Nanofluids in Thermal Energy Storage Systems: A Comprehensive Review
Abstract
1. Introduction
2. Thermal Energy Storage Systems
2.1. Sensible Heat Storage (SHS)
- solid-based SHS can store less heat per unit mass or volume due to inferior specific heat capacity and energy density;
- because of the formation of hotter and colder regions within the storage medium, it can be challenging to maintain and achieve constant distribution of temperature;
- factors like conduction and radiation cause self-discharge in solid-based SHS, leading to a gradual loss of heat periodically;
- while solid-based SHS may be less expensive up-front, they often have elevated enduring operational and maintenance expenses compared to liquid-based SHS.
2.2. Latent Heat Storage (LHS)
2.3. Thermo-Chemical Heat Storage (TCHS)
2.4. Challenges in TES
- Low Energy Density: Sensible heat storage typically has lower energy storage density, meaning large volumes of material are needed to store significant amounts of energy [29].
- Thermal Losses: As SHS systems primarily rely on temperature changes for energy storage, maintaining consistent heat transfer efficiency is challenging [52].
- Slow Heat Transfer: The rate of heat absorption and release is often slow due to limited heat conductivity of the storage material [53].
- Supercooling and Phase Separation: Some PCMs exhibit supercooling (cooling below their freezing point without solidifying), and phase separation (where different phases of the material separate), which can hinder performance [48].
- Limited Melting and Freezing Cycles: PCMs degrade over time with repeated melting and freezing cycles, reducing their effectiveness [48].
- Low Energy Density: While TCHS systems can store large amounts of energy, the materials used often suffer from low energy density when compared to SHS and LHS systems [54].
- Kinetics of Chemical Reactions: The rate of the chemical reactions involved in thermochemical storage can be slow, limiting the system’s responsiveness [55].
- Complex System Design: The need to control reaction conditions, such as pressure and temperature, makes TCHS systems complex and potentially costly [56].
- Optimized Energy Density: The addition of nanofluids improves heat transfer rate and thermal response, allowing for higher energy storage density compared to conventional systems [57].
- Improved Heat Transfer Efficiency: The enhanced thermal properties of nanofluids allow for better heat distribution, minimizing thermal losses during the cycles of charging and discharging [58].
- Enhanced Heat Transfer: Nanofluids increase thermal conductivity of the liquid phase, improving heat transfer rate during the phase change process, thus speeding up the energy storage and retrieval processes [58].
- Reduced Supercooling: By adding nanoparticles in PCM, nanofluids can reduce the degree of supercooling, improving the overall efficiency of the system [60].
- Prevention of Phase Separation: Nanoparticles can act as nucleating agents, promoting a more uniform phase change process and reducing the tendency for phase separation [61].
- Improved Thermal Stability: Nanofluids improve the thermal stability and lifespan of PCMs by reducing the repeated thermal cycling effects [62].
- Enhanced Reaction Rates: Nanoparticles can provide a large surface area that promotes faster chemical reactions, improving the rate of energy storage and release [63].
3. Nanofluids
3.1. Synthesis Techniques
3.1.1. One-Step Method
3.1.2. Two-Step Method
3.2. Thermophysical Properties
3.2.1. Thermal Conductivity
3.2.2. Density
3.2.3. Viscosity
3.2.4. Specific Heat Capacity
3.2.5. Stability
3.3. Heat Transfer
- Increased thermal conductivity: The nanoparticles possess greater thermal conductivity compared to most base fluids, allowing for more efficient heat transfer.
- Enhanced turbulence: The presence of nanoparticles can initiate fluid flow turbulence, promoting heat transfer by disrupting thermal boundary layers.
- Brownian motion: The random motion of nanoparticles within fluid can increase the rate of heat transfer by promoting energy exchange.
3.3.1. Natural Convection
3.3.2. Forced Convection
3.3.3. Mixed Convection
3.3.4. Turbulent Heat Transfer Using Nanofluids
- Enhanced Thermal Conductivity—Nanoparticles have higher thermal conductivity compared to normal base fluids, like water or ethylene glycol. When added to a base fluid, even at low concentrations, these nanoparticles significantly increase the thermal conductivity, which enables more efficient conduction of heat in the system. This improvement is significant at higher temperatures, at which the thermal conductivity of the base fluid increases.
- Boundary Layer Disruption—In turbulent flow, the thermal boundary layer plays a critical role in heat transfer. The introduction of nanoparticles alters the flow characteristics near the boundary layer, increasing turbulence intensity and reducing its thickness. This disruption enhances the temperature gradient at the wall-fluid interface, leading to improved convective heat transfer coefficients.
- Brownian Motion-Induced Convection—Nanoparticles in suspension exhibit continuous random motion (Brownian motion) due to collisions with surrounding fluid molecules. This motion generates localized micro-convection, which enhances the overall convective heat transfer process. The intensity of Brownian motion increases with smaller particle size and higher temperatures.
- Particle Aggregation Effects—Nanoparticles may form clusters or aggregates, especially at higher concentrations. While aggregation is often considered a limitation, small-scale clusters can create secondary turbulence structures within the flow, which further enhance the mixing of fluid layers and increase heat transfer rates.
- Thermophoresis and Diffusiophoresis—Thermophoresis refers to the movement of nanoparticles under temperature gradients, while diffusiophoresis results from concentration gradients. Both phenomena contribute to the redistribution of nanoparticles, leading to improved thermal performance in turbulent systems.
- Turbulence Intensification—The addition of nanoparticles modifies the flow behavior, increasing Reynolds stresses and intensifying turbulence. This effect becomes more pronounced in high Reynolds number flows, where turbulence dominates the heat transfer mechanism.
- Reduced Thermal Boundary Resistance—At the nanoparticle–fluid interface, thermal boundary resistance is reduced due to the high surface area-to-volume ratio of nanoparticles. This reduction facilitates efficient energy exchange between the particles and the surrounding fluid.
- Advantages of Nanofluids in Turbulent Systems—Nanofluids exhibit significantly higher Nusselt numbers and convective heat transfer coefficients compared to base fluids under turbulent conditions, due to enhanced thermal conductivity, boundary layer disruption, and increased turbulence. This results in improved heat dissipation capabilities, making nanofluids ideal for high-performance applications such as heat exchangers, cooling systems, and energy systems. Additionally, nanofluids show consistent performance improvements across various flow velocities and turbulence intensities, offering versatility for a wide range of engineering applications.
- Limitations of Nanofluids in Turbulent Systems—The addition of nanoparticles to fluids increases their effective viscosity, leading to higher pumping power requirements, creating a trade-off between enhanced heat transfer and increased flow resistance. Furthermore, the stability of nanofluids can be compromised over time as nanoparticles aggregate or settle, requiring stabilizing agents or surfactants to maintain thermal performance. Additionally, existing predictive models, such as the Dittus-Boelter and Gnielinski equations, may not accurately capture nanofluid behavior under turbulent conditions, highlighting the need for the development of more accurate models to ensure reliable performance predictions.
3.3.5. Heat Transfer Behavior of Hybrid Nanofluids
3.3.6. Role of Nanoparticle Concentration in Heat Transfer Optimization
4. Nanofluids in Thermal Energy Storage Systems
4.1. Effect on Thermal Conductivity
4.2. Effect on the Latent Heat
4.3. Effect on the Phase Change Temperature
- Enhanced heat transfer because of the presence of nanoparticles that provide a larger surface area for conduction [239].
- Nanoparticles can generate a network that facilitates thermal energy transfer [249]. No evidence of decrease in thermal conductivity has been found from previous studies.
- Improved microstructural properties of the PCM due to nanoparticles can lead to a higher latent heat of fusion [248].
- Some nanoparticles can enhance the energy storage capability of the PCM by stabilizing the phase change process [250].
- Increased thermal conductivity may result in faster phase transitions, reducing the amount of energy stored as latent heat [223].
- Nanoparticles can modify the nucleation sites and promote a more uniform phase change, shifting the melting temperature upwards [240].
- Improved thermal stability of the PCM due to the incorporation of nanoparticles can lead to a higher phase change temperature [255].
- The interaction between nanoparticles and the PCM can result in enhanced thermal stability under varying temperature conditions [256].
- Certain nanoparticles can introduce impurities that destabilize the solid phase, leading to a lower melting point [240].
4.4. Influence of Nanoparticles on Latent Heat and Temperature Control in PCM Systems: Beneficial and Adverse Effects
- Premature Phase Change: Nanoparticles can act as nucleation sites, which may cause the PCM to solidify or melt prematurely. This disrupts the desired phase change process, leading to a reduction in the amount of energy that can be stored or retrieved.
- Increased Viscosity: Higher concentrations of nanoparticles can increase the viscosity of the PCM, impeding heat transfer by slowing down the movement of the material. This can result in less efficient charging and discharging cycles, reducing the overall performance of the PCM.
- Thermal Conductivity vs. Latent Heat: While nanoparticles enhance thermal conductivity, this does not always correlate with an increase in energy storage capacity. In some cases, the higher thermal conductivity can lead to faster melting or solidification, thus reducing the time the PCM stays in the phase change state and impacting its overall latent heat capacity.
- Systems with Beneficial Temperature Control: In systems where temperature control is less critical, the improved thermal conductivity from nanoparticles can enhance PCM performance by accelerating the melting and solidification processes. This is particularly useful in applications like thermal energy storage in solar power plants or industrial heat recovery systems, where quicker response times are beneficial.
- Systems Requiring Precise Temperature Control: In applications where maintaining a stable temperature is crucial, such as in electronic cooling, the addition of nanoparticles may cause instability. The faster melting and freezing induced by higher thermal conductivity could lead to unwanted temperature fluctuations, which can impair the effectiveness of the PCM in controlling temperature. In such cases, nanoparticle concentration must be carefully optimized to avoid these adverse effects.
5. Challenges
- The release of nanoparticles into the environment can have serious detrimental effects, posing significant risks to human health and harming ecosystems [262]. These nanoparticles may exhibit toxic properties, potentially leading to respiratory issues, neurological damage, and other health concerns. Moreover, the introduction of heavy metals, often found in some nanoparticle formulations, can result in severe damage to vital organs. Given these consequences, it is imperative to explore sustainable methods for recycling and repurposing nanoparticles [262]. This not only helps mitigate the negative environmental impacts but also allows for the recovery of valuable materials. By prioritizing the development of recycling techniques, the scientific community can contribute to safer nanotechnology practices while enhancing the overall sustainability of nanomaterials [263].
- Preparing mono and hybrid nanofluids comes with several challenges. One of the key issues is achieving a stable and uniform dispersion of nanoparticles, as they tend to clump together due to Van der Waals forces, which can affect their stability and thermal performance. Another challenge is finding the right combination of nanoparticles and base fluids, as some combinations may react chemically, leading to instability. It is also tricky to determine the ideal nanoparticle concentration—higher concentrations can boost thermal conductivity, but they also make the fluid thicker, reducing flow efficiency. On top of this, advanced synthesis methods like the one-step technique are expensive and not always practical for large-scale applications. Preparing hybrid nanofluids, which use two or more types of nanoparticles, is even more complex, requiring careful methods to ensure stability, prevent separation, and maximize the combined benefits of the different materials.
- The disposal of nanomaterials poses significant environmental challenges, especially when these materials are deposited in landfills. The incineration of such materials can release harmful emissions into the atmosphere, contributing to air pollution and potentially affecting human health and local ecosystems [264]. Furthermore, the leaching of nanoparticles from landfills into soil and groundwater can lead to contamination, which poses long-term risks to environmental quality and public safety [265]. Research into alternative disposal methods and the development of biodegradable nanomaterials could play a vital role in addressing these issues and promoting a sustainable approach to the lifecycle of nanofluids in energy applications [266].
- Maintaining an optimal concentration of nanoparticles is vital for achieving high thermal conductivity in nanofluids, as excessive concentrations can lead to agglomeration. This clustering reduces the effective surface area for heat transfer and can increase viscosity, resulting in higher pumping power requirements and energy losses. Therefore, careful selection of nanoparticle concentration is essential, along with strategies to prevent agglomeration, such as using surfactants or stabilizers. By optimizing both concentration and dispersion, it becomes possible to enhance thermal conductivity effectively while minimizing the negative impacts of agglomeration, thereby improving the efficiency of TES systems and heat exchangers [267,268].
- Numerical studies of nanofluids come with their own set of challenges, largely due to the complex interactions between nanoparticles and the base fluid. This becomes even more difficult in turbulent or multiphase flows, where traditional models struggle to accurately capture the detailed dynamics. Adding to the complexity, factors like Brownian motion, thermophoresis, and sedimentation require advanced physical models, which not only increase computational demands but also make simulations harder to manage. Another hurdle is the temperature-dependent nature of nanofluid properties, like viscosity and thermal conductivity, which introduces uncertainties—especially since experimental data for validation are often limited. These studies are also highly sensitive to assumptions and boundary conditions, meaning that oversimplified models might not reflect real-world scenarios. Lastly, most numerical simulations are done on a lab scale, making it tough to apply the results to industrial systems, as experimental validation at larger scales is both expensive and complicated.
- The widespread application of nanoparticles is constrained by their high purchasing cost, which can deter manufacturers and investors from adopting this technology [266]. The cost of nanofluid PCMs increases with increasing nanoparticle concentration. The nanofluid’s total cost (EUR) CT is calculated by previous researchers [269,270] as (Equation (8)):
6. Limitations and Future Directions in Nanoparticle-Based PCM Systems and Thermal Storage Systems
6.1. Limitations of Nanoparticle-Based Phase Change Material (NPPCM) Systems
- Thermal Conductivity Enhancement: While adding nanoparticles improves thermal conductivity, this enhancement is often limited and highly dependent on particle size, shape, and concentration.
- Agglomeration and Stability: Nanoparticles in PCM systems tend to aggregate over time, reducing the overall effectiveness and stability of the material.
- Cost and Scalability: The preparation and integration of NPPCMs are cost-intensive, limiting large-scale adoption in industrial applications.
- Environmental Concerns: Many nanoparticle materials are non-biodegradable and pose environmental and health risks during production, use, and disposal.
- PCM Compatibility: Ensuring compatibility between nanoparticles and the PCM matrix without altering its phase change properties is a persistent challenge.
6.2. Limitations of Nanoparticles in Thermal Storage Systems
- Optimal Nanoparticle Concentration: The enhancement in thermal performance is concentration-dependent, but high nanoparticle volumes can lead to increased viscosity, sedimentation, and reduced system efficiency.
- Heat Transfer Limitations: While nanoparticles improve thermal conductivity, the actual enhancement in heat transfer rate may not always justify the added complexity and cost.
- Sedimentation Issues: In fluid-based systems, nanoparticles tend to sediment over time, requiring additional stabilization mechanisms.
- Compatibility with Existing Systems: Retrofitting thermal storage systems to accommodate nanoparticle-based fluids can be challenging due to potential chemical reactions, wear, or clogging issues.
6.3. Limitations of Volumetric Nanoparticle/Volume Fraction in Nanofluids
- Viscosity Increase: Higher concentrations of nanoparticles significantly increase viscosity, leading to higher pumping power requirements and reduced flow efficiency.
- Clustering and Agglomeration: Beyond a certain threshold (often 5–10% by volume), nanoparticles begin to cluster, transitioning the nanofluid into a slurry state, which adversely affects heat transfer properties.
- Thermal Saturation: The thermal conductivity improvement plateaus at higher concentrations, reducing the benefit of adding more nanoparticles.
6.4. Numerical and Theoretical Developments in Thermal Storage Systems
- Predictive Insights: Numerical models provide valuable insights into nanoparticle behavior, phase change dynamics, and thermal properties, enabling optimization without costly experiments.
- Customizable Simulations: Advanced numerical tools allow researchers to simulate specific conditions and configurations, reducing the time and resources required for experimental validation.
- Complex Interactions: Capturing the full range of nanoparticle-fluid interactions (e.g., Brownian motion, thermophoresis) is computationally expensive and often requires simplifying assumptions.
- Validation Challenges: Numerical results are often based on idealized conditions, which may not match real-world scenarios, leading to discrepancies with experimental findings.
- Scalability Issues: Translating results from small-scale numerical studies to industrial-scale systems is fraught with uncertainties and requires extensive testing.
6.5. Future Directions
- A major challenge in using NEPCMs is the high cost of preparation and synthesis. To mitigate this issue, the authors suggest adopting the economical two-step process, where readily available nanoparticles are added into PCM and base fluid. However, this method can lead to sedimentation problems, which can be addressed through techniques like magnetic stirring, surfactant addition and ultrasonication, enhancing the stability of the dispersion.
- Research findings indicate that incorporating nanoparticles into PCM improves thermal conductivity, although in some instances, a higher concentration of nanoparticles resulted in reduction of latent heat. Therefore, extensive investigation is necessary to establish the optimal nanoparticle concentration, ensuring that the benefits of enhanced thermal conductivity outweigh any adverse effects, such as decreased latent heat.
- A key concern regarding the use of nanoparticles is their environmental impact, which can be mitigated through recycling processes. This issue is critical and requires further research to fully understand the risks associated with nanofluids and NEPCM. To ensure environmental sustainability, it is essential to develop and implement mitigation techniques before applying these materials in TES systems.
