Nanofluids for Heat Transfer: Advances in Thermo-Physical Properties, Theoretical Insights, and Engineering Applications
Abstract
1. Introduction
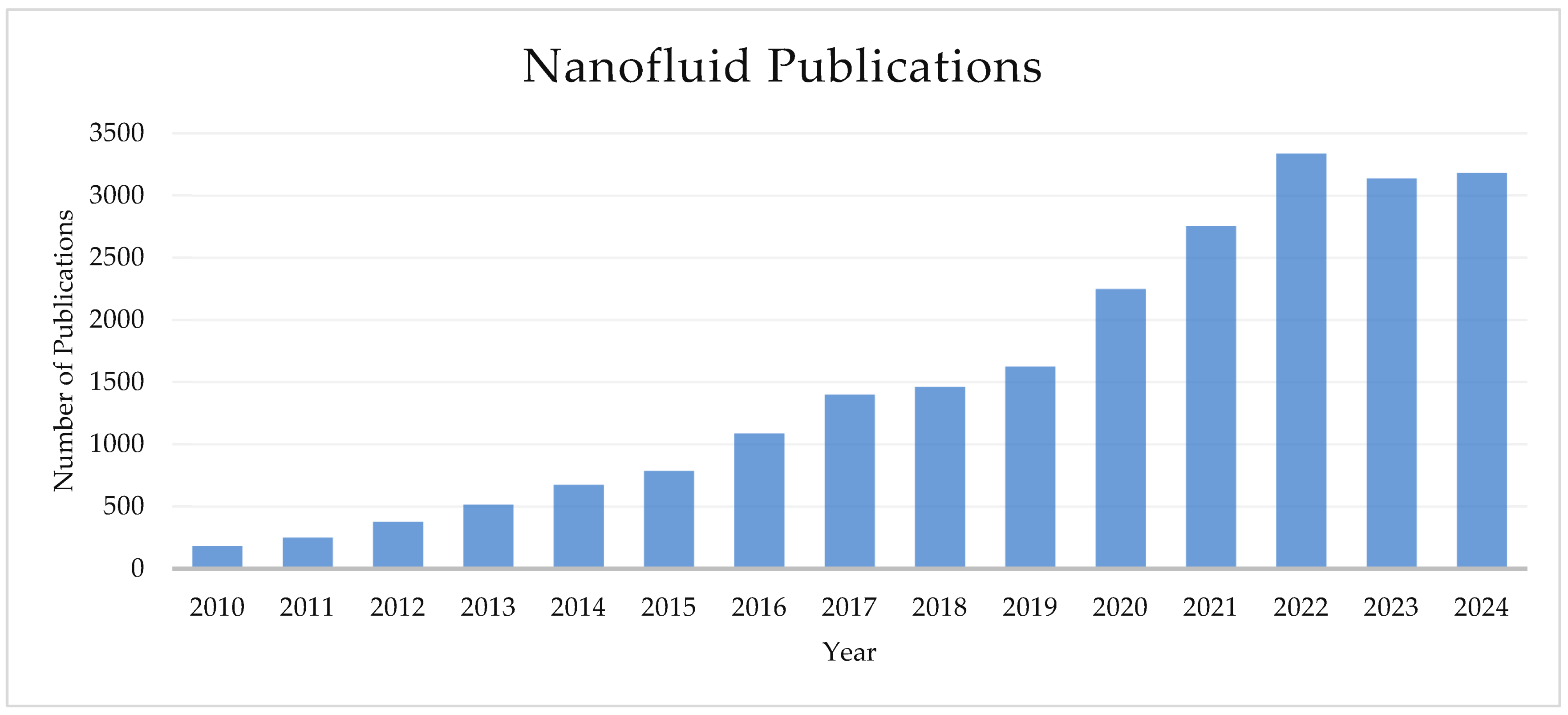
1.1. The Field of Nanofluids
1.2. Effects and Importance of Thermophysical Properties of Nanofluids
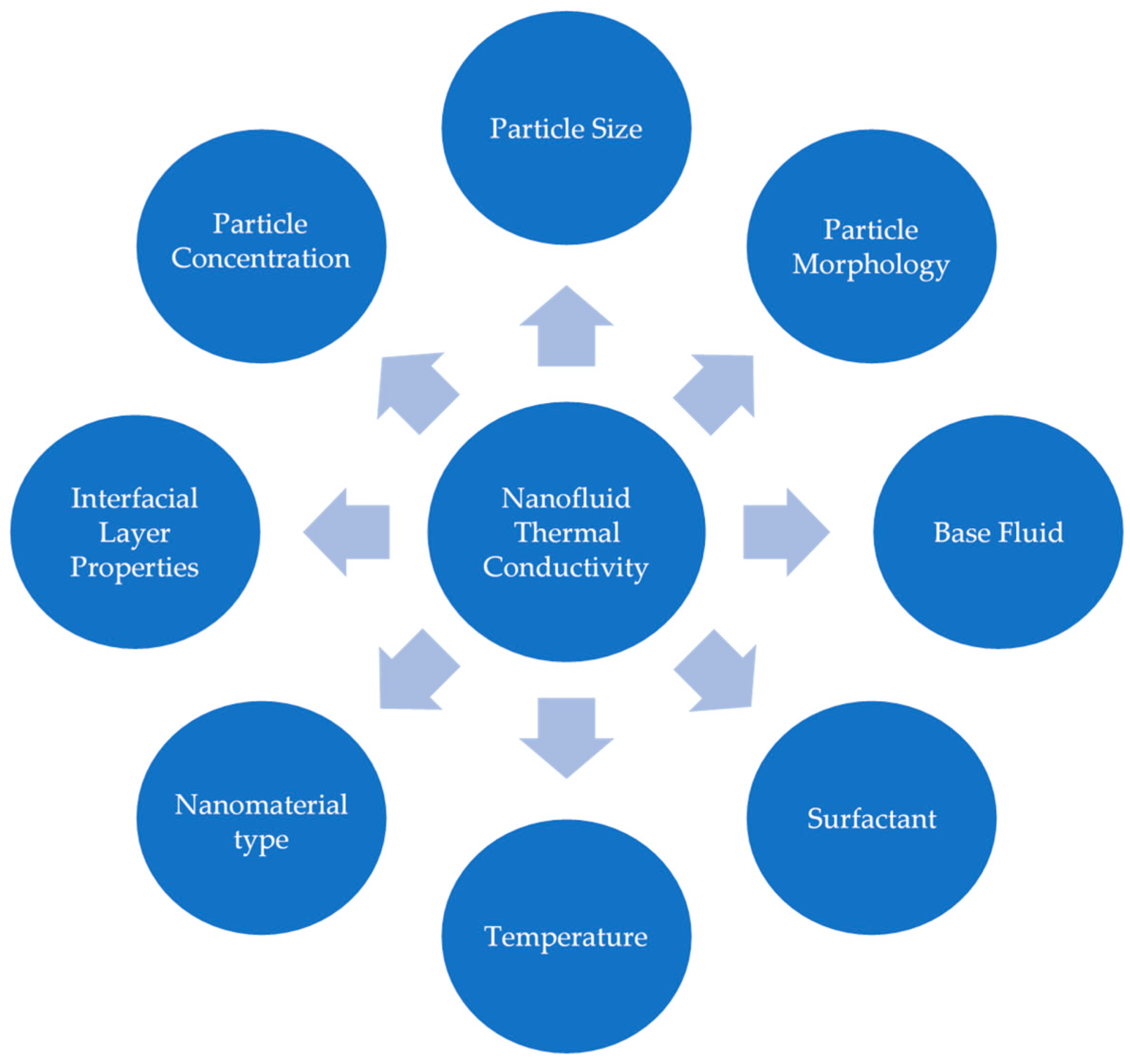
2. Thermal Conductivity
2.1. Introduction
2.2. Nanofluid’s Thermal Conductivity Models/Theories
2.2.1. Effective Medium Theory
2.2.2. Interfacial Layering Theory
2.2.3. Percolation Theory
2.2.4. Brownian Motion
2.2.5. Phonon Theory
2.3. Nanofluids’ Thermal Conductivity Theories/Models
2.4. Nanofluids’ Thermal Conductivity Measurements
3. Viscosity
3.1. Introduction
3.2. Nanofluid’s Viscosity Models/Theories
3.3. Viscosity Measurements
4. Specific Heat Capacity
4.1. Introduction
4.2. Specific Heat Capacity Measurements
5. Flash Point
5.1. Introduction
5.2. Flash Point Measurements
6. Comparative Studies of Nanofluids with the Theoretical Models
7. Effect of Surfactants on Thermal Properties of Nanofluids
7.1. Introduction
7.2. Recent Studies of Surfactants on Nanofluids
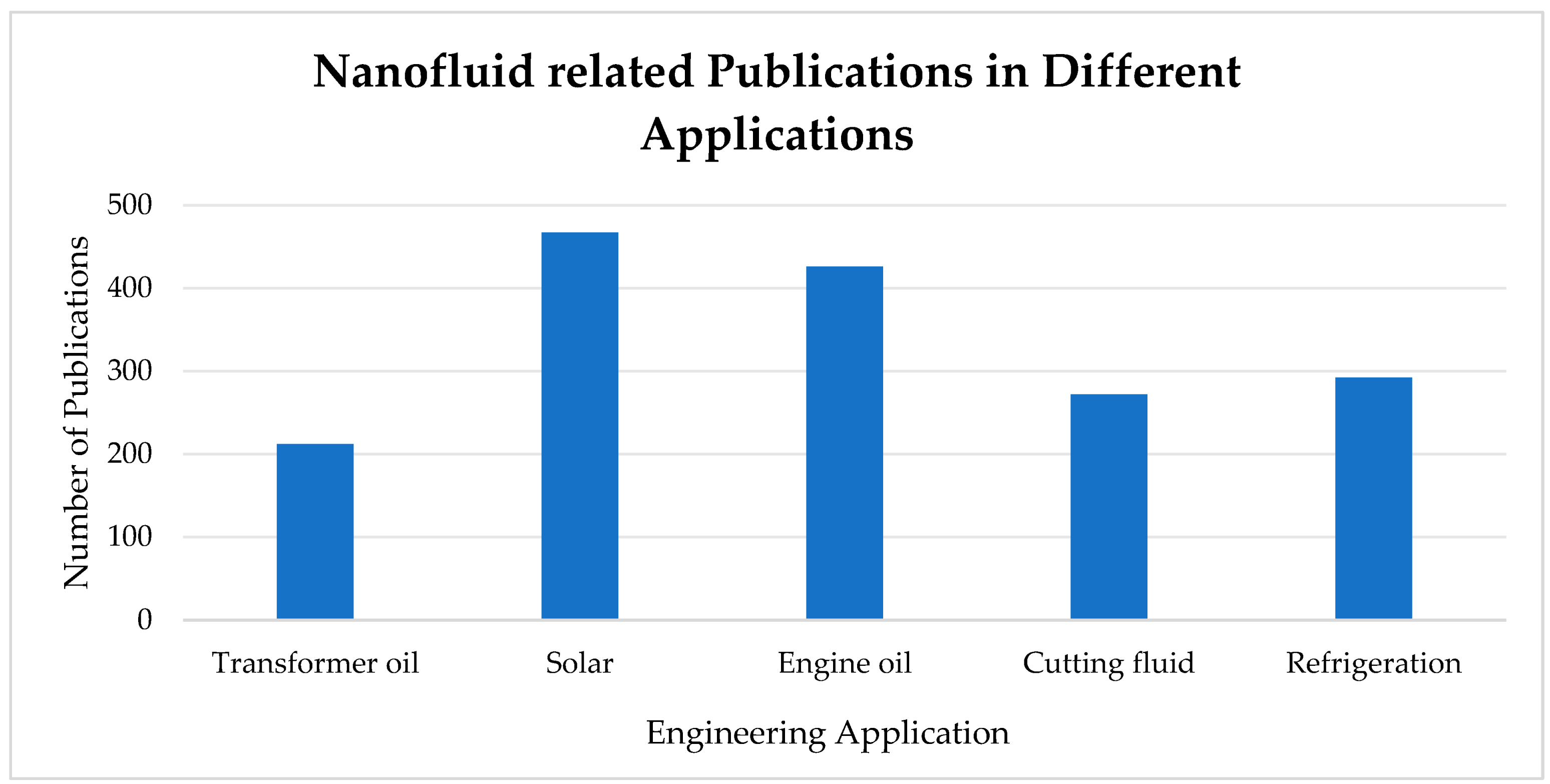
8. Heat Transfer Applications of Nanofluids
8.1. Transformer Oil-Based Nanofluids
8.2. Solar PV System Cooling and Energy Storage of Nanofluids
8.3. Engine Oil-Based Nanofluids
8.4. Radiator Cooling
8.5. Electronic Cooling
8.6. Machining Fluids
8.7. Refrigerator Systems
8.8. Industrial Manufacturing of Nanofluids
9. Molecular Dynamics of Nanofluids
10. Nanofluids and Artificial Intelligence
11. Discussion
- Many research articles have focused on the thermal properties of nanofluids, such as their thermal conductivity, viscosity, and specific heat capacity. However, there is lack of studies in the research articles focused on other properties, such as flash point, which is a crucial property in many applications. The number of theoretical studies is also not at a satisfactory level.
