Phase Change Materials in Electrothermal Conversion Systems: A Review
Abstract
1. Introduction
2. Energy Conversion and Storage Mechanism in PCM-Based Materials
3. Overview of PCMs Capable of Electrothermal Conversion and Storage
- Nano-enhanced PCMs: Adding nanoparticles (e.g., carbon nanotubes or metal oxides) increases the thermal conductivity, improving heat transfer rates. Studies show significant increases in conductivity (up to several hundred percent) with certain nanoparticle loadings;
- Composite PCMs: Combining PCMs with supporting matrices (e.g., metal foams or porous materials) improves structural stability, prevents leakage, and can improve thermal conductivity. Evidence includes improved shape stability and reduced supercooling in composite PCMs.
3.1. PCMs Modified with Carbon-Based Materials
3.1.1. PCMs Modified with CNTs
3.1.2. PCMs Modified with Graphene
3.1.3. PCMs Modified with Graphite
3.1.4. PCMs Modified with Carbon Aerogel
3.1.5. PCMs Modified with Biomass-Derived Carbon
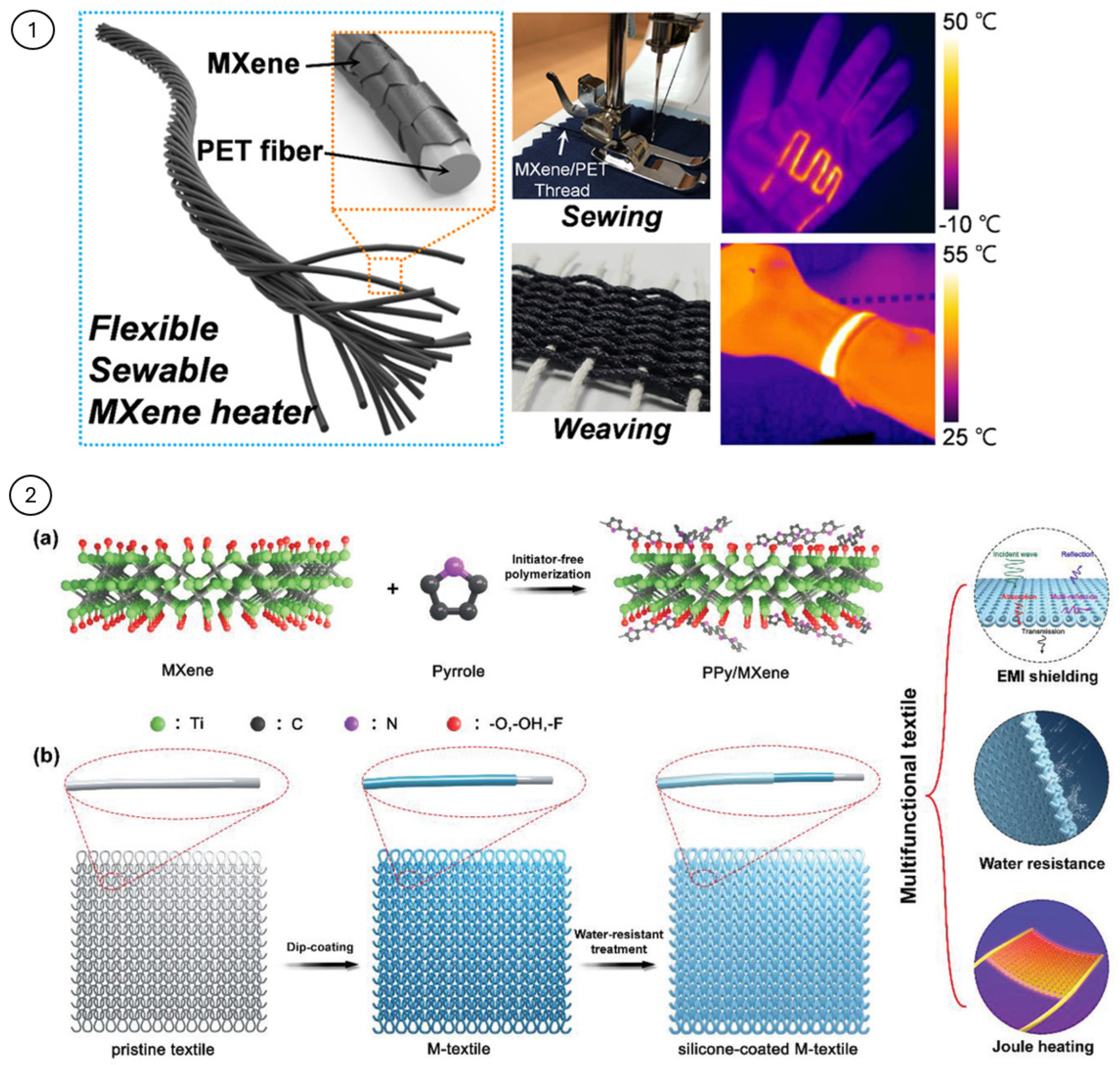
3.2. PCMs Modified with MXene
4. Applications and Future Trends
4.1. Solar Energy
4.2. Automotive and Electoronic
4.3. Textiles
4.4. Building Industry
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| EMI | electromagnetic interference |
| GO | graphene oxide |
| MOF | metal–organic framework |
| MWCNTs | multi-walled carbon nanotubes |
| PCC | phase change composite |
| PCM | phase change materials |
| PEG | poly(ethylene glycol) |
| RGNP | reticulated graphite nanoplatelets |
| SEBS | styrene-b-(ethylene-co-butylene)-b-styrene |
| TES | thermal energy storage |
References
- Gao, D.; Kwan, T.H.; Hu, M.; Pei, G. The Energy, Exergy, and Techno-Economic Analysis of a Solar Seasonal Residual Energy Utilization System. Energy 2022, 248, 123626. [Google Scholar] [CrossRef]
- Berger, M.; Worlitschek, J. A Novel Approach for Estimating Residential Space Heating Demand. Energy 2018, 159, 294–301. [Google Scholar] [CrossRef]
- Polcyn, J.; Us, Y.; Lyulyov, O.; Pimonenko, T.; Kwilinski, A. Factors Influencing the Renewable Energy Consumption in Selected European Countries. Energies 2021, 15, 108. [Google Scholar] [CrossRef]
- IEA. Tracking Clean Energy Progress 2023; IEA: Paris, France, 2023; Available online: https://www.iea.org/reports/tracking-clean-energy-progress-2023 (accessed on 11 October 2024).
- Cole, W.; Gates, N.; Mai, T. Exploring the Cost Implications of Increased Renewable Energy for the U.S. Power System. Electr. J. 2021, 34, 106957. [Google Scholar] [CrossRef]
- Saqib, M.; Andrzejczyk, R. A Review of Phase Change Materials and Heat Enhancement Methodologies. Wiley Interdiscip. Rev. Energy Environ. 2023, 12, e467. [Google Scholar] [CrossRef]
- Elkhatat, A.; Al-Muhtaseb, S.A. Combined “Renewable Energy–Thermal Energy Storage (RE–TES)” Systems: A Review. Energies 2023, 16, 4471. [Google Scholar] [CrossRef]
- Stutz, B.; Le Pierres, N.; Kuznik, F.; Johannes, K.; Palomo Del Barrio, E.; Bédécarrats, J.-P.; Gibout, S.; Marty, P.; Zalewski, L.; Soto, J.; et al. Storage of Thermal Solar Energy. Comptes Rendus Phys. 2017, 18, 401–414. [Google Scholar] [CrossRef]
- Hasan, M.M.; Hossain, S.; Mofijur, M.; Kabir, Z.; Badruddin, I.A.; Yunus Khan, T.M.; Jassim, E. Harnessing Solar Power: A Review of Photovoltaic Innovations, Solar Thermal Systems, and the Dawn of Energy Storage Solutions. Energies 2023, 16, 6456. [Google Scholar] [CrossRef]
- ASIF, M.; MUNEER, T. Energy Supply, Its Demand and Security Issues for Developed and Emerging Economies. Renew. Sustain. Energy Rev. 2007, 11, 1388–1413. [Google Scholar] [CrossRef]
- Alva, G.; Liu, L.; Huang, X.; Fang, G. Thermal Energy Storage Materials and Systems for Solar Energy Applications. Renew. Sustain. Energy Rev. 2017, 68, 693–706. [Google Scholar] [CrossRef]
- Esen, M.; Yuksel, T. Experimental Evaluation of Using Various Renewable Energy Sources for Heating a Greenhouse. Energy Build 2013, 65, 340–351. [Google Scholar] [CrossRef]
- Enescu, D.; Chicco, G.; Porumb, R.; Seritan, G. Thermal Energy Storage for Grid Applications: Current Status and Emerging Trends. Energies 2020, 13, 340. [Google Scholar] [CrossRef]
- Musiał, M.; Lichołai, L.; Katunský, D. Modern Thermal Energy Storage Systems Dedicated to Autonomous Buildings. Energies 2023, 16, 4442. [Google Scholar] [CrossRef]
- Cieśliński, J.T.; Fabrykiewicz, M. Thermal Energy Storage with PCMs in Shell-and-Tube Units: A Review. Energies 2023, 16, 936. [Google Scholar] [CrossRef]
- Fantini, P. Phase Change Memory Applications: The History, the Present and the Future. J. Phys. D Appl. Phys. 2020, 53, 283002. [Google Scholar] [CrossRef]
- Pielichowska, K.; Nowicka-Dunal, K.; Pielichowski, K. Bio-Based Polymers for Environmentally Friendly Phase Change Materials. Polymers 2024, 16, 328. [Google Scholar] [CrossRef]
- Elias, C.N.; Stathopoulos, V.N. A Comprehensive Review of Recent Advances in Materials Aspects of Phase Change Materials in Thermal Energy Storage. Energy Procedia 2019, 161, 385–394. [Google Scholar] [CrossRef]
- Radomska, E.; Mika, L.; Sztekler, K. The Impact of Additives on the Main Properties of Phase Change Materials. Energies 2020, 13, 3064. [Google Scholar] [CrossRef]
- Zhou, D.; Zhao, C.Y.; Tian, Y. Review on Thermal Energy Storage with Phase Change Materials (PCMs) in Building Applications. Appl. Energy 2012, 92, 593–605. [Google Scholar] [CrossRef]
- Bunton, G.V.; Quilliam, R.M. Switching and Memory Effects in Amorphous Chalcogenide Thin Films. IEEE Trans. Electron. Devices 1973, ED-20, 140–144. [Google Scholar] [CrossRef]
- Ovshinsky, S.R. Reversible Electrical Switching Phenomena in Disordered Structures. Phys. Rev. Lett. 1968, 21, 1450. [Google Scholar] [CrossRef]
- Behzadi, S.; Farid, M.M. Long Term Thermal Stability of Organic PCMs. Appl. Energy 2014, 122, 11–16. [Google Scholar] [CrossRef]
- Liu, H.; Yang, G.; Ji, M.; Zhu, C.; Xu, J. Phase Change Materials with Multiple Energy Conversion and Storage Abilities Based on Large-Scale Carbon Felts. Compos. Sci. Technol. 2022, 221, 109177. [Google Scholar] [CrossRef]
- Yuan, K.; Shi, J.; Aftab, W.; Qin, M.; Usman, A.; Zhou, F.; Lv, Y.; Gao, S.; Zou, R. Engineering the Thermal Conductivity of Functional Phase-Change Materials for Heat Energy Conversion, Storage, and Utilization. Adv. Funct. Mater. 2020, 30, 1904228. [Google Scholar] [CrossRef]
- Meschke, M.; Guichard, W.; Pekola, J.P. Single-Mode Heat Conduction by Photons. Nature 2006, 444, 187–190. [Google Scholar] [CrossRef]
- Chen, X.; Tang, Z.; Gao, H.; Chen, S.; Wang, G. Phase Change Materials for Electro-Thermal Conversion and Storage: From Fundamental Understanding to Engineering Design. iScience 2020, 23, 101208. [Google Scholar] [CrossRef]
- Kadivar, M.R.; Moghimi, M.A.; Sapin, P.; Markides, C.N. Annulus Eccentricity Optimisation of a Phase-Change Material (PCM) Horizontal Double-Pipe Thermal Energy Store. J. Energy Storage 2019, 26, 101030. [Google Scholar] [CrossRef]
