Sustainable Valorization of Forest Waste Hydrolysis Residues to Solid Biofuel: Insights into Conversion Mechanisms and Fuel Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pretreatment and Enzymatic Hydrolysis
2.3. Composition Analysis of LRR
2.4. Hydrothermal Carbonization
2.5. HTC Product Characterization
2.5.1. Mass Yield, Energy Density, and Energy Yield of Hydrochars
2.5.2. Elemental Analysis
2.5.3. Higher Heating Value
2.5.4. Fourier Transform Infrared Spectroscopy
2.5.5. Scanning Electron Microscopy
2.5.6. Thermogravimetric Analysis
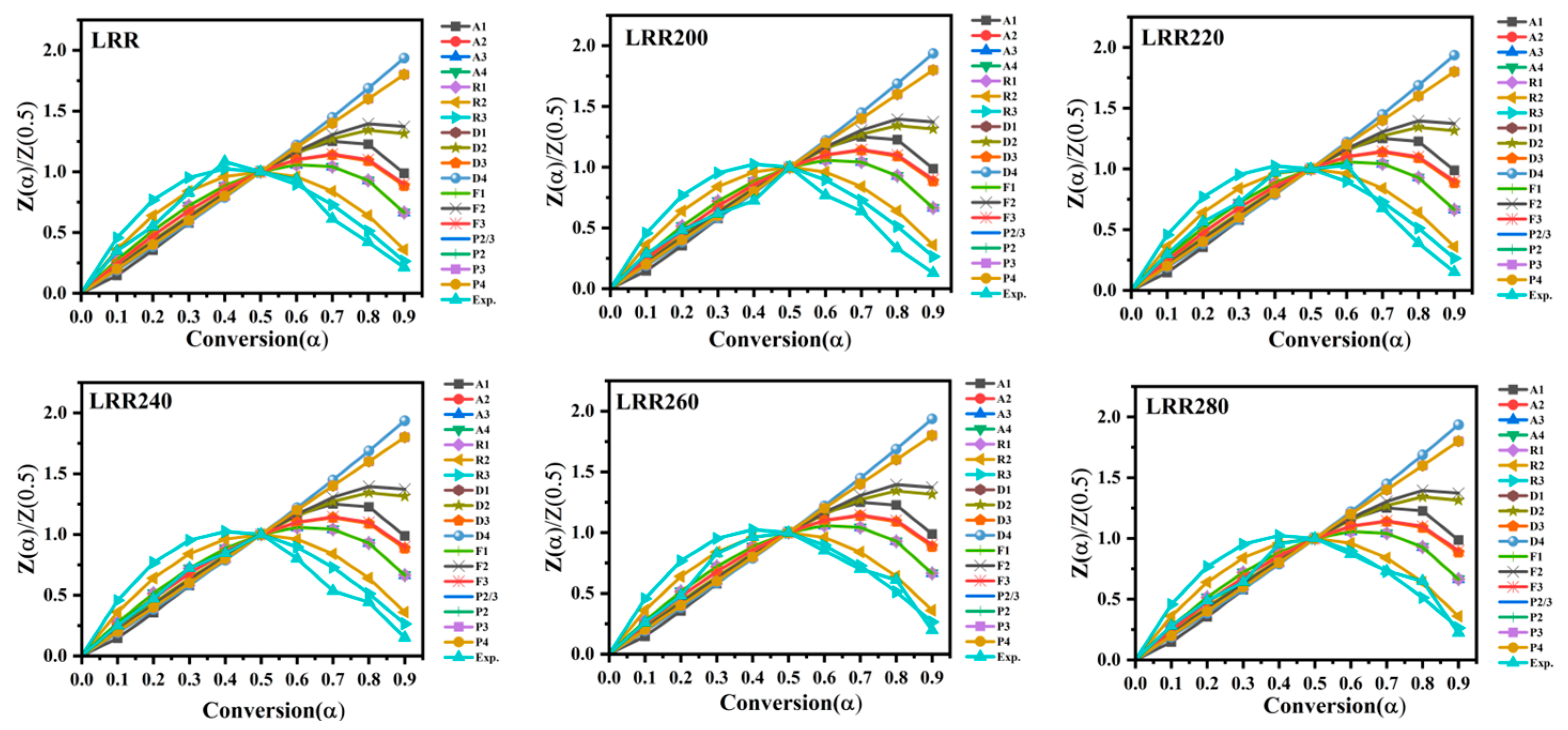
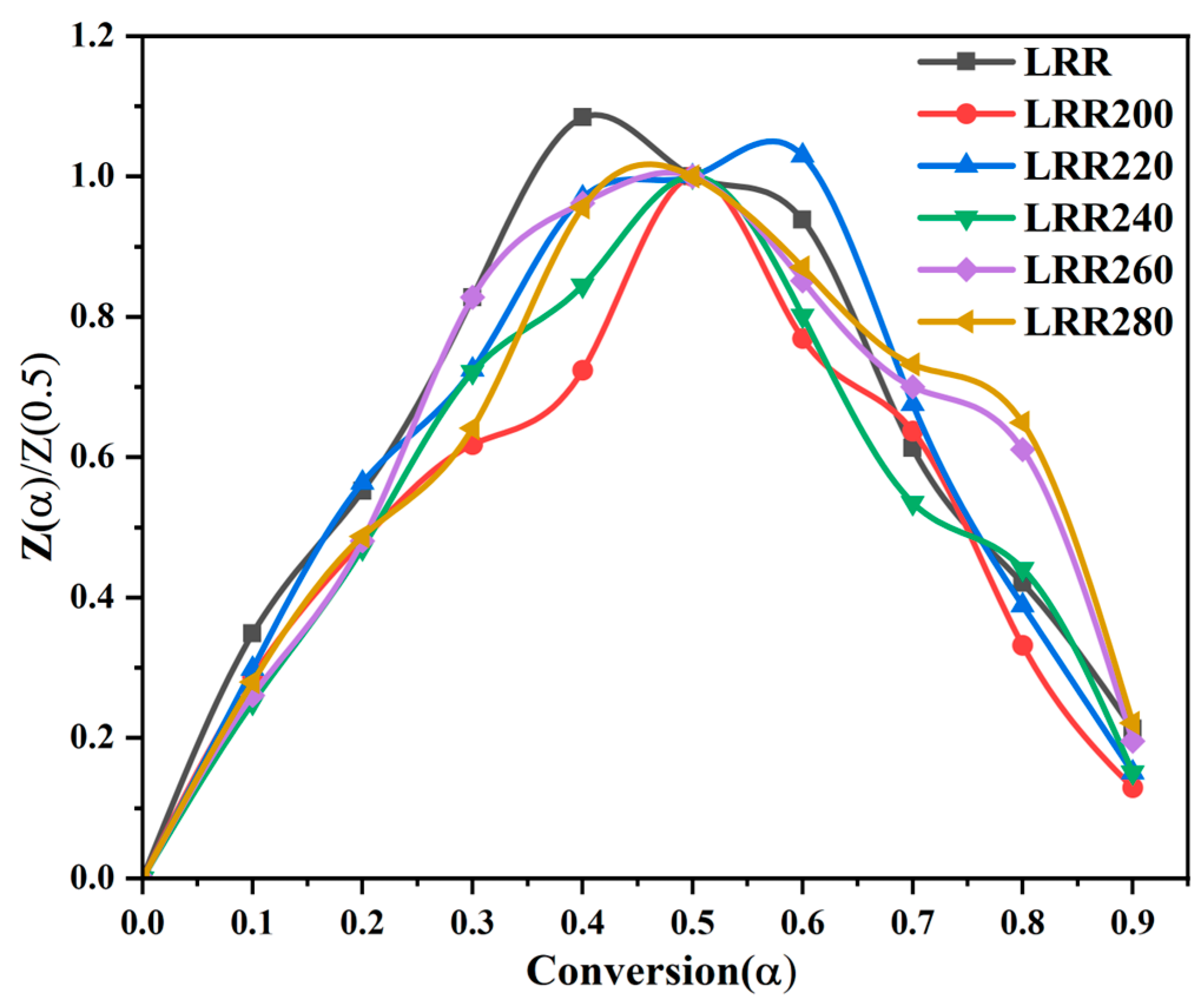
2.6. Master Plot
2.7. Statistical Analysis
3. Results and Discussion
3.1. Composition of LRR and Hydrolysate
3.2. The Appearances of Hydrochars
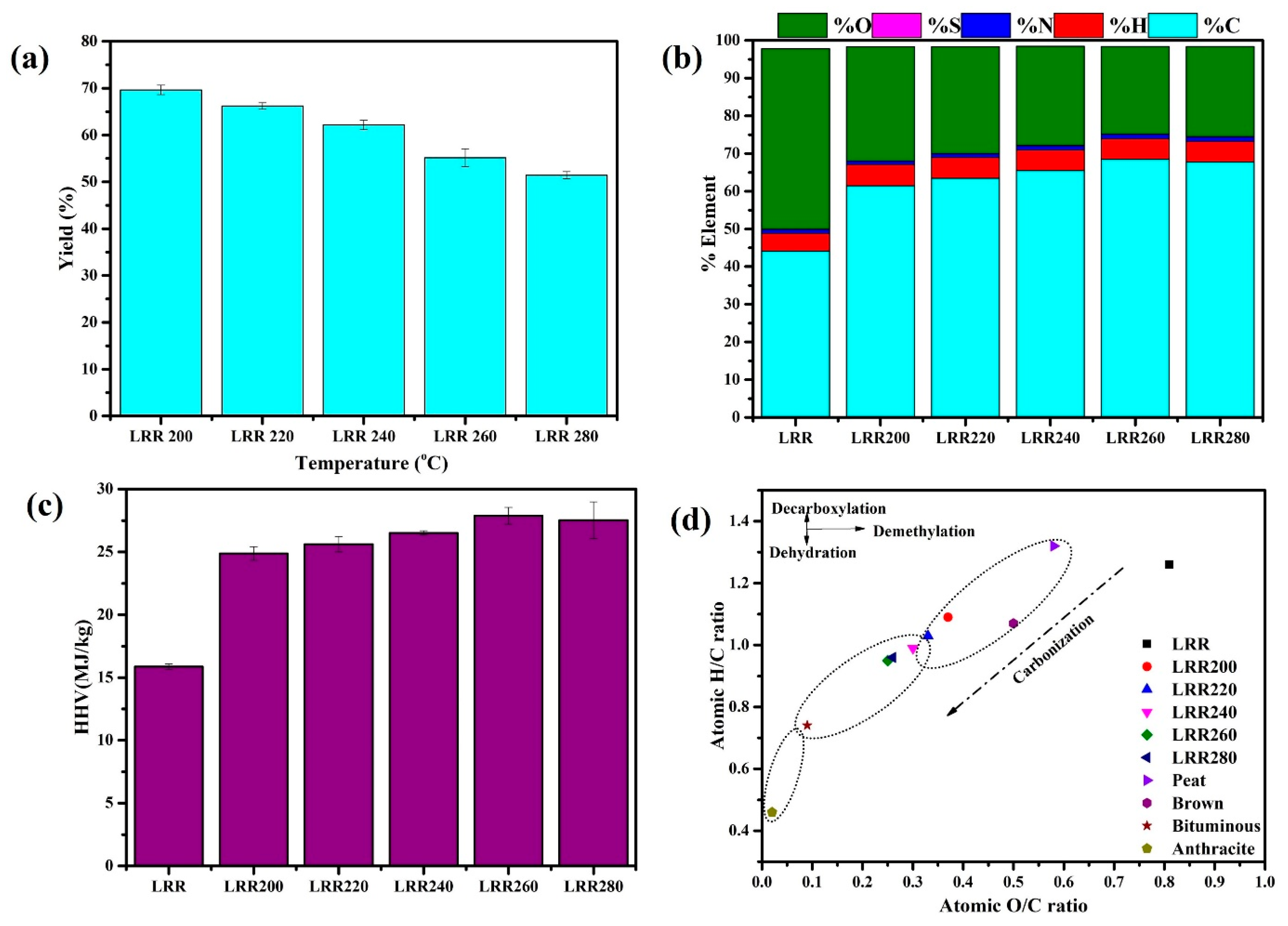
3.3. Hydrochar Yield, Energy Densification, and Energy Yield
3.4. Fuel Properties
3.4.1. High Heating Value
3.4.2. Ultimate Analysis
3.4.3. Van Krevelen Plot
3.4.4. Proximate Analysis
3.5. Effect of Hydrothermal Carbonization on the Microstructure of LRR
3.6. Surface Morphology and Structural Modifications from SEM Analysis
3.7. Textural Properties and BET Surface Area Analysis
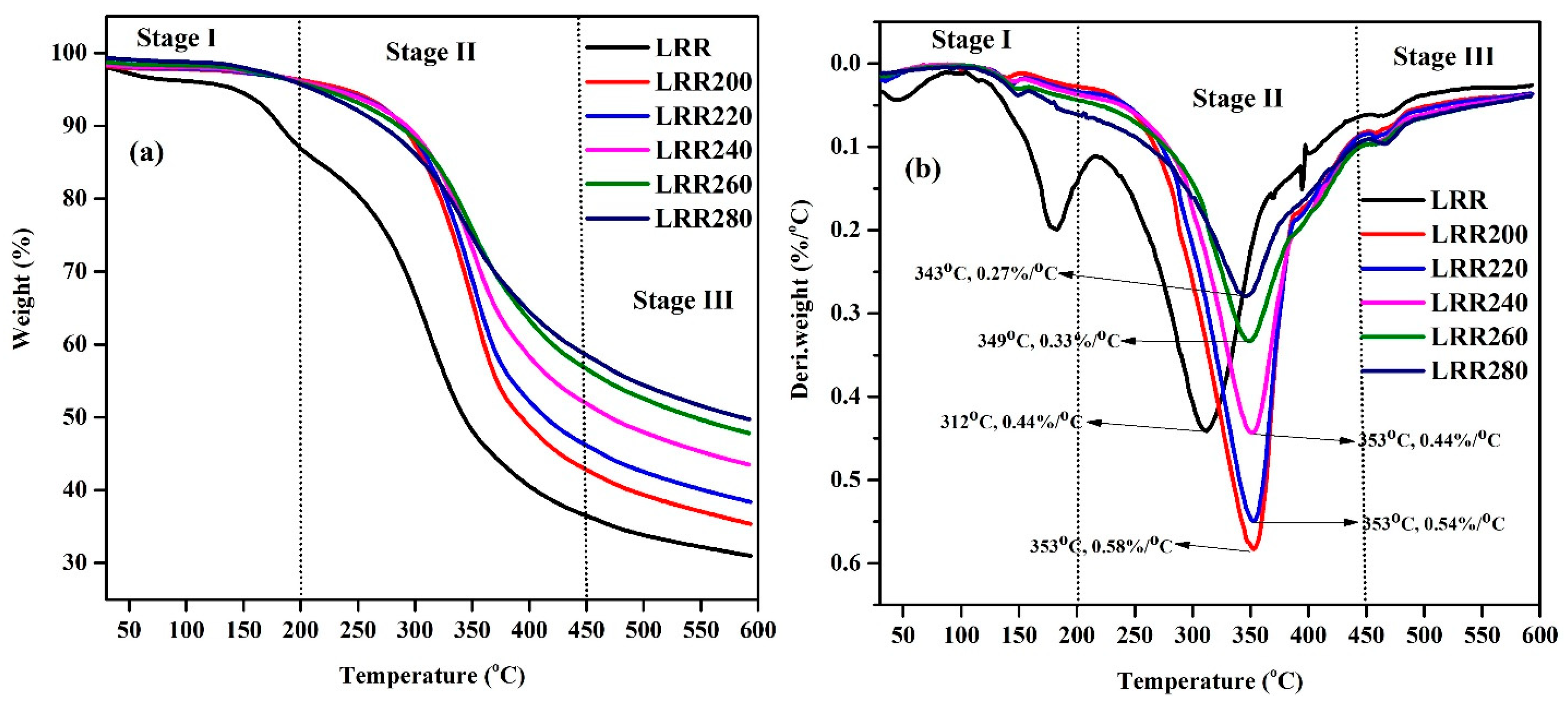
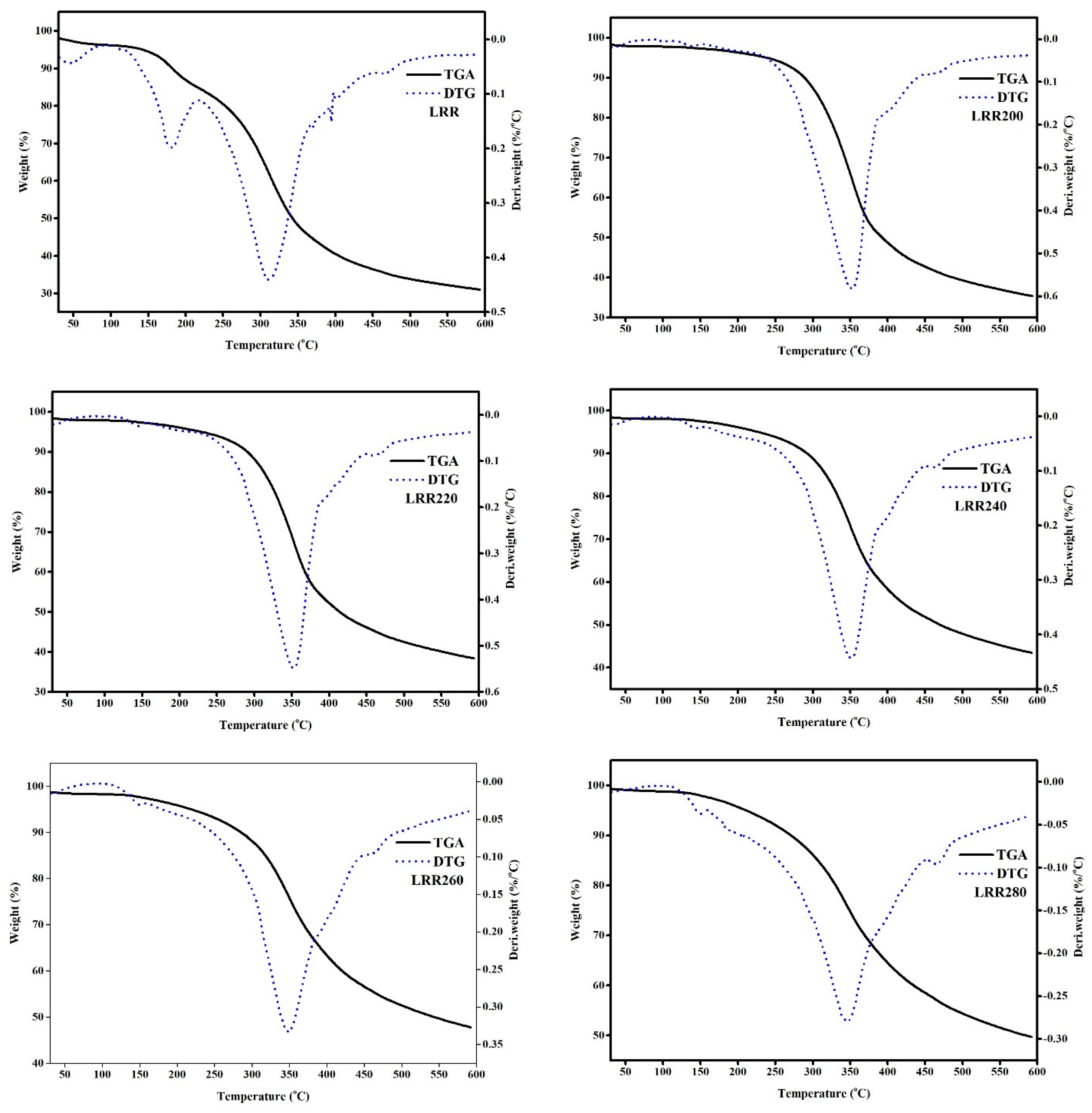
3.8. TGA and DTG Analysis
3.9. Deconvolution
3.10. Reaction Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luo, J.; Lin, Z.; Xie, T.; Chen, J.; Zhang, Y.; Li, P. Valorization of Kitchen Waste Hydrolysis Residue for Green Solid Fuel Application: Conversion Mechanism and Fuel Characteristics. Fuel 2024, 375, 132556. [Google Scholar] [CrossRef]
- Sekoai, P.T.; Ouma, C.N.M.; du Preez, S.P.; Modisha, P.; Engelbrecht, N.; Bessarabov, D.G.; Ghimire, A. Application of Nanoparticles in Biofuels: An Overview. Fuel 2019, 237, 380–397. [Google Scholar] [CrossRef]
- Liu, H.; Li, J. Environmental Consequences of Fuel Price Shocks in China. China Econ. Rev. 2023, 80, 102018. [Google Scholar] [CrossRef]
- Xu, Z.; Qi, R.; Xiong, M.; Zhang, D.; Gu, H.; Chen, W. Conversion of Cotton Textile Waste to Clean Solid Fuel via Surfactant-Assisted Hydrothermal Carbonization: Mechanisms and Combustion Behaviors. Bioresour. Technol. 2021, 321, 124450. [Google Scholar] [CrossRef]
- Huang, W.; Zheng, D.; Chen, X.; Shi, L.; Dai, X.; Chen, Y.; Jing, X. Standard Thermodynamic Properties for the Energy Grade Evaluation of Fossil Fuels and Renewable Fuels. Renew. Energy 2020, 147, 2160–2170. [Google Scholar] [CrossRef]
