Bombyx mori Silk Fibroin Scaffolds with Antheraea pernyi Silk Fibroin Micro/Nano Fibers for Promoting EA. hy926 Cell Proliferation
Abstract
:1. Introduction
2. Results
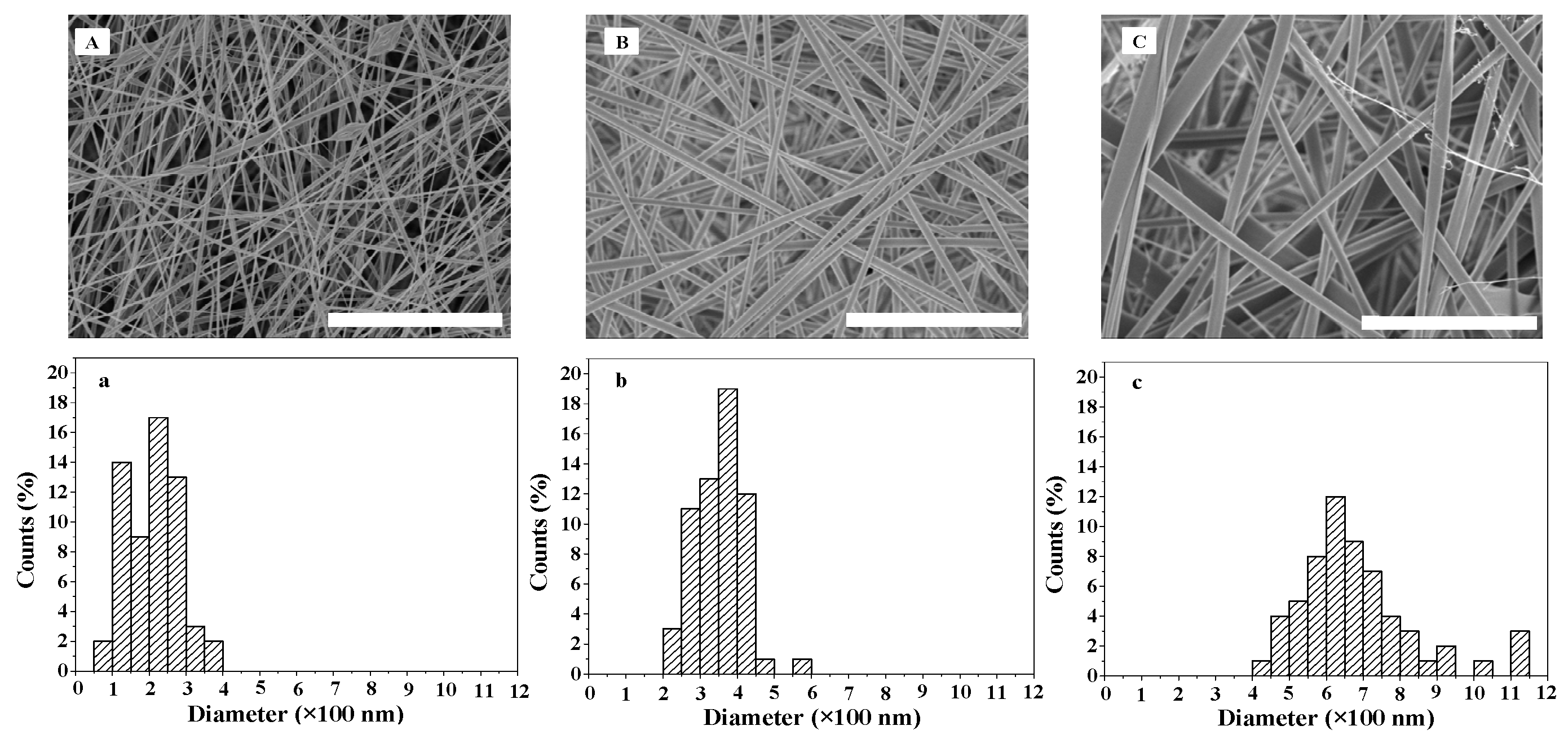
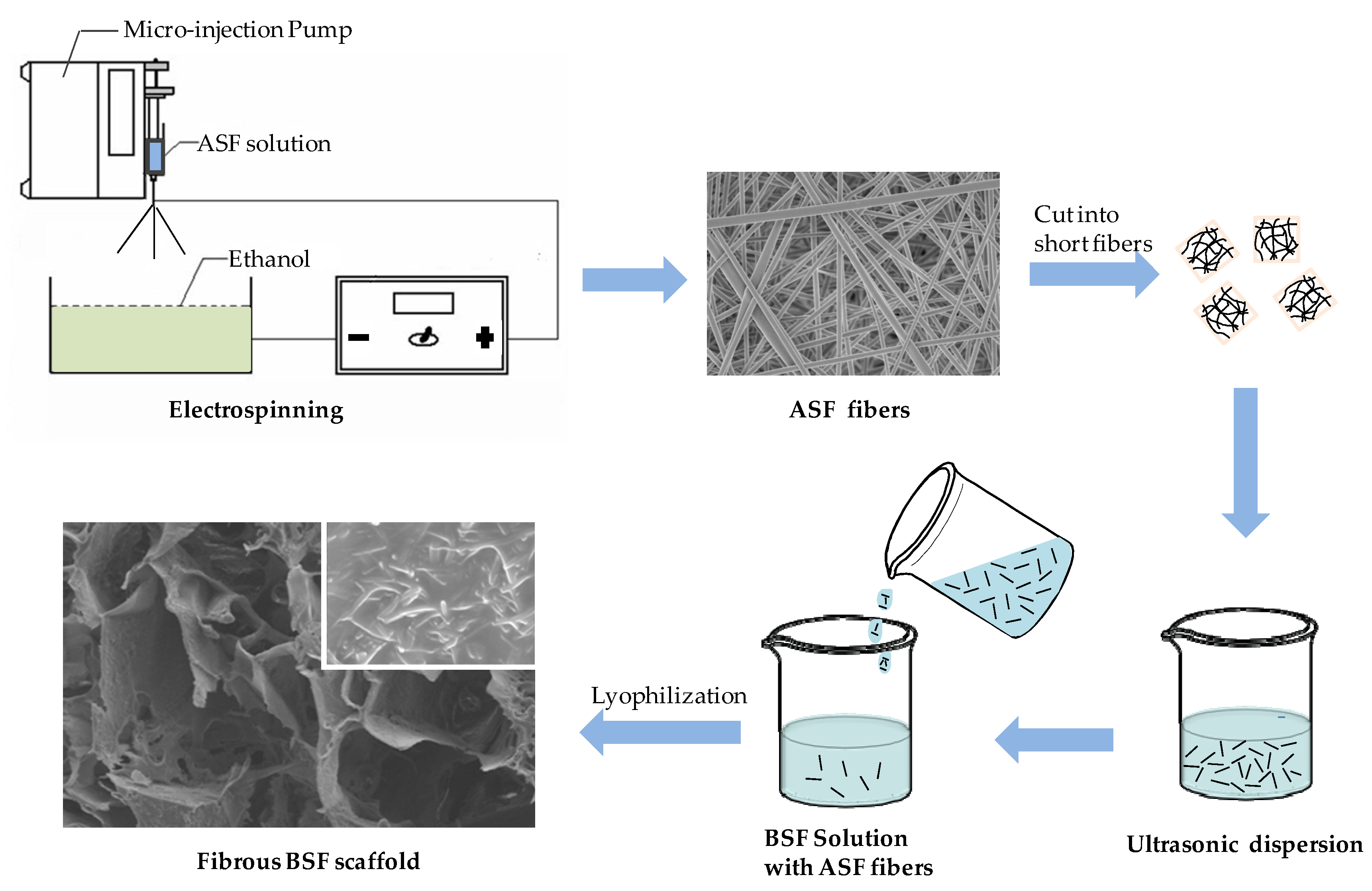
2.1. Morphology of ASF Micro/Nano Fibers
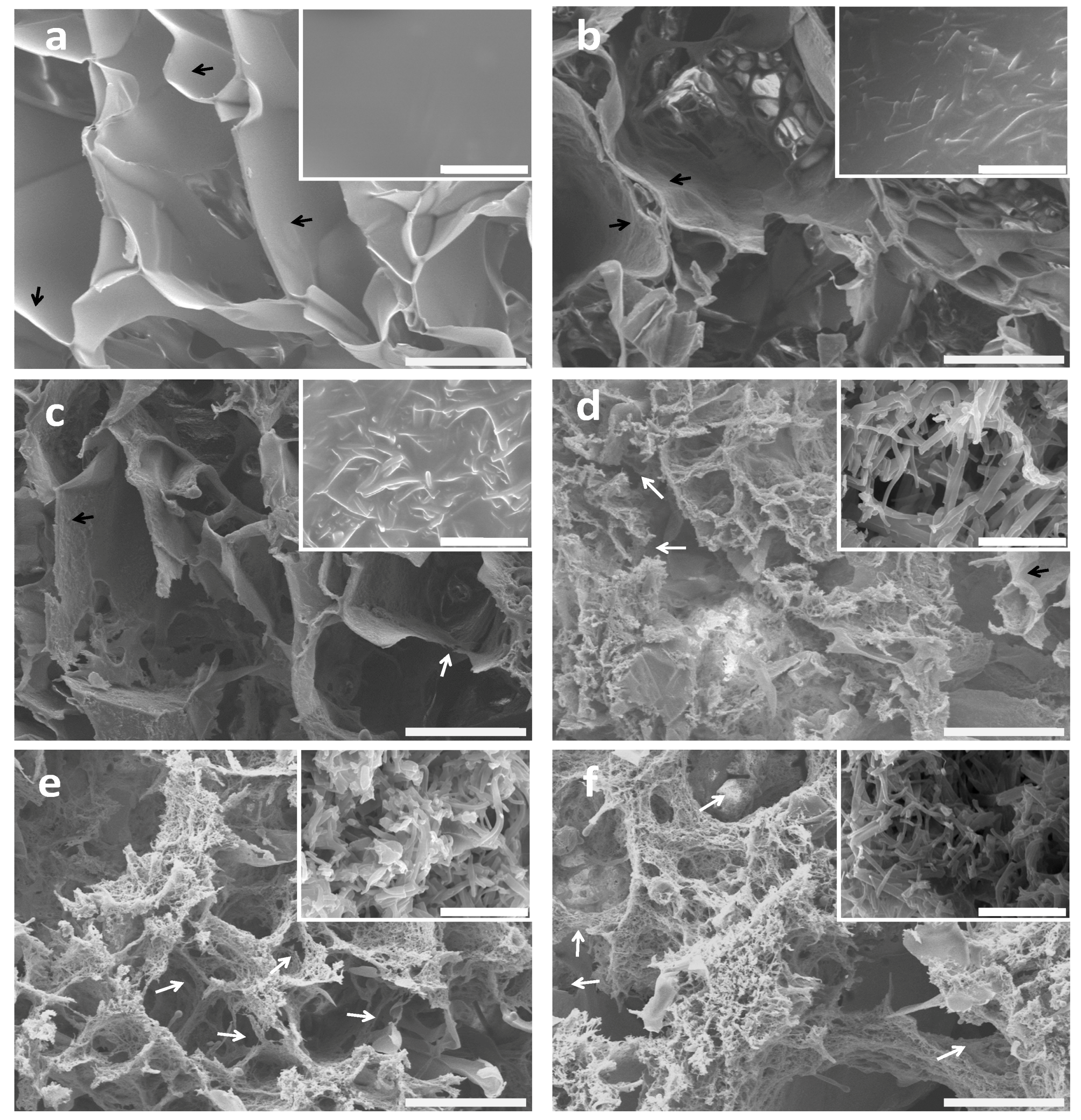
2.2. Morphology of BSF Scaffolds Containing ASF Micro/Nano Fibers
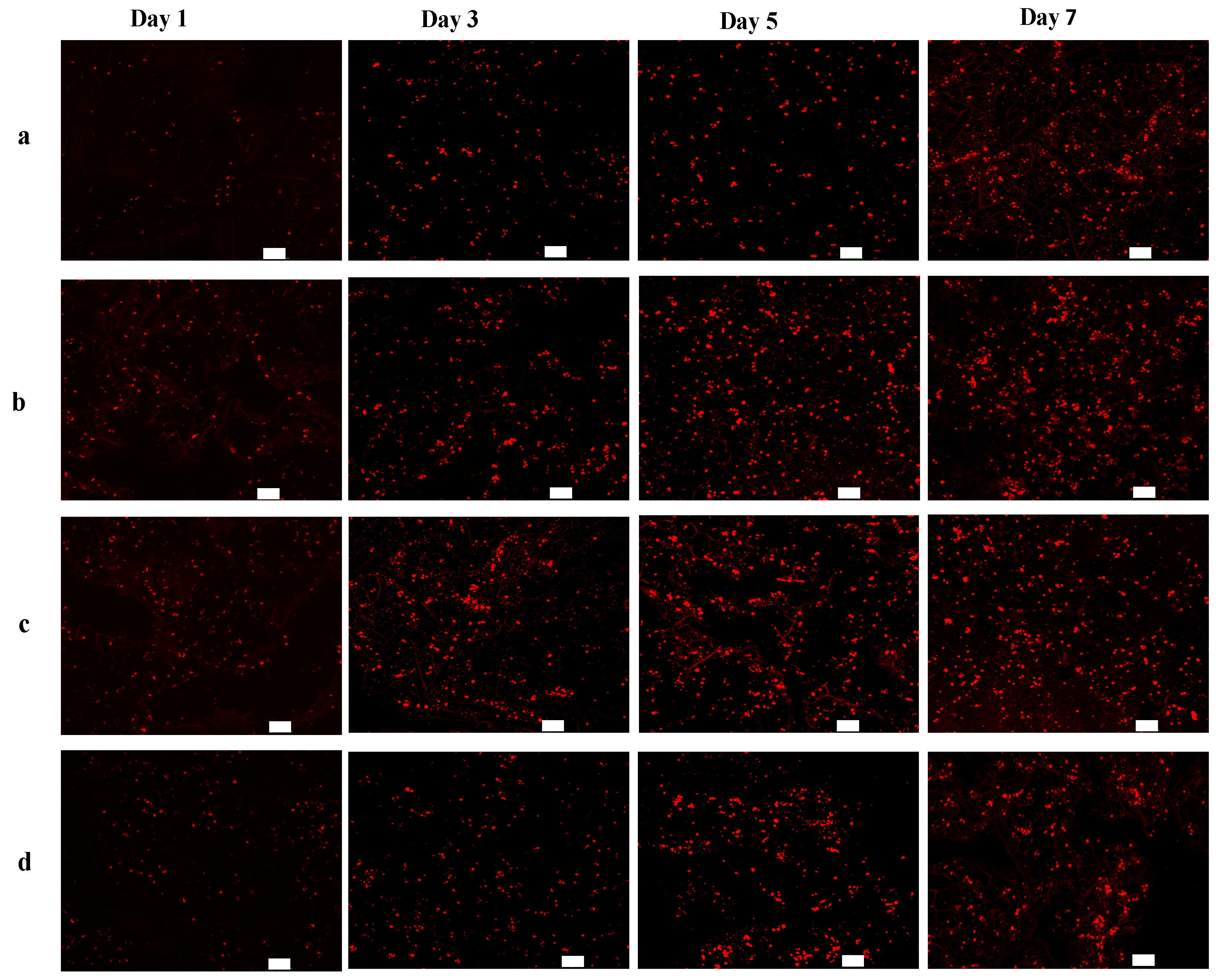
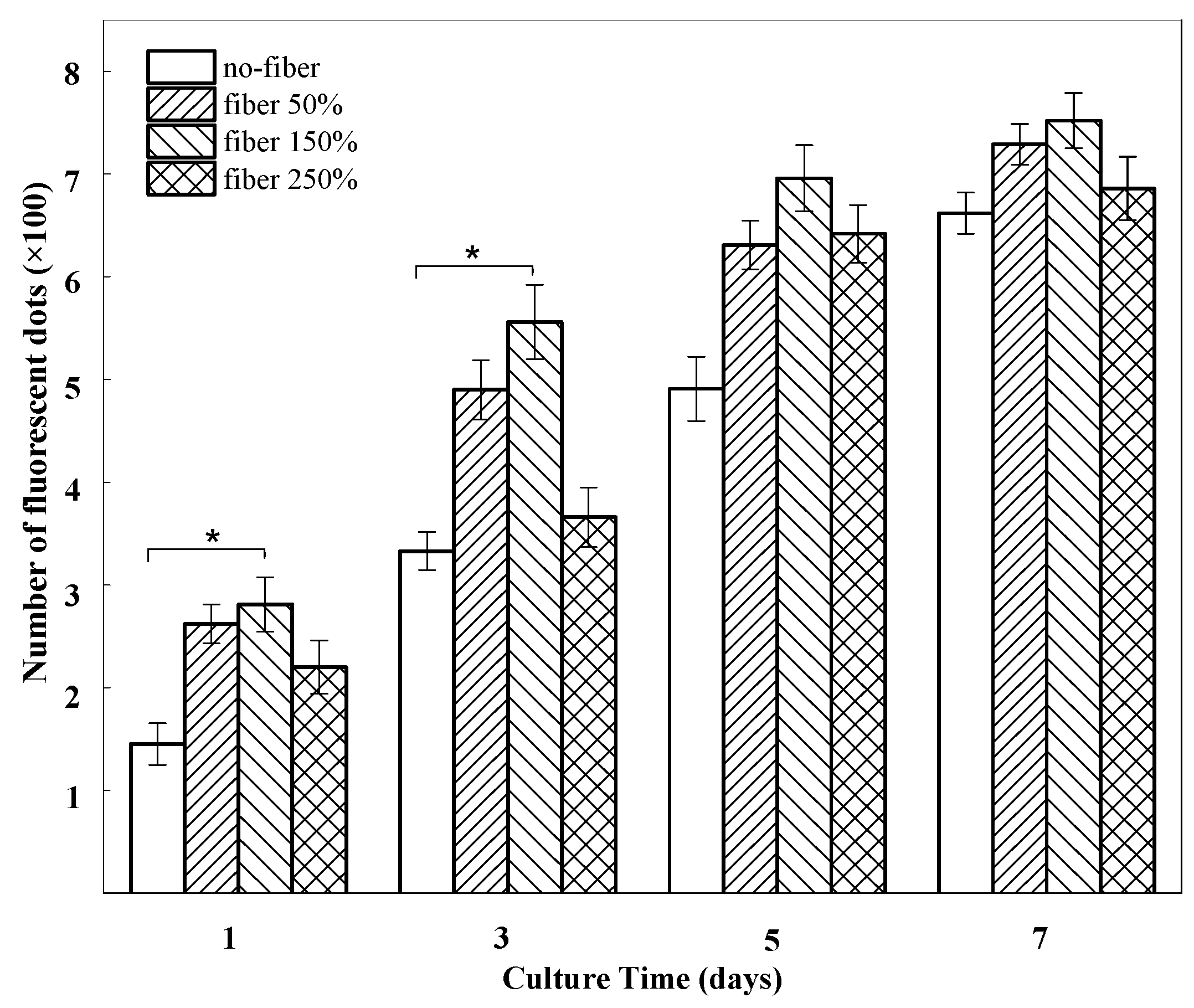
2.3. In Vitro Cell Growth within BSF Scaffolds
3. Discussion
4. Materials and Methods
4.1. Preparation of BSF Solution
4.2. Preparation of ASF Solution and Solids
4.3. Electrospinning of ASF Fibers
4.4. Preparation of BSF Scaffolds Containing ASF Micro/Nano Fibers
4.5. Scanning Electron Microscopy
4.6. Cell Culture and Observation
4.7. Cell Proliferation
4.8. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhou, H.L.; You, C.J.; Wang, X.G.; Jin, R.G.; Wu, P.; Li, Q.; Han, C.M. The progress and challenges for dermal regeneration in tissue engineering. J. Biomed. Mater. Res. 2017, 105, 1208–1218. [Google Scholar] [CrossRef] [PubMed]
- Utzinger, U.; Baggett, B.; Weiss, J.A.; Hoying, J.B.; Edgar, L.T. Large-scale time series microscopy of neovessel growth during angiogenesis. Angiogenesis 2015, 18, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Novosel, E.C.; Kleinhans, C.; Kluger, P.J. Vascularization is the key challenge in tissue engineering. Adv. Drug Delivery Rev. 2011, 63, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.W.; Chang, Y.H.; Yu, J.L.; Hsu, H.W.; Rau, L.R.; Hsu, F.Y. Preparation of nanofibrous structure of mesoporous bioactive glass microbeads for biomedical applications. Materials 2016, 9. