Modeling Calculation and Synthesis of Alumina Whiskers Based on the Vapor Deposition Process
Abstract
1. Introduction
2. Simulation and Experimentation
3. Results and Discussion
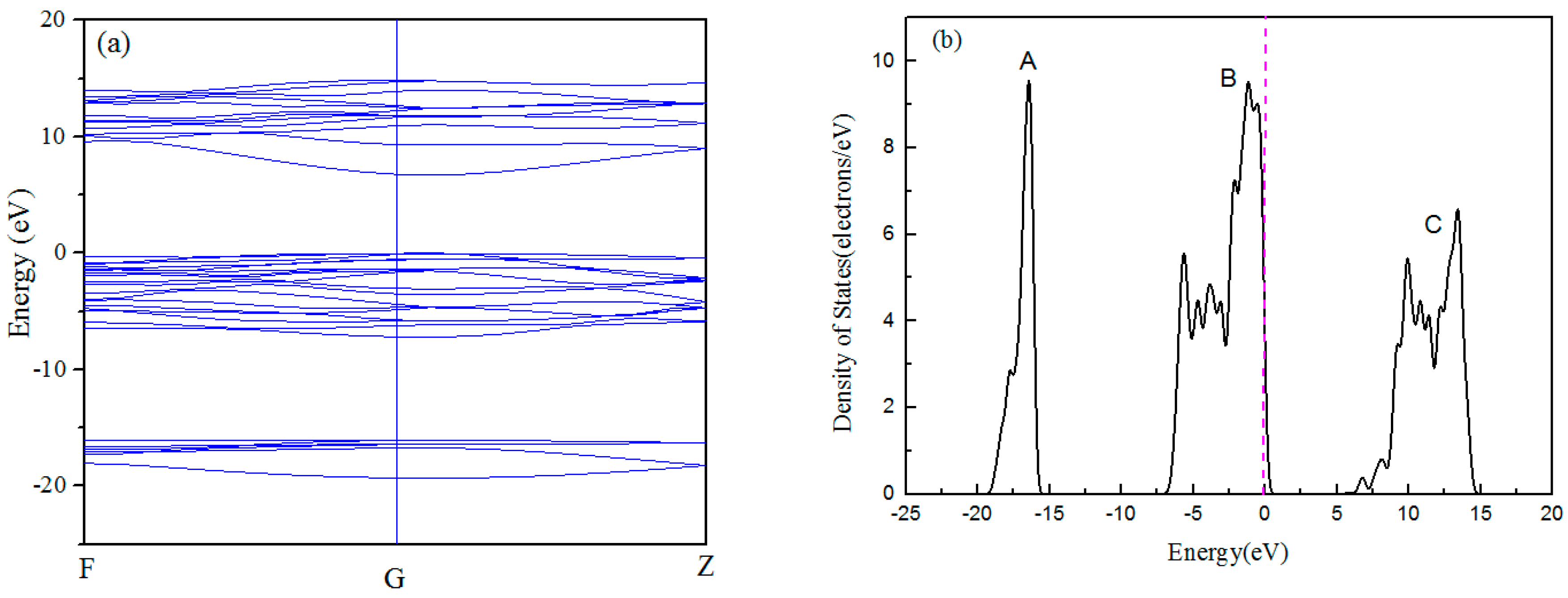
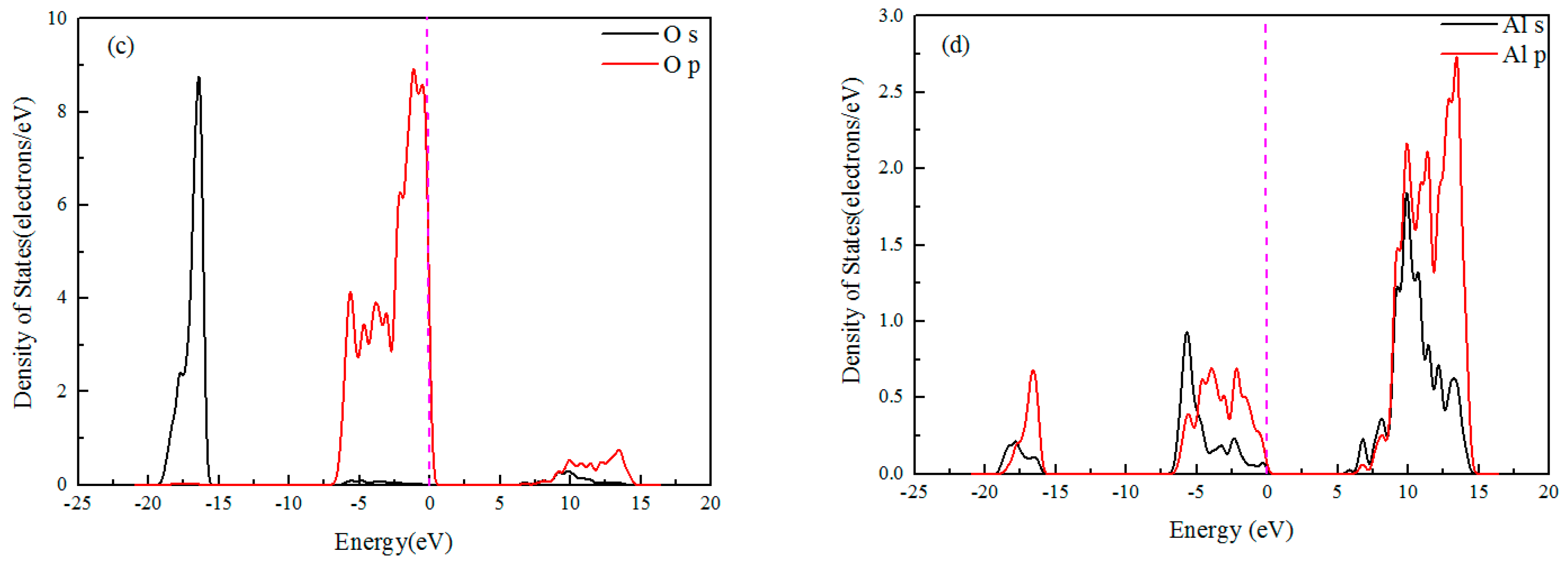
3.1. Band Structure and Density of States
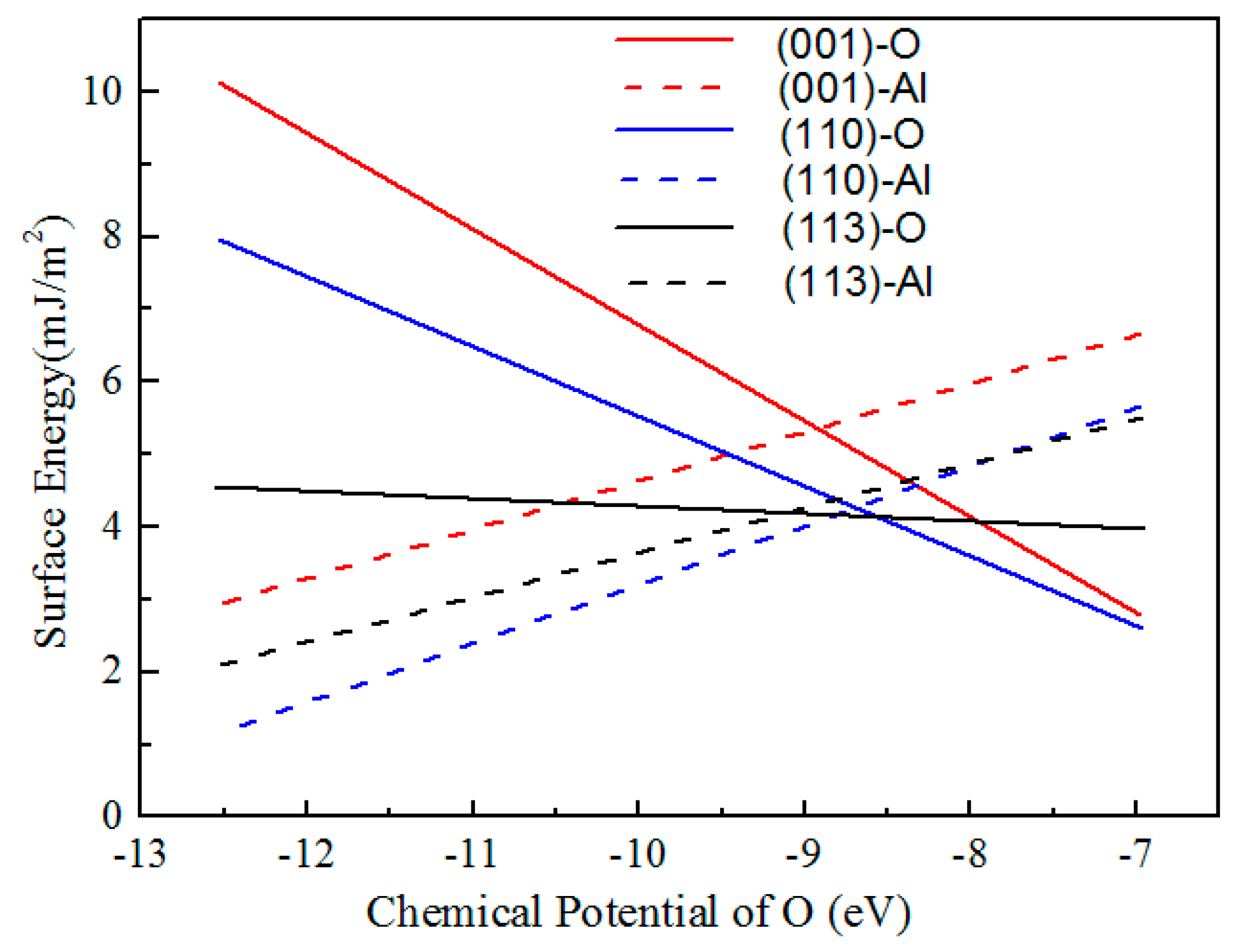
3.2. Surface Energies Calculation
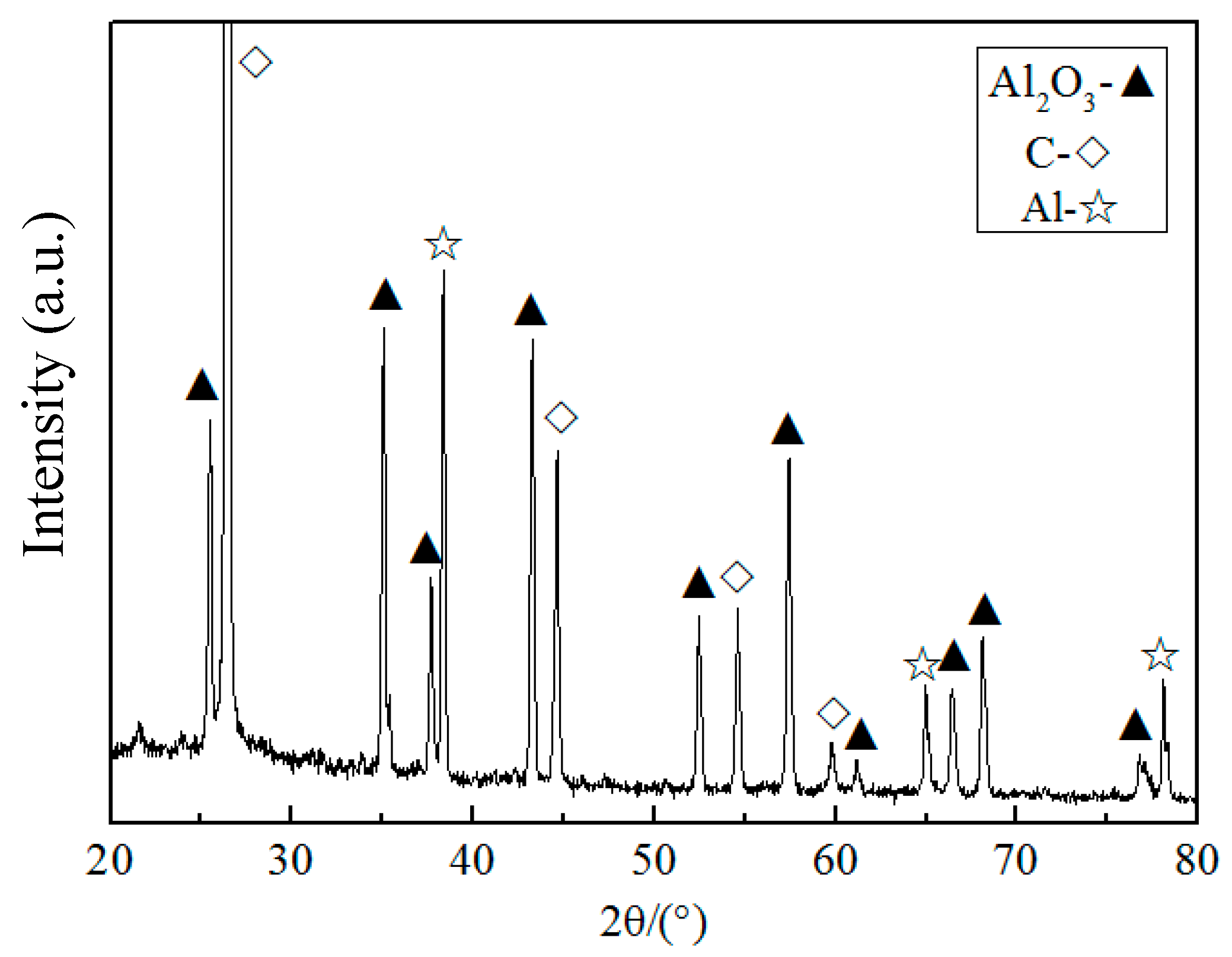
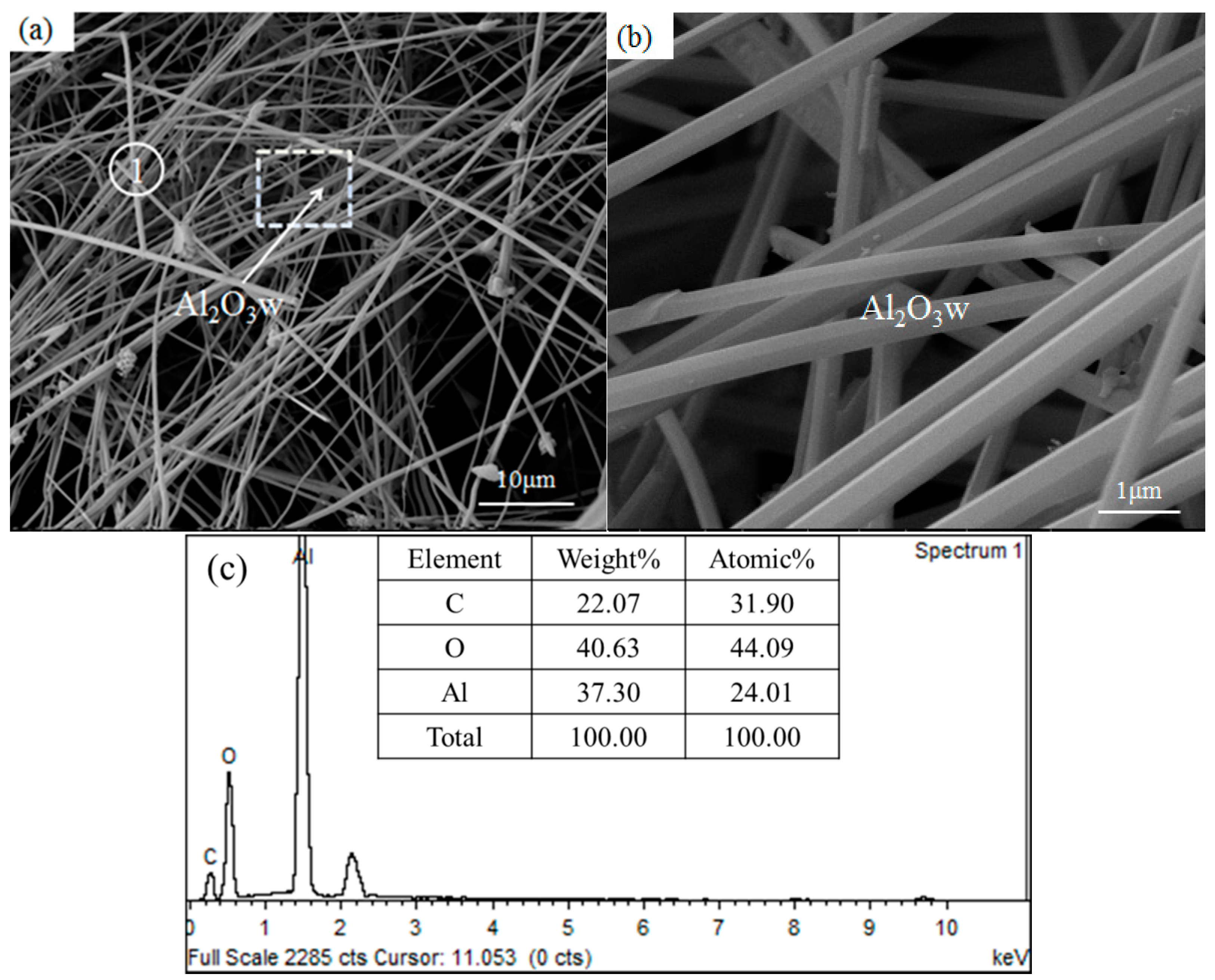
3.3. Phase Composition and Microstructure of the As-Synthesized Sample
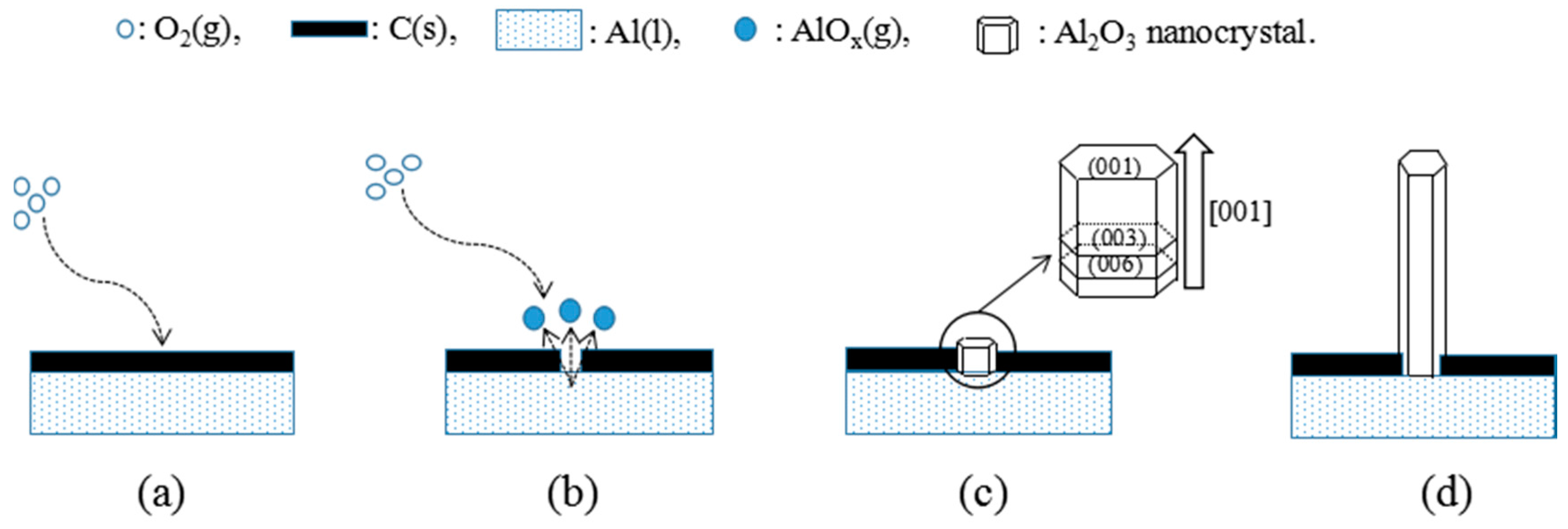
3.4. The Generation Mechanism for Al2O3 Whiskers at High Temperatures
4. Conclusions
- (1)
- Density of States calculation of Al2O3 showed that Al-O bonding exhibited strong ionic characteristics and the direct band gap between the top of the valence band and the bottom of the conduction band is 6.704 eV. The surfaces of (001), (110), and (113) would be terminated and determined by the Al or O layer.