- Nanofluids and NEPCMs show promise in improving TES systems. However, their long-term stability remains a concern, limiting their widespread application. Further research is needed to ensure their durability and effectiveness in these systems.
7. Conclusions and Future Perspectives
- Few studies indicate that the addition of nanoparticles can enhance latent heat of enthalpy, improving the efficiency of TES systems. This enhancement is often attributed to the increased surface area, and better thermal conductivity provided by the nanoparticles. However, in certain cases, particularly with the inclusion of carbon-based nanoparticles, a decline in latent heat has been observed. This reduction may be linked to factors such as nanoparticle agglomeration or changes in the microstructure of the PCM. Understanding these contrasting outcomes is essential for optimizing the formulation of nanofluids and ensuring the effective performance of TES systems. Further investigation into the interactions between nanoparticles and PCMs is needed to develop strategies that maximize the benefits while minimizing any potential drawbacks.
- The incorporation of nanoparticles has demonstrated significant improvements in the thermal conductivity of PCMs, making them more effective for TES applications. This enhancement in thermal conductivity allows for faster heat transfer, which is crucial for the efficiency of systems utilizing PCMs. Also, the addition of nanoparticles can help optimize the phase change process by facilitating quicker melting and solidification, ultimately leading to improved energy management. As research in this area progresses, the continued exploration of different types and concentrations of nanoparticles will be essential for maximizing the benefits of PCMs in various applications.
- The nanoparticles used in conjunction with the PCMs can either be fabricated in-house or sourced from commercial suppliers. It is important to assess the costs associated with integrating these nanoparticles with PCM, as this can lead to significant expenses. Nonetheless, a comprehensive economic analysis should be conducted, taking into account not only the initial investment but also the potential energy savings and overall efficiency improvements that the system may achieve. By evaluating these factors, one can determine the long-term financial viability of using nanoparticles in TES applications.
- Economic flexibility is significantly influenced by the market availability of nanoparticles. The accessibility of these materials directly impacts their pricing; when nanoparticles are readily available, costs tend to be lower, which suggests greater economic sustainability. This situation encourages the adoption of technologies that utilize nanoparticles, as it enhances reliability while making them more financially feasible for various applications. Thus, a stable supply chain for these materials is essential for promoting innovations in TES and other related technologies.
- Nano-based technology is currently experiencing widespread adoption across various fields, including medicine and solar applications, due to its significant potential. However, its extensive use also presents environmental challenges. The entire lifecycle of nanomaterials, from laboratory fabrication to disposal, involves multiple stages and can be costly. Improper disposal can lead to toxicity and harm ecosystems. Consequently, substantial research efforts are being directed towards developing methods for recycling used nanoparticles instead of discarding them. Ultimately, while the benefits of nano-based technology are considerable, addressing the associated risks through thorough research and innovation is essential to safely harness its full potential.
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| Abbreviations | |
| AR | Aspect Ratio |
| BS | Benzene Sulfonate |
| CA | Capric Acid |
| CFD | Computational Fluid Dynamics |
| CNF | Cyanuric fluoride |
| CNT | Carbon Nano Tube |
| CHF | Constant heat flux |
| CSP | Concentrated Solar Power |
| CTAB | Cetyl Trimethyl Ammonium Bromide |
| CWT | Constant wall temperature (K) |
| DDC | Distearyl Dimethylammonium Chloride |
| DW | Distilled Water |
| EG | Ethylene Glycol |
| EG-CNF | Expanded Graphite-Carbon Nano-fiber |
| EG-MWCNT | Expanded Graphite-Multi-walled Carbon Nano-tubes |
| GNP | Graphene Nanoplates |
| GO | Graphene Oxide |
| HTF | Heat Transfer Fluid |
| LA | Lauric acid |
| LHS | Latent Heat Storage |
| MWCNT | Multi-Walled Carbon Nanotube |
| MS | Magnetic Stirring |
| NEPCM | Nano-Enhanced Phase Change Materials |
| NF | Nanofluid |
| NG | Nano graphene |
| PCM | Phase Change Material |
| PCT | Phase Change Temperature |
| PD | Particle Diameter (µm) |
| PV | Photovoltaic |
| PVP | Polyvinylpyrrolidone |
| PW | Paraffin Wax |
| RES | Renewable Energy Sources |
| SDS | Sodium Dodecyl Sulphate |
| SDBS | Sodium Dodecyl Benzene Sulfonate |
| SHS | Sensible Heat Storage |
| SWCNT | Single-Walled Carbon Nanotube |
| TES | Thermal Energy Storage |
| TCHS | Thermo-Chemical Heat Storage |
| US | Ultrasonication |
| USB | Ultrasonic Bath |
| Dimensionless Numbers | |
| Gr | Grashoff number |
| Nu | Nusselt number |
| Pr | Prandtl number |
| Ra | Rayleigh number |
| Re | Reynolds number |
| Symbols | |
| D | Diameter (m) |
| L | Length (m) |
| ζ | Zeta potential (volts) |
| β | Coefficient of thermal expansion (1/°C or 1/K) |
| ε | Eccentricity (no units) |
| ϕ | Volume concentration (%) |
| ψ | Mass fraction (%) |
| µ | Viscosity (N·s/m2) |
| Object density (kg/m3) | |
| Liquid density (kg/m3) | |
| Acceleration due to gravity (m/s2) | |
| Spherical object radius (m) | |
| h | Convective heat transfer coefficient (W/m2·K) |
| k | Thermal conductivity (W/m·K) |
| ΔH | Change in enthalpy (J) |
| T | Temperature (K) |
| R | Radius (m) |
| m | Mass (kg) |
| Cp | Specific capacity (J/kg.K) |
| Subscripts | |
| bf | Base fluid |
| hw | Hot wall |
| nf | Nanofluid |
| p | Particle |
| np | Nanoparticle |
| f | Fluid |
| min | Minimum |
| max | Maximum |
| s | Channel/tube surface |
| con | Convection |
| Nt | Thermophoresis parameter |
| Nb | Brownian diffusion parameter |
References
- Petrollese, M.; Cascetta, M.; Tola, V.; Cocco, D.; Cau, G. Pumped thermal energy storage systems integrated with a concentrating solar power section: Conceptual design and performance evaluation. Energy 2022, 247, 123516. [Google Scholar] [CrossRef]
- Energy Institute—2024 Statistical Review of World Energy. June 2024. 73rd Edition. Available online: https://www.energyinst.org/statistical-review (accessed on 3 January 2025).
- Cascetta, M.; Petrollese, M.; Oyekale, J.; Cau, G. Thermocline vs. two-tank direct thermal storage system for concentrating solar power plants: A comparative techno-economic assessment. Int. J. Energy Res. 2021, 45, 17721–17737. [Google Scholar] [CrossRef]
- Cascetta, M.; Licheri, F.; Merchán, R.P.; Petrollese, M. Operating performance of a Joule-Brayton pumped thermal energy storage system integrated with a concentrated solar power plant. J. Energy Storage 2023, 73, 108865. [Google Scholar] [CrossRef]
- Javadi, F.S.; Metselaar, H.S.C.; Ganesan, P. Performance improvement of solar thermal systems integrated with phase change materials (PCM): A review. Sol. Energy 2020, 206, 330–352. [Google Scholar] [CrossRef]
- Zhang, P.; Li, K.; Liu, Q.; Zou, Q.; Liang, R.; Qin, L.; Wang, Y. Thermal stratification characteristics and cooling water shortage risks for pumped storage reservoir–green data centers under extreme climates. Renew. Energy 2024, 229, 120697. [Google Scholar] [CrossRef]
- Vahidhosseini, S.M.; Rashidi, S.; Hsu, S.-H.; Yan, W.-M.; Rashidi, A. Integration of solar thermal collectors and heat pumps with thermal energy storage systems for building energy demand reduction: A comprehensive review. J. Energy Storage 2024, 95, 112568. [Google Scholar] [CrossRef]
- Alva, G.; Liu, L.; Huang, X.; Fang, G. Thermal energy storage materials and systems for solar energy applications. Renew. Sustain. Energy Rev. 2017, 68, 693–706. [Google Scholar] [CrossRef]
- Abolghasemi, M.; Keshavarz, A.; Mehrabian, M.A. Thermodynamic analysis of a thermal storage unit under the influence of nanoparticles added to the phase change material and/or the working fluid. Heat Mass Transf. 2012, 48, 1961–1970. [Google Scholar] [CrossRef]
- Abdulateef, A.M.; Mat, S.; Abdulateef, J.; Sopian, K.; Al-Abidi, A.A. Thermal performance enhancement of triplex tube latent thermal storage using fins-nano-phase change material technique. Heat Transf. Eng. 2017, 39, 1067–1080. [Google Scholar] [CrossRef]
- Akeiber, H.; Nejat, P.; Majid MZ, A.; Wahid, M.A.; Jomehzadeh, F.; Famileh, I.Z.; Zaki, S.A. A review on phase change material (PCM) for sustainable passive cooling in building envelopes. Renew. Sustain. Energy Rev. 2016, 60, 1470–1497. [Google Scholar] [CrossRef]
- Peng, G.; Dou, G.; Hu, Y.; Sun, Y.; Chen, Z. Phase change material (PCM) microcapsules for thermal energy storage. Adv. Polym. Technol. 2020, 2020, 9490873. [Google Scholar] [CrossRef]
- Sarbu, I.; Sebarchievici, C. A comprehensive review of thermal energy storage. Sustainability 2018, 10, 191. [Google Scholar] [CrossRef]
- Al-Jethelah, M.; Tasnim, S.H.; Mahmud, S.; Dutta, A. Nano-PCM filled energy storage system for solar-thermal applications. Renew. Energy 2018, 126, 137–155. [Google Scholar] [CrossRef]
- Al-Waeli, A.H.; Sopian, K.; Kazem, H.A.; Yousif, J.H.; Chaichan, M.T.; Ibrahim, A.; Ruslan, M.H. Comparison of prediction methods of PV/T nanofluid and nano-PCM system using a measured dataset and artificial neural network. Sol. Energy 2018, 162, 378–396. [Google Scholar] [CrossRef]
- Magendran, S.S.; Khan, F.S.A.; Mubarak, N.M.; Vaka, M.; Walvekar, R.; Khalid, M.; Karri, R.R. Synthesis of organic phase change materials (PCM) for energy storage applications: A review. Nano-Struct. Nano-Objects 2019, 20, 100399. [Google Scholar] [CrossRef]
- Das, N.; Takata, Y.; Kohno, M.; Harish, S. Effect of carbon nano inclusion dimensionality on the melting of phase change nanocomposites in vertical shell-tube thermal energy storage unit. Int. J. Heat Mass Transf. 2017, 113, 423–431. [Google Scholar] [CrossRef]
- Nazir, H.; Batool, M.; Osorio FJ, B.; Isaza-Ruiz, M.; Xu, X.; Vignarooban, K.; Kannan, A.M. Recent developments in phase change materials for energy storage applications: A review. Int. J. Heat Mass Transf. 2019, 129, 491–523. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, K.; Wei, Q.; Ma, L.; Ye, W.; Li, H.; Gan, X. Thermal conductivity enhancement of phase change materials with 3D porous diamond foam for thermal energy storage. Appl. Energy 2019, 233–234, 208–219. [Google Scholar] [CrossRef]
- Shojaeefard, M.H.; Jourabian, M.; Ali Rabienataj Darzi, A.; Bayat, A. Inward melting inside a horizontal multilobed capsule with conductive wall affected by Ag-MgO/water hybrid and MgO/water nanofluids. J. Heat Mass Transf. Res. 2021, 8, 205–223. [Google Scholar] [CrossRef]
- Rahman, M.M.; Khan, I.; Alameh, K. The role of energy storage technologies for sustainability in developing countries. In Renewable Energy and Sustainability; Elsevier: Amsterdam, The Netherlands, 2022; pp. 347–376. [Google Scholar] [CrossRef]
- Alva, G.; Lin, Y.; Fang, G. An overview of thermal energy storage systems. Energy 2018, 144, 341–378. [Google Scholar] [CrossRef]
- Dahash, A.; Ochs, F.; Janetti, M.B.; Streicher, W. Advances in seasonal thermal energy storage for solar district heating applications: A critical review on large-scale hot-water tank and pit thermal energy storage systems. Appl. Energy 2019, 239, 296–315. [Google Scholar] [CrossRef]
- Mohan, G.; Venkataraman, M.; Gomez-Vidal, J.; Coventry, J. Assessment of a novel ternary eutectic chloride salt for next generation high-temperature sensible heat storage. Energy Convers. Manag. 2018, 167, 156–164. [Google Scholar] [CrossRef]
- Nallusamy, N.; Sampath, S.; Velraj, R. Experimental investigation on a combined sensible and latent heat storage system integrated with constant/varying (solar) heat sources. Renew. Energy 2007, 32, 1206–1227. [Google Scholar] [CrossRef]
- Barbhuiya, S.; Das, B.B.; Idrees, M. Thermal energy storage in concrete: A comprehensive review on fundamentals, technology and sustainability. J. Build. Eng. 2024, 82, 108302. [Google Scholar] [CrossRef]
- El Alami, K.; Asbik, M.; Agalit, H. Identification of natural rocks as storage materials in thermal energy storage (TES) system of concentrated solar power (CSP) plants—A review. Sol. Energy Mater. Sol. Cells 2020, 217, 110599. [Google Scholar] [CrossRef]
- Calderón-Vásquez, I.; Segovia, V.; Cardemil, J.M.; Barraza, R. Assessing the use of copper slags as thermal energy storage material for packed-bed systems. Energy 2021, 227, 120370. [Google Scholar] [CrossRef]
