Bandgap Engineering of CIGS: Active Control of Composition Gradient
Abstract
1. Introduction
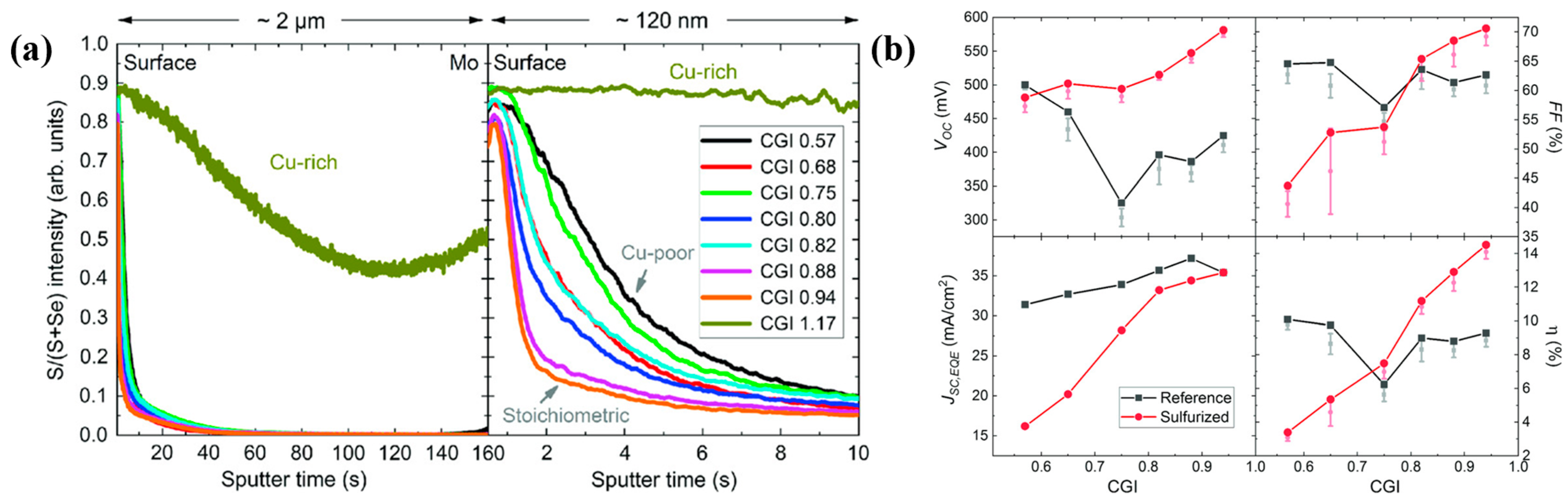
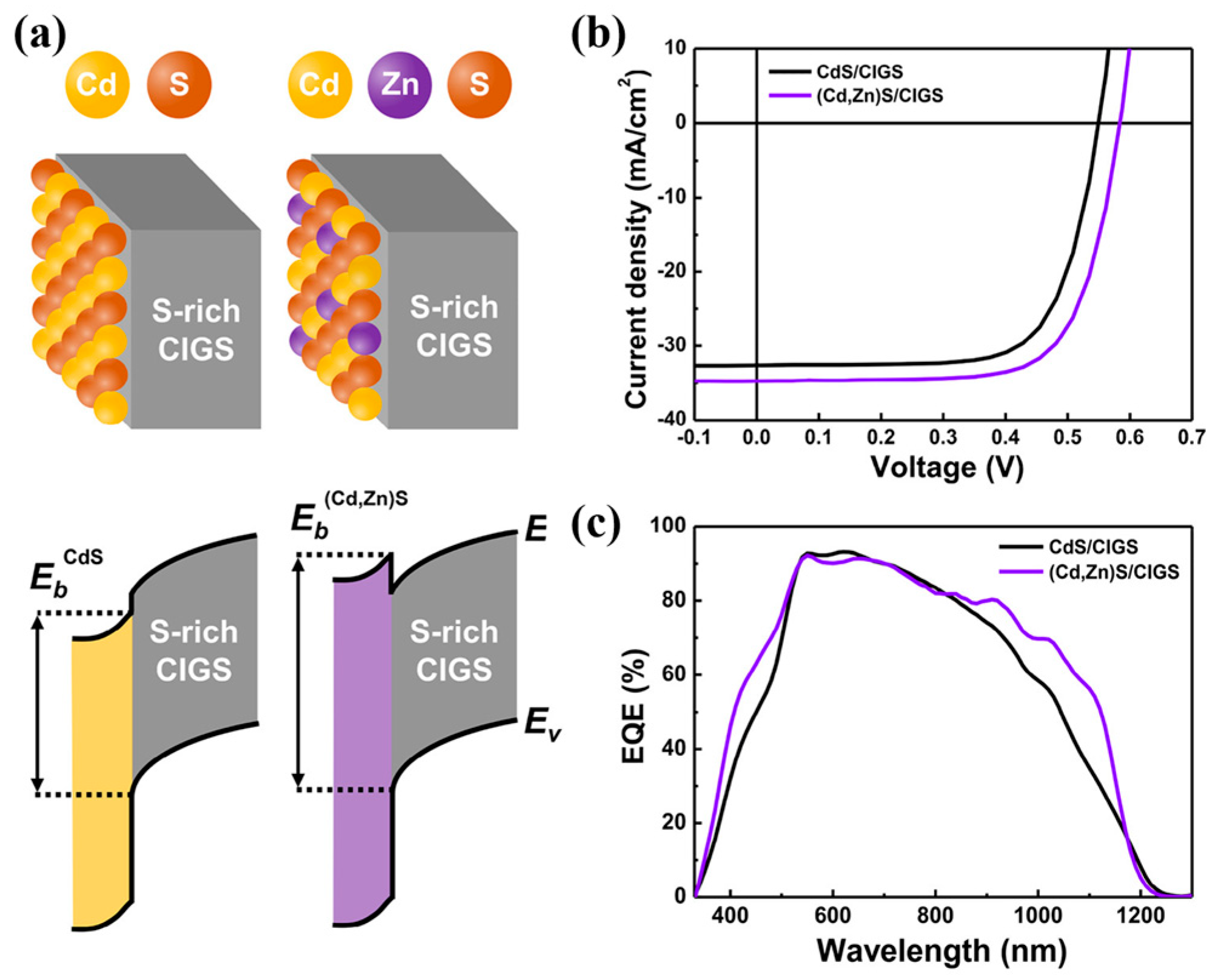
2. Surface Sulfurization
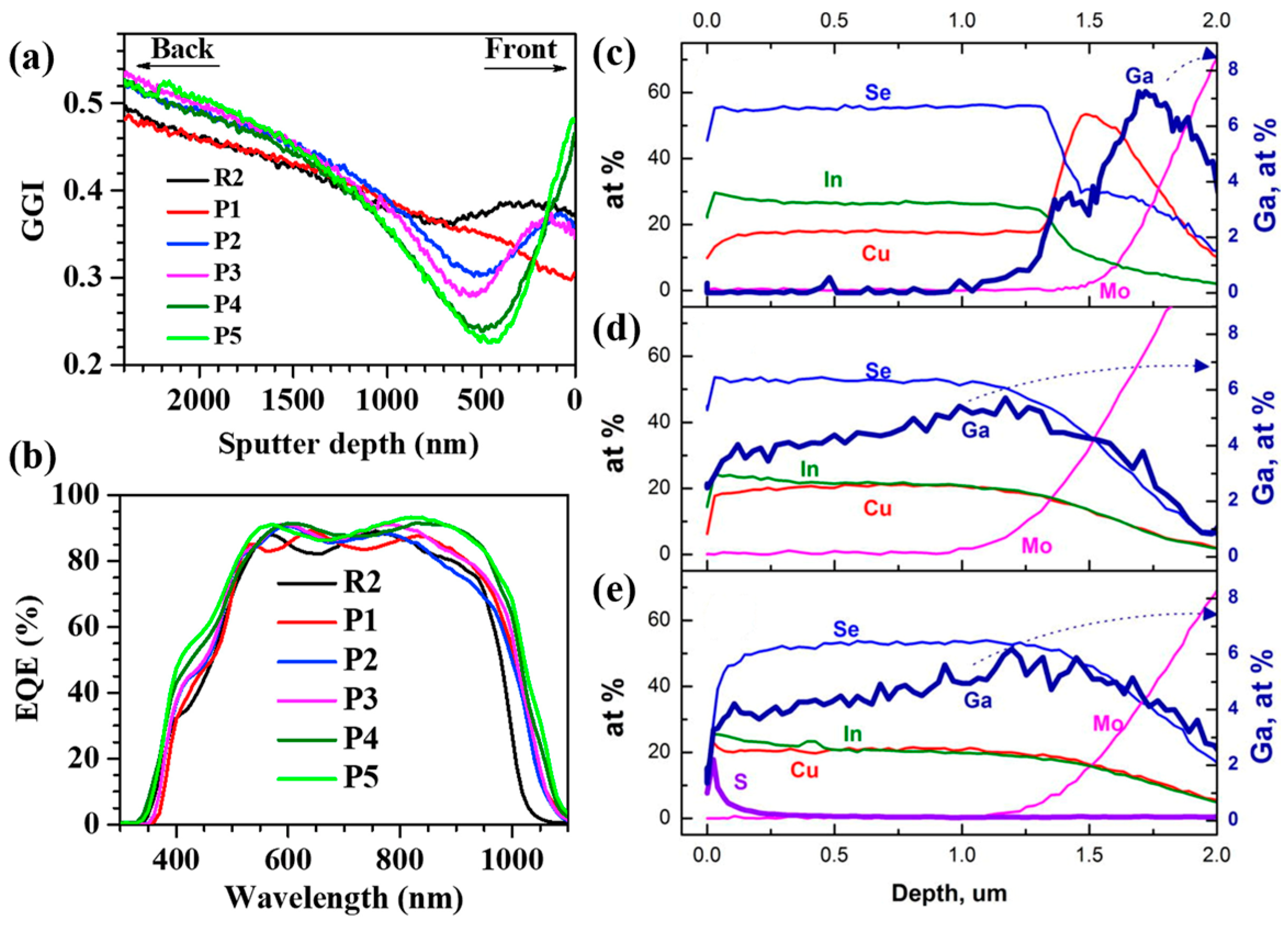
3. Ga Gradient
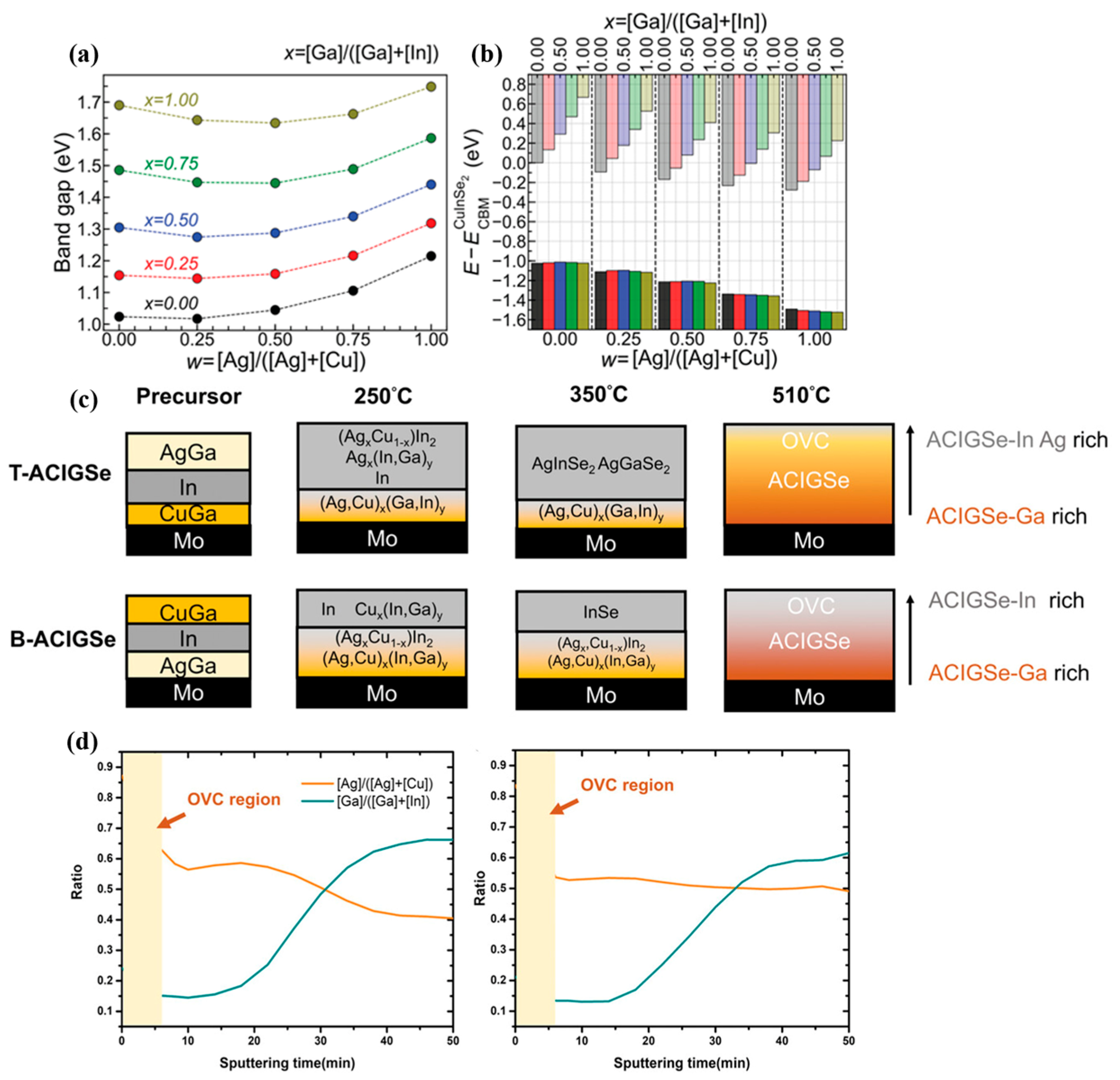
4. Ag Alloying
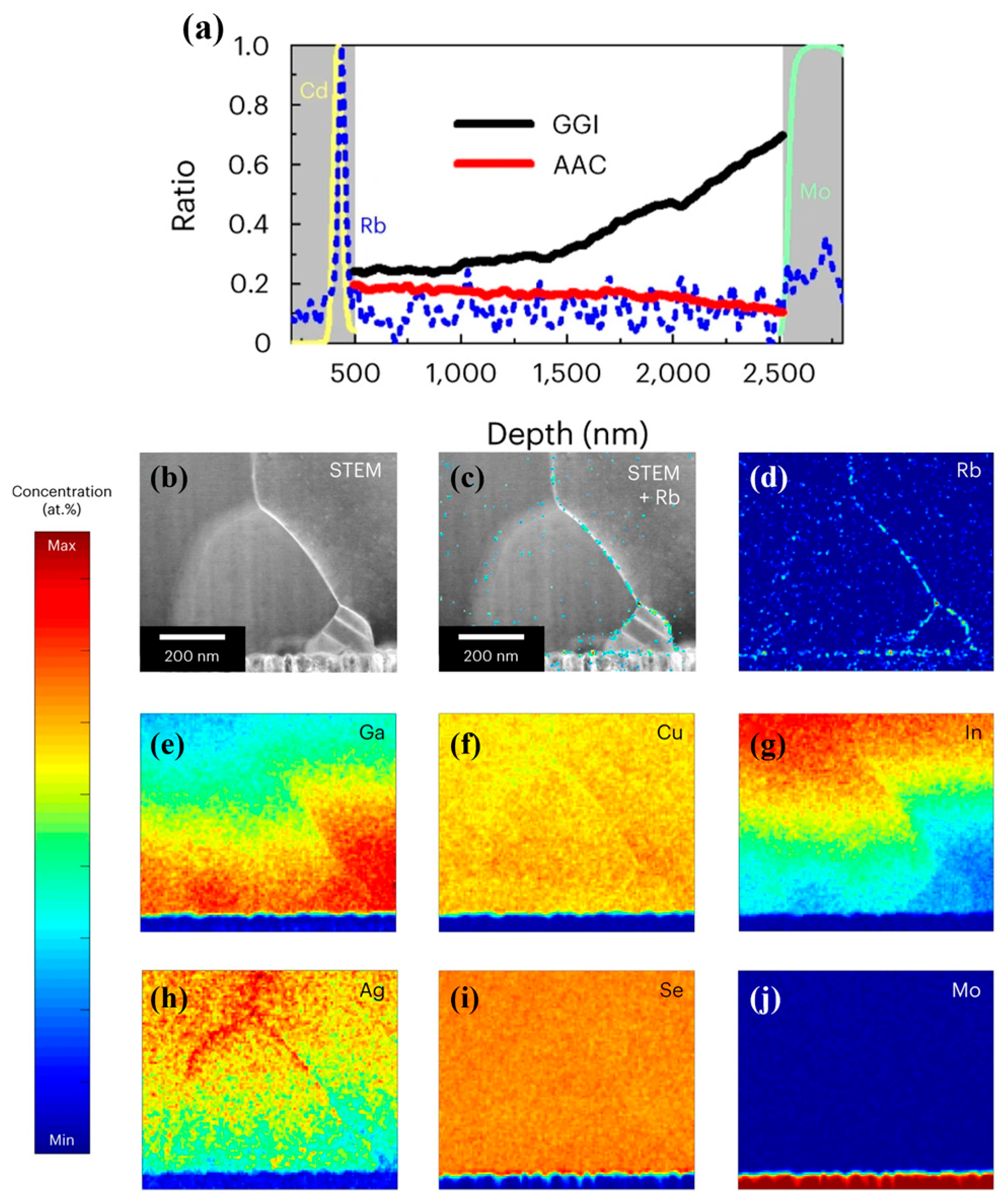
5. Analysis of V-Shaped vs. ‘Hockey Stick’-like Bandgap Distributions
6. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gu, Y.; Zhou, C.; Chen, W.; Zhang, Y.; Yao, Y.; Zhou, Z.; Sun, Y.; Liu, W. Silver (Ag) Substitution in Cu(In,Ga)Se2 Solar Cell: Insights into Current Challenges and Future Prospects. Appl. Phys. A 2024, 130, 636. [Google Scholar] [CrossRef]
- Seok, M.-G.; Kim, Y.; Kim, S.M. Reaction Kinetics Analysis of Treatment Process on Light-Induced Degradation for p-Type Passivated Emitter and Rear Contact Solar Cell Module with Gallium Cz-Si Wafer. Energies 2022, 15, 3563. [Google Scholar] [CrossRef]
- Lee, C.; Hyun, J.; Nam, J.; Jeong, S.-H.; Song, H.; Bae, S.; Lee, H.; Seol, J.; Kim, D.; Kang, Y.; et al. Amorphous Silicon Thin Film Deposition for Poly-Si/SiO2 Contact Cells to Minimize Parasitic Absorption in the Near-Infrared Region. Energies 2021, 14, 8199. [Google Scholar] [CrossRef]
- Keller, J.; Kiselman, K.; Donzel-Gargand, O.; Martin, N.M.; Babucci, M.; Lundberg, O.; Wallin, E.; Stolt, L.; Edoff, M. High-Concentration Silver Alloying and Steep Back-Contact Gallium Grading Enabling Copper Indium Gallium Selenide Solar Cell with 23.6% Efficiency. Nat. Energy 2024, 9, 467–478. [Google Scholar] [CrossRef]
- Nakamura, M.; Yamaguchi, K.; Kimoto, Y.; Yasaki, Y.; Kato, T.; Sugimoto, H. Cd-Free Cu(In,Ga)(Se,S)2 Thin-Film Solar Cell With Record Efficiency of 23.35%. IEEE J. Photovolt. 2019, 9, 1863–1867. [Google Scholar] [CrossRef]
- Saeed, A.; Salah, M.M.; Zekry, A.; Mousa, M.; Shaker, A.; Abouelatta, M.; Amer, F.Z.; Mubarak, R.I.; Louis, D.S. Investigation of High-Efficiency and Stable Carbon-Perovskite/Silicon and Carbon-Perovskite/CIGS-GeTe Tandem Solar Cells. Energies 2023, 16, 1676. [Google Scholar] [CrossRef]
- Boukortt, N.E.I.; Triolo, C.; Santangelo, S.; Patanè, S. All-Perovskite Tandem Solar Cells: From Certified 25% and Beyond. Energies 2023, 16, 3519. [Google Scholar] [CrossRef]
- Hasnain, S.M. Examining the Advances, Obstacles, and Achievements of Tin-Based Perovskite Solar Cells: A Review. Solar Energy 2023, 262, 111825. [Google Scholar] [CrossRef]
