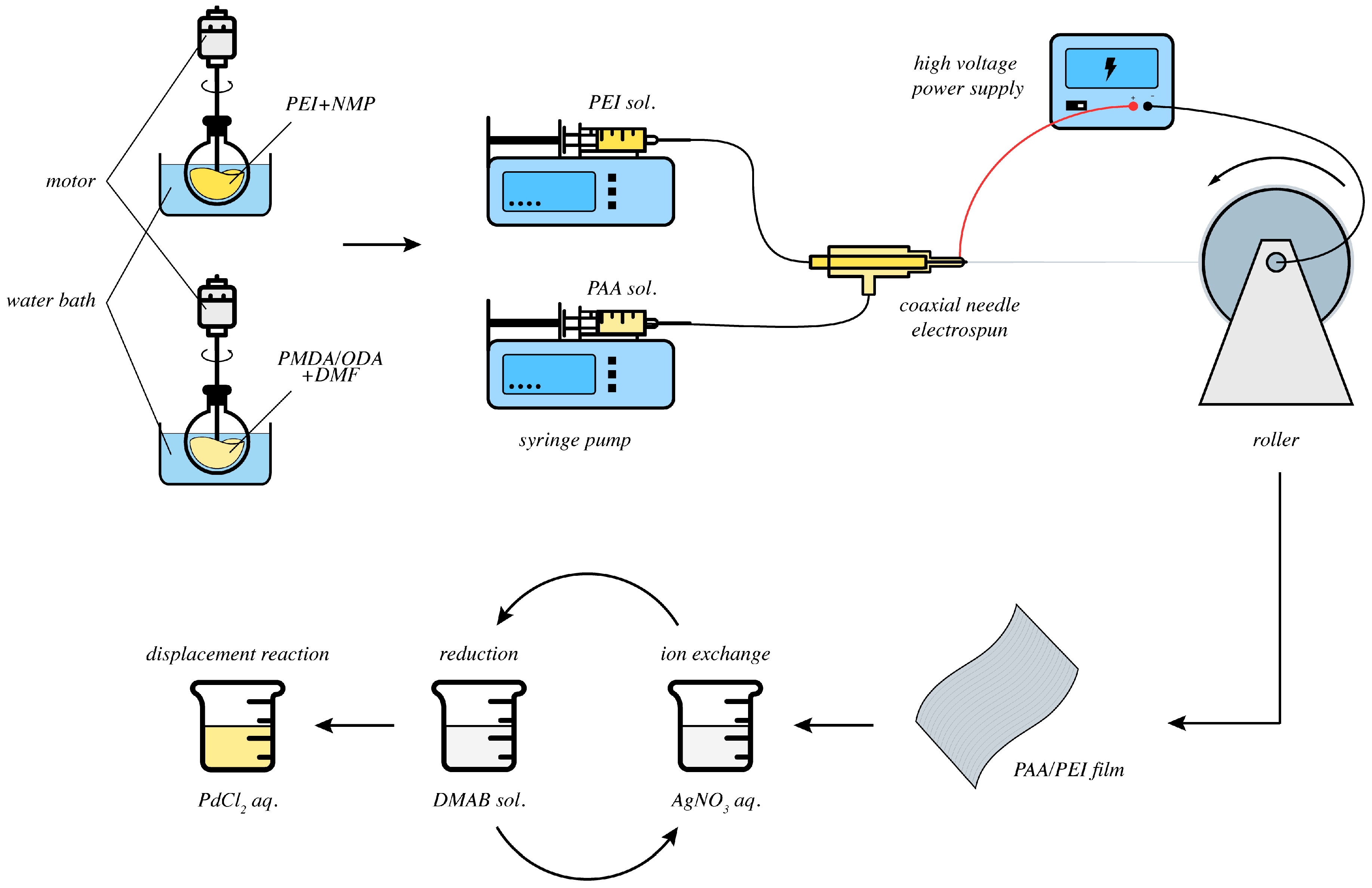
2. Results and Discussion
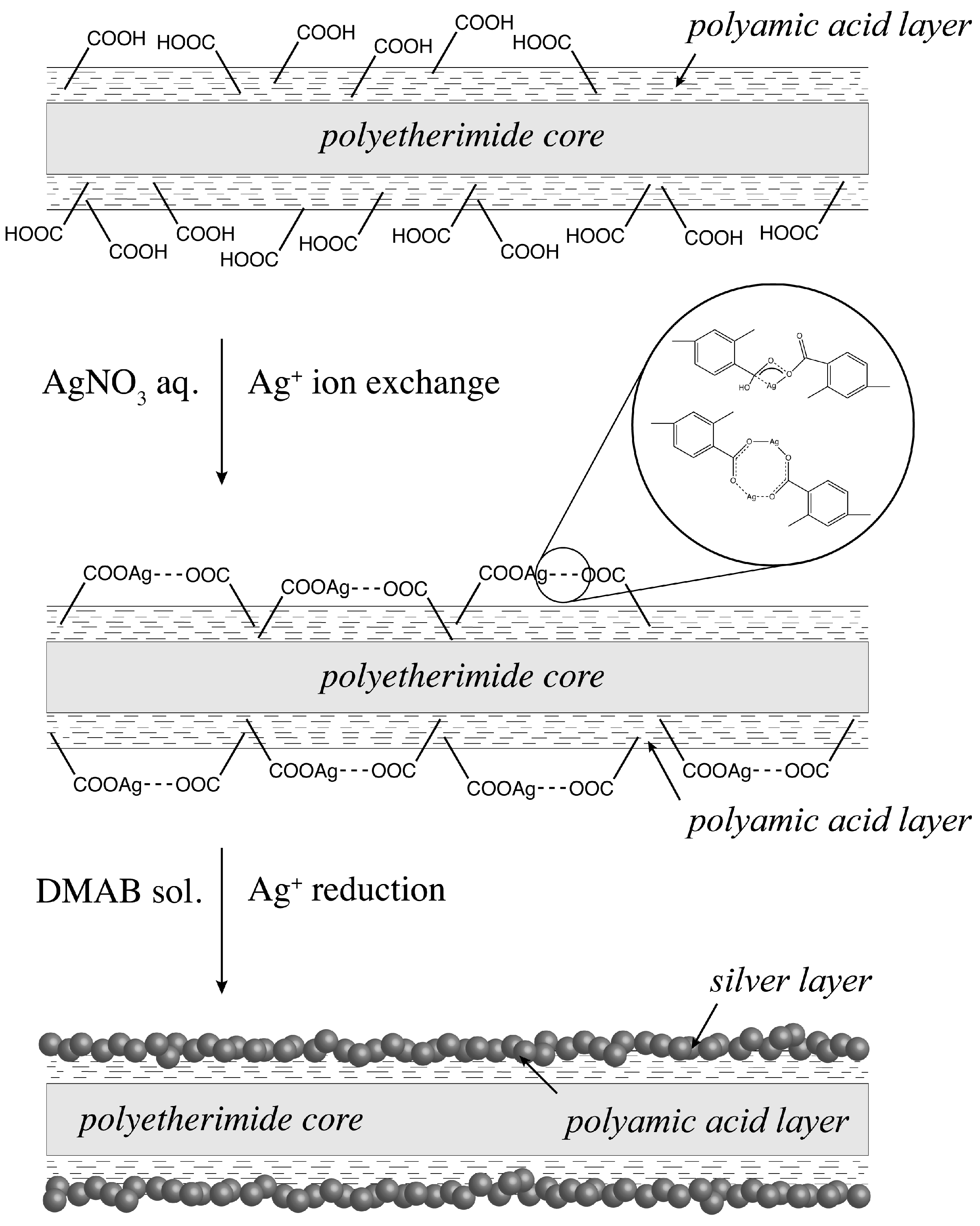
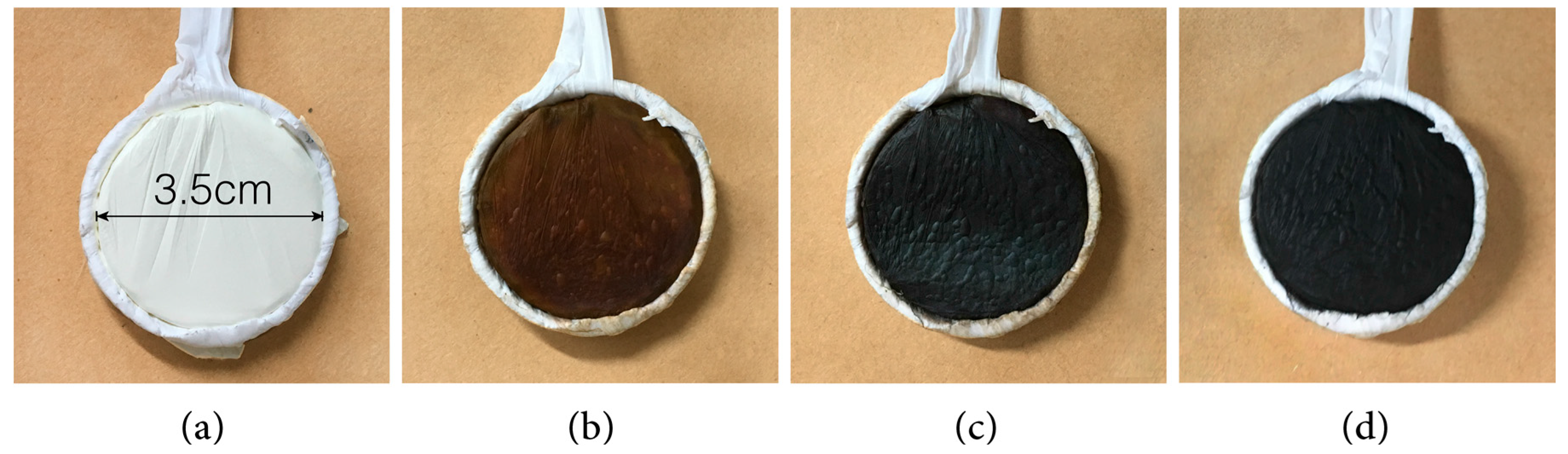
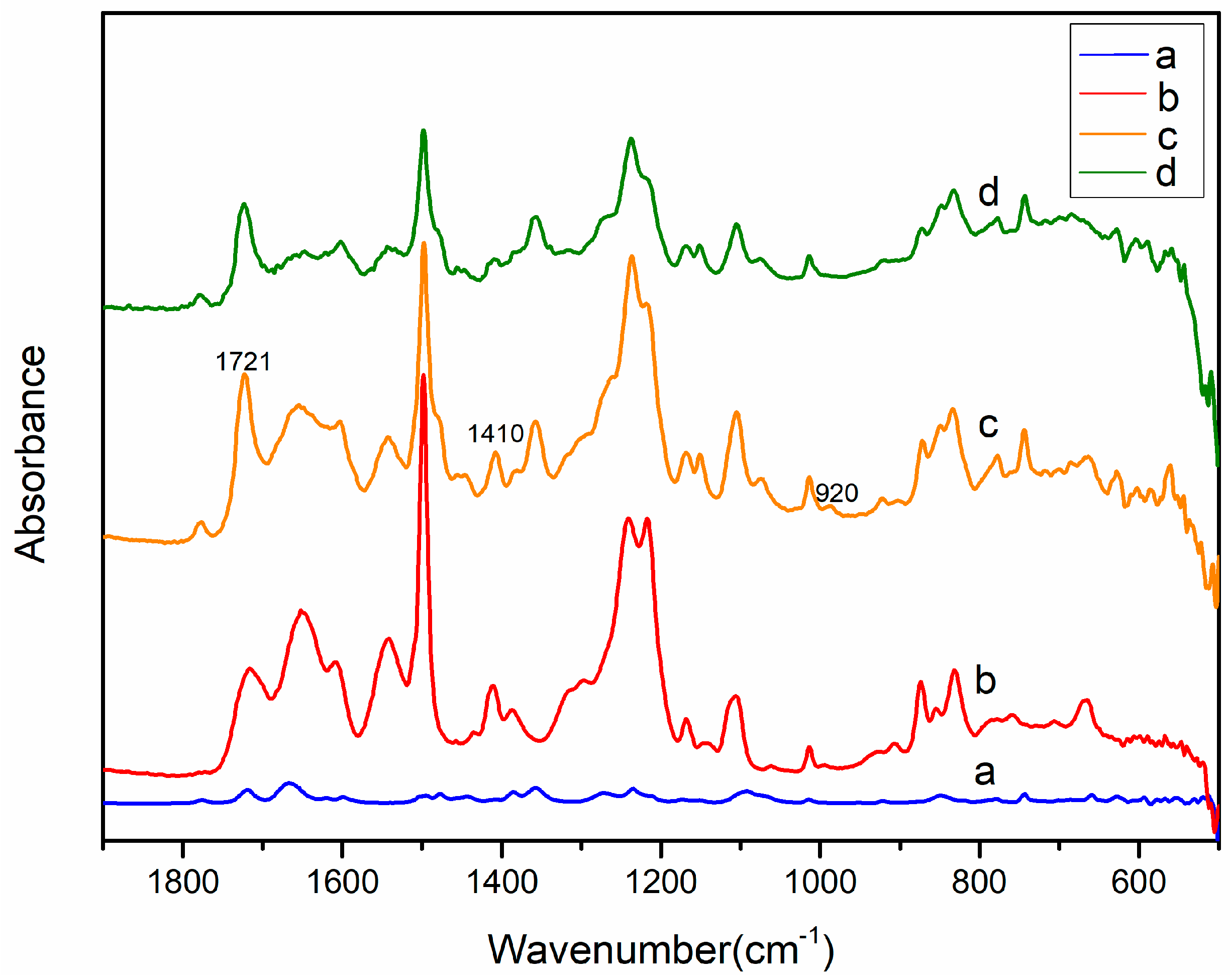
2.1. Ion Exchange and Reduction
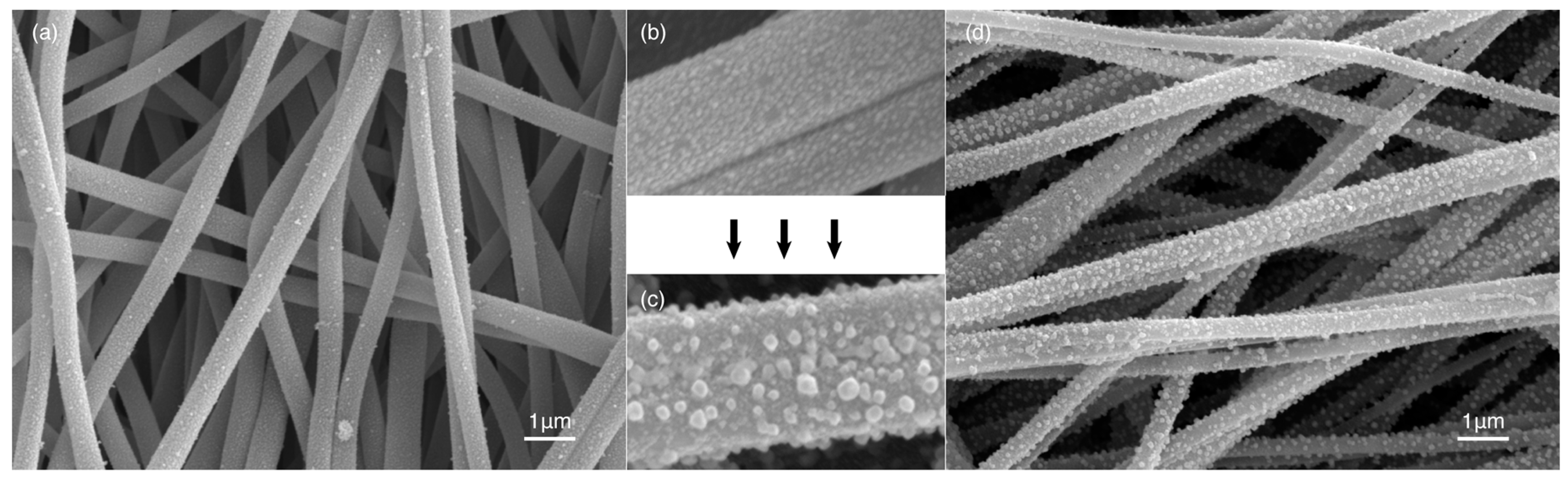
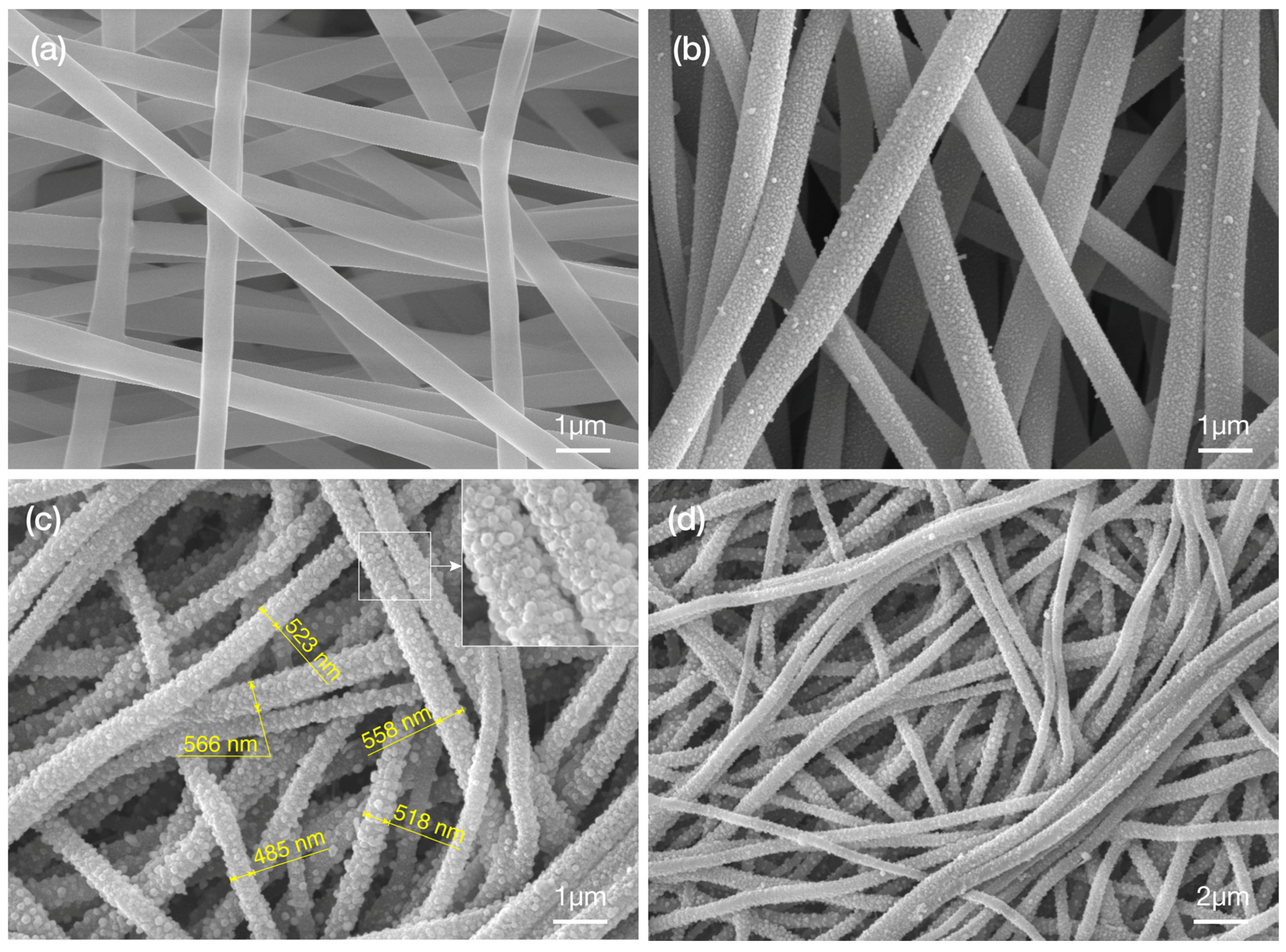
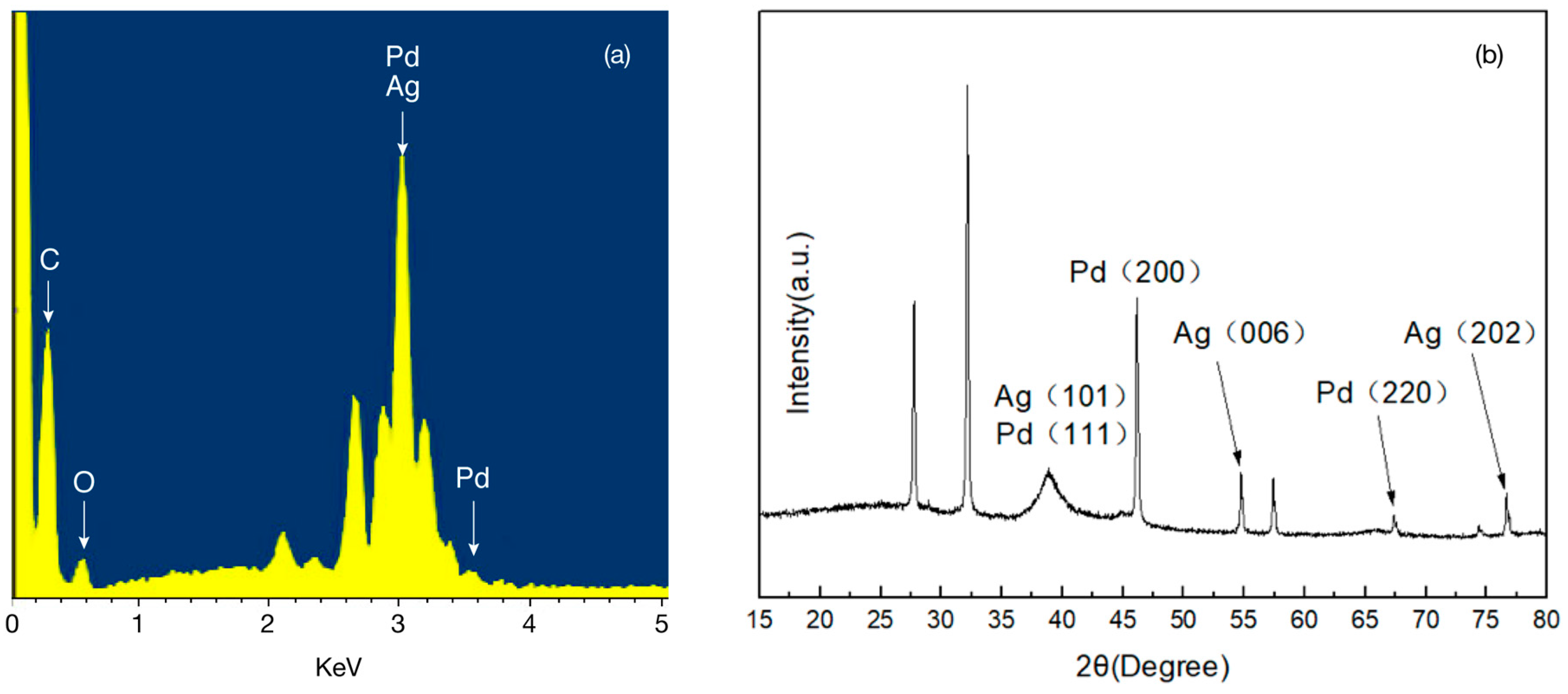
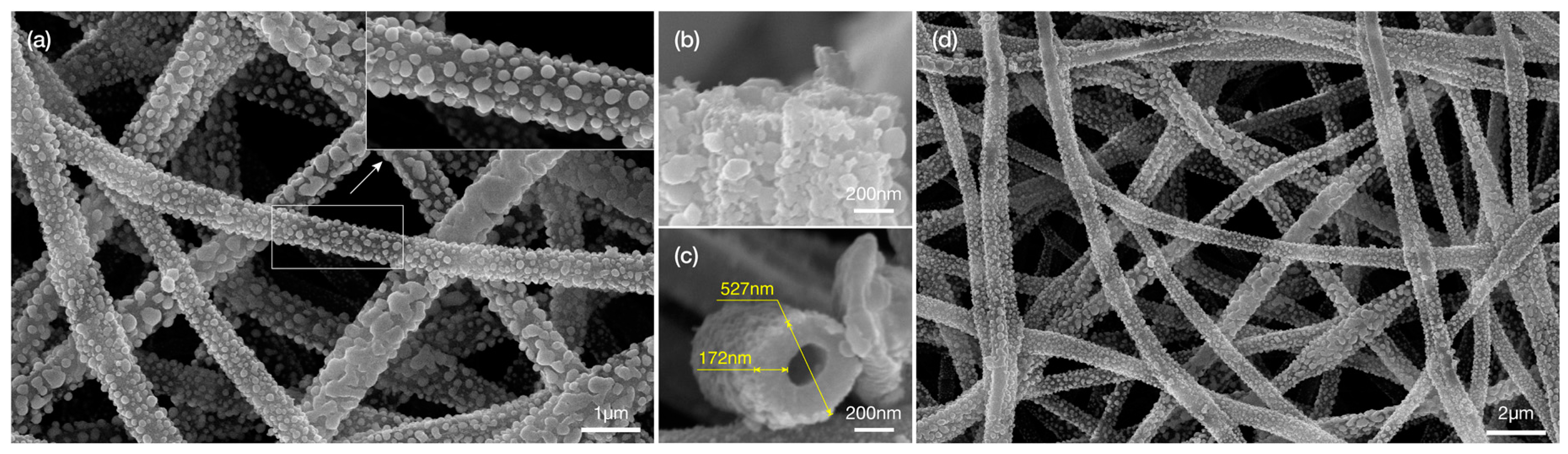
2.2. Metal Displacement Reaction
2.3. Core Displacement and Imidze Reaction
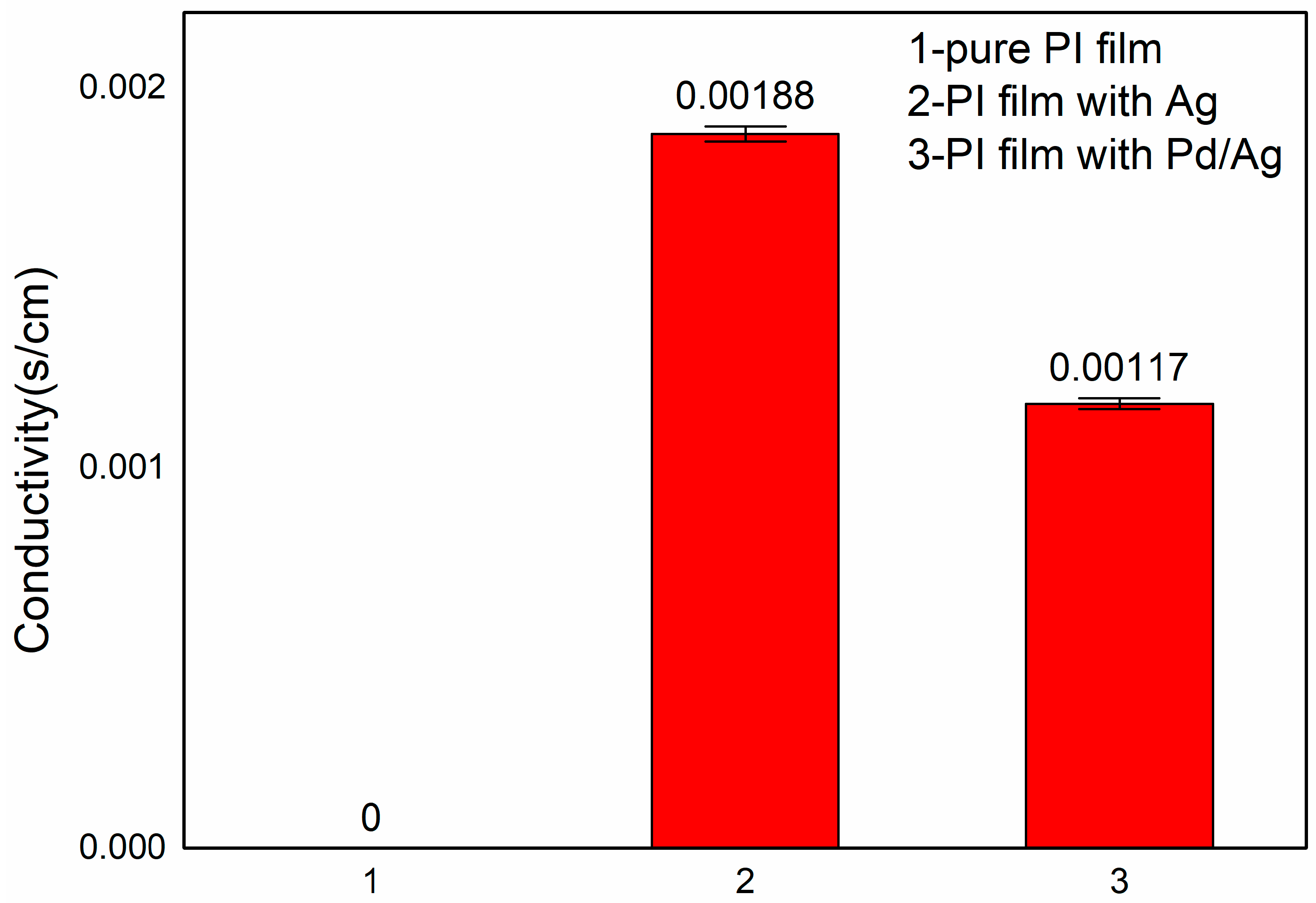
2.4. Electrical Conductivity of Pd/Ag Polyimide Nanotubes
3. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, D.; Miao, Y.E.; Liu, T. Electrically Conductive Polyaniline/Polyimide Nanofiber Membranes Prepared via a Combination of Electrospinning and Subsequent In situ Polymerization Growth. ACS Appl. Mater. Interfaces 2013, 5, 1206–1212. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.J.; Chen, C.J.; Liou, G.S. Novel high-efficiency PL polyimide nanofiber containing aggregation-induced emission (AIE)-active cyanotriphenylamine luminogen. Chem. Commun. 