1. Introduction
The world’s largest dental implant markets are in Europe and the United States. According to Global Markets Direct’s estimates, the U.S. dental implant market in 2001 was approximately US
$1.4 billion; in 2008, it had grown to
$3.9 billion, which constitutes a compound annual growth rate of approximately 8.4%. It is estimated that by 2025, the market demand may reach approximately
$17 billion [
1]. Commercially-pure titanium (CP Ti) has been used as a dental implant material for the past 40 years because it is anti-corrosive, exhibits suitable mechanical properties, and has biocompatibility [
2]. Fast osseointegration after implantation and strong new bone formation on the implant surface during the healing process constitute high biocompatibility. To achieve a high level of success in clinical surgery, the surface quality and topography of CP Ti are among the most critical factors influencing the results of implant surgery. A substantial majority of the developed and investigated implant surfaces, such as SLA (sandblasted with large grit and acid-etched), are smoother than the turning surfaces. Straumann AG proposed an SLA patent in 1992 [
3]. The implant surface is sandblasted using large grit and etched in a boiling mixture of hydrochloric acid or sulfuric acid. The main novel characteristic of this patent is the surface features of Ra = 1–2 μm, Rt = 20–30 μm, and Rs = 1–5 μm. The surface is not microporous and, therefore, provides no enclosed volumes, which reduces vulnerability to bacteria. In recent years, it has become a mainstream and commercially available implant product. Ti implant surfaces have been modified using multiple methods (additive or subtractive processes) such as (additive) hydroxylapatite coating, calcium phosphate coating, Ti plasma spraying, ion deposition, (subtractive) electropolishing, mechanical polishing, blasting, etching, and oxidation. These different techniques are employed to both smoothen and roughen the implant surfaces. Regarding bone integration, smoother surfaces, such as electrically- or mechanically-polished surfaces, may be excessively smooth for adequate osseointegration; however, they may still be used for research purposes if an investigation of certain surface properties is required. Spark erosion techniques can produce a relatively rough surface, but this method is unstable and does not guarantee bone integration, possibly because of the impurities that are incorporated into the surface [
4]. Wennerberg [
5] discussed the possible relationships between Ti surface topography and the bone integration found in numerous studies. Bone integration has been reported in the results of numerous experiments, and bone response has been reported as influenced by implant surface topography.
Gintaras et al. [
6] proposed using a different surface treatment for metals—HCl etching—which did not produce pores on the metal surface. Although HCl/H
2SO
4 etching generated sporadic holes, the holes were not obvious. When an H
2SO
4/HCl/H
3PO