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.T.; Sathe, S.R.; Yim, E.K.F. From nano to micro: Topographical scale and its impact on cell adhesion, morphology and contact guidance. J. Phys. Condens. Matter. 2016, 28. [Google Scholar] [CrossRef] [PubMed]
- Mattila, P.K.; Lappalainen, P. Filopodia: Molecular architecture and cellular functions. Nat. Rev. Mol. Cell Biol. 2008, 9, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J.; Lu, H.; Richmond, J.; Kaplan, D.L. Silk-based biomaterials. Biomaterials 2003, 24, 401–416. [Google Scholar] [CrossRef]
- Zhu, J.; Huang, W.; Zhang, Q.; Ling, S.; Chen, Y.; Kaplan, D.L. Aqueous-based coaxial electrospinning of genetically engineered silk elastin core-shell nanofibers. Materials 2016, 9. [Google Scholar] [CrossRef] [PubMed]
- Dinerman, A.A.; Cappello, J.; Ghandehari, H.; Hoag, S.W. Solute diffusion in genetically engineered silk-elastinlike protein polymer hydrogels. J. Control Release 2002, 82, 277–287. [Google Scholar] [CrossRef]
- Thurber, A.E.; Omenetto, F.G.; Kaplan, D.L. In vivo bioresponses to silk proteins. Biomaterials 2015, 71, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, B.C. Biodegradation of silk biomaterials. Int. J. Mol. Sci. 2009, 10, 1514–1524. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.J.; Di, L.; Ren, Q.S.; Wang, J.Y. Applications and degradation of proteins used as tissue engineering materials. Materials 2009, 2, 613–635. [Google Scholar] [CrossRef]
- You, R.C.; Xu, Y.M.; Liu, G.Z.; Liu, Y.; Li, X.F.; Li, M.Z. Regulating the degradation rate of silk fibroin films through changing the genipin crosslinking degree. Polym. Degrad. Stab. 2014, 109, 226–232. [Google Scholar] [CrossRef]
