The Catalytic Valorization of Lignin from Biomass for the Production of Liquid Fuels
Abstract
1. Introduction
2. Lignin: Formation, Structure and Type
2.1. Formation
2.2. Structure: Building Blocks, Functional Groups and Interunit Linkages
2.2.1. Building Blocks and Functional Groups
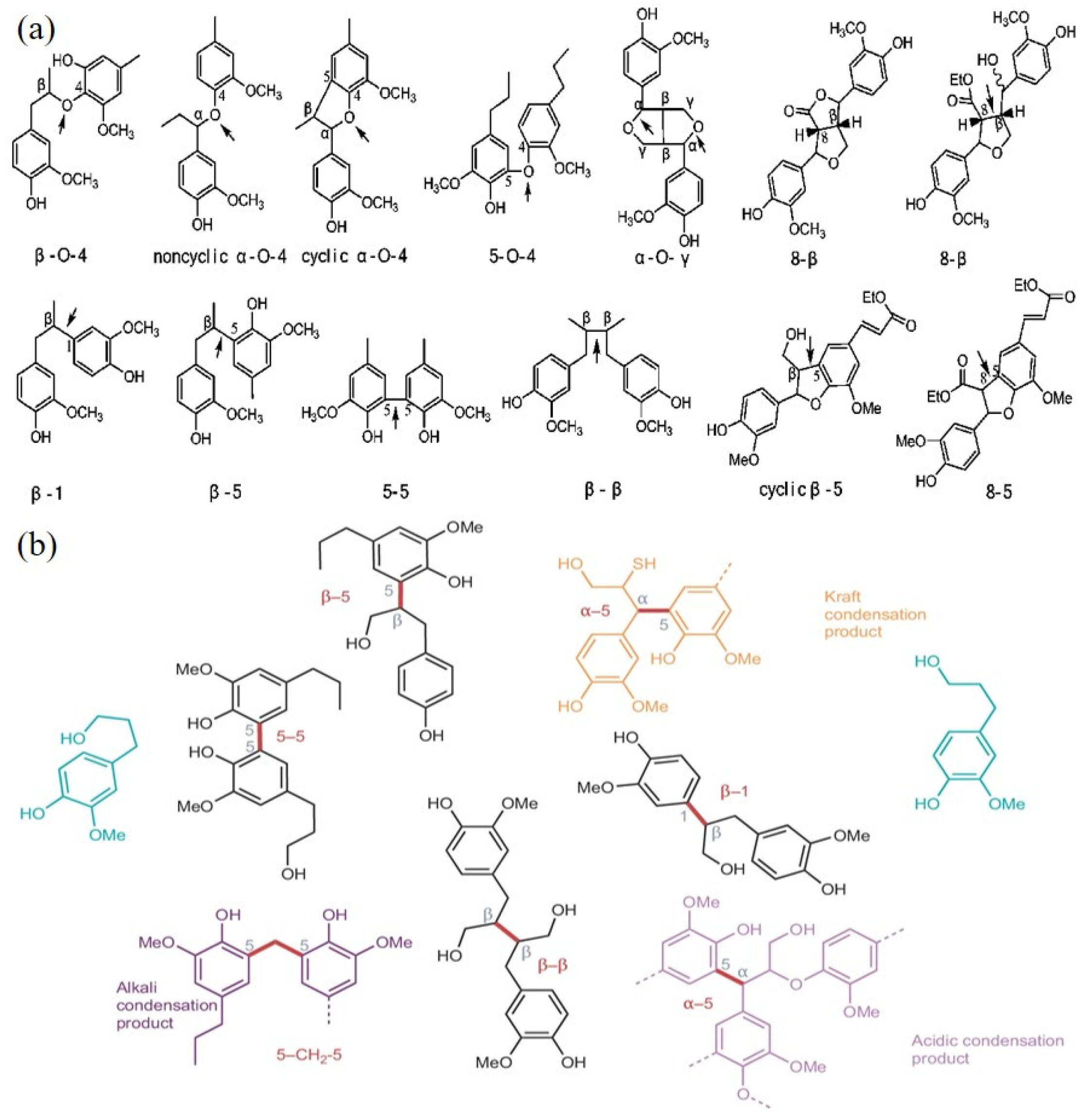
2.2.2. Interunit Linkages
2.2.3. Types
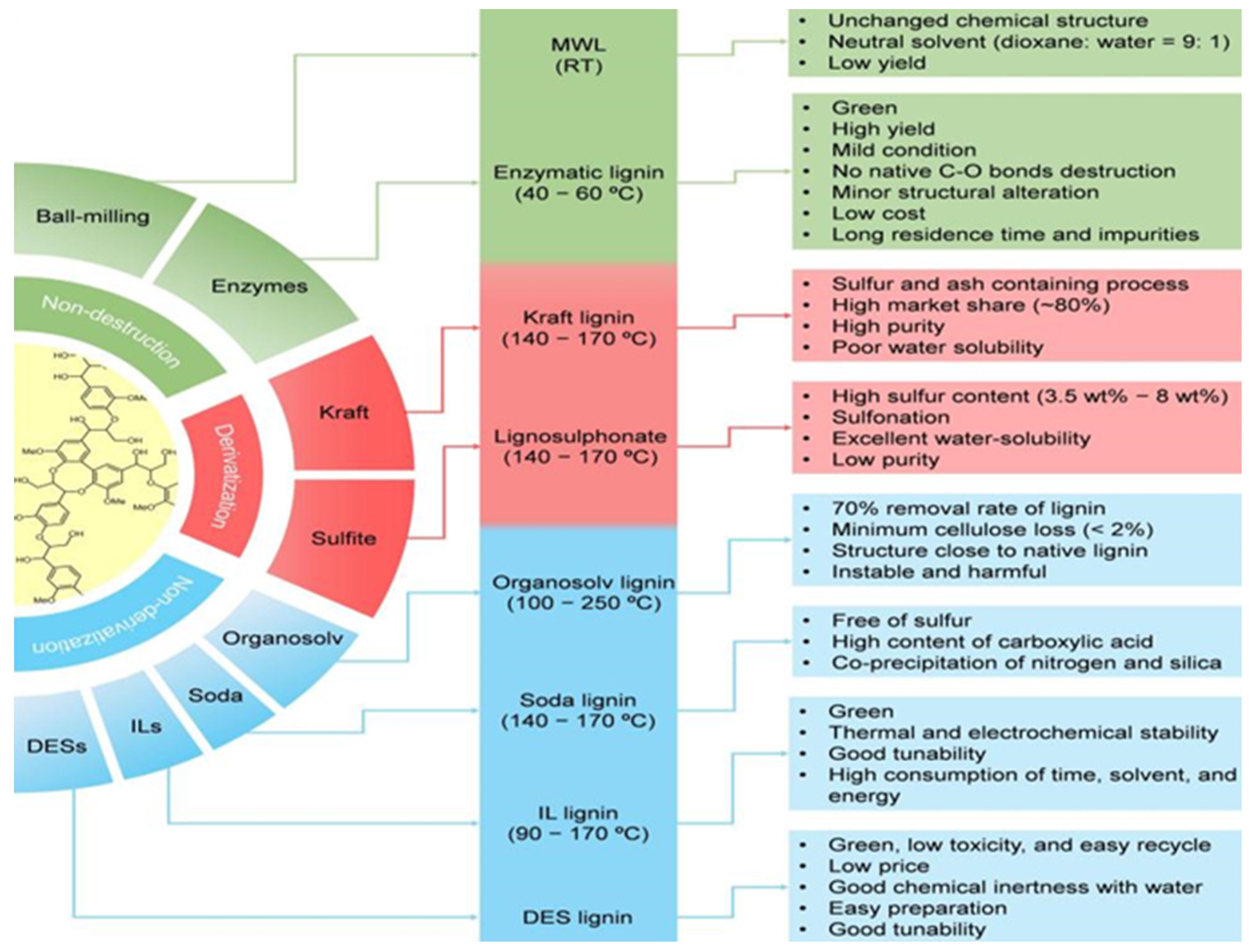
3. Lignin Isolation
3.1. Acid Pretreatment
3.2. Alkaline Pretreatment
3.3. Organsolv Pretreatment
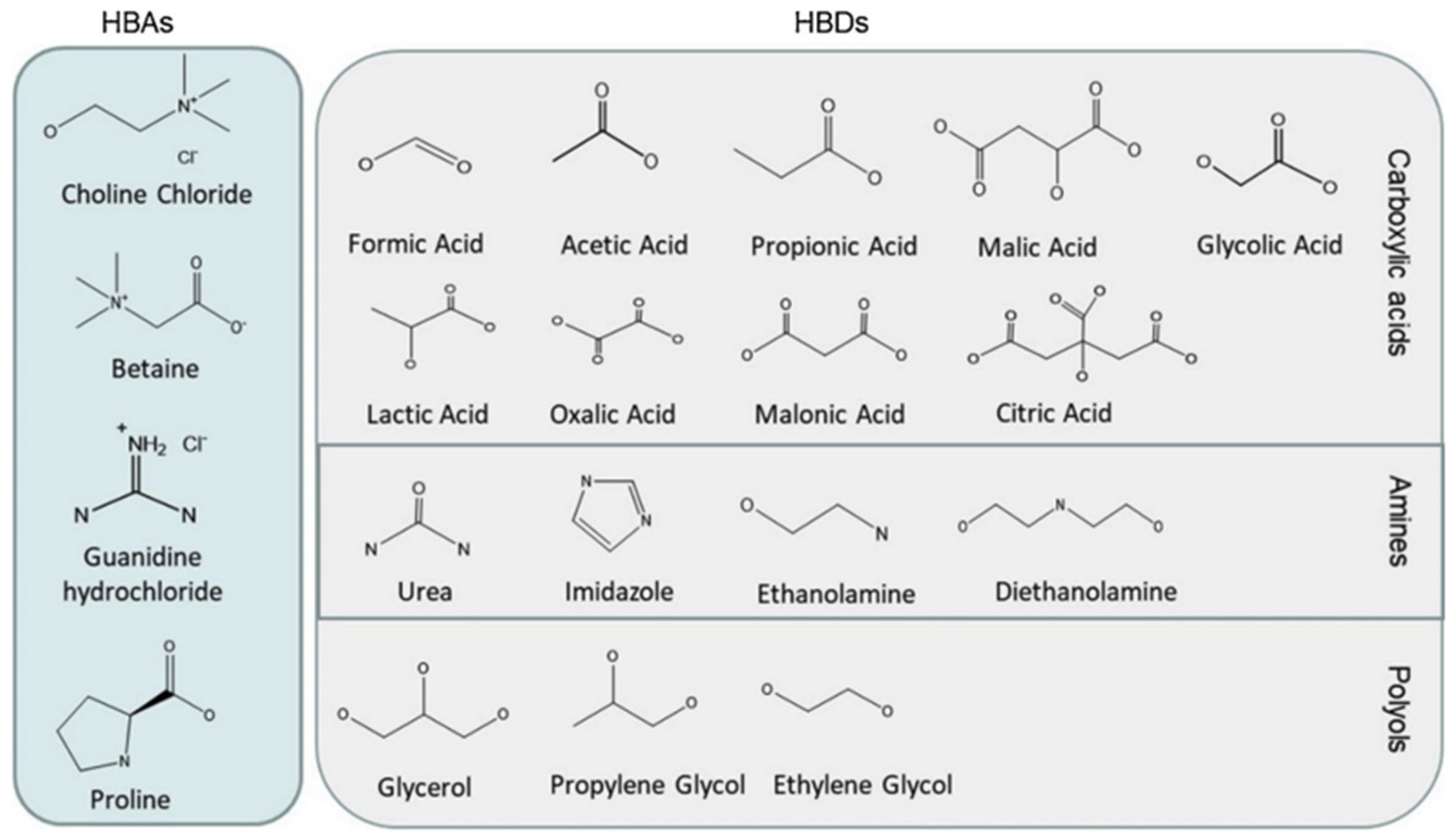
3.4. Deep Eutectic Solvents Pretreatment
3.5. Ionic Liquid Pretreatment
3.6. Other Traditional Methods
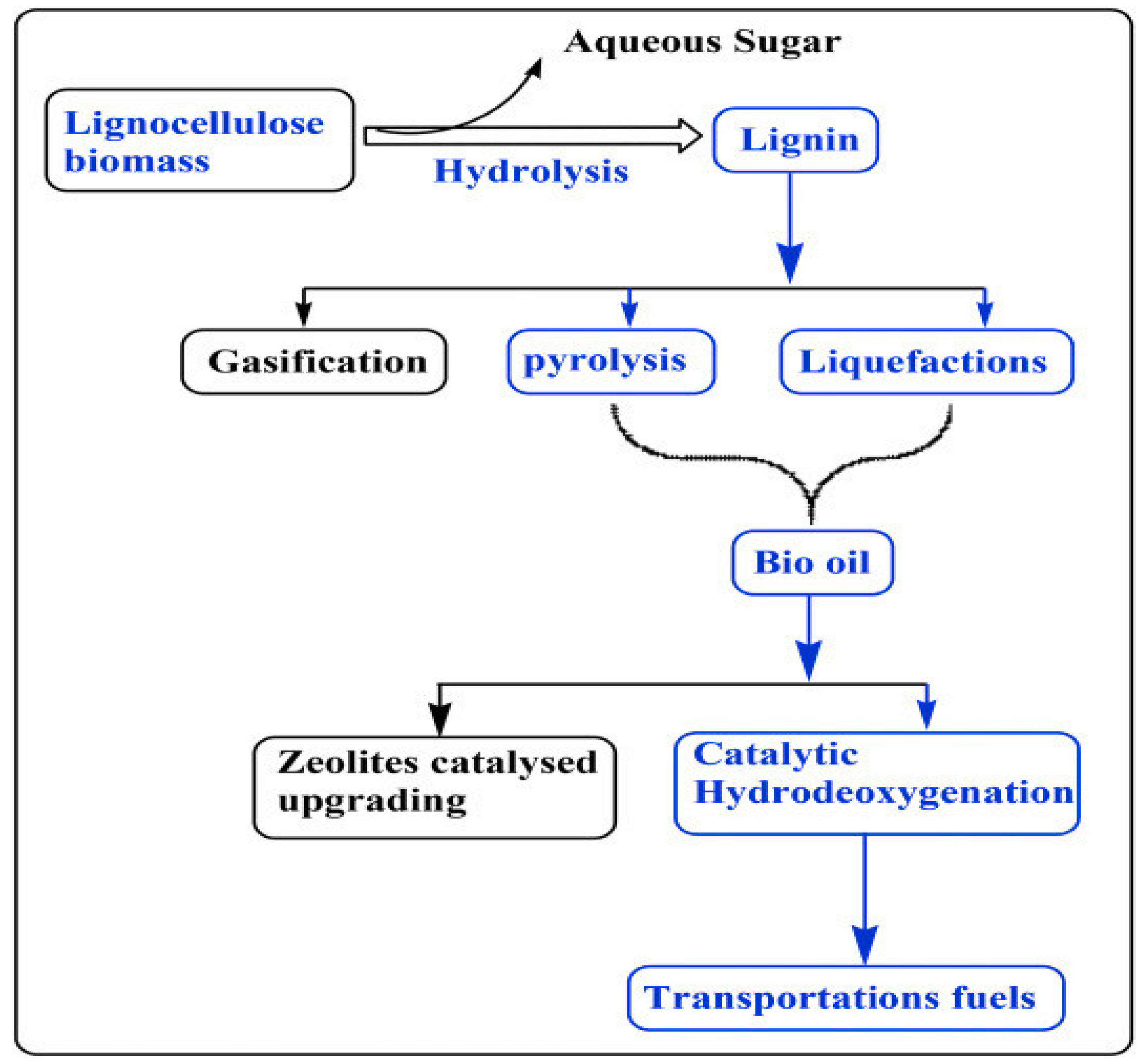
4. Depolymerization and Valorization of Lignin
4.1. Pyrolysis
4.1.1. Thermal Pyrolysis
4.1.2. Catalytic Pyrolysis
- 1.
