Magnetic Force-Driven Graphene Patterns to Direct Synaptogenesis of Human Neuronal Cells
Abstract
:1. Introduction
2. Results and Discussion
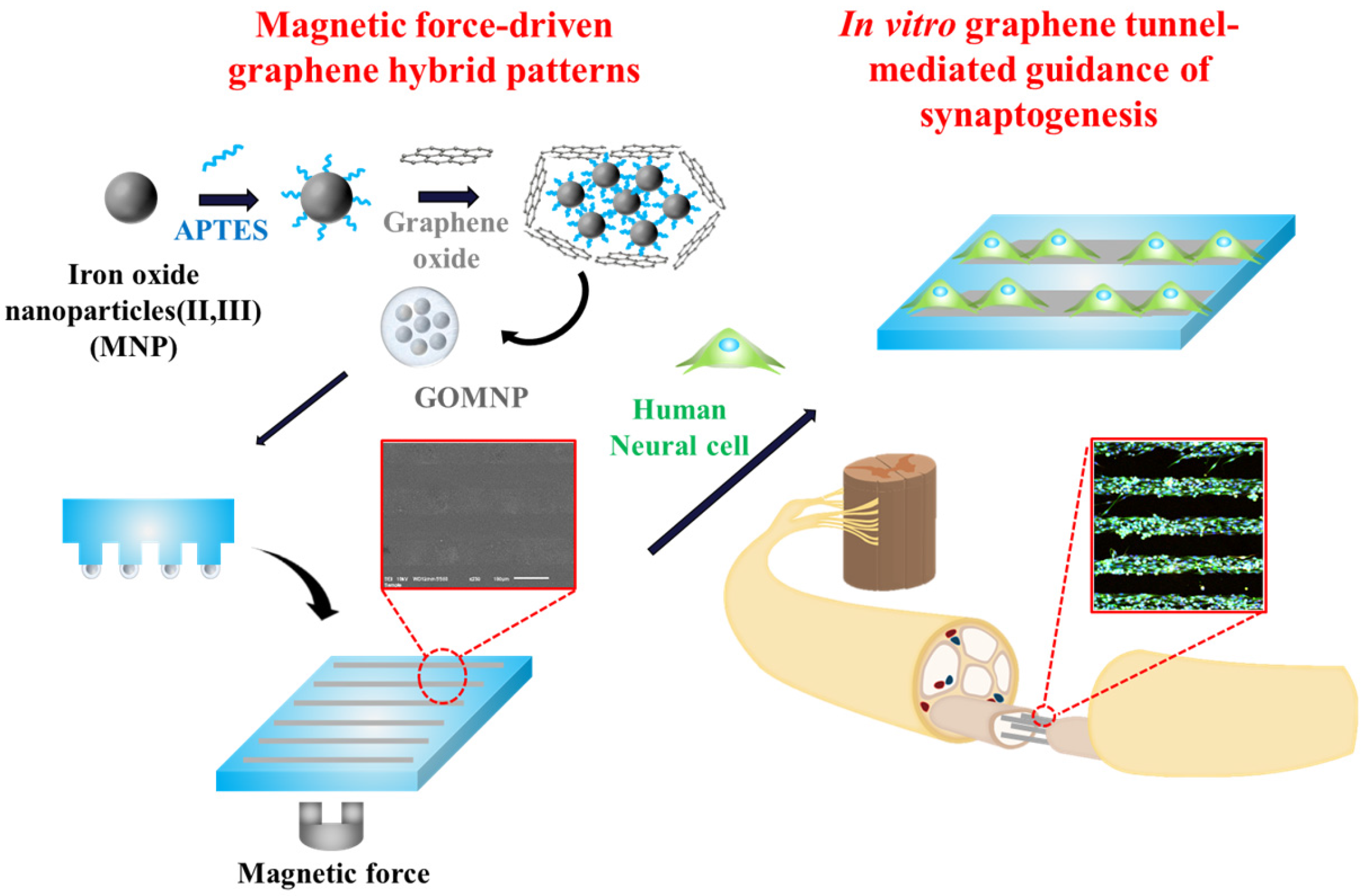
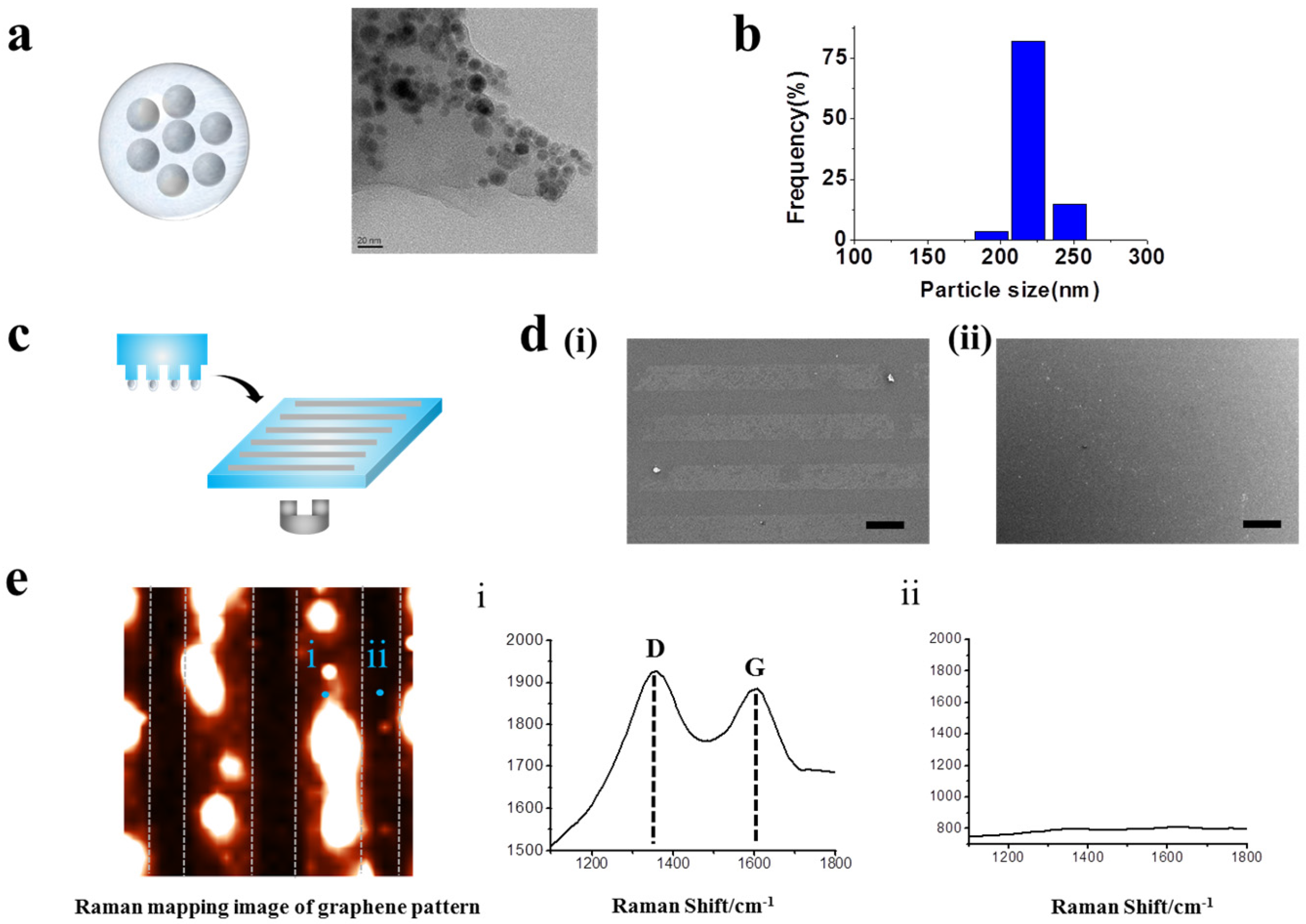
2.1. Generation of GO Hybrid Patterns with Different Geometries
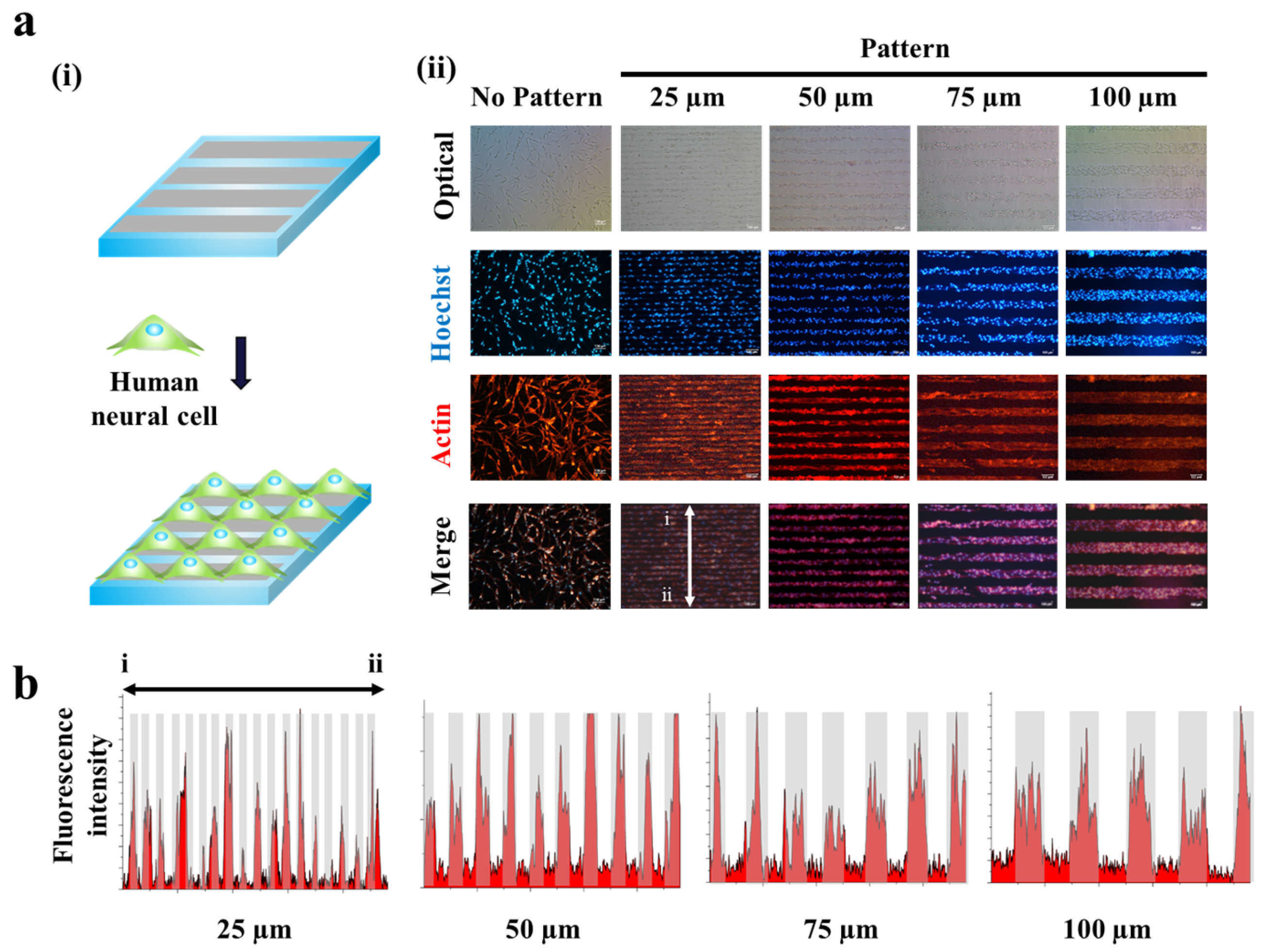
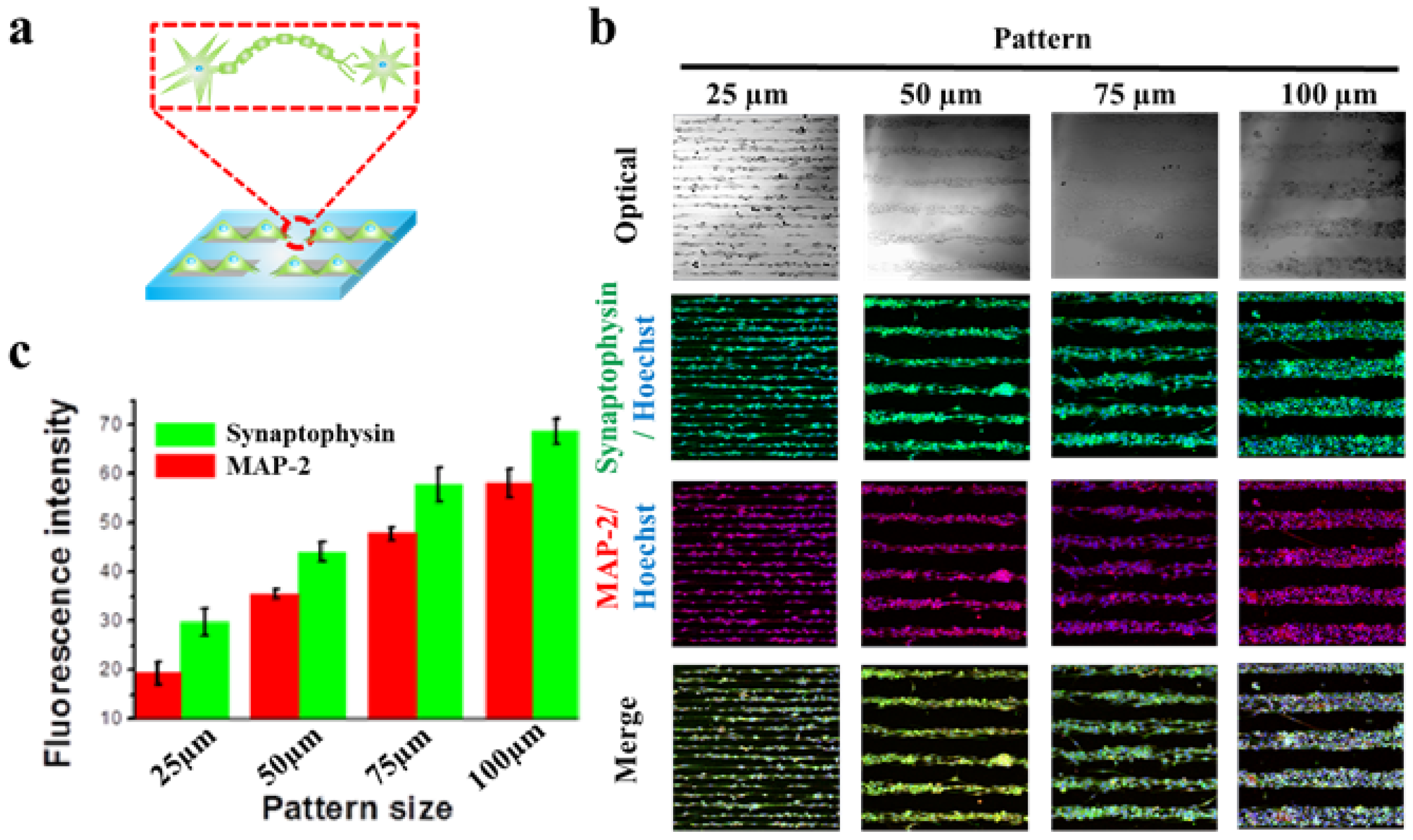
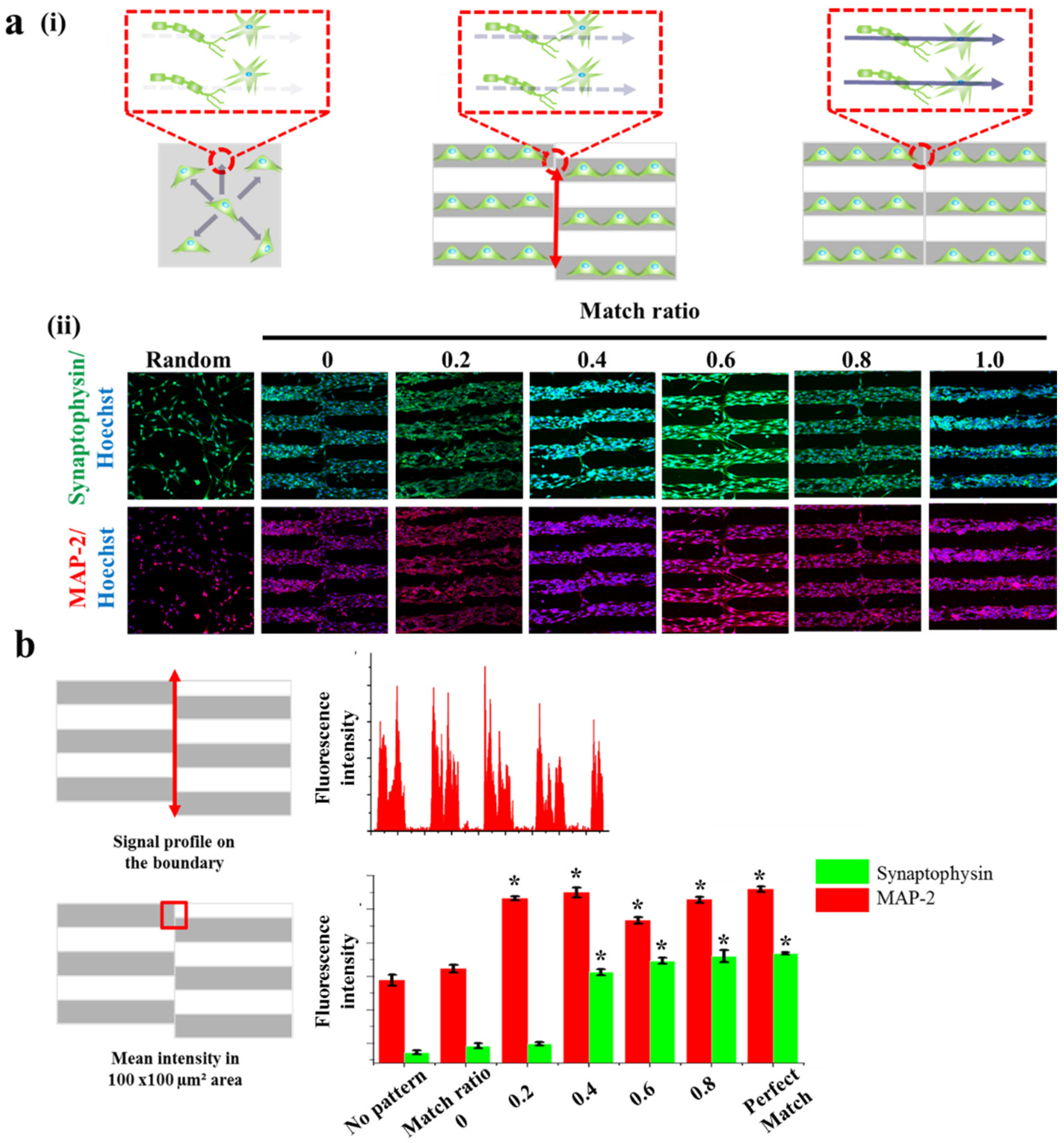
2.2. Guided Synaptogenesis of Human Neuronal Cells (SH-SY5Y) on MF-Driven GO Hybrid Patterns
2.3. Synaptogenesis Guidance Due to Pattern Effect
3. Materials and Methods
3.1. Materials
3.2. Cell Culture
3.3. Fluorescence Imaging
3.4. Confocal Imaging
3.5. Synthesis of GO-Encapsulated Magnetic Nanoparticles
3.6. Generation of GO Hybrid Patterns
3.7. Raman Spectroscopy
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mandel, R.J.; Manfredsson, F.P.; Foust, K.D.; Rising, A.; Reimsnider, S.; Nash, K.; Burger, C. Recombinant adeno-associated viral vectors as therapeutic agents to treat neurological disorders. Mol. Ther. 2006, 13, 463–483. [Google Scholar] [CrossRef] [PubMed]
- Jacoby, A.; Snape, D.; Baker, G.A. Epilepsy and social identity: The stigma of a chronic neurological disorder. Lancet Neurol. 2005, 4, 171–178. [Google Scholar] [CrossRef]
- Lindvall, O.; Kokaia, Z. Stem cells for the treatment of neurological disorders. Nature 2006, 441, 1094. [Google Scholar] [CrossRef] [PubMed]
- Pereira, F.C.; Marcillo, A.E. cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat. Med. 2004, 10, 610–616. [Google Scholar]