4-etched surface displayed numerous holes, they were undulating and uneven. Gintaras et al. proved that a uniform surface can be fabricated after etching Ti in H
2SO
4 for 72 h or through HCl etching for 30 h. However, they did not specify the etchant concentration. Szmukler-Moncler et al. (2003) [
7] investigated the surface characteristics of four implant systems: osseotite (3i) (etching in 15% hydrogen fluoride (HF) and a mixture of H
2SO
4 and HCl (6:1) at 60–80 °C for 3–10 min), SLA (Straumann, Basel, Switzerland), DPS (Friatec, Mannheim, Germany; DPS implant is processed using sand-blasting and acid-etching), and HaTi (HaTi Dental, Solothurn, Switzerland). The roughness values of the four implant types were measured and ranked in the following order: SLA (Straumann) > DPS (Friatech) > HaTi (HaTi Dental) > osseotite (3i). Le Guehennec et al. [
8] reported that numerous holes could be etched by using a mixture of HCl and H
2SO
4 at a temperature exceeding 100 °C to the surface of a substrate; this enhanced the adhesion of osteoblasts and fibrin. Ferguson et al. [
9] proposed that the difference between SLA and SLA using hydrophilic treatment (SLActive) is the hydrophilicity implant. SLActive displayed superior performance relating to biocompatibility, cell activity, healing time, and mechanical properties compared with SLA. Ban et al. [
10] claimed that the surface roughness of CP Ti was strongly related to acid temperature, etching time (0.25–8.00 h), weight loss, and its activation energy in concentrated sulfuric acid (48%).
Surface modification technology, combined with Ti or Ti alloys, can retain the original corrosion resistance and tensile strength of Ti or the Ti alloys and, thus, improve the clinical benefits of implants. However, the existing research on the detailed parameters of the etching recipe, concentration, temperature, and time is limited, and the Ti metal etching methodologies that have been proposed are substantially different. Therefore, the purpose of this paper is to explore the process parameters of the surface treatment, such as the etchant used, acid concentration, reaction temperature, and reaction time, the optimal values of which can be used to obtain uniform nanopores suitable for bone cell growth. In addition, some uncertainties regarding these parameters were addressed, and unified biocompatibility mechanisms of CP Ti acid etching are proposed.
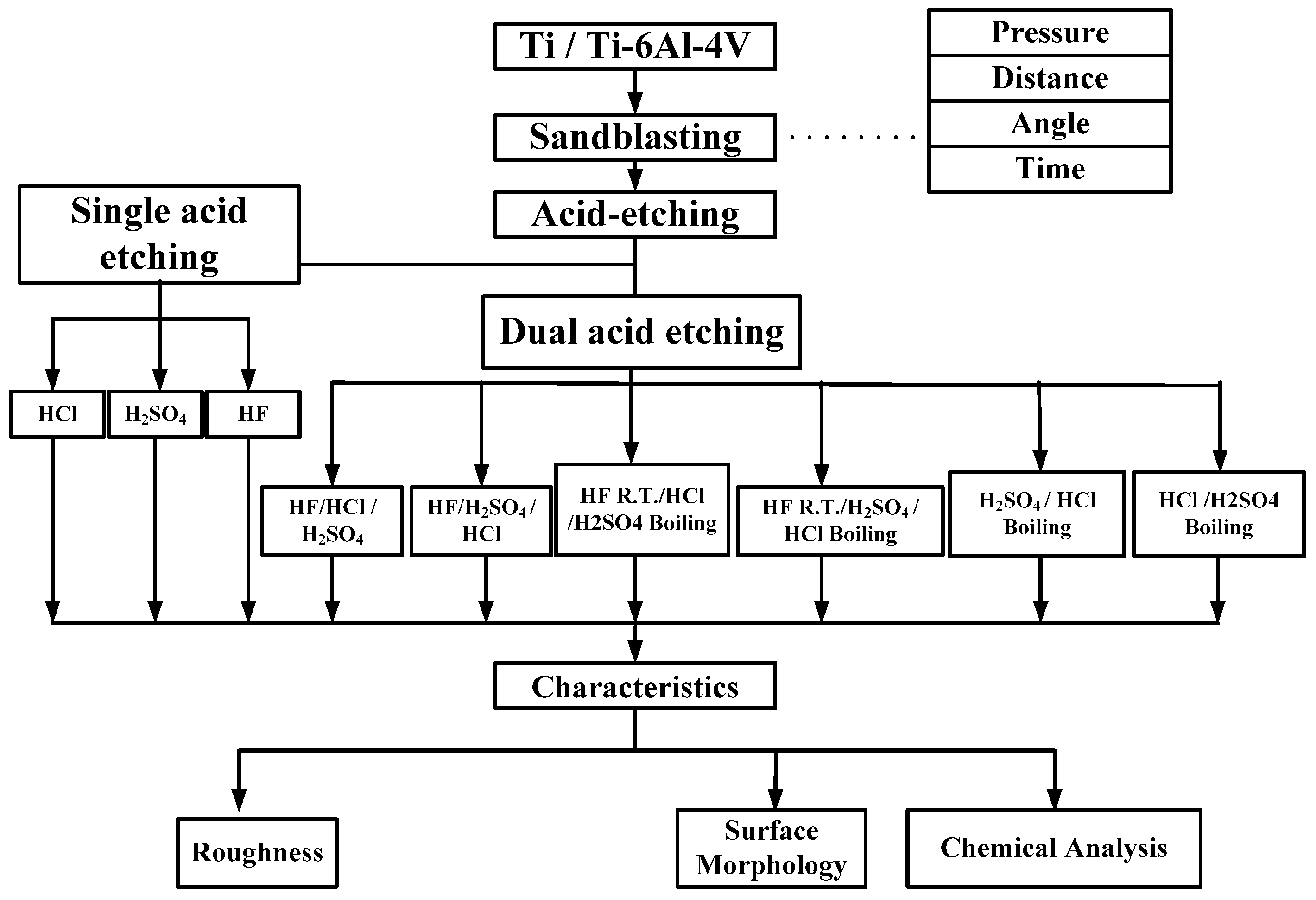
2. Materials and Methods
Circular CP Ti and Ti alloy disks (Grade 5 Ti-6Al-4V, ASTM-F136; 0.375″ in diameter and 2 mm thick) cut from a circular bar using wire electrical discharge machining were used to verify the surface characteristics and in etching experiments in this study. Sandblasting and magnetic grinding were performed to clean the disk surface after the wire electrical discharge machining. The experimental procedure is represented in
