An Evaluation of the Energy Potential of Agri-Food Waste: Green Residues from Tomato (Solanum lycopersicum L.) and Shea Nutshells (Vitellaria paradoxa)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Technical and Elemental Analysis of Biomass
Volatile Solids Content
Ash Content
Calorific Value
C, N, S Content
Total Phosphorus Content
Selected Metals Content
2.2.2. Determination of Digestible Components
Protein Content
Fat Content
Crude Fiber Content
2.2.3. Estimating Biogas and Biomethane Production Capacity
The Baserga Method
Fermentable Organic Matter
2.2.4. Statistical Analysis
3. Results
3.1. Characterization of Waste for Energy Purposes
3.2. Estimating Biogas and Biomethane Production Capacity
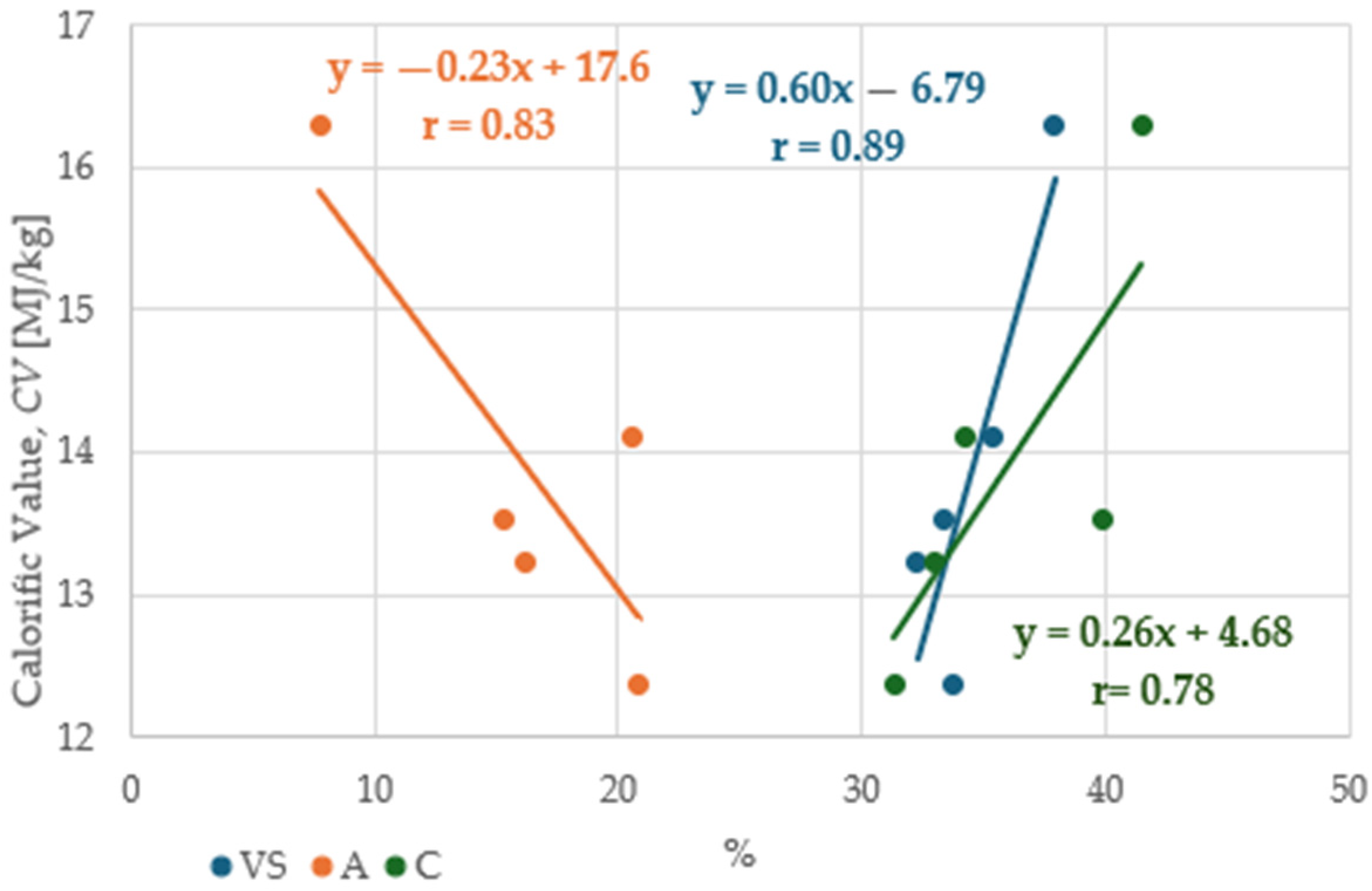
3.3. The Effect of Fertilization of Tomato Waste on Energy Potential
4. Discussion
4.1. Characterization of Waste for Energy Purposes
4.1.1. Thermal Treatment
4.1.2. Biochemical Treatment
4.2. Estimating of Biogas and Biomethane Production
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bracmort, K. Biomass: Comparison of Definitions in Legislation; Congressional Research Service: Washington, DC, USA, 2013.