- Vijjapu, R.; Tiwari, S. Thermodynamics of sensible thermal energy storage systems. Encycl. Energy Storage 2022, 1, 171–185. [Google Scholar] [CrossRef]
- Rahjoo, M.; Goracci, G.; Gaitero, J.J.; Martauz, P.; Rojas, E.; Dolado, J.S. Thermal energy storage (TES) prototype based on Geopolymer concrete for high-temperature applications. Materials 2022, 15, 7086. [Google Scholar] [CrossRef]
- Hua, W.; Lv, X.; Zhang, X.; Ji, Z.; Zhu, J. Research progress of seasonal thermal energy storage technology based on supercooled phase change materials. J. Energy Storage 2023, 67, 107378. [Google Scholar] [CrossRef]
- Ren, Q.; Xu, H.; Luo, Z. PCM charging process accelerated with combination of optimized triangle fins and nanoparticles. Int. J. Therm. Sci. 2019, 140, 466–479. [Google Scholar] [CrossRef]
- Sharma, A.; Chauhan, R.; Ali Kallioğlu, M.; Chinnasamy, V.; Singh, T. A review of phase change materials (PCMs) for thermal storage in solar air heating systems. Mater. Today Proc. 2021, 44, 4357–4363. [Google Scholar] [CrossRef]
- Malik, F.K.; Khan, M.M.; Ahmed, H.F.; Irfan, M.; Ahad, I.U. Performance characteristics of PCM based thermal energy storage system for fluctuating waste heat sources. Therm. Eng. 2022, 34, 102012. [Google Scholar] [CrossRef]
- Yang, H.; Bai, Y.; Ge, C.; He, L.; Liang, W.; Zhang, X. Polyethylene glycol-based phase change materials with high photothermal conversion efficiency and shape stability in an aqueous environment for solar water heater. Compos. Part A Appl. Sci. Manuf. 2022, 154, 106778. [Google Scholar] [CrossRef]
- Lingayat, A.; Das, P.; Gilago, M.C.; Chandramohan, V.P. A detailed assessment of paraffin waxed thermal energy storage medium for solar dryers. Sol. Energy 2023, 261, 14–27. [Google Scholar] [CrossRef]
- Xie, Z.; Yan, H.; Dai, H.; Kou, Y.; Yan, X.; Tian, Y.; Shi, Q. Heat capacity study of fatty acids as phase change materials for thermal energy storage. J. Chem. Thermodyn. 2024, 197, 107338. [Google Scholar] [CrossRef]
- Anand, A.; Mansor, M.; Sharma, K.; Shukla, A.; Sharma, A.; Siddiqui, M.I.H.; Sadasivuni, K.K.; Priyadarshi, N.; Twala, B. A comprehensive review on eutectic phase change materials: Development, thermophysical properties, thermal stability, reliability, and applications. Alex. Eng. J. 2025, 112, 254–280. [Google Scholar] [CrossRef]
- Lee, S.H.; Luvnish, A.; Su, X.; Meng, Q.; Liu, M.; Kuan, H.C.; Saman, W.; Bostrom, M.; Ma, J. Advancements in polymer (Nano)composites for phase change material-based thermal storage: A focus on thermoplastic matrices and ceramic/carbon fillers. Smart Mater. Manuf. 2024, 2, 100044. [Google Scholar] [CrossRef]
- Sarı, A.; Karaipekli, A. Fatty acid esters-based composite phase change materials for thermal energy storage in buildings. Appl. Therm. Eng. 2012, 37, 208–216. [Google Scholar] [CrossRef]
- Liu, H.; Jing, J.; Liu, J.; Wang, X. Sugar alcohol-based phase change materials for thermal energy storage: Optimization design and applications. Renew. Sust. Energ. Rev. 2024, 199, 114528. [Google Scholar] [CrossRef]
- Ensari, Ö.F.; Alkan, C. 1,12-dodecanediol among similar fatty alcohols as a phase change material for thermal energy storage. Sol. Energy Adv. 2025, 5, 100079. [Google Scholar] [CrossRef]
- Wong-Pinto, L.S.; Milian, Y.; Ushak, S. Progress on use of nanoparticles in salt hydrates as phase change materials. Renew. Sust. Energy Rev. 2020, 122, 109727. [Google Scholar] [CrossRef]
- Shamberger, P.J.; Bruno, N.M. Review of metallic phase change materials for high heat flux transient thermal management applications. Appl. Energy 2020, 258, 113955. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Al-Sulaiman, F.A.; Ibrahim, N.I.; Zahir, M.H.; Al-Ahmed, A.; Saidur, R.; Yılbaş, B.S.; Sahin, A.Z. A review on current status and challenges of inorganic phase change materials for thermal energy storage systems. Renew. Sust. Energy Rev. 2017, 70, 1072–1089. [Google Scholar] [CrossRef]
- Liu, M.; Saman, W.; Bruno, F. Review on storage materials and thermal performance enhancement techniques for high temperature phase change thermal storage systems. Renew. Sust. Energ. Rev. 2012, 16, 2118–2132. [Google Scholar] [CrossRef]
- Liu, H.; Awbi, H.B. Performance of phase change material boards under natural convection. Build. Environ. 2009, 44, 1788–1793. [Google Scholar] [CrossRef]
- Zalba, B.; Marín, J.M.; Cabeza, L.F.; Mehling, H. Review on thermal energy storage with phase change: Materials, heat transfer analysis and applications. Appl. Therm. Eng. 2003, 23, 251–283. [Google Scholar] [CrossRef]
- Aydin, D.; Casey, S.P.; Riffat, S. The latest advancements on thermochemical heat storage systems. Renew. Sust. Energ. Rev. 2015, 41, 356–367. [Google Scholar] [CrossRef]
- Kuznik, F.; Opel, O.; Osterland, T.; Ruck, W.K.L. Thermal energy storage for space heating and domestic hot water in individual residential buildings. In Advances in Thermal Energy Storage Systems, 2nd ed.; Woodhead Publishing: Cambridge, UK, 2021; pp. 567–594. [Google Scholar] [CrossRef]
- Sarbu, I.; Sebarchievici, C. General review of solar-powered closed sorption refrigeration systems. Energy Convers. Manag. 2015, 105, 403–422. [Google Scholar] [CrossRef]
- Li, G. Sensible heat thermal storage energy and exergy performance evaluations. Renew. Sustain. Energy Rev. 2016, 53, 897–923. [Google Scholar] [CrossRef]
- Malinauskaite, J.; Jouhara, H. A theoretical analysis of waste heat recovery technologies. In Sustainable Energy Technology, Business Models, and Policies: Theoretical Peripheries and Practical Implications; Elsevier: Amsterdam, The Netherlands, 2024; pp. 99–144. [Google Scholar] [CrossRef]
- Wu, S.; Zhou, C.; Doroodchi, E.; Nellore, R.; Moghtaderi, B. A review on high-temperature thermochemical energy storage based on metal oxides redox cycle. Energy Convers. Manag. 2018, 168, 421–453. [Google Scholar] [CrossRef]
- Long, X.F.; Dai, L.; Lou, B.; Wu, J. The kinetics research of thermochemical energy storage system Ca(OH)2/CaO. Int. J. Energy Res. 2017, 41, 1004–1013. [Google Scholar] [CrossRef]
- Mukherjee, A.; Majumdar, R.; Saha, S.K.; Kumar, L.; Subramaniam, C. Assessment of open thermochemical energy storage system performance for low temperature heating applications. Appl. Therm. Eng. 2019, 156, 453–470. [Google Scholar] [CrossRef]
- Ma, Z.; Lin, W.; Sohel, M.I. Nano-enhanced phase change materials for improved building performance. Renew. Sustain. Energy Rev. 2016, 58, 1256–1268. [Google Scholar] [CrossRef]
- Alshuhail, L.A.; Shaik, F.; Sundar, L.S. Thermal efficiency enhancement of mono and hybrid nanofluids in solar thermal applications—A review. Alex. Eng. J. 2023, 68, 365–404. [Google Scholar] [CrossRef]
- Satti, J.R.; Das, D.K.; Ray, D. Investigation of the thermal conductivity of propylene glycol nanofluids and comparison with correlations. Int. J. Heat Mass Transf. 2017, 107, 871–881. [Google Scholar] [CrossRef]
- He, Q.; Wang, S.; Tong, M.; Liu, Y. Experimental study on thermophysical properties of nanofluids as phase-change material (PCM) in low temperature cool storage. Energy Convers. Manag. 2012, 64, 199–205. [Google Scholar] [CrossRef]
- Said, Z.; Pandey, A.K.; Tiwari, A.K.; Kalidasan, B.; Jamil, F.; Thakur, A.K.; Tyagi, V.V.; Sarı, A.; Ali, H.M. Nano-enhanced phase change materials: Fundamentals and applications. Prog. Energy Combust. Sci. 2024, 104, 101162. [Google Scholar] [CrossRef]
- Pereira, J.; Souza, R.; Moreira, A.; Moita, A. A review on the nanofluids-PCMs integrated solutions for solar thermal heat transfer enhancement purposes. Technologies 2023, 11, 166. [Google Scholar] [CrossRef]
- Saleh, H.M.; Hassan, A.I. Synthesis and characterization of nanomaterials for application in cost-effective electrochemical devices. Sustainability 2023, 15, 10891. [Google Scholar] [CrossRef]
- Moreira, T.A.; Moreira, D.C.; Ribatski, G. Nanofluids for heat transfer applications: A review. J. Braz. Soc. Mech. Sci. Eng. 2018, 40, 303. [Google Scholar] [CrossRef]
- Liu, F.; Cai, Y.; Wang, L.; Zhao, J. Effects of nanoparticle shapes on laminar forced convective heat transfer in curved ducts using two-phase model. Int. J. Heat Mass Transf. 2018, 116, 292–305. [Google Scholar] [CrossRef]
- Choi, S.U.S.; Eastman, J.A. Enhancing thermal conductivity of fluids with nanoparticles. ASME FED 1995, 231, 99–105. Available online: https://www.researchgate.net/publication/236353373_Enhancing_thermal_conductivity_of_fluids_with_nanoparticles/references (accessed on 3 January 2025).
- Özerinç, S.; Kakaç, S.; Yazıcıoğlu, A.G. Enhanced thermal conductivity of nanofluids: A state-of-the-art review. Microfluid Nanofluid 2010, 8, 145–170. [Google Scholar] [CrossRef]
- Papuga, K.; Kaszubkiewicz, J.; Kawałko, D. Do we have to use suspensions with low concentrations in determination of particle size distribution by sedimentation methods? Powder Technol. 2021, 388, 92–99. [Google Scholar] [CrossRef]
- Lo, C.H.; Tsung, T.T.; Chen, L.C.; Su, C.H.; Lin, H.M. Fabrication of copper oxide nanofluid using submerged arc nanoparticle synthesis system (SANSS). J. Nanopart. Res. 2005, 7, 313–320. [Google Scholar] [CrossRef]
- Teng, T.P.; Wang, W.P.; Hsu, Y.C. Fabrication and characterization of nanocarbon-based nanofluids by using an oxygen–acetylene flame synthesis system. Nanoscale Res. Lett. 2016, 11, 266. [Google Scholar] [CrossRef]
- Bhat, D.; Kumar, S.P.; Shenoy, U.S. Enhancing the thermal conductivity and stability of cuprous oxide nanofluids: Ribose-mediated single-step chemical synthesis for solar energy applications. Nano Trends 2025, 9, 100071. [Google Scholar] [CrossRef]
- Botha, S.S.; Ndungu, P.; Bladergroen, B.J. Physicochemical properties of oil-based nanofluids containing hybrid structures of silver nanoparticles supported on silica. Ind. Eng. Chem. Res. 2011, 50, 3071–3077. [Google Scholar] [CrossRef]
- Aberoumand, S.; Jafarimoghaddam, A.; Moravej, M.; Aberoumand, H.; Javaherdeh, K. Experimental study on the rheological behavior of silver-heat transfer oil nanofluid and suggesting two empirical based correlations for thermal conductivity and viscosity of oil based nanofluids. Appl. Therm. Eng. 2016, 101, 362–372. [Google Scholar] [CrossRef]
- Ma, B.; Shin, D.; Banerjee, D. One-step synthesis of molten salt nanofluid for thermal energy storage application: A comprehensive analysis on thermophysical property, corrosion behavior, and economic benefit. J. Energy Storage 2021, 35, 102278. [Google Scholar] [CrossRef]
- Aberoumand, S.; Jafarimoghaddam, A. Experimental study on synthesis, stability, thermal conductivity, and viscosity of Cu–engine oil nanofluid. J. Taiwan Inst. Chem. Eng. 2017, 71, 315–322. [Google Scholar] [CrossRef]
- Zheng, N.; Wang, L.; Wan, M.; Fan, R.; Sun, Z. Experimental investigation on the static and dynamic stability of water-based graphene nanofluids prepared by one-step liquid phase shear exfoliation of graphite. J. Mol. Liq. 2023, 381, 121848. [Google Scholar] [CrossRef]
- Kumar, S.P.; Shenoy, U.S.; Bhat, D.K. A direct approach towards synthesis of copper nanofluid by one-step solution phase method. J. Cryst. Growth 2024, 630, 127591. [Google Scholar] [CrossRef]
- Alrowaili, Z.A.; Ezzeldien, M.; Shaaalan, N.M.; Hussein, E.; Sharafeldin, M.A. Investigation of the effect of hybrid CuO-Cu/water nanofluid on the solar thermal energy storage system. J. Energy Storage 2022, 50, 104675. [Google Scholar] [CrossRef]
- Chen, X.; Xiong, Z.; Chen, M.; Zhou, P. Ultra-stable carbon quantum dot nanofluids for direct absorption solar collectors. Sol. Energy Mater. Sol. Cells 2022, 240, 111720. [Google Scholar] [CrossRef]
- RM Mostafizur, M.G.; Rasul, M.N. Nabi. Effect of surfactant on stability, thermal conductivity, and viscosity of aluminium oxide–methanol nanofluids for heat transfer applications. Therm. Sci. Eng. Progr. 2022, 31, 101302. [Google Scholar] [CrossRef]
- Parsa, S.M.; Yazdani, A.; Aberoumand, H.; Farhadi, Y.; Ansari, A.; Aberoumand, S.; Ali, H.M. A critical analysis on the energy and exergy performance of photovoltaic/thermal (PV/T) system: The role of nanofluids stability and synthesizing method. Sustain. Energy Technol. Assess. 2022, 51, 101887. [Google Scholar] [CrossRef]
- Roy, A.; Venkitaraj, K.P.; Vigneshwaran, P.; Saboor, S.; Cuce, E.; Saxena, K.K. Enhanced convective heat transfer with Al2O3-water nanofluid in a PCM-based thermal energy storage system. J. Energy Storage 2024, 97, 112853. [Google Scholar] [CrossRef]
- Sepehrnia, M.; Farrokh, M.J.; Karimi, M.; Mohammadzadeh, K. Experimental study and development of mathematical model using surface response method to predict the rheological performance of CeO2-CuO/10W40 hybrid nanolubricant. Arab. J. Chem. 2023, 16, 104721. [Google Scholar] [CrossRef]
- Srinivasan, N.K.; Ponnusamy, C. Stability, thermal and solidification behaviour of oxygen functionalized GNPs–CuO/water-based hybrid nanofluid phase change materials for cool thermal energy storage system. J. Energy Storage 2024, 98, 113107. [Google Scholar] [CrossRef]