2013, 49, 630–632. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, S.; Al-Marzouki, F.; Obaid, A.; Gamal, S. Effect of Addition of Colloidal Silica to Films of Polyimide, Polyvinylpyridine, Polystyrene, and Polymethylmethacrylate Nano-Composites. Materials 2016, 9, 104. [Google Scholar] [CrossRef] [PubMed]
- Zope, I.S.; Dasari, A.; Yu, Z.Z. Influence of Polymer-Clay Interfacial Interactions on the Ignition Time of Polymer/Clay Nanocomposites. Materials 2017, 10, 935. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnanm, S.; Dhakshnamoorthy, M.; Jelmy, E.J.; Vasanthakumari, R.; Kothurkar, N.K. Synthesis and characterization of graphene oxide-polyimide nanofiber composites. RSC Adv. 2014, 4, 9743–9749. [Google Scholar] [CrossRef]
- Feng, T.; Mao, D.; Cui, X.; Li, M.; Song, K.; Jiang, B.; Lu, H.; Quan, W. A Filmy Black-Phosphorus Polyimide Saturable Absorber for Q-Switched Operation in an Erbium-Doped Fiber Laser. Materials 2016, 9, 917. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Han, D.; Chen, J.; Ding, Y.; Jiang, S.; Hu, C.; Chen, S.; Hou, H. Highly strong and highly tough electrospun polyimide/polyimide composite nanofibers from binary blend of polyamic acids. RSC Adv. 2014, 4, 59936–59942. [Google Scholar] [CrossRef]
- Gong, G.; Gao, K.; Wu, J.; Sun, N.; Zhou, C.; Zhao, Y.; Jiang, L. A highly durable silica/polyimide superhydrophobic nanocomposite film with excellent thermal stability and abrasion-resistant performance. J. Mater. Chem. A 2015, 3, 713–718. [Google Scholar] [CrossRef]
- Peng, X.; Xu, W.; Chen, L.; Ding, Y.; Chen, S.; Wang, X.; Hou, H. Polyimide complexes with high dielectric performance: Toward polymer film capacitor applications. J. Mater. Chem. C 2016, 4, 6452–6456. [Google Scholar] [CrossRef]
- Li, S.; Wu, D.; Yan, X.; Guan, Y. Acetone-activated polyimide electrospun nanofiber membrane for thin-film microextraction and thermal desorption-gas chromatography–mass spectrometric