- Molecular sieve catalyst
- 2.
- Metal oxide catalysts
- 3.
- Metal salt catalysts
4.2. Hydro-Processing
4.2.1. Hydrogenolysis
- Homogeneous catalyst-based hydrogenolysis
- 2.
- Heterogeneous catalyst-catalyzed hydrogenolysis
4.2.2. Hydrodeoxygenation
4.2.3. Catalytic Hydrogenation
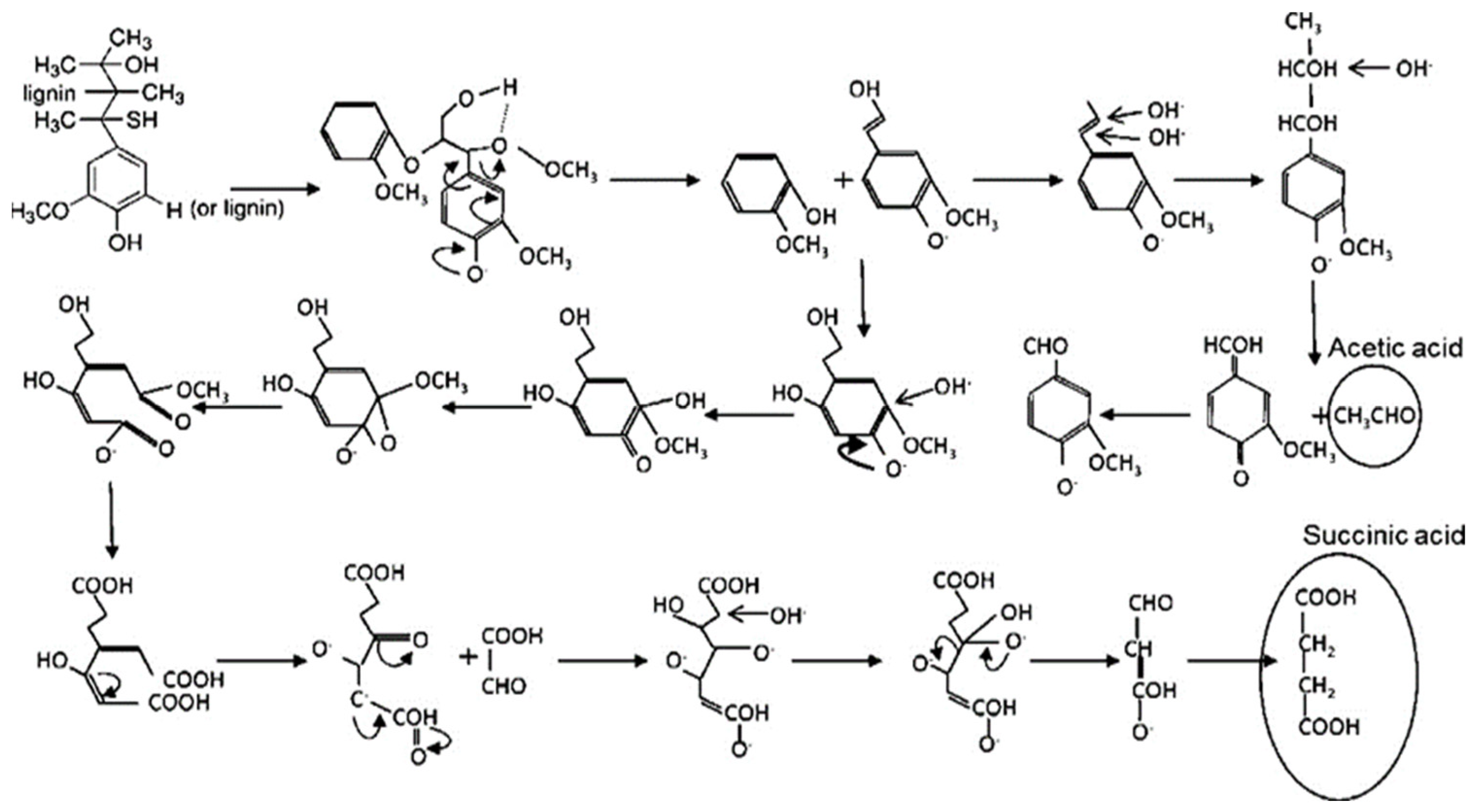
4.3. Oxidation
4.3.1. Organometallic Catalytic Oxidation
4.3.2. Metal-Free Organic Catalytic Oxidation
4.3.3. Base-Catalyzed Oxidation
4.3.4. Acid-Catalyzed Oxidation
4.3.5. Photocatalytic Oxidation
4.3.6. Electrocatalytic Oxidation
4.4. Liquid-Phase Reforming
5. Conclusions and Outlook
- The inherent heterogeneity and complex structure of lignin require suitable catalysts to accomplish effective depolymerization. In contrast, the current development and utilization of catalysts still have difficulties, such as high cost and easy deactivation. In the future, we should continue to improve the characterization instruments and techniques, analyze the depolymerization mechanism of lignin by deciphering the complex structure of lignin, and, using molecular design, synthesize new multifunctional catalysts with strong anti-deactivation activity, high selectivity, and low cost to realize the directional and efficient depolymerization of lignin.
- Hydrogen, commonly used in the preparation of liquid fuels from lignin, is extracted during petroleum refining. However, due to the non-renewable nature of petroleum, the future should focus on exploring alternative methods of producing renewable hydrogen. Thus, attention should be paid to increasing the yield of hydrogen through green and safe methods.
- The current lignin-catalyzed preparation of high-density bio-liquid fuels is still mainly dominated by model studies. The development of efficient catalytic systems, that couple the multiple processes mentioned above, such as lignin depolymerization and HDO, using real biomass or lignin as a substrate is the main point of focus for future research in this field.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, C.; Zhao, X.; Wang, A.; Huber, G.W.; Zhang, T. Catalytic Transformation of Lignin for the Production of Chemicals and Fuels. Chem. Rev. 2015, 115, 11559–11624. [Google Scholar] [CrossRef] [PubMed]
- Amidon, T.E.; Liu, S. Water-based woody biorefinery. Biotechnol. Adv. 2009, 27, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, U.M.; Ji, N.; Li, H.; Wu, Q.; Song, C.; Liu, Q.; Ma, D.; Lu, X. Can lignin be transformed into agrochemicals? Recent advances in the agricultural applications of lignin. Ind. Crops Prod. 2021, 170, 113646. [Google Scholar] [CrossRef]
- Chakar, F.S.; Ragauskas, A.J. Review of current and future softwood kraft lignin process chemistry. Ind. Crops Prod. 2004, 20, 131–141. [Google Scholar] [CrossRef]
- Cassoni, A.C.; Costa, P.; Vasconcelos, M.W.; Pintado, M. Systematic review on lignin valorization in the agro-food system: From sources to applications. J. Environ. Manag. 2022, 317, 115258. [Google Scholar] [CrossRef]
- Zakzeski, J.; Bruijnincx, P.C.A.; Jongerius, A.L.; Weckhuysen, B.M. The Catalytic Valorization of Lignin for the Production of Renewable Chemicals. Chem. Rev. 2010, 110, 3552–3599. [Google Scholar] [CrossRef] [PubMed]
- Gillet, S.; Aguedo, M.; Petitjean, L.; Morais, A.R.C.; da Costa Lopes, A.M.; Łukasik, R.M.; Anastas, P.T. Lignin transformations for high value applications: Towards targeted modifications using green chemistry. Green Chem. 2017, 19, 4200–4233. [Google Scholar] [CrossRef]
- Azadi, P.; Inderwildi, O.R.; Farnood, R.; King, D.A. Liquid fuels, hydrogen and chemicals from lignin: A critical review. Renew. Sustain. Energy Rev. 2013, 21, 506–523. [Google Scholar] [CrossRef]
- Demirbaş, A. Relationships between lignin contents and heating values of biomass. Energy Convers. Manag. 2001, 42, 183–188. [Google Scholar] [CrossRef]
- Composition and Structure of Wood Cells. In Forest Products and Wood Science An Introduction; Wiley: Hoboken, NJ, USA, 2011; pp. 45–63. [CrossRef]
- Sun, P.; Wang, Z.; Li, C.; Tang, B.; Peng, C. Catalytic conversion of lignin and its derivatives to alkanes over multifunctional catalysts: A review. Fuel 2024, 361, 130726. [Google Scholar] [CrossRef]
- Barros, J.; Escamilla-Trevino, L.; Song, L.; Rao, X.; Serrani-Yarce, J.C.; Palacios, M.D.; Engle, N.; Choudhury, F.K.; Tschaplinski, T.J.; Venables, B.J.; et al. 4-Coumarate 3-hydroxylase in the lignin biosynthesis pathway is a cytosolic ascorbate peroxidase. Nat. Commun. 2019, 10, 1994. [Google Scholar] [CrossRef] [PubMed]
- SjöströM, E. Chapter 4—LIGNIN. In Wood Chemistry, 2nd ed.; SjöströM, E., Ed.; Academic Press: San Diego, CA, USA, 1993; pp. 71–89. [Google Scholar] [CrossRef]
- Rajesh Banu, J.; Kavitha, S.; Yukesh Kannah, R.; Poornima Devi, T.; Gunasekaran, M.; Kim, S.-H.; Kumar, G. A review on biopolymer production via lignin valorization. Bioresour. Technol. 2019, 290, 121790. [Google Scholar] [CrossRef]
- Ogunbiyi, A.T.; Li, W.; Zhang, B. 6—Chemo-catalytic conversion of lignin. In Biomass, Biofuels, Biochemicals; Bhaskar, T., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 109–128. [Google Scholar] [CrossRef]
- Shimizu, K.; Matsushita, Y.; Aoki, D.; Mitsuda, H.; Fukushima, K. Reactivity of a benzylic lignin-carbohydrate model compound during enzymatic dehydrogenative polymerisation of coniferyl alcohol. Holzforschung 2021, 75, 773–777. [Google Scholar] [CrossRef]
- Deng, W.; Feng, Y.; Fu, J.; Guo, H.; Guo, Y.; Han, B.; Jiang, Z.; Kong, L.; Li, C.; Liu, H.; et al. Catalytic conversion of lignocellulosic biomass into chemicals and fuels. Green Energy Environ. 2023, 8, 10–114. [Google Scholar] [CrossRef]
- del Río, J.C.; Rencoret, J.; Gutiérrez, A.; Elder, T.; Kim, H.; Ralph, J. Lignin Monomers from beyond the Canonical Monolignol Biosynthetic Pathway: Another Brick in the Wall. ACS Sustain. Chem. Eng. 2020, 8, 4997–5012. [Google Scholar] [CrossRef]
- Ralph, J.; Lapierre, C.; Boerjan, W. Lignin structure and its engineering. Curr. Opin. Biotechnol. 2019, 56, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, R.; Jastrzebski, R.; Clough, M.T.; Ralph, J.; Kennema, M.; Bruijnincx, P.C.A.; Weckhuysen, B.M. Paving the Way for Lignin Valorisation: Recent Advances in Bioengineering, Biorefining and Catalysis. Angew. Chem. Int. Ed. 2016, 55, 8164–8215. [Google Scholar] [CrossRef]
- Liu, C.; Wang, S.; Wang, B.; Song, G. Catalytic hydrogenolysis of castor seeds C-lignin in deep eutectic solvents. Ind. Crops Prod. 2021, 169, 113666. [Google Scholar] [CrossRef]
- Huang, D.; Li, R.; Xu, P.; Li, T.; Deng, R.; Chen, S.; Zhang, Q. The cornerstone of realizing lignin value-addition: Exploiting the native structure and properties of lignin by extraction methods. Chem. Eng. J. 2020, 402, 126237. [Google Scholar] [CrossRef]
- Li, Z. Research on renewable biomass resource—Lignin. J. Nanjing For. Univ. 2012, 36, 1. [Google Scholar]
- Zhang, C.; Wang, F. Sell a dummy: Adjacent functional group modification strategy for the catalytic cleavage of lignin β–O–4 linkage. Chin. J. Catal. 2017, 38, 1102–1107. [Google Scholar] [CrossRef]
- Siau, J.F. Wood Structure and Chemical Composition. In Transport Processes in Wood; Siau, J.F., Ed.; Springer: Berlin/Heidelberg, Germany, 1984; pp. 35–72. [Google Scholar] [CrossRef]
- Vanholme, R.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin engineering. Curr. Opin. Plant Biol. 2008, 11, 278–285. [Google Scholar] [CrossRef]
- Shah, S.W.A.; Xu, Q.; Ullah, M.W.; Zahoor; Sethupathy, S.; Morales, G.M.; Sun, J.; Zhu, D. Lignin-based additive materials: A review of current status, challenges, and future perspectives. Addit. Manuf. 2023, 74, 103711. [Google Scholar] [CrossRef]
- Xiong, F.; Han, Y.; Wang, S.; Li, G.; Qin, T.; Chen, Y.; Chu, F. Preparation and formation mechanism of size-controlled lignin nanospheres by self-assembly. Ind. Crops Prod. 2017, 100, 146–152. [Google Scholar] [CrossRef]
- Li, Y.; Meng, X.; Meng, R.; Cai, T.; Pu, Y.; Zhao, Z.-M.; Ragauskas, A.J. Valorization of homogeneous linear catechyl lignin: Opportunities and challenges. RSC Adv. 2023, 13, 12750–12759. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.-H.; Xia, X.; Lin, C.-X.; Tong, D.-S.; Beltramini, J. Catalytic conversion of lignocellulosic biomass to fine chemicals and fuels. Chem. Soc. Rev. 2011, 40, 5588–5617. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, C.T.; Ouellette, E.T.; Zhu, J.; Román-Leshkov, Y.; Stahl, S.S.; Beckham, G.T. Accessing monomers from lignin through carbon–carbon bond cleavage. Nat. Rev. Chem. 2024, 8, 799–816. [Google Scholar] [CrossRef]
- Mottiar, Y.; Vanholme, R.; Boerjan, W.; Ralph, J.; Mansfield, S.D. Designer lignins: Harnessing the plasticity of lignification. Curr. Opin. Biotechnol. 2016, 37, 190–200. [Google Scholar] [CrossRef]
- Ralph, J.; Lundquist, K.; Brunow, G.; Lu, F.; Kim, H.; Schatz, P.F.; Marita, J.M.; Hatfield, R.D.; Ralph, S.A.; Christensen, J.H.; et al. Lignins: Natural polymers from oxidative coupling of 4-hydroxyphenyl- propanoids. Phytochem. Rev. 2004, 3, 29–60. [Google Scholar] [CrossRef]
- Zhang, B.; Qiang, G.; Barta, K.; Sun, Z. Bio–based polymers from lignin. Innov. Mater. 2024, 2, 100062. [Google Scholar] [CrossRef]
- Chapter 2—Structure and Characteristics of Lignin. In Lignin Chemistry and Applications; Huang, J., Fu, S., Gan, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 25–50. [Google Scholar] [CrossRef]