- Yang, Y.M.; Ding, F.; Wu, J.; Hu, W.; Liu, W.; Liu, J.; Gu, S.X. Development and evaluation of silk fibroin-based nerve grafts used for peripheral nerve regeneration. Biomaterials 2007, 28, 5526–5535. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Baughman, C.B.; Kaplan, D.L. In vitro evaluation of electrospun silk fibroin scaffolds for vascular cell growth. Biomaterials 2008, 29, 2217–2227. [Google Scholar] [CrossRef] [PubMed]
- Wharram, S.E.; Zhang, X.; Kaplan, D.L.; McCarthy, S.P. Electrospun silk material systems for wound healing. Macromol. Biosci. 2010, 10, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Elahi, M.F.; Guan, G.; Wang, L.; King, M.W. Influence of Layer-by-layer polyelectrolyte deposition and EDC/NHS activated heparin immobilization onto silk fibro in fabric. Materials 2014, 7, 2956–2977. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, S.S.; Xing, T.L.; Kundu, B.; Li, M.Z.; Kundu, S.C.; Lu, S.Z. Ion-induced fabrication of silk fibroin nanoparticles from Chinese oak tasar Antheraea pernyi. Int. J. Biol. Macromol. 2015, 79, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Kweon, H.Y.; Um, I.C.; Park, Y.H. Structural and thermal characteristics of Antheraea pernyi silk fibroin/chitosan blend film. Polymer 2001, 42, 6651–6656. [Google Scholar] [CrossRef]
- Luan, X.Y.; Wang, Y.; Duan, X.; Duan, Q.Y.; Li, M.Z.; Lu, S.Z.; Zhang, H.X.; Zhang, X.G. Attachment and growth of human bone marrow derived mesenchymal stem cells on regenerated Antheraea pernyi silk fibroin films. Biomed. Mater. 