- Most of the theoretical models are based solely on the nanoparticle volume fraction. However, the extensions of these models have considered the effect of other important properties, such as interfacial layer and particle shape. However, there is lack of models which consider the effect of temperature, which has a significant effect of thermal properties of nanofluids.
- In the nanofluid theoretical models, there are several limitations, such as concentration and particle size and shape. It can be seen that not enough research has been conducted to investigate suitable nanofluid types with which to use these models without significant deviations.
- Many research studies have been conducted considering the applications of nanofluids; however, there has been a lack of financial analysis concerning the use of nanofluids in such applications.
- The use of ANNs in thermal property predicting can be identified as a positive trend in the recent past, especially due to their higher accuracy compared to theoretical models.
12. Conclusions
- The thermal conductivity, viscosity, and flash point of nanofluids increase with the nanoparticle concentration.
- A clear relationship between the specific heat capacity of nanofluids and the nanoparticle concentration or nanoparticle type was not observed. Both increments and decrements in the specific heat capacity of nanoparticles can be seen with the increase in nanoparticle concentration.
- The concentration and type of surfactants negatively and positively affected the increased thermal conductivity depending on the type of nanofluid.
- The applications of nanofluids have made a significant impact on several engineering and scientific disciplines in improving the performance of heat transfer applications.
- Molecular dynamics simulations demonstrated the need for a new field in the nanofluid research area in order to understand the nano-level impact of the nanoparticles in nanofluids.
- The collaboration of artificial intelligence can make a significant impact on nanofluid studies by providing a data-driven approach to predict important thermo-physical properties.
Funding
Conflicts of Interest
References
- Stephan, U.S.; Eastman, J.A. Enhancing Thermal Conductivity of Fluids with Nanoparticles; Argonne National Lab (ANL): Argonne, IL, USA, 1995. [Google Scholar]
- Choi, S.U.S. Nanofluids: A New Field of Scientific Research and Innovative Applications. Heat Transf. Eng. 2008, 29, 429–431. [Google Scholar] [CrossRef]
- Jama, M.; Singh, T.; Gamaleldin, S.M.; Koc, M.; Samara, A.; Isaifan, R.J.; Atieh, M.A. Critical Review on Nanofluids: Preparation, Characterization, and Applications. J. Nanomater. 2016, 2016, 6717624. [Google Scholar] [CrossRef]
- Kanti, P.; Sharma, K.V.; Ramachandra, C.G.; Panitapu, B. Stability and Thermophysical Properties of Fly Ash Nanofluid for Heat Transfer Applications. Heat Transfer 2020, 49, 4722–4737. [Google Scholar] [CrossRef]
- Shafiq, A.; Rasool, G.; Khalique, C.M.; Aslam, S. Second Grade Bioconvective Nanofluid Flow with Buoyancy Effect and Chemical Reaction. Symmetry 2020, 12, 621. [Google Scholar] [CrossRef]
- Qu, D.; Cheng, L.; Bao, Y.; Gao, Y.; Zheng, X.; Qin, G. Enhanced Optical Absorption and Solar Steam Generation of CB-ATO Hybrid Nanofluids. Renew. Energy 2022, 199, 509–516. [Google Scholar] [CrossRef]
- Amiri, A.; Shanbedi, M.; Ahmadi, G.; Rozali, S. Transformer Oils-Based Graphene Quantum Dots Nanofluid as a New Generation of Highly Conductive and Stable Coolant. Int. Commun. Heat Mass Transf. 2017, 83, 40–47. [Google Scholar] [CrossRef]
- Ben Said, L.; Kolsi, L.; Ghachem, K.; Almeshaal, M.; Maatki, C. Application of Nanofluids as Cutting Fluids in Machining Operations: A Brief Review. Appl Nanosci 2023, 13, 4247–4278. [Google Scholar] [CrossRef]
- Milanese, M.; Micali, F.; Colangelo, G.; De Risi, A. Experimental Evaluation of a Full-Scale HVAC System Working with Nanofluid. Energies 2022, 15, 2902. [Google Scholar] [CrossRef]
- Koçak Soylu, S.; Atmaca, İ.; Asiltürk, M.; Doğan, A. Improving Heat Transfer Performance of an Automobile Radiator Using Cu and Ag Doped TiO2 Based Nanofluids. Appl. Therm. Eng. 2019, 157, 113743. [Google Scholar] [CrossRef]
- Valdés, L.; Hernández, D.; De Ménorval, L.C.; Pérez, I.; Altshuler, E.; Fossum, J.O.; Rivera, A. Incorporation of Tramadol Drug into Li-Fluorohectorite Clay: A Preliminary Study of a Medical Nanofluid. Eur. Phys. J. Spec. Top. 2016, 225, 767–771. [Google Scholar] [CrossRef]
- Rashidi, M.M.; Nazari, M.A.; Mahariq, I.; Assad, M.E.H.; Ali, M.E.; Almuzaiqer, R.; Nuhait, A.; Murshid, N. 11- Thermophysical Properties of Hybrid Nanofluids and the Proposed Models: An Updated Comprehensive Study. Nanomaterials 2021, 11, 3084. [Google Scholar] [CrossRef] [PubMed]
- Xian, H.W.; Sidik, N.A.C.; Saidur, R. Impact of Different Surfactants and Ultrasonication Time on the Stability and Thermophysical Properties of Hybrid Nanofluids. Int. Commun. Heat Mass Transf. 2020, 110, 104389. [Google Scholar] [CrossRef]
- Khairul, M.A.; Shah, K.; Doroodchi, E.; Azizian, R.; Moghtaderi, B. Effects of Surfactant on Stability and Thermo-Physical Properties of Metal Oxide Nanofluids. Int. J. Heat Mass Transf. 2016, 98, 778–787. [Google Scholar] [CrossRef]
- Ali, N. Graphene-Based Nanofluids: Production Parameter Effects on Thermophysical Properties and Dispersion Stability. Nanomaterials 2022, 12, 357. [Google Scholar] [CrossRef]
- Gupta, S.K.; Sharma, A. A Brief Review of Nanofluids Utilization in Heat Transfer Devices for Energy Saving. Mater. Today Proc. 2023, S2214785323014116. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Dawood, H.I.; Onyeaka, H.N. A Review Paper on Properties and Applications of Nanofluids. IOP Conf. Ser. Earth Environ. Sci. 2023, 1232, 012009. [Google Scholar] [CrossRef]
- Majstorovic, D.; Zivkovic, E. Nanofluids: Why We Love Them? Hem Ind 2024, 78, 105–111. [Google Scholar] [CrossRef]
- Simpson, S.; Schelfhout, A.; Golden, C.; Vafaei, S. Nanofluid Thermal Conductivity and Effective Parameters. Appl. Sci. 2018, 9, 87. [Google Scholar] [CrossRef]
- Lenin, R.; Joy, P.A.; Bera, C. A Review of the Recent Progress on Thermal Conductivity of Nanofluid. J. Mol. Liq. 2021, 338, 116929. [Google Scholar] [CrossRef]
- Maxwell, J.C. A Treatise on Electricity and Magnetism. Vol. I. Macmillan and Co. Publishers to the University of Oxford: Oxford, UK, 1873. [Google Scholar]
- Bruggeman, D.A.G. Berechnung verschiedener physikalischer Konstanten von heterogenen Substanzen. I. Dielektrizitätskonstanten und Leitfähigkeiten der Mischkörper aus isotropen Substanzen. Ann. Der Phys. 1935, 416, 636–664. [Google Scholar] [CrossRef]
- Hamilton, R.L.; Crosser, O.K. Thermal Conductivity of Heterogeneous Two-Component Systems. Ind. Eng. Chem. Fund. 1962, 1, 187–191. [Google Scholar] [CrossRef]
- Yu, W.; Choi, S.U.S. The Role of Interfacial Layers in the Enhanced Thermal Conductivity of Nanofluids: A Renovated Maxwell Model. J. Nanopart. Res. 2003, 5, 167–171. [Google Scholar] [CrossRef]
- Wang, B.-X.; Zhou, L.-P.; Peng, X.-F. A Fractal Model for Predicting the Effective Thermal Conductivity of Liquid with Suspension of Nanoparticles. Int. J. Heat Mass Transf. 2003, 46, 2665–2672. [Google Scholar] [CrossRef]
- Avsec, J. The Combined Analysis of Phonon and Electron Heat Transfer Mechanism on Thermal Conductivity for Nanofluids. Int. J. Heat Mass Transf. 2008, 51, 4589–4598. [Google Scholar] [CrossRef]
- Ceylan, A.; Jastrzembski, K.; Shah, S.I. Enhanced Solubility Ag-Cu Nanoparticles and Their Thermal Transport Properties. Met. Mater. Trans. A 2006, 37, 2033–2038. [Google Scholar] [CrossRef]
- Xuan, Y.; Li, Q. Heat Transfer Enhancement of Nanofluids. Int. J. Heat Fluid Flow 2000, 21, 58–64. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, S.-H.; Choi, C.; Jang, S.; Choi, S. A Review of Thermal Conductivity Data, Mechanisms and Models for Nanofluids. Int. J. Micro-Nano Scale Transp. 2010, 1, 269–322. [Google Scholar] [CrossRef]
- Xuan, Y.; Li, Q.; Hu, W. Aggregation Structure and Thermal Conductivity of Nanofluids. AIChE J. 2003, 49, 1038–1043. [Google Scholar] [CrossRef]
- Pak, B.C.; Cho, Y.I. HYDRODYNAMIC AND HEAT TRANSFER STUDY OF DISPERSED FLUIDS WITH SUBMICRON METALLIC OXIDE PARTICLES. Exp. Heat Transf. 1998, 11, 151–170. [Google Scholar] [CrossRef]
- Lu, S.-Y.; Lin, H.-C. Effective Conductivity of Composites Containing Aligned Spheroidal Inclusions of Finite Conductivity. J. Appl. Phys. 1996, 79, 6761–6769. [Google Scholar] [CrossRef]
- Zadkhast, M.; Toghraie, D.; Karimipour, A. Developing a New Correlation to Estimate the Thermal Conductivity of MWCNT-CuO/Water Hybrid Nanofluid via an Experimental Investigation. J. Therm. Anal. Calorim. 2017, 129, 859–867. [Google Scholar] [CrossRef]
- Bakhtiari, R.; Kamkari, B.; Afrand, M.; Abdollahi, A. Preparation of Stable TiO2-Graphene/Water Hybrid Nanofluids and Development of a New Correlation for Thermal Conductivity. Powder Technol. 2021, 385, 466–477. [Google Scholar] [CrossRef]
- Esfahani, M.R.; Languri, E.M.; Nunna, M.R. Effect of Particle Size and Viscosity on Thermal Conductivity Enhancement of Graphene Oxide Nanofluid. Int. Commun. Heat Mass Transf. 2016, 76, 308–315. [Google Scholar] [CrossRef]
- Iacobazzi, F.; Milanese, M.; Colangelo, G.; Lomascolo, M.; De Risi, A. An Explanation of the Al2O3 Nanofluid Thermal Conductivity Based on the Phonon Theory of Liquid. Energy 2016, 116, 786–794. [Google Scholar] [CrossRef]
- Afrand, M.; Toghraie, D.; Sina, N. Experimental Study on Thermal Conductivity of Water-Based Fe3O4 Nanofluid: Development of a New Correlation and Modeled by Artificial Neural Network. Int. Commun. Heat Mass Transf. 2016, 75, 262–269. [Google Scholar] [CrossRef]