- (2)
- Under oxygen-rich conditions, the surface energies of low Miller index surfaces of Al2O3 followed the sequence of (001)-Al > (110)-Al > (113)-Al > (113)-O > (001)-O > (110)-O. Under oxygen-deficient conditions, the sequence was converted to the trend of (001)-O > (110)-O > (113)-O > (001)-Al > (113)-Al > (110)-Al. The (001) surface possessed the higher surface energy.
- (3)
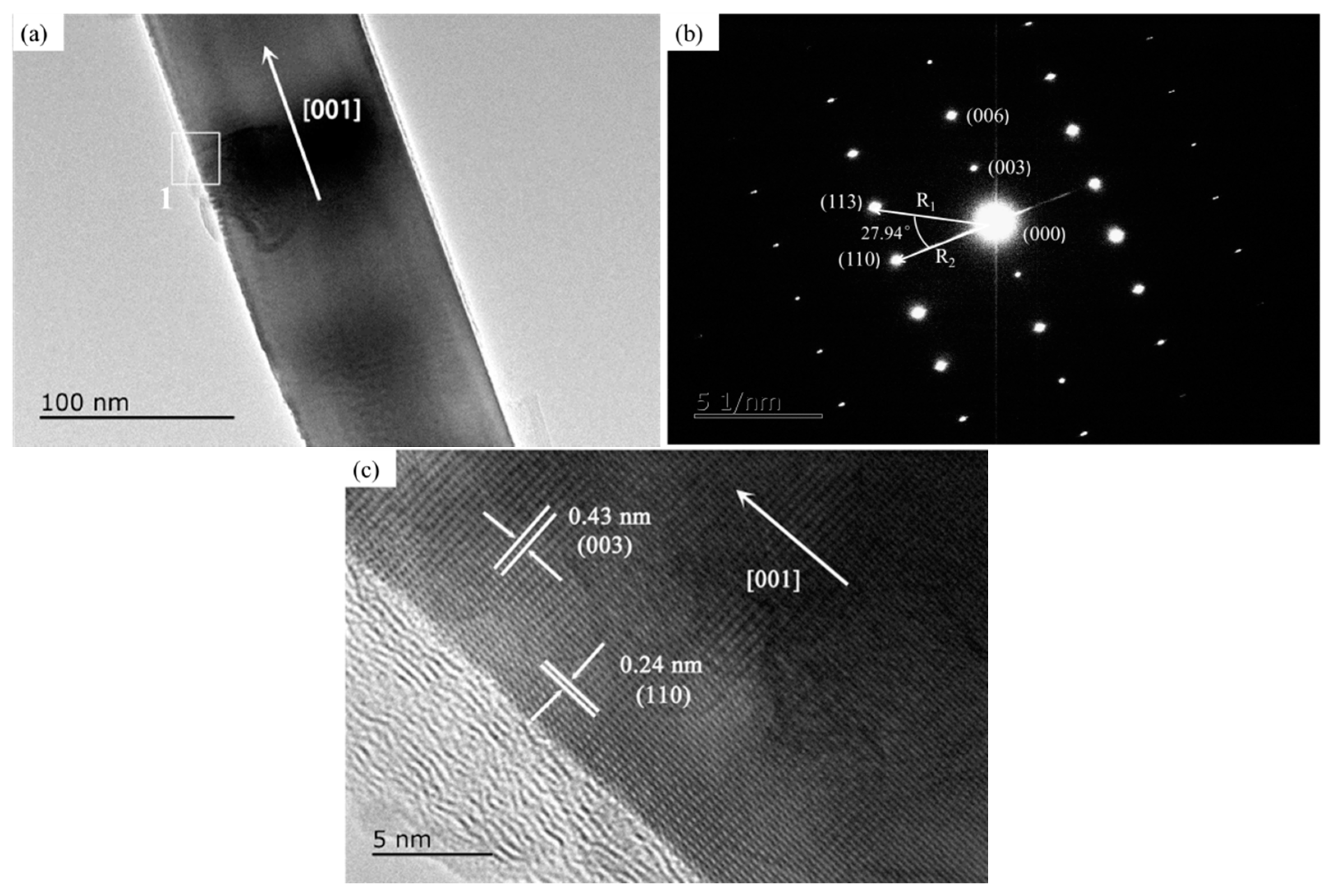
- The growth experiment of Al2O3 whiskers showed that the whiskers were 50–500 nm in diameter and hundreds of microns in length. The diffraction spots along the alumina whiskers’ axis was the crystal face (003), and its growth direction was parallel to [001], namely, along the c-axis. This analysis was in agreement with the calculation and modeling results based on DOS.