Figure 1. The CP Ti and Ti alloy specimens were sandblasted to roughen the surface and were then cleaned using ultrasonic oscillation. Various etching mechanisms were applied to form a uniform surface with micro- to nano-sized holes. Finally, the results were analyzed to investigate the influences of the process parameters on the roughness, surface morphology, and chemical composition of the materials. The specimens were treated and examined using the following equipment: a sandblasting machine (quad disc blasting machine CS-1000RT, Zhaoshun, Taipei, Taiwan), etching equipment, and an ultrasonic oscillator (Delta Ultrasonic, DC300H, New Taipei City, Taiwan, frequency: 40 kHz). The chemical composition of the specimens was investigated using X-ray diffractometry (X-ray diffractometer, XRD, X’Pert Pro, PHILIPS, Eindhoven, The Netherlands; Cu-K_P Å, 40 kV, 30 mA, scan speed 0.1 s/step). The surface morphologies of the disks were observed using a scanning electron microscope (field emission scanning electron microscope, FE-SEM, JSM-6701F, JEOL, Tokyo, Japan). The surface roughness of the dried specimens was analyzed using a white-light interferometer (Chroma 7502, New Taipei City, Taiwan, vertical resolution: 1 nm, Sa measurement area: 730 × 730 μm
2). Precision microbalances (RADWAG, MYA 21, Radom, Poland) were used to measure the weight lost by the materials, which was derived from measuring the weight difference before and after the etching was performed.
In this study, the blasting parameters, namely, pressure (A), blasting time (B), blasting distance (C), blasting angle (D), and grain size (E), were tested to assess the surface roughness and characteristics of the implant. Then, the predominant characteristics of Ti or Ti alloy after monoacid etching were determined by calculating the etching rate and observing the surface morphology. In this study, HF, H2SO4, and HCl were first tested at different temperatures. Whereas Ti has a strong affinity reaction with oxygen, nitrogen, and hydrogen at a high temperature, only the hydrogen absorption is reversible. The Ti neither reacts with cold mineral acids (HF is the exception) nor with a hot alkali solution. However, it can be dissolved in hot HF, HCl, H2SO4, and H3PO4. In general, the dissolution accelerates as the acid concentration increases.
2.1. Sandblasting
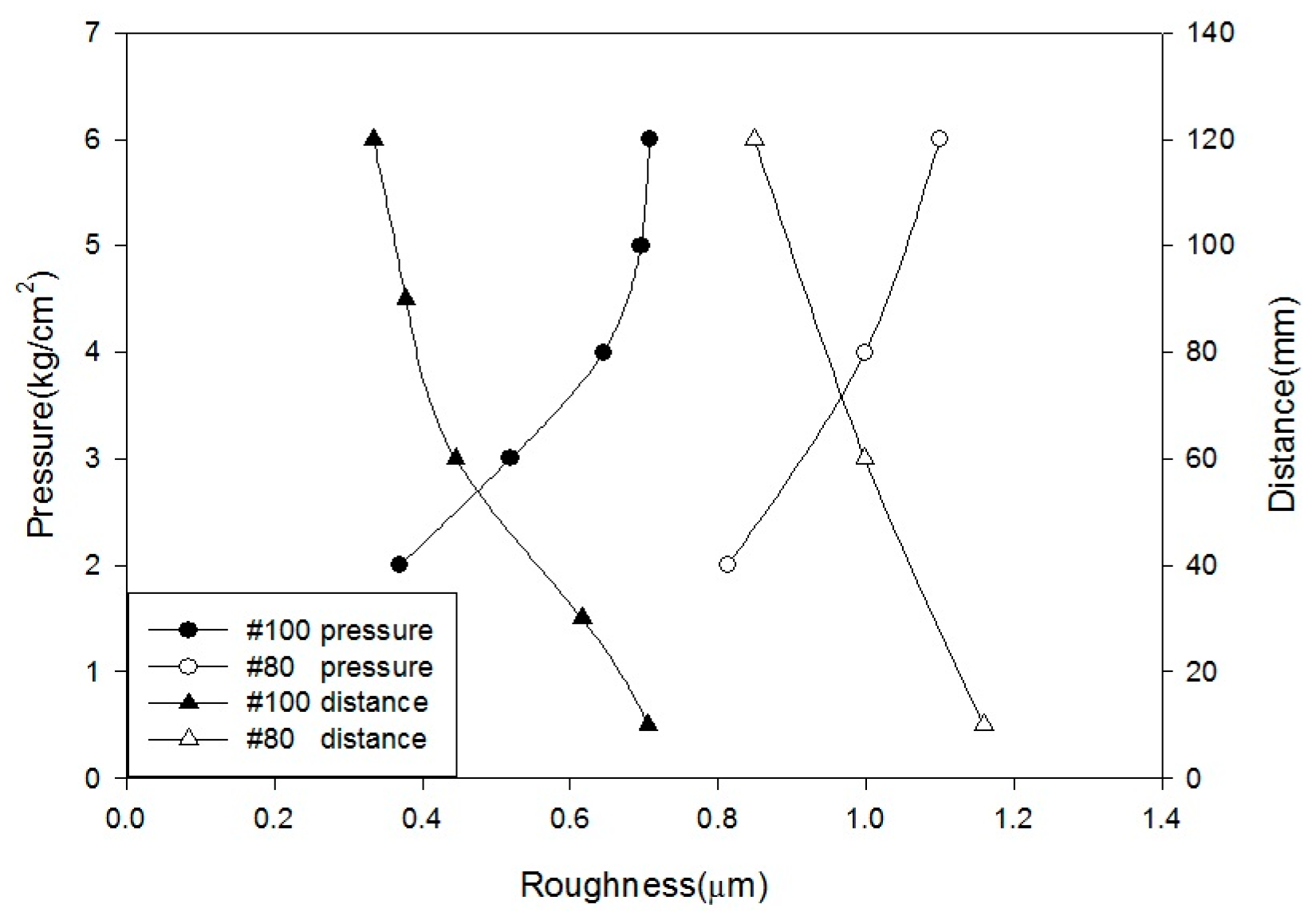
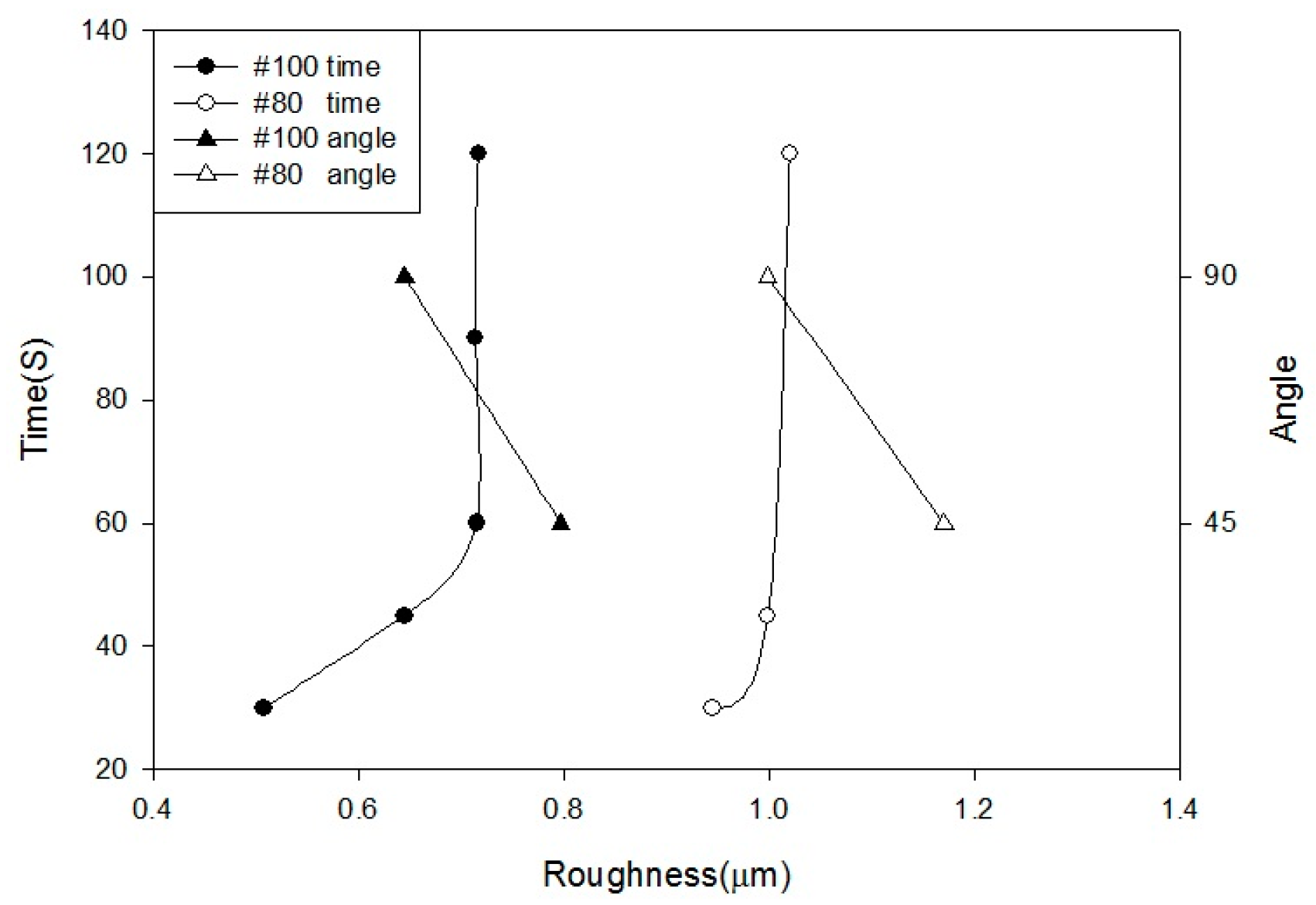
Alumina (Al2O3) was used as a blasting material and produced various levels of surface roughness according to different parameters: pressure at 2, 3, 4, 5, and 6 kg/cm2; distances of 10, 30, 60, 90, and 120 mm; periods of 30, 45, 60, 90, and 120 s; angles at 45° and 90°; and grain sizes of #80 (180–212 µm) and #100 (120–150 µm).
2.2. Acid Etching
The etching (acid etching) process is often used to remove the surface oxide layer and the contaminants of Ti to obtain a clean and uniform surface. The etchant of Ti is often dissolved in 10% to 30% nitric acid (HNO
3) and in 1% to 3% HF in water. Hydrogen fluoride reacts readily with Ti and forms Ti fluoride and hydrogen gas. To prevent hydrogen embrittlement [
11], a 10:1 mixture of nitric acid and hydrogen fluoride were normally used to reduce hydrogen generation.