2006, 1, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.L.; He, J.X.; Sang, F.; Chen, L.; Cui, S.Z.; Ding, B. Enhanced bone formation in electrospun poly(l-lactic-co-glycolic acid)-tussah silk fibroin ultrafine nanofiber scaffolds incorporated with grapheme oxide. Mater. Sci. Eng. C 2016, 62, 823–834. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.H.; Chen, Y.W.; Shie, M.Y.; Fang, H.Y. Poly(dopamine)-assisted immobilization of Xu Duan on 3D printed poly(lactic acid) scaffolds to up-regulate osteogenic and angiogenic markers of bone marrow stem cells. Materials 2015, 8, 4299–4315. [Google Scholar] [CrossRef] [PubMed]
- Kim, U.J.; Jaehyung, P.; Kim, H.J.; Masahisa, C.; Kaplana, D.L. Three-dimensional aqueous-derived biomaterial scaffolds from silk fibroin. Biomaterials 2005, 26, 2775–2785. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.H.; Lin, H.; Yao, J.R.; Chen, Z.G.; Shao, J.J. Preparation of 3D Fibroin/Chitosan Blend Porous Scaffold for Tissue Engineering Via a Simplified Method. Macromol. Biosci. 2011, 11, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Liu, J.M.J.; Chua, C.K.; Chou, S.M.; Shyu, V.B.H.; Chen, J.P. Cartilage tissue engineering with silk fibroin scaffolds fabricated by indirect additive manufacturing technology. Materials 2014, 7, 2104–2119. [Google Scholar] [CrossRef] [PubMed]
- Schieker, M.; Seitz, H.; Drosse, I.; Seitz, S.; Mutschler, F. Biomaterials as Scaffold for Bone Tissue Engineering. Eur. J. Trauma 2006, 32, 114–124. [Google Scholar] [CrossRef]
- Li, M.Z.; Wu, Z.Y.; Zhang, C.S.; Lu, S.Z.; Yan, H.J.; Huang, D.; Ye, H.L. Study on porous silk fibroin materials. II. Preparation and characteristics of spongy porous silk fibroin materials. Appl. Polym. Sci. 2001, 79, 2192–2199. [Google Scholar] [CrossRef]