- Zeng, Z.; Lu, L.; Cao, X.; Xie, T.; Lu, X.; Zhou, L.; Cheng, J.; Ma, L.; Jing, D. Influence of particle morphology on solar thermal conversion performance and sensible heat storage capacity: A case study of TiO2@Go binary nanofluid. J. Colloid Interface Sci. 2025, 682, 502–518. [Google Scholar] [CrossRef] [PubMed]
- Hadadian, M.; Goharshadi, E.K.; Youssefi, A. Electrical conductivity, thermal conductivity, and rheological properties of graphene oxide-based nanofluids. J. Nanopart. Res. 2014, 16, 2788. [Google Scholar] [CrossRef]
- Asadi, A.; Pourfattah, F. Heat transfer performance of two oil-based nanofluids containing ZnO and MgO nanoparticles; a comparative experimental investigation. Powder Technol. 2019, 343, 296–308. [Google Scholar] [CrossRef]
- Giwa, S.O.; Sharifpur, M.; Ahmadi, M.H.; Sohel Murshed, S.M.; Meyer, J.P. Experimental investigation on stability, viscosity, and electrical conductivity of water-based hybrid nanofluid of MWCNT-Fe2O3. Nanomaterials 2021, 11, 136. [Google Scholar] [CrossRef]
- Yılmaz Aydın, D.; Gürü, M. Nanofluids: Preparation, stability, properties, and thermal performance in terms of thermo-hydraulic, thermodynamics and thermo-economic analysis. J. Therm. Anal. Calorim. 2022, 147, 7631–7664. [Google Scholar] [CrossRef]
- Kumar, P.G.; Sakthivadivel, D.; Thangapandian, N.; Salman, M.; Thakur, A.K.; Sathyamurthy, R.; Kim, S.C. Effects of ultasonication and surfactant on the thermal and electrical conductivity of water–solar glycol mixture based Al2O3 nanofluids for solar-thermal applications. Sustain. Energy Technol. Assess. 2021, 47, 101371. [Google Scholar] [CrossRef]
- Mehryan, S.A.M.; Ghalambaz, M.; Kalantar Feeoj, R.; Hajjar, A.; Izadi, M. Free convection in a trapezoidal enclosure divided by a flexible partition. Int. J. Heat Mass Transf. 2020, 149, 119186. [Google Scholar] [CrossRef]
- Mansour, M.A.; Siddiqa, S.; Gorla, R.S.R.; Rashad, A.M. Effects of heat source and sink on entropy generation and MHD natural convection of Al2O3-Cu/water hybrid nanofluid filled with square porous cavity. Therm. Sci. Eng. Prog. 2018, 6, 57–71. [Google Scholar] [CrossRef]
- Takabi, B.; Salehi, S. Augmentation of the heat transfer performance of a sinusoidal corrugated enclosure by employing hybrid nanofluid. Adv. Mech. Eng. 2014, 6, 147059. [Google Scholar] [CrossRef]
- Hemmat Esfe, M.; Abbasian Arani, A.A.; Rezaie, M.; Yan, W.M.; Karimipour, A. Experimental determination of thermal conductivity and dynamic viscosity of Ag–MgO/water hybrid nanofluid. Int. Commun. Heat Mass Transf. 2015, 66, 189–195. [Google Scholar] [CrossRef]
- Wole-Osho, I.; Okonkwo, E.C.; Adun, H.; Kavaz, D.; Abbasoglu, S. An intelligent approach to predicting the effect of nanoparticle mixture ratio, concentration and temperature on thermal conductivity of hybrid nanofluids. J. Therm. Anal. Calorim. 2021, 144, 671–688. [Google Scholar] [CrossRef]
- Amani, M.; Amani, P.; Kasaeian, A.; Mahian, O.; Wongwises, S. Thermal conductivity measurement of spinel-type ferrite MnFe2O4 nanofluids in the presence of a uniform magnetic field. J. Mol. Liquids 2017, 230, 121–128. [Google Scholar] [CrossRef]
- Esfe, M.H.; Rejvani, M.; Karimpour, R.; Abbasian Arani, A.A. Estimation of thermal conductivity of ethylene glycol-based nanofluid with hybrid suspensions of SWCNT–Al2O3 nanoparticles by correlation and ANN methods using experimental data. J. Therm. Anal. Calorim. 2017, 128, 1359–1371. [Google Scholar] [CrossRef]
- Pourrajab, R.; Noghrehabadi, A.; Behbahani, M.; Hajidavalloo, E. An efficient enhancement in thermal conductivity of water-based hybrid nanofluid containing MWCNTs-COOH and Ag nanoparticles: Experimental study. J. Therm. Anal. Calorim. 2021, 143, 3331–3343. [Google Scholar] [CrossRef]
- Yu, W.; Choi, S. The role of interfacial layers in the enhanced thermal conductivity of nanofluids: A renovated Maxwell model. J. Nanopart. Res. 2003, 5, 167–171. [Google Scholar] [CrossRef]
- Parsian, A.; Akbari, M. New experimental correlation for the thermal conductivity of ethylene glycol containing Al2O3–Cu hybrid nanoparticles. J. Therm. Anal. Calorim. 2018, 131, 1605–1613. [Google Scholar] [CrossRef]
- Roy, G.; Nguyen, C.T.; Lajoie, P.-R. Numerical investigation of laminar flow and heat transfer in a radial flow cooling system with the use of nanofluids. Superlattices Microstruct. 2004, 35, 497–511. [Google Scholar] [CrossRef]
- Ho, C.J.; Huang, J.B.; Tsai, P.S.; Yang, Y.M. Preparation and properties of hybrid water-based suspension of Al2O3 nanoparticles and MEPCM particles as functional forced convection fluid. Int. Commun. Heat Mass Transf. 2010, 37, 490–494. [Google Scholar] [CrossRef]
- Murali Krishna, V.; Sandeep Kumar, M.; Mahesh, O.; Senthil, K.P. Numerical investigation of heat transfer and pressure drop for cooling of microchannel heat sink using MWCNT-CuO-water hybrid nanofluid with different mixture ratio. Mater. Today Proc. 2021, 42, 969–974. [Google Scholar] [CrossRef]
- Alnaqi, A.A. Numerical analysis of pressure drop and heat transfer of non-Newtonian nanofluids in a Li-ion battery thermal management system (BTMS) using bionic geometries. J. Energy Storage 2022, 45, 103670. [Google Scholar] [CrossRef]
- Baghbanzadeh, M.; Rashidi, A.; Soleimanisalim, A.H.; Rashtchian, D. Investigating the rheological properties of nanofluids of water/hybrid nanostructure of spherical silica/MWCNT. Thermochim. Acta 2014, 578, 53–58. [Google Scholar] [CrossRef]
- Bellos, E.; Tzivanidis, C. Thermal analysis of parabolic trough collector operating with mono and hybrid nanofluids. Sustain. Energy Technol. Assess. 2018, 26, 105–115. [Google Scholar] [CrossRef]
- Alklaibi, A.M.; Syam Sundar, L.; Chandra Mouli, K.V.V. Experimental investigation on the performance of hybrid Fe3O4 coated MWCNT/water nanofluid as a coolant of a plate heat exchanger. Int. J. Therm. Sci. 2022, 171, 107249. [Google Scholar] [CrossRef]
- Okonkwo, E.C.; Wole-Osho, I.; Kavaz, D.; Abid, M. Comparison of experimental and theoretical methods of obtaining the thermal properties of alumina/iron mono and hybrid nanofluids. J. Mol. Liq. 2019, 292, 111377. [Google Scholar] [CrossRef]
- Sundar, L.S.; Mesfin, S.; Ramana, E.V.; Said, Z.; Sousa, A.C.M. Experimental investigation of thermo-physical properties, heat transfer, pumping power, entropy generation, and exergy efficiency of nanodiamond + Fe3O4/60:40% water-ethylene glycol hybrid nanofluid flow in a tube. Therm. Sci. Eng. Prog. 2021, 21, 100799. [Google Scholar] [CrossRef]
- Zahan, I.; Nasrin, R.; Alim, M.A. Hybrid nanofluid flow in combined convective lid-driven sinusoidal triangular enclosure. AIP Conf. Proc. 2019, 2121, 070001. [Google Scholar] [CrossRef]
- Saleh, B.; Syam Sundar, L. Entropy generation and exergy efficiency analysis of ethylene glycol-water-based nanodiamond + Fe3O4 hybrid nanofluids in a circular tube. Powder Technol. 2021, 380, 430–442. [Google Scholar] [CrossRef]
- Ghalambaz, M.; Doostani, A.; Izadpanahi, E.; Chamkha, A.J. Conjugate natural convection flow of Ag–MgO/water hybrid nanofluid in a square cavity. J. Therm. Anal. Calorim. 2020, 139, 2321–2336. [Google Scholar] [CrossRef]
- Ghadikolaei, S.S.; Yassari, M.; Sadeghi, H.; Hosseinzadeh, K.H.; Ganji, D.D. Investigation on thermophysical properties of TiO2–Cu/H2O hybrid nanofluid transport dependent on shape factor in MHD stagnation point flow. Powder Technol. 2017, 322, 428–438. [Google Scholar] [CrossRef]
- Aghili Yegane, S.P.; Kasaeian, A. Thermal performance assessment of a flat-plate solar collector considering porous media, hybrid nanofluid, and magnetic field effects. J. Therm. Anal. Calorim. 2020, 141, 1969–1980. [Google Scholar] [CrossRef]
- Dalkılıç, A.S.; Türk, O.A.; Mercan, H.; Nakkaew, S.; Wongwises, S. An experimental investigation on heat transfer characteristics of graphite-SiO2/water hybrid nanofluid flow in horizontal tube with various quad-channel twisted tape inserts. Int. Commun. Heat Mass Transf. 2019, 107, 1–13. [Google Scholar] [CrossRef]
- Said, Z.; Sundar, L.S.; Tiwari, A.K.; Ali, H.M.; Sheikholeslami, M.; Bellos, E.; Babar, H. Recent advances on the fundamental physical phenomena behind stability, dynamic motion, thermophysical properties, heat transport, applications, and challenges of nanofluids. Phys. Rep. 2022, 946, 1–94. [Google Scholar] [CrossRef]
- Li, H.; Wang, L.; He, Y.; Hu, Y.; Zhu, J.; Jiang, B. Experimental investigation of thermal conductivity and viscosity of ethylene glycol based ZnO nanofluids. Appl. Therm. Eng. 2015, 88, 363–368. [Google Scholar] [CrossRef]
- Loulijat, H.; Moustabchir, H. Numerical study of the effects of Brownian motion and interfacial layer on the viscosity of nanofluid (Au-H2O). J. Mol. Liq. 2022, 350, 118221. [Google Scholar] [CrossRef]
- Zhang, H.; Qing, S.; Zhai, Y.; Zhang, X.; Zhang, A. The changes induced by pH in TiO2/water nanofluids: Stability, thermophysical properties and thermal performance. Powder Technol. 2021, 377, 748–759. [Google Scholar] [CrossRef]
- Hossein Karimi Darvanjooghi, M.; Nasr, E.M. Experimental investigation of the effect of nanoparticle size on thermal conductivity of in-situ prepared silica–ethanol nanofluid. Int. Commun. Heat Mass Transf. 2016, 77, 148–154. [Google Scholar] [CrossRef]
- Afrand, M.; Nazari Najafabadi, K.; Akbari, M. Effects of temperature and solid volume fraction on viscosity of SiO2-MWCNTs/SAE40 hybrid nanofluid as a coolant and lubricant in heat engines. Appl. Therm. Eng. 2016, 102, 45–54. [Google Scholar] [CrossRef]
- Hemmat Esfe, M.; Afrand, M.; Rostamian, S.H.; Toghraie, D. Examination of rheological behavior of MWCNTs/ZnO-SAE40 hybrid nano-lubricants under various temperatures and solid volume fractions. Exp. Therm. Fluid. Sci. 2017, 80, 384–390. [Google Scholar] [CrossRef]
- Sahoo, R.R.; Kumar, V. Development of a new correlation to determine the viscosity of ternary hybrid nanofluid. Int. Commun. Heat Mass Transf. 2020, 111, 104451. [Google Scholar] [CrossRef]
- Dalkılıç, A.S.; Açıkgöz, Ö.; Küçükyıldırım, B.O.; Akdoğan Eker, A.; Lüleci, B.; Jumpholkul, C.; Wongwises, S. Experimental investigation on the viscosity characteristics of water-based SiO2-graphite hybrid nanofluids. Int. Commun. Heat Mass Transf. 2018, 97, 30–38. [Google Scholar] [CrossRef]
- Esfe, M.H.; Afrand, M.; Yan, W.M.; Yarmand, H.; Toghraie, D.; Dahari, M. Effects of temperature and concentration on rheological behavior of MWCNTs/SiO2(20–80)-SAE40 hybrid nano-lubricant. Int. Commun. Heat Mass Transf. 2016, 76, 133–138. [Google Scholar] [CrossRef]
- Nabil, M.F.; Azmi, W.H.; Abdul Hamid, K.; Mamat, R.; Hagos, F.Y. An experimental study on the thermal conductivity and dynamic viscosity of TiO2-SiO2 nanofluids in water: Ethylene glycol mixture. Int. Commun. Heat Mass Transf. 2017, 86, 181–189. [Google Scholar] [CrossRef]
- Zareie, A.; Akbari, M. Hybrid nanoparticles effects on rheological behavior of water-EG coolant under different temperatures: An experimental study. J. Mol. Liquids 2017, 230, 408–414. [Google Scholar] [CrossRef]
- Asadi, M.; Asadi, A. Dynamic viscosity of MWCNT/ZnO–engine oil hybrid nanofluid: An experimental investigation and new correlation in different temperatures and solid concentrations. Int. Commun. Heat Mass Transf. 2016, 76, 41–45. [Google Scholar] [CrossRef]
- Alade, I.O.; Abd Rahman, M.A.; Saleh, T.A. Modeling and prediction of the specific heat capacity of Al2O3/water nanofluids using hybrid genetic algorithm/support vector regression model. Nano-Struct. Nano-Objects 2019, 17, 103–111. [Google Scholar] [CrossRef]
- Li, X.; Chen, W.; Zou, C. An experimental study on β-cyclodextrin modified carbon nanotubes nanofluids for the direct absorption solar collector (DASC): Specific heat capacity and photo-thermal conversion performance. Sol. Energy Mater. Sol. Cells 2020, 204, 110240. [Google Scholar] [CrossRef]
- Rizvi, S.M.M.; Shin, D. Specific heat capacity, viscosity, and thermal stability of carbonate-based molten salt nanofluids. J. Energy Storage 2021, 43, 103192. [Google Scholar] [CrossRef]
- Yarmand, H.; Gharehkhani, S.; Shirazi SF, S.; Amiri, A.; Montazer, E.; Arzani, H.K.; Kazi, S.N. Nanofluid based on activated hybrid of biomass carbon/graphene oxide: Synthesis, thermo-physical and electrical properties. Int. Commun. Heat Mass Transf. 2016, 72, 10–15. [Google Scholar] [CrossRef]
- Khanafer, K.; Vafai, K. A critical synthesis of thermophysical characteristics of nanofluids. Int. J. Heat Mass Transfer. 2011, 54, 4410–4428. [Google Scholar] [CrossRef]
- Barbés, B.; Páramo, R.; Blanco, E.; Pastoriza-Gallego, M.J.; Pineiro, M.M.; Legido, J.L.; Casanova, C. Thermal conductivity and specific heat capacity measurements of Al2O3 nanofluids. J. Therm. Anal. Calorim. 2013, 111, 1615–1625. [Google Scholar] [CrossRef]