- Liu, W.I.; Malekahmadi, O.; Bagherzadeh, S.A.; Ghashang, M.; Karimipour, A.; Hasani, S.; Tlili, I.; Goodarzi, M. A Novel Comprehensive Experimental Study Concerned Graphene Oxide Nanoparticles Dispersed in Water: Synthesise, Characterisation, Thermal Conductivity Measurement and Present a New Approach of RLSF Neural Network. Int. Commun. Heat Mass Transf. 2019, 109, 104333. [Google Scholar] [CrossRef]
- Kanti, P.; Korada, V.S.; Ramachandra, C.G.; Sesha Talpa Sai, P.H.V. Experimental Study on Density and Thermal Conductivity Properties of Indian Coal Fly Ash Water-Based Nanofluid. Int. J. Ambient Energy 2022, 43, 2557–2562. [Google Scholar] [CrossRef]
- Kamel, M.S.; Al-Oran, O.; Lezsovits, F. Thermal Conductivity of Al2O3 and CeO2 Nanoparticles and Their Hybrid Based Water Nanofluids: An Experimental Study. Period. Polytech. Chem. Eng. 2020, 65, 50–60. [Google Scholar] [CrossRef]
- Ouikhalfan, M.; Labihi, A.; Belaqziz, M.; Chehouani, H.; Benhamou, B.; Sarı, A.; Belfkira, A. Stability and Thermal Conductivity Enhancement of Aqueous Nanofluid Based on Surfactant-Modified TiO2. J. Dispers. Sci. Technol. 2020, 41, 374–382. [Google Scholar] [CrossRef]
- Pavithra, K.S.; Fasiulla; Yashoda, M.P.; Prasannakumar, S. Synthesis, Characterisation and Thermal Conductivity of CuO—Water Based Nanofluids with Different Dispersants. Part. Sci. Technol. 2020, 38, 559–567. [Google Scholar] [CrossRef]
- Aberoumand, S.; Jafarimoghaddam, A. Experimental Study on Synthesis, Stability, Thermal Conductivity and Viscosity of Cu–Engine Oil Nanofluid. J. Taiwan Inst. Chem. Eng. 2017, 71, 315–322. [Google Scholar] [CrossRef]
- Ali, M.K.A.; Xianjun, H. Improving the Heat Transfer Capability and Thermal Stability of Vehicle Engine Oils Using Al2O3/TiO2 Nanomaterials. Powder Technol. 2020, 363, 48–58. [Google Scholar] [CrossRef]
- Sukkar, K.A.; Karamalluh, A.A.; Jaber, T.N. Rheological and Thermal Properties of Lubricating Oil Enhanced by the Effect of CuO and TiO2 Nano-Additives. Al-Khwarizmi Eng. J. 2019, 15, 24–33. [Google Scholar] [CrossRef]
- Yang, L.; Mao, M.; Huang, J.; Ji, W. Enhancing the Thermal Conductivity of SAE 50 Engine Oil by Adding Zinc Oxide Nano-Powder: An Experimental Study. Powder Technol. 2019, 356, 335–341. [Google Scholar] [CrossRef]
- Kamel, B.M.; El-Kashif, E.; Hoziefa, W.; Shiba, M.S.; Elshalakany, A.B. The Effect of MWCNTs/GNs Hybrid Addition on the Tribological and Rheological Properties of Lubricating Engine Oil. J. Dispers. Sci. Technol. 2021, 42, 1811–1819. [Google Scholar] [CrossRef]
- Tian, X.-X.; Kalbasi, R.; Qi, C.; Karimipour, A.; Huang, H.-L. Efficacy of Hybrid Nano-Powder Presence on the Thermal Conductivity of the Engine Oil: An Experimental Study. Powder Technol. 2020, 369, 261–269. [Google Scholar] [CrossRef]
- Alqahtani, B.; Hoziefa, W.; Abdel Moneam, H.M.; Hamoud, M.; Salunkhe, S.; Elshalakany, A.B.; Abdel-Mottaleb, M.; Davim, J.P. Tribological Performance and Rheological Properties of Engine Oil with Graphene Nano-Additives. Lubricants 2022, 10, 137. [Google Scholar] [CrossRef]
- Soltani, F.; Toghraie, D.; Karimipour, A. Experimental Measurements of Thermal Conductivity of Engine Oil-Based Hybrid and Mono Nanofluids with Tungsten Oxide (WO3) and MWCNTs Inclusions. Powder Technol. 2020, 371, 37–44. [Google Scholar] [CrossRef]
- Aberoumand, S.; Jafarimoghaddam, A. Tungsten (III) Oxide (WO3)—Silver/Transformer Oil Hybrid Nanofluid: Preparation, Stability, Thermal Conductivity and Dielectric Strength. Alex. Eng. J. 2018, 57, 169–174. [Google Scholar] [CrossRef]
- Qing, S.H.; Rashmi, W.; Khalid, M.; Gupta, T.C.S.M.; Nabipoor, M.; Hajibeigy, M.T. Thermal Conductivity and Electrical Properties of Hybrid SiO2-Graphene Naphthenic Mineral Oil Nanofluid as Potential Transformer Oil. Mater. Res. Express 2017, 4, 015504. [Google Scholar] [CrossRef]
- Bhunia, M.M.; Panigrahi, K.; Das, S.; Chattopadhyay, K.K.; Chattopadhyay, P. Amorphous Graphene—Transformer Oil Nanofluids with Superior Thermal and Insulating Properties. Carbon 2018, 139, 1010–1019. [Google Scholar] [CrossRef]
- Koutras, K.N.; Peppas, G.D.; Fetsis, T.T.; Tegopoulos, S.N.; Charalampakos, V.P.; Kyritsis, A.; Yiotis, A.G.; Gonos, I.F.; Pyrgioti, E.C. Dielectric and Thermal Response of TiO2 and SiC Natural Ester Based Nanofluids for Use in Power Transformers. IEEE Access 2022, 10, 79222–79236. [Google Scholar] [CrossRef]
- Yao, W.; Huang, Z.; Li, J.; Wu, L.; Xiang, C. Enhanced Electrical Insulation and Heat Transfer Performance of Vegetable Oil Based Nanofluids. J. Nanomater. 2018, 2018, 4504208. [Google Scholar] [CrossRef]
- Farade, R.A.; Abdul Wahab, N.I.; Mansour, D.-E.A.; Azis, N.B.; Jasni, J.B.; Soudagar, M.E.M.; Siddappa, V. Development of Graphene Oxide-Based Nonedible Cottonseed Nanofluids for Power Transformers. Materials 2020, 13, 2569. [Google Scholar] [CrossRef]
- Van Trinh, P.; Anh, N.N.; Hong, N.T.; Hong, P.N.; Minh, P.N.; Thang, B.H. Experimental Study on the Thermal Conductivity of Ethylene Glycol-Based Nanofluid Containing Gr-CNT Hybrid Material. J. Mol. Liq. 2018, 269, 344–353. [Google Scholar] [CrossRef]
- Bagherzadeh, S.A.; D’Orazio, A.; Karimipour, A.; Goodarzi, M.; Bach, Q.-V. A Novel Sensitivity Analysis Model of EANN for F-MWCNTs–Fe3O4/EG Nanofluid Thermal Conductivity: Outputs Predicted Analytically Instead of Numerically to More Accuracy and Less Costs. Phys. A: Stat. Mech. Its Appl. 2019, 521, 406–415. [Google Scholar] [CrossRef]
- Li, Z.; Asadi, S.; Karimipour, A.; Abdollahi, A.; Tlili, I. Experimental Study of Temperature and Mass Fraction Effects on Thermal Conductivity and Dynamic Viscosity of SiO2-Oleic Acid/Liquid Paraffin Nanofluid. Int. Commun. Heat Mass Transf. 2020, 110, 104436. [Google Scholar] [CrossRef]
- Zheng, Y.; Shahsavar, A.; Afrand, M. Sonication Time Efficacy on Fe3O4-Liquid Paraffin Magnetic Nanofluid Thermal Conductivity: An Experimental Evaluation. Ultrason. Sonochem. 2020, 64, 105004. [Google Scholar] [CrossRef] [PubMed]
- Hameed, A.; Mukhtar, A.; Shafiq, U.; Qizilbash, M.; Khan, M.S.; Rashid, T.; Bavoh, C.B.; Rehman, W.U.; Guardo, A. Experimental Investigation on Synthesis, Characterization, Stability, Thermo-Physical Properties and Rheological Behavior of MWCNTs-Kapok Seed Oil Based Nanofluid. J. Mol. Liq. 2019, 277, 812–824. [Google Scholar] [CrossRef]
- Aslfattahi, N.; Samylingam, L.; Abdelrazik, A.S.; Arifutzzaman, A.; Saidur, R. MXene Based New Class of Silicone Oil Nanofluids for the Performance Improvement of Concentrated Photovoltaic Thermal Collector. Sol. Energy Mater. Sol. Cells 2020, 211, 110526. [Google Scholar] [CrossRef]
- Rasheed, H.S. Improvement in Lubricating Properties of Engine Oil by Blending with Palm Oil, Trimethylolpropane Ester and Nanoparticles. Ph.D. Thesis, Universiti Putra Malaysia, Serdang, Malaysia, 2019. [Google Scholar]
- Einstein, A. Investigations on the Theory of the Brownian Movement; Dover Publications: Mineola, NY, USA, 1956. [Google Scholar]
- Brinkman, H.C. The Viscosity of Concentrated Suspensions and Solutions. J. Chem. Phys. 1952, 20, 571. [Google Scholar] [CrossRef]
- Krieger, I.M.; Dougherty, T.J. A Mechanism for Non-Newtonian Flow in Suspensions of Rigid Spheres. Trans. Soc. Rheol. 1959, 3, 137–152. [Google Scholar] [CrossRef]
- Batchelor, G.K. The Effect of Brownian Motion on the Bulk Stress in a Suspension of Spherical Particles. J. Fluid Mech. 1977, 83, 97–117. [Google Scholar] [CrossRef]
- Lundgren, T.S. Slow Flow through Stationary Random Beds and Suspensions of Spheres. J. Fluid Mech. 1972, 51, 273–299. [Google Scholar] [CrossRef]
- Michael Joseph Stalin, P.; Arjunan, T.V.; Matheswaran, M.M.; Manoj Kumar, P.; Sadanandam, N. Investigations on Thermal Properties of CeO2/Water Nanofluids for Heat Transfer Applications. Mater. Today Proc. 2021, 47, 6815–6820. [Google Scholar] [CrossRef]
- Hu, Y.; He, Y.; Zhang, Z.; Wen, D. Enhanced Heat Capacity of Binary Nitrate Eutectic Salt-Silica Nanofluid for Solar Energy Storage. Sol. Energy Mater. Sol. Cells 2019, 192, 94–102. [Google Scholar] [CrossRef]
- Kanti, P.; Sharma, K.V.; Ramachandra, C.G.; Azmi, W.H. Experimental Determination of Thermophysical Properties of Indonesian Fly-Ash Nanofluid for Heat Transfer Applications. Part. Sci. Technol. 2021, 39, 597–606. [Google Scholar] [CrossRef]
- Afzal, A.; Khan, S.A.; Ahamed Saleel, C. Role of Ultrasonication Duration and Surfactant on Characteristics of ZnO and CuO Nanofluids. Mater. Res. Express 2019, 6, 1150d8. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Esmaeilzadeh, F.; Wang, X.P. A Detailed Investigation on the Thermo-Physical and Rheological Behavior of MgO/TiO2 Aqueous Dual Hybrid Nanofluid. J. Mol. Liq. 2019, 282, 323–339. [Google Scholar] [CrossRef]
- Almanassra, I.W.; Manasrah, A.D.; Al-Mubaiyedh, U.A.; Al-Ansari, T.; Malaibari, Z.O.; Atieh, M.A. An Experimental Study on Stability and Thermal Conductivity of Water/CNTs Nanofluids Using Different Surfactants: A Comparison Study. J. Mol. Liq. 2020, 304, 111025. [Google Scholar] [CrossRef]
- Wole-Osho, I.; Okonkwo, E.C.; Kavaz, D.; Abbasoglu, S. An Experimental Investigation into the Effect of Particle Mixture Ratio on Specific Heat Capacity and Dynamic Viscosity of Al2O3-ZnO Hybrid Nanofluids. Powder Technol. 2020, 363, 699–716. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Pandya, N.S.; Shah, H.; Said, Z. Experimental Comparison of Specific Heat Capacity of Three Different Metal Oxides with MWCNT/Water-Based Hybrid Nanofluids: Proposing a New Correlation. Appl. Nanosci. 2023, 13, 189–199. [Google Scholar] [CrossRef]
