Microstructural Modification and Characterization of Sericite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation
2.3. Characterization
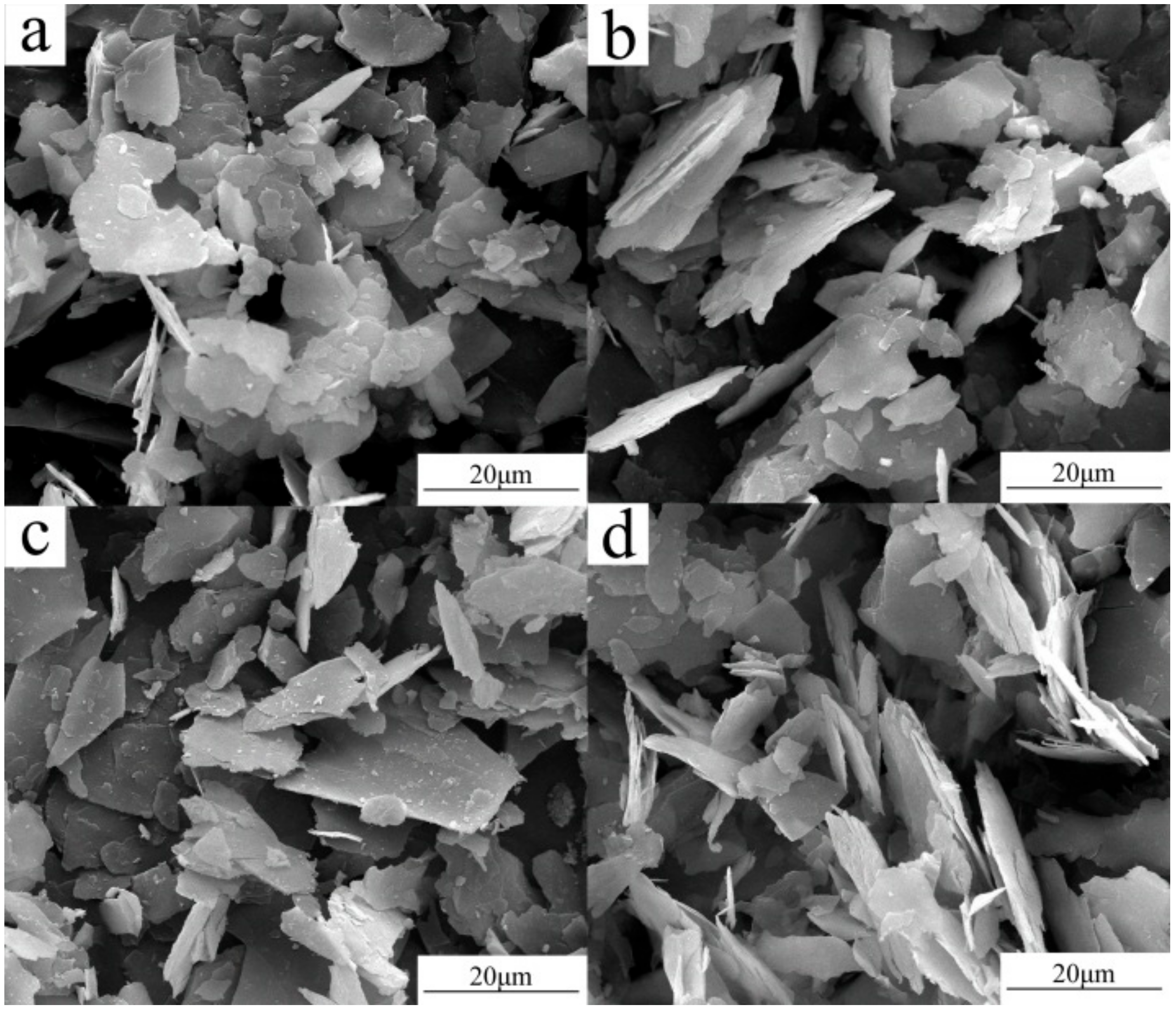
3. Results and Discussion
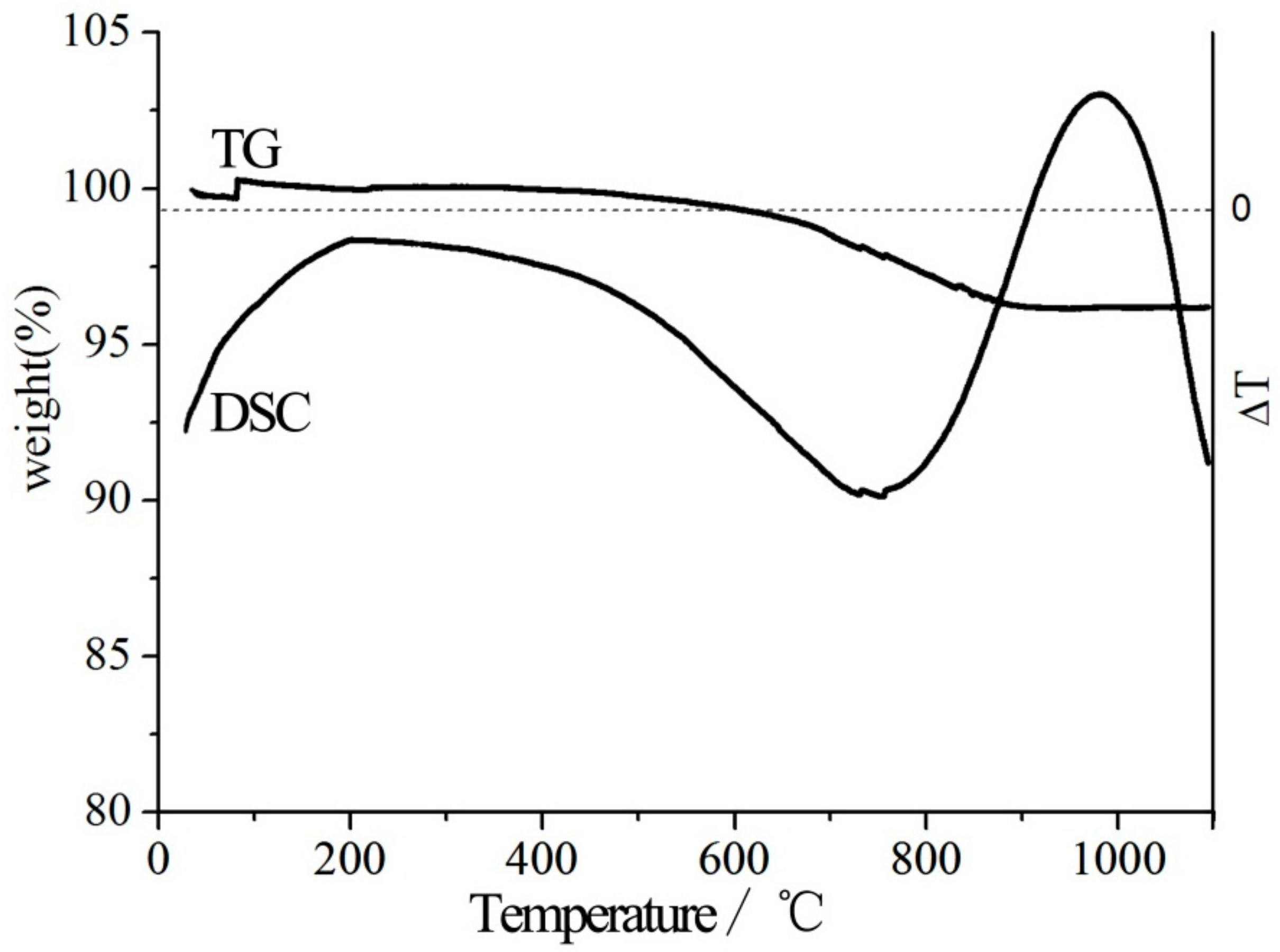
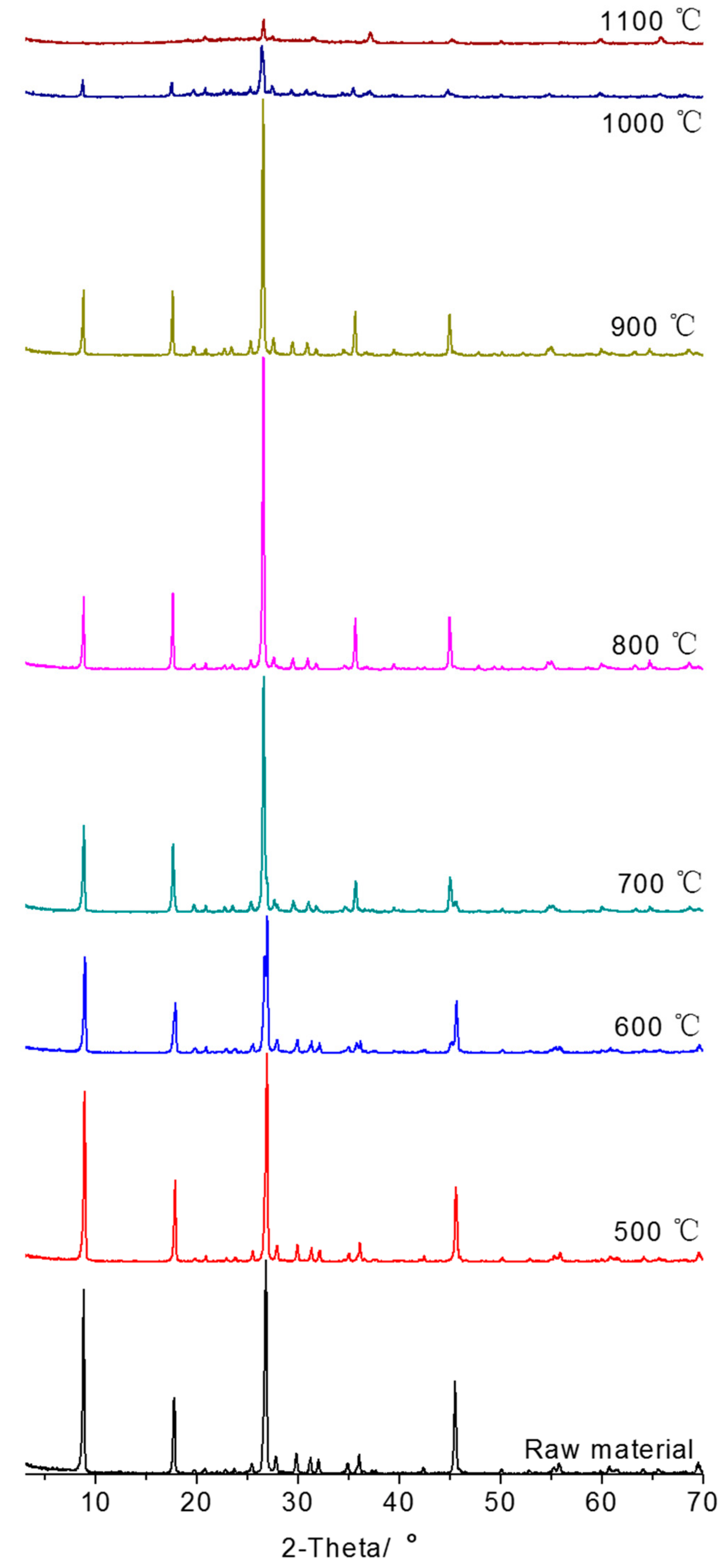
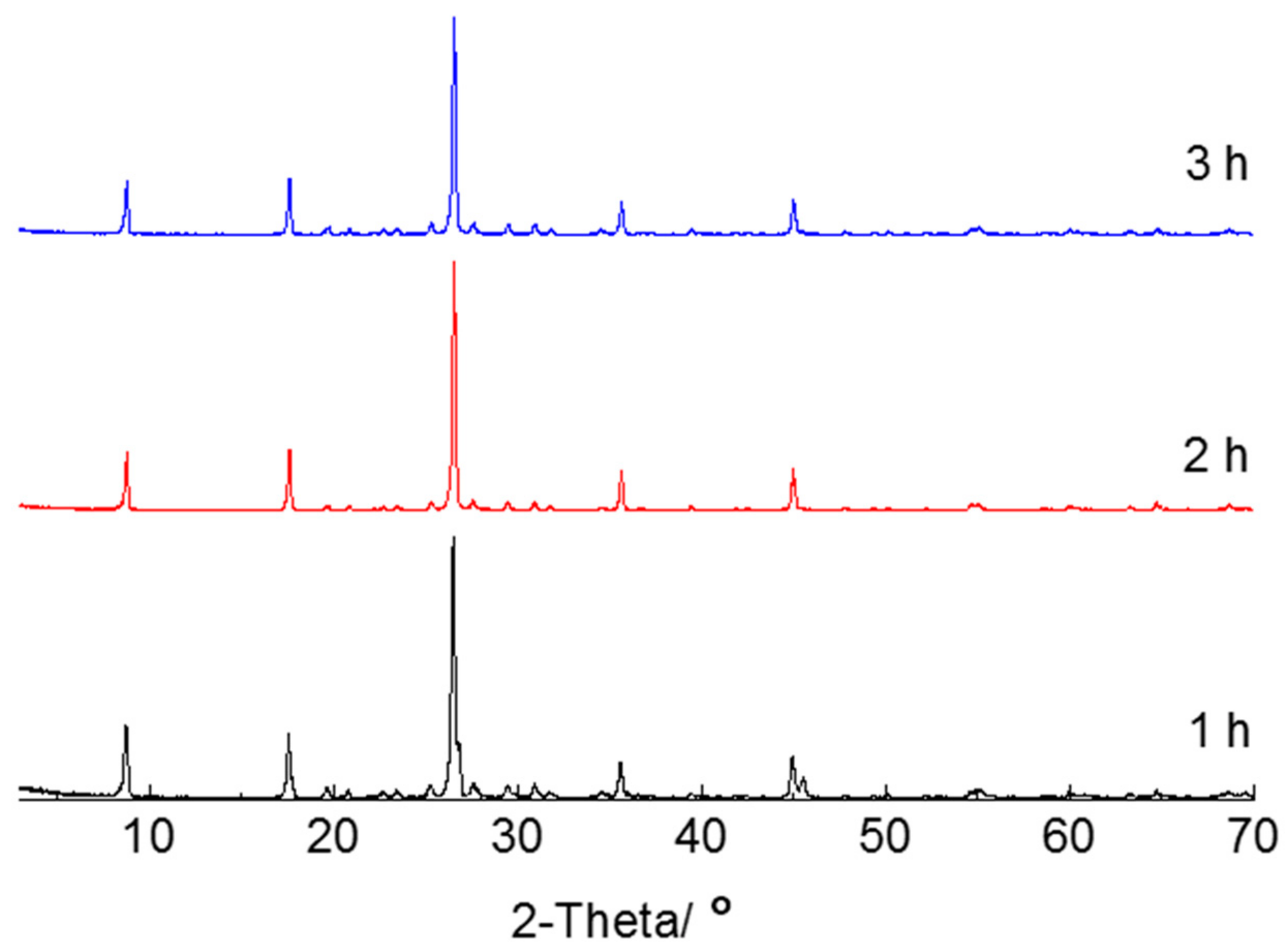
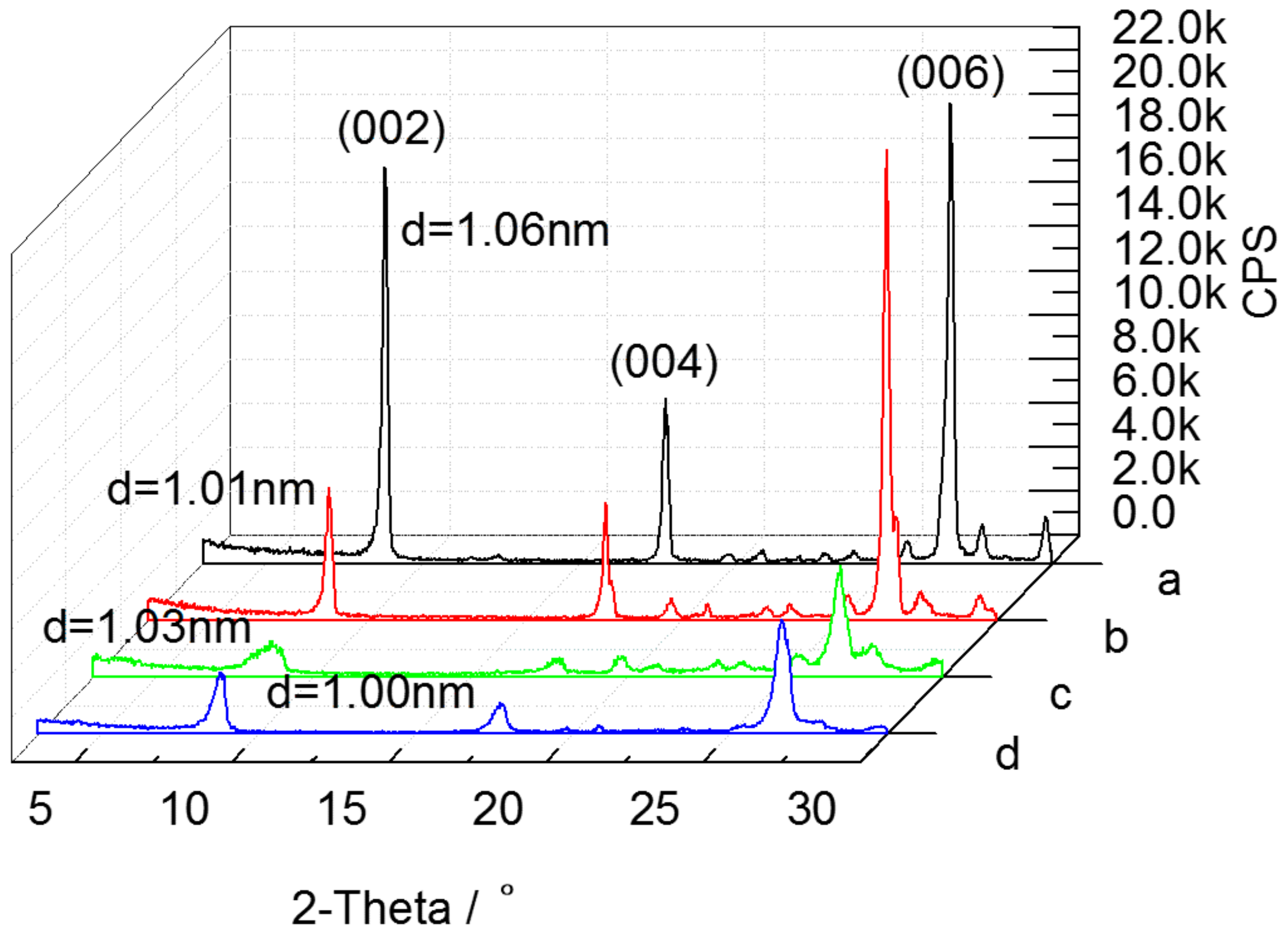
3.1. Thermal Modification
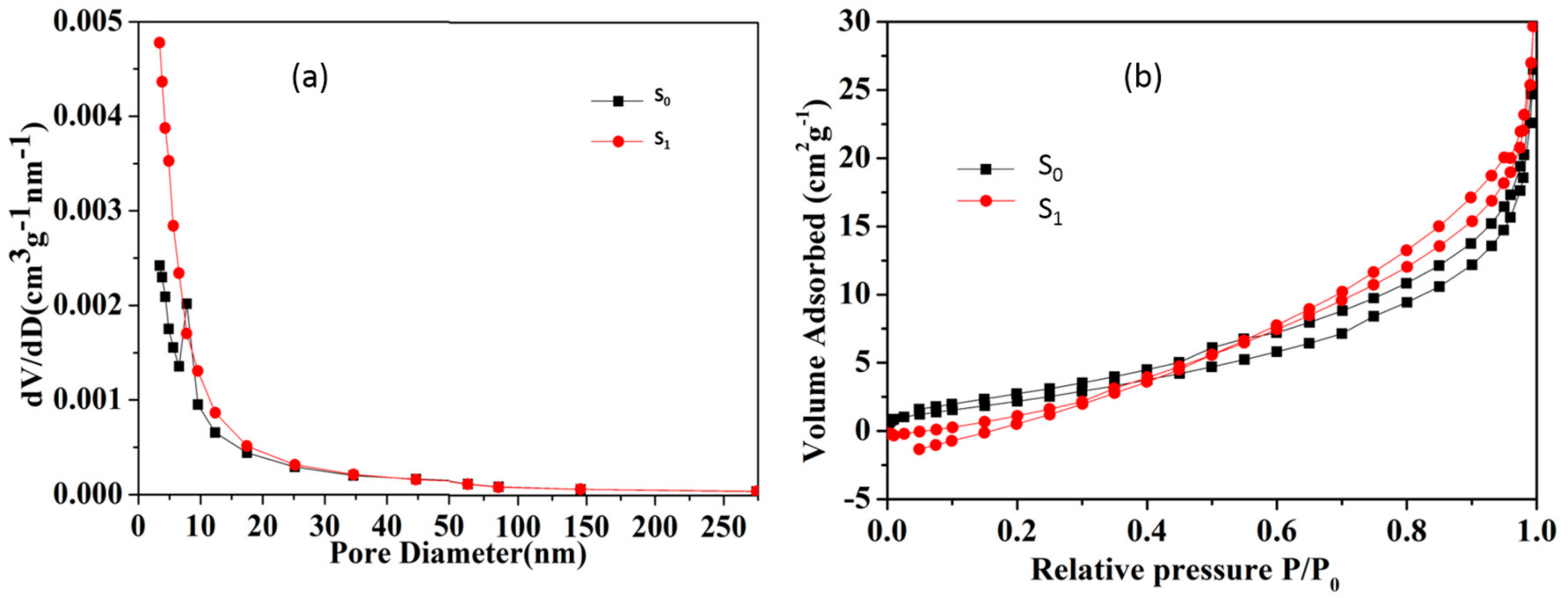
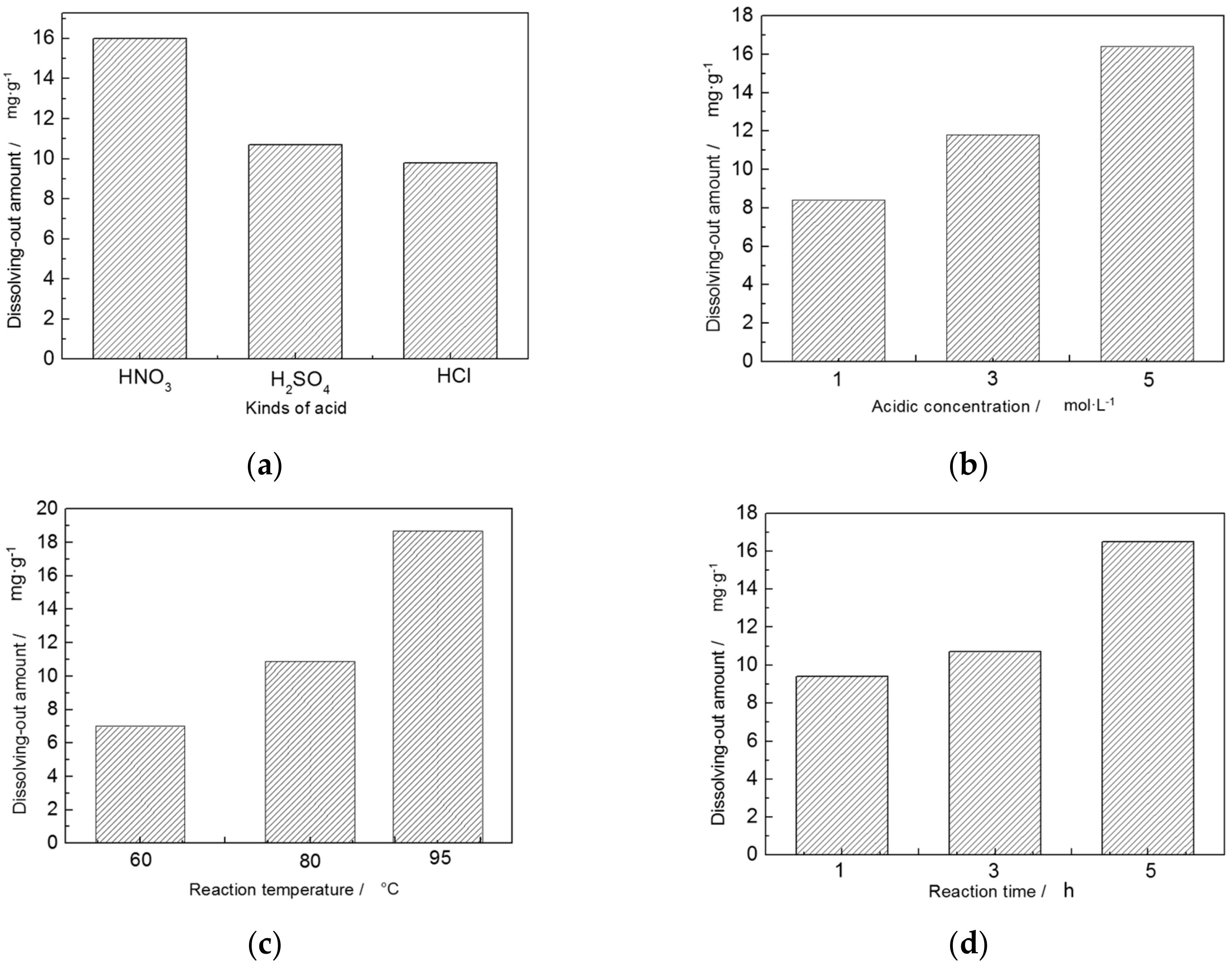
3.2. Acid Activation
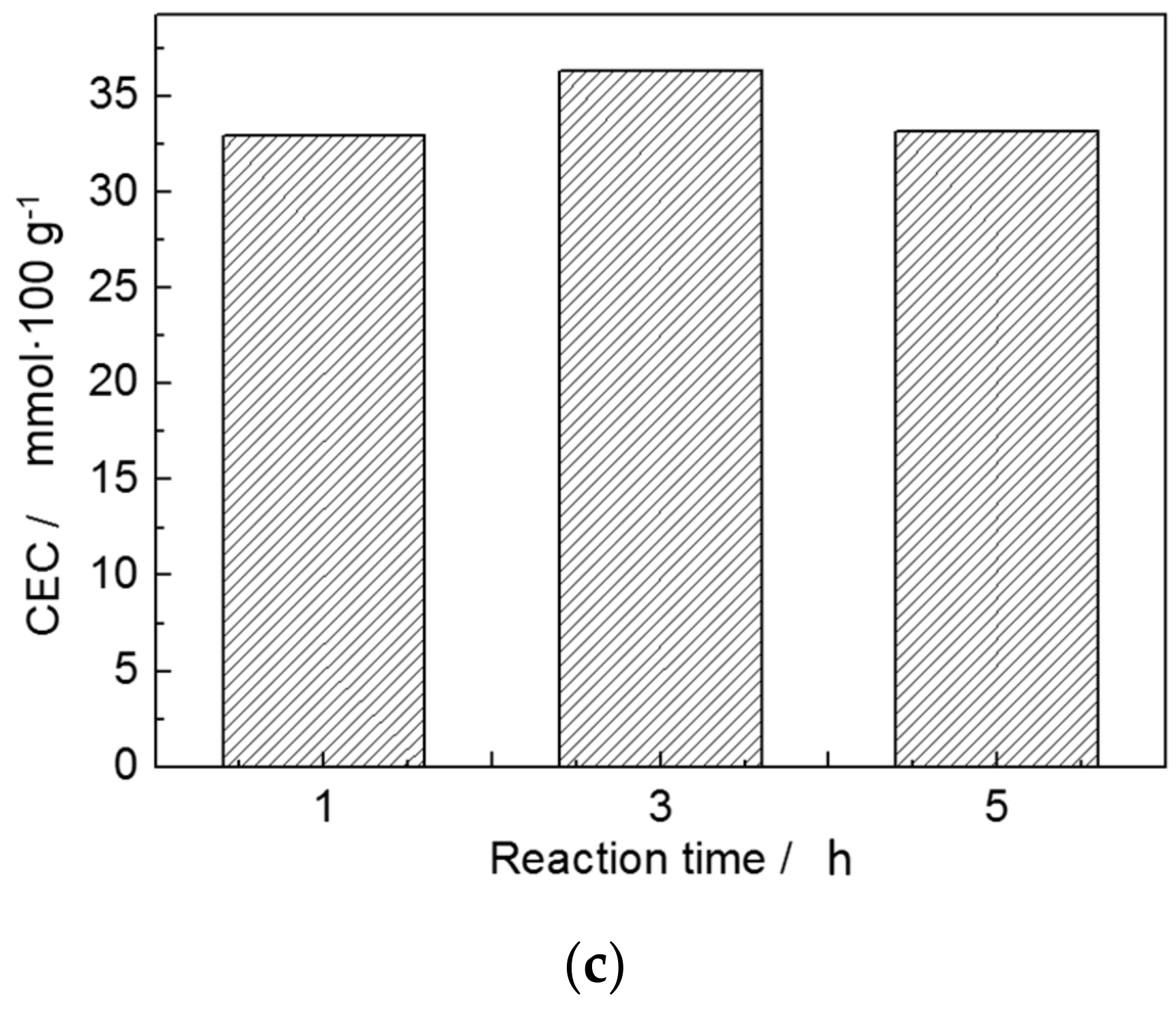
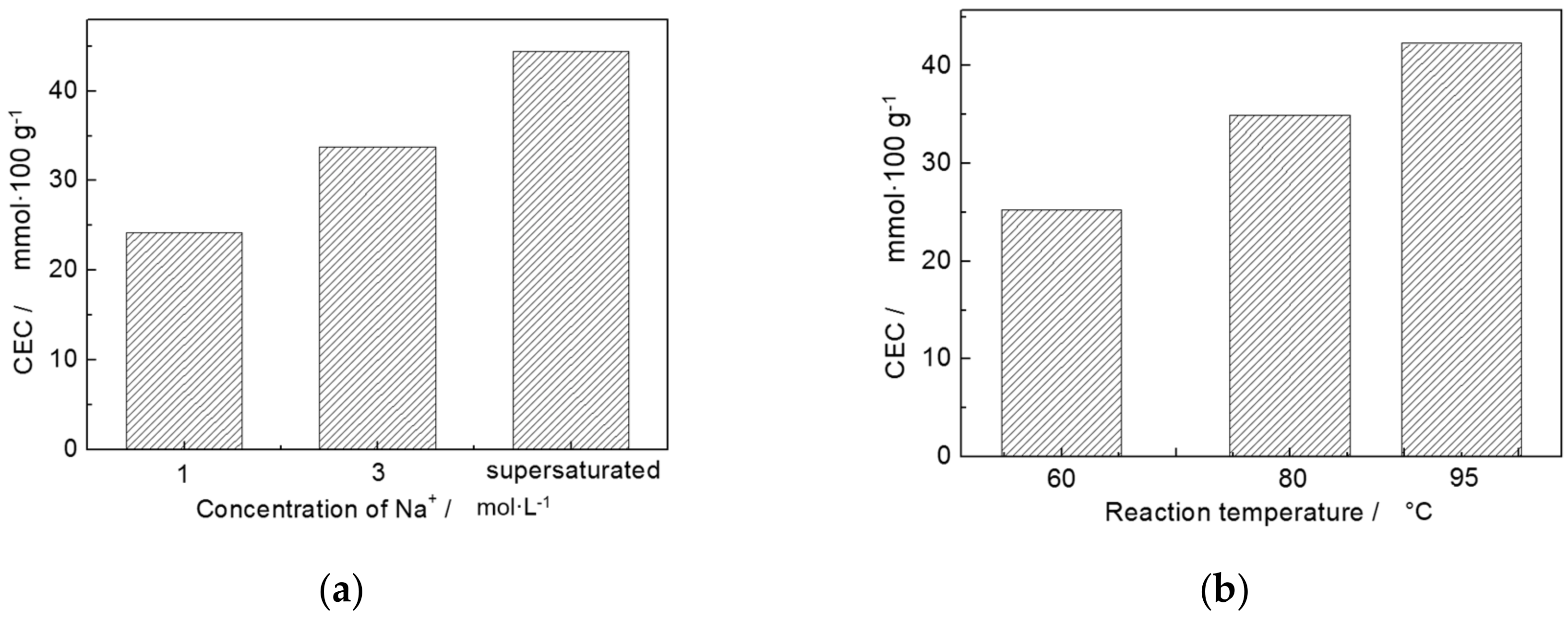
3.3. Sodium Modification
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Kojima, Y.; Usuki, A.; Kawasumi, M.; Okada, A.; Fukushima, Y.; Kurauchi, T.; Kamigaito, O. Mechanical properties of nylon 6-nlay hybrid. J. Mater. Res. 1993, 8, 1185–1189. [Google Scholar]
- Lebaron, P.C.; Wang, Z.; Pinnavaia, T.J. Polymer-layered silicate nanocomposites: An overview. Appl. Clay Sci. 1999, 15, 11–29. [Google Scholar]
- Vaia, R.A.; Price, G.; Ruth, P.N.; Nguyen, H.T.; Lichtenhan, J. Polymer/layered silicate nanocomposites as high performance ablative materials. Appl. Clay Sci. 1999, 15, 67–92. [Google Scholar]
- Giannelis, E.P. Polymer-layered silicate nanocomposites: Synthesis, properties and applications. Appl. Organomet. Chem. 1998, 12, 675–680. [Google Scholar]
- Xu, R.; Manias, E.; Snyder, A.J.; Runt, J. New biomedical poly(urethane urea)—Layered silicate nanocomposites. Macromolecules 2001, 34, 337–339. [Google Scholar]