- Zhang, Y.; Umair, M.M.; Zhang, S.; Tang, B. Phase Change Materials for Electron-Triggered Energy Conversion and Storage: A Review. J. Mater. Chem. A Mater. 2019, 7, 22218–22228. [Google Scholar] [CrossRef]
- Yu, J.-K.; Mitrovic, S.; Tham, D.; Varghese, J.; Heath, J.R. Reduction of Thermal Conductivity in Phononic Nanomesh Structures. Nat. Nanotechnol. 2010, 5, 718–721. [Google Scholar] [CrossRef]
- Chen, X.; Gao, H.; Hai, G.; Jia, D.; Xing, L.; Chen, S.; Cheng, P.; Han, M.; Dong, W.; Wang, G. Carbon Nanotube Bundles Assembled Flexible Hierarchical Framework Based Phase Change Material Composites for Thermal Energy Harvesting and Thermotherapy. Energy Storage Mater. 2020, 26, 129–137. [Google Scholar] [CrossRef]
- Wang, X.; Li, G.; Hong, G.; Guo, Q.; Zhang, X. Graphene Aerogel Templated Fabrication of Phase Change Microspheres as Thermal Buffers in Microelectronic Devices. ACS Appl. Mater. Interfaces 2017, 9, 41323–41331. [Google Scholar] [CrossRef] [PubMed]
- Jurczyk, M.; Spietz, T.; Czardybon, A.; Dobras, S.; Ignasiak, K.; Bartela, Ł.; Uchman, W.; Ochmann, J. Review of Thermal Energy Storage Materials for Application in Large-Scale Integrated Energy Systems—Methodology for Matching Heat Storage Solutions for Given Applications. Energies 2024, 17, 3544. [Google Scholar] [CrossRef]
- Ahmad, A.; Navarro, H.; Ghosh, S.; Ding, Y.; Roy, J.N. Evaluation of New PCM/PV Configurations for Electrical Energy Efficiency Improvement through Thermal Management of PV Systems. Energies 2021, 14, 4130. [Google Scholar] [CrossRef]
- Wu, C.; Zhao, Y.; Li, W.; Fan, J.; Xu, H.; Yuan, D.; Ling, Z. Layered Operation Optimization Methods for Concentrated Solar Power (CSP) Technology and Multi-Energy Flow Coupling Systems. Energies 2024, 17, 6297. [Google Scholar] [CrossRef]
- Meng, F.; Che, C.; Wu, Y.; Wei, J.; Rong, J.; Yang, X.; Li, D.; Yang, R.; Wang, Z. Thermal Storage Performance of a Shell and Tube Phase Change Heat Storage Unit with Different Thermophysical Parameters of the Phase Change Material. Processes 2024, 12, 123. [Google Scholar] [CrossRef]
- AL-Saadi, S.N.; Zhai, Z. (John) Modeling Phase Change Materials Embedded in Building Enclosure: A Review. Renew. Sustain. Energy Rev. 2013, 21, 659–673. [Google Scholar] [CrossRef]
- Karbowniczak, A.; Latała, H.; Nęcka, K.; Kurpaska, S.; Bergel, T. Modelling of the Electric Energy Storage Process in a PCM Battery. Energies 2022, 15, 735. [Google Scholar] [CrossRef]
- Hu, Y.; Guo, R.; Heiselberg, P.K.; Johra, H. Modeling PCM Phase Change Temperature and Hysteresis in Ventilation Cooling and Heating Applications. Energies 2020, 13, 6455. [Google Scholar] [CrossRef]
- Diaconu, B.M.; Cruceru, M. Novel Concept of Composite Phase Change Material Wall System for Year-Round Thermal Energy Savings. Energy Build. 2010, 42, 1759–1772. [Google Scholar] [CrossRef]
- de Gracia, A.; Navarro, L.; Castell, A.; Cabeza, L.F. Numerical Study on the Thermal Performance of a Ventilated Facade with PCM. Appl. Therm. Eng. 2013, 61, 372–380. [Google Scholar] [CrossRef]
- Yang, H.; He, Y. Solving Heat Transfer Problems with Phase Change via Smoothed Effective Heat Capacity and Element-Free Galerkin Methods. Int. Commun. Heat Mass Transf. 2010, 37, 385–392. [Google Scholar] [CrossRef]
- Musiał, M.; Lichołai, L.; Pękala, A. Analysis of the Thermal Performance of Isothermal Composite Heat Accumulators Containing Organic Phase-Change Material. Energies 2023, 16, 1409. [Google Scholar] [CrossRef]
- Pielichowska, K.; Pielichowski, K. Phase Change Materials for Thermal Energy Storage. Prog. Mater. Sci. 2014, 65, 67–123. [Google Scholar] [CrossRef]
- Dinçer, I.; Rosen, M. Thermal Energy Storage Systems and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2021. [Google Scholar]
- Farid, M.M.; Khudhair, A.M.; Razack, S.A.K.; Al-Hallaj, S. A Review on Phase Change Energy Storage: Materials and Applications. Energy Convers. Manag. 2004, 45, 1597–1615. [Google Scholar] [CrossRef]
- Rathod, M.K.; Banerjee, J. Thermal Stability of Phase Change Materials Used in Latent Heat Energy Storage Systems: A Review. Renew. Sustain. Energy Rev. 2013, 18, 246–258. [Google Scholar] [CrossRef]
- Reddy, M.; Nallusamy, N.; Prasad, A.; Reddy, H. Thermal Energy Storage System Using Phase Change Materials: Constant Heat Source. Therm. Sci. 2012, 16, 1097–1104. [Google Scholar] [CrossRef]
- Pointner, H.; Steinmann, W.-D.; Eck, M. Introduction of the PCM Flux Concept for Latent Heat Storage. Energy Procedia 2014, 57, 643–652. [Google Scholar] [CrossRef]
- Regin, A.F.; Solanki, S.C.; Saini, J.S. Heat Transfer Characteristics of Thermal Energy Storage System Using PCM Capsules: A Review. Renew. Sustain. Energy Rev. 2008, 12, 2438–2458. [Google Scholar] [CrossRef]
- Royo, P.; Acevedo, L.; Ferreira, V.J.; García-Armingol, T.; López-Sabirón, A.M.; Ferreira, G. High-Temperature PCM-Based Thermal Energy Storage for Industrial Furnaces Installed in Energy-Intensive Industries. Energy 2019, 173, 1030–1040. [Google Scholar] [CrossRef]
- Liu, M.; Saman, W.; Bruno, F. Review on Storage Materials and Thermal Performance Enhancement Techniques for High Temperature Phase Change Thermal Storage Systems. Renew. Sustain. Energy Rev. 2012, 16, 2118–2132. [Google Scholar] [CrossRef]
- Agyenim, F.; Hewitt, N.; Eames, P.; Smyth, M. A Review of Materials, Heat Transfer and Phase Change Problem Formulation for Latent Heat Thermal Energy Storage Systems (LHTESS). Renew. Sustain. Energy Rev. 2010, 14, 615–628. [Google Scholar] [CrossRef]
- Khanna, S.; Reddy, K.S.; Mallick, T.K. Optimization of Solar Photovoltaic System Integrated with Phase Change Material. Solar Energy 2018, 163, 591–599. [Google Scholar] [CrossRef]
- Talukdar, S.; Afroz, H.M.M.; Hossain, M.A.; Aziz, M.A.; Hossain, M.M. Heat Transfer Enhancement of Charging and Discharging of Phase Change Materials and Size Optimization of a Latent Thermal Energy Storage System for Solar Cold Storage Application. J. Energy Storage 2019, 24, 100797. [Google Scholar] [CrossRef]
- Kumar, S.K.; Muniamuthu, S.; Mohan, A.; Amirthalingam, P.; Anbu Muthuraja, M. Effect of Charging and Discharging Process of PCM with Paraffin and Al2O3 Additive Subjected to Three Point Temperature Locations. J. Ecol. Eng. 2022, 23, 34–42. [Google Scholar] [CrossRef]
- Frahat, N.B.; Ustaoglu, A.; Gencel, O.; Sarı, A.; Hekimoğlu, G.; Yaras, A.; del Coz Díaz, J.J. Fuel, Cost, Energy Efficiency and CO2 Emission Performance of PCM Integrated Wood Fiber Composite Phase Change Material at Different Climates. Sci. Rep. 2023, 13, 7714. [Google Scholar] [CrossRef]
- Almonti, D.; Mingione, E.; Tagliaferri, V.; Ucciardello, N. Design and Analysis of Compound Structures Integrated with Bio-Based Phase Change Materials and Lattices Obtained through Additive Manufacturing. Int. J. Adv. Manuf. Technol. 2022, 119, 149–161. [Google Scholar] [CrossRef]