- Binda, C.; Newton-Vinson, P.; Hubálek, F.; Edmondson, D.E.; Mattevi, A. Structure of human monoamine oxidase B, a drug target for the treatment of neurological disorders. Nat. Struct. Mol. Biol. 2002, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Vernino, S.; O’Neill, B.P.; Marks, R.S.; O’Fallon, J.R.; Kimmel, D.W. Immunomodulatory treatment trial for paraneoplastic neurological disorders. Neuro Oncol. 2004, 6, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Gold, B.G. Neuroimmunophilin ligands: Evaluation of their therapeutic potential for the treatment of neurological disorders. Expert Opin. Investig. Drugs 2000, 9, 2331–2342. [Google Scholar] [CrossRef] [PubMed]
- Wulff, H.; Zhorov, B.S. K+ channel modulators for the treatment of neurological disorders and autoimmune diseases. Chem. Rev. 2008, 108, 1744–1773. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, J.N.; Lisak, R.P.; Bashir, R.M.; Fitch, O.H.; Seyer, J.M.; Krance, R.; Lawrence, J.A.; Ch’ien, L.T.; O’Sullivan, P. Immunoreactive myelin basic protein in the cerebrospinal fluid in neurological disorders. Ann. Neurol. 1980, 7, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Yermakova, A.; O’banion, M.K. Cyclooxygenases in the central nervous system: Implications for treatment of neurological disorders. Curr. Pharm. Des. 2000, 6, 1755–1776. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.-T.; Svendsen, C.N. Stem cells as a potential treatment of neurological disorders. Curr. Opin. Pharmacol. 2004, 4, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Woodruff, G.; Wang, Y.; Graham, L.; Hunt, M.; Wu, D.; Boehle, E.; Ahmad, R.; Poplawski, G.; Brock, J. Long-distance axonal growth from human induced pluripotent stem cells after spinal cord injury. Neuron 2014, 83, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Jones, L.L.; Snyder, E.Y.; Tuszynski, M.H. Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Exp. Neurol. 2003, 181, 115–129. [Google Scholar] [CrossRef]