- Messersmith, P.; Giannelis, E. Synthesis and barrier properties of poly(ε-caprolactone)—layered silicate nanocomposites. J. Polym. Sci. A Polym. Chem. 1995, 33, 1047–1057. [Google Scholar]
- Akin, O.; Tihminlioglu, F. Effects of organo-modified clay addition and temperature on the water vapor barrier properties of polyhydroxy butyrate homo and copolymer nanocomposite films for packaging applications. J. Polym. Environ. 2017, 25, 1–12. [Google Scholar] [CrossRef]
- Nguyen, Q.; Ngo, T.; Tran, P.; Mendis, P.; Bhattacharyya, D. Influences of clay and manufacturing on fire resistance of organoclay/thermoset nanocomposites. Compos. Part A 2015, 74, 26–37. [Google Scholar]
- Gilman, J.; Jackson, C.; Morgan, A.; Harris, R.; Manias, E.; Giannelis, E.; Wuthenow, M.; Hilton, D.; Phillips, S. Flammability properties of polymer-layered-silicate nanocomposites. Polypropylene and polystyrene nanocomposites. Chem. Mater. 2000, 12, 1866–1873. [Google Scholar]
- Aranda, P.; Mosqueda, Y.; Perez-Cappe, E.; Ruiz-Hitzky, E. Electrical characterization of poly(ethylene oxide)—clay nanocomposites prepared by microwave irradiation. J. Polym. Sci. B Polym. Phys. 2003, 41, 3249–3263. [Google Scholar]
- Ray, S.S.; Yamada, K.; Okamoto, M.; Ueda, K. Polylactide-layered silicate nanocomposite: A novel biodegradable material. Nano Lett. 2002, 2, 1093–1096. [Google Scholar]