- Wang, H.; Zhuang, D.; Zhao, M.; Jia, M.; Wu, Z.; Han, J.; Gong, Q.; Wei, J. Recombination and Performance Analysis of Wide Bandgap CIGSe Devices for Efficiency Solar Cell. Chem. Eng. J. 2025, 507, 160435. [Google Scholar] [CrossRef]
- Han, J.; Zhuang, D.; Zhao, M.; Tao, S.; Wang, H.; Jia, M.; Wu, Z.; Zhou, J.; Baranova, M.; Gong, Q. Trade-Offs in Morphology and Electrical Performance of Selenium-Deficient Cu(In,Ga)Se2 Precursors: Systematic Analysis of In2Se Volatilization. Sol. Energy Mater. Sol. Cells 2026, 294, 113876. [Google Scholar] [CrossRef]
- Tong, H.; Kou, Z.; Zhao, M.; Zhuang, D.; Wang, C.; Li, Y. Effects of Annealing Temperature and Atmosphere on Performances of Zn0.9Mg0.1O Buffer Layers for CIGS Solar Cell. Ceram. Int. 2022, 48, 24523–24530. [Google Scholar] [CrossRef]
- Wang, X.; Gao, W.; Zhang, N.; Zuo, K.; Wu, L.; Li, M. The Study on Photoelectric Response Properties of CIGS Film With V-Shaped Bandgap Regulated by Adjustment of Cu and Se Concentrations. IEEE Sens. J. 2024, 24, 38935–38944. [Google Scholar] [CrossRef]
- Xin, W.; Yuan, M.; Zeng, C.; Lin, R.; Li, D.; Hong, R. Investigation on the Diffusion Mechanism of Ga Element and Regulation of the V-Shaped Bandgap in CIGS Thin Films Based on Magnetron Sputtering with a Quaternary Target. Sol. Energy 2024, 273, 112510. [Google Scholar] [CrossRef]
- Lontchi, J.; Dufoulon, V.; Crossay, A.; Gamet, D.; Tom, T.; Rebai, A.; Posada, J.; Donsanti, F.; Lincot, D.; Guillemoles, J.-F.; et al. Achieving High-Performance in Flexible CIGS Solar Cells Through Advanced Deposition Optimization. In Proceedings of the 2025 IEEE 53rd Photovoltaic Specialists Conference (PVSC), Montreal, QC, Canada, 8–13 June 2025; pp. 0438–0440. [Google Scholar]
- Jeon, D.-H.; Park, S.-N.; Lee, J.-B.; Kim, Y.-I.; Yang, K.-J.; Kang, J.-K.; Kim, D.-H.; Sung, S.-J.; Hwang, D.-K. Enhancing the Open-Circuit Voltage in Narrow-Bandgap CuInSe2 Solar Cells via Local Contact Passivation with Al2O3. J. Sci. Adv. Mater. Devices 2024, 9, 100648. [Google Scholar] [CrossRef]
- Knecht, R.; Hammer, M.S.; Parisi, J.; Riedel, I. Impact of Varied Sulfur Incorporation on the Device Performance of Sequentially Processed Cu(In,Ga)(Se,S)2 Thin Film Solar Cells. Phys. Status Solidi (A) 2013, 210, 1392–1399. [Google Scholar] [CrossRef]
- Bauer, G.H.; Brüggemann, R.; Tardon, S.; Vignoli, S.; Kniese, R. Quasi-Fermi Level Splitting and Identification of Recombination Losses from Room Temperature Luminescence in Cu(In1-xGax)Se2 Thin Films versus Optical Band Gap. Thin Solid Film. 2005, 480–481, 410–414. [Google Scholar] [CrossRef]