- Hekimoğlu, G.; Sarı, A. A Review on Phase Change Materials (PCMs) for Thermal Energy Storage Implementations. Mater. Today Proc. 2022, 58, 1360–1367. [Google Scholar] [CrossRef]
- Sharma, R.; Jang, J.-G.; Hu, J.-W. Phase-Change Materials in Concrete: Opportunities and Challenges for Sustainable Construction and Building Materials. Materials 2022, 15, 335. [Google Scholar] [CrossRef]
- Łach, M.; Pławecka, K.; Bąk, A.; Adamczyk, M.; Bazan, P.; Kozub, B.; Korniejenko, K.; Lin, W.-T. Review of Solutions for the Use of Phase Change Materials in Geopolymers. Materials 2021, 14, 6044. [Google Scholar] [CrossRef]
- Singh, P.; Sharma, R.K.; Ansu, A.K.; Goyal, R.; Sarı, A.; Tyagi, V.V. A Comprehensive Review on Development of Eutectic Organic Phase Change Materials and Their Composites for Low and Medium Range Thermal Energy Storage Applications. Sol. Energy Mater. Sol. Cells 2021, 223, 110955. [Google Scholar] [CrossRef]
- Dixit, P.; Reddy, V.J.; Parvate, S.; Balwani, A.; Singh, J.; Maiti, T.K.; Dasari, A.; Chattopadhyay, S. Salt Hydrate Phase Change Materials: Current State of Art and the Road Ahead. J. Energy Storage 2022, 51, 104360. [Google Scholar] [CrossRef]
- Choo, Y.M.; Wei, W. Salt Hydrates as Phase Change Materials for Photovoltaics Thermal Management. Energy Sci. Eng. 2022, 10, 1630–1642. [Google Scholar] [CrossRef]
- Xie, N.; Huang, Z.; Luo, Z.; Gao, X.; Fang, Y.; Zhang, Z. Inorganic Salt Hydrate for Thermal Energy Storage. Appl. Sci. 2017, 7, 1317. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, X.; Ji, J.; Lv, Y. A Review of the Application of Hydrated Salt Phase Change Materials in Building Temperature Control. J. Energy Storage 2022, 56, 106157. [Google Scholar] [CrossRef]
- Styś-Maniara, M.; Porowski, R. Inorganic Salt Hydrates as Phase Change Materials (PCM) for Thermal Energy Storage in Solar Installations. Struct. Environ. 2022, 14, 161–172. [Google Scholar] [CrossRef]
- Wang, S.; Lei, K.; Wang, Z.; Wang, H.; Zou, D. Metal-Based Phase Change Material (PCM) Microcapsules/Nanocapsules: Fabrication, Thermophysical Characterization and Application. Chem. Eng. J. 2022, 438, 135559. [Google Scholar] [CrossRef]
- Junaid, M.F.; Rehman, Z.U.; Čekon, M.; Čurpek, J.; Farooq, R.; Cui, H.; Khan, I. Inorganic Phase Change Materials in Thermal Energy Storage: A Review on Perspectives and Technological Advances in Building Applications. Energy Build. 2021, 252, 111443. [Google Scholar] [CrossRef]
- Yao, Y.; Li, W.; Liu, J.; Deng, Z. Liquid Metal Phase Change Materials for Thermal Management of Electronics. Adv. Phys. X 2024, 9, 2324910. [Google Scholar] [CrossRef]
- Ostrý, M.; Bantová, S.; Struhala, K. Compatibility of Phase Change Materials and Metals: Experimental Evaluation Based on the Corrosion Rate. Molecules 2020, 25, 2823. [Google Scholar] [CrossRef]
- Hirschey, J.R.; Kumar, N.; Turnaoglu, T.; Gluesenkamp, K.R.; Graham, S. Review of Low-Cost Organic and Inorganic Phase Change Review of Low-Cost Organic and Inorganic Phase Change Materials with Phase Change Temperature between 0 °C and 65 °C. In Proceedings of the International High Performance Buildings Conference, West Lafayette, IN, USA, 24–28 May 2021. [Google Scholar]
- Min, K.-E.; Jang, J.-W.; Kim, J.-K.; Wern, C.; Yi, S. Thermophysical Properties of Inorganic Phase-Change Materials Based on MnCl2·4H2O. Appl. Sci. 2022, 12, 6338. [Google Scholar] [CrossRef]
- Hassan, H.M.A.; Lund, I. Inorganic PCMs Applications in Passive Cooling of Buildings—A Review. J. Phys. Conf. Ser. 2021, 2116, 012103. [Google Scholar] [CrossRef]
- Chen, J.; Yang, D.; Jiang, J.; Ma, A.; Song, D. Research Progress of Phase Change Materials (PCMs) Embedded with Metal Foam (a Review). Procedia Mater. Sci. 2014, 4, 389–394. [Google Scholar] [CrossRef]
- Maiti, T.K.; Dixit, P.; Suhag, A.; Bhushan, S.; Yadav, A.; Talapatra, N.; Chattopadhyay, S. Advancements in Organic and Inorganic Shell Materials for the Preparation of Microencapsulated Phase Change Materials for Thermal Energy Storage Applications. RSC Sustain. 2023, 1, 665–697. [Google Scholar] [CrossRef]
- Yang, G.; Yim, Y.-J.; Lee, J.W.; Heo, Y.-J.; Park, S.-J. Carbon-Filled Organic Phase-Change Materials for Thermal Energy Storage: A Review. Molecules 2019, 24, 2055. [Google Scholar] [CrossRef] [PubMed]
- Zare, M.; Mikkonen, K.S. Phase Change Materials for Life Science Applications. Adv. Funct. Mater. 2023, 33, 2213455. [Google Scholar] [CrossRef]
- Magendran, S.S.; Khan, F.S.A.; Mubarak, N.M.; Vaka, M.; Walvekar, R.; Khalid, M.; Abdullah, E.C.; Nizamuddin, S.; Karri, R.R. Synthesis of Organic Phase Change Materials (PCM) for Energy Storage Applications: A Review. Nano-Struct. Nano-Objects 2019, 20, 100399. [Google Scholar] [CrossRef]
- Liu, M.; Qian, R.; Yang, Y.; Lu, X.; Huang, L.; Zou, D. Modification of Phase Change Materials for Electric-Thermal, Photo-Thermal, and Magnetic-Thermal Conversions: A Comprehensive Review. Adv. Funct. Mater. 2024, 34, 2400038. [Google Scholar] [CrossRef]
- Jia, Z.; Hu, C.; Zhang, Y.; Zhang, S.; Tang, B. Exploring Electro-Thermal Conversion in Phase Change Materials: A Review. Compos. Part A Appl. Sci. Manuf. 2023, 175, 107809. [Google Scholar] [CrossRef]
- Prabhathan, P.; Sreekanth, K.V.; Teng, J.; Ko, J.H.; Yoo, Y.J.; Jeong, H.-H.; Lee, Y.; Zhang, S.; Cao, T.; Popescu, C.-C.; et al. Roadmap for Phase Change Materials in Photonics and Beyond. iScience 2023, 26, 107946. [Google Scholar] [CrossRef]
- NematpourKeshteli, A.; Iasiello, M.; Langella, G.; Bianco, N. Using Metal Foam and Nanoparticle Additives with Different Fin Shapes for PCM-Based Thermal Storage in Flat Plate Solar Collectors. Therm. Sci. Eng. Prog. 2024, 52, 102690. [Google Scholar] [CrossRef]
- NematpourKeshteli, A.; Iasiello, M.; Langella, G.; Bianco, N. Thermal Enhancement Techniques for a Lobed-Double Pipe PCM Thermal Storage System. Appl. Therm. Eng. 2023, 233, 121139. [Google Scholar] [CrossRef]
- Chen, X.; Cheng, P.; Tang, Z.; Xu, X.; Gao, H.; Wang, G. Carbon-Based Composite Phase Change Materials for Thermal Energy Storage, Transfer, and Conversion. Adv. Sci. 2021, 8, 2001274. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Gao, H.; Yang, M.; Dong, W.; Huang, X.; Li, A.; Dong, C.; Wang, G. Highly Graphitized 3D Network Carbon for Shape-Stabilized Composite PCMs with Superior Thermal Energy Harvesting. Nano Energy 2018, 49, 86–94. [Google Scholar] [CrossRef]
- Wu, T.; Wang, C.; Hu, Y.; Zeng, X.; Song, M. Flexible Solid-Solid Phase Change Materials with High Stability for Thermal Management. Int. J. Heat Mass Transf. 2023, 211, 124202. [Google Scholar] [CrossRef]
- Wang, J.; Xie, H.; Xin, Z. Thermal Properties of Paraffin Based Composites Containing Multi-Walled Carbon Nanotubes. Thermochim. Acta 2009, 488, 39–42. [Google Scholar] [CrossRef]