- Kaur, M.; Datta, M. Synthesis and characterization of biodegradable clay-polymer nanocomposites for oral sustained release of anti-inflammatory drug. Eur. Chem. Bull. 2013, 2, 670–678. [Google Scholar]
- Lilichenko, N.; Maksimov, R.D.; Zicans, J.; Meri, R.M.; Plume, E. A biodegradable polymer nanocomposite: Mechanical and barrier properties. Mech. Compos. Mater. 2008, 44, 45–56. [Google Scholar]
- Pavlidou, S.; Papaspyrides, C.D. A review on polymer-layered silicate nanocomposites. Prog. Polym. Sci. 2008, 22, 1119–1198. [Google Scholar]
- Sahoo, S.; Manjaiah, K.M.; Datta, S.C.; Shabeer, T.P.A.; Kumar, J. Kinetics of metribuzin release from bentonite-polymer composites in water. J. Environ. Sci. Health Part B Pestic. Food Contam. Agric. Wastes 2014, 49, 591–600. [Google Scholar]
- El-Hamshary, H.; Selim, A.I.; Salahuddin, N.A.; Mandour, H.S. Clay-polymer nanocomposite-supported brominating agent. Clays Clay Miner. 2015, 63, 328–336. [Google Scholar]
- Choi, H.; Kim, S.; Hyun, Y.; Jhon, M. Preparation and rheological characteristics of solvent-cast poly(ethylene oxide)/montmorillonite nanocomposites. Macromol. Rapid Commun. 2001, 22, 320–325. [Google Scholar]
- Hackett, E.; Manias, E.; Giannelis, E.P. Molecular dynamics simulations of organically modified layered silicates. J. Chem. Phys. 1998, 108, 7410–7415. [Google Scholar]
- Strawhecker, K.E.; Manias, E. Structure and properties of poly(vinyl alcohol)/Na+ montmorillonite nanocomposites. Chem. Mater. 2000, 12, 2943–2949. [Google Scholar]