- Bakari, R.; Asha, R.; Hossein, M.; Huang, X.; Islam, N.F.; Liew, R.K.; Narayan, M.; Lam, S.S.; Sarma, H. Converting food waste to biofuel: A sustainable energy solution for Sub-Saharan Africa. Sustain. Chem. Environ. 2024, 7, 100126. [Google Scholar] [CrossRef]
- Mao, G.; Huang, N.; Chen, L.; Wang, H. Research on biomass energy and environment from the past to the future: A bibliometric analysis. Sci. Total Environ. 2018, 635, 1081–1090. [Google Scholar] [CrossRef]
- Sher, F.; Smječanin, N.; Hrnjić, H.; Karadža, A.; Omanović, R.; Šehović, E.; Sulejmanović, J. Emerging technologies for biogas production: A critical review on recent progress, challenges and future perspectives. Process Saf. Environ. Prot. 2024, 188, 834–859. [Google Scholar] [CrossRef]
- Jha, S.; Nanda, S.; Acharya, B.; Dalai, A.K. A Review of Thermochemical Conversion of Waste Biomass to Biofuels. Energies 2022, 15, 6352. [Google Scholar] [CrossRef]
- Department of Economic and Social Affairs. Energy Statistics Pocketbook 2024, 1st ed.; Energy Statistics Pocketbook Series; United Nations Research Institute for Social Development: Geneva, Switzerland, 2024. [Google Scholar]
- CEWEP—The Confederation of European Waste-to-Energy Plants. Available online: https://www.cewep.eu/waste-to-energy-plants-in-europe-in-2021/ (accessed on 5 December 2024).
- Curkowski, A.; Mroczkowski, P.; Oniszk-Popławska, A.; Wiśniewski, G. Biogaz Rolniczy—Produkcja i Wykorzystanie; Mazowiecka Agencja Energetyczna: Warszawa, Poland, 2009. [Google Scholar]
- Wiącek, D.; Tys, J. Biogaz—Wytwarzanie i możliwości jego wykorzystania. Acta Agrophysica 2015, 1, 1–92. [Google Scholar]
- Pavičić, J.; Novak Mavar, K.; Brkić, V.; Simon, K. Biogas and Biomethane Production and Usage: Technology Development, Advantages and Challenges in Europe. Energies 2022, 15, 2940. [Google Scholar] [CrossRef]
- Teraz Środowisko. Biogaz i biometan w Polsce 2024. Available online: https://www.teraz-srodowisko.pl/publikacje/biogaz-biometan-insight-Polska-2024/teraz-srodowisko-publikacja-biogaz-biometan-insight-Polska-2024.pdf (accessed on 8 October 2024).
- Kuboń, M.; Skibko, Z.; Borusiewicz, A.; Romaniuk, W.; Gajda, J.S.; Kłosowska, O.; Wasąg, Z. Influence of the Parameters of an Agricultural Biogas Plant on the Amount of Power Generated. Appl. Sci. 2024, 14, 4200. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Sar, T.; Gowd, S.C.; Rajendran, K.; Kumar, V.; Sarsaiya, S.; Li, Y.; Sindhu, R.; Binod, P.; Zhang, Z.; et al. A comprehensive review on thermochemical, and biochemical conversion methods of lignocellulosic biomass into valuable end product. Fuel 2023, 342, 127790. [Google Scholar] [CrossRef]
- Fachagentur Nachwachsende Rohstoffe; Deutsches Biomasseforschungszentrum; Kuratorium für Technik und Bauwesen in der Landwirtschaft; Institut für Agrartechnologie und Biosystemtechnik. Leitfaden Biogas: Von der Gewinnung zur Nutzung. Bioenergie. 2016. Available online: https://www.fnr.de/fileadmin/allgemein/pdf/broschueren/Leitfaden_Biogas_web_V01.pdf (accessed on 7 October 2024).