- Gan, M.J.; Niu, Y.Q.; Qu, X.J.; Zhou, C.H. Lignin to value-added chemicals and advanced materials: Extraction, degradation, and functionalization. Green Chem. 2022, 24, 7705–7750. [Google Scholar] [CrossRef]
- Schutyser, W.; Renders, T.; Van den Bosch, S.; Koelewijn, S.F.; Beckham, G.T.; Sels, B.F. Chemicals from lignin: An interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem. Soc. Rev. 2018, 47, 852–908. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-J. Deciphering the Enigma of Lignification: Precursor Transport, Oxidation, and the Topochemistry of Lignin Assembly. Mol. Plant 2012, 5, 304–317. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, S.; Nishida, T.; Tsutsumi, Y.; Kondo, R. Lignin dehydrogenative polymerization mechanism: A poplar cell wall peroxidase directly oxidizes polymer lignin and produces in vitro dehydrogenative polymer rich in β-O-4 linkage. FEBS Lett. 2004, 562, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Önnerud, H.; Zhang, L.; Gellerstedt, G.r.; Henriksson, G. Polymerization of Monolignols by Redox Shuttle–Mediated Enzymatic Oxidation: A New Model in Lignin Biosynthesis I. Plant Cell 2002, 14, 1953–1962. [Google Scholar] [CrossRef]
- Sharma, V.; Tsai, M.-L.; Nargotra, P.; Chen, C.-W.; Sun, P.-P.; Singhania, R.R.; Patel, A.K.; Dong, C.-D. Journey of lignin from a roadblock to bridge for lignocellulose biorefineries: A comprehensive review. Sci. Total Environ. 2023, 861, 160560. [Google Scholar] [CrossRef]
- Liao, J.J.; Latif, N.H.A.; Trache, D.; Brosse, N.; Hussin, M.H. Current advancement on the isolation, characterization and application of lignin. Int. J. Biol. Macromol. 2020, 162, 985–1024. [Google Scholar] [CrossRef]
- Gong, Z.; Shuai, L. Lignin condensation, an unsolved mystery. Trends Chem. 2023, 5, 163–166. [Google Scholar] [CrossRef]
- Yu, S.; Chen, L.; Xie, Y.; Feng, Q.; Chen, C. Lignin/polysaccharide composite: A nature-made match toward multifunctional bio-based materials. Prog. Mater. Sci. 2025, 148, 101383. [Google Scholar] [CrossRef]
- Fodil Cherif, M.; Trache, D.; Brosse, N.; Benaliouche, F.; Tarchoun, A.F. Comparison of the Physicochemical Properties and Thermal Stability of Organosolv and Kraft Lignins from Hardwood and Softwood Biomass for Their Potential Valorization. Waste Biomass Valorization 2020, 11, 6541–6553. [Google Scholar] [CrossRef]
- Moretti, C.; Corona, B.; Hoefnagels, R.; Vural-Gürsel, I.; Gosselink, R.; Junginger, M. Review of life cycle assessments of lignin and derived products: Lessons learned. Sci. Total Environ. 2021, 770, 144656. [Google Scholar] [CrossRef] [PubMed]
- Beig, B.; Riaz, M.; Raza Naqvi, S.; Hassan, M.; Zheng, Z.; Karimi, K.; Pugazhendhi, A.; Atabani, A.E.; Thuy Lan Chi, N. Current challenges and innovative developments in pretreatment of lignocellulosic residues for biofuel production: A review. Fuel 2021, 287, 119670. [Google Scholar] [CrossRef]
- Gnansounou, E.; Dauriat, A. Techno-economic analysis of lignocellulosic ethanol: A review. Bioresour. Technol. 2010, 101, 4980–4991. [Google Scholar] [CrossRef]
- Dionísio, S.R.; Santoro, D.C.J.; Bonan, C.I.D.G.; Soares, L.B.; Biazi, L.E.; Rabelo, S.C.; Ienczak, J.L. Second-generation ethanol process for integral use of hemicellulosic and cellulosic hydrolysates from diluted sulfuric acid pretreatment of sugarcane bagasse. Fuel 2021, 304, 121290. [Google Scholar] [CrossRef]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M.; Ladisch, M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef]
- Haghighi Mood, S.; Hossein Golfeshan, A.; Tabatabaei, M.; Salehi Jouzani, G.; Najafi, G.H.; Gholami, M.; Ardjmand, M. Lignocellulosic biomass to bioethanol, a comprehensive review with a focus on pretreatment. Renew. Sustain. Energy Rev. 2013, 27, 77–93. [Google Scholar] [CrossRef]
- Taherzadeh, M.J.; Karimi, K. Pretreatment of Lignocellulosic Wastes to Improve Ethanol and Biogas Production: A Review. Int. J. Mol. Sci. 2008, 9, 1621–1651. [Google Scholar] [CrossRef]
- Alvira, P.; Tomás-Pejó, E.; Ballesteros, M.; Negro, M.J. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review. Bioresour. Technol. 2010, 101, 4851–4861. [Google Scholar] [CrossRef]
- Chiaramonti, D.; Prussi, M.; Ferrero, S.; Oriani, L.; Ottonello, P.; Torre, P.; Cherchi, F. Review of pretreatment processes for lignocellulosic ethanol production, and development of an innovative method. Biomass Bioenergy 2012, 46, 25–35. [Google Scholar] [CrossRef]
- Weil, J.; Westgate, P.; Kohlmann, K.; Ladisch, M.R. Cellulose pretreaments of lignocellulosic substrates. Enzym. Microb. Technol. 1994, 16, 1002–1004. [Google Scholar] [CrossRef]
- Wagner, A.; Lackner, N.; Mutschlechner, M.; Prem, E.; Markt, R.; Illmer, P. Biological Pretreatment Strategies for Second-Generation Lignocellulosic Resources to Enhance Biogas Production. Energies 2018, 11, 1797. [Google Scholar] [CrossRef]
- Xiang, Q.; Lee, Y.Y. Production of oxychemicals from precipitated hardwood lignin. Appl. Biochem. Biotechnol. 2001, 91, 71–80. [Google Scholar] [CrossRef]
- Hernández-Beltrán, J.U.; Hernández-De Lira, I.O.; Cruz-Santos, M.M.; Saucedo-Luevanos, A.; Hernández-Terán, F.; Balagurusamy, N. Insight into Pretreatment Methods of Lignocellulosic Biomass to Increase Biogas Yield: Current State, Challenges, and Opportunities. Appl. Sci. 2019, 9, 3721. [Google Scholar] [CrossRef]
- Tanis, M.H.; Wallberg, O.; Galbe, M.; Al-Rudainy, B. Lignin Extraction by Using Two-Step Fractionation: A Review. Molecules 2024, 29, 98. [Google Scholar] [CrossRef]
- Xue, F.; Li, W.; An, S.; Li, C.; Li, X.; Wu, M.; Wei, X. Ethylene glycol based acid pretreatment of corn stover for cellulose enzymatic hydrolysis. RSC Adv. 2021, 11, 14140–14147. [Google Scholar] [CrossRef]
- Harris, E.E.; Mitchell, R.L. Effect of Various Pre-extractions on the Lignin Determination of Wood. Ind. Eng. Chem. Anal. Ed. 1939, 11, 153–155. [Google Scholar] [CrossRef]
- Casimiro, F.M.; Costa, C.A.E.; Vega-Aguilar, C.; Rodrigues, A.E. Hardwood and softwood lignins from sulfite liquors: Structural characterization and valorization through depolymerization. Int. J. Biol. Macromol. 2022, 215, 272–279. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.; Li, W.; Wu, W.; Tang, B.; Zhu, C. Efficient pretreatment of cornstalks for lignin valorization using p-toluene sulfonic acid coupling ethylene glycol. Biomass Convers. Biorefinery 2024, 14, 18707–18720. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, L.; Lu, X.; Zhang, S. Pretreatment of corn stover with diluted acetic acid for enhancement of acidogenic fermentation. Bioresour. Technol. 2014, 158, 12–18. [Google Scholar] [CrossRef]
- Chen, Y.; Yan, Z.; Liang, L.; Ran, M.; Wu, T.; Wang, B.; Zou, X.; Zhao, M.; Fang, G.; Shen, K. Comparative Evaluation of Organic Acid Pretreatment of Eucalyptus for Kraft Dissolving Pulp Production. Materials 2020, 13, 361. [Google Scholar] [CrossRef]
- Chen, L.; Dou, J.; Ma, Q.; Li, N.; Wu, R.; Bian, H.; Yelle, D.J.; Vuorinen, T.; Fu, S.; Pan, X.; et al. Rapid and near-complete dissolution of wood lignin at ≤80 °C by a recyclable acid hydrotrope. Sci. Adv. 2017, 3, e1701735. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Zhu, J.; Cao, L.; Yan, L.; Qin, C.; Liang, C.; Yao, S. Acidolysis mechanism of lignin from bagasse during p-toluenesulfonic acid treatment. Ind. Crops Prod. 2022, 176, 114374. [Google Scholar] [CrossRef]
- Zhai, Q.; Han, S.; Hse, C.-Y.; Jiang, J.; Xu, J. 5-Sulfosalicylic acid as an acid hydrotrope for the rapid and green fractionation of woody biomass. Ind. Crops Prod. 2022, 177, 114435. [Google Scholar] [CrossRef]
- Solarte-Toro, J.C.; Romero-García, J.M.; Martínez-Patiño, J.C.; Ruiz-Ramos, E.; Castro-Galiano, E.; Cardona-Alzate, C.A. Acid pretreatment of lignocellulosic biomass for energy vectors production: A review focused on operational conditions and techno-economic assessment for bioethanol production. Renew. Sustain. Energy Rev. 2019, 107, 587–601. [Google Scholar] [CrossRef]
- Barr, M.R.; Forster, L.; D’Agostino, C.; Volpe, R. Alkaline pretreatment of walnut shells increases pore surface hydrophilicity of derived biochars. Appl. Surf. Sci. 2022, 571, 151253. [Google Scholar] [CrossRef]
- Shin, J.; Kwak, J.; Lee, Y.-G.; Kim, S.; Choi, M.; Bae, S.; Lee, S.-H.; Park, Y.; Chon, K. Competitive adsorption of pharmaceuticals in lake water and wastewater effluent by pristine and NaOH-activated biochars from spent coffee wastes: Contribution of hydrophobic and π-π interactions. Environ. Pollut. 2021, 270, 116244. [Google Scholar] [CrossRef]
- Gordobil, O.; Moriana, R.; Zhang, L.; Labidi, J.; Sevastyanova, O. Assesment of technical lignins for uses in biofuels and biomaterials: Structure-related properties, proximate analysis and chemical modification. Ind. Crops Prod. 2016, 83, 155–165. [Google Scholar] [CrossRef]
- Oriez, V.; Peydecastaing, J.; Pontalier, P.-Y. Lignocellulosic Biomass Mild Alkaline Fractionation and Resulting Extract Purification Processes: Conditions, Yields, and Purities. Clean Technol. 2020, 2, 91–115. [Google Scholar] [CrossRef]
- Bleaching of Non-Wood Pulps. Can Sodium Hydroxide Be Replaced with Sodium Carbonate in the Alkaline Extraction of Non-Wood Pulps? 1990 Pulping Conference Proceedings. Available online: https://imisrise.tappi.org/TAPPI/Products/PUL/PULP90487.aspx (accessed on 13 February 2025).