- Hossain, M.S.; Therasme, O.; Volk, T.A.; Kumar, V.; Kumar, D. Optimization of Combined Hydrothermal and Mechanical Refining Pretreatment of Forest Residue Biomass for Maximum Sugar Release during Enzymatic Hydrolysis. Energies 2024, 17, 4929. [Google Scholar] [CrossRef]
- Liang, W.; Wang, G.; Xu, R.; Ning, X.; Zhang, J.; Guo, X.; Ye, L.; Li, J.; Jiang, C.; Wang, P.; et al. Hydrothermal Carbonization of Forest Waste into Solid Fuel: Mechanism and Combustion Behavior. Energy 2022, 246, 123343. [Google Scholar] [CrossRef]
- Stafford, W.; De Lange, W.; Nahman, A.; Chunilall, V.; Lekha, P.; Andrew, J.; Johakimu, J.; Sithole, B.; Trotter, D. Forestry Biorefineries. Renew. Energy 2020, 154, 461–475. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, J. Hydrolysis of Lignocellulosic Materials for Ethanol Production: A Review. Bioresource 2002, 83, 1–11. [Google Scholar] [CrossRef]
- Jørgensen, H.; Kristensen, J.B.; Felby, C. Enzymatic Conversion of Lignocellulose into Fermentable Sugars: Challenges and Opportunities. Biofuels Bioprod. Biorefin. 2007, 1, 119–134. [Google Scholar] [CrossRef]
- Hendriks, A.T.W.M.; Zeeman, G. Pretreatments to Enhance the Digestibility of Lignocellulosic Biomass. Bioresour. Technol. 2009, 100, 10–18. [Google Scholar] [CrossRef]
- Vázquez, V.; Giorgi, V.; Bonfiglio, F.; Menéndez, P.; Gioia, L.; Ovsejevi, K. Lignocellulosic Residues from Bioethanol Production: A Novel Source of Biopolymers for Laccase Immobilization. RSC Adv. 2023, 13, 13463–13471. [Google Scholar] [CrossRef]
- Zhuang, X.; Zhan, H.; Huang, Y.; Song, Y.; Yin, X.; Wu, C. Conversion of Industrial Biowastes to Clean Solid Fuels via Hydrothermal Carbonization (HTC): Upgrading Mechanism in Relation to Coalification Process and Combustion Behavior. Bioresour. Technol. 2018, 267, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Li, B.; Wen, H.; Zhang, X.; Wang, L.; Ye, J. Co-Hydrothermal Treatment of Fallen Leaves with Iron Sludge to Prepare Magnetic Iron Product and Solid Fuel. Bioresour. Technol. 2018, 257, 229–237. [Google Scholar] [CrossRef]
- Pstrowska, K.; Łużny, R.T.; Fałtynowicz, H.; Jaroszewska, K.; Postawa, K.; Pyshyev, S.; Witek-Krowiak, A. Unlocking Sustainability: A Comprehensive Review of Up-Recycling Biomass Waste into Biochar for Environmental Solutions. Chem. Chem. Technol. 2024, 18, 211–231. [Google Scholar] [CrossRef]