- Ishii, D.; Ying, T.H.; Yamaoka, T.; Iwata, T. Characterization and biocompatibility of biopolyester nanofibers. Materials 2009, 2, 1520–1546. [Google Scholar] [CrossRef]
- Yoshimoto, H.; Shin, Y.M.; Terai, H.; Vacanti, J.P. A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials 2003, 24, 2077–2082. [Google Scholar] [CrossRef]
- Li, M.Z.; Lu, S.Z.; Wu, Z.Y.; Yan, H.J.; Mo, J.Y.; Wang, L.H. Study on porous silk fibroin materials. I. Fine structure of freeze dried silk fibroin. Appl. Polym. Sci. 2001, 79, 2185–2191. [Google Scholar] [CrossRef]
- Zhang, Q.; Yan, S.Q.; Li, M.Z. Silk fibroin based porous materials. Materials 2009, 2, 2276–2295. [Google Scholar] [CrossRef]
- Guan, G.P.; Bai, L.; Zuo, B.Q.; Li, M.Z.; Wu, Z.Y.; Li, Y.L.; Wang, L. Promoted dermis healing from full-thickness skin defect by porous silk fibroin scaffolds (PSFSs). Biomed. Mater. Eng. 2010, 20, 295–380. [Google Scholar] [PubMed]
- You, R.C.; Xu, Y.M.; Liu, Y.; Li, X.F.; Li, M.Z. Comparison of the in vitro and in vivo degradations of silk fibroin scaffolds from mulberry and nonmulberry silkworms. Biomed. Mater. 2014, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.R.; Pui, D.Y.H.; Kaufman, S.L. Electrospraying of conducting liquids for monodisperse aerosol generation in the 4 nm to 1.8 μm diameter range. J. Aerosol. Sci. 1995, 26, 963–977. [Google Scholar] [CrossRef]
- Li, X.F.; You, R.C.; Luo, Z.W.; Chen, G.; Li, M.Z. Silk fibroin scaffolds with micro/nano fibrous architecture for dermal regeneration. J. Mater. Chem. B 2016, 4, 2903–2912. [Google Scholar] [CrossRef]
- Wu, J.; Meredith, J.C. Assembly of Chitin Nanofibers into Porous Biomimetic Structures via Freeze Drying. ACS Macro. Lett. 2014, 3, 185–190. [Google Scholar] [CrossRef]