- Bhutto, Y.A.; Pandey, A.K.; Saidur, R.; Laghari, I.A.; Buddhi, D.; Tyagi, V.V. Thermal Behaviour of Paraffin-MWCNT Stabilized by Sodium Dodecylbenzene Sulphonate Nano-Enhanced Phase Change Material for Energy Storage Applications. IOP Conf Ser Earth Environ. Sci. 2023, 1261, 012023. [Google Scholar] [CrossRef]
- Kuziel, A.W.; Dzido, G.; Turczyn, R.; Jędrysiak, R.G.; Kolanowska, A.; Tracz, A.; Zięba, W.; Cyganiuk, A.; Terzyk, A.P.; Boncel, S. Ultra-Long Carbon Nanotube-Paraffin Composites of Record Thermal Conductivity and High Phase Change Enthalpy among Paraffin-Based Heat Storage Materials. J. Energy Storage 2021, 36, 102396. [Google Scholar] [CrossRef]
- Li, M.; Guo, Q.; Su, Y. The Thermal Conductivity Improvements of Phase Change Materials Using Modified Carbon Nanotubes. Diam. Relat. Mater. 2022, 125, 109023. [Google Scholar] [CrossRef]
- Zhang, K.; Han, B.; Yu, X. Electrically Conductive Carbon Nanofiber/Paraffin Wax Composites for Electric Thermal Storage. Energy Convers. Manag. 2012, 64, 62–67. [Google Scholar] [CrossRef]
- Chen, L.; Zou, R.; Xia, W.; Liu, Z.; Shang, Y.; Zhu, J.; Wang, Y.; Lin, J.; Xia, D.; Cao, A. Electro- and Photodriven Phase Change Composites Based on Wax-Infiltrated Carbon Nanotube Sponges. ACS Nano 2012, 6, 10884–10892. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, H.; Qi, H. High Efficiency Electro- and Photo-Thermal Conversion Cellulose Nanofiber-Based Phase Change Materials for Thermal Management. J. Colloid Interface Sci. 2023, 629, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Zhang, N.; Zhang, H.; Yu, X.; Ding, Y.; Yuan, Y. Functional Phase Change Composites with Highly Efficient Electrical to Thermal Energy Conversion. Renew Energy 2020, 145, 2629–2636. [Google Scholar] [CrossRef]
- Aftab, W.; Mahmood, A.; Guo, W.; Yousaf, M.; Tabassum, H.; Huang, X.; Liang, Z.; Cao, A.; Zou, R. Polyurethane-Based Flexible and Conductive Phase Change Composites for Energy Conversion and Storage. Energy Storage Mater 2019, 20, 401–409. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Z.; Xie, H.; Wang, T.; Wang, Y.; Huang, Y.; Dong, L. Graphene Oxide/Polyurethane-based Composite Solid–Solid Phase Change Materials with Enhanced Energy Storage Capacity and Photothermal Performance. Int. J. Energy Res. 2022, 46, 22744–22756. [Google Scholar] [CrossRef]
- Liu, Z.; Zou, R.; Lin, Z.; Gui, X.; Chen, R.; Lin, J.; Shang, Y.; Cao, A. Tailoring Carbon Nanotube Density for Modulating Electro-to-Heat Conversion in Phase Change Composites. Nano Lett. 2013, 13, 4028–4035. [Google Scholar] [CrossRef]
- Sudeep, P.M.; Narayanan, T.N.; Ganesan, A.; Shaijumon, M.M.; Yang, H.; Ozden, S.; Patra, P.K.; Pasquali, M.; Vajtai, R.; Ganguli, S.; et al. Covalently Interconnected Three-Dimensional Graphene Oxide Solids. ACS Nano 2013, 7, 7034–7040. [Google Scholar] [CrossRef]
- Guo, X.; Liu, C.; Li, N.; Zhang, S.; Wang, Z. Electrothermal Conversion Phase Change Composites: The Case of Polyethylene Glycol Infiltrated Graphene Oxide/Carbon Nanotube Networks. Ind. Eng. Chem. Res. 2018, 57, 15697–15702. [Google Scholar] [CrossRef]
- Ruiz-Calleja, T.; Bonet-Aracil, M.; Gisbert-Payá, J.; Bou-Belda, E. Analysis of the Influence of Graphene and Phase Change Microcapsules on Thermal Behavior of Cellulosic Fabrics. Mater. Today Commun. 2020, 25, 101557. [Google Scholar] [CrossRef]
- Zhu, Q.; Ong, P.J.; Goh, S.H.A.; Yeo, R.J.; Wang, S.; Liu, Z.; Loh, X.J. Recent Advances in Graphene-Based Phase Change Composites for Thermal Energy Storage and Management. Nano Mater. Sci. 2024, 6, 115–138. [Google Scholar] [CrossRef]
- Mokhena, T.C.; Mochane, M.J.; Mtibe, A.; Sigonya, S.; Ntsendwana, B.; Masibi, E.G.; Sikhwivhilu, L.; Motsoeneng, T.S. Recent Advances on Nanocellulose-Graphene Oxide Composites: A Review. Cellulose 2024, 31, 7207–7249. [Google Scholar] [CrossRef]
- Li, J.; Meng, L.; Chen, J.; Chen, X.; Wang, Y.; Xiao, Z.; Wang, H.; Liang, D.; Xie, Y. Encapsulation of Polyethylene Glycol in Cellulose-Based Porous Capsules for Latent Heat Storage and Light-to-Thermal Conversion. Front. Chem. Sci. Eng. 2023, 17, 1038–1050. [Google Scholar] [CrossRef]
- Yang, G.; Zhao, L.; Shen, C.; Mao, Z.; Xu, H.; Feng, X.; Wang, B.; Sui, X. Boron Nitride Microsheets Bridged with Reduced Graphene Oxide as Scaffolds for Multifunctional Shape Stabilized Phase Change Materials. Sol. Energy Mater. Sol. Cells 2020, 209, 110441. [Google Scholar] [CrossRef]
- Nishad, S.; Kasak, P.; Krupa, I. Highly Conductive Phase Change Composites Based on Paraffin-Infiltrated Graphite Panels for Photo/Electrothermal Conversion and Storage. J. Energy Storage 2023, 66, 107449. [Google Scholar] [CrossRef]
- Yu, X.K.; Tao, Y.B. Preparation and Characterization of Paraffin/Expanded Graphite Composite Phase Change Materials with High Thermal Conductivity. Int. J. Heat Mass Transf. 2022, 198, 123433. [Google Scholar] [CrossRef]
- Wu, W.; Huang, X.; Li, K.; Yao, R.; Chen, R.; Zou, R. A Functional Form-Stable Phase Change Composite with High Efficiency Electro-to-Thermal Energy Conversion. Appl. Energy 2017, 190, 474–480. [Google Scholar] [CrossRef]
- Maleki, M.; Karimian, H.; Shokouhimehr, M.; Ahmadi, R.; Valanezhad, A.; Beitollahi, A. Development of Graphitic Domains in Carbon Foams for High Efficient Electro/Photo-to-Thermal Energy Conversion Phase Change Composites. Chem. Eng. J. 2019, 362, 469–481. [Google Scholar] [CrossRef]
- Tabassum, H.; Huang, X.; Chen, R.; Zou, R. Tailoring Thermal Properties via Synergistic Effect in a Multifunctional Phase Change Composite Based on Methyl Stearate. J. Mater. 2015, 1, 229–235. [Google Scholar] [CrossRef]
- Li, T.; Wu, M.; Wu, S.; Xiang, S.; Xu, J.; Chao, J.; Yan, T.; Deng, T.; Wang, R. Highly Conductive Phase Change Composites Enabled by Vertically-Aligned Reticulated Graphite Nanoplatelets for High-Temperature Solar Photo/Electro-Thermal Energy Conversion, Harvesting and Storage. Nano Energy 2021, 89, 106338. [Google Scholar] [CrossRef]
- Chriaa, I.; Karkri, M.; Trigui, A.; Jedidi, I.; Abdelmouleh, M.; Boudaya, C. The Performances of Expanded Graphite on the Phase Change Materials Composites for Thermal Energy Storage. Polymer 2021, 212, 123128. [Google Scholar] [CrossRef]
- Xue, F.; Lu, Y.; Qi, X.; Yang, J.; Wang, Y. Melamine Foam-Templated Graphene Nanoplatelet Framework toward Phase Change Materials with Multiple Energy Conversion Abilities. Chem. Eng. J. 2019, 365, 20–29. [Google Scholar] [CrossRef]
- Cai, Y.; Zhang, N.; Cao, X.; Yuan, Y.; Zhang, Z.; Yu, N. Ultra-Light and Flexible Graphene Aerogel-Based Form-Stable Phase Change Materials for Energy Conversion and Energy Storage. Sol. Energy Mater. Sol. Cells 2023, 252, 112176. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, K.; Min, X.; Xiao, J.; Xu, Z.; Huang, Z.; Liu, Y.; Wu, X.; Fang, M. Graphene Aerogel Stabilized Phase Change Material for Thermal Energy Storage. Case Stud. Therm. Eng. 2022, 40, 102497. [Google Scholar] [CrossRef]