- Pak, B.C.; Cho, Y.I. Hydrodynamic and heat transfer study of dispersed fluids with submicron metallic oxide particles. Exp. Heat Transf. 1998, 11, 151–170. [Google Scholar] [CrossRef]
- Sekhar, Y.R.; Sharma, K.V. Study of viscosity and specific heat capacity characteristics of water-based Al2O3 nanofluids at low particle concentrations. J. Exp. Nanosci. 2013, 10, 86–102. [Google Scholar] [CrossRef]
- Xuan, Y.; Roetzel, W. Conceptions for heat transfer correlation of nanofluids. Int. J. Heat Mass Transfer. 2000, 43, 3701–3707. [Google Scholar] [CrossRef]
- Cabaleiro, D.; Gracia-Fernández, C.; Legido, J.L.; Lugo, L. Specific heat of metal oxide nanofluids at high concentrations for heat transfer. Int. J. Heat Mass Transfer. 2015, 88, 872–879. [Google Scholar] [CrossRef]
- Chakraborty, S.; Panigrahi, P.K. Stability of nanofluid: A review. Appl. Therm. Eng. 2020, 174, 115259. [Google Scholar] [CrossRef]
- Jiang, W.; Song, J.; Jia, T.; Yang, L.; Li, S.; Li, Y.; Du, K. A comprehensive review on the pre-research of nanofluids in absorption refrigeration systems. Energy Rep. 2022, 8, 3437–3464. [Google Scholar] [CrossRef]
- Farzaneh, H.; Behzadmehr, A.; Yaghoubi, M.; Samimi, A.; Sarvari, S.M.H. Stability of nanofluids: Molecular dynamic approach and experimental study. Energy Convers. Manag. 2016, 111, 1–14. [Google Scholar] [CrossRef]
- Lei, J.; Luo, Z.; Qing, S.; Huang, X.; Li, F. Effect of surfactants on the stability, rheological properties, and thermal conductivity of Fe3O4 nanofluids. Powder Technol. 2022, 399, 117197. [Google Scholar] [CrossRef]
- Zare, P.; Keshavarz, P.; Mowla, D. Membrane absorption coupling process for CO2 capture: Application of water-based ZnO, TiO2, and multi-walled carbon nanotube nanofluids. Energy Fuels 2019, 33, 1392–1403. [Google Scholar] [CrossRef]
- Sarsam, W.S.; Amiri, A.; Kazi, S.N.; Badarudin, A. Stability and thermophysical properties of non-covalently functionalized graphene nanoplatelets nanofluids. Energy Convers. Manag. 2016, 116, 101–111. [Google Scholar] [CrossRef]
- Ghadimi, A.; Metselaar, I.H. The influence of surfactant and ultrasonic processing on improvement of stability, thermal conductivity and viscosity of titania nanofluid. Exp. Therm. Fluid. Sci. 2013, 51, 1–9. [Google Scholar] [CrossRef]
- Akash, A.R.; Abraham, S.; Pattamatta, A.; Das, S.K. Experimental assessment of the thermo-hydraulic performance of automobile radiator with metallic and nonmetallic nanofluids. Heat Trans. Eng. 2020, 41, 235–251. [Google Scholar] [CrossRef]
- Elminshawy, A.; Morad, K.; Elminshawy, N.A.S.; Elhenawy, Y. Performance enhancement of concentrator photovoltaic systems using nanofluids. Int. J. Energy Res. 2021, 45, 2959–2979. [Google Scholar] [CrossRef]
- Almanassra, I.W.; Manasrah, A.D.; Al-Mubaiyedh, U.A.; Al-Ansari, T.; Malaibari, Z.O.; Atieh, M.A. An experimental study on stability and thermal conductivity of water/CNTs nanofluids using different surfactants: A comparison study. J. Mol. Liq. 2020, 304, 111025. [Google Scholar] [CrossRef]
- Islam, M.F.; Rojas, E.; Bergey, D.M.; Johnson, A.T.; Yodh, A.G. High weight fraction surfactant solubilization of single-wall carbon nanotubes in water. Nano Lett. 2003, 3, 269–273. [Google Scholar] [CrossRef]
- Sarafraz, M.M.; Safaei, M.R.; Tian, Z.; Goodarzi, M.; Bandarra Filho, E.P.; Arjomandi, M. Thermal assessment of nano-particulate graphene-water/ethylene glycol (WEG 60:40) nano-suspension in a compact heat exchanger. Energies 2019, 12, 1929. [Google Scholar] [CrossRef]
- Abdelrazik, A.S.; Tan, K.H.; Aslfattahi, N.; Saidur, R.; Al-Sulaiman, F.A. Optical properties and stability of water-based nanofluids mixed with reduced graphene oxide decorated with silver and energy performance investigation in hybrid photovoltaic/thermal solar systems. Int. J. Energy Res. 2020, 44, 11487–11508. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Pandya, N.S.; Said, Z.; Öztop, H.F.; Abu-Hamdeh, N. 4S consideration (synthesis, sonication, surfactant, stability) for the thermal conductivity of CeO2 with MWCNT and water based hybrid nanofluid: An experimental assessment. Colloids Surf. Physicochem. Eng. Asp. 2021, 610, 125918. [Google Scholar] [CrossRef]
- Khoshvaght-Aliabadi, M.; Nouri, M.; Sartipzadeh, O.; Salami, M. Performance of agitated serpentine heat exchanger using metallic nanofluids. Chem. Eng. Res. Des. 2016, 109, 53–64. [Google Scholar] [CrossRef]
- Chakraborty, S.; Sarkar, I.; Ashok, A.; Sengupta, I.; Pal, S.K.; Chakraborty, S. Thermophysical properties of Cu-Zn-Al LDH nanofluid and its application in spray cooling. Appl. Therm. Eng. 2018, 141, 339–351. [Google Scholar] [CrossRef]
- Everts, M.; Meyer, J.P. Laminar hydrodynamic and thermal entrance lengths for simultaneously hydrodynamically and thermally developing forced and mixed convective flows in horizontal tubes. Exp. Therm. Fluid. Sci. 2020, 118, 110153. [Google Scholar] [CrossRef]
- Guzman-Urbina, A.; Fukushima, K.; Ohno, H.; Fukushima, Y. Deriving local Nusselt number correlations for heat transfer of nanofluids by genetic programming. Int. J. Therm. Sci. 2023, 192, 108382. [Google Scholar] [CrossRef]
- Khanafer, K.; Vafai, K.; Lightstone, M. Buoyancy-driven heat transfer enhancement in a two-dimensional enclosure utilizing nanofluids. Int. J. Heat Mass Transf. 2003, 46, 3639–3653. [Google Scholar] [CrossRef]
- Mahdy, A.; El-Zahar, E.R.; Rashad, A.M.; Saad, W.; Al-Juaydi, H.S. The magneto-natural convection flow of a micropolar hybrid nanofluid over a vertical plate saturated in a porous medium. Fluids 2021, 6, 202. [Google Scholar] [CrossRef]
- Acharya, N. On the hydrothermal behavior and entropy analysis of buoyancy-driven magnetohydrodynamic hybrid nanofluid flow within an octagonal enclosure fitted with fins: Application to thermal energy storage. J. Energy Storage 2022, 53, 105198. [Google Scholar] [CrossRef]
- Mokaddes, A.; Akhter, R.; Alim, M.A. Hydromagnetic natural convection in a wavy-walled enclosure equipped with hybrid nanofluid and heat generating cylinder. Alex. Eng. J. 2021, 60, 5245–5264. [Google Scholar] [CrossRef]
- Nayak, M.K.; Karimi, N.; Chamkha, A.J.; Dogonchi, A.S.; El-Sapa, S.; Galal, A.M. Efficacy of diverse structures of wavy baffles on heat transfer amplification of double-diffusive natural convection inside a C-shaped enclosure filled with hybrid nanofluid. Sustain. Energy Technol. Assess. 2022, 52, 102180. [Google Scholar] [CrossRef]
- Sheikholeslami, M.; Ziabakhsh, Z.; Ganji, D.D. Transport of Magnetohydrodynamic nanofluid in a porous media. Colloids Surf. A Physicochem. Eng. Asp. 2017, 520, 201–212. [Google Scholar] [CrossRef]
- Rahimpour, N.; Keshavarz Moraveji, M. Free convection of water–Fe3O4 nanofluid in an inclined cavity subjected to a magnetic field: CFD modeling, sensitivity analysis. Adv. Powder Technol. 2017, 28, 1573–1584. [Google Scholar] [CrossRef]
- Ho, C.J.; Liu, W.K.; Chang, Y.S.; Lin, C.C. Natural convection heat transfer of alumina-water nanofluid in vertical square enclosures: An experimental study. Int. J. Therm. Sci. 2010, 49, 1345–1353. [Google Scholar] [CrossRef]
- Khosravi, R.; Rabiei, S.; Khaki, M.; Safaei, M.R.; Goodarzi, M. Entropy generation of graphene–platinum hybrid nanofluid flow through a wavy cylindrical microchannel solar receiver by using neural networks. J. Therm. Anal. Calorim. 2021, 145, 1949–1967. [Google Scholar] [CrossRef]
- Abdelrazek, A.H.; Alawi, O.A.; Kazi, S.N.; Yusoff, N. Thermal performance evaluation for alumina coated MWCNTs composite nanofluid in annular passage of various eccentricities. Powder Technol. 2021, 391, 114–132. [Google Scholar] [CrossRef]
- Xuan, Y.; Li, Q. Investigation on convective heat transfer and flow features of nanofluids. J. Heat Transf. 2003, 125, 151–155. [Google Scholar] [CrossRef]
- Anoop, K.B.; Sundararajan, T.; Das, S.K. Effect of particle size on the convective heat transfer in nanofluid in the developing region. Int. J. Heat Mass Transf. 2009, 52, 2189–2195. [Google Scholar] [CrossRef]
- Hejazian, M.; Moraveji, M.K. A comparative analysis of single and two-phase models of turbulent convective heat transfer in a tube for TiO2 nanofluid with CFD. Numer. Heat Transf. Part A Appl. 2013, 63, 795–806. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Z.G.; Grulke, E.A.; Anderson, W.B.; Wu, G. Heat transfer properties of nanoparticle-in-fluid dispersions (nanofluids) in laminar flow. Int. J. Heat Mass Transf. 2005, 48, 1107–1116. [Google Scholar] [CrossRef]
- Vajjha, R.S.; Das, D.K.; Kulkarni, D.P. Development of new correlations for convective heat transfer and friction factor in turbulent regime for nanofluids. Int. J. Heat Mass Transf. 2010, 53, 4607–4618. [Google Scholar] [CrossRef]
- Ravi Kumar, N.T.; Bhramara, P.; Addis, B.M.; Syam Sundar, L.; Singh, M.K.; Sousa, A.C.M. Heat transfer, friction factor and effectiveness analysis of Fe3O4/water nanofluid flow in a double pipe heat exchanger with return bend. Int. Commun. Heat Mass Transf. 2017, 81, 155–163. [Google Scholar] [CrossRef]
- Asirvatham, L.G.; Raja, B.; Lal, D.M.; Wongwises, S. Convective heat transfer of nanofluids with correlations. Particuology 2011, 9, 626–631. [Google Scholar] [CrossRef]
- Huang, D.; Wu, Z.; Sunden, B. Effects of hybrid nanofluid mixture in plate heat exchangers. Exp. Therm. Fluid. Sci. 2016, 72, 190–196. [Google Scholar] [CrossRef]
- Agarwal, D.K.; Vaidyanathan, A.; Kumar, S.S. Experimental investigation on thermal performance of kerosene–graphene nanofluid. Exp. Therm. Fluid. Sci. 2016, 71, 126–137. [Google Scholar] [CrossRef]
- Hussain, S.; Ahmed, S.E.; Akbar, T. Entropy generation analysis in MHD mixed convection of hybrid nanofluid in an open cavity with a horizontal channel containing an adiabatic obstacle. Int. J. Heat Mass Transf. 2017, 114, 1054–1066. [Google Scholar] [CrossRef]
- Khan, U.; Zaib, A.; Ishak, A. Non-similarity solutions of radiative stagnation point flow of a hybrid nanofluid through a yawed cylinder with mixed convection. Alex. Eng. J. 2021, 60, 5297–5309. [Google Scholar] [CrossRef]
- Jamaludin, A.; Nazar, R.; Naganthran, K.; Pop, I. Mixed convection hybrid nanofluid flow over an exponentially accelerating surface in a porous media. Neural Comput. Appl. 2021, 33, 15719–15729. [Google Scholar] [CrossRef]
- Hussain, S.; Jamal, M.; Maatki, C.; Ghachem, K.; Kolsi, L. MHD mixed convection of Al2O3 –Cu–water hybrid nanofluid in a wavy channel with incorporated fixed cylinder. J. Therm. Anal. Calorim. 2021, 144, 2219–2233. [Google Scholar] [CrossRef]
- Xia, W.F.; Ahmad, S.; Khan, M.N.; Ahmad, H.; Rehman, A.; Baili, J.; Gia, T.N. Heat and mass transfer analysis of nonlinear mixed convective hybrid nanofluid flow with multiple slip boundary conditions. Case Stud. Therm. Eng. 2022, 32, 101893. [Google Scholar] [CrossRef]
- Ahmad, A.; Asghar, S.; Afzal, S. Flow of nanofluid past a Riga plate. J. Magn. Magn. Mater. 2016, 402, 44–48. [Google Scholar] [CrossRef]
- Ben Mansour, R.; Galanis, N.; Nguyen, C.T. Experimental study of mixed convection with water–Al2O3 nanofluid in inclined tube with uniform wall heat flux. Int. J. Therm. Sci. 2011, 50, 403–410. [Google Scholar] [CrossRef]
- Yu, W.; France, D.M.; Timofeeva, E.V.; Singh, D.; Routbort, J.L. Comparative review of turbulent heat transfer of nanofluids. Int. J. Heat Mass Transf. 2012, 55, 5380–5396. [Google Scholar] [CrossRef]
- Fotukian, S.M.; Nasr Esfahany, M. Experimental investigation of turbulent convective heat transfer of dilute γ-Al2O3/water nanofluid inside a circular tube. Int. J. Heat Fluid Flow 2010, 31, 606–612. [Google Scholar] [CrossRef]
- Fotukian, S.M.; Nasr Esfahany, M. Experimental study of turbulent convective heat transfer and pressure drop of dilute CuO/water nanofluid inside a circular tube. Int. Commun. Heat Mass Transf. 2010, 37, 214–219. [Google Scholar] [CrossRef]
- Demir, H.; Dalkilic, A.S.; Kürekci, N.A.; Duangthongsuk, W.; Wongwises, S. Numerical investigation on the single-phase forced convection heat transfer characteristics of TiO2 nanofluids in a double-tube counter flow heat exchanger. Int. Commun. Heat Mass Transf. 2011, 38, 218–228. [Google Scholar] [CrossRef]
- Ajeel, R.K.; Salim, W.S.-I.; Sopian, K.; Yusoff, M.Z.; Hasnan, K.; Ibrahim, A.; Al-Waeli, A.H.A. Turbulent convective heat transfer of silica oxide nanofluid through corrugated channels: An experimental and numerical study. Int. J. Heat Mass Transf. 2019, 145, 118806. [Google Scholar] [CrossRef]
- Albadr, J.; Tayal, S.; Alasadi, M. Heat transfer through heat exchanger using Al2O3 nanofluid at different concentrations. Case Stud. Therm. Eng. 2013, 1, 38–44. [Google Scholar] [CrossRef]
- Montazer, E.; Shafii, M.B.; Salami, E.; Muhamad, M.R.; Yarmand, H.; Gharehkhani, S.; Chowdhury, Z.Z.; Kazi, S.N.; Badarudin, A. Heat transfer in turbulent nanofluids: Separation flow studies and development of novel correlations. Adv. Powder Technol. 2020, 31, 3120–3133. [Google Scholar] [CrossRef]