analysis of phenols in environmental water. J. Chromatogr. A 2015, 1411, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Doradla, P.; Narkhede, M.; Li, L.; Samuelson, L.A.; Giles, R.H.; Kumar, J. A Simple method for fabricating silver nanotubes. RSC Adv. 2014, 4, 36671–36674. [Google Scholar] [CrossRef]
- Kalluri, S.; Seng, K.H.; Guo, Z.; Liu, H.K.; Dou, S.X. Electrospun lithium metal oxide cathode materials for lithium-ion batteries. RSC Adv. 2013, 3, 25576–25601. [Google Scholar] [CrossRef]
- Sun, Y.; Mayers, B.; Xia, Y. Metal Nanostructures with Hollow Interiors. Adv. Mater. 2003, 15, 641–646. [Google Scholar] [CrossRef]
- Cui, G.; Wu, D.; Qi, S.; Jin, S.; Wu, Z.; Jin, R. Preparation SnO2 Nanolayer on Flexible Polyimide Substrates via Direct Ion-Exchange and in situ Oxidation Process. ACS Appl. Mater. Interfaces 2011, 3, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.S.; Chen, T.L.; Mkhitaryan, V.; Pruneri, V. Ultrathin transparent conductive polyimide foil embedding silver nanowires. ACS Appl. Mater. Interfaces 2014, 6, 20943–20948. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.S.; Davis, L.M.; Thompson, D.W.; Southward, R.E. Reflective and Conductive Silver-Polyimide Films Prepared from Silver(I) Complexes with ODPA/4,4’-ODA. ACS Appl. Mater. Interfaces 2009, 1, 1457–1466. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, S.; Yanagimoto, H.; Akamatsu, K.; Nawafune, H. Copper/Polyimide Heterojunctions: Controlling Interfacial Structures through an Additive-Based, All-Wet Chemical Process Using Ion-Doped Precursors. Adv. Funct. Mater. 2007, 17, 889–897. [Google Scholar] [CrossRef]
- Serbezeanu, D.; Popa, A.M.; Sava, I.; Carja, I.D.; Amberg, M.R.; Rossi, M.; Fortunato, G. Design and synthesis of polyimide-Gold nanofibers with tunable optical properties. Eur. Polym. J. 2015, 64, 10–20. [Google Scholar] [CrossRef]
- Zhu, J.; Wei, S.; Chen, X.; Karki, A.B.; Rutman, D.; Young, D.P.; Guo, Z. Electrospun Polyimide Nanocomposite Fibers Reinforced with Core-Shell Fe-FeO nanoparticles. J. Phys. Chem. C 2010, 114, 8844–8850. [Google Scholar] [CrossRef]