- Yano, K.; Usuki, A.; Okada, A.; Kuranchi, T.; Kamigaito, O. Synthesis and properties of polyimide clay hybrid. J. Polym. Sci. A Polym. Chem. 1993, 31, 2493–2498. [Google Scholar]
- Ginzburg, V.; Balazs, A. Calculating phase diagrams for nanocomposites: The effect of adding end-functionalized chains to polymer/clay mixtures. Adv. Mater. 2000, 12, 1805–1809. [Google Scholar]
- Manias, E.; Chen, H.; Krishnamoorti, R.; Genzer, J.; Kramer, E.J.; Giannelis, E.P. Intercalation kinetics of long polymers in 2 nm confinements. Macromolecules 2000, 33, 7955–7966. [Google Scholar]
- Wang, L.; Chen, Z.; Wang, X.; Yan, S.; Wang, J.; Fan, Y. Preparations of organo-vermiculite with large interlayer space by hot solution and ball milling methods: A comparative study. Appl. Clay Sci. 2011, 51, 151–157. [Google Scholar]
- Fonseca, M.G.D.; Wanderley, A.F.; Souea, K.; Arakaki, L.N.H.; Espinola, J.G.P. Interaction of aliphatic diamines with vermiculite in aqueous solution. Appl. Clay Sci. 2006, 32, 94–98. [Google Scholar]
- Rey-Perez-Caballero, F.D.; Poncelet, G. Preparation and characterization of microporous 18 angstrom Al-pillared structures from natural phlogopite micas. Microporous Mesoporous Mater. 2000, 41, 169–181. [Google Scholar]
- Williams-Daryn, S.; Thomas, R.K. The intercalation of a vermiculite by cationic surfactants and its subsequent swelling with organic solvents. J. Colloid Interface Sci. 2002, 255, 303–311. [Google Scholar]