- Fu, R.; Liu, Z.; Chen, Y.; Yan, Y. Experimental investigation of turbulent forced heat transfer of Fe3O4 ethylene glycol–water nanofluid with highly disaggregated particles. Therm. Sci. Eng. Prog. 2019, 10, 1–9. [Google Scholar] [CrossRef]
- Sundar, L.S.; Singh, M.K.; Sousa, A.C.M. Enhanced heat transfer and friction factor of MWCNT–Fe3O4/water hybrid nanofluids. Int. Commun. Heat Mass Transf. 2014, 52, 73–83. [Google Scholar] [CrossRef]
- Adogbeji, V.O.; Sharifpur, M.; Meyer, J.P. Experimental investigation into heat transfer and flow characteristics of magnetic hybrid nanofluid (Fe3O4/TiO2) in turbulent region. Appl. Therm. Eng. 2025, 258, 124630. [Google Scholar] [CrossRef]
- Andreozzi, A.; Manca, O.; Nardini, S.; Ricci, D. Forced convection enhancement in channels with transversal ribs and nanofluids. Appl. Therm. Eng. 2016, 98, 1044–1053. [Google Scholar] [CrossRef]
- Yu, Y.; Li, D.; Meng, H.; Zhang, J.; Xiang, K.; Li, W. Enhancement study of turbulent heat transfer performance of nanofluids in the clover static mixer. Int. J. Therm. Sci. 2024, 198, 108900. [Google Scholar] [CrossRef]
- Sonawane, S. Investigation of turbulent heat transfer performance of aviation turbine fuel multi-wall carbon nanotube nanofluid. Adv. Powder Technol. 2023, 34, 104079. [Google Scholar] [CrossRef]
- Shuvo, M.S.; Ruvo, T.H.; Saha, S. Characteristics of turbulent forced convective nanofluid flow and heat transfer in a 2D axisymmetric corrugated pipe. Therm. Sci. Eng. Prog. 2023, 41, 101838. [Google Scholar] [CrossRef]
- Fujimoto, K.; Shibata, A.; Torii, S. An experimental and numerical study of turbulent heat transfer enhancement for graphene nanofluids produced by pulsed discharge. Int. J. Thermofluids 2022, 16, 100219. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, L.; Wen, T.; Dong, C. Turbulent heat transfer and flow analysis of hybrid Al2O3-CuO/water nanofluid: An experiment and CFD simulation study. Appl. Therm. Eng. 2021, 188, 116589. [Google Scholar] [CrossRef]
- Karami, F.; Abbasian Arani, A.A.; Akbari, O.A.; Pourfattah, F.; Toghraie, D. Numerical study of location and depth of rectangular grooves on the turbulent heat transfer performance and characteristics of CuO-water nanofluid flow. Heliyon 2023, 9, e14239. [Google Scholar] [CrossRef]
- Ahmad, F.; Waqas, H.; Ayed, H.; Hussain, S.; Farooq, S.; Khan, S.A.; Almatroud, A.O. Numerical treatment with Lobatto-IIIa scheme magneto-thermo-natural convection flow of casson nanofluid (MoS2-Cu/SA) configured by a stretching cylinder in porous medium with multiple slips. Case Stud. Therm. Eng. 2021, 26, 101132. [Google Scholar] [CrossRef]
- Rashad, A.M.; Chamkha, A.J.; Ismael, M.A.; Salah, T. Magnetohydrodynamics natural convection in a triangular cavity filled with a Cu-Al2O3/water hybrid nanofluid with localized heating from below and internal heat generation. J. Heat Transf. 2018, 140, 093701. [Google Scholar] [CrossRef]
- Yarmand, H.; Gharehkhani, S.; Ahmadi, G.; Shirazi, S.F.; Baradaran, S.; Montazer, E.; Zubir, M.N.M.; Alehashem, M.S.; Kazi, S.N.; Dahari, M. Graphene nanoplatelets-silver hybrid nanofluids for enhanced heat transfer. Energy Convers. Manag. 2015, 100, 419–428. [Google Scholar] [CrossRef]
- Dinarvand, S.; Rostami, M.N.; Pop, I. A novel hybridity model for TiO2-CuO/water hybrid nanofluid flow over a static/moving wedge or corner. Sci. Rep. 2019, 9, 16290. [Google Scholar] [CrossRef]
- Madhesh, D.; Parameshwaran, R.; Kalaiselvam, S. Experimental investigation on convective heat transfer and rheological characteristics of Cu-TiO2 hybrid nanofluids. Exp. Therm. Fluid. Sci. 2014, 52, 104–115. [Google Scholar] [CrossRef]
- Moghadassi, A.; Ghomi, E.; Parvizian, F. A numerical study of water-based Al2O3 and Al2O3-Cu hybrid nanofluid effect on forced convective heat transfer. Int. J. Therm. Sci. 2015, 92, 50–57. [Google Scholar] [CrossRef]
- Ferrouillat, S.; Bontemps, A.; Poncelet, O.; Soriano, O.; Gruss, J.-A. Influence of nanoparticle shape factor on convective heat transfer and energetic performance of water-based SiO2 and ZnO nanofluids. Appl. Therm. Eng. 2013, 51, 839–851. [Google Scholar] [CrossRef]
- Li, S.; Yang, Q.; Ye, L.; Du, H.; Zhang, Z.; Huang, X.; Xu, J. Effect of nanoparticle concentration on physical and heat-transfer properties and evaporation characteristics of graphite/n-decane nanofluid fuels. ACS Omega 2022, 7, 3284–3292. [Google Scholar] [CrossRef] [PubMed]
- Sujith, S.V.; Kim, H.; Lee, J. A Review on Thermophysical Property Assessment of Metal Oxide-Based Nanofluids: Industrial Perspectives. Metals 2022, 12, 165. [Google Scholar] [CrossRef]
- Ali, N.; Teixeira, J.A.; Addali, A. A review on nanofluids: Fabrication, stability, and thermophysical properties. J. Nanomater. 2018, 2018, 6978130. [Google Scholar] [CrossRef]
- Szczyglewska, P.; Feliczak-Guzik, A.; Nowak, I. Nanotechnology–General Aspects: A Chemical Reduction Approach to the Synthesis of Nanoparticles. Molecules 2023, 28, 4932. [Google Scholar] [CrossRef]
- Moradi, A.; Zareh, M.; Afrand, M.; Khayat, M. Effects of temperature and volume concentration on thermal conductivity of MWCNTs (70-30)/EG-water hybrid nano-fluid. Powder Technol. 2020, 362, 578–585. [Google Scholar] [CrossRef]
- Tahmooressi, H.; Kasaeian, A.; Tarokh, A.; Rezaei, R.; Hoorfar, M. Numerical simulation of aggregation effect on nanofluids thermal conductivity using the lattice Boltzmann method. Int. Commun. Heat Mass Transf. 2020, 110, 104408. [Google Scholar] [CrossRef]
- Li, Q.; Wang, J.; Wang, J.; Baleta, J.; Min, C.; Sundén, B. Effects of gravity and variable thermal properties on nanofluid convective heat transfer using connected and unconnected walls. Energy Convers. Manag. 2018, 171, 1440–1448. [Google Scholar] [CrossRef]
- Cieśliński, J.T.; Smolen, S.; Sawicka, D. Effect of Temperature and Nanoparticle Concentration on Free Convective Heat Transfer of Nanofluids. Energies 2021, 14, 3566. [Google Scholar] [CrossRef]
- Ben Bacha, H.; Ullah, N.; Hamid, A.; Shah, N.A. A comprehensive review on nanofluids: Synthesis, cutting-edge applications, and future prospects. Int. J. Thermofluids 2024, 22, 100595. [Google Scholar] [CrossRef]
- Bellos, E.; Said, Z.; Tzivanidis, C. Nanofluids in Linear Fresnel Reflector. In Nanotechnology Applications for Solar Energy Systems, 1st ed.; Wiley: Hoboken, NJ, USA, 2023; pp. 99–124. [Google Scholar] [CrossRef]
- Yang, Z.L.; Walvekar, R.; Wong, W.P.; Sharma, R.K.; Dharaskar, S.; Khalid, M. Advances in phase change materials, heat transfer enhancement techniques, and their applications in thermal energy storage: A comprehensive review. J. Energy Storage 2024, 87, 111329. [Google Scholar] [CrossRef]
- Harikrishnan, S.; Imran Hussain, S.; Devaraju, A.; Sivasamy, P.; Kalaiselvam, S. Improved performance of a newly prepared nano-enhanced phase change material for solar energy storage. J. Mech. Sci. Technol. 2017, 31, 4903–4910. [Google Scholar] [CrossRef]
- Qu, Y.; Wang, S.; Zhou, D.; Tian, Y. Experimental study on thermal conductivity of paraffin-based shape-stabilized phase change material with hybrid carbon nano-additives. Renew. Energy 2020, 146, 2637–2645. [Google Scholar] [CrossRef]
- Colla, L.; Fedele, L.; Mancin, S.; Danza, L.; Manca, O. Nano-PCMs for enhanced energy storage and passive cooling applications. Appl. Therm. Eng. 2017, 110, 584–589. [Google Scholar] [CrossRef]
- Lin, S.C.; Al-Kayiem, H.H. Evaluation of copper nanoparticles–paraffin wax compositions for solar thermal energy storage. Sol. Energy 2016, 132, 267–278. [Google Scholar] [CrossRef]
- Singh, R.P.; Xu, H.; Kaushik, S.C.; Rakshit, D.; Romagnoli, A. Charging performance evaluation of finned conical thermal storage system encapsulated with nanoenhanced phase change material. Appl. Therm. Eng. 2019, 151, 176–190. [Google Scholar] [CrossRef]
- Li, C.; Zhang, B.; Xie, B.; Zhao, X.; Chen, J.; Chen, Z.; Long, Y. Stearic acid/expanded graphite as a composite phase change thermal energy storage material for tankless solar water heater. Sustain. Cities Soc. 2019, 44, 458–464. [Google Scholar] [CrossRef]
- Bahiraei, F.; Fartaj, A.; Nazri, G.A. Experimental and numerical investigation on the performance of carbon-based nanoenhanced phase change materials for thermal management applications. Energy Convers. Manag. 2017, 153, 115–128. [Google Scholar] [CrossRef]
- Kibria, M.G.; Mohtasim, M.S.; Paul, U.K.; Das, B.K.; Saidur, R. Impact of hybrid nano PCM (paraffin wax with Al2O3 and ZnO nanoparticles) on photovoltaic thermal system: Energy, exergy, exergoeconomic and enviroeconomic analysis. J. Clean. Prod. 2024, 436, 140577. [Google Scholar] [CrossRef]
- Krishna, J.; Kishore, P.; Solomon, A.B. Heat pipe with nano enhanced-PCM for electronic cooling application. Exp. Therm. Fluid Sci. 2017, 81, 84–92. [Google Scholar] [CrossRef]
- Soni, V.; Kumar, A.; Jain, V.K. Performance evaluation of nano-enhanced phase change materials during discharge stage in waste heat recovery. Renew. Energy 2018, 127, 587–601. [Google Scholar] [CrossRef]
- Parameshwaran, R.; Deepak, K.; Saravanan, R.; Kalaiselvam, S. Preparation, thermal and rheological properties of hybrid nanocomposite phase change material for thermal energy storage. Appl. Energy 2014, 115, 320–330. [Google Scholar] [CrossRef]
- Singh, S.K.; Verma, S.K.; Kumar, R. Thermal performance and behavior analysis of SiO2, Al2O3, and MgO based nano-enhanced phase-changing materials, latent heat thermal energy storage system. J. Energy Storage 2022, 48, 103977. [Google Scholar] [CrossRef]
- Moein-Jahromi, M.; Rahmanian-Koushkaki, H.; Rahmanian, S.; Pilban Jahromi, S. Evaluation of nanostructured GNP and CuO compositions in PCM-based heat sinks for photovoltaic systems. J. Energy Storage 2022, 53, 105240. [Google Scholar] [CrossRef]
- Ali, M.A.; Fayaz Viegas, R.F.; Kumar, M.B.S.; Kannapiran, R.K.; Feroskhan, M. Enhancement of heat transfer in paraffin wax PCM using nano graphene composite for industrial helmets. J. Energy Storage 2019, 26, 100982. [Google Scholar] [CrossRef]
- Maher, H.; Rocky, K.A.; Bassiouny, R.; Saha, B.B. Synthesis and thermal characterization of paraffin-based nanocomposites for thermal energy storage applications. Therm. Sci. Eng. Prog. 2021, 22, 100797. [Google Scholar] [CrossRef]
- Qian, T.; Li, J.; Feng, W.; Nian, H.E. Enhanced thermal conductivity of form-stable phase change composite with single-walled carbon nanotubes for thermal energy storage. Sci. Rep. 2017, 7, 44710. [Google Scholar] [CrossRef]
- Paul, U.K.; Mohtasim, M.S.; Kibria, M.G.; Das, B.K. Nano-material based composite phase change materials and nanofluid for solar thermal energy storage applications: Featuring numerical and experimental approaches. J. Energy Storage 2024, 98, 113032. [Google Scholar] [CrossRef]
- Rufuss, D.D.W.; Kumar, V.R.; Suganthi, L.; Iniyan, S.; Davies, P. Techno-economic analysis of solar stills using integrated fuzzy analytical hierarchy process and data envelopment analysis. Sol. Energy 2018, 159, 820–833. [Google Scholar] [CrossRef]
- Saeed, R.M.; Schlegel, J.P.; Castano, C.; Sawafta, R. Preparation and enhanced thermal performance of novel (solid to gel) form-stable eutectic PCM modified by nano-graphene platelets. J. Energy Storage 2018, 15, 91–102. [Google Scholar] [CrossRef]
- Salyan, S.; Suresh, S. Study of thermo-physical properties and cycling stability of D-Mannitol-copper oxide nanocomposites as phase change materials. J. Energy Storage 2018, 15, 245–255. [Google Scholar] [CrossRef]
- Warzoha, R.J.; Fleischer, A.S. Improved heat recovery from paraffin-based phase change materials due to the presence of percolating graphene networks. Int. J. Heat. Mass. Transf. 2014, 79, 314–323. [Google Scholar] [CrossRef]
- Pethurajan, V.; Sivan, S. Fabrication, characterisation and heat transfer study on microencapsulation of nano-enhanced phase change material. Chem. Eng. Process. 2018, 133, 12–23. [Google Scholar] [CrossRef]
- Arshad, A.; Jabbal, M.; Shi, L.; Yan, Y. Thermophysical characteristics and enhancement analysis of carbon-additives phase change mono and hybrid materials for thermal management of electronic devices. J. Energy Storage 2021, 34, 102231. [Google Scholar] [CrossRef]
- Karthikeyan, K.; Mariappan, V.; Kalidoss, P.; Mohana Jai Ganesh, J.; Nanda Kishore, P.V.R.; Prathiban, S.; Anish, R. Characterization and thermal properties of lauryl alcohol-capric acid binary mixture with hybrid-nanoparticles as phase change material for vaccine storage applications. J. Energy Storage 2023, 74, 109442. [Google Scholar] [CrossRef]
- Han, D.; Guene Lougou, B.; Xu, Y.; Shuai, Y.; Huang, X. Thermal properties characterization of chloride salts/nanoparticles composite phase change material for high-temperature thermal energy storage. Appl. Energy 2020, 264, 114674. [Google Scholar] [CrossRef]