- Nabae, Y.; Nagata, S.; Hayakawa, T.; Niwa, H.; Harada, Y.; Oshima, M.; Isoda, A.; Matsunaga, A.; Tanaka, K.; Aoki, T. Pt-free carbon-based fuel cell catalyst prepared from spherical polyimide for enhanced oxygen diffusion. Sci. Rep. 2016, 6, 23276. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Nabae, Y.; Hayakawa, T.; Kakimoto, M. Hyperbranched aromatic poly(ether ketone) functionalized with TEMPO as a heterogeneous catalyst for aerobic oxidation of alcohols. RSC Adv. 2015, 5, 1923–1928. [Google Scholar] [CrossRef]
- Qi, S.; Shen, X.; Lin, Z.; Tian, G.; Wu, D.; Jin, R. Synthesis of silver nanocubes with controlled size using water-soluble poly(amic acid) salt as the intermediate via a novel ion-exchange self-assembly technique. Nanoscale 2013, 5, 12132–12135. [Google Scholar] [CrossRef] [PubMed]
- Han, E.; Wu, D.; Qi, S.; Tian, G.; Niu, H.; Shang, G.; Yan, X.; Yang, X. Incorporation of Silver Nanoparticles into the Bulk of the Electrospun Ultrafine Polyimide Nanofibers via a Direct Ion Exchange Self-Metallization Process. ACS Appl. Mater. Interfaces 2012, 4, 2583–2590. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Wu, Z.; Wu, D.; Yang, W.; Jin, R. The chemistry involved in the loading of silver (I) into poly(amic acid) via ion exchange: A metal-ion-induced crosslinking behavior. Polymer 2009, 50, 845–854. [Google Scholar] [CrossRef]
- Yang, T.; Yu, Y.Z.; Zhu, L.S.; Wu, X.; Wang, X.H.; Zhang, J. Fabrication of silver interdigitated electrodes on polyimide films via surface modification and ion-exchange technique and its flexible humidity sensor application. Sens. Actuators B 2015, 208, 327–333. [Google Scholar] [CrossRef]
- Peng, H.C.; Xie, S.; Park, J.; Xia, X.; Xia, Y. Quantitative Analysis of the Coverage Density of Br− Ions on Pd{100} Facets and Its Role in Controlling the Shape of Pd Nanocrystals. Chem. Soc. 2013, 135, 3780–3783. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, L.; Rui, G.; Wang, G.; Huang, R.; Li, R.; Yu, J.; Qi, S.; Wu, D. Preparation of Palladium/Silver-Coated Polyimide Nanotubes: Flexible, Electrically Conductive Fibers. Materials 2017, 10, 1263. https://doi.org/10.3390/ma10111263
Kong L, Rui G, Wang G, Huang R, Li R, Yu J, Qi S, Wu D. Preparation of Palladium/Silver-Coated Polyimide Nanotubes: Flexible, Electrically Conductive Fibers. Materials. 2017; 10(11):1263. https://doi.org/10.3390/ma10111263
Chicago/Turabian StyleKong, Lushi, Guanchun Rui, Guangyu Wang, Rundong Huang, Ran Li, Jiajie Yu, Shengli Qi, and Dezhen Wu. 2017. "Preparation of Palladium/Silver-Coated Polyimide Nanotubes: Flexible, Electrically Conductive Fibers" Materials 10, no. 11: 1263. https://doi.org/10.3390/ma10111263
APA StyleKong, L., Rui, G., Wang, G., Huang, R., Li, R., Yu, J., Qi, S., & Wu, D. (2017). Preparation of Palladium/Silver-Coated Polyimide Nanotubes: Flexible, Electrically Conductive Fibers. Materials, 10(11), 1263. https://doi.org/10.3390/ma10111263