- Pyshyev, S.; Miroshnichenko, D.; Malik, I.; Bautista Contreras, A.; Hassan, N.; Abd Elrasoul, A. State of the Art in the Production of Charcoal: A Review. Chem. Chem. Technol. 2021, 15, 61–73. [Google Scholar] [CrossRef]
- Liu, F.; Yu, R.; Guo, M. Hydrothermal Carbonization of Forestry Residues: Influence of Reaction Temperature on Holocellulose-Derived Hydrochar Properties. J. Mater. Sci. 2017, 52, 1736–1746. [Google Scholar] [CrossRef]
- Lee, J.; Lee, K.; Sohn, D.; Kim, Y.M.; Park, K.Y. Hydrothermal Carbonization of Lipid Extracted Algae for Hydrochar Production and Feasibility of Using Hydrochar as a Solid Fuel. Energy 2018, 153, 913–920. [Google Scholar] [CrossRef]
- Funke, A.; Ziegler, F. Hydrothermal Carbonization of Biomass: A Summary and Discussion of Chemical Mechanisms for Process Engineering. Biofuels Bioprod. Biorefin. 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Kannan, S.; Gariepy, Y.; Raghavan, V. Optimization of Enzyme Hydrolysis of Seafood Waste for Microwave Hydrothermal Carbonization. Energy Fuels 2015, 29, 8006–8016. [Google Scholar] [CrossRef]
- Phang, F.J.F.; Tiong, S.I.X.; Wang, Y.S.; Soh, M.; Chew, J.J.; Khaerudini, D.S.; Thangalazhy-Gopakumar, S.; How, B.S.; Loh, S.K.; Yusup, S.; et al. Hydrochars Derived via Wet Torrefaction of Empty Fruit Bunches: Effect of Temperature and Time, Comparison to Oil Palm Trunks Counterpart, and Their Pyrolysis Behavior. J. Anal. Appl. Pyrolysis 2024, 179, 106441. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; NREL/TP-510-42618; Laboratory Analytical Procedure (LAP), National Renewable Energy Laboratory: Golden, CO, USA, 2008. Available online: https://www.nrel.gov/docs/gen/fy13/42618.pdf (accessed on 21 October 2025).
- Kojić, M.M.; Petrović, J.T.; Petrović, M.S.; Stanković, S.M.; Porobić, S.J.; Marinović-Cincović, M.T.; Mihajlović, M.L. Hydrothermal Carbonization of Spent Mushroom Substrate: Physicochemical Characterization, Combustion Behavior, Kinetic and Thermodynamic Study. J. Anal. Appl. Pyrolysis 2021, 155, 105028. [Google Scholar] [CrossRef]
- Yu, Y.; Lei, Z.; Yang, X.; Yang, X.; Huang, W.; Shimizu, K.; Zhang, Z. Hydrothermal Carbonization of Anaerobic Granular Sludge: Effect of Process Temperature on Nutrients Availability and Energy Gain from Produced Hydrochar. Appl. Energy 2018, 229, 88–95. [Google Scholar] [CrossRef]