- Gütay, L.; Bauer, G.H. Non-Uniformities of Opto-Electronic Properties in Cu(In,Ga)Se2 Thin Films and Their Influence on Cell Performance Studied with Confocal Photoluminescence. In Proceedings of the 2009 34th IEEE Photovoltaic Specialists Conference (PVSC), Philadelphia, PA, USA, 7–12 June 2009; pp. 000874–000877. [Google Scholar]
- Contreras, M.A.; Mansfield, L.M.; Egaas, B.; Li, J.; Romero, M.; Noufi, R.; Rudiger-Voigt, E.; Mannstadt, W. Wide Bandgap Cu(In,Ga)Se2 Solar Cells with Improved Energy Conversion Efficiency. Prog. Photovolt. Res. Appl. 2012, 20, 843–850. [Google Scholar] [CrossRef]
- Kwok, C.K.G.; Tangara, H.; Masuko, N.; Scheer, R.; Ishizuka, S.; Monirul Islam, M.; Sakurai, T. Effects of Quasi-Fermi Level Splitting and Band Tail States on Open Circuit Voltage towards High-Efficiency Cu(In,Ga)Se2 Solar Cells. Sol. Energy Mater. Sol. Cells 2024, 269, 112767. [Google Scholar] [CrossRef]
- Maeda, T.; Nakanishi, R.; Yanagita, M.; Wada, T. Control of Electronic Structure in Cu(In, Ga)(S, Se)2 for High-Efficiency Solar Cells. Jpn. J. Appl. Phys. 2020, 59, SGGF12. [Google Scholar] [CrossRef]
- Wada, T.; Nakamura, S.; Maeda, T. Ternary and Multinary Cu-Chalcogenide Photovoltaic Materials from CuInSe2 to Cu2ZnSnS4 and Other Compounds. Prog. Photovolt. Res. Appl. 2012, 20, 520–525. [Google Scholar] [CrossRef]
- Singh, U.P.; Shafarman, W.N.; Birkmire, R.W. Surface Sulfurization Studies of Cu(InGa)Se2 Thin Film. Sol. Energy Mater. Sol. Cells 2006, 90, 623–630. [Google Scholar] [CrossRef]
- Turcu, M.; Kötschau, I.M.; Rau, U. Composition Dependence of Defect Energies and Band Alignments in the Cu(In1-xGax)(Se1-ySy)2 Alloy System. J. Appl. Phys. 2002, 91, 1391–1399. [Google Scholar] [CrossRef]
- Yuan, S.; Wang, X.; Zhao, Y.; Chang, Q.; Xu, Z.; Kong, J.; Wu, S. Solution Processed Cu(In,Ga)(S,Se)2 Solar Cells with 15.25% Efficiency by Surface Sulfurization. ACS Appl. Energy Mater. 2020, 3, 6785–6792. [Google Scholar] [CrossRef]
- Rau, U.; Schmitt, M.; Engelhardt, F.; Seifert, O.; Parisi, J.; Riedl, W.; Rimmasch, J.; Karg, F. Impact of Na and S Incorporation on the Electronic Transport Mechanisms of Cu(In, Ga)Se2 Solar Cells. Solid. State Commun. 1998, 107, 59–63. [Google Scholar] [CrossRef]
- Ohashi, D.; Nakada, T.; Kunioka, A. Improved CIGS Thin-Film Solar Cells by Surface Sulfurization Using In2S3 and Sulfur Vapor. Sol. Energy Mater. Sol. Cells 2001, 67, 261–265. [Google Scholar] [CrossRef]
- Tai, K.F.; Kamada, R.; Yagioka, T.; Kato, T.; Sugimoto, H. From 20.9 to 22.3% Cu(In,Ga)(S,Se)2 Solar Cell: Reduced Recombination Rate at the Heterojunction and the Depletion Region Due to K-Treatment. Jpn. J. Appl. Phys. 2017, 56, 08MC03. [Google Scholar] [CrossRef]