- Gao, Y.; Xi, Y.; Zhenzhong, Y.; Sasmito, A.; Mujumdar, A.; Wang, L. Experimental Investigation of Specific Heat of Aqueous Graphene Oxide Al2O3 Hybrid Nanofluid. Therm. Sci. 2021, 25, 515–525. [Google Scholar] [CrossRef]
- Khalil, W.; Mohamed, A.; Bayoumi, M.; Osman, T.A. Thermal and Rheological Properties of Industrial Mineral Gear Oil and Paraffinic Oil/CNTs Nanolubricants. Iran. J. Sci. Technol. Trans. Mech. Eng. 2018, 42, 355–361. [Google Scholar] [CrossRef]
- ASTM D93-20; Test Methods for Flash Point by Pensky-Martens Closed Cup Tester. ASTM International: West Conshohocken, PA, USA, 2020. [CrossRef]
- Viswanathan, P.K.; Chandrasekar, S. Influence of Carbon Quantum Dots Modified Silica Nanoparticles on Insulation Properties of Mineral Oil. In Proceedings of the 2019 International Conference on High Voltage Engineering and Technology (ICHVET), Hyderabad, India, 7–8 February 2019; pp. 1–5. [Google Scholar]
- Ma, J.; Shahsavar, A.; Al-Rashed, A.A.A.A.; Karimipour, A.; Yarmand, H.; Rostami, S. Viscosity, Cloud Point, Freezing Point and Flash Point of Zinc Oxide/SAE50 Nanolubricant. J. Mol. Liq. 2020, 298, 112045. [Google Scholar] [CrossRef]
- Chandrasekar, S.; Kasi Viswanathan, P.; Uthirakumar, P.; Montanari, G.C. Investigations on Novel Carbon Quantum Dots Covered Nanofluid Insulation for Medium Voltage Applications. J. Electr. Eng. Technol. 2020, 15, 269–278. [Google Scholar] [CrossRef]
- Abid, M.A.; Khan, I.; Ullah, Z.; Ullah, K.; Haider, A.; Ali, S.M. Dielectric and Thermal Performance Up-Gradation of Transformer Oil Using Valuable Nano-Particles. IEEE Access 2019, 7, 153509–153518. [Google Scholar] [CrossRef]
- Baruah, N.; Maharana, M.; Nayak, S.K. Performance Analysis of Vegetable Oil-based Nanofluids Used in Transformers. IET Sci. Meas. Technol. 2019, 13, 995–1002. [Google Scholar] [CrossRef]
- Sumathi, S.; Rajesh, R. Improvement on the Characteristics of Transformer Oil Using Nanofluids. Curr. Sci. 2020, 118, 29–33. [Google Scholar]
- Pourpasha, H.; Zeinali Heris, S.; Mahian, O.; Wongwises, S. The Effect of Multi-Wall Carbon Nanotubes/Turbine Meter Oil Nanofluid Concentration on the Thermophysical Properties of Lubricants. Powder Technol. 2020, 367, 133–142. [Google Scholar] [CrossRef]
- Ranjbarzadeh, R.; Chaabane, R. Experimental Study of Thermal Properties and Dynamic Viscosity of Graphene Oxide/Oil Nano-Lubricant. Energies 2021, 14, 2886. [Google Scholar] [CrossRef]
- Suresh Kumar, V.P.; Subramanian, K.M.; Stalin, B.; Vairamuthu, J. Influence of ZnO Nanoparticles on Thermophysical and Tribological Properties of Polyolester Oil. Mater. Res. Express 2021, 8, 045502. [Google Scholar] [CrossRef]
- Desai, N.; Nagaraj, A.M.; Sabnis, N. Analysis of Thermo-Physical Properties of SAE20W40 Engine Oil by the Addition of SiO2 Nanoparticles. Mater. Today Proc. 2021, 47, 5646–5651. [Google Scholar] [CrossRef]
- Awad, A.; Sukkar, K.; Jaed, D. Deep Understanding of the Mechanism and Thermophysical Properties of Prepared Nanofluids Lube Oil Stock-60 with Al2O3 NPs. J. Am. Soc. Nephrol. 2022, 2, 37–51. [Google Scholar] [CrossRef]
- Ghislain, M.M.; Jean-Bernard, A.; Adolphe, M.I. Effect of FeO3 Nanoparticles on the Thermodynamic and Physico-Chemical Properties of Nanofluid Based on Kernel Palm Oil Methyl Ester (KPOME). Fuel Commun. 2022, 12, 100076. [Google Scholar] [CrossRef]
- Okonkwo, E.C.; Wole-Osho, I.; Kavaz, D.; Abid, M. Comparison of Experimental and Theoretical Methods of Obtaining the Thermal Properties of Alumina/Iron Mono and Hybrid Nanofluids. J. Mol. Liq. 2019, 292, 111377. [Google Scholar] [CrossRef]
- Esfahani, J.A.; Safaei, M.R.; Goharimanesh, M.; De Oliveira, L.R.; Goodarzi, M.; Shamshirband, S.; Filho, E.P.B. Comparison of Experimental Data, Modelling and Non-Linear Regression on Transport Properties of Mineral Oil Based Nanofluids. Powder Technol. 2017, 317, 458–470. [Google Scholar] [CrossRef]
- Sonawane, S.S.; Khedkar, R.S.; Wasewar, K.L. Effect of Sonication Time on Enhancement of Effective Thermal Conductivity of Nano TiO2–Water, Ethylene Glycol, and Paraffin Oil Nanofluids and Models Comparisons. J. Exp. Nanosci. 2015, 10, 310–322. [Google Scholar] [CrossRef]
- Bovesecchi, G.; Corasaniti, S.; Costanza, G.; Piccotti, F.; Potenza, M.; Tata, M.E. Heat Conduction and Microconvection in Nanofluids: Comparison between Theoretical Models and Experimental Results. Aerospace 2022, 9, 608. [Google Scholar] [CrossRef]
- Xia, G.; Jiang, H.; Liu, R.; Zhai, Y. Effects of Surfactant on the Stability and Thermal Conductivity of Al2O3/de-Ionized Water Nanofluids. Int. J. Therm. Sci. 2014, 84, 118–124. [Google Scholar] [CrossRef]
- Huminic, A.; Huminic, G.; Fleaca, C.; Dumitrache, F.; Morjan, I. Thermal Conductivity, Viscosity and Surface Tension of Nanofluids Based on FeC Nanoparticles. Powder Technol. 2015, 284, 78–84. [Google Scholar] [CrossRef]
- Das, P.K.; Islam, N.; Santra, A.K.; Ganguly, R. Experimental Investigation of Thermophysical Properties of Al2O3–Water Nanofluid: Role of Surfactants. J. Mol. Liq. 2017, 237, 304–312. [Google Scholar] [CrossRef]
- Das, P.K.; Mallik, A.K.; Ganguly, R.; Santra, A.K. Synthesis and Characterization of TiO2–Water Nanofluids with Different Surfactants. Int. Commun. Heat Mass Transf. 2016, 75, 341–348. [Google Scholar] [CrossRef]
- Murshed, S.M.S.; Leong, K.C.; Yang, C. Enhanced Thermal Conductivity of TiO2—Water Based Nanofluids. Int. J. Therm. Sci. 2005, 44, 367–373. [Google Scholar] [CrossRef]
- Seyhan, M.; Altan, C.L.; Gurten, B.; Bucak, S. The Effect of Functionalized Silver Nanoparticles over the Thermal Conductivity of Base Fluids. AIP Adv. 2017, 7, 045101. [Google Scholar] [CrossRef]
- Colangelo, G.; Favale, E.; Miglietta, P.; Milanese, M.; De Risi, A. Thermal Conductivity, Viscosity and Stability of Al2O3 -Diathermic Oil Nanofluids for Solar Energy Systems. Energy 2016, 95, 124–136. [Google Scholar] [CrossRef]
- Asadi, A.; Asadi, M.; Siahmargoi, M.; Asadi, T.; Gholami Andarati, M. The Effect of Surfactant and Sonication Time on the Stability and Thermal Conductivity of Water-Based Nanofluid Containing Mg(OH)2 Nanoparticles: An Experimental Investigation. Int. J. Heat Mass Transf. 2017, 108, 191–198. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, D.; Yang, S. Investigation of pH and SDBS on Enhancement of Thermal Conductivity in Nanofluids. Chem. Phys. Lett. 2009, 470, 107–111. [Google Scholar] [CrossRef]
- Birleanu, C.; Pustan, M.; Cioaza, M.; Molea, A.; Popa, F.; Contiu, G. Effect of TiO2 Nanoparticles on the Tribological Properties of Lubricating Oil: An Experimental Investigation. Sci. Rep. 2022, 12, 5201. [Google Scholar] [CrossRef]
- Abdullah, M.I.H.C.; Abdollah, M.F.B.; Amiruddin, H.; Nuri, N.R.M.; Tamaldin, N.; Hassan, M.; Rafeq, S.A. Effect of hBNAl2O3 Nanoparticles on Engine Oil Properties. Energy Educ. Sci. Technol. Part A Energy Sci. Res. 2014, 32, 3261–3268. [Google Scholar]
- Kaplan, I.R.; Rasco, J.; Lu, S.-T. Chemical Characterization of Transformer Mineral-Insulating Oils. Environ. Forensics 2010, 11, 117–145. [Google Scholar] [CrossRef]
- Bhunia, M.M.; Panigrahi, K.; Naskar, C.B.; Bhattacharjee, S.; Chattopadhyay, K.K.; Chattopadhyay, P. 2D Square Nanosheets of Anatase TiO2: A Surfactant Free Nanofiller for Transformer Oil Nanofluids. J. Mol. Liq. 2021, 325, 115000. [Google Scholar] [CrossRef]
- Lv, Y.; Du, Q.; Wang, L.; Sun, Q.; Huang, M.; Li, C.; Qi, B. Effect of TiO2 Nanoparticles on the Ion Mobilities in Transformer Oil-Based Nanofluid. AIP Adv. 2017, 7, 105022. [Google Scholar] [CrossRef]
- Induranga, A.; Galpaya, C.; Vithanage, V.; Koswattage, K.R. Thermal Properties of TiO2 Nanoparticle-Treated Transformer Oil and Coconut Oil. Energies 2023, 17, 49. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, S.; Lin, Y.; Feng, M.; Wu, Q. Stability, Thermal Conductivity, and Rheological Properties of Controlled Reduced Graphene Oxide Dispersed Nanofluids. Appl. Therm. Eng. 2017, 119, 132–139. [Google Scholar] [CrossRef]
- Wanatasanappan, V.V.; Rezman, M.; Abdullah, M.Z. Thermophysical Properties of Vegetable Oil-Based Hybrid Nanofluids Containing Al2O3-TiO2 Nanoparticles as Insulation Oil for Power Transformers. Nanomaterials 2022, 12, 3621. [Google Scholar] [CrossRef]
- Hemmat Esfe, M.; Kamyab, M.H.; Valadkhani, M. Application of Nanofluids and Fluids in Photovoltaic Thermal System: An Updated Review. Sol. Energy 2020, 199, 796–818. [Google Scholar] [CrossRef]
- Wole-osho, I.; Okonkwo, E.C.; Abbasoglu, S.; Kavaz, D. Nanofluids in Solar Thermal Collectors: Review and Limitations. Int. J. Thermophy. 2020, 41, 157. [Google Scholar] [CrossRef]
- Manigandan, S.; Kumar, V. Comparative Study to Use Nanofluid ZnO and CuO with Phase Change Material in Photovoltaic Thermal System. Int. J. Energy Res. 2019, 43, 1882–1891. [Google Scholar] [CrossRef]
- Ebaid, M.S.Y.; Ghrair, A.M.; Al-Busoul, M. Experimental Investigation of Cooling Photovoltaic (PV) Panels Using (TiO2) Nanofluid in Water-Polyethylene Glycol Mixture and (Al2O3) Nanofluid in Water- Cetyltrimethylammonium Bromide Mixture. Energy Convers. Manag. 2018, 155, 324–343. [Google Scholar] [CrossRef]
- Tong, Y.; Boldoo, T.; Ham, J.; Cho, H. Improvement of Photo-Thermal Energy Conversion Performance of MWCNT/Fe3O4 Hybrid Nanofluid Compared to Fe3O4 Nanofluid. Energy 2020, 196, 117086. [Google Scholar] [CrossRef]
- Al-Waeli, A.H.A.; Kazem, H.A.; Chaichan, M.T.; Sopian, K. Experimental Investigation of Using Nano-PCM/Nanofluid on a Photovoltaic Thermal System (PVT): Technical and Economic Study. Therm. Sci. Eng. Prog. 2019, 11, 213–230. [Google Scholar] [CrossRef]