- ASTM D1762-84; Standard Test Method for Chemical Analysis of Wood Charcoal. ASTM International: West Conshohocken, PA, USA, 2021.
- Kambo, H.S.; Dutta, A. Strength, Storage, and Combustion Characteristics of Densified Lignocellulosic Biomass Produced via Torrefaction and Hydrothermal Carbonization. Appl. Energy 2014, 135, 182–191. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Q.; Wang, B.; Yang, G.; Lucia, L.A.; Chen, J. Hydrothermal Carbonization of Corncob Residues for Hydrochar Production. Energy Fuels 2015, 29, 872–876. [Google Scholar] [CrossRef]
- Criado, J.M.; Málek, J.; Ortega, A. Applicability of the Master Plots in Kinetic Analysis of Non-Isothermal Data. Thermochim. Acta 1989, 147, 377–385. [Google Scholar] [CrossRef]
- Mishra, R.K.; Mohanty, K. Thermocatalytic Conversion of Non-Edible Neem Seeds towards Clean Fuel and Chemicals. J. Anal. Appl. Pyrolysis 2018, 134, 83–92. [Google Scholar] [CrossRef]
- Kumar, M.; Upadhyay, S.N.; Mishra, P.K. Effect of Montmorillonite Clay on Pyrolysis of Paper Mill Waste. Bioresour. Technol. 2020, 307, 123161. [Google Scholar] [CrossRef]
- He, Q.; Gong, Y.; Ding, L.; Guo, Q.; Yoshikawa, K.; Yu, G. Reactivity Prediction and Mechanism Analysis of Raw and Demineralized Coal Char Gasification. Energy 2021, 229, 120724. [Google Scholar] [CrossRef]
- Eom, I.Y.; Yu, J.H. Structural Characterization of the Solid Residue Produced by Hydrothermal Treatment of Sunflower Stalks and Subsequent Enzymatic Hydrolysis. J. Ind. Eng. Chem. 2015, 23, 72–78. [Google Scholar] [CrossRef]
- Ul Saqib, N.; Sarmah, A.K.; Baroutian, S. Effect of Temperature on the Fuel Properties of Food Waste and Coal Blend Treated under Co-Hydrothermal Carbonization. Waste Manag. 2019, 89, 236–246. [Google Scholar] [CrossRef]
- Reza, M.T.; Uddin, M.H.; Lynam, J.G.; Hoekman, S.K.; Coronella, C.J. Hydrothermal Carbonization of Loblolly Pine: Reaction Chemistry and Water Balance. Biomass Convers. Biorefinery 2014, 4, 311–321. [Google Scholar] [CrossRef]
- Pińkowska, H.; Wolak, P.; Złocińska, A. Hydrothermal Decomposition of Xylan as a Model Substance for Plant Biomass Waste—Hydrothermolysis in Subcritical Water. Biomass Bioenergy 2011, 35, 3902–3912. [Google Scholar] [CrossRef]
- Fan, F.; Yang, Z.; Li, H.; Shi, Z.; Kan, H. Preparation and Properties of Hydrochars from Macadamia Nut Shell via Hydrothermal Carbonization. R. Soc. Open Sci. 2018, 5, 181126. [Google Scholar] [CrossRef]