- Sun, K.; Kou, Y.; Dong, H.; Ye, S.; Zhao, D.; Liu, J.; Shi, Q. The Design of Phase Change Materials with Carbon Aerogel Composites for Multi-Responsive Thermal Energy Capture and Storage. J. Mater. Chem. A Mater. 2021, 9, 1213–1220. [Google Scholar] [CrossRef]
- Li, G.; Zhang, X.; Zhang, X. Graphene Aerogel-Directed Fabrication of Phase Change Composites. In Phase Change Materials and Their Applications; InTech: London, UK, 2018. [Google Scholar]
- Li, G.; Hong, G.; Dong, D.; Song, W.; Zhang, X. Multiresponsive Graphene-Aerogel-Directed Phase-Change Smart Fibers. Adv. Mater. 2018, 30, e1801754. [Google Scholar] [CrossRef]
- Li, G.; Zhang, X.; Wang, J.; Fang, J. From Anisotropic Graphene Aerogels to Electron- and Photo-Driven Phase Change Composites. J. Mater. Chem. A Mater. 2016, 4, 17042–17049. [Google Scholar] [CrossRef]
- Min, P.; Liu, J.; Li, X.; An, F.; Liu, P.; Shen, Y.; Koratkar, N.; Yu, Z. Thermally Conductive Phase Change Composites Featuring Anisotropic Graphene Aerogels for Real-Time and Fast-Charging Solar-Thermal Energy Conversion. Adv. Funct. Mater. 2018, 28, 1805365. [Google Scholar] [CrossRef]
- Wang, J.; Jia, X.; Atinafu, D.G.; Wang, M.; Wang, G.; Lu, Y. Synthesis of “Graphene-like” Mesoporous Carbons for Shape-Stabilized Phase Change Materials with High Loading Capacity and Improved Latent Heat. J. Mater. Chem. A Mater. 2017, 5, 24321–24328. [Google Scholar] [CrossRef]
- Yang, J.; Qi, G.-Q.; Liu, Y.; Bao, R.-Y.; Liu, Z.-Y.; Yang, W.; Xie, B.-H.; Yang, M.-B. Hybrid Graphene Aerogels/Phase Change Material Composites: Thermal Conductivity, Shape-Stabilization and Light-to-Thermal Energy Storage. Carbon 2016, 100, 693–702. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhou, M.; Huang, F.; Lin, T.; Wan, D. Effect of Graphene Aerogel on Thermal Behavior of Phase Change Materials for Thermal Management. Sol. Energy Mater. Sol. Cells 2013, 113, 195–200. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, X.; Liu, X.; Sheng, D.; Ji, F.; Dong, L.; Xu, S.; Wu, H.; Yang, Y. Polyurethane-Based Solid-Solid Phase Change Materials with Halloysite Nanotubes-Hybrid Graphene Aerogels for Efficient Light- and Electro-Thermal Conversion and Storage. Carbon 2019, 142, 558–566. [Google Scholar] [CrossRef]
- Jiang, T.; Zhang, Y.; Olayiwola, S.; Lau, C.; Fan, M.; Ng, K.; Tan, G. Biomass-Derived Porous Carbons Support in Phase Change Materials for Building Energy Efficiency: A Review. Mater. Today Energy 2022, 23, 100905. [Google Scholar] [CrossRef]
- Atinafu, D.G.; Yun, B.Y.; Wi, S.; Chang, S.J.; Kim, S. Unveiling Sustainable Nano-Enabled Phase Change Materials for High Thermal Stability and Energy Storage Capacity. J. Energy Storage 2023, 60, 106650. [Google Scholar] [CrossRef]
- Tyagi, V.V.; Panwar, N.L.; Rahim, N.A.; Kothari, R. Review on Solar Air Heating System with and without Thermal Energy Storage System. Renew. Sustain. Energy Rev. 2012, 16, 2289–2303. [Google Scholar] [CrossRef]
- Hanif, F.; Imran, M.; Zhang, Y.; Jia, Z.; Lu, X.; Lu, R.; Tang, B. Form-Stable Phase Change Material with Wood-Based Materials as Support. Polymers 2023, 15, 942. [Google Scholar] [CrossRef]
- Angel-López, A.; Norambuena, Á.; Arriaza-Echanes, C.; Terraza, C.A.; Tundidor-Camba, A.; Coll, D.; Ortiz, P.A. Development of Novel Phase-Change Materials Derived from Methoxy Polyethylene Glycol and Aromatic Acyl Chlorides. Polymers 2023, 15, 3069. [Google Scholar] [CrossRef]
- Gao, N.; Du, J.; Yang, W.; Li, Y.; Chen, N. Biomass-Based Shape-Stabilized Composite Phase-Change Materials with High Solar–Thermal Conversion Efficiency for Thermal Energy Storage. Polymers 2023, 15, 3747. [Google Scholar] [CrossRef]
- Fabiani, C.; Santini, C.; Barbanera, M.; Giannoni, T.; Rubino, G.; Cotana, F.; Pisello, A.L. Phase Change Materials-Impregnated Biomass for Energy Efficiency in Buildings: Innovative Material Production and Multiscale Thermophysical Characterization. J. Energy Storage 2023, 58, 106223. [Google Scholar] [CrossRef]
- Song, S.; Ai, H.; Zhu, W.; Lv, L.; Feng, R.; Dong, L. Carbon Aerogel Based Composite Phase Change Material Derived from Kapok Fiber: Exceptional Microwave Absorbility and Efficient Solar/Magnetic to Thermal Energy Storage Performance. Compos. B Eng. 2021, 226, 109330. [Google Scholar] [CrossRef]
- Wei, Y.; Li, J.; Sun, F.; Wu, J.; Zhao, L. Leakage-Proof Phase Change Composites Supported by Biomass Carbon Aerogels from Succulents. Green Chem. 2018, 20, 1858–1865. [Google Scholar] [CrossRef]
- Li, Y.; Samad, Y.A.; Polychronopoulou, K.; Alhassan, S.M.; Liao, K. From Biomass to High Performance Solar–Thermal and Electric–Thermal Energy Conversion and Storage Materials. J. Mater. Chem. A 2014, 2, 7759–7765. [Google Scholar] [CrossRef]
- Umair, M.M.; Zhang, Y.; Tehrim, A.; Zhang, S.; Tang, B. Form-Stable Phase-Change Composites Supported by a Biomass-Derived Carbon Scaffold with Multiple Energy Conversion Abilities. Ind. Eng. Chem. Res. 2020, 59, 1393–1401. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Y.; Yu, Q.; Cao, G.; Sun, X.; Yang, R.; Zhang, Q.; Liu, F.; Di, X.; Li, J.; et al. Low-Cost, Three-Dimension, High Thermal Conductivity, Carbonized Wood-Based Composite Phase Change Materials for Thermal Energy Storage. Energy 2018, 159, 929–936. [Google Scholar] [CrossRef]
- Pogorielov, M.; Smyrnova, K.; Kyrylenko, S.; Gogotsi, O.; Zahorodna, V.; Pogrebnjak, A. MXenes—A New Class of Two-Dimensional Materials: Structure, Properties and Potential Applications. Nanomaterials 2021, 11, 3412. [Google Scholar] [CrossRef]
- Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y. 25th Anniversary Article: MXenes: A New Family of Two-Dimensional Materials. Adv. Mater. 2014, 26, 992–1005. [Google Scholar] [CrossRef]
- Verger, L.; Xu, C.; Natu, V.; Cheng, H.-M.; Ren, W.; Barsoum, M.W. Overview of the Synthesis of MXenes and Other Ultrathin 2D Transition Metal Carbides and Nitrides. Curr. Opin. Solid State Mater. Sci. 2019, 23, 149–163. [Google Scholar] [CrossRef]
- Alhabeb, M.; Maleski, K.; Anasori, B.; Lelyukh, P.; Clark, L.; Sin, S.; Gogotsi, Y. Guidelines for Synthesis and Processing of Two-Dimensional Titanium Carbide (Ti3C2TxMXene). Chem. Mater. 2017, 29, 7633–7644. [Google Scholar] [CrossRef]
- Lim, K.R.G.; Shekhirev, M.; Wyatt, B.C.; Anasori, B.; Gogotsi, Y.; Seh, Z.W. Fundamentals of MXene Synthesis. Nat. Synth. 2022, 1, 601–614. [Google Scholar] [CrossRef]
- Oyehan, T.A.; Salami, B.A.; Abdulrasheed, A.A.; Hambali, H.U.; Gbadamosi, A.; Valsami-Jones, E.; Saleh, T.A. MXenes: Synthesis, Properties, and Applications for Sustainable Energy and Environment. Appl. Mater. Today 2023, 35, 101993. [Google Scholar] [CrossRef]
- Awan, H.T.A.; Kumar, L.; Wong, W.P.; Walvekar, R.; Khalid, M. Recent Progress and Challenges in MXene-Based Phase Change Material for Solar and Thermal Energy Applications. Energies 2023, 16, 1977. [Google Scholar] [CrossRef]