- Fudholi, A.; Razali, N.F.M.; Yazdi, M.H.; Ibrahim, A.; Ruslan, M.H.; Othman, M.Y.; Sopian, K. TiO2/Water-Based Photovoltaic Thermal (PVT) Collector: Novel Theoretical Approach. Energy 2019, 183, 305–314. [Google Scholar] [CrossRef]
- Hooshmandzade, N.; Motevali, A.; Reza Mousavi Seyedi, S.; Biparva, P. Influence of Single and Hybrid Water-Based Nanofluids on Performance of Microgrid Photovoltaic/Thermal System. Appl. Energy 2021, 304, 117769. [Google Scholar] [CrossRef]
- Karaaslan, I.; Menlik, T. Numerical Study of a Photovoltaic Thermal (PV/T) System Using Mono and Hybrid Nanofluid. Sol. Energy 2021, 224, 1260–1270. [Google Scholar] [CrossRef]
- Xiao, Y.; Bao, Y.; Yu, L.; Zheng, X.; Qin, G.; Chen, M.; He, M. Ultra-Stable Carbon Quantum Dot Nanofluids as Excellent Spectral Beam Splitters in PV/T Applications. Energy 2023, 273, 127159. [Google Scholar] [CrossRef]
- Dmour, A.; Xiao, Y.; Tian, W.; Qin, G.; Zheng, X. CQD-ATO Hybrid Nanofluid with Good Stability in the Application of Spectral Beam Splitters. Sol. Energy Mater. Sol. Cells 2023, 261, 112536. [Google Scholar] [CrossRef]
- Bao, Y.; Huang, A.; Zheng, X.; Qin, G. Enhanced Photothermal Conversion Performance of MWCNT/SiC Hybrid Aqueous Nanofluids in Direct Absorption Solar Collectors. J. Mol. Liq. 2023, 387, 122577. [Google Scholar] [CrossRef]
- Bai, X.; Lam, S.H.; Hu, J.; Chui, K.K.; Zhu, X.-M.; Shao, L.; Chow, T.H.; Wang, J. Colloidal Plasmonic TiN Nanoparticles for Efficient Solar Seawater Desalination. ACS Appl. Mater. Interfaces 2023, 15, 55856–55869. [Google Scholar] [CrossRef]
- Sanchez, A.E.; Goel, N.; Otanicar, T. Novel Hybrid Solar Nanophotonic Distillation Membrane with Photovoltaic Module for Co-Production of Electricity and Water. Appl. Energy 2022, 305, 117944. [Google Scholar] [CrossRef]
- Kuzmenkov, D.M.; Struchalin, P.G.; Olkhovskii, A.V.; Yunin, V.S.; Kutsenko, K.V.; Balakin, B.V. Solar-Driven Desalination Using Nanoparticles. Energies 2021, 14, 5743. [Google Scholar] [CrossRef]
- Rahman, S.; Said, Z.; Issa, S.; Haj Assad, M.E.; Sharma, P.; Hachicha, A.A. Performance Evaluation of Evacuated Tube Solar Collector Using Al2O3/Water Nanofluid: Experiment, Modelling, Life Cycle and Cost Analysis in the UAE Context. Sustain. Energy Technol. Assess. 2025, 76, 104261. [Google Scholar] [CrossRef]
- Amar, M.; Akram, N.; Chaudhary, G.Q.; Kazi, S.N.; Soudagar, M.E.M.; Mubarak, N.M.; Kalam, M.A. Energy, Exergy and Economic (3E) Analysis of Flat-Plate Solar Collector Using Novel Environmental Friendly Nanofluid. Sci. Rep. 2023, 13, 411. [Google Scholar] [CrossRef] [PubMed]
- Galpaya, C.; Induranga, A.; Vithanage, V.; Mantilaka, P.; Koswattage, K.R. Comparative Study on the Thermal Properties of Engine Oils and Their Nanofluids Incorporating Fullerene-C60, TiO2 and Fe2O3 at Different Temperatures. Energies 2024, 17, 732. [Google Scholar] [CrossRef]
- Ukueje, W.E.; Abam, F.I.; Obi, A. A Perspective Review on Thermal Conductivity of Hybrid Nanofluids and Their Application in Automobile Radiator Cooling. J. Nanotechnol. 2022, 2022, 2187932. [Google Scholar] [CrossRef]
- Jadeja, K.M.; Bumataria, R.; Chavda, N. Nanofluid as a Coolant in Internal Combustion Engine—A Review. Int. J. Ambient Energy 2023, 44, 363–380. [Google Scholar] [CrossRef]
- Bhattad, A.; Atgur, V.; Rao, B.; Banapurmath, N.; Yunus Khan, T.; Vadlamudi, C.; Krishnappa, S.; Sajjan, A.; Shankara, R.; Ayachit, N. Review on Mono and Hybrid Nanofluids: Preparation, Properties, Investigation, and Applications in IC Engines and Heat Transfer. Energies 2023, 16, 3189. [Google Scholar] [CrossRef]
- Aditi; Farooque, Z.; Chauhan, N.R. Comparative Study of Nano-Fluids as Coolants in a Car Radiator. IOP Conf. Ser. Mater. Sci. Eng. 2022, 1228, 012011. [Google Scholar] [CrossRef]
- Tetik, T.; Karagoz, Y. Enhancing Radiator Cooling Capacity: A Comparative Study of Nanofluids and Water/EG Mixtures. Heliyon 2024, 10, e38352. [Google Scholar] [CrossRef]
- Hassaan, A.M. An Experimental Investigation Examining the Usage of a Hybrid Nanofluid in an Automobile Radiator. Sci. Rep. 2024, 14, 27597. [Google Scholar] [CrossRef]
- Yaw, C.T.; Koh, S.P.; Sandhya, M.; Kadirgama, K.; Tiong, S.K.; Ramasamy, D.; Sudhakar, K.; Samykano, M.; Benedict, F.; Tan, C.H. Heat Transfer Enhancement by Hybrid Nano Additives—Graphene Nanoplatelets/Cellulose Nanocrystal for the Automobile Cooling System (Radiator). Nanomaterials 2023, 13, 808. [Google Scholar] [CrossRef] [PubMed]
- Ali, W.; Hussain, A.; Usman, A.; Mahmood, K.; Iqbal, M.M.; Khan, H. Heat Transfer Enhancement in Louvered Fin Flat Tube Radiator Using Hybrid Nanofluids. In Proceedings of the ICAME 2023, Shah Alam, Malaysia, 19 September 2023; p. 51. [Google Scholar]
- Hajiakbari, M.; Mahdavi Nejad, A.; Houshfar, E. Enhancing Diesel Engine Cooling Efficiency: A Comprehensive Numerical Study on Nanofluid Coolants with Exergy and Economic Analysis. Case Stud. Therm. Eng. 2024, 56, 104217. [Google Scholar] [CrossRef]
- Balaji, T.; Selvam, C.; Mohan Lal, D. A Review on Electronics Cooling Using Nanofluids. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1130, 012007. [Google Scholar] [CrossRef]
- Moita, A.; Moreira, A.; Pereira, J. Nanofluids for the Next Generation Thermal Management of Electronics: A Review. Symmetry 2021, 13, 1362. [Google Scholar] [CrossRef]
- Mahmoud, E.E.; Algehyne, E.A.; Alqarni, M.M.; Afzal, A.; Ibrahim, M. Investigating the Thermal Efficiency and Pressure Drop of a Nanofluid within a Micro Heat Sink with a New Circular Design Used to Cool Electronic Equipment. Chem. Eng. Commun. 2022, 209, 1035–1047. [Google Scholar] [CrossRef]
- Aglawe, K.; Yadav, R.; Thool, S. Experimental Investigation of Al2O3 Nanofluid for Thermal Energy Management of Microchannel Heat Sink. Transdiscipl. J. Eng. Sci. 2022, 13. [Google Scholar] [CrossRef]
- Rangasamy, S.; Raghavan, R.R.V.; Elavarasan, R.M.; Kasinathan, P. Energy Analysis of Flattened Heat Pipe with Nanofluids for Sustainable Electronic Cooling Applications. Sustainability 2023, 15, 4716. [Google Scholar] [CrossRef]
- Saad, I.; Maalej, S.; Zaghdoudi, M.C. Investigation of the Thermal Performance of a Nanofluid-Filled Grooved Cylindrical Heat Pipe for Electronics Cooling. J. Adv. Res. Fluid Mech. Therm. Sci. 2022, 99, 135–154. [Google Scholar] [CrossRef]
- Waqas, H.; Khan, S.A.; Farooq, U.; Muhammad, T.; Alshehri, A.; Yasmin, S. Thermal Transport Analysis of Six Circular Microchannel Heat Sink Using Nanofluid. Sci. Rep. 2022, 12, 8035. [Google Scholar] [CrossRef]
- Hwang, S.-G.; Garud, K.S.; Seo, J.-H.; Lee, M.-Y. Heat Flow Characteristics of Ferrofluid in Magnetic Field Patterns for Electric Vehicle Power Electronics Cooling. Symmetry 2022, 14, 1063. [Google Scholar] [CrossRef]
- Souayeh, B.; Bhattacharyya, S.; Hdhiri, N.; Hammami, F.; Yasin, E.; Raju, S.S.K.; Alam, M.W.; Alsheddi, T.; Al Nuwairan, M. Effect of Magnetic Baffles and Magnetic Nanofluid on Thermo-Hydraulic Characteristics of Dimple Mini Channel for Thermal Energy Applications. Sustainability 2022, 14, 10419. [Google Scholar] [CrossRef]
- Chu, A.; Li, C.; Zhou, Z.; Liu, B.; Zhang, Y.; Yang, M.; Gao, T.; Liu, M.; Zhang, N.; Dambatta, Y.S.; et al. Nanofluids Minimal Quantity Lubrication Machining: From Mechanisms to Application. Lubricants 2023, 11, 422. [Google Scholar] [CrossRef]
- Dalke, P.A.; Karanjkar, A.V.; Deshmukh, G.P. A Review: Nanofluids in Machining for Performance and Sustainability. J. Phys. Conf. Ser. 2024, 2763, 012012. [Google Scholar] [CrossRef]
- Patole, P.B.; Kulkarni, V.V.; Bhatwadekar, S.G. MQL Machining with Nano Fluid: A Review. Manuf. Rev. 2021, 8, 13. [Google Scholar] [CrossRef]
- Yücel, A.; Yıldırım, Ç.V.; Sarıkaya, M.; Şirin, Ş.; Kıvak, T.; Gupta, M.K.; Tomaz, Í.V. Influence of MoS2 Based Nanofluid-MQL on Tribological and Machining Characteristics in Turning of AA 2024 T3 Aluminum Alloy. J. Mater. Res. Technol. 2021, 15, 1688–1704. [Google Scholar] [CrossRef]
- Eltaggaz, A.; Nouzil, I.; Deiab, I. Machining Ti-6Al-4V Alloy Using Nano-Cutting Fluids: Investigation and Analysis. J. Manuf. Mater. Process. 2021, 5, 42. [Google Scholar] [CrossRef]
- Khan, A.M.; Anwar, S.; Jamil, M.; Nasr, M.M.; Gupta, M.K.; Saleh, M.; Ahmad, S.; Mia, M. Energy, Environmental, Economic, and Technological Analysis of Al-GnP Nanofluid- and Cryogenic LN2-Assisted Sustainable Machining of Ti-6Al-4V Alloy. Metals 2021, 11, 88. [Google Scholar] [CrossRef]
- Wang, H.; Bai, Q.; Chen, S.; Dou, Y.; Guo, W.; Wang, T. Performance Evaluation of Graphene Nanofluid to Mitigate the Wear of a Diamond Tool in Micro-Machining of Ti6Al4V Alloy. J. Manuf. Mater. Process. 2023, 7, 131. [Google Scholar] [CrossRef]
- Saravanan, R.; Sathish, T.; Vijayan, V.; Rajkumar, S.; Sharma, S.; Li, C.; Zhang, Y.; Sharma, K.; Eldin, S.M. Eco-Friendly MoS2 /Waste Coconut Oil Nanofluid for Machining of Magnesium Implants. Rev. Adv. Mater. Sci. 2023, 62, 20220296. [Google Scholar] [CrossRef]
- Talib, N.; Jamaluddin, N.A.; Sheng, T.K.; Kiow, L.W.; Abdullah, H.; Ahmad, S.; Saleh, A. Tribological Study of Activated Carbon Nanoparticle in Nonedible Nanofluid for Machining Application. Evergreen 2021, 8, 454–460. [Google Scholar] [CrossRef]
- Makhesana, M.A.; Patel, K.M. Performance Assessment of Vegetable Oil-Based Nanofluid in Minimum Quantity Lubrication (MQL) during Machining of Inconel 718. Adv. Mater. Process. Technol. 2022, 8, 3182–3198. [Google Scholar] [CrossRef]
- Su, Y.; Chu, Z.; Gong, L.; Wang, B.; Liu, Z. Assessment of Lubrication Property and Machining Performance of Nanofluid Composite Electrostatic Spraying (NCES) Using Different Types of Vegetable Oils as Base Fluids of External Fluid. Chin. J. Mech. Eng. 2023, 36, 94. [Google Scholar] [CrossRef]