- Lin, Y.; Ma, X.; Peng, X.; Hu, S.; Yu, Z.; Fang, S. Effect of Hydrothermal Carbonization Temperature on Combustion Behavior of Hydrochar Fuel from Paper Sludge. Appl. Therm. Eng. 2015, 91, 574–582. [Google Scholar] [CrossRef]
- Li, H.; Wang, S.; Yuan, X.; Xi, Y.; Huang, Z.; Tan, M.; Li, C. The Effects of Temperature and Color Value on Hydrochars’ Properties in Hydrothermal Carbonization. Bioresour. Technol. 2018, 249, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Quek, A.; Kent Hoekman, S.; Balasubramanian, R. Production of Solid Biochar Fuel from Waste Biomass by Hydrothermal Carbonization. Fuel 2013, 103, 943–949. [Google Scholar] [CrossRef]
- Reza, M.T.; Coronella, C.; Holtman, K.M.; Franqui-Villanueva, D.; Poulson, S.R. Hydrothermal Carbonization of Autoclaved Municipal Solid Waste Pulp and Anaerobically Treated Pulp Digestate. ACS Sustain. Chem. Eng. 2016, 4, 3649–3658. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Broch, A.; Robbins, C. Hydrothermal Carbonization (HTC) of Lignocellulosic Biomass. Energy Fuels 2011, 25, 1802–1810. [Google Scholar] [CrossRef]
- Yang, W.; Wang, H.; Zhang, M.; Zhu, J.; Zhou, J.; Wu, S. Fuel Properties and Combustion Kinetics of Hydrochar Prepared by Hydrothermal Carbonization of Bamboo. Bioresour. Technol. 2016, 205, 199–204. [Google Scholar] [CrossRef]
- Wang, Q.; Sarkar, J. Pyrolysis Behaviors of Waste Coconut Shell and Husk Biomasses. Int. J. Energy Prod. Mgmt. 2018, 3, 34–43. [Google Scholar] [CrossRef]
- Wang, Z.; Bui, Q.; Zhang, B.; Pham, T.L.H. Biomass Energy Production and Its Impacts on the Ecological Footprint: An Investigation of the G7 Countries. Sci. Total Environ. 2020, 743, 140741. [Google Scholar] [CrossRef]
- He, Q.; Cheng, C.; Raheem, A.; Ding, L.; Shiung Lam, S.; Yu, G. Effect of Hydrothermal Carbonization on Woody Biomass: From Structure to Reactivity. Fuel 2022, 330, 125586. [Google Scholar] [CrossRef]
- Santos Santana, M.; Pereira Alves, R.; da Silva Borges, W.M.; Francisquini, E.; Guerreiro, M.C. Hydrochar Production from Defective Coffee Beans by Hydrothermal Carbonization. Bioresour. Technol. 2020, 300, 122653. [Google Scholar] [CrossRef]
- Thiru, S.; Kola, R.; Thimmaraju, M.K.; Dhanalakshmi, C.S.; Sharma, V.; Sakthi, P.; Maguluri, L.P.; Ranganathan, L.; Lalvani, J.I.J.R. An Analytical Characterization Study on Biofuel Obtained from Pyrolysis of Madhuca longifolia Residues. Sci. Rep. 2024, 14, 14745. [Google Scholar] [CrossRef] [PubMed]