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tamura, Y.; Moshtaghioun, B.M.; Gomez-Garcia, D.; Rodríguez, A.D. Spark plasma sintering of fine-grained alumina ceramics reinforced with alumina whiskers. Ceram. Int. 2017, 43, 658–663. [Google Scholar] [CrossRef]
- Mudra, E.; Streckova, M.; Pavlinak, D.; Medvecka, V.; Kovacik, D.; Kovalcikova, A.; Zubko, P.; Girman, V.; Dankova, Z.; Koval, V.; et al. Development of Al2O3 electrospun fibers prepared by conventional sintering method or plasma assisted surface calcination. Appl. Surf. Sci. 2017, 415, 90–98. [Google Scholar] [CrossRef]
- Rao, C.; Gundiah, G.; Deepak, F.; Govindaraj, A.; Cheetham, A. Carbon-assisted synthesis of inorganic nanowires. J. Mater. Chem. 2004, 14, 440–450. [Google Scholar] [CrossRef]
- Xu, M.; Wang, S.; Shen, F.; Ji, J.; Dai, W.J. Study on the influential factors for preparing transition alumina whiskers. Alloys Compd. 2017, 695, 2865–2869. [Google Scholar] [CrossRef]
- Nevarez-Rascon, A.; Gonzalez-Lopez, S.; ACOSTA-TORRES, L.S.; NEVAREZ-RASCON, M.M.; Orrantia-Borunda, E. Synthesis, biocompatibility and mechanical properties of ZrO2-Al2O3 ceramics composites. Dent. Mater. J. 2016, 35, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Ares, A.; Lasagabaster, A.; Abad, M.; Noguerol, R.; Cerecedo, C.; Valcárcel, V.; Caamano, J.; Guitián, F. Effects of silane functionalization of alumina whiskers on high-density polyethylene composites. J. Compos. Mater. 2014, 48, 3141–3151. [Google Scholar] [CrossRef]
- Corrochano, J.; Cerecedo, C.; Valcárcel, V.; Lieblich, M.; Guitián, F. Whiskers of Al2O3 as reinforcement of a powder metallurgical 6061 aluminium matrix composite. Mater. Lett. 2008, 62, 103–105. [Google Scholar] [CrossRef]
- Abdullah, M.; Ahmad, J.; Mehmood, M. Influence of Al2O3 whisker concentration on flexural strength of Al2O3 (w)–ZrO2 (TZ-3Y) composite. Ceram. Int. 2012, 38, 6517–6523. [Google Scholar] [CrossRef]