- Park, H.J.; Lee, O.J.; Lee, M.C.; Moon, B.M.; Ju, H.W.; Lee, J.M.; Kim, J.H.; Kim, D.W.; Park, C.H. Fabrication of 3D porous silk scaffolds by particulate (salt/sucrose) leaching for bone tissue reconstruction. Int. J. Biol. Macromol. 2015, 78, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhu, R.; Sun, L.; Xue, Y.; Hao, Z.; Xie, Z.; Fan, X.; Fan, H. Effect of thickness of HA-coating on microporous silk scaffolds using alternate soaking technology. BioMed. Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Ercan, B.; Roy, A.K.; Webster, T.J. Addition of selenium nanoparticles to electrospun silk scaffold improves the mammalian cell activity while reducing bacterial growth. Front. Physiol. 2016, 7, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Deitzel, J.M.; Kleinmeyer, J.; Harris, D.E.A.; Tan, N.B. The effect of processing variables on the morphology of electrospun nanofibers and textiles. Polymer 2001, 42, 261–272. [Google Scholar] [CrossRef]
- McKee, M.G.; Wilkes, G.L.; Colby, R.H.; Long, T.E. Correlations of solution rheology with electrospun fiber formation of linear and branched polyesters. Macromolecules 2004, 37, 1760–1767. [Google Scholar] [CrossRef]
- Han, F.; Liu, S.; Liu, X.; Pei, Y.; Bai, S.; Zhao, H.; Lu, Q.; Ma, F.; Kaplan, D.L.; Zhu, H. Woven silk fabric-reinforced silk nanofibrous scaffolds for regenerating load-bearing soft tissues. Acta Biomater. 2014, 10, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Mandal, B.B.; Grinberg, A.; Gil, E.S.; Panilaitis, B.; Kaplan, D.L. High-strength silk protein scaffolds for bone repair. Proc. Natl. Acad. Sci. 2012, 109, 7699–7704. [Google Scholar] [CrossRef] [PubMed]