- Kalbande, V.P.; Walke, P.V.; Untawale, S.; Mohan, M. Performance evaluation of novel heat pipe-assisted thermal storage system with parabolic trough solar collector using nanofluid. Energy Technol. 2022, 10, 2200118. [Google Scholar] [CrossRef]
- Wang, J.; Xie, H.; Xin, Z. Thermal properties of paraffin based composites containing multi-walled carbon nanotubes. Thermochim. Acta 2009, 488, 39–42. [Google Scholar] [CrossRef]
- Harikrishnan, S.; Devaraju, A.; Rajesh Kumar, G.; Kalaiselvam, S. Improved thermal energy storage behavior of a novel nanofluid as phase change material (PCM). Mater. Today Proc. 2019, 9, 410–421. [Google Scholar] [CrossRef]
- Mohamed, N.H.; Soliman, F.S.; El Maghraby, H.; Moustfa, Y. Thermal conductivity enhancement of treated petroleum waxes, as phase change material, by α nano alumina: Energy storage. Renew. Sustain. Energy Rev. 2017, 70, 1052–1058. [Google Scholar] [CrossRef]
- Kalbande, V.P.; Fating, G.; Mohan, M.; Rambhad, K.; Sinha, A.K. Experimental and theoretical study for suitability of hybrid nano enhanced phase change material for thermal energy storage applications. J. Energy Storage 2022, 51, 104431. [Google Scholar] [CrossRef]
- Amin, M.; Putra, N.; Kosasih, E.A.; Prawiro, E.; Luanto, R.A.; Mahlia, T.M.I. Thermal properties of beeswax/graphene phase change material as energy storage for building applications. Appl. Therm. Eng. 2017, 112, 273–280. [Google Scholar] [CrossRef]
- Arshad, A.; Jabbal, M.; Shi, L.; Darkwa, J.; Weston, N.J.; Yan, Y. Development of TiO2/RT–35HC based nanocomposite phase change materials (NCPCMs) for thermal management applications. Sustain. Energy Technol. Assess. 2021, 43, 100865. [Google Scholar] [CrossRef]
- Gupta, N.; Kumar, A.; Dhasmana, H.; Kumar, V.; Kumar, A.; Shukla, P.; Verma, A.; Nutan, G.V.; Dhawan, S.K.; Jain, V.K. Enhanced thermophysical properties of metal oxide nanoparticles embedded magnesium nitrate hexahydrate based nanocomposite for thermal energy storage applications. J. Energy Storage 2020, 32, 101773. [Google Scholar] [CrossRef]
- Pugalenthi, S.; Chellapandian, M.; Dharmaraj, J.J.; Devaraj, J.; Arunachelam, N.; Singh, S.B. Enhancing the thermal transport property of eutectic lauric-stearic acid based phase change material with silicon carbide nanoparticles for usage in battery thermal management system. J. Energy Storage 2024, 84, 110890. [Google Scholar] [CrossRef]
- Mayilvelnathan, V.; Arasu, A.V. Characterisation and thermophysical properties of graphene nanoparticles dispersed erythritol PCM for medium temperature thermal energy storage applications. Thermochim. Acta 2019, 676, 94–103. [Google Scholar] [CrossRef]
- Yu, Q.; Zhang, C.; Lu, Y.; Kong, Q.; Wei, H.; Yang, Y.; Sciacovelli, A. Comprehensive performance of composite phase change materials based on eutectic chloride with SiO2 nanoparticles and expanded graphite for thermal energy storage system. Renew. Energy 2021, 172, 1120–1132. [Google Scholar] [CrossRef]
- Tian, H.; Du, L.; Wei, X.; Deng, S.; Wang, W.; Ding, J. Enhanced thermal conductivity of ternary carbonate salt phase change material with Mg particles for solar thermal energy storage. Appl. Energy 2017, 204, 525–530. [Google Scholar] [CrossRef]
- Islam, A.; Pandey, A.K.; Saidur, R.; Tyagi, V.V. Shape stable composite phase change material with improved thermal conductivity for electrical-to-thermal energy conversion and storage. Mater. Today Sustain. 2024, 25, 100678. [Google Scholar] [CrossRef]
- Yin, S.; Lu, M.; Liu, C.; Tong, L.; Wang, L.; Ding, Y. Fabrication and thermal properties of capric–stearic acid eutectic/nano-SiO2 phase change material with expanded graphite and CuO for thermal energy storage. J. Energy Storage 2024, 77, 110025. [Google Scholar] [CrossRef]
- He, M.; Yang, L.; Lin, W.; Chen, J.; Mao, X.; Ma, Z. Preparation, thermal characterization and examination of phase change materials (PCMs) enhanced by carbon-based nanoparticles for solar thermal energy storage. J. Energy Storage 2019, 25, 100874. [Google Scholar] [CrossRef]
- Kumar, P.M.; Sudarvizhi, D.; Michael Joseph Stalin, P.; Aarif, A.; Abhinandhana, R.; Renuprasanth, A.; Sathya, V.; Thirukkural Ezhilan, N. Thermal characteristics analysis of a phase change material under the influence of nanoparticles. Mater. Today Proc. 2021, 45, 7876–7880. [Google Scholar] [CrossRef]
- Hayat, M.A.; Yang, Y.; Li, L.; Bevilacqua, M.; Chen, Y. Preparation and thermophysical characterisation analysis of potential nano-phase transition materials for thermal energy storage applications. J. Mol. Liq. 2023, 376, 121464. [Google Scholar] [CrossRef]
- Bharathiraja, R.; Ramkumar, T.; Selvakumar, M. Studies on the thermal characteristics of nano-enhanced paraffin wax phase change material (PCM) for thermal storage applications. J. Energy Storage 2023, 73, 109216. [Google Scholar] [CrossRef]
- Pasupathi, M.K.; Alagar, K.; Joseph Stalin, P.M.; Matheswaran, M.M.; Ghosh, A. Characterization of hybrid-nano/paraffin organic phase change material for thermal energy storage applications in solar thermal systems. Energies 2020, 13, 5079. [Google Scholar] [CrossRef]
- Anika, U.A.; Kibria, M.G.; Kanka, S.D.; Mohtasim, M.S.; Paul, U.K.; Das, B.K. Exergy, exergo-economic, environmental and sustainability analysis of pyramid solar still integrated hybrid nano-PCM, black sand, and sponge. Sol. Energy 2024, 274, 112559. [Google Scholar] [CrossRef]
- Hemmatian, A.; Kargarsharifabad, H.; Abedini Esfahlani, A.; Rahbar, N.; Shoeibi, S. Improving solar still performance with heat pipe/pulsating heat pipe evacuated tube solar collectors and PCM: An experimental and environmental analysis. Sol. Energy 2024, 269, 112371. [Google Scholar] [CrossRef]
- Sathish, T.; Suresh, P.; Sharma, K.; Saravanan, R.; Saleel, C.A.; Shaik, S.; Khan, S.A.; Panchal, H. Zero emission/energy building heating through parabolic dish collector focused KNO3–NaNO3 and KNO3–NaNO3–NaNO2 PCM absorber: A case study. Case Stud. Therm. Eng. 2023, 44, 102854. [Google Scholar] [CrossRef]
- Nourizadeh, F.; Shekaari, H.; Mokhtarpour, M. High performance nano-enhanced phase change composites based on 2-hydroxyethylammonium stearate for efficient and environmentally friendly thermal energy storage and thermoelectric conversion. J. Power Sources 2024, 614, 235042. [Google Scholar] [CrossRef]
- Tyagi, P.K.; Kumar, R. Comprehensive performance assessment of photovoltaic/thermal system using MWCNT/water nanofluid and novel finned multi-block nano-enhanced phase change material-based thermal collector: Energy, exergy, economic, and environmental (4E) perspectives. Energy 2024, 312, 133575. [Google Scholar] [CrossRef]
- Yu, X.; He, G.; Wu, J.; Wu, Z.; Wang, Y.; Dong, L.; Xie, H. Enhancement of solar-to-thermal properties of multiple-point Cu2O/TiN plasmonic nanofluids. Chem. Eng. J. 2024, 498, 155523. [Google Scholar] [CrossRef]
- Aligholami, M.; Shafiei, S.; Akbari, M.; Moodley, M.K.; Veeredhi, V.R.; Mthunzi, P.; Maaza, M. MoS2-based nanofluid using pulsed laser ablation in liquid for concentrating solar power plant: Thermophysical study. Sol. Energy 2024, 280, 112876. [Google Scholar] [CrossRef]
- Seyyedi, S.M.; Dogonchi, A.S.; Hashemi-Tilehnoee, M.; Waqas, M.; Ganji, D.D. Entropy generation and economic analyses in a nanofluid filled L-shaped enclosure subjected to an oriented magnetic field. Appl. Therm. Eng. 2020, 168, 114789. [Google Scholar] [CrossRef]
- Tayebi, T.; Dogonchi, A.S.; Karimi, N.; Hu, G.-J.; Chamkha, A.J.; Elmasry, Y. Thermo-economic and entropy generation analyses of magnetic natural convective flow in a nanofluid-filled annular enclosure fitted with fins. Sustain. Energy Technol. Assess. 2021, 46, 101274. [Google Scholar] [CrossRef]
- Hu, J.-T.; Mei, S.-J. Natural convection in nanofluid enclosure under magnetic field: Entropy generation and economic analysis. Propuls. Power Res. 2024, 13, 273–293. [Google Scholar] [CrossRef]



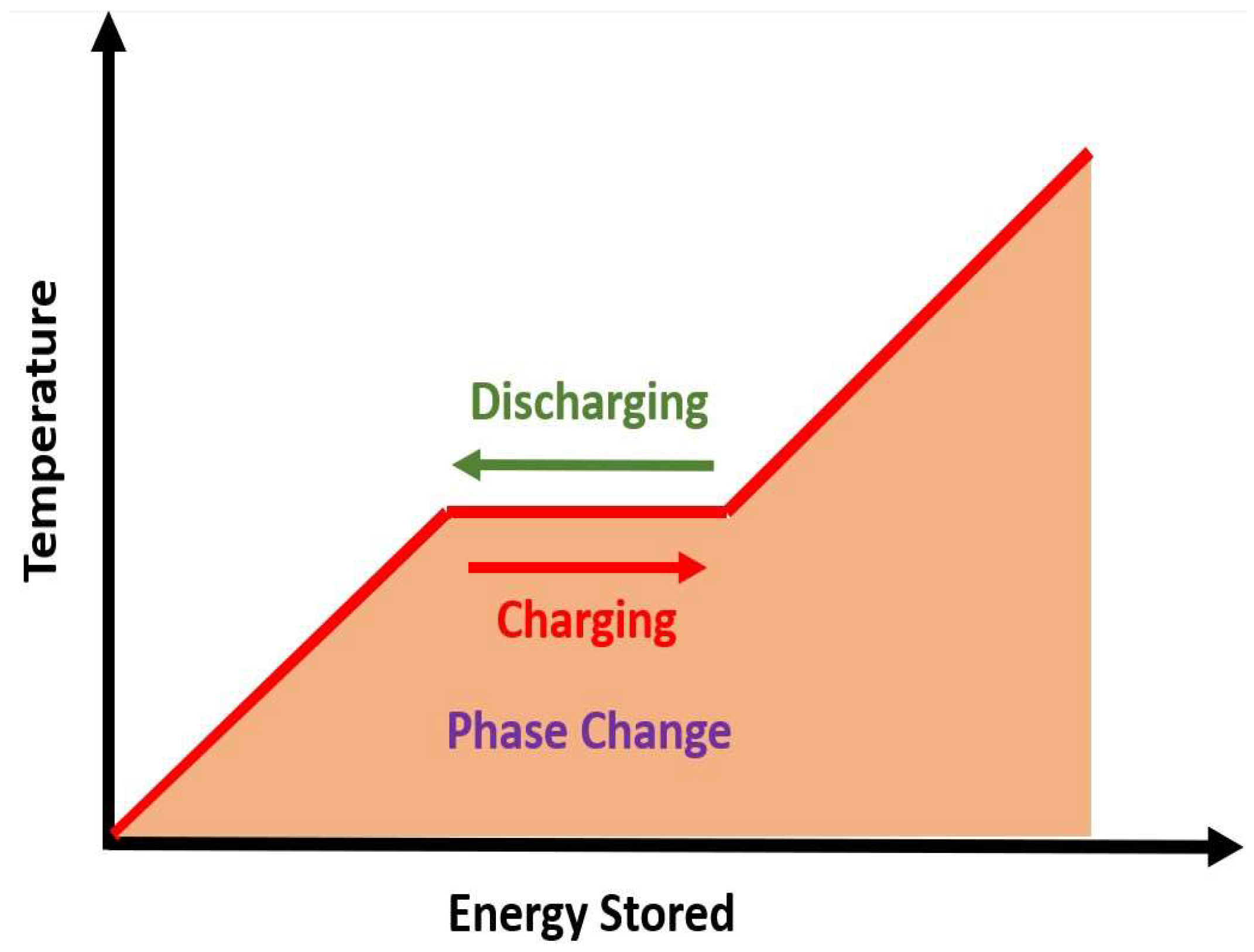
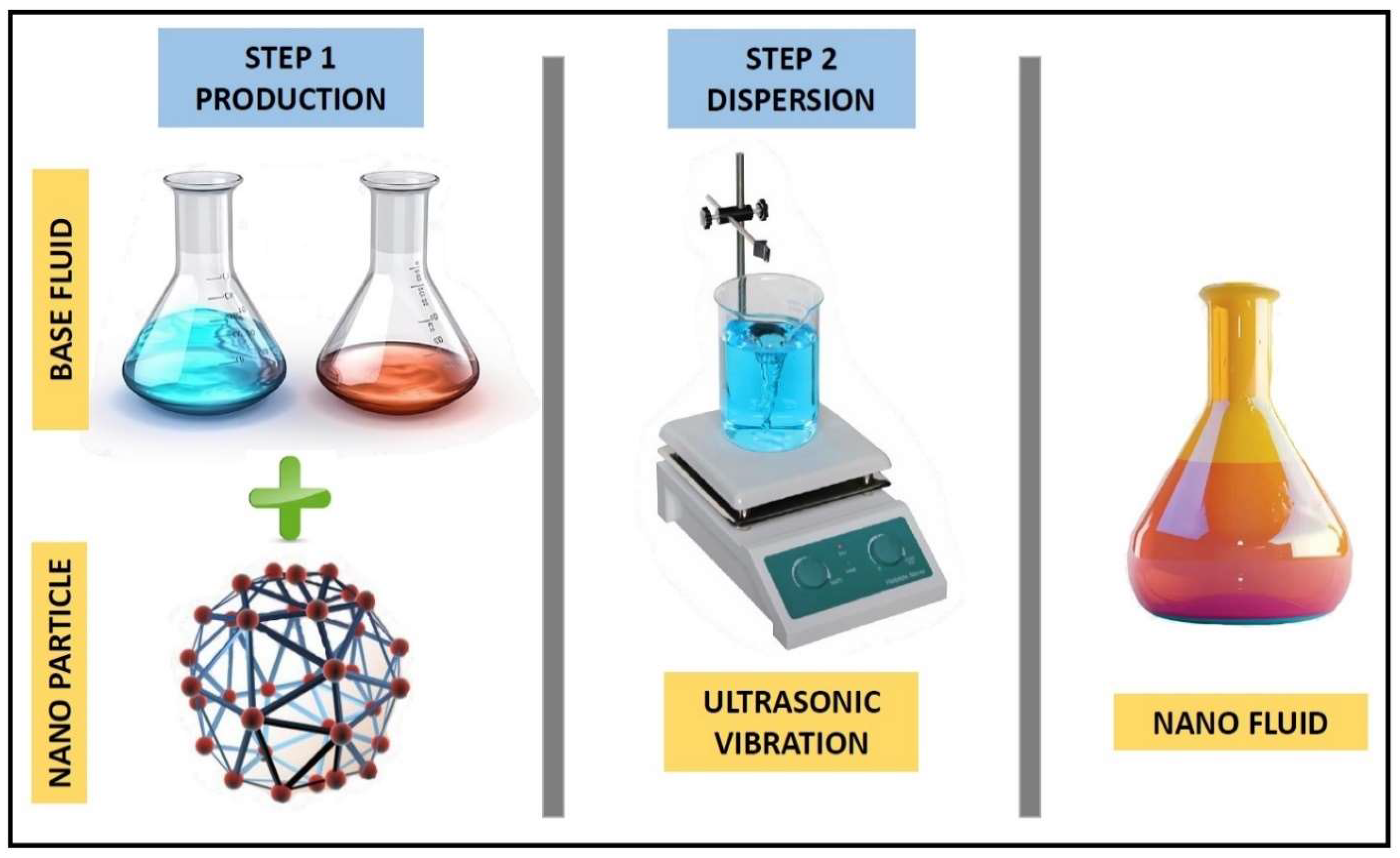

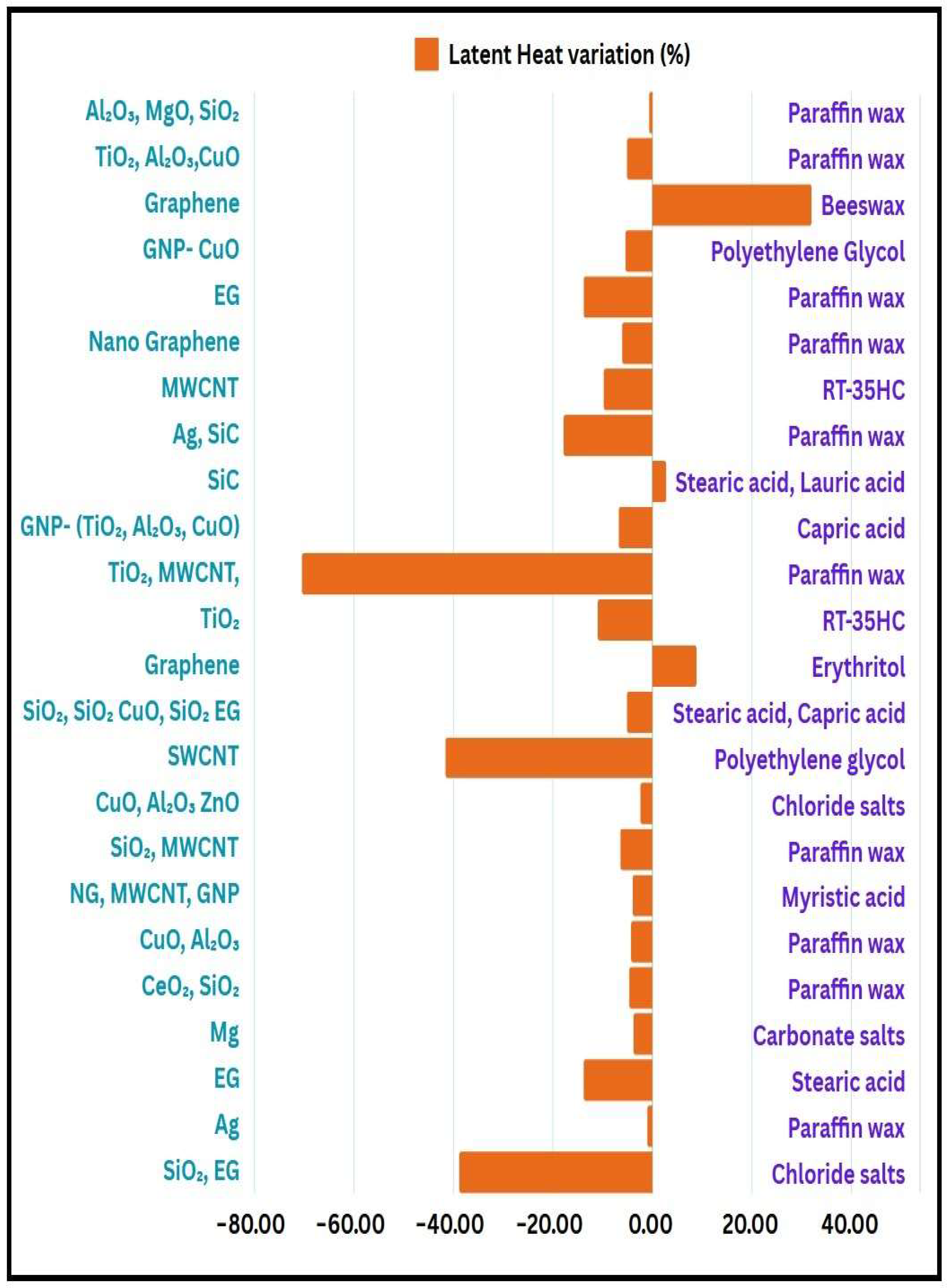


| Organic PCMs | Inorganic PCMs |
|---|---|
| Benefits | Benefits |
| Low supercooling | High latent heat |
| Non-corrosive | High phase change enthalpy |
| Stable thermal performance | |
| Wide range of available melting points | |
| Drawbacks | Drawbacks |
| Inflammability | Prone to supercooling |
| Lower thermal conductivity | Risk of phase separation and phase segregation |
| Potential for degradation over time | Can be corrosive |
| Low phase change enthalpy | Lack of thermal stability |
| Ref | Base Fluids | Nanomaterial | Key Findings |
|---|---|---|---|
| [70] | Water | Nanocarbon | The one-step synthesis of nanocarbon utilizes an O2–C2H2 flame to generate carbon-based nanoparticles, which are then mixed and condensed with water. The process includes precise control of flame ratios and water flow, followed by continuous stirring, homogenization, and ultrasonic treatment to ensure optimal dispersion and suspension of the nanoparticles. |
| [71] | EG | Cuprous oxide | Cuprous oxide nanofluid was synthesized via reduction of copper sulfate in ethylene glycol with ammonium hydroxide and ribose, followed by pH adjustment and heating at 70 °C. Key reaction parameters like reducing agent concentration and microwave irradiation were optimized. |
| [72] | Oil | Ag/Si | Silica-based nanofluids were prepared using a one-step method by magnetic stirring at 130 °C for 2 h, with silica concentrations ranging from 0.07 to 4.4 wt%. Silver nanoparticles supported on silica were similarly synthesized by adding silver nitrate and silica to the base fluid, followed by stirring at 130 °C for 2 h to prevent oxidation and facilitate nanoparticle formation. |
| [73] | Oil | SiC | Nanofluids in this study were prepared using the Electrical Explosion Welding (EEW) method, where electrical explosion of thin wire electrodes in liquid media (e.g., water, oil, glycerin) simultaneously produces and distributes nanoparticles. The process allows for the use of various liquids and surfactants, ensuring long-term stability of the nanofluid, and is particularly cost-effective for large-scale nanoparticle production. |
| [74] | Distilled Water | NaNO3-KNO3 | Metal oxide nanoparticles were synthesized directly in molten NaNO3-KNO3 eutectic by thermal decomposition of metal nitrate precursors, ensuring no contamination due to shared anions with the solvent. Aluminum nitrate was identified as the optimal precursor. |
| [75] | Oil | CuO | EEW method involves using high voltage and current to convert thin conductive wires into nanoparticles under liquid, offering the ability to produce a wide range of metallic nanocolloids. This environmentally friendly method ensures excellent dispersion of nanoparticles in various liquids like water, oil, and glycerin, without the need for surfactants. |
| [76] | Deionized water | Graphene | Graphene nanofluids were prepared by dispersing graphite powder in deionized water and exfoliating the mixture using a high-speed homogenizer at 10,000 rpm for one hour. After centrifugation, the supernatant was collected as the final nanofluid. |
| [77] | EG | Cu | Copper nanofluid was synthesized by reducing copper sulfate in ethylene glycol with fructose, followed by pH adjustment and heating to 70 °C. Microwave heating was also tested to examine its effect on synthesis. Copper nanoparticles were separated by centrifugation, washed, and dried. |
| Ref | Base Fluids | Nanomaterial | Key Findings |
|---|---|---|---|
| [78] | Water | Cu/CuO | Hybrid nanofluids were prepared using a two-step method by mixing copper and copper oxide nanoparticles with water as the base fluid. Various weight fractions of nanoparticles (Cu and CuO) were tested, with concentrations ranging from 2 g/L to 4 g/L to achieve optimal stability for low-temperature applications. |
| [82] | Water | Al2O3 | The two-step method involves dispersing nanoparticles like aluminum oxide (Al2O3) in deionized water using sonication, homogenization, or magnetic stirring for uniform distribution. Surfactants may be used to enhance stability, with Al2O3 concentrations ranging from 0.1% to 0.6% in the base fluid. |
| [83] | Oil | CeO2/CuO | The CeO2-CuO hybrid nanolubricant was prepared using the two-step method by dispersing nanoparticles in base fluid to achieve various volume fractions (0.25% to 1.5%). The mixture was stirred with a magnetic stirrer for 1 h and exposed to ultrasonic waves for 1.5 h to prevent agglomeration and ensure stability. |
| [84] | DI water | CuO/GNP | The two-step method was used to prepare hybrid nanofluid-based phase change materials (NFPCMs) by adding oxygen-functionalized GNPs and CuO nanoparticles to the base PCM. The mixture was stirred and ultrasonicated, followed by probe sonication to enhance stability. A nucleating agent and surfactant were also added to improve homogeneity and stability, with no visible sedimentation observed after three days. |