- Webb, W.; Forgeng, W. Growth and defect structure of sapphire microcrystals. J. Appl. Phys. 1957, 28, 1449–1454. [Google Scholar] [CrossRef]
- Chu, Y.; Chen, P.; Tang, J.; Rao, P. Engineer in situ growth of α-Al2O3 whiskers by axial screw dislocations. Cryst. Growth Des. 2017, 17, 1999–2005. [Google Scholar] [CrossRef]
- Zhang, P.; Li, X.; Zhu, B. Influence of carbon source on the morphology of Al2O3 whiskers synthesized with a carbon assisted method. Rare Met. Mater. Eng. 2013, 42, 501–504. [Google Scholar]
- Heid, R.; Strauch, D.; Bohnen, K.P.; Bohnen, K.P. Ab initio lattice dynamics of sapphire. Phys. Rev. B 2000, 61, 8625. [Google Scholar] [CrossRef]
- Marmier, A.; Parker, S.C. Ab initio morphology and surface thermodynamics of α− Al2O 3. Phys. Rev. B 2004, 69, 115409. [Google Scholar] [CrossRef]
- Batyrev, I.G.; Alavi, A.; Finnis, M.W. Equilibrium and adhesion of Nb/sapphire: The effect of oxygen partial pressure. Phys. Rev. B 2000, 62, 4698. [Google Scholar] [CrossRef]