- Keller, J.; Bilousov, O.V.; Wallin, E.; Lundberg, O.; Neerken, J.; Heise, S.; Riekehr, L.; Edoff, M.; Platzer-Björkman, C. Effect of Cu Content on Post-Sulfurization of Cu(In,Ga)Se2 Films and Corresponding Solar Cell Performance. Phys. Status Solidi (A) 2019, 216, 1900472. [Google Scholar] [CrossRef]
- Kobayashi, T.; Yamaguchi, H.; Jehl Li Kao, Z.; Sugimoto, H.; Kato, T.; Hakuma, H.; Nakada, T. Impacts of Surface Sulfurization on Cu(In1-x,Gax)Se2 Thin-Film Solar Cells. Prog. Photovolt. Res. Appl. 2015, 23, 1367–1374. [Google Scholar] [CrossRef]
- Mueller, B.J.; Mock, M.; Haug, V.; Hergert, F.; Koehler, T.; Zweigart, S.; Herr, U. Ex- and in-Situ Investigations of Sulfur Diffusion into Cu(In,Ga)Se2 Thin Films. Thin Solid Film. 2015, 582, 284–289. [Google Scholar] [CrossRef]
- Niki, S.; Contreras, M.; Repins, I.; Powalla, M.; Kushiya, K.; Ishizuka, S.; Matsubara, K. CIGS Absorbers and Processes. Prog. Photovolt. Res. Appl. 2010, 18, 453–466. [Google Scholar] [CrossRef]
- Kato, T. Cu(In,Ga)(Se,S)2 Solar Cell Research in Solar Frontier: Progress and Current Status. Jpn. J. Appl. Phys. 2017, 56, 04CA02. [Google Scholar] [CrossRef]
- Yang, J.; Nam, J.; Kim, D.; Kim, G.; Jo, W.; Kang, Y.; Lee, D. Enhancement of the Photo Conversion Efficiencies in Cu(In,Ga)(Se,S)2 Solar Cells Fabricated by Two-Step Sulfurization Process. Appl. Phys. Lett. 2015, 107, 193901. [Google Scholar] [CrossRef]
- Başol, B.M.; Halani, A.; Leidholm, C.; Norsworthy, G.; Kapur, V.K.; Swartzlander, A.; Matson, R. Studies on Sulfur Diffusion into Cu(In,Ga)Se2 Thin Films. Prog. Photovolt. Res. Appl. 2000, 8, 227–235. [Google Scholar] [CrossRef]
- Chen, C.-W.; Tsai, H.-W.; Wu, T.-T.; Yen, Y.-T.; Wang, Y.-C.; Hsu, C.-H.; Tsai, W.-C.; Tsai, H.-S.; Shen, C.-H.; Shieh, J.-M.; et al. Enhanced Solar Performance of Chemical Bath Deposited-Zn(O,S)/Cu(In,Ga)Se2 Solar Cells via Interface Engineering by a Wet Soaking Process. J. Mater. Chem. A 2015, 3, 14985–14990. [Google Scholar] [CrossRef]
- Nakada, T.; Matsumoto, K.; Okumura, M. Improved Efficiency of Cu(In,Ga)Se2 Thin Film Solar Cells by Surface Sulfurization Using Wet Process. In Proceedings of the Conference Record of the Twenty-Ninth IEEE Photovoltaic Specialists Conference, New Orleans, LA, USA, 19–24 May 2002; pp. 527–530. [Google Scholar]