The chemical reaction of Ti and HF is as follows:
H
2SO
4 oxidizes if its concentration exceeds 85% at any temperature. The reactions of Ti in concentrated H
2SO
4 are the following [
10]:
When Titanium reacts with HCl [
12], possible reaction are:
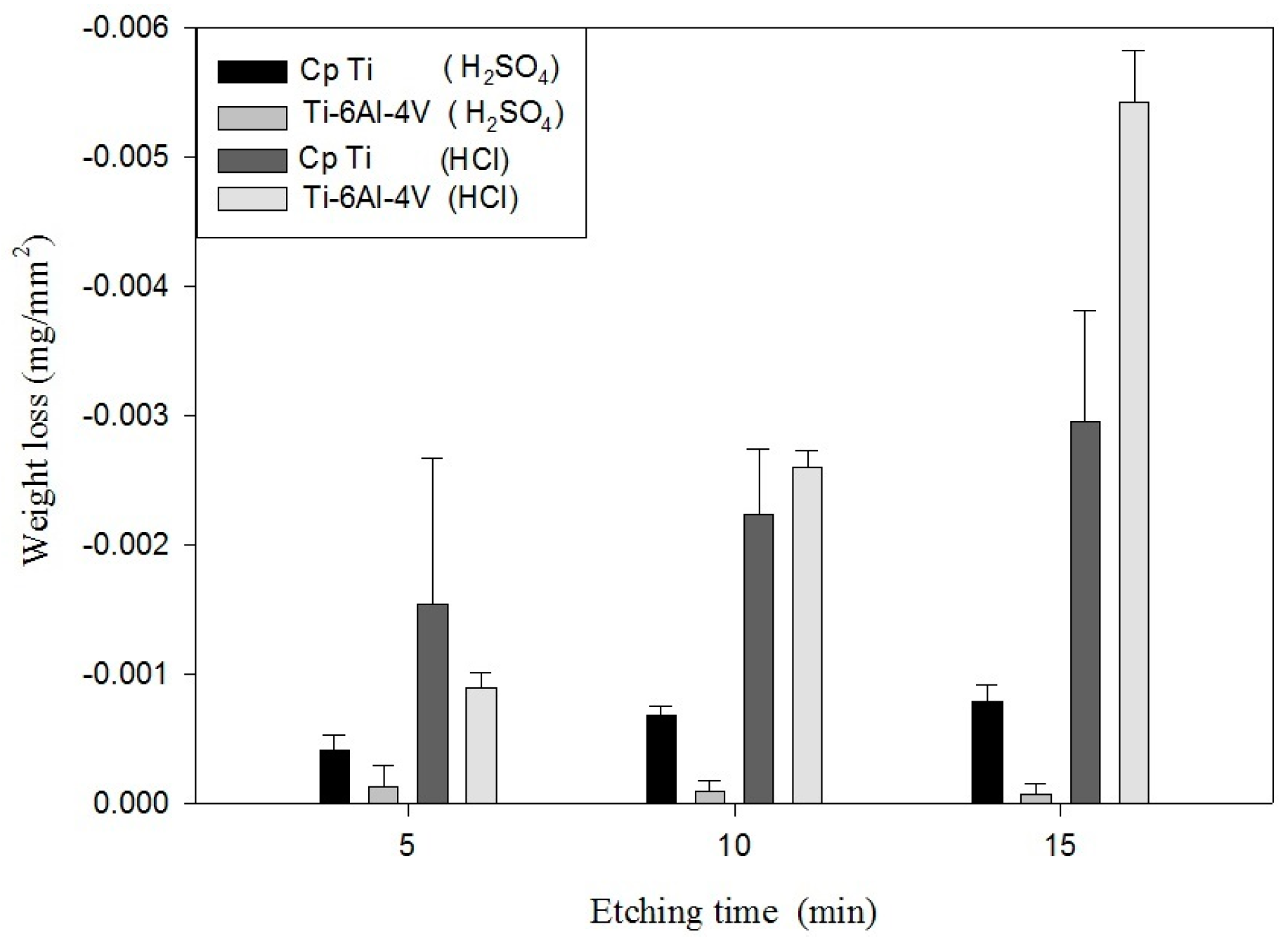
The initial period, in which no weight changes were observed, might represent the time required to dissolve the passive oxide film and expose the metallic Ti to the acid.
Table 1 shows the concentration of the acid used for the study.
Table 2 shows the etching parameters for a single type of acid at different temperature.
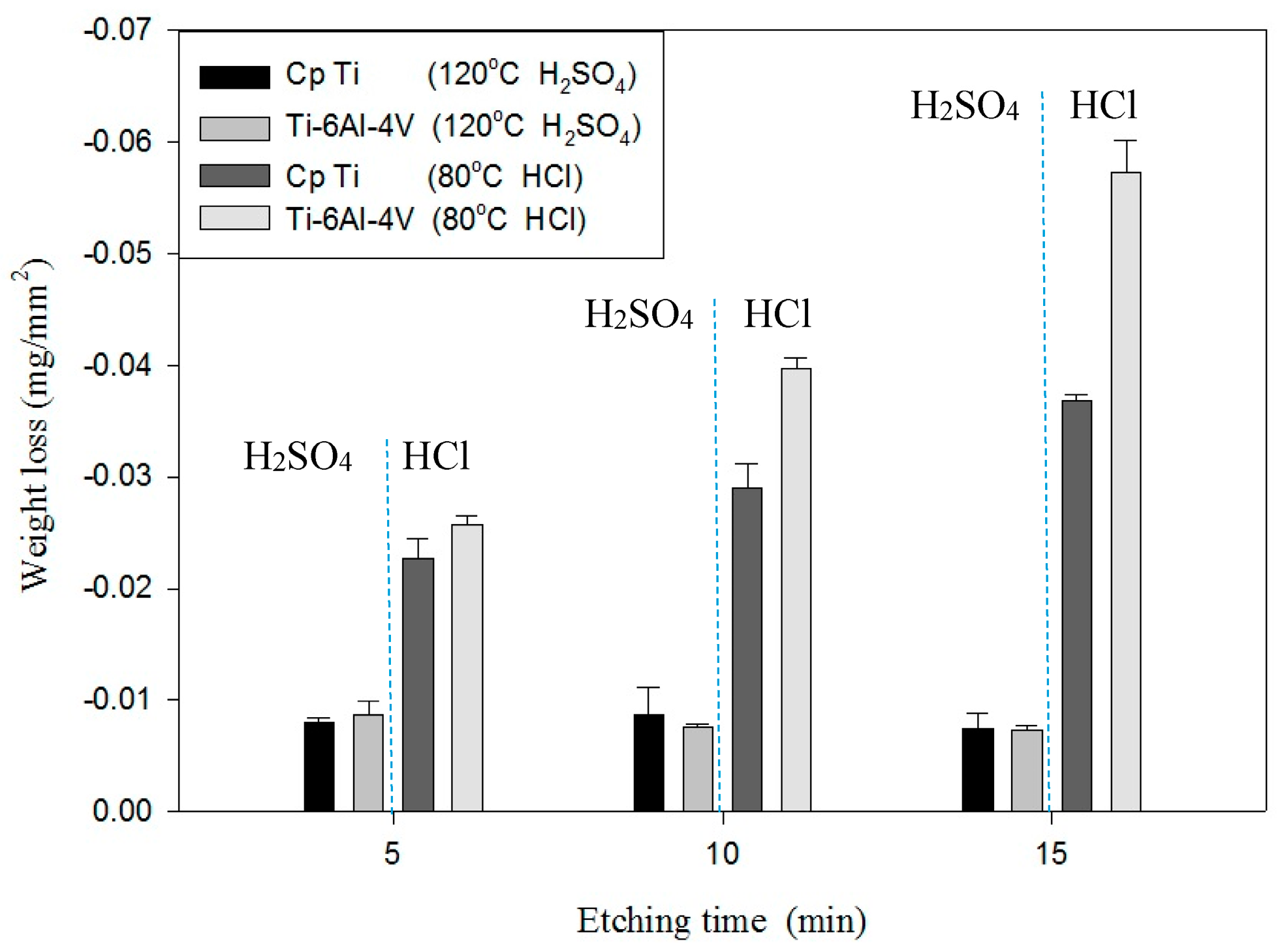
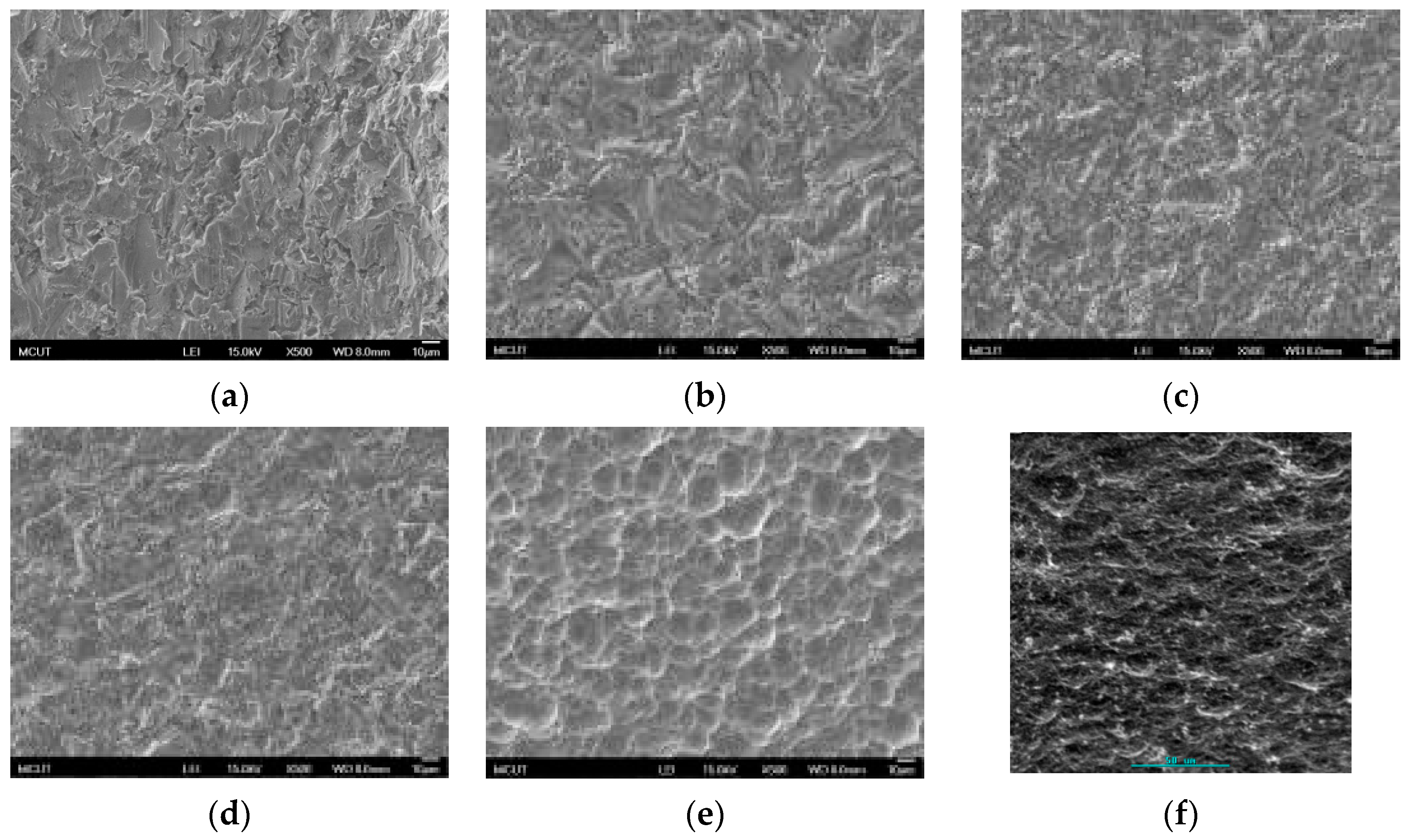
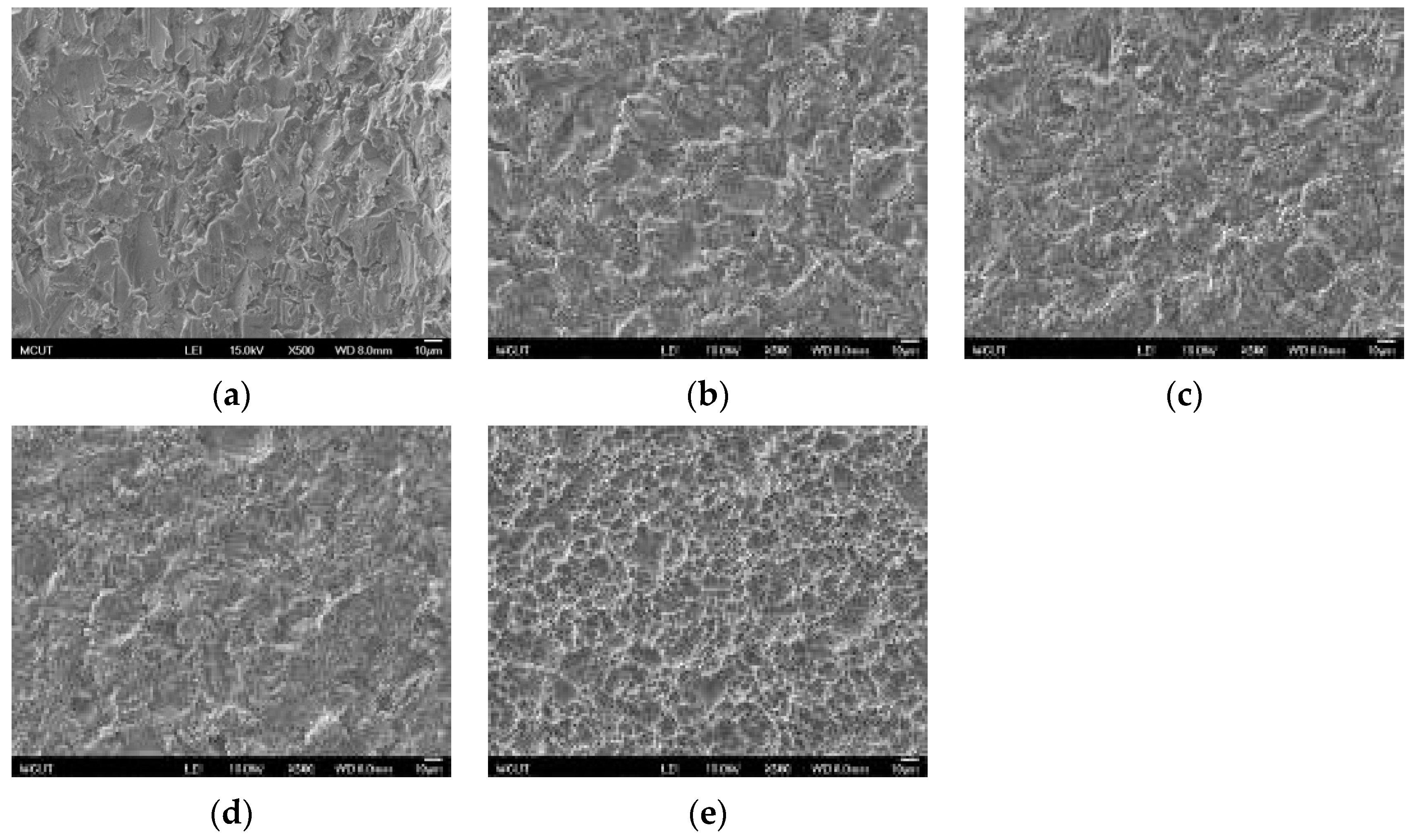
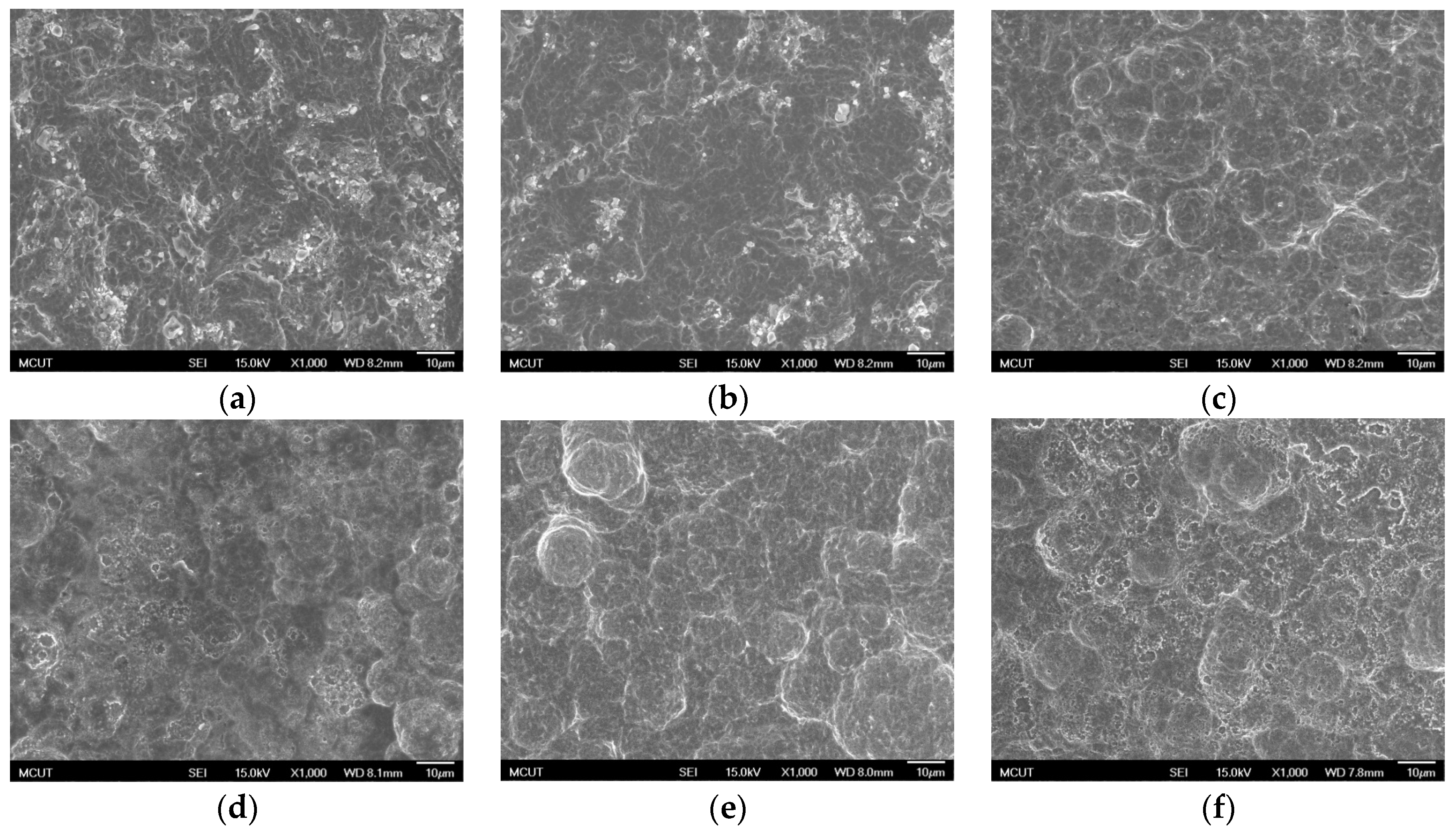
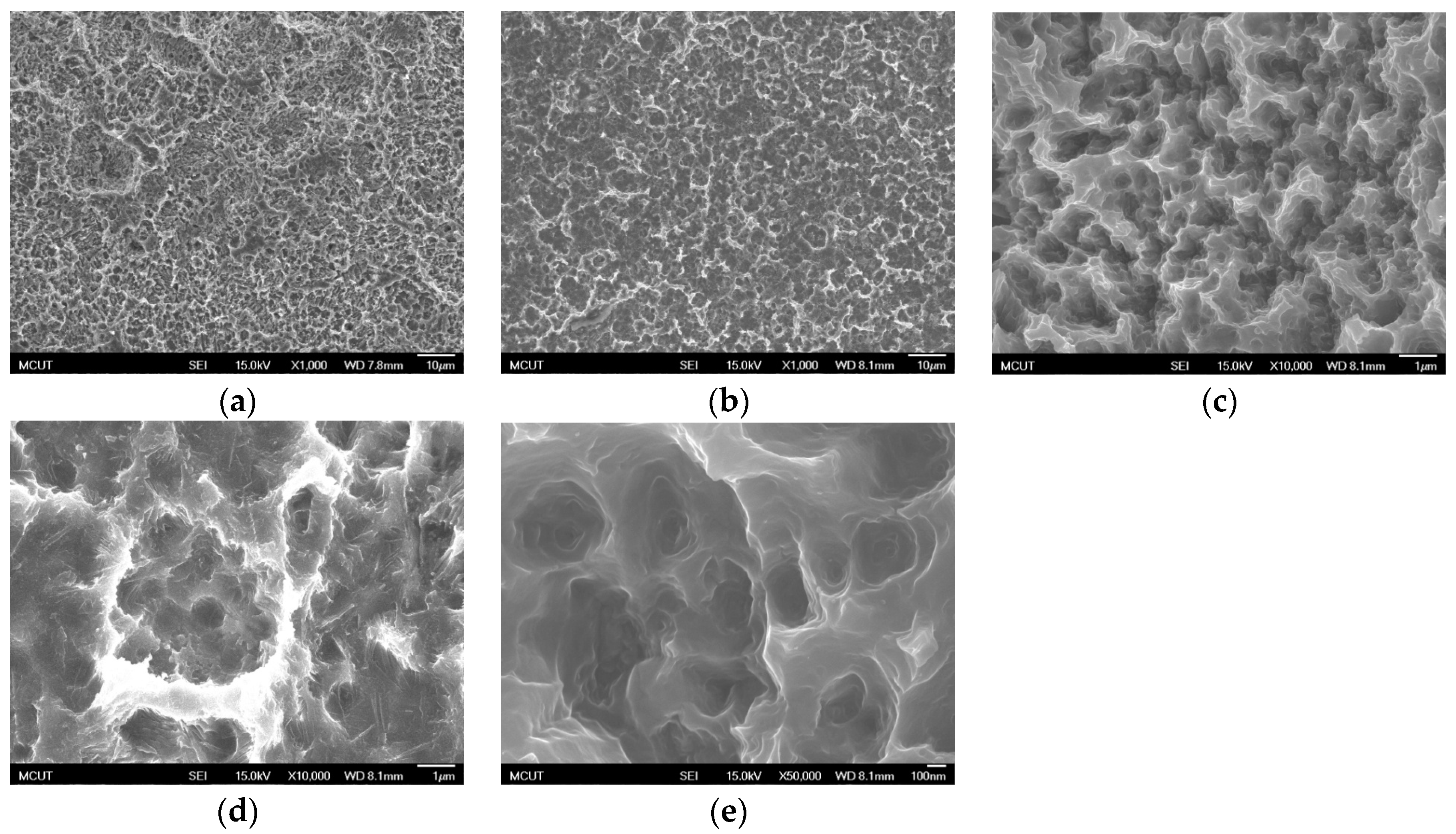