- Sivasubramanian, K.; Raju, S.; Mohandas, E. Estimating enthalpy and bulk modulus from thermal expansion data—A case study with α-Al2O3 and SiC. J. Eur. Ceram. Soc. 2001, 21, 1229–1235. [Google Scholar] [CrossRef]
- Liu, Q.J.; Liu, Z.T.; Chen, J.C.; Feng, L.P.; Tian, H.; Zeng, W. Structural and electronic properties of cubic SrHfO3 surface: First-principles calculations. Appl. Surf. Sci. 2012, 258, 3455–3461. [Google Scholar] [CrossRef]
- Chen, G.; Hou, Z.; Gong, X. Structural and electronic properties of cubic HfO2 surfaces. Comput. Mater. Sci. 2008, 44, 46–52. [Google Scholar] [CrossRef]
- Li, X.; Hui, Q.; Shao, D.Y.; Chen, J.J.; Li, C.M.; Cheng, N.P. Stability and electronic structure of MgAl2O4 (111) surfaces: A first-principles study. Comput. Mater. Sci. 2016, 112, 8–17. [Google Scholar] [CrossRef]
- Liu, P.L.; Siao, Y.J. Ab initio study on preferred growth of ZnO. Scr. Mater. 2011, 64, 483–485. [Google Scholar] [CrossRef]