- Tjong, S.C.; Meng, Y.Z. Preparation and characterization of melt-compounded polyethylene/vermiculite nanocomposites. J. Polym. Sci. B Polym. Phys. 2003, 41, 1476–1484. [Google Scholar]
- Tjong, S.C.; Meng, Y.Z.; Xu, Y. Structure and properties of polyamide-6/vermiculite nanocomposites prepared by direct melt compounding. J. Polym. Sci. B Polym. Phys. 2002, 40, 2860–2870. [Google Scholar]
- Xu, J.; Meng, Y.Z.; Li, R.K.Y.; Rajulu, A.V. Preparation and properties of poly(vinyl alcohol)-vermiculite nanocomposites. J. Polym. Sci. B Polym. Phys. 2003, 41, 749–755. [Google Scholar]
- Yu, X.; Ram, B.; Jiang, X. Parameter setting in a bio-inspired model for dynamic flexible job shop scheduling with sequence-dependent setups. Eur. J. Ind. Eng. 2007, 1, 182–199. [Google Scholar]
- Ding, H.; Xu, X.; Liang, N.; Wang, Y. Preparation sericite nanoflakes by exfoliation of wet ultrafine grinding. Adv. Mater. Res. 2011, 178, 242–247. [Google Scholar]
- Shih, Y.-J.; Shen, Y.-H. Swelling of sericite by LiNO(3)-hydrothermal treatment. Appl. Clay Sci. 2009, 43, 282–288. [Google Scholar]
- Kim, J.-O.; Lee, S.-M.; Jeon, C. Adsorption characteristics of sericite for cesium ions from an aqueous solution. Chem. Eng. Res. Des. 2014, 92, 368–374. [Google Scholar]
- Vaia, R.A.; Teukolsky, R.K.; Giannelis, E.P. Interlayer structure and molecular environment of alkylammonium layered silicates. Chem. Mater. 1994, 6, 1017–1022. [Google Scholar]