- Buffière, M.; Mel, A.-A.E.; Lenaers, N.; Brammertz, G.; Zaghi, A.E.; Meuris, M.; Poortmans, J. Surface Cleaning and Passivation Using (NH4)2S Treatment for Cu(In,Ga)Se2 Solar Cells: A Safe Alternative to KCN. Adv. Energy Mater. 2015, 5, 1401689. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, Q.; Ao, J.; Zhang, Y.; Bi, J.; Guo, J.; Han, Y.; Sun, G.; Zhang, Y.; Liu, W.; et al. Enhancing Surface Properties for Electrodeposited Cu(In,Ga)Se2 Films by (NH4)2S Solution at Room Temperature. ACS Appl. Energy Mater. 2021, 4, 3822–3831. [Google Scholar] [CrossRef]
- Lin, S.-H.; Sung, J.-C.; Lu, C.-H. Effects of the Surface Sulfurization Reactions on the Structural and Photovoltaic Properties of Cu(In,Ga)(Se,S)2 Solar Cells. Thin Solid Film. 2016, 616, 746–753. [Google Scholar] [CrossRef]
- Kato, T.; Wu, J.-L.; Hirai, Y.; Sugimoto, H.; Bermudez, V. Record Efficiency for Thin-Film Polycrystalline Solar Cells Up to 22.9% Achieved by Cs-Treated Cu(In,Ga)(Se,S)2. IEEE J. Photovolt. 2019, 9, 325–330. [Google Scholar] [CrossRef]
- Kim, G.Y.; Yang, J.; Nguyen, T.T.T.; Yoon, S.; Nam, J.; Lee, D.; Kim, D.; Kwon, M.; Jeon, C.-W.; Kim, Y.-K.; et al. High Photo-Conversion Efficiency in Double-Graded Cu(In,Ga)(S,Se)2 Thin Film Solar Cells with Two-Step Sulfurization Post-Treatment. Prog. Photovolt. Res. Appl. 2017, 25, 139–148. [Google Scholar] [CrossRef]
- Li, W.; Song, Q.; Zhao, C.; Qi, T.; Zhang, C.; Wang, W.; Gao, C.; Zheng, X.; Ning, D.; Ma, M.; et al. Toward High-Efficiency Cu(In,Ga)(S,Se)2 Solar Cells by a Simultaneous Selenization and Sulfurization Rapid Thermal Process. ACS Appl. Energy Mater. 2021, 4, 14546–14553. [Google Scholar] [CrossRef]
- Kodalle, T.; Schmidt, S.S.; Wolf, C.; Greiner, D.; Bloeck, U.; Schubert-Bischoff, P.; Kaufmann, C.A.; Schlatmann, R. Investigating Sulfur Distribution and Corresponding Bandgap Grading in Cu(In,Ga)(S,Se)2 Absorber Layers Processed by Fast Atmospheric Chalcogenization of Metal Precursors. J. Alloys Compd. 2017, 703, 600–604. [Google Scholar] [CrossRef]
- Huang, Y.; Han, A.; Wang, X.; Liu, X.; Liu, Z.; Meng, F. Tuning the Band Gap of Cu(In,Ga)Se2 Thin Films by Simultaneous Selenization/Sulfurization. Mater. Lett. 2016, 182, 114–117. [Google Scholar] [CrossRef]
- Siew, J.H.; Chen, Y.-H.; Chang, Y.-L.; Lai, C.-H.; Lin, T.-Y. Heat Soaking for Improving Rollover from S at the Back of CIGSSe Solar Cells. IEEE J. Photovolt. 2023, 13, 503–509. [Google Scholar] [CrossRef]
- Sozzi, G.; Troni, F.; Menozzi, R. On the Combined Effects of Window/Buffer and Buffer/Absorber Conduction-Band Offsets, Buffer Thickness and Doping on Thin-Film Solar Cell Performance. Sol. Energy Mater. Sol. Cells 2014, 121, 126–136. [Google Scholar] [CrossRef]