- Myczko, A.; Myczko, R.; Kołodziejczyk, T.; Golimowska, R.; Lenarczyk, J.; Janas, Z.; Kliber, A.; Karłowski, J.; Dolska, M. Budowa i eksploatacja biogazowni rolniczych: Poradnik dla inwestorów zainteresowanych budową biogazowni rolniczych; Publishing of the Institute of Technology and Life Sciences: Warszawa, Poland; Poznań, Poland, 2011. [Google Scholar]
- Szilágyi, Á.; Bodor, A.; Tolvai, N.; Kovacs, K.L.; Bodai, L.; Wirth, R.; Bagi, Z.; Szepesi, A.; Marko, V.; Kakuk, B.; et al. A comparative analysis of biogas production from tomato bio-waste in mesophilic batch and continuous anaerobic digestion systems. PLoS ONE 2021, 16, e0248654. [Google Scholar] [CrossRef]
- Saghouri, M.; Mansoori, Y.; Rohani, A.; Hossein, M.; Khodaparast, H.; Sheikhdavoodi, M.J. Modelling and evaluation of anaerobic digestion process of tomato processing wastes for biogas generation. J. Mater. Cycles Waste Manag. 2017, 20, 561–567. [Google Scholar] [CrossRef]
- Almeida, P.V.; Rodrigues, R.P.; Teixeira, L.M.; Santos, A.F.; Martins, R.C.; Quina, M.J. Bioenergy Production through Mono and Co-Digestion of Tomato Residues. Energies 2021, 14, 5563. [Google Scholar] [CrossRef]
- Saev, M.; Koumanova, B.; Simeonov, I. Anaerobic co-digestion of wasted tomatoes and cattle dung for biogas production. J. Univ. Chem. Technol. Metall. 2009, 44, 55–60. [Google Scholar]
- Li, Y.; Li, Y.; Zhang, D.; Li, G.; Lu, J.; Li, S. Solid state anaerobic co-digestion of tomato residues with dairy manure and corn stover for biogas production. Bioresour. Technol. 2016, 217, 50–55. [Google Scholar] [CrossRef]
- Alharbi, M.; Alseroury, F.; Alkthami, B. Biogas Production from Manure of Camel and Sheep Using Tomato and Rumen as Co-Substrate. J. Ecol. Eng. 2023, 24, 54–61. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Yao, L.; Fu, L.; Liu, Z. The Biogas Production Potential and Community Structure Characteristics of the Co-Digestion of Dairy Manure and Tomato Residues. Agronomy 2024, 14, 881. [Google Scholar] [CrossRef]
- Ofosu, M.A.; Aklaku, E.D. Determining the optimum proportion of Shea waste in anaerobic co-fermentation process. J. Sci. Technol. 2010, 20, 119–128. [Google Scholar] [CrossRef][Green Version]
- Ofosu, M.A.; Adonadaga, M.-G.; Sackey, I.; Ampadu, B. Substrate-induced pH changes and process stability of anaerobic digestion of shea waste. J. Appl. Sci. Environ. Manag. 2021, 24, 2035–2042. [Google Scholar] [CrossRef]
- PN-EN ISO 18134-3:2023-12; Solid Biofuels—Determination of Moisture Content—Part 3: Moisture in General Analysis Sample, 2023. Available online: https://www.pkn.pl/ (accessed on 5 October 2024).
- PN-EN ISO 18123:2023-10; Solid Biofuels—Determination of Volatile Matter, 2023. Available online: https://www.pkn.pl/ (accessed on 5 October 2024).
- PN-EN ISO 18122:2023-05; Solid Biofuels—Determination of Ash Content, 2023. Available online: https://www.pkn.pl/ (accessed on 5 October 2024).
- PN-81/G-04513; Paliwa stałe—Oznaczanie ciepła spalania i obliczanie wartości opałowej. 1981. Available online: https://sip.lex.pl/akty-prawne/dzu-dziennik-ustaw/metody-badania-jakosci-paliw-stalych-18761768 (accessed on 5 October 2024).