- Liu, S.; Qu, Y.; Stewart, T.J.; Howard, M.J.; Chakrabortty, S.; Holekamp, T.F.; McDonald, J.W. Embryonic stem cells differentiate into oligodendrocytes and myelinate in culture and after spinal cord transplantation. Proc. Natl. Acad. Sci. USA 2000, 97, 6126–6131. [Google Scholar] [CrossRef] [PubMed]
- Cummings, B.J.; Uchida, N.; Tamaki, S.J.; Salazar, D.L.; Hooshmand, M.; Summers, R.; Gage, F.H.; Anderson, A.J. Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proc. Natl. Acad. Sci. USA 2005, 102, 14069–14074. [Google Scholar] [CrossRef] [PubMed]
- Nori, S.; Okada, Y.; Yasuda, A.; Tsuji, O.; Takahashi, Y.; Kobayashi, Y.; Fujiyoshi, K.; Koike, M.; Uchiyama, Y.; Ikeda, E. Grafted human-induced pluripotent stem-cell-derived neurospheres promote motor functional recovery after spinal cord injury in mice. Proc. Natl. Acad. Sci. USA 2011, 108, 16825–16830. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.D.; Lavik, E.B.; Qu, X.; Park, K.I.; Ourednik, J.; Zurakowski, D.; Langer, R.; Snyder, E.Y. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proc. Natl. Acad. Sci. USA 2002, 99, 3024–3029. [Google Scholar] [CrossRef] [PubMed]
- Keirstead, H.S.; Nistor, G.; Bernal, G.; Totoiu, M.; Cloutier, F.; Sharp, K.; Steward, O. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J. Neurosci. 2005, 25, 4694–4705. [Google Scholar] [CrossRef] [PubMed]
- Di Lullo, E.; Kriegstein, A.R. The use of brain organoids to investigate neural development and disease. Nat. Rev. Neurosci. 2017, 18, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, M.A.; Corsini, N.S.; Wolfinger, S.; Gustafson, E.H.; Phillips, A.W.; Burkard, T.R.; Otani, T.; Livesey, F.J.; Knoblich, J.A. Guided self-organization and cortical plate formation in human brain organoids. Nat. Biotechnol. 