- Segall, M.; Lindan, P.J.; Probert, M.A.; Pickard, C.; Hasnip, P.; Clark, S.; Payne, M. First-principles simulation: Ideas, illustrations and the CASTEP code. J. Phys. 2002, 14, 2717. [Google Scholar] [CrossRef]
- Søndergård, E.; Kerjan, O.; Barreteau, C.; Jupille, J. Structure and growth of titanium buffer layers on Al2O3 (0001). Surf. Sci. 2004, 559, 131–140. [Google Scholar] [CrossRef]
- Kenny, S. Philos. Ab initio modelling of alumina. Mag. Lett. 1998, 78, 469–476. [Google Scholar] [CrossRef]
- Gundiah, G.; Deepak, F.; Govindaraj, A.; Rao, C. Carbothermal synthesis of the nanostructures of Al2O3 and ZnO. Top. Catal. 2003, 24, 137–146. [Google Scholar] [CrossRef]
- Gundiah, G.; Govindaraj, A.; Rao, C. Nanowires, nanobelts and related nanostructures of Ga2O3. Chem. Phys. Lett. 2002, 351, 189–194. [Google Scholar] [CrossRef]
 Al atoms,
Al atoms,  O atoms.
O atoms.
 Al atoms,
Al atoms,  O atoms.