- Institut für Energetik und Umwelt gGmbH; Bundesforschungsanstalt für Landwirtschaft; Kuratorium für Technik und Bauwesen in der Landwirtschaft e.V. Biogaz produkcja i wykorzystywanie; Institut für Energetik und Umwelt gGmbH: Leipzig, Germany, 2005. [Google Scholar]
- Podkówka, W. Biogaz rolniczy—Odnawialne źródło energii. Teoria, praktyczne zastosowanie; Powszechne Wydawnictwo Rolnicze i Leśne: Warszawa, Poland, 2012. [Google Scholar]
- Podkówka, Z.; Podkówka, W. Zasady obliczania wartości “biogazowej” substratu na podstawie składu chemicznego. Przegląd Hod. 2008, 7, 28–29. [Google Scholar]
- Baserga, U. Landwirtschaftliche Co-Vergarungs Biogasanlagen. Biogas aus organischen Reststoffen und Energiegras. FAT-Berichte 1998, 512, 1–11. [Google Scholar]
- Weißbach, F. Zur Bewertung des Gasbildungspotenzials von nachwachsenden Rohstoffen. Agric. Eng. 2008, 63, 356–358. [Google Scholar] [CrossRef]
- Weißbach, F. Das Gasbildungspotenzial von Halm- und Körnerfrüchten bei der Biogasgewinnung. Agric. Eng. 2009, 64, 317–321. [Google Scholar] [CrossRef]
- Piskier, T. A method of estimation of the caloric value of the biomass. Part I—Biomass energy potential. J. Mech. Energy Eng. 2017, 1, 189–194. [Google Scholar]
- Lunguleasa, A.; Spirchez, C.; Zeleniuc, O. Evaluation of the calorific values of wastes from some tropical wood species. Maderas Cienc. Tecnol. 2020, 22, 269–280. [Google Scholar] [CrossRef]
- Esteves, B.; Sen, U.; Pereira, H. Influence of Chemical Composition on Heating Value of Biomass: A Review and Bibliometric Analysis. Energies 2023, 16, 4226. [Google Scholar] [CrossRef]
- Ruiz-Aquino, F.; Ruiz-Angel, S.; Feria-Reyes, R.; Santiago-Garcia, W.; Suarez-Mota, M.E.; Rutiaga-Quinones, J.G. Wood Chemical Composition of Five Tree Species from Oaxaca, Mexico. Bioresources 2019, 14, 9826–9839. [Google Scholar] [CrossRef]
- Ismaila, A.; Zakari, I.Y.; Nasiru, R.; Tijjani, B.I.; Abdullahi, I.; Garba, N.N. Investigation on biomass briquettes as energy source in relation to their calorific values and measurement of their total carbon and elemental contents for efficient biofuel utilization. Adv. Appl. Sci. Res. 2013, 4, 303–309. [Google Scholar]
- Aliah, H.; Winarti, I.; Iman, R.N.; Setiawan, A.; Safarina, R.; Sawitri, A. Influence of Sieve Size on Calorific Value and Proximate Properties of Bio-Briquette Composites. J. Ecol. Eng. 2023, 24, 25–34. [Google Scholar] [CrossRef]
- Mézes, L.; Bai, A.; Nagy, D.; Cinka, I.; Gabnai, Z. Optimization of Raw Material Composition in an Agricultural Biogas Plant. Trends Renew. Energy 2017, 3, 61–75. [Google Scholar] [CrossRef]
- Ingabire, H.; Ntambara, B.; Mazimpaka, E. Characterization and analysis of fish waste as feedstock for biogas production. Int. J. Low-Carbon Technol. 2023, 18, 212–217. [Google Scholar] [CrossRef]
- Orhorhoro, E.K.; Ebunilo, P.O.; Sadjere, G.E. Experimental Determination of Effect of Total Solid (TS) and Volatile Solid (VS) on Biogas Yield. Am. J. Mod. Energy 2017, 3, 131–135. [Google Scholar] [CrossRef]
- Steffen, F.; Requejo, A.; Ewald, C.; Janzon, R.; Saake, B. Anaerobic digestion of fines from recovered paper processing—Influence of fiber source, lignin and ash content on biogas potential. Bioresour. Technol. 2016, 200, 506–513. [Google Scholar] [CrossRef]