- Solangi, N.H.; Mubarak, N.M.; Karri, R.R.; Mazari, S.A.; Jatoi, A.S.; Koduru, J.R.; Dehghani, M.H. MXene-Based Phase Change Materials for Solar Thermal Energy Storage. Energy Convers. Manag. 2022, 273, 116432. [Google Scholar] [CrossRef]
- Cui, Y.; Zhu, J.; Tong, H.; Zou, R. Advanced Perspectives on MXene Composite Nanomaterials: Types Synthetic Methods, Thermal Energy Utilization and 3D-Printed Techniques. iScience 2023, 26, 105824. [Google Scholar] [CrossRef]
- Aslfattahi, N.; Saidur, R.; Arifutzzaman, A.; Sadri, R.; Bimbo, N.; Sabri, M.F.M.; Maughan, P.A.; Bouscarrat, L.; Dawson, R.J.; Said, S.M.; et al. Experimental Investigation of Energy Storage Properties and Thermal Conductivity of a Novel Organic Phase Change Material/MXene as A New Class of Nanocomposites. J. Energy Storage 2020, 27, 101115. [Google Scholar] [CrossRef]
- Krishna, Y.; Saidur, R.; Aslfattahi, N.; Faizal, M.; Ng, K.C. Enhancing the Thermal Properties of Organic Phase Change Material (Palmitic Acid) by Doping MXene Nanoflakes. AIP Conf. Proc. 2020, 2233, 020013. [Google Scholar]
- Wang, L.; Liang, W.; Wang, C.; Fan, Y.; Liu, Y.; Xiao, C.; Sun, H.; Zhu, Z.; Li, A. Dodecylamine/Ti3C2-Pectin Form-Stable Phase Change Composites with Enhanced Light-to-Thermal Conversion and Mechanical Properties. Renew. Energy 2021, 176, 663–674. [Google Scholar] [CrossRef]
- Ferrara, C.; Gentile, A.; Marchionna, S.; Quinzeni, I.; Fracchia, M.; Ghigna, P.; Pollastri, S.; Ritter, C.; Vanacore, G.M.; Ruffo, R. The Missing Piece: The Structure of the Ti3C2Tx MXene and Its Behavior as Negative Electrode in Sodium Ion Batteries. Nano Lett. 2021, 21, 8290–8297. [Google Scholar] [CrossRef]
- Benchakar, M.; Loupias, L.; Garnero, C.; Bilyk, T.; Morais, C.; Canaff, C.; Guignard, N.; Morisset, S.; Pazniak, H.; Hurand, S.; et al. One MAX Phase, Different MXenes: A Guideline to Understand the Crucial Role of Etching Conditions on Ti3C2Tx Surface Chemistry. Appl. Surf. Sci. 2020, 530, 147209. [Google Scholar] [CrossRef]
- Jena, D.P.; Anwar, S.; Parida, R.K.; Parida, B.N.; Nayak, N.C. Structural, Thermal and Dielectric Behavior of Two-Dimensional Layered Ti3C2Tx(MXene) Filled Ethylene–Vinyl Acetate (EVA) Nanocomposites. J. Mater. Sci. Mater. Electron. 2021, 32, 8081–8091. [Google Scholar] [CrossRef]
- Mo, Z.; Mo, P.; Yi, M.; Hu, Z.; Tan, G.; Selim, M.S.; Chen, Y.; Chen, X.; Hao, Z.; Wei, X. Ti3C2Tx@Polyvinyl Alcohol Foam-Supported Phase Change Materials with Simultaneous Enhanced Thermal Conductivity and Solar-Thermal Conversion Performance. Sol. Energy Mater. Sol. Cells 2021, 219, 110813. [Google Scholar] [CrossRef]
- Wu, H.; Hu, X.; Li, X.; Sheng, M.; Sheng, X.; Lu, X.; Qu, J. Large-Scale Fabrication of Flexible EPDM/MXene/PW Phase Change Composites with Excellent Light-to-Thermal Conversion Efficiency via Water-Assisted Melt Blending. Compos. Part A Appl. Sci. Manuf. 2022, 152, 106713. [Google Scholar] [CrossRef]
- Lu, X.; Huang, H.; Zhang, X.; Lin, P.; Huang, J.; Sheng, X.; Zhang, L.; Qu, J. Novel Light-Driven and Electro-Driven Polyethylene Glycol/Two-Dimensional MXene Form-Stable Phase Change Material with Enhanced Thermal Conductivity and Electrical Conductivity for Thermal Energy Storage. Compos. B Eng. 2019, 177, 107372. [Google Scholar] [CrossRef]
- Shao, Y.; Hu, W.; Gao, M.; Xiao, Y.; Huang, T.; Zhang, N.; Yang, J.; Qi, X.; Wang, Y. Flexible MXene-Coated Melamine Foam Based Phase Change Material Composites for Integrated Solar-Thermal Energy Conversion/Storage, Shape Memory and Thermal Therapy Functions. Compos. Part A Appl. Sci. Manuf. 2021, 143, 106291. [Google Scholar] [CrossRef]
- Du, Y.; Huang, H.; Hu, X.; Liu, S.; Sheng, X.; Li, X.; Lu, X.; Qu, J. Melamine Foam/Polyethylene Glycol Composite Phase Change Material Synergistically Modified by Polydopamine/MXene with Enhanced Solar-to-Thermal Conversion. Renew Energy 2021, 171, 1–10. [Google Scholar] [CrossRef]
- Gong, S.; Ding, Y.; Li, X.; Liu, S.; Wu, H.; Lu, X.; Qu, J. Novel Flexible Polyurethane/MXene Composites with Sensitive Solar Thermal Energy Storage Behavior. Compos. Part A Appl. Sci. Manuf. 2021, 149, 106505. [Google Scholar] [CrossRef]
- Lin, P.; Xie, J.; He, Y.; Lu, X.; Li, W.; Fang, J.; Yan, S.; Zhang, L.; Sheng, X.; Chen, Y. MXene Aerogel-Based Phase Change Materials toward Solar Energy Conversion. Sol. Energy Mater. Sol. Cells 2020, 206, 110229. [Google Scholar] [CrossRef]
- Luo, Y.; Xie, Y.; Jiang, H.; Chen, Y.; Zhang, L.; Sheng, X.; Xie, D.; Wu, H.; Mei, Y. Flame-Retardant and Form-Stable Phase Change Composites Based on MXene with High Thermostability and Thermal Conductivity for Thermal Energy Storage. Chem. Eng. J. 2021, 420, 130466. [Google Scholar] [CrossRef]
- Wang, X.; Yu, W.; Wang, L.; Xie, H. Vertical Orientation Graphene/MXene Hybrid Phase Change Materials with Anisotropic Properties, High Enthalpy, and Photothermal Conversion. Sci. China Technol. Sci. 2022, 65, 882–892. [Google Scholar] [CrossRef]
- Islam, A.; Pandey, A.K.; Saidur, R.; Tyagi, V.V. Shape Stable Composite Phase Change Material with Improved Thermal Conductivity for Electrical-to-Thermal Energy Conversion and Storage. Mater. Today Sustain. 2024, 25, 100678. [Google Scholar] [CrossRef]
- Li, C.; Zhang, B.; Liu, Q. N-Eicosane/Expanded Graphite as Composite Phase Change Materials for Electro-Driven Thermal Energy Storage. J. Energy Storage 2020, 29, 101339. [Google Scholar] [CrossRef]
- Wu, H.; Deng, S.; Shao, Y.; Yang, J.; Qi, X.; Wang, Y. Multiresponsive Shape-Adaptable Phase Change Materials with Cellulose Nanofiber/Graphene Nanoplatelet Hybrid-Coated Melamine Foam for Light/Electro-to-Thermal Energy Storage and Utilization. ACS Appl. Mater. Interfaces 2019, 11, 46851–46863. [Google Scholar] [CrossRef]
- Chen, R.; Yao, R.; Xia, W.; Zou, R. Electro/Photo to Heat Conversion System Based on Polyurethane Embedded Graphite Foam. Appl. Energy 2015, 152, 183–188. [Google Scholar] [CrossRef]
- Ding, Y.; Lu, X.; Liu, S.; Wu, H.; Sheng, X.; Li, X.; Qu, J. Sandwich-Structured Multifunctional Composite Films with Excellent Electromagnetic Interference Shielding and Light/Electro/Magnetic-to-Thermal Conversion and Storage Capabilities. Compos. Part A Appl. Sci. Manuf. 2022, 163, 107178. [Google Scholar] [CrossRef]
- Nejat, P.; Jomehzadeh, F.; Taheri, M.M.; Gohari, M.; Majid, M.Z.A. A Global Review of Energy Consumption, CO2 Emissions and Policy in the Residential Sector (with an Overview of the Top Ten CO2 Emitting Countries). Renew. Sustain. Energy Rev. 2015, 43, 843–862. [Google Scholar] [CrossRef]
- Wu, S.; Li, T.; Tong, Z.; Chao, J.; Zhai, T.; Xu, J.; Yan, T.; Wu, M.; Xu, Z.; Bao, H.; et al. High-Performance Thermally Conductive Phase Change Composites by Large-Size Oriented Graphite Sheets for Scalable Thermal Energy Harvesting. Adv. Mater. 2019, 31, e1905099. [Google Scholar] [CrossRef] [PubMed]