- Sarwar Jahan, M.; Shamsuzzaman, M.; Rahman, M.M.; Iqbal Moeiz, S.M.; Ni, Y. Effect of pre-extraction on soda-anthraquinone (AQ) pulping of rice straw. Ind. Crops Prod. 2012, 37, 164–169. [Google Scholar] [CrossRef]
- Song, D.-Y.; Lee, J.-W. Valorization of biochar and lignin derived from the NaOH pretreatment of lignocellulosic biomass for applications as an adsorbent and antioxidant. Biomass Bioenergy 2024, 182, 107103. [Google Scholar] [CrossRef]
- Tao, L.; Ren, J.; Yu, F.K.; Ni, T.R. Effects of Liquid-to-Solid Ratio and Reaction Temperature on NaOH Pretreatment of Achnatherum splendens. Asian J. Chem. 2013, 25, 3545–3548. [Google Scholar] [CrossRef]
- Nitsos, C.; Stoklosa, R.; Karnaouri, A.; Vörös, D.; Lange, H.; Hodge, D.B.; Crestini, C.; Rova, U.S.L.; Christakopoulos, P.J.J.W.; Sons, L. Isolation and Characterization of Organosolv and Alkaline Lignins from Hardwood and Softwood Biomass. ACS Sustain. Chem. Eng. 2016, 4, 5181–5193. [Google Scholar] [CrossRef]
- Zheng, M.; Li, X.; Li, L.; Yang, X.; He, Y. Enhancing anaerobic biogasification of corn stover through wet state NaOH pretreatment. Bioresour. Technol. 2009, 100, 5140–5145. [Google Scholar] [CrossRef]
- Lou, H.; Hu, Q.; Qiu, X.; Li, X.; Lin, X. Pretreatment of Miscanthus by NaOH/Urea Solution at Room Temperature for Enhancing Enzymatic Hydrolysis. BioEnergy Res. 2016, 9, 335–343. [Google Scholar] [CrossRef]
- Zhu, Q.; Wei, W.; Sun, J.; Wang, Q. College of Natural Resources. One-Pot NaOH/Urea Pretreatment and Saccharification of Corn Stover for Fermentable Sugar Production. BioResources 2018, 13, 5875–5882. [Google Scholar] [CrossRef]
- Li, C.; Fan, M.; Xie, J.; Zhang, H. Effect of NaOH-catalyzed organosolv pretreatment on the co-production of ethanol and xylose from poplar. Ind. Crops Prod. 2023, 200, 116774. [Google Scholar] [CrossRef]
- Vishtal, A.; Kraslawski, A. Challenges in industrial applications of technical lignins. BioResources 2011, 6, 3547–3568. [Google Scholar] [CrossRef]
- Alam, M.M.; Greco, A.; Rajabimashhadi, Z.; Corcione, C.E. Efficient and environmentally friendly techniques for extracting lignin from lignocellulose biomass and subsequent uses: A review. Clean. Mater. 2024, 13, 100253. [Google Scholar] [CrossRef]
- Zhang, K.; Pei, Z.; Wang, D. Organic solvent pretreatment of lignocellulosic biomass for biofuels and biochemicals: A review. Bioresour. Technol. 2016, 199, 21–33. [Google Scholar] [CrossRef]
- Parot, M.; Rodrigue, D.; Stevanovic, T. High purity softwood lignin obtained by an eco-friendly organosolv process. Bioresour. Technol. Rep. 2022, 17, 100880. [Google Scholar] [CrossRef]
- Pye, E.K.; Berlin, A.; Winner, S.R.; Armiger, W.B. High Yield Biorefining Using Organosolv Processing. In Proceedings of the 2008 AIChE Annual Meeting, Philadelphia, PA, USA, 16–21 November 2008. [Google Scholar]
- Rodrigues Gurgel da Silva, A.; Errico, M.; Rong, B.-G. Techno-economic analysis of organosolv pretreatment process from lignocellulosic biomass. Clean Technol. Environ. Policy 2018, 20, 1401–1412. [Google Scholar] [CrossRef]
- Bujanovic, B.M.; Hirth, K.; Ralph, S.; Reiner, R.S.; Dongre, P.; Mickles, C.; Karlen, S.D.; Baez, C.; Clemons, C. Use of Renewable Alcohols in Autocatalytic Production of Aspen Organosolv Lignins. ACS Omega 2024, 9, 38227–38247. [Google Scholar] [CrossRef]
- Oh, Y.S.; Sellers, T.; Kim, M.G.; Strickland, R.C. Evaluation of phenol-formaldehyde osb resins modified with lignin residues from acid-hydrolyzed waste newsprint. For. Prod. J. 1994, 44, 25–29. [Google Scholar]
- Das, A.; Mohanty, K. Optimization of lignin extraction from bamboo by ultrasound-assisted organosolv pretreatment. Bioresour. Technol. 2023, 376, 128884. [Google Scholar] [CrossRef]
- Win, N.N.; Weinwurm, F.; Friedl, A. Investigation of organosolv and hot-compressed water pretreatments of rice straw. Biomass Convers. Biorefinery 2016, 6, 355–364. [Google Scholar] [CrossRef]
- Díaz, M.J.; Huijgen, W.J.J.; Laan, R.R.v.d.; Reith, J.H.; Cara, C.; Castro, E. Organosolv pretreatment of olive tree biomass for fermentable sugars. Holzforschung 2011, 65, 177–183. [Google Scholar] [CrossRef]
- Wei, W.; Wang, B.; Wang, X.; Ling, R.; Jin, Y. Comparison of acid and alkali catalyzed ethylene glycol organosolv pretreatment for sugar production from bagasse. Bioresour. Technol. 2021, 320, 124293. [Google Scholar] [CrossRef]
- Song, G.; Bai, Y.; Pan, Z.; Liu, D.; Qin, Y.; Zhang, Y.; Fan, Z.; Li, Y.; Madadi, M. Enhancing fermentable sugar production from sugarcane bagasse through surfactant-assisted ethylene glycol pretreatment and enzymatic hydrolysis: Reduced temperature and enzyme loading. Renew. Energy 2024, 227, 120515. [Google Scholar] [CrossRef]
- Tang, S.; Dong, Q.; Fang, Z.; Miao, Z.-d. Complete recovery of cellulose from rice straw pretreated with ethylene glycol and aluminum chloride for enzymatic hydrolysis. Bioresour. Technol. 2019, 284, 98–104. [Google Scholar] [CrossRef]
- Ghozatloo, A.; Mohammadi-Rovshandeh, J.; Hashemi, S.J. Optimization of pulp properties by dimethyl formamide pulping of rice straw. Cellul. Chem. Technol. 2006, 40, 659–667. [Google Scholar]
- Lv, Y.; Chen, Z.; Wang, H.; Xiao, Y.; Ling, R.; Gong, M.; Wei, W. Enhancement of glucose production from sugarcane bagasse through an HCl-catalyzed ethylene glycol pretreatment and Tween 80. Renew. Energy 2022, 194, 495–503. [Google Scholar] [CrossRef]
- De Santi, A.; Galkin, M.V.; Lahive, C.W.; Deuss, P.J.; Barta, K. Lignin-First Fractionation of Softwood Lignocellulose Using a Mild Dimethyl Carbonate and Ethylene Glycol Organosolv Process. ChemSusChem 2020, 13, 4468–4477. [Google Scholar] [CrossRef]
- Chen, Z.; Ragauskas, A.; Wan, C. Lignin extraction and upgrading using deep eutectic solvents. Ind. Crops Prod. 2020, 147, 112241. [Google Scholar] [CrossRef]
- Bo, L.; Zhang, X.; Luo, Z.; Saboori, T.; Dehghan, M.; Ghasemizadeh, M.; Karimi-Maleh, H.; Alagumalai, A.; Mahian, O. An overview of the applications of ionic fluids and deep eutectic solvents enhanced by nanoparticles. J. Therm. Anal. Calorim. 2022, 147, 7589–7601. [Google Scholar] [CrossRef]
- Grillo, G.; Gaudino, E.C.; Rosa, R.; Leonelli, C.; Timonina, A.; Grygiškis, S.; Tabasso, S.; Cravotto, G. Green deep eutectic solvents for microwave-assisted biomass delignification and valorisation. Molecules 2021, 26, 798. [Google Scholar] [CrossRef]
- Xiao, T.; Hou, M.; Guo, X.; Cao, X.; Li, C.; Zhang, Q.; Jia, W.; Sun, Y.; Guo, Y.; Shi, H. Recent progress in deep eutectic solvent(DES) fractionation of lignocellulosic components: A review. Renew. Sustain. Energy Rev. 2024, 192, 114243. [Google Scholar] [CrossRef]
- Jafari, K.; Fatemi, M.H.; Estellé, P. Deep eutectic solvents (DESs): A short overview of the thermophysical properties and current use as base fluid for heat transfer nanofluids. J. Mol. Liq. 2021, 321, 114752. [Google Scholar] [CrossRef]
- Almeida, R.O.; Maloney, T.C.; Gamelas, J.A.F. Production of functionalized nanocelluloses from different sources using deep eutectic solvents and their applications. Ind. Crops Prod. 2023, 199, 116583. [Google Scholar] [CrossRef]
- Zhang, C.-W.; Xia, S.-Q.; Ma, P.-S. Facile pretreatment of lignocellulosic biomass using deep eutectic solvents. Bioresour. Technol. 2016, 219, 1–5. [Google Scholar] [CrossRef]
- Ma, H.; Fu, P.; Zhao, J.; Lin, X.; Wu, W.; Yu, Z.; Xia, C.; Wang, Q.; Gao, M.; Zhou, J. Pretreatment of Wheat Straw Lignocelluloses by Deep Eutectic Solvent for Lignin Extraction. Molecules 2022, 27, 7955. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, J.; Xiao, J.; He, X.; Zhang, K.; Yuan, S.; Peng, Z.; Chen, Z.; Lin, X. Enhanced Enzymatic Hydrolysis and Lignin Extraction of Wheat Straw by Triethylbenzyl Ammonium Chloride/Lactic Acid-Based Deep Eutectic Solvent Pretreatment. ACS Omega 2019, 4, 19829–19839. [Google Scholar] [CrossRef]
- Chen, Z.; Wan, C. Ultrafast fractionation of lignocellulosic biomass by microwave-assisted deep eutectic solvent pretreatment. Bioresour. Technol. 2018, 250, 532–537. [Google Scholar] [CrossRef]
- Lobato-Rodríguez, Á.; Gullón, B.; Romaní, A.; Ferreira-Santos, P.; Garrote, G.; Del-Río, P.G. Recent advances in biorefineries based on lignin extraction using deep eutectic solvents: A review. Bioresour. Technol. 2023, 388, 129744. [Google Scholar] [CrossRef]
- Cronin, D.J.; Chen, X.; Moghaddam, L.; Zhang, X. Deep Eutectic Solvent Extraction of High-Purity Lignin from a Corn Stover Hydrolysate. ChemSusChem 2020, 13, 4678–4690. [Google Scholar] [CrossRef]
- Tribot, A.; Amer, G.; Abdou Alio, M.; de Baynast, H.; Delattre, C.; Pons, A.; Mathias, J.-D.; Callois, J.-M.; Vial, C.; Michaud, P.; et al. Wood-lignin: Supply, extraction processes and use as bio-based material. Eur. Polym. J. 2019, 112, 228–240. [Google Scholar] [CrossRef]
- Margarida Martins, M.; Carvalheiro, F.; Gírio, F. An overview of lignin pathways of valorization: From isolation to refining and conversion into value-added products. Biomass Convers. Biorefinery 2024, 14, 3183–3207. [Google Scholar] [CrossRef]