- Dioha, I.J.; Ikeme, C.H.; Nafi’u, T.; Soba, N.I.; Yusuf, M.B.S. Effect of carbon to nitrogen ratio on biogas production. Int. Res. J. Nat. Sci. 2013, 1, 1–10. [Google Scholar]
- Manyi-Loh, C.E.; Lues, R. Anaerobic Digestion of Lignocellulosic Biomass: Substrate Characteristics (Challenge) and Innovation. Fermentation 2023, 9, 755. [Google Scholar] [CrossRef]
- Zieliński, M.; Kisielewska, M.; Dębowski, M.; Elbruda, K. Effects of Nutrients Supplementation on Enhanced Biogas Production from Maize Silage and Cattle Slurry Mixture. Water. Air. Soil Pollut. 2019, 230, 117. [Google Scholar] [CrossRef]
- Alrawashdeh, K.A.b.; Gul, E.; Yang, Q.; Yang, H.; Bartocci, P.; Fantozzi, F. Effect of Heavy Metals in the Performance of Anaerobic Digestion of Olive Mill Waste. Processes 2020, 8, 1146. [Google Scholar] [CrossRef]
- Economou, E.A.; Dimitropoulou, G.; Prokopidou, N.; Dalla, I.; Sfetsas, T. Anaerobic Digestion Remediation in Three Full-Scale Biogas Plants through Supplement Additions. Methane 2023, 2, 265–278. [Google Scholar] [CrossRef]
- Owczarek, M.; Siwek, H.; Buchwał, A.; Bojko, K. Zawartość wybranych makro- i mikroelementów odpadów pomidora zwyczajnego (Solanum lycopersicum) w aspekcie ich zagospodarowania. In Postępy w Technologii i Inżynierii Chemicznej 2024; Wydawnictwo Uczelniane Zachodniopomorskiego Uniwersytetu Technologicznego w Szczecinie: Szczecin, Poland, 2024; pp. 84–98. [Google Scholar]
- Rodrigues, M.; da Silva Junges, L.F.; Mozorovicz, C.; Seidel Ziemmer, G.; Kosera Neto, C.; Américo de Andrade, E.; Izabel dos Passos, A.; Palczewski Pacheco, F.; Cezar, E.; de Melo Teixeira, L. Paraná basin basalt powder: A multinutrient soil amendment for enhancing soil chemistry and microbiology. J. South Am. Earth Sci. 2024, 141, 104957. [Google Scholar] [CrossRef]
- Conceição, L.T.; Silva, G.N.; Holsback, H.M.S.; Oliveira, C.d.F.; Marcante, N.C.; Martins, É.d.S.; Santos, F.L.d.S.; Santos, E.F. Potential of basalt dust to improve soil fertility and crop nutrition. J. Agric. Food Res. 2022, 10, 100443. [Google Scholar] [CrossRef]
- Hassan, M.S.; Mohamed, E.T. The Effect of Gabbroic Rock on Vegetative Growth, and Nutrient Status of Sesame. Asian J. Agric. Food Sci. 2017, 5, 113–125. [Google Scholar]
- Silva, F.J.P.; Carvalho, A.M.X.; Borges, P.H.C. The gabbro dacite blend as soil remineralizer. Rev. Bras. Ciênc. Agrár.—Braz. J. Agric. Sci. 2022, 17, 1–9. [Google Scholar] [CrossRef]
- Al-Ajlouni, M.G.; Othman, Y.A.; Abu-Shanab, N.S.; Alzyoud, L.F. Evaluating the Performance of Cocopeat and Volcanic Tuff in Soilless Cultivation of Roses. Plants 2024, 13, 2293. [Google Scholar] [CrossRef]
- Al-Zboon, K.K.; Al-Tabbal, J.A.; Al-Kharabsheh, N.M.; Al-Mefleh, N.K. Natural volcanic tuff as a soil mulching: Effect on plant growth and soil chemistry under water stress. Appl. Water Sci. 2019, 9, 123. [Google Scholar] [CrossRef]
- Roß, C.-L.; Nielsen, K.; Krieger, J.; Hoffmann, M.; Sensel-Gunke, K.; Ellmer, F.; Kautz, T. Monitoring N:P Ratio and Cd, Cu, Pb, and Zn Contents in Different Types of Anaerobic Digestates: A Six-Year Study Case. Int. J. Agron. 2020, 2020, 8816088. [Google Scholar] [CrossRef]
- Borowski, S.; Cieciura-Włoch, W.; Liczbiński, P. Enhancement of Biogas Production from Vegetable Waste by Application of Mineral Fertilizers. BioEnergy Res. 2024, 17, 972–982. [Google Scholar] [CrossRef]