2017, 35, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Fatehullah, A.; Tan, S.H.; Barker, N. Organoids as an in vitro model of human development and disease. Nat. Cell Biol. 2016, 18, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-H.; Lee, J.; Shin, W.; Choi, J.-W.; Kim, H.J. Priming nanoparticle-guided diagnostics and therapeutics towards human organs-on-chips microphysiological system. Nano Converg. 2016, 3, 24. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-H.; Lee, K.-B.; Choi, J.-W. 3D graphene oxide-encapsulated gold nanoparticles to detect neural stem cell differentiation. Biomaterials 2013, 34, 8660–8670. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.-S.; Kim, D.-S.; Suhito, I.R.; Choo, S.-S.; Kim, S.-J.; Song, I.; Kim, T.-H. Guiding osteogenesis of mesenchymal stem cells using carbon-based nanomaterials. Nano Converg. 2017, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-H.; Lee, T.; El-Said, W.A.; Choi, J.-W. Graphene-based materials for stem cell applications. Materials 2015, 8, 8674–8690. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Park, J.; Sim, S.H.; Sung, M.G.; Kim, K.S.; Hong, B.H.; Hong, S. Enhanced differentiation of human neural stem cells into neurons on graphene. Adv. Mater. 2011, 23, H236–H267. [Google Scholar] [CrossRef] [PubMed]
- Nayak, T.R.; Andersen, H.; Makam, V.S.; Khaw, C.; Bae, S.; Xu, X.; Ee, P.-L.R.; Ahn, J.-H.; Hong, B.H.; Pastorin, G. Graphene for controlled and accelerated osteogenic differentiation of human mesenchymal stem cells. ACS Nano 2011, 5, 4670–4678. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.W.; Lee, J.H.; Kang, S.H.; Hwang, E.Y.; Hwang, Y.-S.; Lee, M.H.; Han, D.-W.; Park, J.-C. Enhanced neural cell adhesion and neurite outgrowth on graphene-based biomimetic substrates. Biomed. Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Solanki, A.; Chueng, S.D.; Yin, P.T.; Kappera, R.; Chhowalla, M.; Lee, K. Axonal alignment and enhanced neuronal differentiation of neural stem cells on graphene-nanoparticle hybrid structures. Adv. Mater. 2013, 25, 5477–5482. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-Y.; Pang, D.-P.; Hwang, S.-M.; Tuan, H.-Y.; Hu, Y.-C. A graphene-based platform for induced pluripotent stem cells culture and differentiation. Biomaterials 2012, 33, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.C.; Lim, C.H.Y.X.; Shi, H.; Tang, L.A.L.; Wang, Y.; Lim, C.T.; Loh, K.P. Origin of enhanced stem cell growth and differentiation on graphene and graphene oxide. ACS Nano 2011, 5, 7334–7341. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Yin, P.T.; Uehara, T.M.; Chueng, S.D.; Yang, L.; Lee, K. Guiding stem cell differentiation into oligodendrocytes using graphene-nanofiber hybrid scaffolds. Adv. Mater. 2014, 26, 3673–3680. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-H.; Shah, S.; Yang, L.; Yin, P.T.; Hossain, M.K.; Conley, B.; Choi, J.-W.; Lee, K.-B. Controlling differentiation of adipose-derived stem cells using combinatorial graphene hybrid-pattern arrays. ACS Nano 2015, 9, 3780–3790. [Google Scholar] [CrossRef] [PubMed]
- Hamon, C.; Henriksen-Lacey, M.; La Porta, A.; Rosique, M.; Langer, J.; Scarabelli, L.; Montes, A.B.S.; González-Rubio, G.; de Pancorbo, M.M.; Liz-Marzán, L.M. Tunable nanoparticle and cell assembly using combined self-powered microfluidics and microcontact printing. Adv. Funct. Mater. 2016, 26, 8053–8061. [Google Scholar] [CrossRef]
- Yin, P.T.; Shah, S.; Chhowalla, M.; Lee, K.-B. Design, synthesis, and characterization of graphene-nanoparticle hybrid materials for bioapplications. Chem. Rev. 2015, 115, 2483–2531. [Google Scholar] [CrossRef] [PubMed]