- Usluer, E.; Emiroğlu, U.; Yapan, Y.F.; Uysal, A.; Sarıkaya, M.; Kshitij, G.; Khanna, N. Investigation of the Effects of Hybrid Nanofluid-Mql Conditions in Orthogonal Turning and a Sustainability Assessment. Sustain. Mater. Technol. 2023, 36, e00618. [Google Scholar] [CrossRef]
- Ikumapayi, O.M.; Ogedengbe, T.S.; Laseinde, O.T.; Kazeem, R.A.; Afolalu, S.A.; Ogundipe, A.T.; Akinlabi, S.A.; Akinlabi, E.T. A Concise Review on the Suitability of Nano-Refrigerants for Residential Refrigeration Systems (RRS). E3S Web Conf. 2023, 391, 01084. [Google Scholar] [CrossRef]
- Katoch, A.; Abdul Razak, F.; Suresh, A.; Bibin, B.S.; Gundabattini, E.; Yusoff, M.Z. Performance of Nanoparticles in Refrigeration Systems: A Review. J. Nanofluids 2022, 11, 469–486. [Google Scholar] [CrossRef]
- Ponticorvo, E.; Iuliano, M.; Cirillo, C.; Maiorino, A.; Aprea, C.; Sarno, M. Fouling Behavior and Dispersion Stability of Nanoparticle-Based Refrigeration Fluid. Energies 2022, 15, 3059. [Google Scholar] [CrossRef]
- Gobane, S.; Dama, T.; Hasan, N.; Yanmaz, E. Characterization of Copper Oxide–Jatropha Oil Nanofluid as a Secondary Refrigerant. J. Nanomater. 2023, 2023, 7612959. [Google Scholar] [CrossRef]
- Afolalu, S.A.; Ikumapayi, O.M.; Ogedengbe, T.S.; Adegbenjo, A.; Jen, T.-C. Evaluation and Analysis of an Agro-Based Nano Refrigerant to Improve the Performance of a Domestic Refrigeration System. Int. J. Heat Technol. 2022, 40, 1305–1310. [Google Scholar] [CrossRef]
- Dilawar, M.; Qayoum, A. Simulation of Vapour Compression Air Conditioning System Using Al2O3 Based Nanofluid Refrigerant. J. Therm. Eng. 2023, 9, 1307–1323. [Google Scholar] [CrossRef]
- Meliorum Technologies. Silicon Nanoparticles. Gold Nanoparticles. Available online: https://www.meliorum.com (accessed on 31 March 2025).
- Tool, X. Available online: https://www.tool-x.net (accessed on 31 March 2025).
- Heat Transfer Nanofluid. Hydromx® Nanofluid for Heating & Cooling. Available online: https://www.hydromx.com/our-solution/ (accessed on 31 March 2025).
- Empowering Tomorrow’s Technology. Available online: https://synano.eu/ (accessed on 31 March 2025).
- Jabbari, F.; Rajabpour, A.; Saedodin, S. Thermal Conductivity and Viscosity of Nanofluids: A Review of Recent Molecular Dynamics Studies. Chem. Eng. Sci. 2017, 174, 67–81. [Google Scholar] [CrossRef]
- Mosavi, A.; Hekmatifar, M.; Alizadeh, A.; Toghraie, D.; Sabetvand, R.; Karimipour, A. The Molecular Dynamics Simulation of Thermal Manner of Ar/Cu Nanofluid Flow: The Effects of Spherical Barriers Size. J. Mol. Liq. 2020, 319, 114183. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, X.; Yu, J.; Wang, S.; Yang, F. Measurement and Modeling of Surface Tension of Binary Mixtures Containing N, N-Dimethyl cyclohexylamine and Alcohols. J. Mol. Liq. 2023, 392, 123435. [Google Scholar] [CrossRef]
- Abir, F.M.; Shin, D. Specific Heat Capacity of Solar Salt-Based Nanofluids: Molecular Dynamics Simulation and Experiment. Materials 2024, 17, 506. [Google Scholar] [CrossRef]
- Liang, Q.; Valizadeh, K.; Bateni, A.; Patra, I.; Abdul-Fattah, M.N.; Kandeel, M.; Zahra, M.M.A.; Bashar, B.S.; Baghaei, S.; Esmaeili, S. 150- The Effect of Type and Size of Nanoparticles and Porosity on the Pool Boiling Heat Transfer of Water/Fe Nanofluid: Molecular Dynamics Approach. J. Taiwan Inst. Chem. Eng. 2022, 136, 104409. [Google Scholar] [CrossRef]
- Khamliche, T.; Khamlich, S.; Moodley, M.K.; Mothudi, B.M.; Henini, M.; Maaza, M. Laser Fabrication of Cu Nanoparticles Based Nanofluid with Enhanced Thermal Conductivity: Experimental and Molecular Dynamics Studies. J. Mol. Liq. 2021, 323, 114975. [Google Scholar] [CrossRef]
- Dehkordi, K.G.; Karimipour, A.; Afrand, M.; Toghraie, D.; Isfahani, A.H.M. Molecular Dynamics Simulation Concerning Nanofluid Boiling Phenomenon Affected by the External Electric Field: Effects of Number of Nanoparticles through Pt, Fe, and Au Microchannels. J. Mol. Liq. 2021, 324, 114775. [Google Scholar] [CrossRef]
- Jha, N.K.; Ivanova, A.; Lebedev, M.; Barifcani, A.; Cheremisin, A.; Iglauer, S.; Sangwai, J.S.; Sarmadivaleh, M. Interaction of Low Salinity Surfactant Nanofluids with Carbonate Surfaces and Molecular Level Dynamics at Fluid-Fluid Interface at ScCO2 Loading. J. Colloid Interface Sci. 2021, 586, 315–325. [Google Scholar] [CrossRef]
- Khetib, Y.; Abo-Dief, H.M.; Alanazi, A.K.; Cheraghian, G.; Sajadi, S.M.; Sharifpur, M. Simulation of Nanofluid Flow in a Micro-Heat Sink With Corrugated Walls Considering the Effect of Nanoparticle Diameter on Heat Sink Efficiency. Front. Energy Res. 2021, 9, 769374. [Google Scholar] [CrossRef]
- Abu-Hamdeh, N.H.; Bantan, R.A.R.; Golmohammadzadeh, A.; Toghraie, D. The Thermal Properties of Water-Copper Nanofluid in the Presence of Surfactant Molecules Using Molecular Dynamics Simulation. J. Mol. Liq. 2021, 325, 115149. [Google Scholar] [CrossRef]
- Hassanloo, H.; Sadeghzadeh, S.; Ahmadi, R. Reactive Molecular Dynamics Simulation of Thermo-Physicochemical Properties of Non- Covalent Functionalized Graphene Nanofluids. Mater. Today Commun. 2022, 32, 103869. [Google Scholar] [CrossRef]
- Meijuan, C. Application of ANN Technique to Predict the Thermal Conductivity of Nanofluids: A Review. J. Therm. Anal. Calorim. 2021, 145, 2021–2032. [Google Scholar] [CrossRef]
- Ewim, D.R.E.; Okwu, M.O.; Onyiriuka, E.J.; Abiodun, A.S.; Abolarin, S.M.; Kaood, A. A Quick Review of the Applications of Artificial Neural Networks (ANN) in the Modelling of Thermal Systems. Eng. Appl. Sci. Res. 2022, 49, 444458. [Google Scholar] [CrossRef]
- Basu, A.; Saha, A.; Banerjee, S.; Roy, P.C.; Kundu, B. A Review of Artificial Intelligence Methods in Predicting Thermophysical Properties of Nanofluids for Heat Transfer Applications. Energies 2024, 17, 1351. [Google Scholar] [CrossRef]
- Wang, N.; Maleki, A.; Alhuyi Nazari, M.; Tlili, I.; Safdari Shadloo, M. Thermal Conductivity Modeling of Nanofluids Contain MgO Particles by Employing Different Approaches. Symmetry 2020, 12, 206. [Google Scholar] [CrossRef]
- Esfe, M.H.; Eftekhari, S.A.; Hekmatifar, M.; Toghraie, D. A Well-Trained Artificial Neural Network for Predicting the Rheological Behavior of MWCNT–Al2O3 (30–70%)/Oil SAE40 Hybrid Nanofluid. Sci. Rep. 2021, 11, 17696. [Google Scholar] [CrossRef]
- Nasir, S.; Berrouk, A.S.; Gul, T.; Ali, A. Develop the Artificial Neural Network Approach to Predict Thermal Transport Analysis of Nanofluid inside a Porous Enclosure. Sci. Rep. 2023, 13, 21039. [Google Scholar] [CrossRef]
- Islam, T.; Gama, S.; Afonso, M.M. Artificial Neural Network and Response Surface Methodology-Driven Optimization of Cu–Al2O3/Water Hybrid Nanofluid Flow in a Wavy Enclosure with Inclined Periodic Magnetohydrodynamic Effects. Mathematics 2024, 13, 78. [Google Scholar] [CrossRef]
- Alqaed, S.; Mustafa, J.; Sharifpur, M.; Alharthi, M.A. Numerical Simulation and Artificial Neural Network Modeling of Exergy and Energy of Parabolic Trough Solar Collectors Equipped with Innovative Turbulators Containing Hybrid Nanofluids. J. Therm. Anal. Calorim. 2023, 148, 8611–8626. [Google Scholar] [CrossRef]





| Refs. | Model Name | Formula | Remarks |
|---|---|---|---|
| [21,29] | Maxwell | Basic thermal conductivity formula for most of the thermal conductivity models. This model is based on the effective medium theory.The Maxwell model is most suitable for low-concentration (<5%) nanofluids with spherical nanoparticles. | |
| [22] | Bruggeman | Δ = [ | This model is also based on the effective medium theory of nanofluids. Compared to the other models, this one specifically focuses on higher concentrations of nanoparticles. The Bruggeman model does not depend on the nanoparticle concentration; however, at low concentrations, the Maxwell model and Bruggeman model display identical results. |
| [23] | Hamilton–Crosser | Developed by considering the shape factor of the nanoparticles. n = 3 for spheres, n = 6 for cylinders. | |
| [28,30] | Wasp | This is the derived form of the Hamilton–Crosser model by using n = 3. | |
| [31] | Pak and Cho | Developed under the assumption that all the nanoparticles contribute to enhancing the thermal conductivity of the nanofluid. | |
| [32] | Lu-Lin | 1 + aϕ + bϕ2 | Here, a and b are empirical coefficients that depend on the type of nanoparticles and the base fluid. |
| [21] | Maxwell–Garnett | This model is also based on the effective medium theory of nanofluids. | |
| [24] | Yu and Choi | This model is a modified version of the Maxwell model. This one considers the effect of the nanolayer on the thermal conductivity. |
| Ref. | Nanomaterial and Particle Size | Base Fluid/Surfactant | Concentration | Temp. Range | Maximum Thermal Conductivity Improvement |
|---|---|---|---|---|---|
| [33] | MWCNT (multi-walled carbon nanotubes): 5–15 nm (outer diameter) 3–5 nm (inner diameter) CuO: 30 nm–50 nm | Water | 0.05% to 0.6 vol.% | 25–50 °C | 30.38% at 50 °C and 0.6% volume concentration. |
| [34] | Graphene: 2–18 nm TiO2: 10–25 nm Graphene/TiO2—Hybrid | Water | 0.005% to 0.5 vol.% | 25–75 °C | 27.84% of enhancement for TiO2–graphene/water hybrid nanofluid was observed at a volume fraction of 0.5% and a temperature of 75 °C. |
| [35] | Graphene oxide | Water | 0.01 to 0.5 wt.% | 25–60 °C | 19.9% enhancement at 25 °C for 0.5 wt.%. |
| [36] | Al2O3—45 nm Al2O3—500 nm | Water Ice Diathermic oil | 0.01% to 0.1 vol. % 0.01% to 0.1 vol. % 0.01% to 0.04 vol. % | 293 K (Water) 253 K (Ice) 293 K (Oil) | The highest thermal conductivity for micro-sized and nano-sized alumina particles was discovered at the highest volume ratio in addition to a 0.01% vol. ratio for nanosized Alumina dispersed in ice. |