- Hasanov, I.; Raud, M.; Kikas, T. The Role of Ionic Liquids in the Lignin Separation from Lignocellulosic Biomass. Energies 2020, 13, 4864. [Google Scholar] [CrossRef]
- Norfarhana, A.S.; Ilyas, R.A.; Ngadi, N.; Othman, M.H.D.; Misenan, M.S.M.; Norrrahim, M.N.F. Revolutionizing lignocellulosic biomass: A review of harnessing the power of ionic liquids for sustainable utilization and extraction. Int. J. Biol. Macromol. 2024, 256, 128256. [Google Scholar] [CrossRef]
- Azimi, B.; Maleki, H.; Gigante, V.; Bagherzadeh, R.; Mezzetta, A.; Milazzo, M.; Guazzelli, L.; Cinelli, P.; Lazzeri, A.; Danti, S. Cellulose-based fiber spinning processes using ionic liquids. Cellulose 2022, 29, 3079–3129. [Google Scholar] [CrossRef]
- Ullah, Z.; Bustam, M.A.; Man, Z.; Muhammad, N.; Khan, A.S. Synthesis, characterization and the effect of temperature on different physicochemical properties of protic ionic liquids. RSC Adv. 2015, 5, 71449–71461. [Google Scholar] [CrossRef]
- Financie, R.; Moniruzzaman, M.; Uemura, Y. Enhanced enzymatic delignification of oil palm biomass with ionic liquid pretreatment. Biochem. Eng. J. 2016, 110, 1–7. [Google Scholar] [CrossRef]
- Eqbalpour, M.; Andooz, A.; Kowsari, E.; Ramakrishna, S.; Cheshmeh, Z.A.; Chinnappan, A. A comprehensive review on how ionic liquids enhance the pyrolysis of cellulose, lignin, and lignocellulose toward a circular economy. Wiley Interdiscip. Rev. Energy Environ. 2023, 12, e473. [Google Scholar] [CrossRef]
- Bernardo, J.R.; Gírio, F.M.; Łukasik, R.M. The effect of the chemical character of ionic liquids on biomass pre-treatment and posterior enzymatic hydrolysis. Molecules 2019, 24, 808. [Google Scholar] [CrossRef]
- Muhammad, N.; Man, Z.; Bustam, M.A.; Mutalib, M.I.A.; Rafiq, S. Investigations of novel nitrile-based ionic liquids as pre-treatment solvent for extraction of lignin from bamboo biomass. J. Ind. Eng. Chem. 2013, 19, 207–214. [Google Scholar] [CrossRef]
- Ullah, Z.; Man, Z.; Khan, A.S.; Muhammad, N.; Mahmood, H.; Ben Ghanem, O.; Ahmad, P.; Hassan Shah, M.-U.; Mamoon Ur, R.; Raheel, M. Extraction of valuable chemicals from sustainable rice husk waste using ultrasonic assisted ionic liquids technology. J. Clean. Prod. 2019, 220, 620–629. [Google Scholar] [CrossRef]
- Fu, D.; Mazza, G. Aqueous ionic liquid pretreatment of straw. Bioresour. Technol. 2011, 102, 7008–7011. [Google Scholar] [CrossRef]
- Pu, Y.; Jiang, N.; Ragauskas, A.J. Ionic Liquid as a Green Solvent for Lignin. J. Wood Chem. Technol. 2007, 27, 23–33. [Google Scholar] [CrossRef]
- Weerachanchai, P.; Lee, J.-M. Recyclability of an ionic liquid for biomass pretreatment. Bioresour. Technol. 2014, 169, 336–343. [Google Scholar] [CrossRef]
- Jardim, J.M.; Hart, P.W.; Lucia, L.; Jameel, H. Insights into the potential of hardwood kraft lignin to be a green platform material for emergence of the biorefinery. Polymers 2020, 12, 1795. [Google Scholar] [CrossRef]
- Hu, R.; Zhan, J.; Zhao, Y.; Xu, X.; Luo, G.; Fan, J.; Clark, J.H.; Zhang, S. Bio-based platform chemicals synthesized from lignin biorefinery. Green Chem. 2023, 25, 8970–9000. [Google Scholar] [CrossRef]
- Lahtinen, M.H.; Mikkilä, J.; Mikkonen, K.S.; Kilpeläinen, I. Kraft Process—Formation of Secoisolariciresinol Structures and Incorporation of Fatty Acids in Kraft Lignin. J. Agric. Food Chem. 2021, 69, 5955–5965. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, W.; Zhang, D.; Li, X.; Shi, J. Structural Characteristics–Reactivity Relationships for Catalytic Depolymerization of Lignin into Aromatic Compounds: A Review. Int. J. Mol. Sci. 2023, 24, 8330. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Yadama, V. Effects of hot water extraction (HWE) of Douglas fir as a pre-process for the sulfite pretreatment to overcome recalcitrance of lignocellulose (SPORL). Holzforschung 2017, 71, 91–98. [Google Scholar] [CrossRef]
- Zhou, H.; Gleisner, R.; Zhu, J.Y.; Tian, Y.; Qiao, Y. SPORL Pretreatment Spent Liquors Enhance the Enzymatic Hydrolysis of Cellulose and Ethanol Production from Glucose. Energy Fuels 2018, 32, 7636–7642. [Google Scholar] [CrossRef]
- Wang, G.S.; Pan, X.J.; Zhu, J.Y.; Gleisner, R.; Rockwood, D. Sulfite Pretreatment to Overcome Recalcitrance of Lignocellulose (SPORL) for Robust Enzymatic Saccharification of Hardwoods. Biotechnol. Prog. 2009, 25, 1086–1093. [Google Scholar] [CrossRef] [PubMed]
- Brienza, F.; Cannella, D.; Montesdeoca, D.; Cybulska, I.; Debecker, D.P. A guide to lignin valorization in biorefineries: Traditional, recent, and forthcoming approaches to convert raw lignocellulose into valuable materials and chemicals. RSC Sustain. 2024, 2, 37–90. [Google Scholar] [CrossRef]
- Shevchenko, A.R.; Mayorova, K.A.; Chukhchin, D.G.; Malkov, A.V.; Toptunov, E.A.; Telitsin, V.D.; Rozhkova, A.M.; Zorov, I.N.; Rodicheva, M.A.; Plakhin, V.A.; et al. Enzymatic Hydrolysis of Kraft and Sulfite Pulps: What Is the Best Cellulosic Substrate for Industrial Saccharification? Fermentation 2023, 9, 936. [Google Scholar] [CrossRef]
- Fernández-Rodríguez, J.; Erdocia, X.; Hernández-Ramos, F.; Alriols, M.G.; Labidi, J. Chapter 7—Lignin Separation and Fractionation by Ultrafiltration. In Separation of Functional Molecules in Food by Membrane Technology; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 229–265. [Google Scholar] [CrossRef]
- Rathore, A.S.; Singh, A. Biomass to fuels and chemicals: A review of enabling processes and technologies. J. Chem. Technol. Biotechnol. 2021, 97, 597–607. [Google Scholar] [CrossRef]
- Shu, R.; Lin, B.; Wang, C.; Chen, Y. Hydrogenolysis of dealkaline lignin catalyzed by noble metal cooperated with metal chloride. Energy Procedia 2019, 158, 406–411. [Google Scholar] [CrossRef]
- Roy, P.; Dias, G. Prospects for pyrolysis technologies in the bioenergy sector: A review. Renew. Sustain. Energy Rev. 2017, 77, 59–69. [Google Scholar] [CrossRef]
- Howe, D.; Westover, T.; Carpenter, D.; Santosa, D.; Emerson, R.; Deutch, S.; Starace, A.; Kutnyakov, I.; Lukins, C. Field-to-Fuel Performance Testing of Lignocellulosic Feedstocks: An Integrated Study of the Fast Pyrolysis–Hydrotreating Pathway. Energy Fuels 2015, 29, 3188–3197. [Google Scholar] [CrossRef]
- Crombie, K.; Mašek, O. Investigating the potential for a self-sustaining slow pyrolysis system under varying operating conditions. Bioresour. Technol. 2014, 162, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Patwardhan, P.R.; Satrio, J.A.; Brown, R.C.; Shanks, B.H. Influence of inorganic salts on the primary pyrolysis products of cellulose. Bioresour. Technol. 2010, 101, 4646–4655. [Google Scholar] [CrossRef]
- Kanaujia, P.K.; Sharma, Y.K.; Garg, M.O.; Tripathi, D.; Singh, R. Review of analytical strategies in the production and upgrading of bio-oils derived from lignocellulosic biomass. J. Anal. Appl. Pyrolysis 2014, 105, 55–74. [Google Scholar] [CrossRef]
- Iribarren, D.; Peters, J.F.; Dufour, J. Life cycle assessment of transportation fuels from biomass pyrolysis. Fuel 2012, 97, 812–821. [Google Scholar] [CrossRef]
- Dang, Q.; Yu, C.; Luo, Z. Environmental life cycle assessment of bio-fuel production via fast pyrolysis of corn stover and hydroprocessing. Fuel 2014, 131, 36–42. [Google Scholar] [CrossRef]
- Kosa, M.; Ben, H.; Theliander, H.; Ragauskas, A.J. Pyrolysis oils from CO2 precipitated Kraft lignin. Green Chem. 2011, 13, 3196–3202. [Google Scholar] [CrossRef]
- Kibet, J.; Khachatryan, L.; Dellinger, B. Molecular Products and Radicals from Pyrolysis of Lignin. Environ. Sci. Technol. 2012, 46, 12994–13001. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Fan, J.; Chang, J. Effect of reaction conditions on hydrothermal degradation of cornstalk lignin. J. Anal. Appl. Pyrolysis 2012, 94, 190–195. [Google Scholar] [CrossRef]
- Yu, Y.; Li, X.; Su, L.; Zhang, Y.; Wang, Y.; Zhang, H. The role of shape selectivity in catalytic fast pyrolysis of lignin with zeolite catalysts. Appl. Catal. A Gen. 2012, 447, 115–123. [Google Scholar] [CrossRef]
- Kurnia, I.; Karnjanakom, S.; Bayu, A.; Yoshida, A.; Rizkiana, J.; Prakoso, T.; Abudula, A.; Guan, G. In-situ catalytic upgrading of bio-oil derived from fast pyrolysis of lignin over high aluminum zeolites—ScienceDirect. Fuel Process. Technol. 2017, 167, 730–737. [Google Scholar] [CrossRef]
- Ma, Z.; Troussard, E.; Van Bokhoven, J.A. Controlling the selectivity to chemicals from lignin via catalytic fast pyrolysis. Appl. Catal. A Gen. 2012, 423–424, 130–136. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Kumar, J.; Bhaskar, T. Catalytic pyrolysis of soda lignin over zeolites using pyrolysis gas chromatography-mass spectrometry. Bioresour. Technol. 2019, 291, 121822. [Google Scholar] [CrossRef]
- Kim, J.Y.; Moon, J.; Lee, J.H.; Jin, X.; Choi, J.W. Conversion of phenol intermediates into aromatic hydrocarbons over various zeolites during lignin pyrolysis. Fuel 2020, 279, 118484. [Google Scholar] [CrossRef]