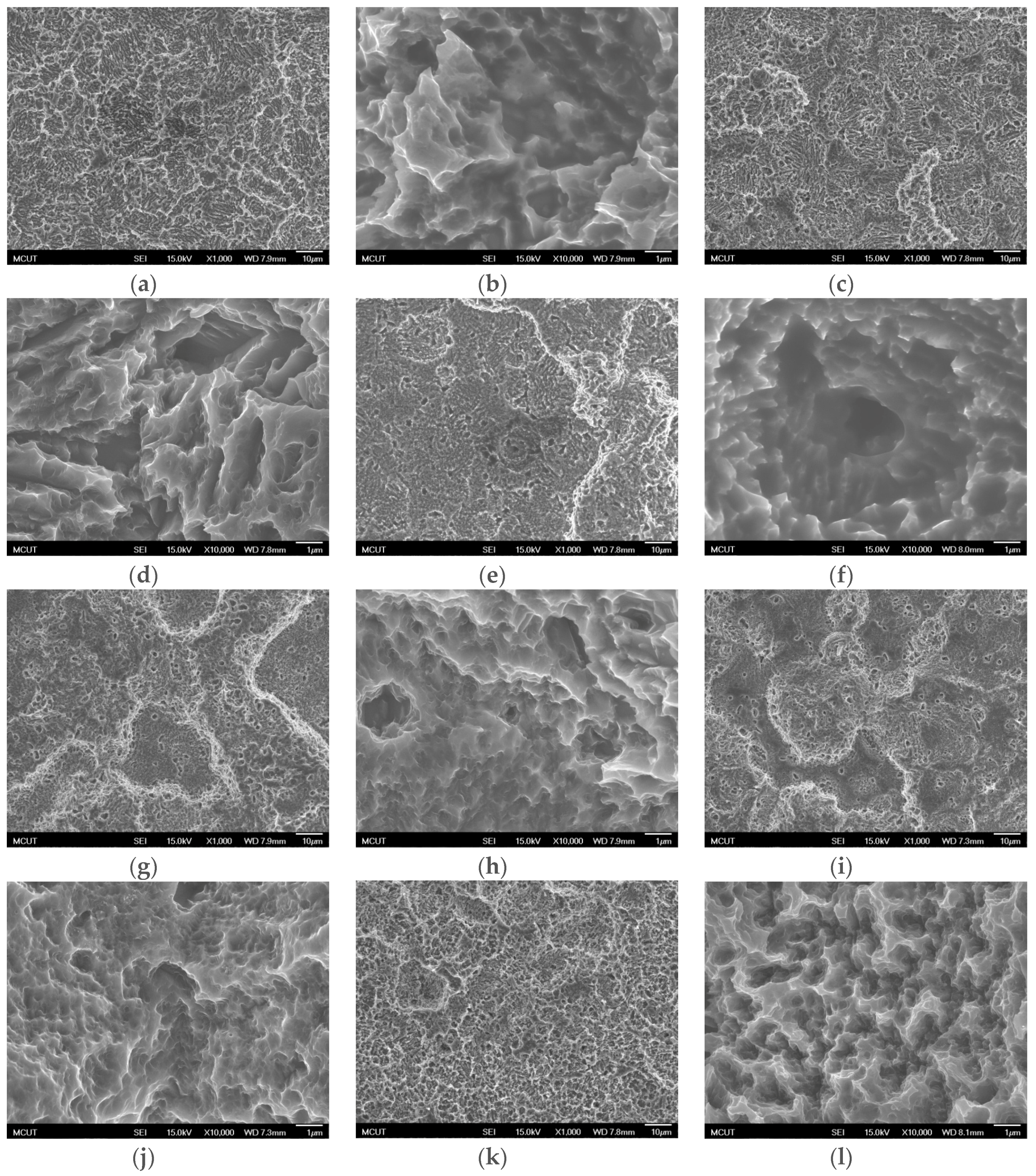
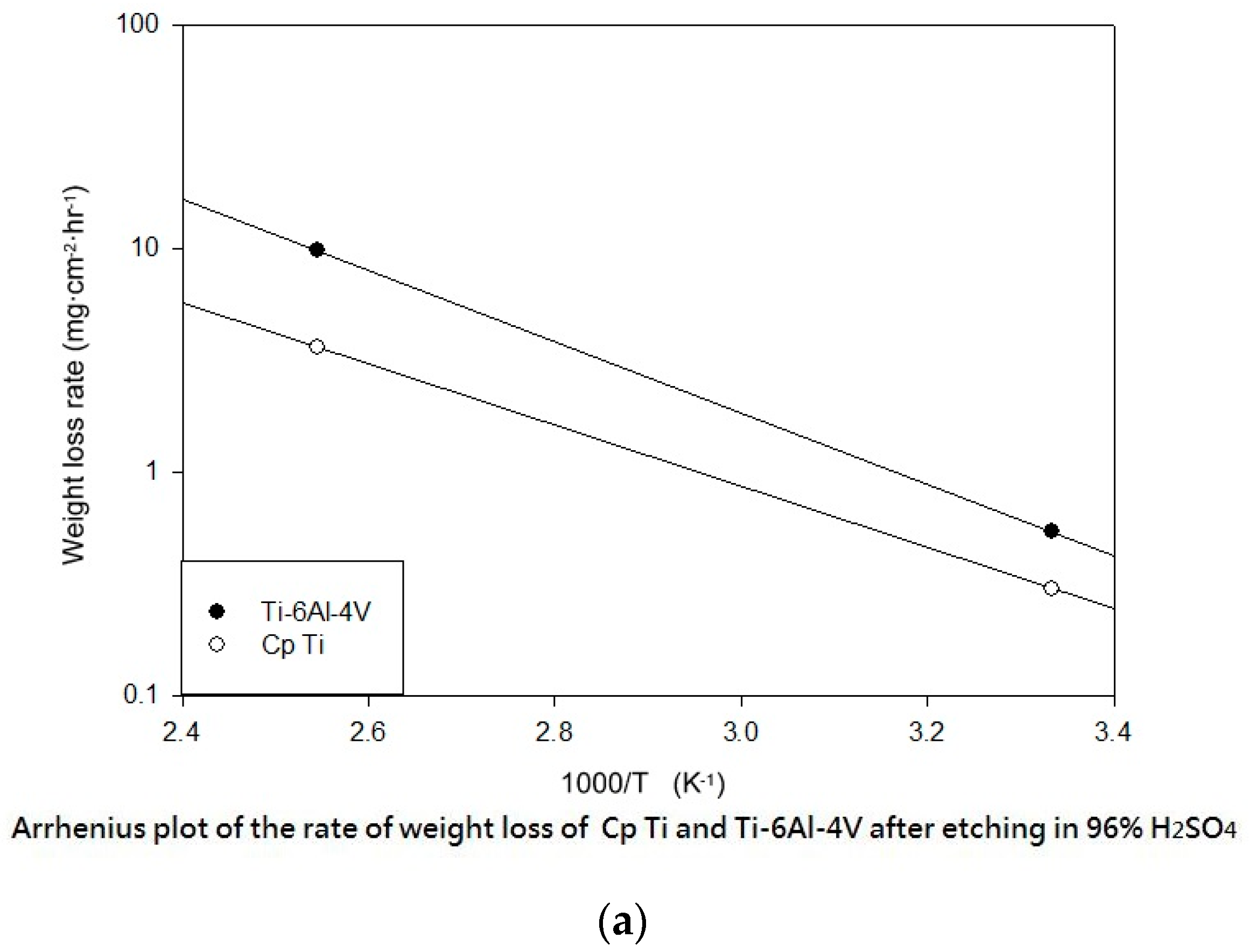
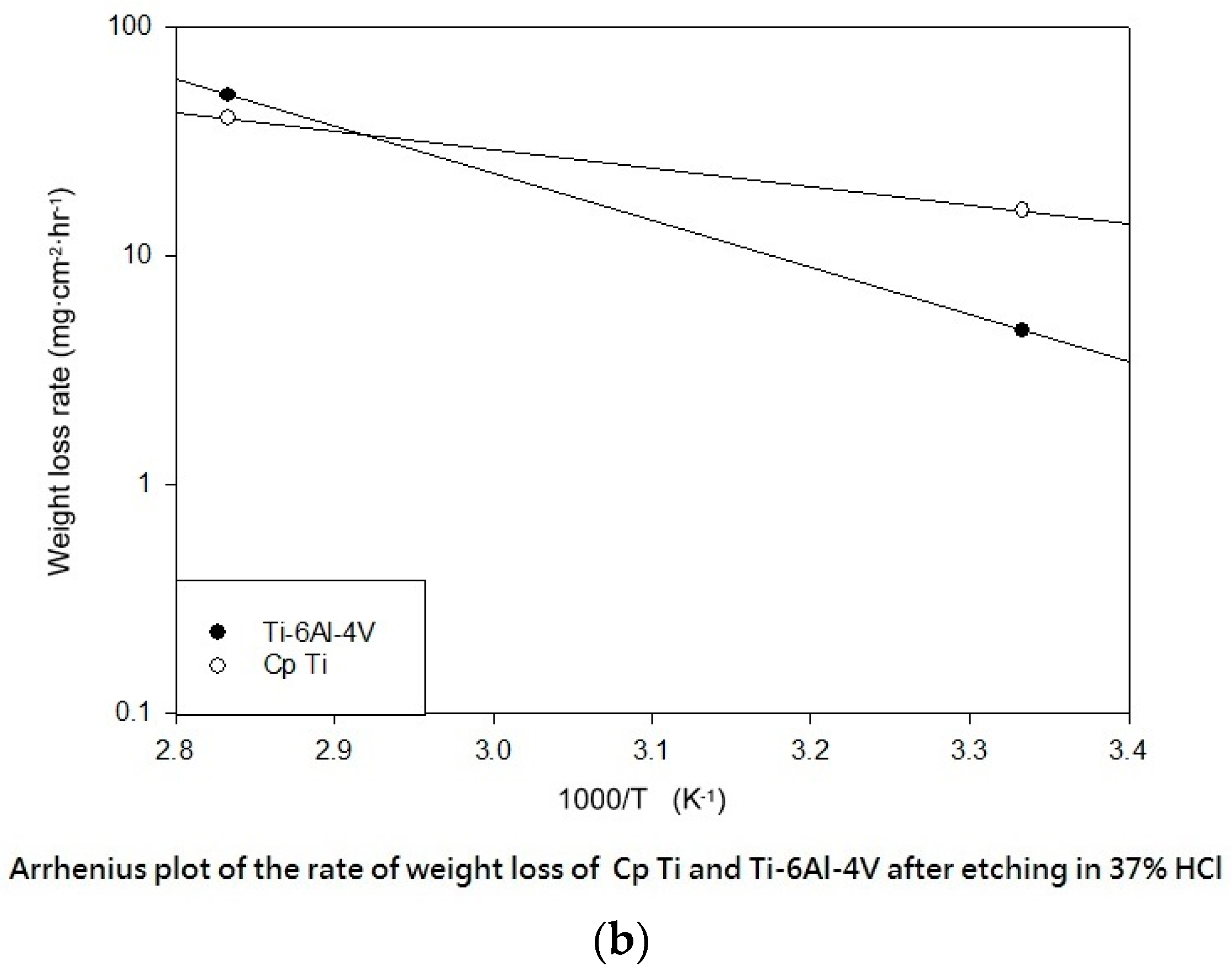
Table 3 shows the etching parameters for multiple types of acid at boiling. According to the weight loss, etching time, and temperature during acid etching, the activation energy can be estimated for the dissolution of CP Ti or Ti6Al4V in a different etchant solution by using the Arrhenius theory. The surface roughness was also measured after the etching process.
The Arrhenius equation [
12] is:
where
R is the universal gas constant (8.314 J/mol K) and
k is the number of collisions that result in a reaction per second.
A is the total number of collisions (both leading to and not leading to a reaction) per second and
is the probability that any given collision results in a reaction.