- Guo, Q.; Majeed, S.; Xu, R.; Zhang, K.; Kakade, A.; Khan, A.; Hafeez, F.Y.; Mao, C.; Liu, P.; Li, X. Heavy metals interact with the microbial community and affect biogas production in anaerobic digestion: A review. J. Environ. Manag. 2019, 240, 266–272. [Google Scholar] [CrossRef]
- Abdel-Shafy, H.I.; Mansour, M.S.M. Biogas production as affected by heavy metals in the anaerobic digestion of sludge. Egypt. J. Pet. 2014, 23, 409–417. [Google Scholar] [CrossRef]
- Łagocka, A.; Kamiński, M.; Cholewiński, M.; Pospolita, W. Korzyści ekologiczne ze stosowania pofermentu z biogazowni rolniczych jako nawozu organicznego. Kosm. Ser. Biol. Pol. Tow. Przyr. Kopernika 2016, 65, 601–607. [Google Scholar]
- Gołaszewski, J. Wykorzystanie substratów pochodzenia rolniczego w biogazowniach w Polsce. Postępy Nauk Rol. 2011, 2, 69–94. [Google Scholar]
- Abubakar, A.M. Biodigester and Feedstock Type: Characteristic, Selection, and Global Biogas Production. J. Eng. Res. Sci. 2022, 1, 170–187. [Google Scholar] [CrossRef]
- Kovács, E.; Wirth, R.; Maróti, G.; Bagi, Z.; Nagy, K.; Minárovits, J.; Rákhely, G.; Kovács, K.L. Augmented biogas production from protein-rich substrates and associated metagenomic changes. Bioresour. Technol. 2015, 178, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Klimiuk, E.; Pokój, T.; Budzyński, W.; Dubis, B. Theoretical and observed biogas production from plant biomass of different fibre contents. Bioresour. Technol. 2010, 101, 9527–9535. [Google Scholar] [CrossRef] [PubMed]
- Nakhate, S.P.; Gupta, R.K.; Poddar, B.J.; Singh, A.K.; Tikariha, H.; Pandit, P.D.; Khardenavis, A.A.; Purohit, H.J. Influence of lignin level of raw material on anaerobic digestion process in reorganization and performance of microbial community. Int. J. Environ. Sci. Technol. 2021, 19, 1819–1836. [Google Scholar] [CrossRef]
- Xue, S.; Wang, Y.; Lyu, X.; Zhao, N.; Song, J.; Wang, X.; Yang, G. Interactive effects of carbohydrate, lipid, protein composition and carbon/nitrogen ratio on biogas production of different food wastes. Bioresour. Technol. 2020, 312, 123566. [Google Scholar] [CrossRef]
- Gruber, W.L. Biogasanlagen—Lohnt der Einsatz von Kofermenten? Mais 2003, 3, 4–7. [Google Scholar]
- Speckmaier, M.; Schlattmann, M.; Metzner, T.; Gronauer, A. Bestimmung des Biogasertrags aus Co-Substraten im diskontinuierlichen Durchflussverfahren. Agric. Eng. 2005, 60, 340–341. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, R.; Liu, G.; Chen, C.; He, Y.; Liu, X. Comparison of methane production potential, biodegradability, and kinetics of different organic substrates. Bioresour. Technol. 2013, 149, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Yasim, N.S.E.M.; Buyong, F. Comparative of experimental and theoretical biochemical methane potential generated by municipal solid waste. Environ. Adv. 2023, 11, 100345. [Google Scholar] [CrossRef]
- Llanos-Lizcano, R.; Senila, L.; Modoi, O.C. Evaluation of Biochemical Methane Potential and Kinetics of Organic Waste Streams for Enhanced Biogas Production. Agronomy 2024, 14, 2546. [Google Scholar] [CrossRef]
- Fischer, E.; Postel, J.; Ehrendreich, F.; Nelles, M. Using the mean fuel efficiency to energetically assess agricultural biogas plants. Landtechnik 2016, 71, 139–154. [Google Scholar] [CrossRef]
| Material | Parameter | ||||
|---|---|---|---|---|---|
| DM [%] | DOM [%] | VS [%] | A [%] | CV [MJ/kg] | |