- Park, G.S.; Chu, V.B.; Kim, B.W.; Kim, D.-W.; Oh, H.-S.; Hwang, Y.J.; Min, B.K. Achieving 14.4% Alcohol-Based Solution-Processed Cu(In,Ga)(S,Se)2 Thin Film Solar Cell through Interface Engineering. ACS Appl. Mater. Interfaces 2018, 10, 9894–9899. [Google Scholar] [CrossRef] [PubMed]
- Contreras, M.; Tuttle, J.; Du, D.; Qi, Y.; Swartzlander, A.; Tennant, A.; Noufi, R. Graded Band-gap Cu(In,Ga)Se2 Thin-film Solar Cell Absorber with Enhanced Open-circuit Voltage. Appl. Phys. Lett. 1993, 63, 1824–1826. [Google Scholar] [CrossRef]
- Keller, J.; Aboulfadl, H.; Stolt, L.; Donzel-Gargand, O.; Edoff, M. Rubidium Fluoride Absorber Treatment for Wide-Gap (Ag,Cu)(In,Ga)Se2 Solar Cells. Sol. RRL 2022, 6, 2200044. [Google Scholar] [CrossRef]
- Yao, Y.; Ma, J.; Liu, Y.; Yang, X.; Meng, H.; Liu, F.; Zhang, Y.; Liu, W. Achieving over 860 mV Open-Circuit Voltage in Low-Ag Wide-Bandgap Cu(In,Ga)Se2 Solar Cells Through Ion Diffusion and Band Structure Optimization. Adv. Funct. Mater. 2025, 35, 2423228. [Google Scholar] [CrossRef]
- Li, J.V.; Grover, S.; Contreras, M.A.; Ramanathan, K.; Kuciauskas, D.; Noufi, R. A Recombination Analysis of Cu(In,Ga)Se2 Solar Cells with Low and High Ga Compositions. Sol. Energy Mater. Sol. Cells 2014, 124, 143–149. [Google Scholar] [CrossRef]
- Jung, S.; Ahn, S.; Yun, J.H.; Gwak, J.; Kim, D.; Yoon, K. Effects of Ga Contents on Properties of CIGS Thin Films and Solar Cells Fabricated by Co-Evaporation Technique. Curr. Appl. Phys. 2010, 10, 990–996. [Google Scholar] [CrossRef]
- Hanket, G.M.; Shafarman, W.N.; McCandless, B.E.; Birkmire, R.W. Incongruent Reaction of Cu–(InGa) Intermetallic Precursors in H2Se and H2S. J. Appl. Phys. 2007, 102, 074922. [Google Scholar] [CrossRef]
- Caballero, R.; Guillén, C.; Gutiérrez, M.T.; Kaufmann, C.A. CuIn1-xGaxSe2-Based Thin-Film Solar Cells by the Selenization of Sequentially Evaporated Metallic Layers. Prog. Photovolt. Res. Appl. 2006, 14, 145–153. [Google Scholar] [CrossRef]
- Marudachalam, M.; Birkmire, R.W.; Hichri, H.; Schultz, J.M.; Swartzlander, A.; Al-Jassim, M.M. Phases, Morphology, and Diffusion in CuInxGa1-xSe2 Thin Films. J. Appl. Phys. 1997, 82, 2896–2905. [Google Scholar] [CrossRef]
- Witte, W.; Abou-Ras, D.; Albe, K.; Bauer, G.H.; Bertram, F.; Boit, C.; Brüggemann, R.; Christen, J.; Dietrich, J.; Eicke, A.; et al. Gallium Gradients in Cu(In,Ga)Se2 Thin-Film Solar Cells. Prog. Photovolt. Res. Appl. 2015, 23, 717–733. [Google Scholar] [CrossRef]
- Schroeder, D.J.; Berry, G.D.; Rockett, A.A. Gallium Diffusion and Diffusivity in CuInSe2 Epitaxial Layers. Appl. Phys. Lett. 1996, 69, 4068–4070. [Google Scholar] [CrossRef]