- Vallee, R. Structure and phosphorylation of microtubule-associated protein 2 (MAP-2). Proc. Natl. Acad. Sci. USA 1980, 77, 3206–3210. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.V.W.; Jope, R.S. The role of microtubule-associated protein 2 (MAP-2) in neuronal growth, plasticity, and degeneration. J. Neurosci. Res. 1992, 33, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Bloom, G.S.; Vallee, R.B. Association of microtubule-associated protein 2 (MAP-2) with microtubules and intermediate filaments in cultured brain cells. J. Cell Biol. 1983, 96, 1523–1531. [Google Scholar] [CrossRef] [PubMed]
- Correas, I.; Padilla, R.; Avila, J. The tubulin-binding sequence of brain microtubule-associated proteins, tau and MAP-2, is also involved in actin binding. Biochem. J. 1990, 269, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yan, L.; Altman, M.; Lässle, M.; Nugent, H.; Frankel, F.; Lauffenburger, D.A.; Whitesides, G.M.; Rich, A. Biological surface engineering: A simple system for cell pattern formation. Biomaterials 1999, 20, 1213–1220. [Google Scholar] [CrossRef]
- Kim, D.-N.; Lee, W.; Koh, W.-G. Micropatterning of proteins on the surface of three-dimensional poly (ethylene glycol) hydrogel microstructures. Anal. Chim. Acta 2008, 609, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Guilak, F.; Cohen, D.M.; Estes, B.T.; Gimble, J.M.; Liedtke, W.; Chen, C.S. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell 2009, 5, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Sun, J.; Ding, J. Critical areas of cell adhesion on micropatterned surfaces. Biomaterials 2011, 32, 3931–3938. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Peng, R.; Ding, J. Cell-material interactions revealed via material techniques of surface patterning. Adv. Mater. 2013, 25, 5257–5286. [Google Scholar] [CrossRef] [PubMed]