- Atallah, E.; Zeaiter, J.; Ahmad, M.N.; Leahy, J.J.; Kwapinski, W. Hydrothermal Carbonization of Spent Mushroom Compost Waste Compared against Torrefaction and Pyrolysis. Fuel Process. Technol. 2021, 216, 106795. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, J.; Zhang, M.; Wang, D. Utilizing Hydrolysis Residue from Bioethanol Production as an Additive for Solid Fuel Pellets. Fuel 2023, 348, 128582. [Google Scholar] [CrossRef]
- Ma, L.; Goldfarb, J.L.; Song, J.; Chang, C.; Ma, Q. Enhancing Cleaner Biomass-Coal Co-Combustion by Pretreatment of Wheat Straw via Washing versus Hydrothermal Carbonization. J. Clean. Prod. 2022, 366, 132991. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, J.; Lee, J.Y.; Mao, X.; Ye, L.; Xu, W.; Ning, X.; Zhang, N.; Teng, H.; Wang, C. Hydrothermal Carbonization of Maize Straw for Hydrochar Production and Its Injection for Blast Furnace. Appl. Energy 2020, 266, 114818. [Google Scholar] [CrossRef]
- Patil, Y.; Ku, X. Pyrolysis Kinetics and Thermodynamic Behavior of Pseudo Components of Raw and Torrefied Maple Wood. Energy Sources Part A Recovery Util. Environ. Eff. 2024, 46, 462–474. [Google Scholar] [CrossRef]
- Soh, M.; Chew, J.J.; Liu, S.; Sunarso, J. Comprehensive Kinetic Study on the Pyrolysis and Combustion Behaviours of Five Oil Palm Biomass by Thermogravimetric-Mass Spectrometry (TG-MS) Analyses. Bioenergy Res. 2019, 12, 370–387. [Google Scholar] [CrossRef]
- Lopez-Velazquez, M.A.; Santes, V.; Balmaseda, J.; Torres-Garcia, E. Pyrolysis of Orange Waste: A Thermo-Kinetic Study. J. Anal. Appl. Pyrolysis 2013, 99, 170–177. [Google Scholar] [CrossRef]
- Mallick, D.; Poddar, M.K.; Mahanta, P.; Moholkar, V.S. Discernment of Synergism in Pyrolysis of Biomass Blends Using Thermogravimetric Analysis. Bioresour. Technol. 2018, 261, 294–305. [Google Scholar] [CrossRef]
- Mishra, G.; Kumar, J.; Bhaskar, T. Kinetic Studies on the Pyrolysis of Pinewood. Bioresour. Technol. 2015, 182, 282–288. [Google Scholar] [CrossRef]
- Poletto, M.; Zattera, A.J.; Santana, R.M.C. Thermal Decomposition of Wood: Kinetics and Degradation Mechanisms. Bioresour. Technol. 2012, 126, 7–12. [Google Scholar] [CrossRef]
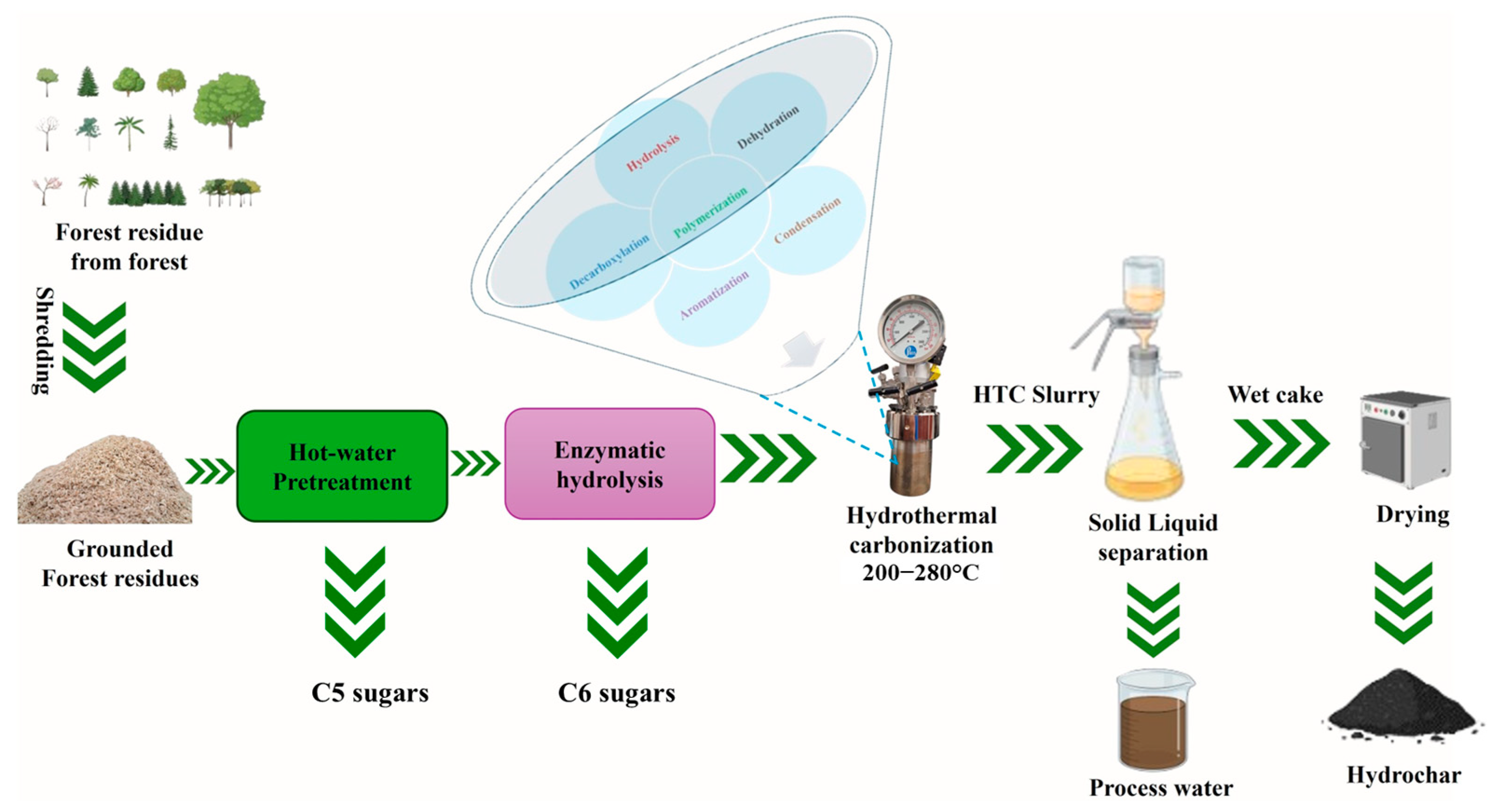




| Samples | Reaction Conditions | |||
|---|---|---|---|---|