O atoms.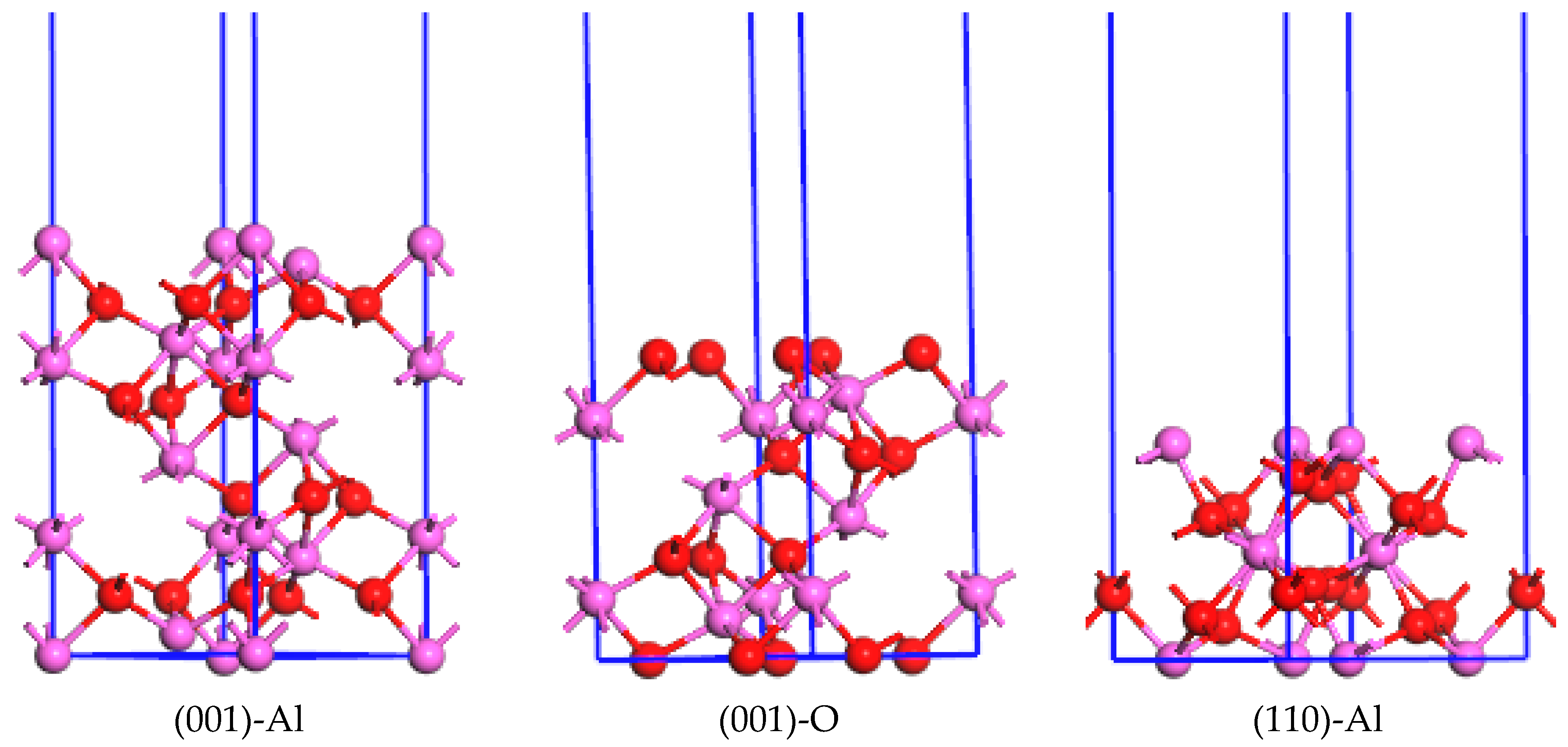

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, W.; Li, X.-C.; Zhu, B.-Q. Modeling Calculation and Synthesis of Alumina Whiskers Based on the Vapor Deposition Process. Materials 2017, 10, 1192. https://doi.org/10.3390/ma10101192
Gong W, Li X-C, Zhu B-Q. Modeling Calculation and Synthesis of Alumina Whiskers Based on the Vapor Deposition Process. Materials. 2017; 10(10):1192. https://doi.org/10.3390/ma10101192
Chicago/Turabian StyleGong, Wei, Xiang-Cheng Li, and Bo-Quan Zhu. 2017. "Modeling Calculation and Synthesis of Alumina Whiskers Based on the Vapor Deposition Process" Materials 10, no. 10: 1192. https://doi.org/10.3390/ma10101192
APA StyleGong, W., Li, X.-C., & Zhu, B.-Q. (2017). Modeling Calculation and Synthesis of Alumina Whiskers Based on the Vapor Deposition Process. Materials, 10(10), 1192. https://doi.org/10.3390/ma10101192