- Valášková, M.; Barabaszová, K.; Hundáková, M.; Ritz, M.; Plevová, E. Effects of brief milling and acid treatment on two ordered and disordered kaolinite structures. Appl. Clay Sci. 2011, 54, 70–76. [Google Scholar]
- Caseri, W.R.; Shelden, R.A.; Suter, U.W. Preparation of muscovite with ultrahigh specific surface-area by chemical cleavage. Colloid Polym.Sci. 1992, 270, 392–398. [Google Scholar]
- Obut, A.; Girgin, I. Hydrogen peroxide exfoliation of vermiculite and phlogopite. Miner. Eng. 2002, 15, 683–687. [Google Scholar]
- Rey-Perez-Caballero, F.D.; Poncelet, G. Microporous 18 angstrom Al-pilared vermiculites: Preparation and characterization. Microporous Mesoporous Mater. 2000, 37, 313–327. [Google Scholar]
- Teng, H.; Zhu, G.; Huang, P.; Liu, P. Example analysis of orthogonal experimental design. Pharm. Care Res. 2008, 1, 75–76. [Google Scholar]
- He, Z.; Liu, Y.; Chen, L.; Cao, M.; Xia, J. Orthogonal design-direct analysis for PCR optimization. Bull. Hunan Med. Univ. 1998, 4, 76–77. [Google Scholar]
- Gates, W.P.; Komadel, P.; Madejova, J.; Bujdak, J.; Stucki, J.W.; Kirkpatrick, R.J. Electronic and structural properties of reduced-charge montmorillonites. Appl. Clay Sci. 2000, 16, 257–271. [Google Scholar]

| Composition | SiO2 | Al2O3 | Fe2O3 | TiO2 | K2O | Na2O | CaO | MgO | SO3 | L.O.I | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Content (mass %) | 45.71 | 28.32 | 3.04 | 0.35 | 8.09 | 0.71 | 0.10 | 2.12 | 0.075 | 4.47 | 99.555 |
| Temperature (°C) | Raw Material | 500 | 600 | 700 | 800 | 900 | 1000 |