- Paysepar, H.; Venkateswara Rao, K.T.; Yuan, Z.; Shui, H.; Xu, C. Production of phenolic chemicals from hydrolysis lignin via catalytic fast pyrolysis. J. Anal. Appl. Pyrolysis 2020, 149, 104842. [Google Scholar] [CrossRef]
- Nair, V.; Vinu, R. Production of guaiacols via catalytic fast pyrolysis of alkali lignin using titania, zirconia and ceria. J. Anal. Appl. Pyrolysis 2016, 119, 31–39. [Google Scholar] [CrossRef]
- Nolte, M.W.; Shanks, B.H. A Perspective on Catalytic Strategies for Deoxygenation in Biomass Pyrolysis. Energy Technol. 2017, 5, 7–18. [Google Scholar] [CrossRef]
- Zhang, C.; Hu, X.; Guo, H.; Wei, T.; Dong, D.; Hu, G.; Hu, S.; Xiang, J.; Liu, Q.; Wang, Y. Pyrolysis of poplar, cellulose and lignin: Effects of acidity and alkalinity of the metal oxide catalysts. J. Anal. Appl. Pyrolysis 2018, 134, 590–605. [Google Scholar] [CrossRef]
- Kaiqi, S.; Shuangxi, S.; Qiang, H.; Xuwen, L.; Lan, J.; Ya, L. Review of catalytic pyrolysis of biomass for bio-oil. In Proceedings of the 2011 International Conference on Materials for Renewable Energy & Environment, Shanghai, China, 20–22 May 2011; pp. 317–321. [Google Scholar]
- Demiral, İ.; Şensöz, S. The effects of different catalysts on the pyrolysis of industrial wastes (olive and hazelnut bagasse). Bioresour. Technol. 2008, 99, 8002–8007. [Google Scholar] [CrossRef]
- Ryu, H.W.; Lee, H.W.; Jae, J.; Park, Y.-K. Catalytic pyrolysis of lignin for the production of aromatic hydrocarbons: Effect of magnesium oxide catalyst. Energy 2019, 179, 669–675. [Google Scholar] [CrossRef]
- Hendry, A.; Åhlén, M.; Fernandes, T.; Cheung, O.; Sanna, A. Catalytic cracking of Etek lignin with zirconia supported metal-oxides for alkyl and alkoxy phenols recovery. Bioresour. Technol. 2020, 317, 124008. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Yang, H.; Chen, P.; Liu, Z.; Chen, Y.; Wang, L.; Wang, X.; Chen, H. Lignin Characterization and Catalytic Pyrolysis for Phenol-Rich Oil with TiO2-Based Catalysts. Energy Fuels 2019, 33, 9934–9941. [Google Scholar] [CrossRef]
- Wang, H.; Han, H.; Sun, E.; Zhang, Y.; Li, J.; Chen, Y.; Song, H.; Zhao, H. Production of aryl oxygen-containing compounds from catalytic pyrolysis of bagasse lignin over LaTixFe1−xO3. Chin. J. Chem. Eng. 2019, 27, 1939–1944. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Guo, F.; Li, X.; Li, T.; Guo, C.; Chang, J. Characterization of the gas releasing behaviors of catalytic pyrolysis of rice husk using potassium over a micro-fluidized bed reactor. Energy Convers. Manag. 2017, 136, 395–403. [Google Scholar] [CrossRef]
- Geng, J.; Wang, W.; Yu, Y.-X.; Chang, J.-m.; Cai, L.; Shi, S.Q. Adding nickel formate in alkali lignin to increase contents of alkylphenols and aromatics during fast pyrolysis. Bioresour. Technol. 2017, 227, 1–6. [Google Scholar] [CrossRef]
- Peng, C.; Zhang, G.; Yue, J.; Xu, G. Pyrolysis of lignin for phenols with alkaline additive. Fuel Process. Technol. 2014, 124, 212–221. [Google Scholar] [CrossRef]
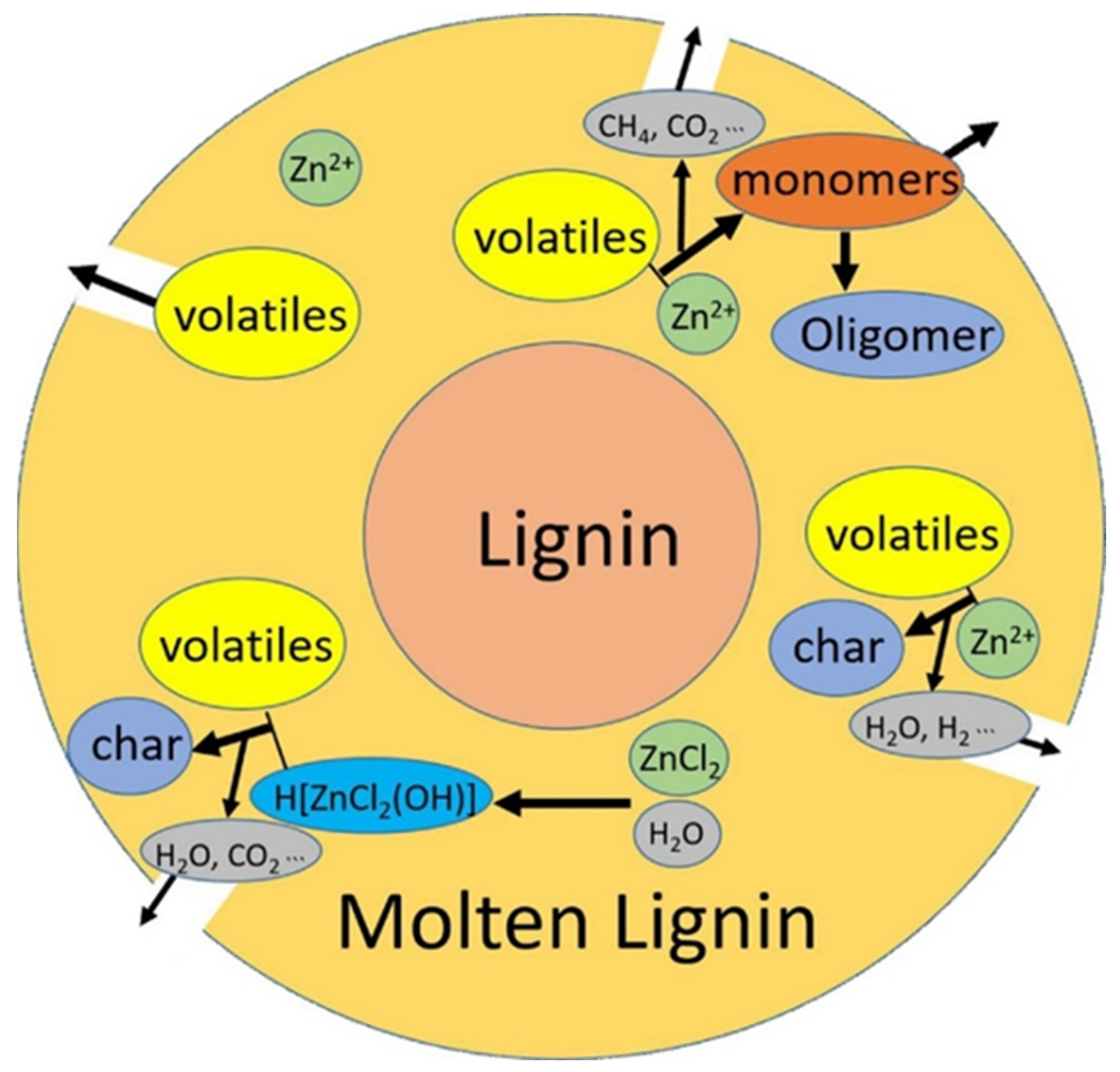
- Hu, J.; Shen, D.; Wu, S.; Xiao, R. Insight into the effect of ZnCl2 on analytical pyrolysis behavior of cellulolytic enzyme corn stover lignin. J. Anal. Appl. Pyrolysis 2017, 127, 444–450. [Google Scholar] [CrossRef]
- Duval, A.; Lawoko, M. A review on lignin-based polymeric, micro- and nano-structured materials. React. Funct. Polym. 2014, 85, 78–96. [Google Scholar] [CrossRef]
- Fu, C.; Mielenz, J.R.; Xiao, X.; Ge, Y.; Hamilton, C.Y.; Rodriguez, M.; Chen, F.; Foston, M.; Ragauskas, A.; Bouton, J.; et al. Genetic manipulation of lignin reduces recalcitrance and improves ethanol production from switchgrass. Proc. Natl. Acad. Sci. USA 2011, 108, 3803–3808. [Google Scholar] [CrossRef]
- Chen, F.; Dixon, R.A. Lignin modification improves fermentable sugar yields for biofuel production. Nat. Biotechnol. 2007, 25, 759–761. [Google Scholar] [CrossRef] [PubMed]
- Gosselink, R.J.; Teunissen, W.; van Dam, J.E.; de Jong, E.; Gellerstedt, G.; Scott, E.L.; Sanders, J.P. Lignin depolymerisation in supercritical carbon dioxide/acetone/water fluid for the production of aromatic chemicals. Bioresour. Technol. 2012, 106, 173–177. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, q.; Long, J.; Xu, Y.; Wang, T.; Ma, L.; Li, Y. Phenolics Production through Catalytic Depolymerization of Alkali Lignin with Metal Chlorides. BioResources 2014, 9, 3347–3360. [Google Scholar] [CrossRef]
- Hidajat, M.J.; Riaz, A.; Park, J.; Insyani, R.; Verma, D.; Kim, J. Depolymerization of concentrated sulfuric acid hydrolysis lignin to high-yield aromatic monomers in basic sub- and supercritical fluids. Chem. Eng. J. 2017, 317, 9–19. [Google Scholar] [CrossRef]
- Sergeev, A.G.; Hartwig, J.F. Selective, Nickel-Catalyzed Hydrogenolysis of Aryl Ethers. Science 2011, 332, 439–443. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, K.; Li, H.; Xiao, L.-P.; Song, G. Selective hydrogenolysis of catechyl lignin into propenylcatechol over an atomically dispersed ruthenium catalyst. Nat. Commun. 2021, 12, 416. [Google Scholar] [CrossRef]
- Karnitski, A.; Choi, J.-w.; Suh, D.J.; Yoo, C.-J.; Lee, H.-Y.; Kim, K.H.; Kim, C.S.; Kim, K.s.; Ha, J.M. Roles of metal and acid sites in the reductive depolymerization of concentrated lignin over supported Pd catalysts. Catal. Today 2022, 411, 113844. [Google Scholar] [CrossRef]
- Bosch, S.V.d.; Schutyser, W.; Vanholme, R.; Driessen, T.; Koelewijn, S.-F.; Renders, T.; Meester, B.D.; Huijgen, W.J.J.; Dehaen, W.; Courtin, C.M.; et al. Reductive lignocellulose fractionation into soluble lignin-derived phenolic monomers and dimers and processable carbohydrate pulps. Energy Environ. Sci. 2015, 8, 1748–1763. [Google Scholar] [CrossRef]
- Shu, R.; Long, J.; Xu, Y.; Ma, L.; Zhang, Q.; Wang, T.; Wang, C.; Yuan, Z.; Wu, Q. Investigation on the structural effect of lignin during the hydrogenolysis process. Bioresour. Technol. 2016, 200, 14–22. [Google Scholar] [CrossRef]
- Barta, K.; Warner, G.; Beach, E.; Anastas, P. Depolymerization of organosolv lignin to aromatic compounds over Cu-doped porous metal oxides. Green Chem. 2013, 16, 191–196. [Google Scholar] [CrossRef]
- Warner, G.R.; Hansen, T.S.; Riisager, A.; Beach, E.S.; Barta, K.; Anastas, P.T. Depolymerization of organosolv lignin using doped porous metal oxides in supercritical methanol. Bioresour. Technol. 2014, 161, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Chen, X.; Wang, L.; Liang, J.; Wei, X.; Nong, W. Anchoring single Ni atoms on CeO2 nanospheres as an efficient catalyst for the hydrogenolysis of lignin to aromatic monomers. Fuel 2022, 324, 124499. [Google Scholar] [CrossRef]
- Yadagiri, J.; Koppadi, K.S.; Enumula, S.S.; Vakati, V.; Kamaraju, S.R.R.; Burri, D.R.; Somaiah, P.V. Ni/KIT-6 catalysts for hydrogenolysis of lignin-derived diphenyl ether. J. Chem. Sci. 2018, 130, 106. [Google Scholar] [CrossRef]
- Rautiainen, S.; Di Francesco, D.; Katea, S.N.; Westin, G.; Tungasmita, D.N.; Samec, J.S.M. Lignin Valorization by Cobalt-Catalyzed Fractionation of Lignocellulose to Yield Monophenolic Compounds. ChemSusChem 2019, 12, 404–408. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Li, L.; Shao, Y.; Yi, Q.; Liu, Z.; Geng, H.; Liu, Y.; Valtchev, V. Recent Advances in Hydrodeoxygenation of Lignin-Derived Phenolics over Metal-Zeolite Bifunctional Catalysts. ChemCatChem 2024, 16, e202301681. [Google Scholar] [CrossRef]
- Ambursa, M.M.; Juan, J.C.; Yahaya, Y.; Taufiq-Yap, Y.H.; Lin, Y.-C.; Lee, H.V. A review on catalytic hydrodeoxygenation of lignin to transportation fuels by using nickel-based catalysts. Renew. Sustain. Energy Rev. 2021, 138, 110667. [Google Scholar] [CrossRef]
- Mukundan, S.; Konarova, M.; Atanda, L.; Ma, Q.; Beltramini, J. Guaiacol hydrodeoxygenation reaction catalyzed by highly dispersed, single layered MoS2/C. Catal. Sci. Technol. 2015, 5, 4422–4432. [Google Scholar] [CrossRef]
- Kay Lup, A.N.; Abnisa, F.; Wan Daud, W.M.A.; Aroua, M.K. A review on reactivity and stability of heterogeneous metal catalysts for deoxygenation of bio-oil model compounds. J. Ind. Eng. Chem. 2017, 56, 1–34. [Google Scholar] [CrossRef]
- Honkela, M.L.; Viljava, T.R.; Gutierrez, A.; Krause, A.O.I. Hydrotreating for bio-oil upgrading. RSC Energy Environ. Ser. 2010, 2010, 288–306. [Google Scholar]
- Gutierrez, A.; Kaila, R.K.; Honkela, M.L.; Slioor, R.; Krause, A.O.I. Hydrodeoxygenation of guaiacol on noble metal catalysts. Catal. Today 2009, 147, 239–246. [Google Scholar] [CrossRef]
- Ruddy, D.A.; Schaidle, J.A.; Ferrell, J.R.; Wang, J.; Moens, L.; Hensley, J.E. Recent advances in heterogeneous catalysts for bio-oil upgrading via “ex situ catalytic fast pyrolysis”: Catalyst development through the study of model compounds. Green Chem. 2014, 16, 454–490. [Google Scholar] [CrossRef]
- Wildschut, J.; Mahfud, F.H.; Venderbosch, R.H.; Heeres, H.J. Hydrotreatment of fast pyrolysis oil using heterogeneous noble-metal catalysts. Ind. Eng. Chem. Res. 2009, 48, 10324–10334. [Google Scholar] [CrossRef]