T is the temperature in absolute kelvin and
Ea (kilojoules per mole) is the activation energy. In the Arrhenius equation, the activation energy is defined as the minimum energy required to initiate the chemical reaction.
Taking the natural logarithm of the Arrhenius equation yields:
This has the same form as the equation for a straight line, namely,
. Therefore, when a reaction has a rate constant that is consistent with the Arrhenius equation, a plot of ln(
k) versus
T−1 generates a straight line, the gradient and intercept of which can be used to determine
Ea and
A. That is, the activation energy is defined as (−
R) times the slope of a plot of ln(
k) vs. (1/
T):
4. Conclusions
This study also successfully explored the process parameters of surface treatment, such as the etching rate, acid concentration, reaction temperature, and reaction time, the optimal values of which can be used to obtain uniform nanopores suitable for bone cell growth. A CP Ti/Ti-6Al-4V ELI surface as tested using shot blasting (pressure, grain size, blasting distance, blasting angle, and time) and acid etching to investigate its topographical characteristics, weight loss, surface roughness, and activation energy. The activation energy of Ti/Ti-6Al-4V ELI after etching using H2SO4 or HCl at different temperatures was determined. In addition, white-light interferometry was applied to measure the surface nanomorphology of the implant to obtain 2D or 3D roughness parameters. The Ti/Ti-6Al-4V ELI etching rate of the HCl or H2SO4 at RT was low, whereas HF exhibited the fastest etching rate 9.28 mg/min. After blasting, the acid etching occurred in a short period without initially requiring the etching of the oxide layer in HF. The surface roughness of the Ti, after etching in boiling HCl and H2SO4, was approximately 1.23 μm. The analysis of the results demonstrated that etching Ti metal in boiling H2SO4 or HCl aided in the formation of nanoporous structures. Since Ti-6Al-4V is an α + β crystal phase material and its etching rate differs, the variability of the surface roughness was more substantial than that found for Ti. The activation energy of the Ti was 76.51 kJ/mol when etched using H2SO4 at BT. The activation energy of Ti-6Al-4V was 88.96 kJ/mol when etched using H2SO4 at BT. This study also indicated that etching with concentrated hydraulic acid results in superior surface modification effects on Ti compared with when H2SO4 is used.