- Gabor, A.M.; Tuttle, J.R.; Albin, D.S.; Contreras, M.A.; Noufi, R.; Hermann, A.M. High-Efficiency CuInxGa1-xSe2 Solar Cells Made from (Inx,Ga1-x)2Se3 Precursor Films. Appl. Phys. Lett. 1994, 65, 198–200. [Google Scholar] [CrossRef]
- Yang, S.; Zhu, J.; Zhang, X.; Ma, X.; Luo, H.; Yin, L.; Xiao, X. Bandgap Optimization of Submicron-Thick Cu(In,Ga)Se2 Solar Cells. Prog. Photovolt. Res. Appl. 2015, 23, 1157–1163. [Google Scholar] [CrossRef]
- Gong, J.; Kong, Y.; Li, J.; Wang, K.; Wang, X.; Zhang, Z.; Ding, Z.; Xiao, X. Enhancing Photocurrent of Cu(In,Ga)Se2 Solar Cells with Actively Controlled Ga Grading in the Absorber Layer. Nano Energy 2019, 62, 205–211. [Google Scholar] [CrossRef]
- Kim, K.; Park, H.; Hanket, G.M.; Kim, W.K.; Shafarman, W.N. Composition and Bandgap Control in Cu(In,Ga)Se2-Based Absorbers Formed by Reaction of Metal Precursors. Prog. Photovolt. Res. Appl. 2015, 23, 765–772. [Google Scholar] [CrossRef]
- Colombara, D.; Conley, K.; Malitckaya, M.; Komsa, H.-P.; Puska, M.J. The Fox and the Hound: In-Depth and in-Grain Na Doping and Ga Grading in Cu(In,Ga)Se2 Solar Cells. J. Mater. Chem. A 2020, 8, 6471–6479. [Google Scholar] [CrossRef]
- Cai, C.-H.; Chen, R.-Z.; Chan, T.-S.; Lu, Y.-R.; Huang, W.-C.; Yen, C.-C.; Zhao, K.; Lo, Y.-C.; Lai, C.-H. Interplay between Potassium Doping and Bandgap Profiling in Selenized Cu(In,Ga)Se2 Solar Cells: A Functional CuGa:KF Surface Precursor Layer. Nano Energy 2018, 47, 393–400. [Google Scholar] [CrossRef]
- Nishiwaki, S.; Satoh, T.; Hashimoto, Y.; Negami, T.; Wada, T. Preparation of Cu(In,Ga)Se2 Thin Films at Low Substrate Temperatures. J. Mater. Res. 2001, 16, 394–399. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Shieh, H.-P.D. Improvement of Bandgap Homogeneity in Cu(In,Ga)Se2 Thin Films Using a Modified Two-Step Selenization Process. Appl. Phys. Lett. 2013, 103, 153502. [Google Scholar] [CrossRef]
- Sim, J.-K.; Ashok, K.; Lee, C.-R. Formation of CIGS Thin Absorption Layer by Sequential Sputtering of CuGa/In/CuGa Precursor on Mo/SLG with Post Selenization. Met. Mater. Int. 2013, 19, 303–308. [Google Scholar] [CrossRef]
- Kim, K.; Hanket, G.M.; Huynh, T.; Shafarman, W.N. Three-Step H2Se/Ar/H2S Reaction of Cu-In-Ga Precursors for Controlled Composition and Adhesion of Cu(In,Ga)(Se,S)2 Thin Films. J. Appl. Phys. 2012, 111, 083710. [Google Scholar] [CrossRef]
- Hanket, G.M.; Kamada, R.; Kim, W.K.; Shafarman, W.N. Effect of Reaction Temperature on Cu(InGa)(SeS)2 Formation by a Sequential H2Se/H2S Precursor Reaction Process. In Proceedings of the 2008 33rd IEEE Photovoltaic Specialists Conference, San Diego, CA, USA, 11–16 May 2008; pp. 1–5. [Google Scholar]