- Gur, I.; Sawyer, K.; Prasher, R. Searching for a Better Thermal Battery. Science 2012, 335, 1454–1455. [Google Scholar] [CrossRef]
- Aneke, M.; Wang, M. Energy Storage Technologies and Real Life Applications—A State of the Art Review. Appl. Energy 2016, 179, 350–377. [Google Scholar] [CrossRef]
- Wang, L.; Guo, L.; Ren, J.; Kong, X. Using of Heat Thermal Storage of PCM and Solar Energy for Distributed Clean Building Heating: A Multi-Level Scale-up Research. Appl. Energy 2022, 321, 119345. [Google Scholar] [CrossRef]
- Mofijur, M.; Mahlia, T.; Silitonga, A.; Ong, H.; Silakhori, M.; Hasan, M.; Putra, N.; Rahman, S.M. Phase Change Materials (PCM) for Solar Energy Usages and Storage: An Overview. Energies 2019, 12, 3167. [Google Scholar] [CrossRef]
- Hussain, B.; Malik, H.W.; Hasnain, F.U.; Irfan, M. Phase Change Material for the Cooling of Solar Panels—An Experimental Study. In Proceedings of the ICAME 2023, Cartagena de Indias, Colombia, 3–8 September 2023; MDPI: Basel, Switzerland, 2023; p. 43. [Google Scholar]
- Xu, Z.; Kong, Q.; Qu, H.; Wang, C. Cooling Characteristics of Solar Photovoltaic Panels Based on Phase Change Materials. Case Stud. Therm. Eng. 2023, 41, 102667. [Google Scholar] [CrossRef]
- Gupta, B.; Bhalavi, J.; Sharma, S.; Bisen, A. Phase Change Materials in Solar Energy Applications: A Review. Mater. Today Proc. 2021, 46, 5550–5554. [Google Scholar] [CrossRef]
- Alghamdi, H.; Maduabuchi, C.; Okoli, K.; Alobaid, M.; Alghassab, M.; Alsafran, A.S.; Makki, E.; Alkhedher, M. Latest Advancements in Solar Photovoltaic-Thermoelectric Conversion Technologies: Thermal Energy Storage Using Phase Change Materials, Machine Learning, and 4E Analyses. Int. J. Energy Res. 2024, 2024, 1050785. [Google Scholar] [CrossRef]
- Awad, M.M.; Ahmed, O.K.; Ali, O.M.; Alwan, N.T.; Yaqoob, S.J.; Nayyar, A.; Abouhawwash, M.; Alrasheedi, A.F. Photovoltaic Thermal Collectors Integrated with Phase Change Materials: A Comprehensive Analysis. Electronics 2022, 11, 337. [Google Scholar] [CrossRef]
- Elfeky, K.E.; Li, X.; Ahmed, N.; Lu, L.; Wang, Q. Optimization of Thermal Performance in Thermocline Tank Thermal Energy Storage System with the Multilayered PCM(s) for CSP Tower Plants. Appl. Energy 2019, 243, 175–190. [Google Scholar] [CrossRef]
- Kalidasan, B.; Pandey, A.K.; Shahabuddin, S.; Samykano, M.; Thirugnanasambandam, M.; Saidur, R. Phase Change Materials Integrated Solar Thermal Energy Systems: Global Trends and Current Practices in Experimental Approaches. J. Energy Storage 2020, 27, 101118. [Google Scholar] [CrossRef]
- Miliozzi, A.; Dominici, F.; Candelori, M.; Veca, E.; Liberatore, R.; Nicolini, D.; Torre, L. Development and Characterization of Concrete/PCM/Diatomite Composites for Thermal Energy Storage in CSP/CST Applications. Energies 2021, 14, 4410. [Google Scholar] [CrossRef]
- Afzal, A.; Saleel, C.A.; Badruddin, I.A.; Khan, T.M.Y.; Kamangar, S.; Mallick, Z.; Samuel, O.D.; Soudagar, M.; Elahi, M. Human Thermal Comfort in Passenger Vehicles Using an Organic Phase Change Material– an Experimental Investigation, Neural Network Modelling, and Optimization. Build. Environ. 2020, 180, 107012. [Google Scholar] [CrossRef]
- Omara, A.A.M.; Abuelnour, A.A.A. Improving the Performance of Air Conditioning Systems by Using Phase Change Materials: A Review. Int. J. Energy Res. 2019, 43, 5175–5198. [Google Scholar] [CrossRef]
- Krishnamoorthi, S.; Prabhu, L.; Kuriakose, G.; Jose Lewis, D.; Harikrishnan, J. Air-Cooling System Based on Phase Change Materials for a Vehicle Cabin. Mater. Today Proc. 2021, 45, 5991–5996. [Google Scholar] [CrossRef]
- Ham, J.; Shin, Y.; Lee, M.; Cho, H. Investigation of Performance and Energy Cost of Automobile Air-Conditioner Using Alternative Refrigerants Based on Thermal Comfort Using Various Cooling Seats. Results Eng. 2023, 20, 101541. [Google Scholar] [CrossRef]
- Wazeer, A.; Das, A.; Abeykoon, C.; Sinha, A.; Karmakar, A. Phase Change Materials for Battery Thermal Management of Electric and Hybrid Vehicles: A Review. Energy Nexus 2022, 7, 100131. [Google Scholar] [CrossRef]
- Ortiz, Y.; Arévalo, P.; Peña, D.; Jurado, F. Recent Advances in Thermal Management Strategies for Lithium-Ion Batteries: A Comprehensive Review. Batteries 2024, 10, 83. [Google Scholar] [CrossRef]
- Cai, S.; Zhang, X.; Ji, J. Recent Advances in Phase Change Materials-Based Battery Thermal Management Systems for Electric Vehicles. J. Energy Storage 2023, 72, 108750. [Google Scholar] [CrossRef]
- Jaguemont, J.; Omar, N.; Van den Bossche, P.; Mierlo, J. Phase-Change Materials (PCM) for Automotive Applications: A Review. Appl. Therm. Eng. 2018, 132, 308–320. [Google Scholar] [CrossRef]
- Faraji, H.; Faraji, M.; El Alami, M.; Hariti, Y.; Arshad, A.; Hader, A.; Benkaddour, A. Cooling of Recent Microprocessors by the Fusion of Nano-Enhanced Phase Change Materials. Mater. Today Proc. 2020, 30, 865–869. [Google Scholar] [CrossRef]
- Wilke, S.; Schweitzer, B.; Khateeb, S.; Al-Hallaj, S. Preventing Thermal Runaway Propagation in Lithium Ion Battery Packs Using a Phase Change Composite Material: An Experimental Study. J. Power Sources 2017, 340, 51–59. [Google Scholar] [CrossRef]
- Schweitzer, B.; Wilke, S.; Khateeb, S.; Al-Hallaj, S. Experimental Validation of a 0-D Numerical Model for Phase Change Thermal Management Systems in Lithium-Ion Batteries. J. Power Sources 2015, 287, 211–219. [Google Scholar] [CrossRef]
- Lin, C.; Xu, S.; Chang, G.; Liu, J. Experiment and Simulation of a LiFePO4 Battery Pack with a Passive Thermal Management System Using Composite Phase Change Material and Graphite Sheets. J. Power Sources 2015, 275, 742–749. [Google Scholar] [CrossRef]
- Luo, M.; Song, J.; Ling, Z.; Zhang, Z.; Fang, X. Phase Change Material Coat for Battery Thermal Management with Integrated Rapid Heating and Cooling Functions from −40 °C to 50 °C. Mater. Today Energy 2021, 20, 100652. [Google Scholar] [CrossRef]
- Salaün, F. Phase Change Materials for Textile Application. In Textile Industry and Environment; IntechOpen: London, UK, 2019. [Google Scholar]
- Peng, Y.; Cui, Y. Thermal Management with Innovative Fibers and Textiles: Manipulating Heat Transport, Storage and Conversion. Natl. Sci. Rev. 2024, 11, nwae295. [Google Scholar] [CrossRef]
- Hossain, M.T.; Shahid, M.A.; Ali, M.Y.; Saha, S.; Jamal MS, I.; Habib, A. Fabrications, Classifications, and Environmental Impact of PCM-Incorporated Textiles: Current State and Future Outlook. ACS Omega 2023, 8, 45164–45176. [Google Scholar] [CrossRef]
- Park, T.H.; Yu, S.; Koo, M.; Kim, H.; Kim, E.H.; Park, J.-E.; Ok, B.; Kim, B.; Noh, S.H.; Park, C.; et al. Shape-Adaptable 2D Titanium Carbide (MXene) Heater. ACS Nano 2019, 13, 6835–6844. [Google Scholar] [CrossRef]