| T0 | 90.44 *a ± 0.18 | 75.16 a ± 4.96 | 33.41 a ± 1.15 | 15.28 a ± 0.03 | 13.53 a ± 0.08 |
| SN | 90.75 a ± 0.27 | 82.99 b ± 4.96 | 37.89 b ± 0.38 | 7.76 b ± 0.19 | 16.29 b ± 0.02 |
| Material | Parameter | ||||
|---|---|---|---|---|---|
| C [%] | N [%] | S [%] | C/N | C/S | |
| T0 | 39.95 a ± 5.05 | 2.10 a ± 0.22 | 1.00 a ± 0.05 | 19.02 | 39.95 |
| SN | 41.50 a ± 0.64 | 2.44 a ± 0.06 | 0.28 b ± 0.04 | 17.01 | 148.21 |
| Element | SN |
|---|---|
| P [mg/g] | 1.69 ± 0.03 |
| K [mg/g] | 23.57 ± 0.04 |
| Mg [mg/g] | 2.26 ± 0.06 |
| Ca [mg/g] | 0.49 ± 0.03 |
| Na [mg/g] | 0.141 ± 0.003 |
| Cu [mg/g] | 0.0015 ± 0.0002 |
| Zn [mg/g] | 0.0287 ± 0.0014 |
| Ni [mg/g] | n.d. |
| Mn [mg/g] | 0.0213 ± 0.0004 |
| Fe [mg/g] | 0.5043 ± 0.0252 |
| Pb [mg/g] | 0.0023 ± 0.0002 |
| Cd [mg/g] | 0.00050 ± 0.00004 |
| Material | Parameter | ||||
|---|---|---|---|---|---|
| Pr [%] | F [%] | CF [%] | NFE [%] | CH [%] | |
| T0 | 0.39 ± 0.02 | 0.74 ± 0.02 | 21.02 ± 0.89 | 53.01 | 74.03 |
| SN | 0.59 ± 0.02 | 0.88 ± 0.03 | 11.41 ± 0.23 | 70.11 | 81.52 |
| Material | Biogas Yield [LN/kg DOM] (CH4 content) | Biogas Yield [LN/kg DM] | Biomethane Yield [LN/kg DOM] | Biomethane Yield [LN/kg DM] |
|---|---|---|---|---|
| T0 | 546.87 (50.37%) | 454.47 | 275.48 | 228.94 |
| SN | 551.32 (50.42%) | 504.18 | 277.99 | 254.22 |
| Material | FOM Content [g/kg DM] | Biogas Yield [LN/kg DM] | Biomethane Yield [LN/kg DM] |
|---|---|---|---|
| T0 | 686.45 | 549.16 | 288.31 |
| SN | 839.23 | 671.39 | 352.48 |
| Material | Parameter | ||||
|---|---|---|---|---|---|
| DM [%] | DOM [%] | VS [%] | A [%] | CV [MJ/kg] | |
| T0 | 90.44 ± 0.18 | 75.16 ± 4.96 | 33.41 ± 1.15 | 15.28 ± 0.03 | 13.53 ± 0.08 |
| T1 | 91.26 ± 0.06 | 70.41 ± 4.96 | 33.69 ± 0.65 | 20.85 ± 0.26 | 12.37 ± 0.06 |
| T2 | 91.94 ± 0.13 | 71.33 ± 4.96 | 35.43 ± 0.50 | 20.61 ± 0.02 | 14.12 ± 0.04 |
| T3 | 90.96 ± 0.05 | 74.80 ± 4.96 | 32.28 ± 0.69 | 16.16 ± 0.20 | 13.22 ± 0.06 |
| Material | Parameter | ||||
|---|---|---|---|---|---|
| C [%] | N [%] | S [%] | C/N | C/S | |
| T0 | 39.95 ± 5.05 | 2.10 ± 0.22 | 1.00 ± 0.05 | 19.02 | 39.95 |
| T1 | 31.34 ± 0.66 | 1.28 ± 0.06 | 1.50 ± 0.24 | 24.48 | 20.89 |
| T2 | 34.22 ± 0.04 | 2.68 ± 0.01 | 1.04 ± 0.10 | 12.77 | 32.90 |
| T3 | 32.96 ± 0.12 | 1.16 ± 0.06 | 1.20 ± 0.41 | 28.41 | 27.47 |
| Material | Parameter | ||||
|---|---|---|---|---|---|
| Pr [%] | F [%] | CF [%] | NFE [%] | CH [%] | |
| T0 | 0.39 ± 0.02 | 0.74 ± 0.02 | 21.02 ± 0.89 | 53.01 | 74.03 |
| T1 | 0.29 ± 0.01 | 1.82 ± 0.03 | 19.43 ± 0.77 | 48.87 | 68.30 |
| T2 | 0.59 ± 0.01 | 1.54 ± 0.03 | 21.16 ± 0.54 | 48.04 | 69.20 |
| T3 | 0.31 ± 0.03 | 2.58 ± 0.01 | 20.92 ± 0.88 | 50.99 | 71.91 |
| Material | Biogas Yield [LN/kg DOM] (CH4 Content) | Biogas Yield [LN/kg DM] | Biomethane Yield [LN/kg DOM] | Biomethane Yield [LN/kg DM] |
|---|---|---|---|---|
| T0 | 546.87 (50.37%) | 454.47 | 275.48 | 228.94 |
| T1 | 552.91 (50.83%) | 426.59 | 281.04 | 216.83 |
| T2 | 550.06 (50.77%) | 426.75 | 279.25 | 216.65 |
| T3 | 555.89 (51.08%) | 457.13 | 283.95 | 233.50 |
| Material | FOM Content [g/kg DM] | Biogas Yield [LN/kg DM] | Biomethane Yield [LN/kg DM] |
|---|---|---|---|
| T0 | 686.45 | 549.16 | 288.31 |
| T1 | 644.92 | 515.93 | 270.86 |
| T2 | 631.88 | 505.50 | 265.39 |
| T3 | 678.56 | 542.85 | 285.00 |
| Element | T0 | T1 | T2 | T3 |
|---|---|---|---|---|