- Wittmer, C.R.; Claudepierre, T.; Reber, M.; Wiedemann, P.; Garlick, J.A.; Kaplan, D.L.; Egles, C. Multifunctionaliz edelectrospun silk fibers promote axon regeneration in the central nervous system. Adv. Funct. Mater. 2011, 21, 4232–4242. [Google Scholar] [CrossRef]
- Kim, J.W.; Shin, K.H.; Koh, Y.H.; Hah, M.J.; Moon, J.Y.; Kim, H.E. Production of poly(ε-caprolactone)/hydroxyapatite composite scaffolds with a tailored macro/micro-porous structure, high mechanical properties, and excellent bioactivity. Materials 2017, 10. [Google Scholar] [CrossRef] [PubMed]
- Mandal, B.B.; Gil, E.S.; Panilaitis, B.; Kaplan, D.L. Laminar silk scaffolds for aligned tissue fabrication. Macromol. Biosci. 2013, 13, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Barnes, C.P.; Sell, S.A.; Boland, E.D.; Simpson, D.G.; Bowlin, G.L. Nanofiber technology: Designing the next generation of tissue engineering scaffolds. Adv. Drug Delivery Rev. 2007, 59, 1413–1433. [Google Scholar] [CrossRef] [PubMed]
- Albuschies, J.; Vogel, V. The role of filopodia in the recognition of nanotopographies. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.J.; Fridrikh, S.V.; Rutledge, G.C.; Kaplan, D.L. Electrospinning Bombyx mori silk with poly (ethylene oxide). Biomacromolecules 2002, 3, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Sakai, S.; Yamada, Y.; Yamaguchi, T.; Kawakami, K. Prospective use of electrospun ultra-fine silicate fibers for bone tissue engineering. Biotechnol. J. 2006, 1, 958–962. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.C.; Atkin, N.; Gunning, P.A.; Granville, N.; Wilson, K.; Wilson, D.; Southgate, J. Characterisation of electrospun polystyrene scaffolds for three-dimensional in vitro biological studies. Biomaterials 2006, 27, 3136–3146. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Shen, J.; Feng, X.; Zhang, H.; Guo, Y.; Chen, J. Fabrication and characterization of bioactive silk fibroin/wollastonite composite scaffolds. Mater. Sci. Eng. C 2010, 30, 132–140. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Gogal, R.M.; Walsh, J.E. A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: An alternaootive to [3H] thymidine incorporation assay. Immunol. Meth. 1994, 170, 211–224. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Yang, W.; Wang, W.; Zhang, M.; Li, M. Bombyx mori Silk Fibroin Scaffolds with Antheraea pernyi Silk Fibroin Micro/Nano Fibers for Promoting EA. hy926 Cell Proliferation. Materials 2017, 10, 1153. https://doi.org/10.3390/ma10101153
Chen Y, Yang W, Wang W, Zhang M, Li M. Bombyx mori Silk Fibroin Scaffolds with Antheraea pernyi Silk Fibroin Micro/Nano Fibers for Promoting EA. hy926 Cell Proliferation. Materials. 2017; 10(10):1153. https://doi.org/10.3390/ma10101153
Chicago/Turabian StyleChen, Yongchun, Weichao Yang, Weiwei Wang, Min Zhang, and Mingzhong Li. 2017. "Bombyx mori Silk Fibroin Scaffolds with Antheraea pernyi Silk Fibroin Micro/Nano Fibers for Promoting EA. hy926 Cell Proliferation" Materials 10, no. 10: 1153. https://doi.org/10.3390/ma10101153
APA StyleChen, Y., Yang, W., Wang, W., Zhang, M., & Li, M. (2017). Bombyx mori Silk Fibroin Scaffolds with Antheraea pernyi Silk Fibroin Micro/Nano Fibers for Promoting EA. hy926 Cell Proliferation. Materials, 10(10), 1153. https://doi.org/10.3390/ma10101153