- Wu, R.; Meng, Q.; Yan, J.; Zhang, Z.; Chen, B.; Liu, H.; Tai, J.; Zhang, G.; Zheng, L.; Zhang, J.; et al. Intermetallic synergy in platinum–cobalt electrocatalysts for selective C–O bond cleavage. Nat. Catal. 2024, 7, 702–718. [Google Scholar] [CrossRef]
- Song, G.-Y. The development of catalytic fractionation and conversion of lignocellulosic biomass under lignin-first strategy. J. For. Eng. 2019, 4, 1–10. [Google Scholar]
- Mondal, A.K.; Qin, C.; Ragauskas, A.J.; Ni, Y.; Huang, F. Hydrogenation of Pyrolysis Oil from Loblolly Pine Residue. Pap. Biomater. 2020, 5, 1–13. [Google Scholar] [CrossRef]
- Jia, W.C.; Ding, Z.D.; Wang, R.H.; Zhou, J.H. Catalytic depolymerization of aspen alcohol lignin in superitical ethanol solvent and analysis of the degraded products. China Pulp Paper 2016, 35, 24–28. [Google Scholar] [CrossRef]
- Munick de Albuquerque Fragoso, D.; Bouxin, F.P.; Montgomery, J.R.D.; Westwood, N.J.; Jackson, S.D. Catalytic depolymerisation of isolated lignin to fine chemicals: Depolymerisation of Kraft lignin. Bioresour. Technol. Rep. 2020, 9, 100400. [Google Scholar] [CrossRef]
- Pepper, J.M.; Lee, Y.W. Lignin and related compounds. I. A comparative study of catalysts for lignin hydrogenolysis. Can. J. Chem. 1969, 47, 723–727. [Google Scholar] [CrossRef]
- Zhang, J.; Asakura, H.; Van Rijn, J.; Yang, J.; Duchesne, P.; Zhang, B.; Chen, X.; Zhang, P.; Saeys, M.; Yan, N. Highly efficient, NiAu-catalyzed hydrogenolysis of lignin into phenolic chemicals. Green Chem. 2014, 16, 2432–2437. [Google Scholar] [CrossRef]
- Liu, Z.-Q.; Yu, Y.-Y.; Liu, Y.-J.; Ying, A.-G.; Zhang, X.-L.; Wang, Y. Unlocking Birch Lignin Hydrocracking through Tandem Catalysis: Unraveling the Role of Moderate Hydrogen Spillover. ACS Catal. 2024, 14, 2115–2126. [Google Scholar] [CrossRef]
- Mei, X.; Liu, H.; Wu, H.; Wu, W.; Zheng, B.; Liu, Y.; Zheng, X.; Wang, Y.; Han, W.; Han, B. Catalytic self-transfer hydrogenolysis of lignin over Ni/C catalysts. Green Chem. 2024, 26, 4544–4551. [Google Scholar] [CrossRef]
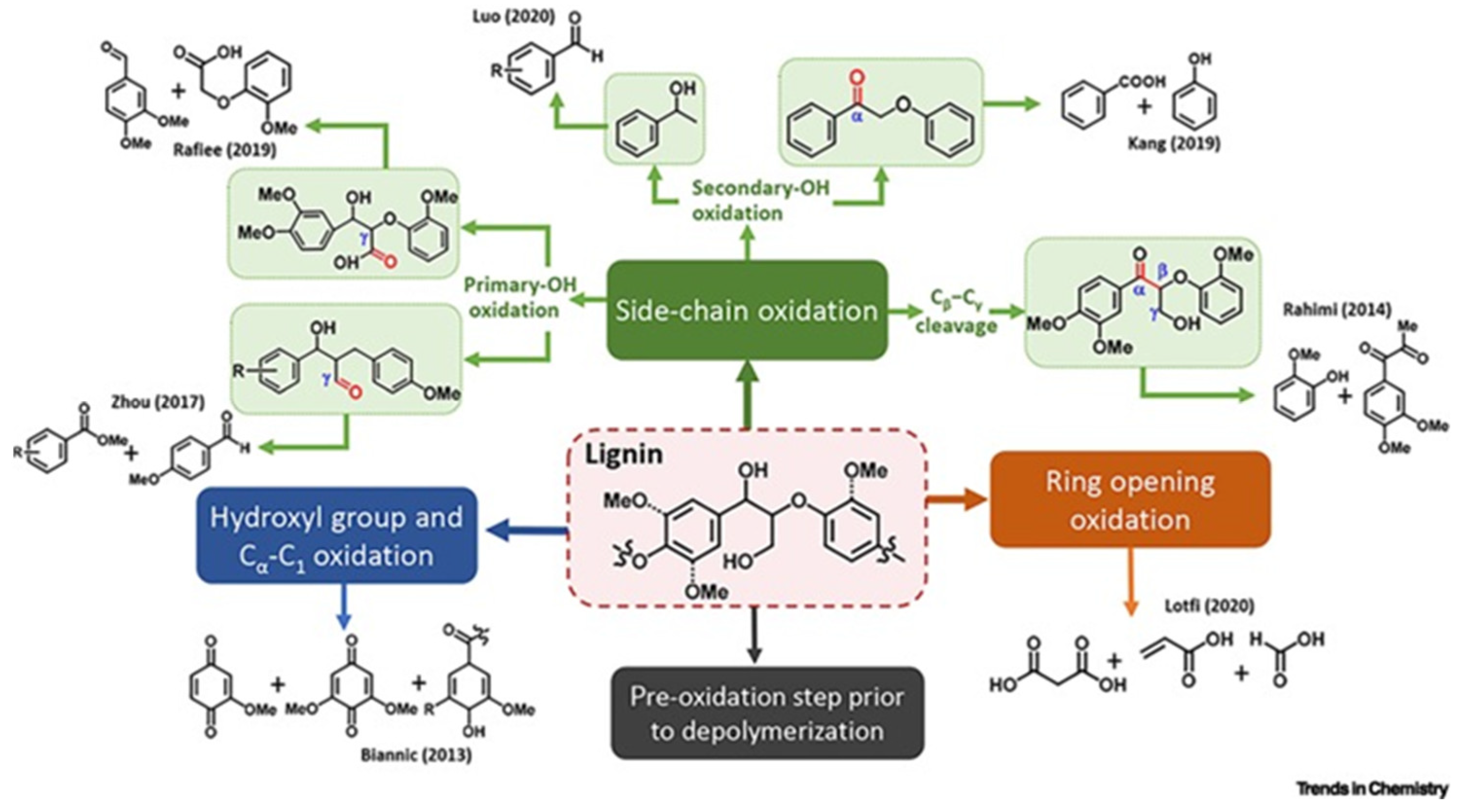
- Ma, C.; Zhang, J.; Yin, Y.; Suo, C.; Liu, S. Free radical theory in lignin oxidation depolymerization. Trends Chem. 2024, 6, 234–247. [Google Scholar] [CrossRef]
- Zhang, Z.; Yin, G.; Andrioletti, B.J.T.M.C. Advances in value-added aromatics by oxidation of lignin with transition metal complexes. Transit. Met. Chem. 2022, 47, 189–211. [Google Scholar] [CrossRef]
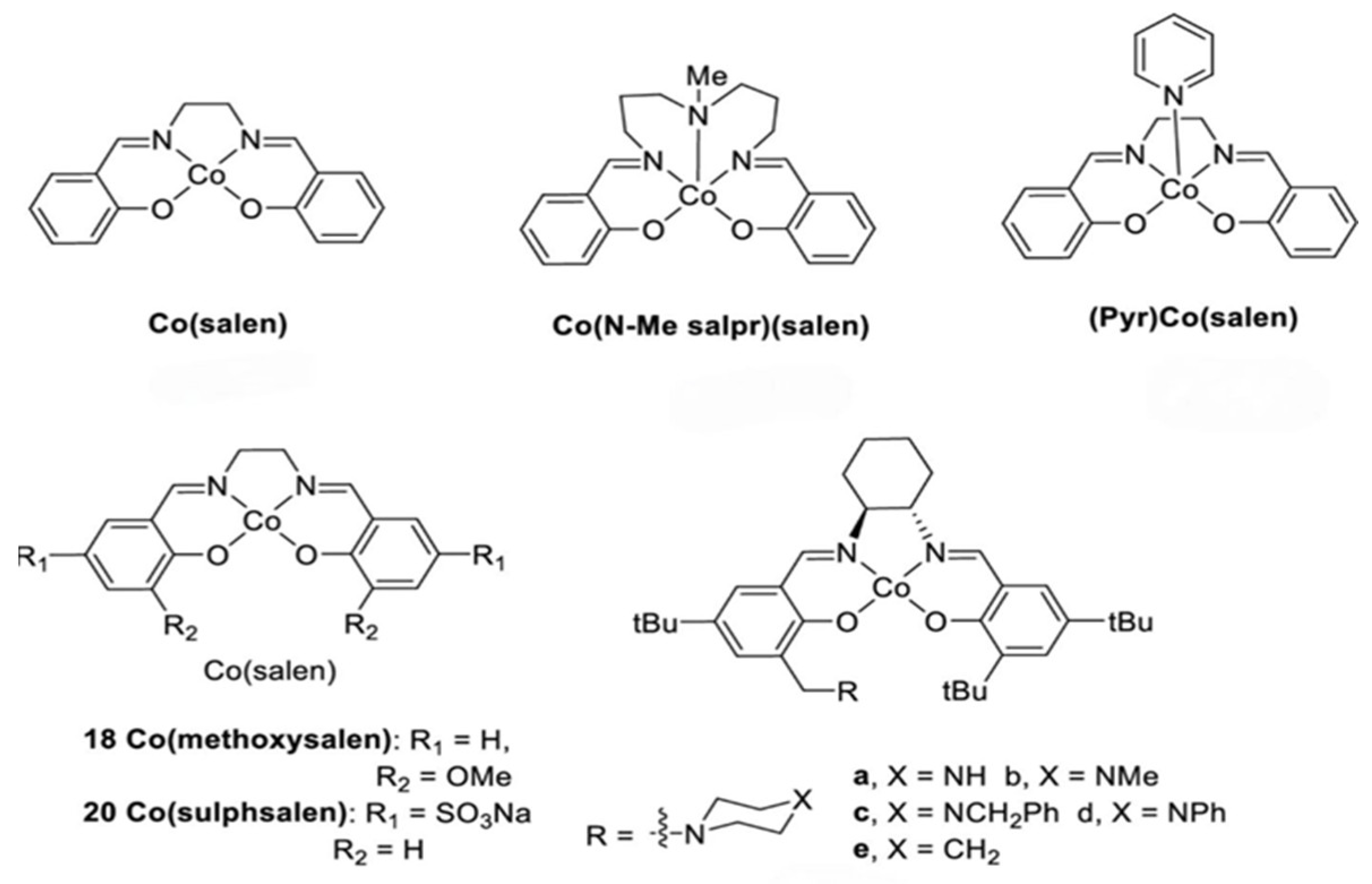
- Haikarainen, A.; Sipilä, J.; Pietikäinen, P.; Pajunen, A.; Mutikainen, I. Synthesis and characterization of bulky salen-type complexes of Co, Cu, Fe, Mn and Ni with amphiphilic solubility properties. J. Chem. Soc. Dalton Trans. 2001, 7, 991–995. [Google Scholar] [CrossRef]
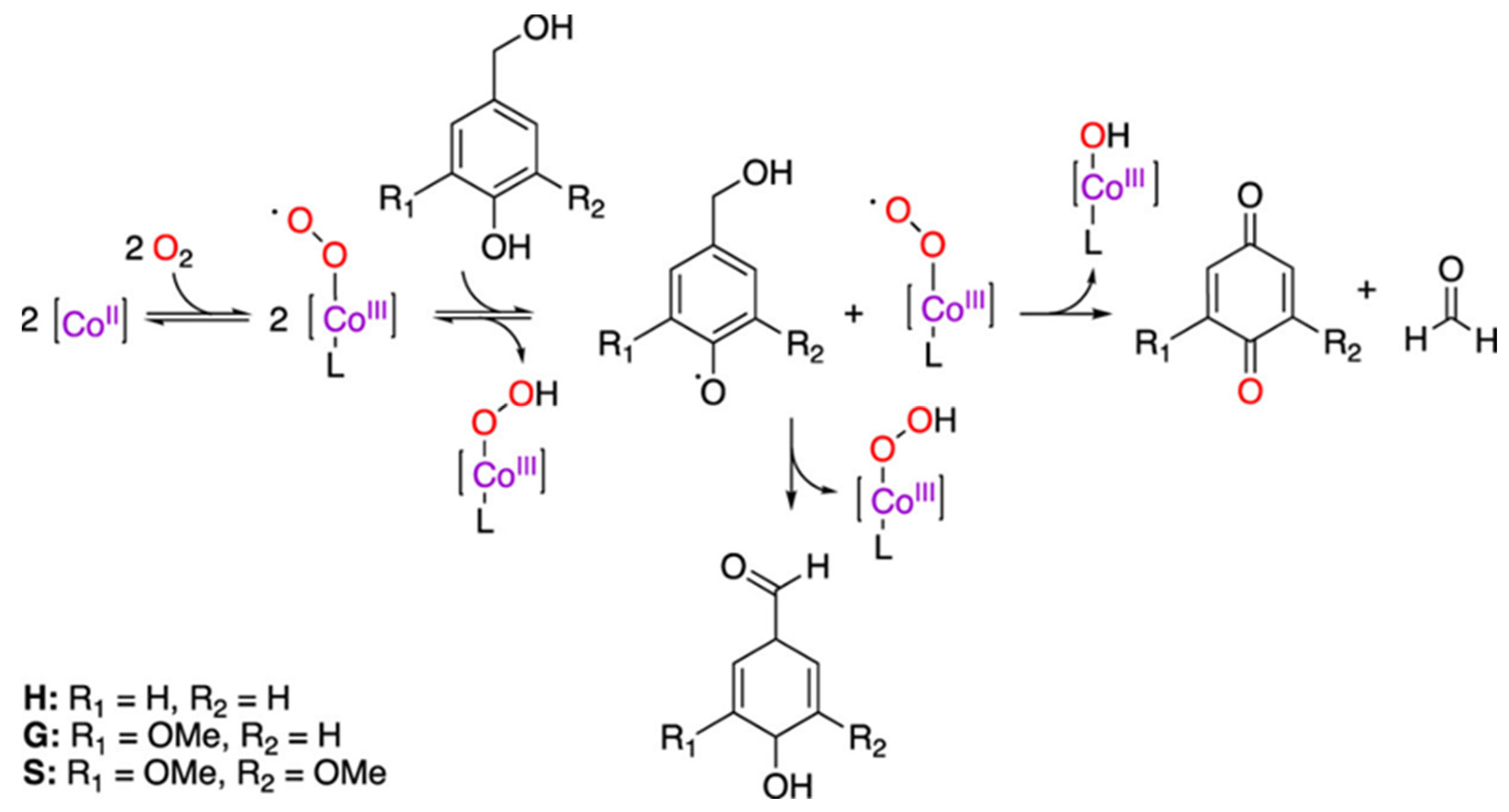
- Biannic, B.; Bozell, J.J. Efficient cobalt-catalyzed oxidative conversion of lignin models to benzoquinones. Org. Lett. 2013, 15, 2730–2733. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.-T.; Zhou, X.-F.; Zhu, Z.-L. Catalytic converison of kraft lignin using paper-like Co (salen) as an effective catalyst. Drew. Pr. Nauk. Doniesienia Komun. 2015, 58, 79–90. [Google Scholar] [CrossRef]
- Zhou, X.F.; Lu, X.J. Co (salen) supported on graphene oxide for oxidation of lignin. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Cooper, C.J.; Alam, S.; Nziko, V.d.P.N.; Johnston, R.C.; Ivanov, A.S.; Mou, Z.; Turpin, D.B.; Rudie, A.W.; Elder, T.J.; Bozell, J.J. Co (salen)-Catalyzed oxidation of lignin models to form benzoquinones and benzaldehydes: A computational and experimental study. ACS Sustain. Chem. Eng. 2020, 8, 7225–7234. [Google Scholar] [CrossRef]
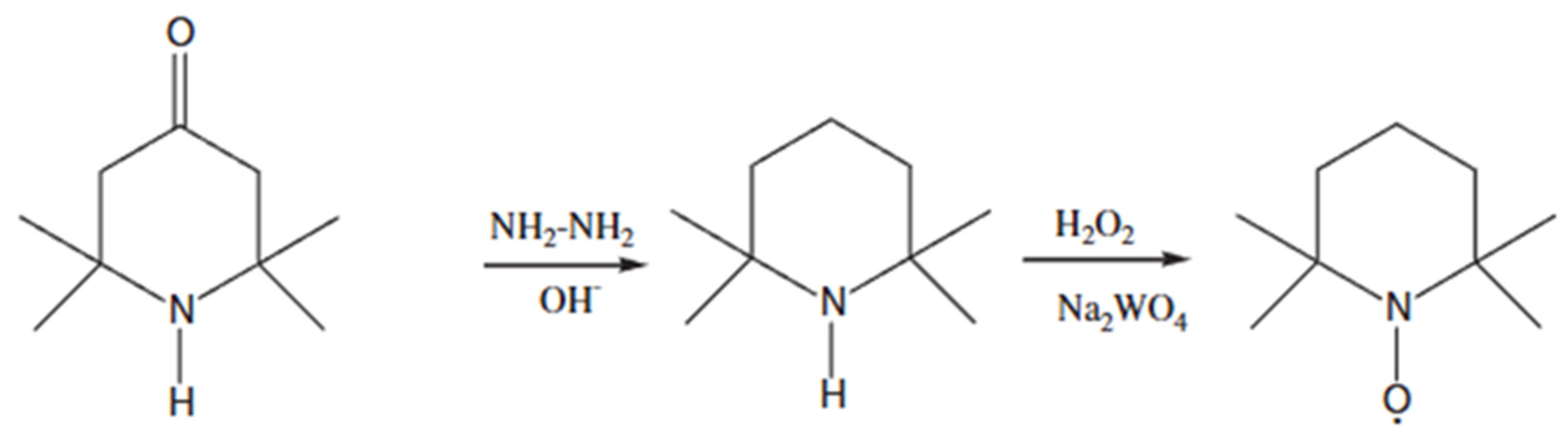
- Wertz, S.; Studer, A. Nitroxide-catalyzed transition-metal-free aerobic oxidation processes. Green Chem. 2013, 15, 3116–3134. [Google Scholar] [CrossRef]
- Bragd, P.; Van Bekkum, H.; Besemer, A. TEMPO-mediated oxidation of polysaccharides: Survey of methods and applications. Top. Catal. 2004, 27, 49–66. [Google Scholar] [CrossRef]
- Patankar, S.C.; Liu, L.-Y.; Ji, L.; Ayakar, S.; Yadav, V.; Renneckar, S. Isolation of phenolic monomers from kraft lignin using a magnetically recyclable TEMPO nanocatalyst. Green Chem. 2019, 21, 785–791. [Google Scholar] [CrossRef]
- Zheng, M.-W.; Lin, K.-Y.A.; Lin, C.-H. TEMPO-functionalized silica as an efficient and recyclable oxidation catalyst for conversion of a lignin model compound to value-added products. Waste Biomass Valorization 2020, 11, 6917–6928. [Google Scholar] [CrossRef]
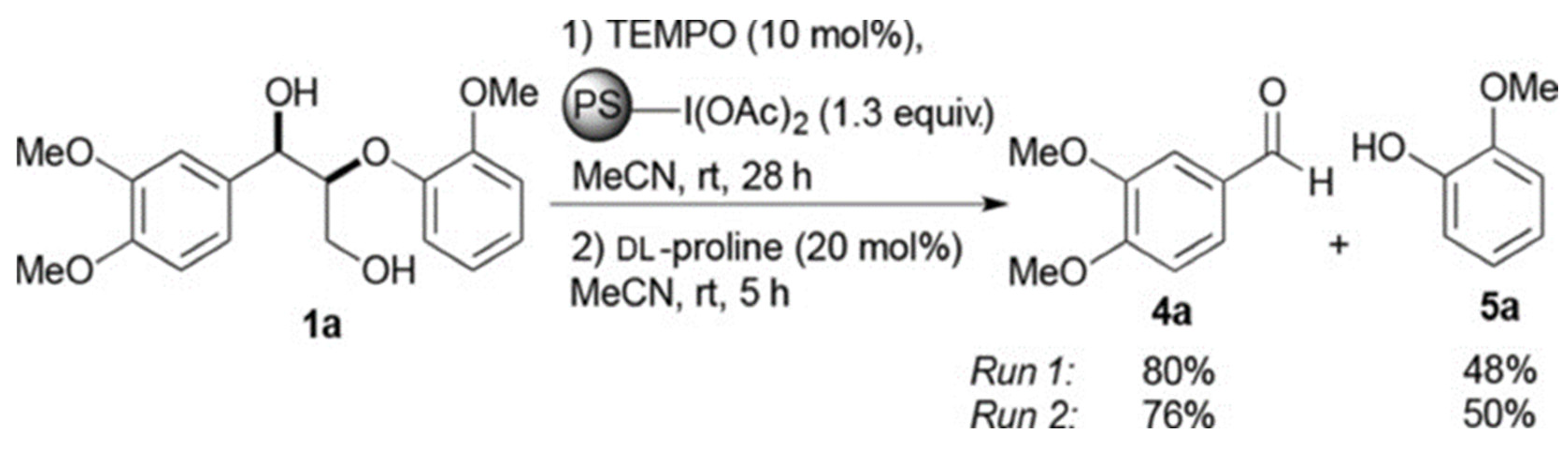
- Dabral, S.; Hernández, J.G.; Kamer, P.C.; Bolm, C. Organocatalytic chemoselective primary alcohol oxidation and subsequent cleavage of lignin model compounds and lignin. ChemSusChem 2017, 10, 2707–2713. [Google Scholar] [CrossRef]
- Lin, J.-Y.; Lin, K.-Y.A. Catalytic conversion of a lignin model compound to value-added products using Cu/TEMPO-catalyzed aerobic oxidation. Biomass Convers. Biorefinery 2019, 9, 617–623. [Google Scholar] [CrossRef]
- Vangeel, T.; Schutyser, W.; Renders, T.; Sels, B.F. Perspective on lignin oxidation: Advances, challenges, and future directions. In Lignin Chemistry; Springer: Cham, Switzerland, 2020; pp. 53–68. [Google Scholar]
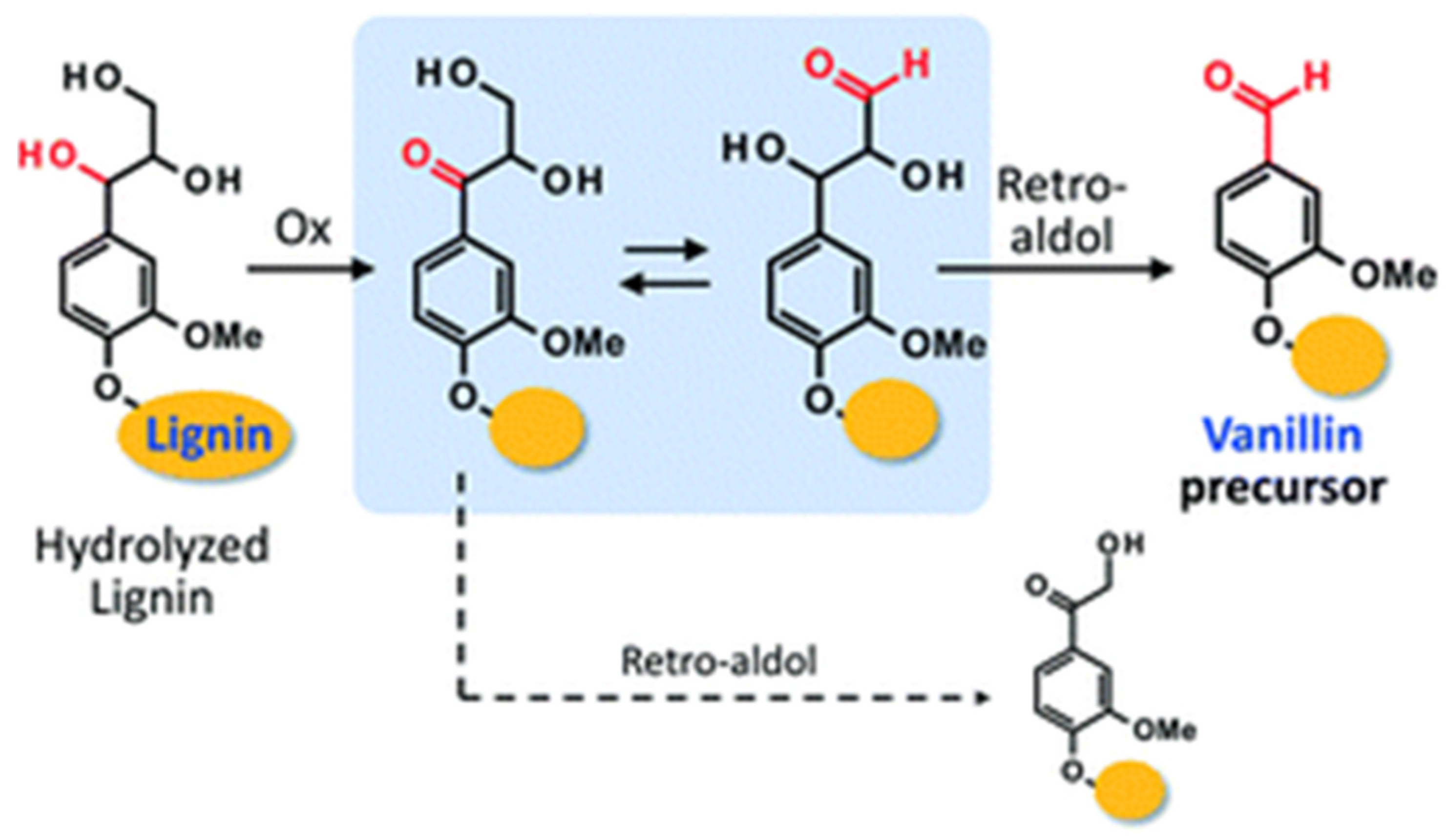
- Hirano, Y.; Izawa, A.; Hosoya, T.; Miyafuji, H. Degradation mechanism of a lignin model compound during alkaline aerobic oxidation: Formation of the vanillin precursor from the β-O-4 middle unit of softwood lignin. React. Chem. Eng. 2022, 7, 1603–1616. [Google Scholar] [CrossRef]
- Santos, S.G.; Marques, A.P.; Lima, D.L.; Evtuguin, D.V.; Esteves, V.I. Kinetics of eucalypt lignosulfonate oxidation to aromatic aldehydes by oxygen in alkaline medium. Ind. Eng. Chem. Res. 2011, 50, 291–298. [Google Scholar] [CrossRef]
- Schutyser, W.; Kruger, J.S.; Robinson, A.M.; Katahira, R.; Brandner, D.G.; Cleveland, N.S.; Mittal, A.; Peterson, D.J.; Meilan, R.; Román-Leshkov, Y. Revisiting alkaline aerobic lignin oxidation. Green Chem. 2018, 20, 3828–3844. [Google Scholar] [CrossRef]
- Kruger, J.S.; Dreiling, R.J.; Wilcox, D.G.; Ringsby, A.J.; Noon, K.L.; Amador, C.K.; Brandner, D.G.; Ramirez, K.J.; Haugen, S.J.; Klein, B.C. Lignin alkaline oxidation using reversibly-soluble bases. Green Chem. 2022, 24, 8733–8741. [Google Scholar] [CrossRef]
- Tarabanko, V.E.; Tarabanko, N. Catalytic oxidation of lignins into the aromatic aldehydes: General process trends and development prospects. Int. J. Mol. Sci. 2017, 18, 2421. [Google Scholar] [CrossRef]
- Chen, Y.; Bai, Y. Research process of preparation of aromatic aldehyde by oxidative depolymerization of lignin. Chem. Ind. Eng. Prog. 2023, 42, 6576. [Google Scholar]
- Voitl, T.; Rohr, P.R.v. Demonstration of a process for the conversion of kraft lignin into vanillin and methyl vanillate by acidic oxidation in aqueous methanol. Ind. Eng. Chem. Res. 2010, 49, 520–525. [Google Scholar] [CrossRef]
- Werhan, H.; Assmann, N.; von Rohr, P.R. Lignin oxidation studies in a continuous two-phase flow microreactor. Chem. Eng. Process. Process Intensif. 2013, 73, 29–37. [Google Scholar] [CrossRef]
- Arturi, K.; Rohrbach, T.; Vogel, F.; Bjelić, S.a. High yields of aromatic monomers from acidolytic oxidation of Kraft lignin in a biphasic system. Ind. Eng. Chem. Res. 2021, 60, 11009–11018. [Google Scholar] [CrossRef]
- More, A.; Elder, T.; Jiang, Z. A review of lignin hydrogen peroxide oxidation chemistry with emphasis on aromatic aldehydes and acids. Holzforschung 2021, 75, 806–823. [Google Scholar] [CrossRef]
- Park, S.-Y.; Choi, J.-H.; Kim, J.-H.; Cho, S.-M.; Yeon, S.; Jeong, H.; Lee, S.M.; Choi, I.-G. Peracetic acid-induced kraft lignin solubilization and its characterization for selective production of macromolecular biopolymers. Int. J. Biol. Macromol. 2020, 161, 1240–1246. [Google Scholar] [CrossRef] [PubMed]
- Li, S.H.; Liu, S.; Colmenares, J.C.; Xu, Y.J. A sustainable approach for lignin valorization by heterogeneous photocatalysis. Green Chem. 2016, 18, 594–607. [Google Scholar] [CrossRef]
- Uğurlu, M.; Karaoğlu, M.H. TiO2 supported on sepiolite: Preparation, structural and thermal characterization and catalytic behaviour in photocatalytic treatment of phenol and lignin from olive mill wastewater. Chem. Eng. J. 2011, 166, 859–867. [Google Scholar] [CrossRef]
- Kamwilaisak, K.; Wright, P.C. Investigating laccase and titanium dioxide for lignin degradation. Energy Fuels 2012, 26, 2400–2406. [Google Scholar] [CrossRef]
- Gong, J.; Imbault, A.; Farnood, R. The promoting role of bismuth for the enhanced photocatalytic oxidation of lignin on Pt-TiO2 under solar light illumination. Appl. Catal. B Environ. 2017, 204, 296–303. [Google Scholar] [CrossRef]
- Kumar, A.; Ghalta, R.; Bal, R.; Srivastava, R. Photocatalytic β-O-4 bond cleavage in lignin models and native lignin through CdS integration on titanium oxide photocatalyst under visible light irradiation. Appl. Catal. B Environ. Energy 2024, 359, 124494. [Google Scholar] [CrossRef]