| P [mg/g] | 1.31 ± 0.03 | 2.00 ± 0.04 | 2.08 ± 0.03 | 1.48 ± 0.01 |
| K [mg/g] | 11.24 ± 0.38 | 14.00 ± 0.35 | 24.33 ± 0.36 | 9.87 ± 0.20 |
| Mg [mg/g] | 6.41 ± 0.21 | 5.95 ± 0.13 | 6.70 ± 0.08 | 7.23 ± 0.07 |
| Ca [mg/g] | 19.01 ± 0.25 | 22.84 ± 0.22 | 22.11 ± 1.89 | 22.49 ± 0.20 |
| Na [mg/g] | 1.40 ± 0.03 | 2.41 ± 0.01 | 1.01 ± 0.004 | 1.07 ± 0.08 |
| Cu [mg/g] | 0.0032 ± 0.0001 | 0.0052 ± 0.0001 | 0.0073 ± 0.0003 | 0.0060 ± 0.0002 |
| Zn [mg/g] | 0.0600 ± 0.0034 | 0.04 ± 0.0011 | 0.03 ± 0.0005 | 0.03 ± 0.0011 |
| Ni [mg/g] | 0.0021 ± 0.0025 | n.d. | n.d. | 0.0011 ± 0.0012 |
| Mn [mg/g] | 0.2300 ± 0.0028 | 0.03 ± 0.0004 | 0.15 ± 0.0013 | 0.19 ± 0.0018 |
| Fe [mg/g] | 0.370 ± 0.017 | 0.30 ± 0.001 | 0.37 ± 0.007 | 0.51 ± 0.014 |
| Pb [mg/g] | 0.0014 ± 0.0002 | 0.0005 ± 0.0002 | 0.0013 ± 0.0009 | n.d. |
| Cd [mg/g] | 0.0014 ± 0.0001 | 0.0008 ± 0.000008 | 0.0016 ± 0.0002 | 0.0013 ± 0.0001 |
| Feedstock | Experimental Biogas Yield [LN/kg DM] (CH4 Content) | Computational Biogas Yield [LN/kg DM] (CH4 Content) | Error * [%] | Estimation Method | References |
| Beetroots | 557.79 (51.80%) | 585.89 (51.00%) | −5.04 | Weende analysis and digestibility of organic constituents | |
| Maize | 541.71 (53.97%) | 595.45 (52.10%) | −9.92 | [68] | |
| White cabbage waste | 705.20 (54.17%) | 669.60 (55.20%) | 5.05 | ||
| Maize silage | 591.09 (53.65%) | 569.05 (52.02%) | 3.73 | Weende analysis and digestibility of organic constituents | |
| Grass silage | 445.59 (53.85%) | 491.59 (53.39%) | −10.32 | [69] | |
| Rape seed oil | 1052.47 (70.75%) | 1199.40 (68.00%) | −13.96 | ||
| Feedstock | Experimental Methane Yield [LN/kg DM] | Computational Methane Yield [LN/kg DM] | Error [%] | Estimation Method | References |
| Kitchen waste | 122.81 | 157.76 | −28.46 | Equation based on organic composition | [70] |
| Fruit and vegetable waste | 11.29 | 15.05 | −33.30 | ||
| Yard waste | 110.53 | 280.26 | −153.56 | ||
| Fruit waste | 165.58 | 238.52 | -44.05 | Buswell equation | [71] |
| Vegetable waste | 105.02 | 201.47 | −91.84 | ||
| Garden waste | 255.67 | 313.05 | −22.44 | ||
| Food waste | 352.12 | 457.27 | −29.86 | Buswell equation | [72] |
| Dairy industry waste | 303.19 | 387.70 | −27.87 | ||
| Brewery waste | 439.89 | 462.82 | −5.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Owczarek, M.; Siwek, H.; Włodarczyk, M. An Evaluation of the Energy Potential of Agri-Food Waste: Green Residues from Tomato (Solanum lycopersicum L.) and Shea Nutshells (Vitellaria paradoxa). Energies 2025, 18, 730. https://doi.org/10.3390/en18030730
Owczarek M, Siwek H, Włodarczyk M. An Evaluation of the Energy Potential of Agri-Food Waste: Green Residues from Tomato (Solanum lycopersicum L.) and Shea Nutshells (Vitellaria paradoxa). Energies. 2025; 18(3):730. https://doi.org/10.3390/en18030730
Chicago/Turabian StyleOwczarek, Maja, Hanna Siwek, and Małgorzata Włodarczyk. 2025. "An Evaluation of the Energy Potential of Agri-Food Waste: Green Residues from Tomato (Solanum lycopersicum L.) and Shea Nutshells (Vitellaria paradoxa)" Energies 18, no. 3: 730. https://doi.org/10.3390/en18030730
APA StyleOwczarek, M., Siwek, H., & Włodarczyk, M. (2025). An Evaluation of the Energy Potential of Agri-Food Waste: Green Residues from Tomato (Solanum lycopersicum L.) and Shea Nutshells (Vitellaria paradoxa). Energies, 18(3), 730. https://doi.org/10.3390/en18030730