| Temperature (°C) | Avg Heating Rate (°C/min) | Avg Pressure (Bar) | Holding Time (min) | |
| LRR | - | - | - | - |
| LRR200 | 200 | 4.13 | 17.00 | 60 |
| LRR220 | 220 | 4.04 | 20.00 | 60 |
| LRR240 | 240 | 3.69 | 35.00 | 60 |
| LRR260 | 260 | 3.66 | 45.50 | 60 |
| LRR280 | 280 | 3.54 | 67.00 | 60 |
| Parameters | LRR | LRR200 | LRR220 | LRR240 | LRR260 | LRR280 |
|---|---|---|---|---|---|---|
| Hydrochar yield (wt.%, dry basis) | - | 69.64 ± 0.85 a | 66.20 ± 0.57 b | 62.16 ± 0.75 c | 55.14 ± 2.09 d | 51.43 ± 0.48 d |
| Organic mass (wt.%, dry basis) | 97.81 | 67.94 | 64.49 | 60.61 | 35.55 | 49.80 |
| Organic yield (wt.%, dry basis) | 100 | 69.46 | 65.93 | 61.97 | 54.75 | 50.91 |
| Ash yield (wt.%, dry basis) | 100 | 77.63 | 78.08 | 70.78 | 73.52 | 74.43 |
| Proximate analysis (wt.%, dry basis) | ||||||
| Volatile matter | 68.90 ± 0.21 | 67.73 ± 0.78 | 63.92 ± 0.07 | 62.22 ± 0.10 | 57.75 ± 1.69 | 56.22 ± 0.73 |
| Fixed carbon | 28.91 ± 0.22 | 30.57 ± 0.78 | 34.37 ± 0.08 | 36.22 ± 0.13 | 40.64 ± 1.68 | 42.15 ± 0.73 |
| Ash content | 2.19 ± 0.04 | 1.70 ± 0.02 | 1.70 ± 0.05 | 1.55 ± 0.08 | 1.61 ± 0.09 | 1.63 ± 0.03 |
| Ultimate analysis (wt.%, dry basis) | ||||||
| C | 44.17 ± 0.21 | 61.46 ± 0.55 | 63.46 ± 0.61 | 65.50 ± 0.16 | 66.98 ± 0.23 | 66.55 ± 2.38 |
| H | 4.64 ± 0.17 | 5.61 ± 0.05 | 5.47 ± 0.14 | 5.45 ± 0.00 | 5.32 ± 0.00 | 5.33 ± 0.11 |
| N | 1.07 ± 0.03 | 0.97 ± 0.02 | 1.00 ± 0.03 | 1.13 ± 0.02 | 1.06 ± 0.00 | 1.05 ± 0.02 |
| O | 48.01 ± 0.13 | 30.26 ± 0.16 | 28.27 ± 0.23 | 26.29 ± 0.8 | 25.07 ± 0.06 | 25.43 ± 0.63 |
| S | 0.08 ± 0.12 | 0.00 ± 0.00 | 0.09 ± 0.12 | 0.09 ± 0.13 | 0.05 ± 0.00 | 0.01 ± 0.02 |
| H/C | 1.26 ± 0.05 | 1.09 ± 0.01 | 1.03 ± 0.03 | 0.99 ± 0.00 | 0.95 ± 0.00 | 0.95 ± 0.04 |
| O/C | 0.81 ± 0.00 | 0.37 ± 0.00 | 0.33 ± 0.00 | 0.30 ± 0.01 | 0.25 ± 0.00 | 0.26 ± 0.01 |
| CHO index | 0.36 | −0.36 | −0.37 | −0.40 | −0.45 | −0.43 |
| Fuel properties | ||||||
| HHV (MJ/kg) | 15.88 | 24.88 | 25.62 | 26.52 | 27.88 | 27.52 |
| Fuel ratio | 0.42 | 0.45 | 0.53 | 0.58 | 0.70 | 0.75 |
| Energy densification | - | 1.57 | 1.61 | 1.67 | 1.76 | 1.73 |
| Energy yield (%) | - | 109.19 | 106.88 | 103.88 | 96.92 | 89.21 |
| BET Surface Area | ||||||
| Specific surface area (SSA), m2/g | 0.20 ± 0.08 | 2.66 ± 0.35 | 1.61 ± 0.14 | 0.15 ± 0.00 | 1.00 ± 0.05 | 1.54 ± 0.13 |
| Samples | Initial Temperature (Ti, °C) | Final Temperature (Tf, °C) | Weight Loss | DTG Peak Temperature (Tm, °C) | DTG, Rmax (%/°C) | Average Rate of Weight Loss (%/°C) | Residual Weight (%) |