- Wiedenmann, B.; Franke, W.W.; Kuhn, C.; Moll, R.; Gould, V.E. Synaptophysin: A marker protein for neuroendocrine cells and neoplasms. Proc. Natl. Acad. Sci. USA 1986, 83, 3500–3504. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, T.L.; Cameron, P.; De Camilli, P.; Banker, G. The distribution of synapsin I and synaptophysin in hippocampal neurons developing in culture. J. Neurosci. 1991, 11, 1617–1626. [Google Scholar] [PubMed]
- Thomas, L.; Hartung, K.; Langosch, D.; Rehm, H.; Bamberg, E. Identification of synaptophysin as a hexameric channel protein of the synaptic vesicle membrane. Science 1988, 242, 1050–1053. [Google Scholar] [CrossRef] [PubMed]
- Calakos, N.; Scheller, R.H. Vesicle-associated membrane protein and synaptophysin are associated on the synaptic vesicle. J. Biol. Chem. 1994, 269, 24534–24537. [Google Scholar] [PubMed]
- Gould, V.E.; Lee, I.; Wiedenmann, B.; Moll, R.; Chejfec, G.; Franke, W.W. Synaptophysin: A novel marker for neurons, certain neuroendocrine cells, and their neoplasms. Hum. Pathol. 1986, 17, 979–983. [Google Scholar] [CrossRef]
- Tarsa, L.; Goda, Y. Synaptophysin regulates activity-dependent synapse formation in cultured hippocampal neurons. Proc. Natl. Acad. Sci. USA 2002, 99, 1012–1016. [Google Scholar] [CrossRef] [PubMed]
- Thiel, G. Synapsin I, synapsin II, and synaptophysin: Marker proteins of synaptic vesicles. Brain Pathol. 1993, 3, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Frank, E.; Fischbach, G.D. Early events in neuromuscular junction formation in vitro: Induction of acetylcholine receptor clusters in the postsynaptic membrane and morphology of newly formed synapses. J. Cell Biol. 1979, 83, 143–158. [Google Scholar] [CrossRef] [PubMed]