- Zhao, X.; Peng, L.-M.; Tang, C.-Y.; Pu, J.-H.; Zha, X.-J.; Ke, K.; Bao, R.-Y.; Yang, M.-B.; Yang, W. All-Weather-Available, Continuous Steam Generation Based on the Synergistic Photo-Thermal and Electro-Thermal Conversion by MXene-Based Aerogels. Mater. Horiz. 2020, 7, 855–865. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, H.; Liu, J.; Zhao, S.; Xie, X.; Liu, L.; Yang, R.; Koratkar, N.; Yu, Z. Multifunctional and Water-Resistant MXene-Decorated Polyester Textiles with Outstanding Electromagnetic Interference Shielding and Joule Heating Performances. Adv. Funct. Mater. 2019, 29. [Google Scholar] [CrossRef]
- Soares, N.; Costa, J.J.; Gaspar, A.R.; Santos, P. Review of Passive PCM Latent Heat Thermal Energy Storage Systems towards Buildings’ Energy Efficiency. Energy Build. 2013, 59, 82–103. [Google Scholar] [CrossRef]
- Jiao, K.; Lu, L.; Zhao, L.; Wang, G. Towards Passive Building Thermal Regulation: A State-of-the-Art Review on Recent Progress of PCM-Integrated Building Envelopes. Sustainability 2024, 16, 6482. [Google Scholar] [CrossRef]
- Elhamy, A.A.; Mokhtar, M. Phase Change Materials Integrated Into the Building Envelope to Improve Energy Efficiency and Thermal Comfort. Future Cities Environ. 2024, 10, 9. [Google Scholar] [CrossRef]
- Kalbasi, R.; Samali, B.; Afrand, M. Taking Benefits of Using PCMs in Buildings to Meet Energy Efficiency Criteria in Net Zero by 2050. Chemosphere 2023, 311, 137100. [Google Scholar] [CrossRef]
- Hwang, R.-L.; Chen, B.-L.; Chen, W.-A. Analysis of Incorporating a Phase Change Material in a Roof for the Thermal Management of School Buildings in Hot-Humid Climates. Buildings 2021, 11, 248. [Google Scholar] [CrossRef]
- Mokhberi, P.; Mokhberi, P.; Izadi, M.; Bagheri Nesaii, M.; Yaici, W.; Minelli, F. Thermal Regulation Enhancement in Multi-Story Office Buildings: Integrating Phase Change Materials into Inter-Floor Void Formers. Case Stud. Therm. Eng. 2024, 60, 104792. [Google Scholar] [CrossRef]
- Entrop, A.G.; Brouwers, H.J.H.; Reinders, A.H.M.E. Experimental Research on the Use of Micro-Encapsulated Phase Change Materials to Store Solar Energy in Concrete Floors and to Save Energy in Dutch Houses. Solar Energy 2011, 85, 1007–1020. [Google Scholar] [CrossRef]
- Kuznik, F.; David, D.; Johannes, K.; Roux, J.-J. A Review on Phase Change Materials Integrated in Building Walls. Renew. Sustain. Energy Rev. 2011, 15, 379–391. [Google Scholar] [CrossRef]
- Huang, J.; Xu, F.; Wang, Z.; Zhang, H. An Experimental Investigation on the Performance of a Water Storage Tank with Sodium Acetate Trihydrate. Energies 2023, 16, 777. [Google Scholar] [CrossRef]
- Li, J.; Wang, W.; Deng, Y.; Gao, L.; Bai, J.; Xu, L.; Chen, J.; Yuan, Z. Thermal Performance Analysis of Composite Phase Change Material of Myristic Acid-Expanded Graphite in Spherical Thermal Energy Storage Unit. Energies 2023, 16, 4527. [Google Scholar] [CrossRef]
- da Cunha, J.P.; Eames, P. Compact Latent Heat Storage Decarbonisation Potential for Domestic Hot Water and Space Heating Applications in the UK. Appl. Therm. Eng. 2018, 134, 396–406. [Google Scholar] [CrossRef]
- Gu, H.; Chen, Y.; Yao, X.; Huang, L.; Zou, D. Review on Heat Pump (HP) Coupled with Phase Change Material (PCM) for Thermal Energy Storage. Chem. Eng. J. 2023, 455, 140701. [Google Scholar] [CrossRef]
- Inkeri, E.; Tynjälä, T.; Nikku, M. Numerical Modeling of Latent Heat Thermal Energy Storage Integrated with Heat Pump for Domestic Hot Water Production. Appl. Therm. Eng. 2022, 214, 118819. [Google Scholar] [CrossRef]
- Kou, X.; Wang, R. Thermodynamic Analysis of Electric to Thermal Heating Pathways Coupled with Thermal Energy Storage. Energy 2023, 284, 129292. [Google Scholar] [CrossRef]


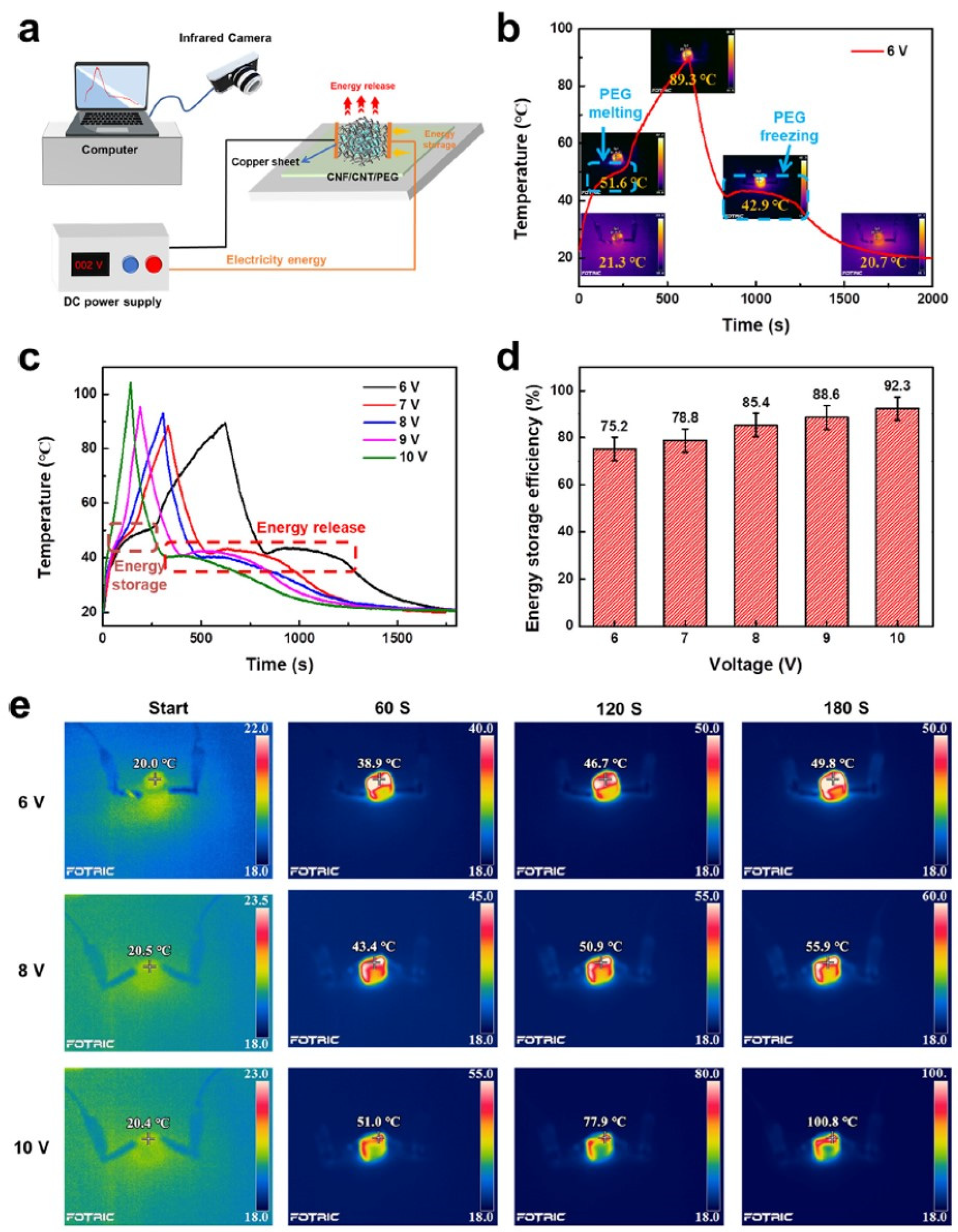
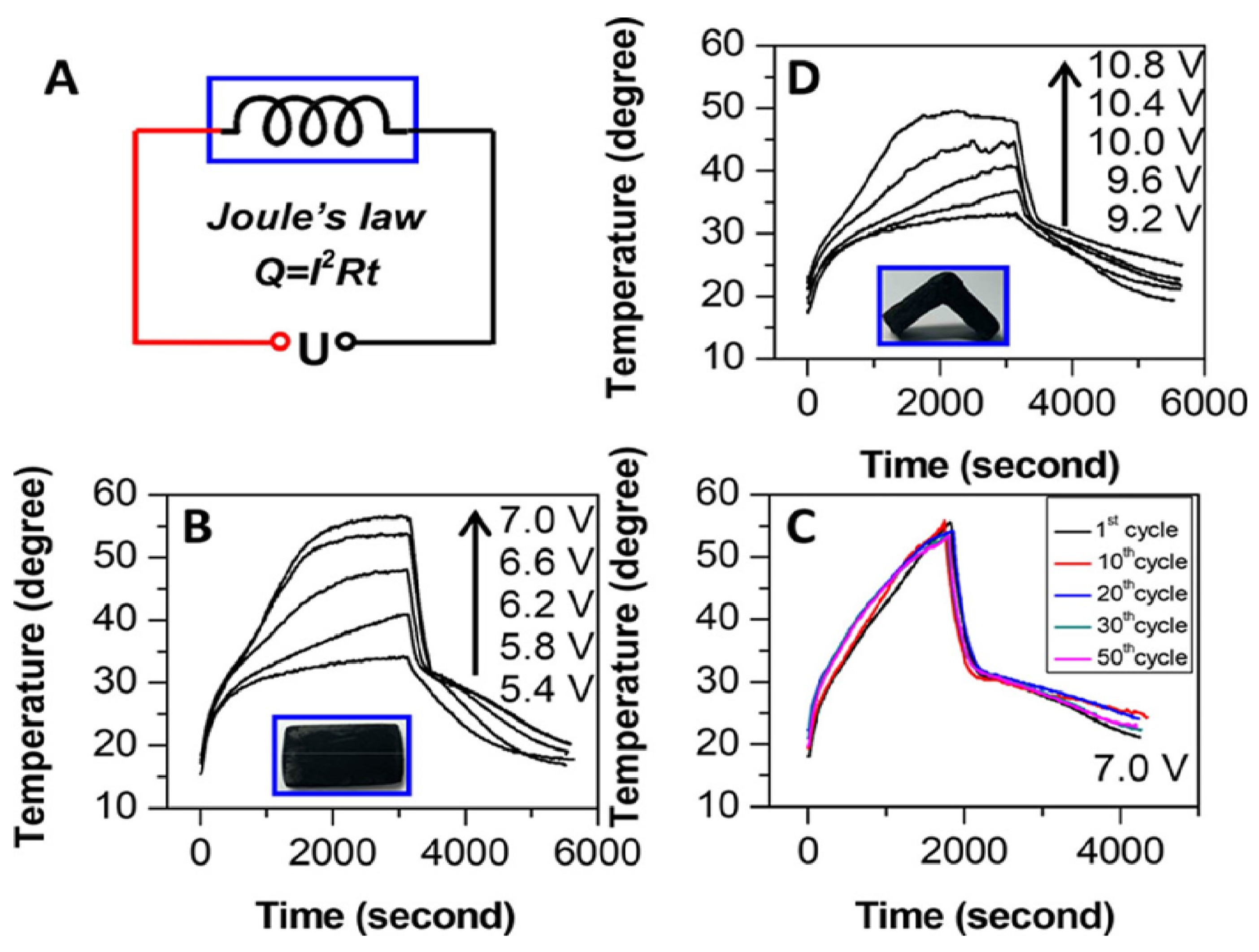
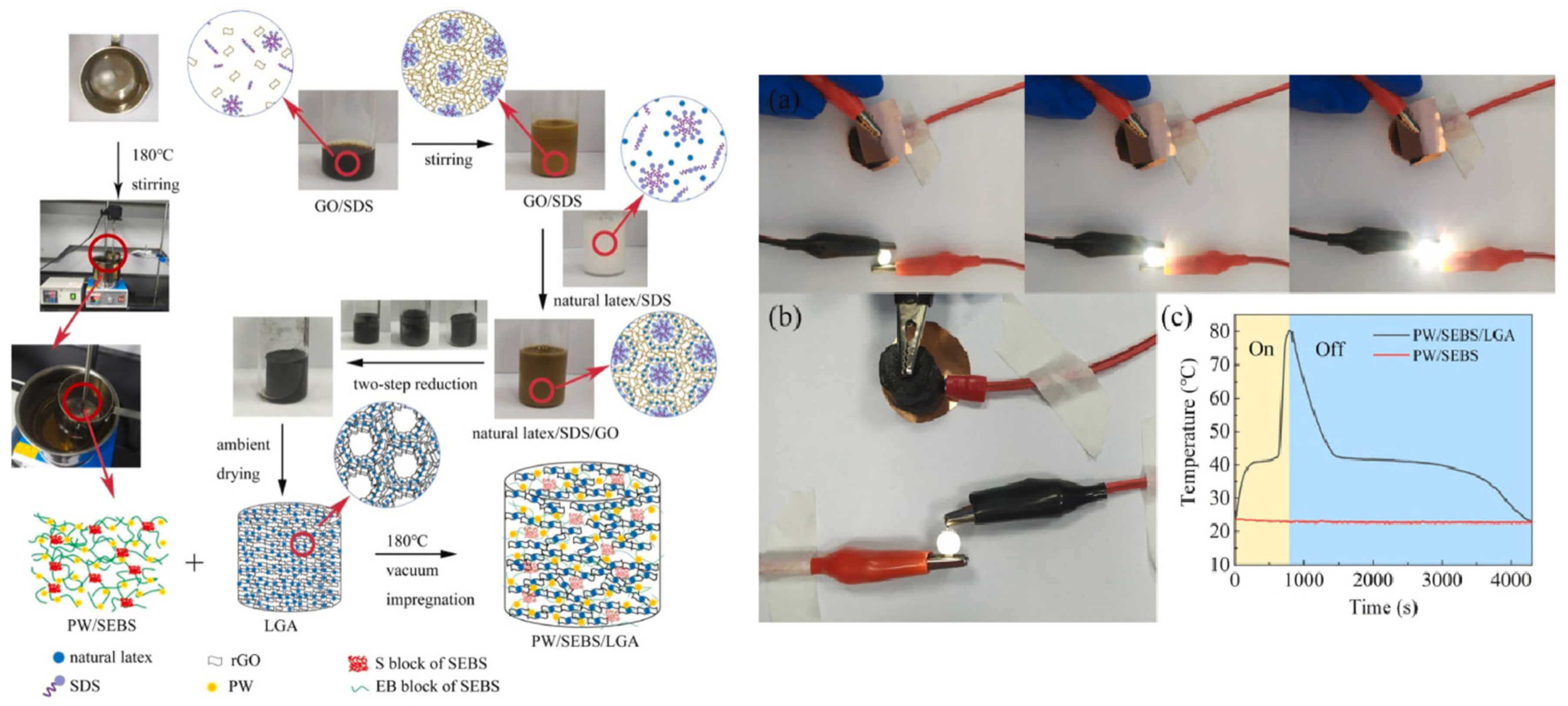
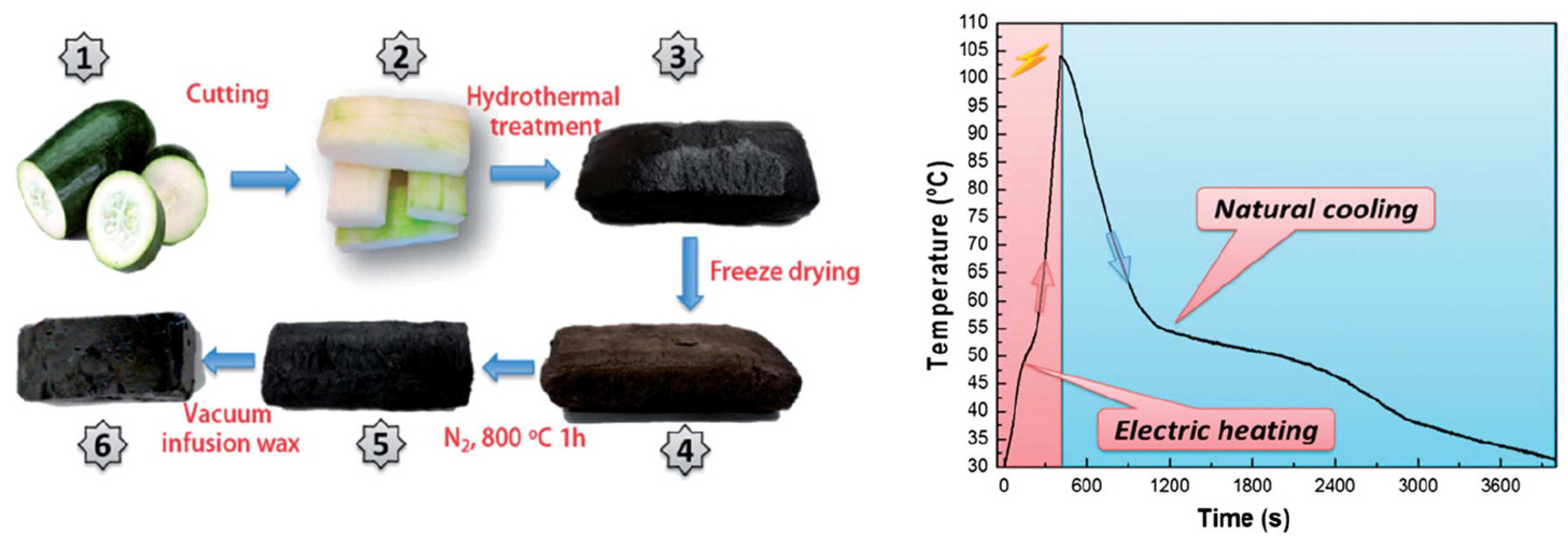
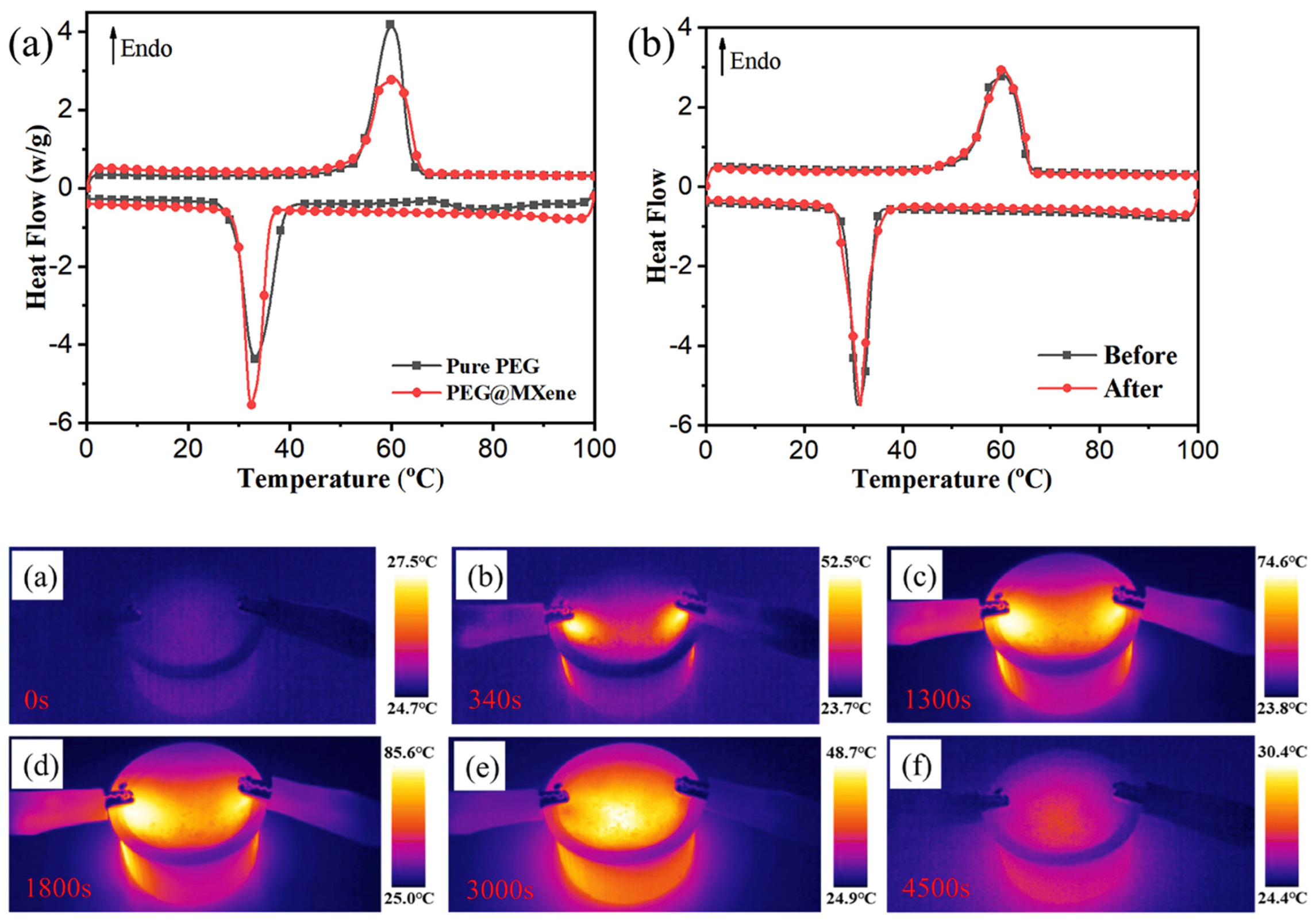

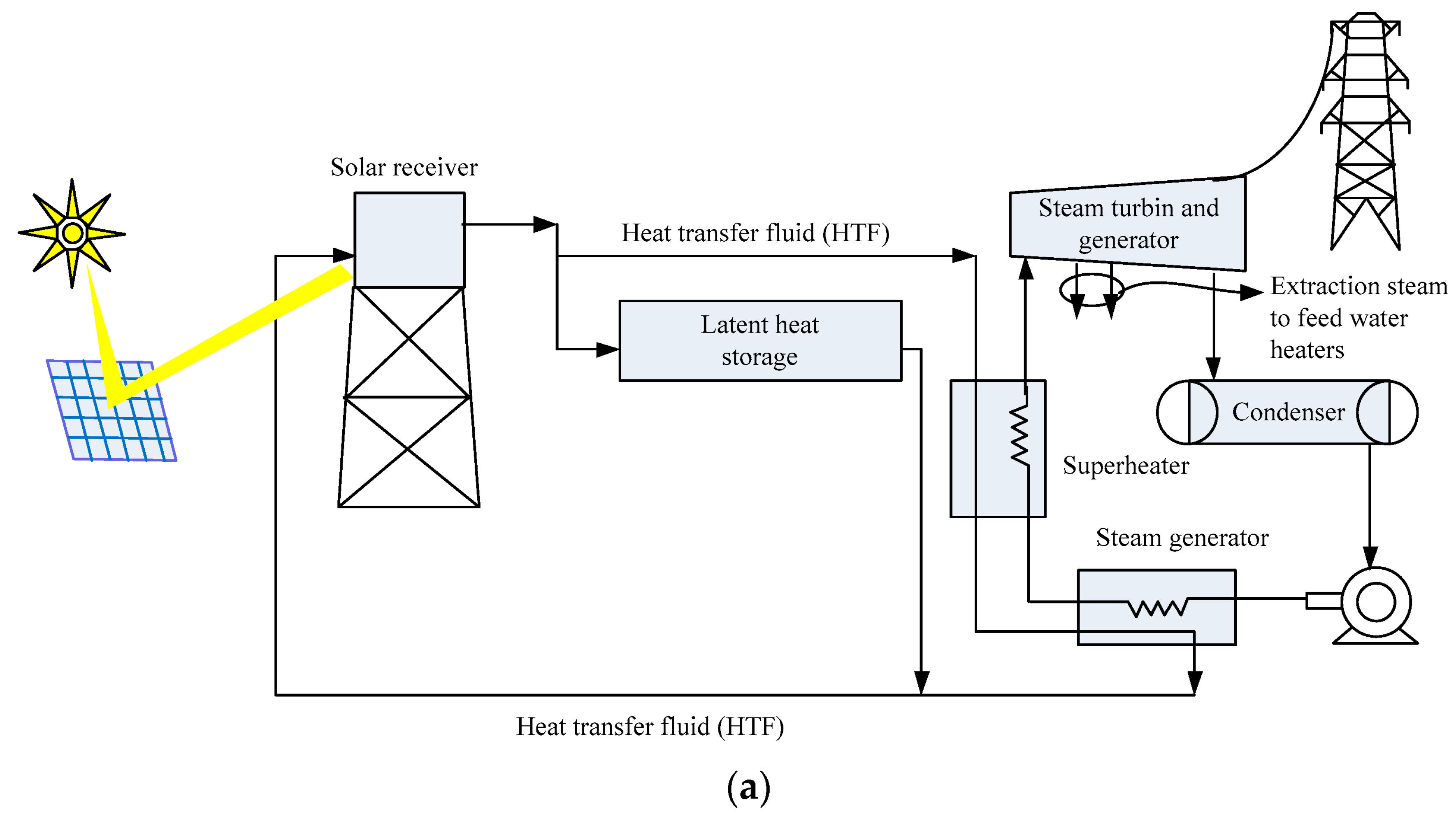
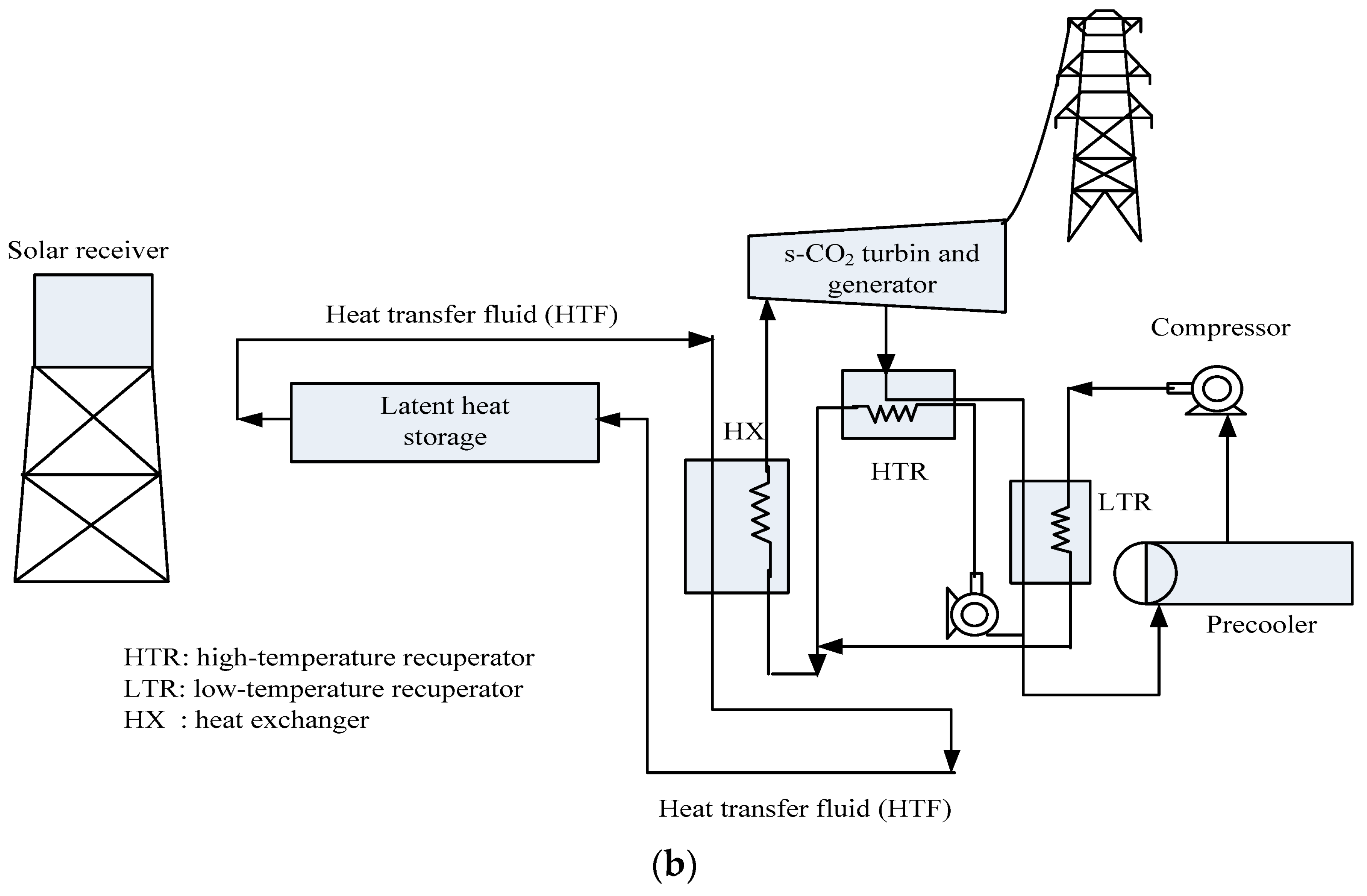
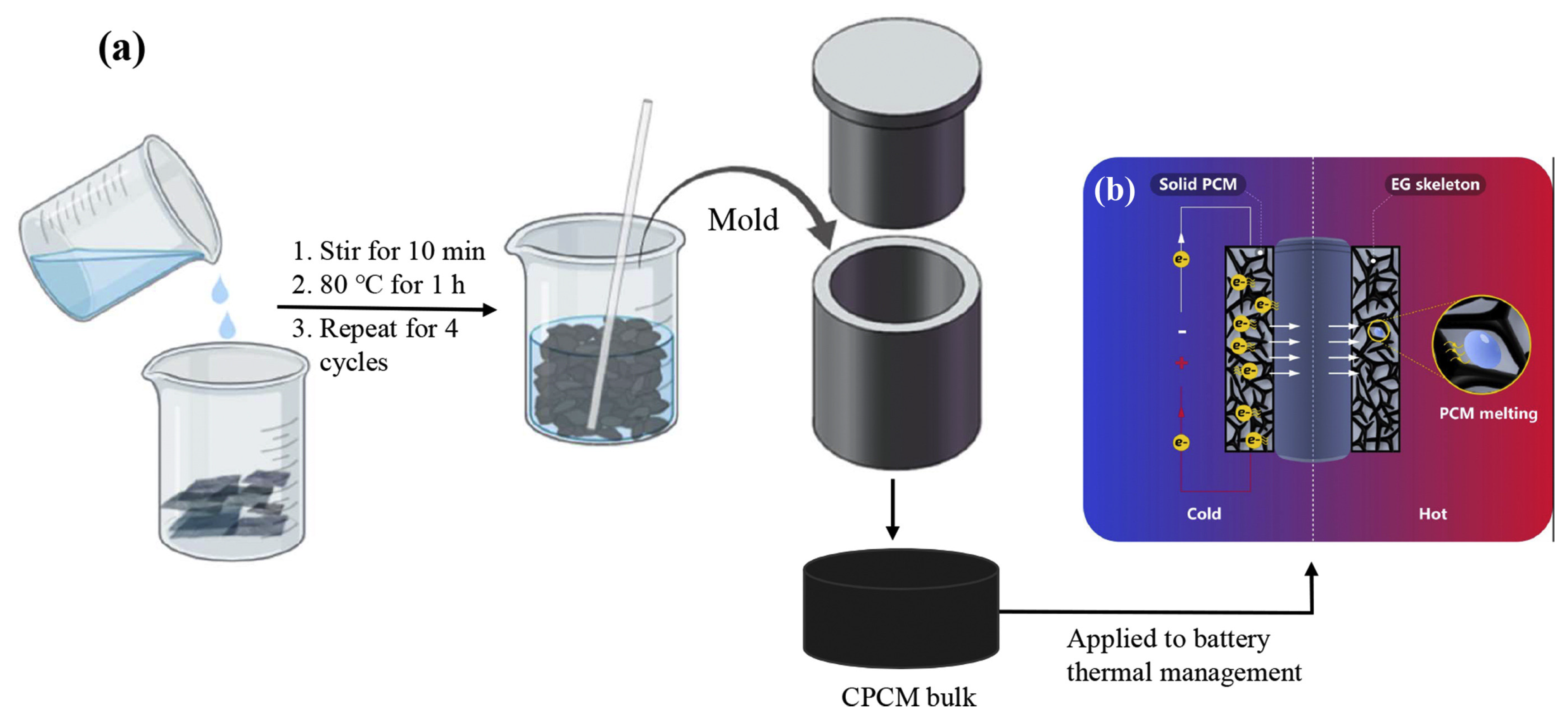
| Type of PCM | Modification Materials | Melting Enthalpy (J/g) | Thermal Conductivity (W/mK) | Input Voltage (V) | Conversion Efficiency (%) | Reference |
|---|---|---|---|---|---|---|
| Paraffin | CNT | 138.2 | 1.2 | 1.50 | 40.6 | [93] |
| Paraffin | CNT | 138.2 | 1.2 | 1.75 | 52.5 | [93] |
| N-eicosane | CNT | 217.3 | 1.89 | 1.3 | 49.0 | [98] |
| N-eicosane | CNT | 217.3 | 1.89 | 1.7 | 74.7 | [98] |
| PEG2000 | CNT | 89.8 | 0.91 | 1.5 | 58.3 | [95] |
| PEG2000 | CNT | 89.8 | 0.91 | 2.0 | 94.0 | [95] |
| Polyurethane | CNT | 132 | 2.40 | 1.5 | 49.0 | [96] |
| PEG4000 | CNT/CNF aerogel | 158.3 | - | 6 | 75.2 | [94] |
| PEG1000 | GO/CNT | 120.7 | 0.37 | 6.6 | 70.0 | [100] |
| PEG10000 | GO/BN | 164.1 | 1.06 | 7 | 87.9 | [105] |
| Paraffin | GO/GNPs | 161.7 | 1.46 | 2.9 | 62.5 | [113] |
| Pentaerythritol | GNPs | 225.3 | 26.63 | 0.22 | 11.33 | [111] |
| Pentaerythritol | GNPs | 222.8 | 26.63 | 0.34 | 92.73 | [111] |
| Methyl Stearate | EG | 147 | 3.6 | 1.7 | 72 | [110] |
| Paraffin | EG | 145.7 | 0.75 | 4.8 | 61.89 | [161] |
| N-eicosane | EG | 199.2 | 3.56 | 2.1 | 65.7 | [162] |
| PEG6000 | Graphene/cellulose | 178.9 | 0.26 | 20 | 66.1 | [163] |
| Paraffin | Graphene aerogel | 193.7 | 2.99 | 1.5 | 50.5 | [119] |
| Paraffin | Graphene aerogel | 193.7 | 2.99 | 3.0 | 85.4 | [119] |
| Paraffin/SEBS | Graphene aerogel | 212.4 | 0.41 | 8 | 73.5 | [114] |
| PEG4000 | Graphene aerogel | 92.1 | - | 10 | 67.2 | [124] |
| Polyurethane | Graphite foam | 60.3 | 10.86 | 1.8 | 85.0 | [108] |
| PEG8000/polyurethane | Graphite foam | 80.3 | 3.40 | 1.4 | 48.0 | [164] |
| PEG6000/polyurethane | Graphite foam | 76.1 | 3.40 | 1.4 | 88.0 | [164] |
| PEG4000/polyurethane | Graphite foam | 64.5 | 3.50 | 1.4 | 69.0 | [164] |
| PEG6000 | Graphite foam | 163.9 | - | 3.0 | 52.0 | [164] |
| Paraffin | Graphitic carbon foam | 120.2 | - | 3.0 | 56.0 | [109] |
| Paraffin | Winter melon-based carbon aerogel | 115.2 | - | 15 | 71.4 | [134] |
| Paraffin | Cotton-derived carbon scaffold | 182.22 | 0.42 | 3 | 81.1 | [135] |
| PEG 4000 | MXene | 131.2 | 2.05 | 7.2 | - | [154] |
| Stearic acid | MXene/GO | 168.25 | 1.21 | - | - | [160] |
| n-octadecane | MXene/Ag nanowire | 165.7 | 0.75 | 1.5 | - | [165] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Twaróg, R.; Szatkowski, P.; Pielichowska, K. Phase Change Materials in Electrothermal Conversion Systems: A Review. Energies 2025, 18, 569. https://doi.org/10.3390/en18030569
Twaróg R, Szatkowski P, Pielichowska K. Phase Change Materials in Electrothermal Conversion Systems: A Review. Energies. 2025; 18(3):569. https://doi.org/10.3390/en18030569
Chicago/Turabian StyleTwaróg, Rafał, Piotr Szatkowski, and Kinga Pielichowska. 2025. "Phase Change Materials in Electrothermal Conversion Systems: A Review" Energies 18, no. 3: 569. https://doi.org/10.3390/en18030569
APA StyleTwaróg, R., Szatkowski, P., & Pielichowska, K. (2025). Phase Change Materials in Electrothermal Conversion Systems: A Review. Energies, 18(3), 569. https://doi.org/10.3390/en18030569