- Shi, N.; Li, Y.; Li, N.; Wen, F.; Liu, D. Acidic-basic-redox trifunctional Cu/P2W17V/{001}-TiO2 promotes photocatalytic lignin model conversion via tunable CC/CO cleavage. Appl. Catal. A Gen. 2025, 689, 120030. [Google Scholar] [CrossRef]
- Xu, E.; Xie, F.; Liu, T.; He, J.; Zhang, Y. Photocatalytic, Oxidative Cleavage of C− C Bond in Lignin Models and Native Lignin. Chem.–Eur. J. 2024, 30, e202304209. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Maldonado, S.; Stephenson, C.R. Electrocatalytic lignin oxidation. ACS Catal. 2021, 11, 10104–10114. [Google Scholar] [CrossRef]
- Rafiee, M.; Alherech, M.; Karlen, S.D.; Stahl, S.S. Electrochemical aminoxyl-mediated oxidation of primary alcohols in lignin to carboxylic acids: Polymer modification and depolymerization. J. Am. Chem. Soc. 2019, 141, 15266–15276. [Google Scholar] [CrossRef]
- Bosque, I.; Magallanes, G.; Rigoulet, M.; Kärkäs, M.D.; Stephenson, C.R. Redox catalysis facilitates lignin depolymerization. ACS Cent. Sci. 2017, 3, 621–628. [Google Scholar] [CrossRef]
- Ko, M.; Pham, L.T.M.; Sa, Y.J.; Woo, J.; Nguyen, T.V.T.; Kim, J.H.; Oh, D.; Sharma, P.; Ryu, J.; Shin, T.J. Unassisted solar lignin valorisation using a compartmented photo-electro-biochemical cell. Nat. Commun. 2019, 10, 5123. [Google Scholar] [CrossRef]
- Jia, Y.; Wen, Y.; Han, X.; Qi, J.; Liu, Z.; Zhang, S.; Li, G. Electrocatalytic degradation of rice straw lignin in alkaline solution through oxidation on a Ti/SnO2–Sb2O3/α-PbO2/β-PbO2 anode and reduction on an iron or tin doped titanium cathode. Catal. Sci. Technol. 2018, 8, 4665–4677. [Google Scholar] [CrossRef]
- Danlu, Z.; Xu, Z.; Sinong, W.; Yan, X.; Qiqi, D.; Fengxia, Y.; Peng, W.; Chuanfu, L.; Wu, L. Electrochemical oxidation of lignin model compounds over metal oxyhydroxides on nickel foam. Green Chem. 2024, 26, 7759–7768. [Google Scholar] [CrossRef]
- Qi, Y.; Liu, B.; Qiu, X.; Zeng, X.; Luo, Z.; Wu, W.; Liu, Y.; Chen, L.; Zu, X.; Dong, H. Simultaneous Oxidative Cleavage of Lignin and Reduction of Furfural via Efficient Electrocatalysis by P-Doped CoMoO4. Adv. Mater. 2023, 35, 2208284. [Google Scholar] [CrossRef]
- Liu, B.; Qi, Y.; Qiu, X.; Zou, H.; Lin, X.; Qin, Y. Photoelectrocatalytic Pathway for the Preparation of Power-Effective Aviation Fuel Precursors from Lignin. Adv. Funct. Mater. 2025, 2421552. [Google Scholar] [CrossRef]
- Cortright, R.D.; Davda, R.R.; Dumesic, J.A. Hydrogen from catalytic reforming of biomass-derived hydrocarbons in liquid water. Nature 2002, 418, 964–967. [Google Scholar] [CrossRef]
- Nie, R.; Tao, Y.; Nie, Y.; Lu, T.; Wang, J.; Zhang, Y.; Lu, X.; Xu, C.C. Recent Advances in Catalytic Transfer Hydrogenation with Formic Acid over Heterogeneous Transition Metal Catalysts. ACS Catal. 2021, 11, 1071–1095. [Google Scholar] [CrossRef]
- Zhang, J.-w.; Lu, G.-p.; Cai, C. Self-hydrogen transfer hydrogenolysis of β-O-4 linkages in lignin catalyzed by MIL-100(Fe) supported Pd–Ni BMNPs. Green Chem. 2017, 19, 4538–4543. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, X.; Guo, Y.; Wang, Y. Self-Hydrogen Supplied Catalytic Fractionation of Raw Biomass into Lignin-Derived Phenolic Monomers and Cellulose-Rich Pulps. JACS Au 2023, 3, 1911–1917. [Google Scholar] [CrossRef]
- Kocaturk, E.; Salan, T.; Ozcelik, O.; Alma, M.H.; Candan, Z. Recent Advances in Lignin-Based Biofuel Production. Energies 2023, 16, 3382. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, X.; Yin, H.; Zheng, Q.; Ma, L.; Li, S.; Zhang, Y.; Fu, P. Production of aromatic hydrocarbons from lignin derivatives by catalytic cracking over a SiO2–Al2O3 catalyst. RSC Adv. 2023, 13, 10830–10839. [Google Scholar] [CrossRef] [PubMed]
- Chou, W.; Lu, P.; Lou, B.; Li, W.; Wu, C.; Wu, P.; Men, Z.; Liu, D. Pt/CeCrO2−x: A multifunctional catalyst for tandem catalysis of lignocellulose hydro-liquefaction and Guerbet reaction. Appl. Catal. B Environ. 2024, 342, 123320. [Google Scholar] [CrossRef]
- Sunil More, G.; Rajendra Kanchan, D.; Banerjee, A.; Srivastava, R. Selective catalytic hydrodeoxygenation of vanillin to 2-Methoxy-4-methyl phenol and 4-Methyl cyclohexanol over Pd/CuFe2O4 and PdNi/CuFe2O4 catalysts. Chem. Eng. J. 2023, 462, 142110. [Google Scholar] [CrossRef]
- Wu, F.-P.; Zhao, Y.-P.; Fu, Z.-P.; Qiu, L.-L.; Xiao, J.; Li, J.; Liu, F.-J.; Cao, J.-P. Catalytic transfer hydrogenolysis mechanism of benzyl phenyl ether over NiCu/Al2O3 using isopropanol as hydrogen source. Fuel Process. Technol. 2023, 250, 107874. [Google Scholar] [CrossRef]
- Gilkey, M.J.; Xu, B. Heterogeneous Catalytic Transfer Hydrogenation as an Effective Pathway in Biomass Upgrading. ACS Catal. 2016, 6, 1420–1436. [Google Scholar] [CrossRef]
- Wang, W.; Niu, M.; Hou, Y.; Wu, W.; Liu, Z.; Liu, Q.; Ren, S.; Marsh, K.N. Catalytic conversion of biomass-derived carbohydrates to formic acid using molecular oxygen. Green Chem. 2014, 16, 2614–2618. [Google Scholar] [CrossRef]
- Tang, B.; Li, W.; Zhang, X.; Zhang, B.; Zhang, H.; Li, C. Depolymerization of Kraft lignin to liquid fuels with MoS2 derived oxygen-vacancy-enriched MoO3 in a hydrogen-donor solvent system. Fuel 2022, 324, 124674. [Google Scholar] [CrossRef]
- Liu, F.; Feng, P.; Yuan, M.; Zhai, G.; Innocent, M.T.; Xiang, H.; Wu, Q.; Lu, Y.; Zhu, M. Continuous Preparation of a Flexible Carbon Nanotube Film from Lignin as a Sulfur Host Material for Lithium–Sulfur Batteries. ACS Sustain. Chem. Eng. 2023, 11, 16544–16553. [Google Scholar] [CrossRef]
- Lu, X.; Gu, X. Efficient lignin conversion over Ni/(Fe/Zn/Co/Mo/Cu)–WO3/Al2O3 for selectively yielding alkyl phenols. Catal. Sci. Technol. 2023, 13, 468–478. [Google Scholar] [CrossRef]





| Types | Lignin (%) | Structure (%) | ||
|---|---|---|---|---|
| Coniferyl Alcohol (G) | Sinapyl Alcohol (S) | p-Coimaryl Alcohol (H) | ||
| Hardwood | 19–28 | 25–50 | 50–75 | — |
| Softwood | 24–33 | 90–95 | 5–10 | — |
| Grass | 17–24 | 25–50 | 25–50 | 10–25 |
| Interunit Linkages | Content (%) | BDE (kJ/mol) | |
|---|---|---|---|
| Hardwood | Softwood | ||
| β-O-4 | 50–65 | 45–50 | 225.378–302.64 |
| α-O-4 | 4–8 | 6–8 | 202.22–239.77 |
| 4-O-5 | 6–9 | 4–8 | 325.41–345.50 |
| 5-5 | 3–10 | 10–25 | 480.95–495.60 |
| β-5 | 3–11 | 9–12 | 524.07–534.11 |
| β-β | 3–12 | 2–6 | 358.31–485.98 |
| β-1 | 3–7 | 1–7 | 270.82–289.41 |
| Methods | Feedback | Reagents | Conditions | Delignification | References |
|---|---|---|---|---|---|
| Acid | Corn stover | Ethylene glycol sulfuric acid | 120 °C 60 min | 80.3% | [61] |
| Corn stover | Ethylene glycol p-toluene sulfonic acid | 110 °C 90 min | 85.37% | [64] | |
| Poplar | p-toluene sulfonic acid | ≤80 °C 20 min | 90% | [67] | |
| Organsolv | Spruce | Water, ethanol erric chloride hexahydrate | 90 °C 180 min | 74% | [87] |
| Bamboo | Water, ethanol, sulfuric acid | 180 °C 55 min | 65.81% | [92] | |
| Rice straw | Water, ethanol | 190 °C 60 min | 64.60% | [93] | |
| Olive | Water, ethanol | 210 °C 60 min | 64% | [94] | |
| Sugarcane bagasse | Ethylene glycol water HCl | 130 °C 60 min | 67.1% | [95] | |
| Sugarcane bagasse | Ethylene glycol water NaOH | 130 °C 60 min | 90.9% | [95] | |
| Sugarcane bagasse | Ethylene glycol water NaOH Tween 80 | 240 °C 60 min | 80.5% | [96] | |
| Rice straw | Ethylene glycol, AlCl3 | 150 °C 30 min | 88% | [97] | |
| Sugarcane bagasse | Ethylene glycol, HCl | 130 °C 60 min | 61.3% | [99] | |
| DES | Corncorb | Choline chloride/oxalic acid | 90 °C 24 h | 98.5% | [107] |
| Wheat straw | Choline chloride/lactic acid | 150 °C 6 h | 81.54% | [108] | |
| Wheat straw | Triethylbenzyl ammonium chloride/lactic acid | 100 °C 10 h | 79.73% | [109] | |
| Switchgrass | Choline chloride, lactic acid, microwave | 152 °C 45 s | 72.23% | [110] | |
| Corn stover | Choline chloride, lactic acid, microwave | 152 °C 45 s | 79.60% | [110] | |
| Miscanthus | Choline chloride lactic acid, microwave | 152 °C 45 s | 65.18% | [110] | |
| Corn stover | Choline chloride/oxalic acid | 150 °C 6 h | 75% | [112] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gui, C.; Wang, L.; Liu, G.; Ogunbiyi, A.T.; Li, W. The Catalytic Valorization of Lignin from Biomass for the Production of Liquid Fuels. Energies 2025, 18, 1478. https://doi.org/10.3390/en18061478
Gui C, Wang L, Liu G, Ogunbiyi AT, Li W. The Catalytic Valorization of Lignin from Biomass for the Production of Liquid Fuels. Energies. 2025; 18(6):1478. https://doi.org/10.3390/en18061478
Chicago/Turabian StyleGui, Chenchen, Lida Wang, Guoshun Liu, Ajibola T. Ogunbiyi, and Wenzhi Li. 2025. "The Catalytic Valorization of Lignin from Biomass for the Production of Liquid Fuels" Energies 18, no. 6: 1478. https://doi.org/10.3390/en18061478
APA StyleGui, C., Wang, L., Liu, G., Ogunbiyi, A. T., & Li, W. (2025). The Catalytic Valorization of Lignin from Biomass for the Production of Liquid Fuels. Energies, 18(6), 1478. https://doi.org/10.3390/en18061478