- Aghajanian, G.K.; Bloom, F.E. The formation of synaptic junctions in developing rat brain: A quantitative electron microscopic study. Brain Res. 1967, 6, 716–727. [Google Scholar] [CrossRef]
- Fannon, A.M.; Colman, D.R. A model for central synaptic junctional complex formation based on the differential adhesive specificities of the cadherins. Neuron 1996, 17, 423–434. [Google Scholar] [CrossRef]
- Cotman, C.W.; Nieto-Sampedro, M.; Harris, E.W. Synapse replacement in the nervous system of adult vertebrates. Physiol. Rev. 1981, 61, 684–784. [Google Scholar] [PubMed]
- Fischbach, G.D. Synapse formation between dissociated nerve and muscle cells in low density cell cultures. Dev. Biol. 1972, 28, 407–429. [Google Scholar] [CrossRef]
- Serpinskaya, A.S.; Feng, G.; Sanes, J.R.; Craig, A.M. Synapse formation by hippocampal neurons from agrin-deficient mice. Dev. Biol. 1999, 205, 65–78. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Min, K.-J.; Kim, T.-H.; Choi, J.-W. Magnetic Force-Driven Graphene Patterns to Direct Synaptogenesis of Human Neuronal Cells. Materials 2017, 10, 1151. https://doi.org/10.3390/ma10101151
Min K-J, Kim T-H, Choi J-W. Magnetic Force-Driven Graphene Patterns to Direct Synaptogenesis of Human Neuronal Cells. Materials. 2017; 10(10):1151. https://doi.org/10.3390/ma10101151
Chicago/Turabian StyleMin, Kyung-Joon, Tae-Hyung Kim, and Jeong-Woo Choi. 2017. "Magnetic Force-Driven Graphene Patterns to Direct Synaptogenesis of Human Neuronal Cells" Materials 10, no. 10: 1151. https://doi.org/10.3390/ma10101151
APA StyleMin, K.-J., Kim, T.-H., & Choi, J.-W. (2017). Magnetic Force-Driven Graphene Patterns to Direct Synaptogenesis of Human Neuronal Cells. Materials, 10(10), 1151. https://doi.org/10.3390/ma10101151