| [37] | Fe3O4 | Water | 0.1% to 3 vol.% | 20–55 °C | 90% enhancement at a solid volume fraction of 3% and at the temperature of 55 °C. |
| [38] | Graphene oxide | Water | 1.0–4.5 mg/mL | 25–50 °C | 25.27% enhancement in 4.5 mg/mL mass fraction at 50 °C. |
| [39] | Fly ash | Water | 0–0.5 vol.% | 30–60 °C | An enhancement of 11.9% was observed at 60 °C. |
| [40] | Al2O3—20 nm CeO2—50 nm Al2O3: CeO2—50:50 Hybrid | Deionized water | 0.01–0.5% vol. 0.01–0.5% vol. 0.01–0.5% vol. | 35–50 °C | Al2O3, CeO2, and their hybrid nanofluids showed 5.3%, 3.3%, and 8.8% maximum enhancements, respectively, at the 0.5% volume concentration at 50 °C. |
| [41] | TiO2—20 nm | Distilled water (CTAB—cetyltrime thylammonium bromide, SDS—sodium dodecyl sulfate) | 0.025–1.25 vol.% 0.025–1.25 vol.% | 20 °C | Maximum enhancement of 10% and 8% at a 1.25% vol. ratio for CTAB-treated and SDS-treated nanofluid samples. |
| [42] | CuO—23 nm CuO—31 nm | Deionized water (SDS, PVP-Polyvinylpyrrolidone) | 0.1–0.5 vol. % | Room temperature | Maximum enhancements of 38% and 34% at 0.4 wt.% of SDS and PVP, while CuO volume concentration was 0.5% vol for 23 nm-sized CuO particles. |
| [43] | Cu | Engine oil | 0.2%, 0.5%, and 1 wt. % | 40–100 °C | The highest enhancement of 49% was observed for a 1% weight fraction. |
| [44] | Al2O3—8 to 12 nm TiO2—10 nm | Engine oil: 5W30 (oleic acid) | 0.05% Al2O3 + 0.05% TiO2 | 18–132 °C | The highest enhancement of 8.6% was observed at 100 °C. |
| [45] | CuO—10 nm TiO2—25 nm | SAE 15W40 engine oil | 0.1–1 wt. % | 25–50 °C | There was a 21.84% enhancement for CuO in 1% wt. and a 20.2% enhancement for TiO2 in 1% wt. |
| [46] | ZnO | SAE 50 engine oil | 0.125–1.5 vol. % | 25–55 °C | The maximum enhancement of 8.74% was obtained at the volume fraction and temperature of 1.5% and 55 °C, respectively, compared to the base fluid at the same temperature. |
| [47] | MWCNT Graphene nanosheets | 15W50 engine oil | 1.5 wt.% 0.5 wt.% | 40 °C | The highest enhancement, 77%, was obtained at 2 wt.%. |
| [48] | Al2O3–MWCNT hybrid | 10W40 engine oil | 0.05–1 vol. % | 25–65 °C | Maximum enhancement of 30.35% was obtained at 1 vol% at 65 °C. |
| [49] | Graphene nanoplate: 10–20 nm | 5W30 engine oil | 0.15 wt. % | - | 29.9% highest enhancement was observed at 0.15 wt.%. |
| [50] | WO3—23 to 65 nm MWCNT—external: 20 to 30 nm Internal: 5 to 10 nm hybrid | 40W10 engine oil | 0.05–0.6 vol. % | 20–60 °C | The maximum enhancement of 19.85% was recorded at 60 °C temperature and 0.6% vol. ratio for the hybrid nanofluid. |
| [51] | Ag–WO3 hybrid | Transformer oil | 1–4 wt.% | 40–100 °C | The maximum enhancement of 41% was reported at 4 wt.% and 100 °C./ |
| [52] | SiO2–graphene hybrid | Transformer oil | 0.01–0.08 wt.% | 20–100 °C | The maximum enhancement of 80% was reported at 0.04 wt.%, pH 9, and 100 °C. |
| [53] | Amorphous graphene sheets | Transformer oil | 0.0012–0.01 wt.% | 35, 45, 55 °C | 30% maximum enhancement was observed at 0.01 wt.%, 55 °C. |
| [54] | TiO2 SiC | Natural ester oil | 0.004 wt.% 0.004 wt.% | 25–90 °C | 25% maximum enhancement at 40 °C; 58% maximum enhancement at 40 °C. |
| [55] | Hexagonal boron nitride (h-Bn) | FR3 Insulating oil | 0–0.1 vol.% | 25 and 90 °C | Enhancement of 14% at 0.1 vol.% at 90 °C. |
| [56] | Graphene oxide (GO) nanosheets | Cottonseed oil (SDS) | 0.01, 0.02, 0.03 and 0.05 wt. % | 45, 60, 75, 90 °C | The highest enhancement is 36.4% at 0.05 wt.% at 65 °C. |
| [57] | Graphene–carbon nanotube (Gr–CNT) hybrid | Ethylene glycol | 0.0175, 0.035, 0.0525, and 0.07 vol.% (1:1 ratio for Gr and MWCNT) | 30 and 50 °C | 0.07 vol.% Gr–CNT hybrid material showed the maximum enhancement of 18 and 50% at 30 and 50 °C. |
| [58] | Functionalized multi-walled carbon nanotubes together with Fe3O4 | Ethylene glycol | 0 to 2.3 vol.% | 25–50 °C | The highest enhancement of 29.7% was reported at 2.3 vol.% at 50 °C. |
| [59] | SiO2 | Liquid paraffin (Oleic acid) | 0.005 to 5 wt.% | 25–70 °C | The highest enhancement was 38% at 5 wt.%, 70 °C. |
| [60] | Fe3O4 | Liquid paraffin (Oleic acid) | 0.005–0.03 vol.% | 20–90 °C | The greatest thermal conductivity enhancement (28.49%) was obtained at 90 °C and 0.03 vol%. |
| [61] | MWCNTs | Kapok seed oil | 0.1 wt.% | 30–90 °C | 6.15% enhancement was observed at 90 °C. |
| [62] | MXene (Ti3C2) | Silicone oil | 0.05, 0.08, and 0.1 wt.% | 25–125 °C | 64% improvement was found for the 0.1 wt.% concentration at 150 °C. |
| Ref. | Model Name | Formula | Remarks |
|---|---|---|---|
| [64] | Einstein | Basic viscosity model for nanofluid viscosity. This model assumes the solid shape of the nanoparticles and the low volume fraction of the nanofluid. | |
| [65] | Brinkman | This is an extended version of the famous Einstein model. This model is also based on the assumption of the spherical shape of the nanoparticles. | |
| [66] | Krieger–Dougherty | This model covers several characteristics of nanoparticles. η is the intrinsic viscosity of nanoparticles, and ϕm is the maximum concentration at which the flow can occur. | |
| [67] | Batchelor | The effect of the interactions among the nanoparticles has been encountered for this model. | |
| [68] | Lundgren | This model considers one of the most important properties of nanofluids, namely the Brownian motion. The bulk stress created by the particles has also been taken into account. |
| Ref. | Nanomaterial and Particle Size | Base Fluid (Surfactant) | Concentration | Temp. Range | Maximum Viscosity Improvement |
|---|---|---|---|---|---|
| [4] | Fly ash— 11.5 nm | Water | 0.1–0.5 vol.% | 30 to 50 °C | Maximum improvement is 13% at a temperature of 30 °C and a 0.5% volume concentration. |
| [7] | Amorphous graphene quantum dots | Transformer oil | 0.001 wt.% | 20 to 80 °C | Maximum viscosity is observed at 20 °C and decreases with temperature. Nanoparticles do not cause an increase or decrease in viscosity. |
| [13] | Graphene nanoplatelets TiO2 | Distilled water and ethylene glycol (CTAB) | 0.1–0.025 wt.% | 30 to 70 °C | Mono nanofluid (graphene nanoplates nanofluid) with 0.1 wt% showed the highest viscosity, 32.54% at 40 °C. |
| [14] | Al2O3 CuO | Water | 0.05–0.15 wt.% | - | Both Al2O3 and CuO increase the viscosity. The concentration of SDBS (sodium dodecylbenzene sulfonate) also improves the viscosity of the nanofluid. |
| [15] | Graphene nanoplatelets | Deionized water (SDS) | 0.01–0.1 vol.% | 10 to 70 °C | The highest enhancement of 4.9% was observed at 0.1 vol.% and 10 °C suspension. |
| [27] | Ag–Cu alloy | Hydrocarbon rotary pump oil | 0.003–0.015 vo.% | - | Viscosity reached 100 mPas at 0.003 and 0.015 vol.% compared to the 98.5 mPas viscosity of the pure oil. |
| [35] | Graphene oxide | Distilled water | 0.01–0.5 wt.% | 25–60 °C | A slight increment of the viscosity from 0.01 wt.% to 0.1 wt.%. A severe increment from 0.1 wt.% to 0.5 wt.% |
| [43] | Cu | Engine oil | 0.2–1 wt.% | 40 to 100 °C | A 37% improvement was observed for a 1% weight fraction at 40 °C. |
| [45] | CuO, TiO2 | SAE 15W40 engine oil | 0.1–1 wt.% | 40 to 100 °C | The highest enhancements are 10.88% and 8.8% for CuO and TiO2, respectively, at 1 vol.%, 40 °C. |
| [47] | MWCNTs/GNs hybrid MWCNTs | 15W50 engine oil | 0.5–2 wt.% 0.5 wt.% | 40 and 100 °C | The highest enhancement in kinematic viscosity was recorded at 2 wt.% as 73.4% and 76.8% at 40 °C and 100 °C, respectively. |
| [49] | Graphene nano-plate Average lateral dimension (x and y) length ≤ 5 µm | SAE 5W30 engine oil | 0.03–0.15 wt.% | 40 and 100 °C | Maximum improvement was 10.5% at 0.15 wt.% of GNs at 40 °C. |
| [52] | Hybrid SiO2–graphene | Transformer oil | 0.01–0.08 wt.% | 40 and 100 °C | The highest enhancement of hybrid SiO2–graphene-based nanofluids was 29.7% at 40 °C.. |
| [59] | SiO2: 20 nm diameter (approximately) | Liquid paraffin (oleic acid) | 0.005–5 wt.% | 25 to 70 °C | The highest enhancement of 495% was reported at 5 wt.% and 70 °C. |
| [61] | MWCNT | Kapok seed oil | 0.1 wt.% | 30 to 90 °C | - |
| [62] | MXene (Ti3C2) | Silicone oil | 0.05–0.1 wt.% | 25 to 125 °C | No noticeable change with respect to nanoparticle weight concentration. |
| Ref. | Nanomaterial and Particle Size | Base Fluid/Surfactant | Concentrations | Specific Heat Capacity Improvement |
|---|---|---|---|---|
| [15] | Graphene nanoplates have a thickness from 2 to 8 nm and a diameter from 4 to 12 µm | Water (SDS) | 0.01, 0.05, and 0.10 vol.% with graphene: SDS ratios are 0.5:1, 1:1, and 1.5:1 | 0.1 vol.% of graphene nanoplatelets with a 1.5:1 surfactant ratio caused the thermal property of the base fluid to be reduced to values between ~28.12% (70 °C). |
| [69] | CeO2 | Water | 0.01, 0.05, 0.1, 0.2, and 0.3 vol.% | 0.3% volume fraction had specific heat of about 5% lesser than the base fluids at the temperature of 35 °C. |
| [70] | SiO2—10 nm, 20 nm, 30 nm | Base salt (NaNO3 and KNO3) | 0.5, 1.0, 1.5, and 2.0 wt.% | Highest enhancement of 26.7% for 20 nm SiO2. |
| [71] | Coal fly ash nanoparticles—14 nm | Water (Triton—X 100) | 0.1, 0.3, and 0.5 vol.% | A 21.19% decrease was observed for 0.5 vol.% at 30 °C. |
| [72] | ZnO CuO | Deionized water (EBT—eriochrome black T, OA—olylamine) | 0.1 wt.% | No comparison has been performed with the base fluids. The parameters considered are temperature and sonication time. However, a recognizable change was not observed with respect to sonication time. |
| [73] | MgO—diameter: 25–45 nm TiO2—diameter: 18–23 nm | Water (SDS) | 0.1–0.5 vol. % with MgO: TiO2 = 50:50, 80:20, 20:80, 60:40, and 40:60 | 80 wt.% MgO—20 wt.% TiO2 decreased by 1.08% with a solid volume concentration of 0.5%. |