|---|---|---|---|---|---|---|---|
| Stage I | |||||||
| LRR | 30 | 200 | 11.06 | 183 | 0.20 | 0.07 | |
| LRR200 | 30 | 200 | 1.94 | 197 | 0.03 | 0.01 | |
| LRR220 | 30 | 200 | 2.31 | 198 | 0.03 | 0.01 | |
| LRR240 | 30 | 200 | 2.28 | 198 | 0.03 | 0.01 | |
| LRR260 | 30 | 200 | 2.84 | 199 | 0.04 | 0.02 | |
| LRR280 | 30 | 200 | 3.61 | 199 | 0.06 | 0.06 | |
| Stage II | |||||||
| LRR | 200 | 450 | 54.46 | 312 | 0.44 | 0.20 | |
| LRR200 | 200 | 450 | 53.49 | 353 | 0.58 | 0.20 | |
| LRR220 | 200 | 450 | 49.94 | 353 | 0.54 | 0.19 | |
| LRR240 | 200 | 450 | 44.25 | 353 | 0.44 | 0.18 | |
| LRR260 | 200 | 450 | 39.26 | 343 | 0.39 | 0.16 | |
| LRR280 | 200 | 450 | 37.15 | 343 | 0.27 | 0.15 | |
| Stage III | |||||||
| LRR | 450 | 600 | 5.50 | 464 | 0.06 | 0.04 | 30.99 |
| LRR200 | 450 | 600 | 7.41 | 458 | 0.08 | 0.05 | 35.39 |
| LRR220 | 450 | 600 | 7.75 | 460 | 0.09 | 0.05 | 38.39 |
| LRR240 | 450 | 600 | 8.41 | 458 | 0.09 | 0.06 | 43.46 |
| LRR260 | 450 | 600 | 8.86 | 458 | 0.10 | 0.06 | 47.77 |
| LRR280 | 450 | 600 | 8.87 | 466 | 0.10 | 0.06 | 49.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, M.; Cheatham, R.; Hossain, M.S.; Reza, T.; Volk, T.A.; Juneja, A.; Kumar, D. Sustainable Valorization of Forest Waste Hydrolysis Residues to Solid Biofuel: Insights into Conversion Mechanisms and Fuel Properties. Energies 2025, 18, 6156. https://doi.org/10.3390/en18236156
Kumar M, Cheatham R, Hossain MS, Reza T, Volk TA, Juneja A, Kumar D. Sustainable Valorization of Forest Waste Hydrolysis Residues to Solid Biofuel: Insights into Conversion Mechanisms and Fuel Properties. Energies. 2025; 18(23):6156. https://doi.org/10.3390/en18236156
Chicago/Turabian StyleKumar, Mohit, Robert Cheatham, Md Shahadat Hossain, Toufiq Reza, Timothy A. Volk, Ankita Juneja, and Deepak Kumar. 2025. "Sustainable Valorization of Forest Waste Hydrolysis Residues to Solid Biofuel: Insights into Conversion Mechanisms and Fuel Properties" Energies 18, no. 23: 6156. https://doi.org/10.3390/en18236156
APA StyleKumar, M., Cheatham, R., Hossain, M. S., Reza, T., Volk, T. A., Juneja, A., & Kumar, D. (2025). Sustainable Valorization of Forest Waste Hydrolysis Residues to Solid Biofuel: Insights into Conversion Mechanisms and Fuel Properties. Energies, 18(23), 6156. https://doi.org/10.3390/en18236156