|---|---|---|---|---|---|---|---|
| kλ/D | 0.2146 | 0.2259 | 0.3059 | 0.3797 | 0.2937 | 0.2128 | 0.1938 |
| 4Δd/d | 0.0118 | −0.0381 | −0.1363 | −0.1631 | −0.1674 | −0.1372 | −0.0407 |
| Samples | FWHM (°) |
|---|---|
| S0 | 0.137 |
| S1 (500 °C) | 0.138 |
| S1 (600 °C) | 0.168 |
| S1 (700 °C) | 0.173 |
| S1 (800 °C) | 0.174 |
| S1 (900 °C) | 0.141 |
| S1 (1000 °C) | 0.132 |
| S1 (1100 °C) | - |
| Holding Time (h) | Raw Material | 1 | 2 | 3 |
|---|---|---|---|---|
| kλ/D | 0.2146 | 0.4287 | 0.2937 | 0.2277 |
| 4Δd/d | 0.0118 | −0.2848 | −0.1674 | −0.1026 |
| Trial No. | Factors | Results Dissolving-Out Amount of Al3+ (mg/g) | |||
|---|---|---|---|---|---|
| Kinds of Acid A | Acid Concentration B (mol/L) | Reaction Temperature C (°C) | Reaction Time D (h) | ||
| 1 | HNO3 | 1 | 60 | 1 | 4.2 |
| 2 | HNO3 | 3 | 80 | 3 | 12.8 |
| 3 | HNO3 | 5 | 95 | 5 | 31.0 |
| 4 | H2SO4 | 1 | 80 | 5 | 9.9 |
| 5 | H2SO4 | 3 | 95 | 1 | 14.0 |
| 6 | H2SO4 | 5 | 60 | 3 | 8.2 |
| 7 | HCl | 1 | 95 | 3 | 11.1 |
| 8 | HCl | 3 | 60 | 5 | 8.5 |
| 9 | HCl | 5 | 80 | 1 | 9.9 |
| K1,j | 48.0 | 25.2 | 20.9 | 28.1 | - |
| K2,j | 32.1 | 35.3 | 32.6 | 32.1 | - |
| K3,j | 29.5 | 49.1 | 56.1 | 49.4 | - |
| k1,j | 16.0 | 8.4 | 7.0 | 9.4 | - |
| k2,j | 10.7 | 11.8 | 10.9 | 10.7 | - |
| k3,j | 9.8 | 16.4 | 18.7 | 16.5 | - |
| Rj | 6.2 | 8.0 | 11.7 | 7.1 | - |
| Trial No. | Factors | Results CEC (mmol/100 g) | ||
|---|---|---|---|---|