| [74] | CNTs (carbon nanotubes)—diameter: 10 to 20 nm. Length ranges from 10 to 30 μm. | Water (SDS, PVP) | 0.1, 0.3, 0.5 and 1 wt.% with CNT: Surfactant = 1:0.5 and 1:1 | At 60 °C, enhancements of 57%, 61%, 63%, and 65% at 0.1, 0.3%, 0.5%, and 1 wt.% were discovered, respectively. Notably, the type of surfactant did not affect the specific heat capacity enhancement. |
| [75] | Al2O3—ZnO Al2O3—29 nm ZnO—70 nm | Water | 0.33, 0.67, 1%, 1.33% and 1.67% with ratio of Al2O3-ZnO = 1:2, 1:1, 2:1 | Nanofluids at a 2:1 mixture ratio have a maximum viscosity increase of 96.37% and maximum specific heat decrease of 30.12% at a temperature of 25 °C and a volume concentration of 1.67% |
| [76] | CuO + MWCNT: average diameter = 20, 30, 40, 50 nm MgO + MWCNT: average diameter = 20, 30, 40, 50 nm SnO2 + MWCNT: average diameter = 20, 30, 40, 50 nm | Deionized water (CTAB) | 0.25, 0.50, 0.75, 1.00, 1.25, 1.50, 80:20 each (by weight ratio) with the addition of the CTAB surfactant at 3:2 | The maximum decrease in specific heat capacity is about 12.84% at φ = 1.50%, 25 °C at an average particle size of 50 nm and 20 nm for CuO + MWCNT. The maximum decrease in specific heat capacity compared to the base fluid has been reported at 25 °C, φ=1.50%, and average size 20 nm h is about 15.09%. The highest decrease of about 13.23% was observed at 1.50 vol%, 25 °C, and 20 nm for SnO2 + MWCNT. |
| [77] | GO: Al2O3 diameter = 30 ± 5 nm | Water (SDS) | 0.05, 0.07, 0.01, 0.12, and 0.15 wt.% | The maximum reduction ratio was almost 7% at 0.15 wt.% at 20 °C |
| [78] | CNT—diameter: 10 to 20 nm. Length ranges from 10 to 30 μm | Water (SDS, PVP) | 0.1, 0.3, 0.5, and 1 wt.% with CNT: surfactant = 1:0.5 and 1:1 | At 60 °C, enhancements of 57%, 61%, 63%, and 65% at 0.1, 0.3%, 0.5%, and 1 wt.% were discovered, respectively. Notably, the type of surfactant did not affect the specific heat capacity enhancement. |
| Ref. | Nanomaterial and Particle Size | Base Fluid | Concentration | Flash Point Improvement |
|---|---|---|---|---|
| [78] | CNT diameter: 10–40 nm; length: 20 μm | Mobil gear 627 paraffinic oils | 0.1, 0.5, 1, and 2 wt.% | The flash point for Mobil gear 627 and paraffinic oils was increased by about 13 and 25%, respectively. |
| [80] | SiO2, CQD (carbon quantum dots) | Mineral oil | 0.01 wt.% | 3.33% improvement. |
| [81] | ZnO—diameter: 30 nm | SAE50 engine oil | 0.1 to 1.5 vol.% | 7.2% improvement at 1.5 vol%. |
| [82] | CQD: SiO2 hybrid, CQD 2.5 nm, SiO2: 15–40 nm | Transformer oil | 0.01, 0.05, and 0.1 wt.% | 6.67% for CQD–SiO2 hybrid nanofluid. |
| [83] | TiO2, ZnO, Al2O3 | Virgin mineral oil | - | TiO2—14.7%, ZnO—12.3%, Al2O3—2.3%. |
| [84] | Exfoliated hexagonal boron nitride (Eh-BN) | Mineral oil Pongamia pinnata oil | 0.01 wt.% | 30% for mineral oil-based nanofluid and 3.6% for Pongamia pinnata oil. |
| [85] | TiO2, Al2O3, MoS2 | Transformer oil | 0.025 wt.% | TiO2—10.56, Al2O3%—7.0%, MoS2—4.2%. |
| [86] | MWCNT | Turbine meter oil | 0.05, 0.1, 0.2, 0.3, and 0.4 wt.% | 4.44% increment at both 0.3 and 0.4 wt.%. |
| [87] | Graphene oxide: 2 μm diameter | Engine oil—SAE-50 | 0.01, 0.25, 0.50, and 1.00 wt.% | 8% improvement at 1 wt.%. |
| [88] | ZnO, diameter: 0.064 nm | Polyol ester oil | 0.1, 0.3, and 0.5 wt.% | 8% improvement at 0.5 wt.%. |
| [89] | SiO2, diameter: 30–50 nm | SAE20W40 engine oil | 0.3, 0.6, 0.9, 1.2, and 1.5 wt.% | Maximum reduction of 6.97% in flash point at 0.6 wt.%. |
| [90] | Al2O3 | Lube oil stock—60 | 0.25, 0.65, 1.05, 1.45, and 1.85 wt.% | 9.73 increment at 1.85 wt.%. |
| [91] | FeO3 | Kernel palm oil methyl ester | 0.10, 0.15, and 0.20 wt.% | The highest decrease of 9.13% observed in the 0.10 wt.% sample. |
| Ref. | Nanomaterial | Base Fluid | Surfactant | Thermal Effects and Stability by Surfactants |
|---|---|---|---|---|
| [14] | Al2O3, CuO | Distilled water | Sodium dodecylbenzene sulfonate (SDBS) | The thermal conductivity increased with surfactant concentration but decreased significantly at greater concentrations. In this study, the higher concentration of nanoparticles decreased the stability (zeta potential), but stability increased with the SDBS concentration. |
| [15] | Graphene nanoplates (thickness from 2 to 8 nm, diameter from 4 to 12 µm) | Water | Sodium dodecyl Sulfate (SDS) | Even though SDS improves the stability of nanofluids, it lowers their thermal conductivity and specific heat capacity. The lower concentration of SDS stabilized the nanofluid for 24 h while the higher concentration maintained the stability for up to 45 days. |
| [73] | MgO–TiO2 | Distilled water | SDS | No identical change was observed since all the samples were prepared with the same surfactant ratio. In this study, the SDS concentration was maintained consistently while varying the MgO and TiO2 ratio. The highest stability was obtained for the MgO–TiO2 = 8:2 sample. |
| [74] | CNT | Water | Gum Arabic (GA), polyvinyl pyrrolidone (PVP), sodium dodecyl sulfate (SDS) | Surfactants were used at the 1:0.2, 1:0.5, and 1:1 ratios. It was discovered that the highest stability existed at the 1:0.5 and 1:1 ratios. Among all the surfactants, SDS displayed the highest stability compared to the other two. These surfactants did not affect the thermal properties. |
| [97] | FeC | Water | Low-viscosity carboxymethyl cellulose sodium salt | There was no effect on thermal conductivity, and the stability of the nanofluid was improved by the surfactant. |
| [98] | Al2O3 | Water | Cetyl trimethyl ammonium bromide (CTAB), SDBS, SDS | Only SDBS produced a stable and greater distribution of nanoparticles in nanofluid at 2:1 ratio. DBS-containing nanofluid showed a slight decrement in thermal conductivity compared to nanofluid containing no surfactant. |
| [99] | TiO2 | Water | CTAB, acetic acid (AA), oleic acid (OA), SDS | Only CTAB and AA produced stable nanofluids for more than 500 h, and the stability was improved with surfactant concentration according to surface tension data. The TEM images also displayed the availability of stable clusters of 147 nm and 207 nm. |
| [100] | TiO2 | Water | CTAB, OA | CTAB produced a more stable and homogeneous nanofluid than OA. It prevented nanoparticle clustering. |
| [101] | Ag | Water, hexane ethylene glycol | GA GA, OA | Gum Arabic lowered the thermal conductivity of water. The stability of the nanofluids were not focused on in this study. |
| [102] | Al2O3 | Therminol | OA | The nanofluid stability was analyzed using Turbiscan LabExpert equipment and FTIR measurements. It was found that the nanofluid samples prepared at 120 °C showed the highest stability. Surfactants did not affect the thermal conductivity of the nanofluid. |
| [103] | Mg(OH)2 | Water | CTAB, SDS, OA | All the nanofluids samples had recorded zeta potential exceeding 45 mV on the 30th day after the preparation, showing that all the surfactants were suitable for the preparation. Among them, CTAB produced the most stable nanofluid. |
| [104] | Cu Al2O3 | Water | SDBS | The effect of the surfactant was investigated along with the pH value for both nanofluids. The highest stability was obtained for Cu at pH = 9 and for Al2O3 at pH = 8. Surfactants have increased thermal conductivity with a surfactant concentration of 0.1 wt.%. |
| [105] | TiO2 | 10W30 engine oil | Triton X | The stability study was performed using a UV–Vis spectrometer, demonstrating an absorbance decrement after 2 h of preparation and electrostatic stability after 168 h. The surfacant improvedthe load-carrying capacity, friction-reducing, and anti-wear abilities of the nanofluid. |
| [106] | h-BN Al2O3 | 15W40 diesel engine oil | OA | The visual recordings of the stability of the nanofluids were obtained after 24 h, 72 h, 168 h, and 720 h. However, both nanofluids displayed better stability at 168 h but not at 720 h. The dispersion of h-BN nanoparticles was better than that of Al2O3 nanoparticles. The total acid number of the nanofluid with added h-BN added showed a slight increment. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Induranga, A.; Galpaya, C.; Vithanage, V.; Indupama, A.; Maduwantha, K.; Gunawardana, N.; Wijesekara, D.; Amarasinghe, P.; Nilmalgoda, H.; Gunasena, K.; et al. Nanofluids for Heat Transfer: Advances in Thermo-Physical Properties, Theoretical Insights, and Engineering Applications. Energies 2025, 18, 1935. https://doi.org/10.3390/en18081935
Induranga A, Galpaya C, Vithanage V, Indupama A, Maduwantha K, Gunawardana N, Wijesekara D, Amarasinghe P, Nilmalgoda H, Gunasena K, et al. Nanofluids for Heat Transfer: Advances in Thermo-Physical Properties, Theoretical Insights, and Engineering Applications. Energies. 2025; 18(8):1935. https://doi.org/10.3390/en18081935
Chicago/Turabian StyleInduranga, Ashan, Chanaka Galpaya, Vimukthi Vithanage, Amalka Indupama, Kaveendra Maduwantha, Niroshan Gunawardana, Dasith Wijesekara, Prasad Amarasinghe, Helitha Nilmalgoda, Kasundi Gunasena, and et al. 2025. "Nanofluids for Heat Transfer: Advances in Thermo-Physical Properties, Theoretical Insights, and Engineering Applications" Energies 18, no. 8: 1935. https://doi.org/10.3390/en18081935
APA StyleInduranga, A., Galpaya, C., Vithanage, V., Indupama, A., Maduwantha, K., Gunawardana, N., Wijesekara, D., Amarasinghe, P., Nilmalgoda, H., Gunasena, K., Perera, H., Hosan, S., & Koswattage, K. (2025). Nanofluids for Heat Transfer: Advances in Thermo-Physical Properties, Theoretical Insights, and Engineering Applications. Energies, 18(8), 1935. https://doi.org/10.3390/en18081935