| [85] | Water | TiO2/Graphene Oxide | TiO2 and GO nanofluids were prepared using the two-step method, where TiO2 nanoparticles at varying concentrations were dispersed in water with cetyltrimethylammonium bromide to prevent agglomeration. The mixture was sonicated and stirred for uniformity, followed by the addition of 20 wt% GO dispersion. The final mixture was heated in an oil bath at 80 °C with magnetic stirring for 6 h. |
| [86] | EG/Oil | MWNT | CNT nanofluids were prepared by dispersing multi-walled carbon nanotubes into ethylene glycol or synthetic engine oil using magnetic agitation and ultrasonication. N-hydroxysuccinimide (NHS) was used as a dispersant in the engine oil-based suspensions. Volume concentrations ranged from 0.2 to 1.0 vol.% in ethylene glycol and 1.0 to 2.0 vol.% in engine oil. |
| [87] | Oil | MgO/ZnO | ZnO and MgO nanofluids were prepared without surfactants using the two-step method, with concentrations ranging from 0.125% to 1.5%. The nanoparticles (40 nm) were dispersed by magnetic stirring for 3 h, followed by ultrasonic processing at 20 kHz for 1 h. The nanofluid showed excellent stability for at least two weeks, with no visible sedimentation observed. |
| [88] | Water | MWCNT/Fe2O3 | Hybrid Fe2O3 and MWCNTs nanofluids (80% Fe2O3, 20% MWCNTs) were prepared using the two-step method. Dispersion was optimized by adjusting pH, conductivity, sonication parameters and dispersion to nanoparticle weight ratios (0.4–1.0) at a 0.1% concentration. Hybrid nanofluids were then formulated with volume concentrations ranging from 0.1% to 1.5%. |
| Ref | Nanomaterial | Models |
|---|---|---|
| [92] | ||
| [93] | ||
| [94] | Ag MgO | |
| [95] | ||
| [96] | ||
| [97] | SWCNT & Al2O3 | |
| [98] | MWCNTs COOH | |
| [99] | ||
| [100] | ||
| [101] | ||
| [102] | Graphite SiO2 |
| Ref | Nanomaterial | Model |
|---|---|---|
| [93] | ||
| [106] | ||
| [107] | MWCNTs & Fe3O4 | |
| [108] | ||
| [109] | Nanodiamond Fe3O4 | |
| [110] | ||
| [111] | Nanodiamond & Fe3O4 | |
| [112] | Ag & MgO | |
| [113] | ||
| [114] | ||
| [115] | Graphite & SiO2 | |
| [116] | - | : |
| Ref | Nanomaterial | Models |
|---|---|---|
| [88] | MWCNT & Fe2O3 | |
| [92] | ||
| [93] | ||
| [94] | Ag & MgO | |
| [106] | ||
| [113] | ||
| [121] | MWCNTs & SiO2 | |
| [122] | MWCNTs & ZnO | |
| [123] | Al2O3, CuO & TiO2 | |
| [124] | Graphite & SiO2 | |
| [125] | MWCNTs & SiO2 | |
| [126] | TiO2 & SiO2 | |
| [127] | MgO & MWCNTs | |
| [128] | MWCNTs & ZnO |
| Ref | Nanomaterial | Models |
|---|---|---|
| [93] | ||
| [105] | Graphite & SiO2 | |
| [106] | ||
| [107] | Nanodiamond | |
| [109] | Nanodiamond & Fe3O4 | |
| [110] | ||
| [133] | ||
| [134] | ||
| [135] | CuO & SiO2 | |
| [136] | ||
| [137] | CuO & SiO2 | |
| [138] | MgO, ZnO & ZrO2 |
| Ref No | Base Fluid/Nanofluid | ϕ (%wt) | PS (nm) | Surfactants | Stabilization Techniques | Stability Indicator |
|---|---|---|---|---|---|---|
| [143] | DW/MWCNT | 0.15 | 10–20 | COOH | MS, US | ζ = 41.40 mV |
| [144] | Water/GNPs | 0.1 | 2 | SDS, CTAB, BS | Water bath, US | >2 months |
| [145] | Water/Al2O3 | 1–3 | 25 | SDS | USB, US | ζ = −47.90 mV |
| [146] | DW/Al & Cu | 0.3 | 80, 40 | SDS | MS, US | ζ = −41.20 and −38.40 mV |
| [147] | Water/Al2O3 | 1–3 | 45 | SDBS | MS, US | ζ = −38.60 mV |
| [148] | Water/CNT | 0.1–1 | 10–20 | PVP, SDS | MS, US | ζ = −63.20 mV |
| [149] | Water/SWCNT | 0.1 | 20–50 | SDBS | US | 2 months |
| [150] | Water-EG (60:40)/Graphene | 0.1–0.3 | 123–424 | HCl + NaOH | US | 3 weeks |
| [151] | DW/rGO-Ag | 0.0005–0.05 | - | SDBS | US | >2 weeks |
| [152] | Water/MWCNT-CeO2 | 0.25–1.5 | <30 | SDBS, SDS, CTAB, DDC, and PVP | US | >1 month |
| [153] | DW/Cu, Fe, Ag | 0.1 | 30–50 | No surfactant | Sonication | 5 days |
| [154] | Water/Cu, Zn, Al | 40–240 ppm | 49 | No surfactant | MS, Sonication | ζ = 34.60 − 38.60 mV |
| Ref No | Nanofluid | ϕ/ψ (%) and PD (nm/μm) | Correlation | Re | Boundary Condition |
|---|---|---|---|---|---|
| [167] | Cu | 0.3 ≤ ϕ ≤ 2.0 (100 nm) | 800 ≤ Re ≤ 25,000 | CHF | |
| [168] | Al2O3 | 1 ≤ ψ ≤ 6 (150 nm) | 500 < Re < 2000 | CHF | |
| [169] | TiO2 | 0.05 ≤ ϕ ≤ 6 (150 nm) | 4.8 × 103 ≤ Re ≤ 3.05 × 104 | CWT | |
| [170] | Graphitic | 2 ≤ ψ ≤ 2.5 (1–2 μm) | 5 ≤ Re ≤ 110 | CWT | |
| [171] | CuO, SiO2 | 1 ≤ ϕ ≤ 10 (20–100 nm) | 3 × 103 ≤ Re ≤ 1.7 × 104 | CHF | |
| [172] | Fe2O3 | 0 ≤ ϕ ≤ 0.06 (30 nm) | 16,000 < Re < 30,000 | CHF | |
| [173] | Ag | 0.3 ≤ ϕ ≤ 0.9 (100 nm) | 900 < Re < 12,000 | CWT | |
| [174] | MWCNT | ϕ = 0.0111% (20 nm) | 182 < Re < 956 | CHF | |
| [175] | GNP | 0.1 ≤ ψ ≤ 2 (2.5 nm) | 6.7 × 103 ≤ Re ≤ 2.8 × 104 | - |
| Ref No | Hybrid Nanofluid | Base Fluid | ϕ (%) | EXP/NUM | Key Findings | |
|---|---|---|---|---|---|---|
| Natural Convection | [93] | Al2O3-Cu | Water | 0 ≤ ϕ ≤ 0.12 | NUM | Compared to the Al2O3/water nanofluid, the Al2O3-Cu/water hybrid nanofluid provides better thermal performance. The buoyancy effect related to the hybrid nanofluid improves the mean Nusselt number, hence enhancing the heat transfer efficiency compared to mono nanofluids. |
| [161] | TiO2-Cu | Water | 0 ≤ ϕ ≤ 0.02 | NUM | The introduction of hybrid nanoparticles to the base fluid enhances its thermal conductivity, resulting in an increment in surface temperature. At high Rayleigh numbers (Ra), the streamline functions deteriorate, indicating a decrease in fluid flow efficiency under these conditions. | |
| [200] | Cu-SWCNT | Sodium alginate | 0.01 ≤ ϕ ≤ 1 | NUM | Hybrid fluid models are appropriate when the nanomaterial concentration reaches 0.3% of the volume. Also, the heat transfer rate for SWCNT-Cu nanofluids increases with increasing nanoparticle volume fraction and Prandtl number, resulting in thermal performance enhancement. | |
| [201] | Al2O3-Cu | Water | 0 ≤ ϕ ≤ 0.2 | NUM | As the size of the heating element decreases, the Nusselt number increases, indicating improved heat transfer efficiency. | |
| [115] | SiO2-Graphite | Water | 0.5 ≤ ϕ ≤ 1 | EXP & NUM | At moderate Reynolds numbers (4500 < Re < 7000), a 1% hybrid nanoparticle concentration increases the Nusselt number by up to 6%, while at higher Reynolds numbers (7500 < Re < 10,000), Nusselt numbers rise consistently with higher concentrations. | |
| [191] | Fe3O4-MWCNT | Water | 0.1 ≤ ϕ ≤ 0.3 | EXP | Experimental testing of MWCNT–Fe3O4 nanofluids showed that Nusselt numbers increased with higher particle concentrations and Reynolds numbers, with enhancements ranging from 9.35% to 31.10%. The improved heat transfer performance was due to the nanoparticles’ high thermal conductivity, Brownian motion, and increased surface area. | |
| [202] | Ag-GNP | Water | 0.5 ≤ ϕ ≤ 1 | EXP | Nusselt number for GNP–Ag nanofluids increased with both Reynolds number and nanoparticle concentration, due to the higher conductivity and Brownian motion of the nanoparticles. The enhancements ranged from 8.29% to 32.70%, depending on particle fraction and Reynolds number. | |
| [203] | TiO2-CuO | Water | 0 ≤ ϕ ≤ 0.06 | NUM | The local Nusselt number increases with the Falkner-Skan power law parameter and nanoparticle mass, with moving wedges enhancing heat transfer more than static ones. The highest nanoparticle mass in hybrid nanofluids results in the best thermal performance, while spherical nanoparticles led to the lowest local Nusselt number. | |
| [204] | Cu-TiO2 | Aqueous | 0.1 ≤ ϕ ≤ 2 | EXP | At 1.0 vol.% concentration of Cu-TiO2, the Nusselt number increased, reaching its maximum, but a decrease was observed at 2.0 vol.%, with a drop ranging from 2.3% to 11.2%. This reduction is attributed to the higher dynamic viscosity and increased thermal boundary layer thickness at higher particle loading. | |
| [205] | Al2O3-Cu | Water | ϕ = 0.1 | NUM | The hybrid nanofluids showed a notable increase in the average Nusselt number, with an enhancement of 4.73% compared to Al2O3/water and 13.46% compared to pure water, demonstrating their superior heat transfer performance. | |
| Mixed Convection | [110] | Al2O3-Cu | Water | 0 ≤ ϕ ≤ 0.05 | NUM | A small rise in the mean Nusselt number was observed in Al2O3-Cu/water hybrid nanofluids compared with regular nanofluids. This augmentation resulted in enhanced heat transfer performance, contributing to higher overall thermal efficiency in heat transfer applications. |
| [179] | Al2O3-Cu | Water | 0.01 ≤ ϕ ≤ 0.05 | NUM | At a volume fraction of 0.05, the maximum heat transmission was observed. |
| One-Step Method | Two-Step Method |
|---|---|
| Benefits | Benefits |
| High stability | Well-suited for large-scale production |
| Reduced agglomeration | Suitable for oxide nanoparticles |
| No need for storage, drying, or oxidation | Cost-effective |
| Greater control over the process | |
| Drawbacks | Drawbacks |
| Not ideal for large-scale production | Prone to rapid agglomeration |
| Lower thermal conductivity | Limited control over dispersion |
| Suitable only for low vapor pressure liquids | High surface energy requiring extra treatment |
| Ref No | EXP/NUM | Nanoparticle | PCM | Variation in Latent Heat | Variation in Phase Change Temperature | Variation in Thermal Conductivity | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Material | Concentration | (J/g) ↑ or ↓ | (%) ↑ or ↓ | (°C) ↑ or ↓ | (%) ↑ or ↓ | (W/m K) ↑ or ↓ | (%) ↑ or ↓ | |||
| [193] | EXP | Graphene | 0.3 wt% | Beeswax | 45.25 ↑ | 31.98 ↑ | 0.14 ↑ | 0.22 ↑ | 2.64 ↑ | ≈150 ↑ |
| [223] | EXP | EGr | - | Stearic acid | 26.2 ↓ | 13.81 ↓ | 0.6 ↑ | 1.13 ↑ | 2.24 ↑ | ≈800 ↑ |
| [229] | EXP & NUM | Al2O3, MgO, SiO2 | 0.5 wt% | Paraffin wax | 1.7 ↓ | 0.65 ↓ | 9.72 ↓ | 22.6 ↓ | 0.06 ↑ | 30 ↑ |
| [230] | EXP | GNP-CuO | 3 wt% | Polyethylene Glycol | 9.65 ↓ | 5.41 ↓ | 4.2 ↓ | 9.74 ↓ | 0.216 ↑ | 92.31 ↑ |
| [231] | EXP | Nano Graphene | 3 wt% | Paraffin wax | 13.98 ↓ | 6.08 ↓ | 1.71 ↓ | 5.73 ↓ | 0.18 ↑ | ≈150 ↑ |
| [232] | EXP | Ag, SiC | 15 wt% | Paraffin wax | 36.94 ↓ | 17.85 ↓ | 3.7 ↓ | 6.98 ↓ | 0.144 ↑ | 58.06 ↑ |
| [233] | EXP | SWCNT | 2 wt% | Polyethylene glycol | 78.3 ↓ | 41.62 ↓ | 2.5 ↓ | 4.02 ↓ | 0.63 ↑ | ≈250 ↑ |
| [239] | EXP & NUM | TiO2, Al2O3,CuO | 3 wt% | Paraffin wax | 5.97 ↓ | 5.11 ↓ | 1.02 ↑ | 1.63 ↑ | 0.348 ↑ | 59.39 ↑ |
| [240] | EXP & NUM | MWCNT | 1 wt% | RT-35HC | 25.06 ↓ | 9.79 ↓ | 0.08 ↑ | 0.2 ↑ | 0.229 ↑ | ≈100 ↑ |
| [241] | EXP | GNP-(TiO2, Al2O3, CuO) | 0.3 wt% | Capric acid | 11.6 ↓ | 6.79 ↓ | 0.2 ↓ | 5 ↓ | 0.09 ↑ | 60.81 ↑ |
| [242] | EXP | CuO, Al2O3 ZnO | 0.5–3 wt% | Chloride salts | 6.8 ↓ | 2.4 ↓ | 0.9 ↓ | 0.23 ↓ | 0.22 ↑ | 62.86 ↑ |
| [248] | EXP & NUM | TiO2 | 2 wt% | RT-35HC | 28.14 ↓ | 10.99 ↓ | 0.04 ↓ | 0.11 ↓ | 0.27 ↑ | ≈100 ↑ |
| [250] | EXP | Ag | 1 wt% | Paraffin wax | 1.6 ↑ | 1.01 ↑ | 0.5 ↓ | 0.9 ↓ | 0.228 ↑ | ≈100 ↑ |
| [251] | EXP | SiC | 0.075 wt% | Stearic acid, Lauric acid | 3.4 ↑ | 2.79 ↑ | No Change | No Change | 0.16 ↑ | 75.83 ↑ |
| [252] | EXP & NUM | Graphene | 1 wt% | Erythritol | 27.6 ↑ | 8.87 ↑ | 7.51 ↓ | 5.89 ↓ | 0.389 ↑ | 53.07 ↑ |
| [253] | EXP | SiO2, EGr | - | Chloride salts | 78.5 ↓ | 38.82 ↓ | 1.8 ↓ | 0.46 ↓ | 5.96 ↑ | ≈1000 ↑ |
| [254] | EXP | Mg | 2 wt% | Carbonate salts | 6.3 ↓ | 3.79 ↓ | 5.99 ↓ | 1.52 ↓ | 0.602 ↑ | 45.33 ↑ |
| [255] | EXP | EGr | 15 wt% | Paraffin wax | 21.3 ↓ | 13.83 ↓ | 0.3 ↑ | 0.64 ↑ | 0.891 ↑ | ≈400 ↑ |
| [256] | EXP | SiO2, SiO2-CuO, SiO2-EGr | 0.2–0.6 g | Stearic acid, Capric acid | 8.5 ↓ | 5.15 ↓ | 1.87 ↑ | 6.5 ↑ | 0.698 ↑ | ≈300 ↑ |
| [257] | EXP | NG, MWCNT, GNP | 3 wt% | Myristic acid | 7.71 ↓ | 3.96 ↓ | 0.1 ↓ | 0.18 ↓ | 0.385 ↑ | ≈150 ↑ |
| [258] | EXP | CuO, Al2O3 | 1 wt% | Paraffin wax | 6 ↓ | 4.3 ↓ | 1.8 ↓ | 2.81 ↓ | 0.109 ↑ | 60.56 ↑ |
| [259] | EXP | TiO2, MWCNT, | 1 wt% | Paraffin wax | 17.5 ↓ | 70.45 ↓ | 0.04 ↓ | 0.14 ↓ | 0.574 ↑ | ≈200 ↑ |
| [260] | EXP | SiO2, MWCNT | 1 wt% | Paraffin wax | 12.7 ↓ | 6.44 ↓ | 3 ↓ | 4.78 ↓ | 0.21 ↑ | 87.5 ↑ |
| [261] | EXP | CeO2, SiO2 | 1 wt% | Paraffin wax | 6.5 ↓ | 4.64 ↓ | 0.93 ↓ | 1.46 ↓ | 0.08 ↑ | 44.44 ↑ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peer, M.S.; Cascetta, M.; Migliari, L.; Petrollese, M. Nanofluids in Thermal Energy Storage Systems: A Comprehensive Review. Energies 2025, 18, 707. https://doi.org/10.3390/en18030707
Peer MS, Cascetta M, Migliari L, Petrollese M. Nanofluids in Thermal Energy Storage Systems: A Comprehensive Review. Energies. 2025; 18(3):707. https://doi.org/10.3390/en18030707
Chicago/Turabian StylePeer, Mohamed Shameer, Mario Cascetta, Luca Migliari, and Mario Petrollese. 2025. "Nanofluids in Thermal Energy Storage Systems: A Comprehensive Review" Energies 18, no. 3: 707. https://doi.org/10.3390/en18030707
APA StylePeer, M. S., Cascetta, M., Migliari, L., & Petrollese, M. (2025). Nanofluids in Thermal Energy Storage Systems: A Comprehensive Review. Energies, 18(3), 707. https://doi.org/10.3390/en18030707