| Concentration of Na+ A (mol/L) | Reaction Temperature B (°C) | Reaction Time C (h) | ||
| 1 | 1 | 60 | 1 | 15.76 |
| 2 | 1 | 80 | 3 | 27.24 |
| 3 | 1 | 95 | 5 | 29.70 |
| 4 | 3 | 60 | 3 | 25.36 |
| 5 | 3 | 80 | 5 | 35.24 |
| 6 | 3 | 95 | 1 | 40.76 |
| 7 | Supersaturated | 60 | 5 | 34.62 |
| 8 | Supersaturated | 80 | 1 | 42.34 |
| 9 | Supersaturated | 95 | 3 | 56.37 |
| K1,j | 72.70 | 75.24 | 98.86 | - |
| K2,j | 101.36 | 104.82 | 108.97 | - |
| K3,j | 133.33 | 126.83 | 99.56 | - |
| k1,j | 24.23 | 25.25 | 32.95 | - |
| k2,j | 33.79 | 34.94 | 36.32 | - |
| k3,j | 44.44 | 42.28 | 32.19 | - |
| Rj | 20.21 | 17.03 | 4.13 | - |
| Samples | FWHM (°) |
|---|---|
| S0 | 0.208 |
| S1 | 0.226 |
| S2 | 0.720 |
| S3 | 0.452 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Y.; Ding, H.; Sun, S.; Chen, Y. Microstructural Modification and Characterization of Sericite. Materials 2017, 10, 1182. https://doi.org/10.3390/ma10101182
Liang Y, Ding H, Sun S, Chen Y. Microstructural Modification and Characterization of Sericite. Materials. 2017; 10(10):1182. https://doi.org/10.3390/ma10101182
Chicago/Turabian StyleLiang, Yu, Hao Ding, Sijia Sun, and Ying Chen. 2017. "Microstructural Modification and Characterization of Sericite" Materials 10, no. 10: 1182. https://doi.org/10.3390/ma10101182
APA StyleLiang, Y., Ding, H., Sun, S., & Chen, Y. (2017). Microstructural Modification and Characterization of Sericite. Materials, 10(10), 1182. https://doi.org/10.3390/ma10101182