- Schmidt, S.S.; Wolf, C.; Rodriguez-Alvarez, H.; Bäcker, J.-P.; Kaufmann, C.A.; Merdes, S.; Ziem, F.; Hartig, M.; Cinque, S.; Dorbandt, I.; et al. Adjusting the Ga Grading during Fast Atmospheric Processing of Cu(In,Ga)Se2 Solar Cell Absorber Layers Using Elemental Selenium Vapor. Prog. Photovolt. Res. Appl. 2017, 25, 341–357. [Google Scholar] [CrossRef]
- Rodriguez-Alvarez, H.; Weber, A.; Lauche, J.; Kaufmann, C.A.; Rissom, T.; Greiner, D.; Klaus, M.; Unold, T.; Genzel, C.; Schock, H.-W.; et al. Formation of CuInSe2 and CuGaSe2 Thin-Films Deposited by Three-Stage Thermal Co-Evaporation: A Real-Time X-Ray Diffraction and Fluorescence Study. Adv. Energy Mater. 2013, 3, 1381–1387. [Google Scholar] [CrossRef]
- Caballero, R.; Kaufmann, C.A.; Efimova, V.; Rissom, T.; Hoffmann, V.; Schock, H.W. Investigation of Cu(In,Ga)Se2 Thin-Film Formation during the Multi-Stage Co-Evaporation Process. Prog. Photovolt. Res. Appl. 2013, 21, 30–46. [Google Scholar] [CrossRef]
- Tu, L.-H.; Cai, C.-H.; Lai, C.-H. Tuning Ga Grading in Selenized Cu(In,Ga)Se2 Solar Cells by Formation of Ordered Vacancy Compound. Sol. RRL 2021, 5, 2000626. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Ho, P.-H.; Huang, W.-C.; Tu, L.-H.; Chang, H.-F.; Cai, C.-H.; Lai, C.-H. Engineering a Ga-Gradient by One-Step Sputtering to Achieve Over 15% Efficiency of Cu(In,Ga)Se2 Flexible Solar Cells without Post-Selenization. ACS Appl. Mater. Interfaces 2020, 12, 28320–28328. [Google Scholar] [CrossRef]
- Boyle, J.H.; McCandless, B.E.; Shafarman, W.N.; Birkmire, R.W. Structural and Optical Properties of (Ag,Cu)(In,Ga)Se2 Polycrystalline Thin Film Alloys. J. Appl. Phys. 2014, 115, 223504. [Google Scholar] [CrossRef]
- Erslev, P.T.; Lee, J.; Hanket, G.M.; Shafarman, W.N.; Cohen, J.D. The Electronic Structure of Cu(In1-xGax)Se2 Alloyed with Silver. Thin Solid Film. 2011, 519, 7296–7299. [Google Scholar] [CrossRef]
- Hanket, G.M.; Boyle, J.H.; Shafarman, W.N. Characterization and Device Performance of (AgCu)(InGa)Se2 Absorber Layers. In Proceedings of the 2009 34th IEEE Photovoltaic Specialists Conference (PVSC), Philadelphia, PA, USA, 7–12 June 2009; pp. 001240–001245. [Google Scholar]
- Kim, K.; Park, J.W.; Yoo, J.S.; Cho, J.; Lee, H.-D.; Yun, J.H. Ag Incorporation in Low-Temperature Grown Cu(In,Ga)Se2 Solar Cells Using Ag Precursor Layers. Sol. Energy Mater. Sol. Cells 2016, 146, 114–120. [Google Scholar] [CrossRef]
- Cheng, K.; Shen, X.; Liu, J.; Liu, X.; Du, Z. Sputtered Ag-Alloyed Cu(In, Ga)(Se, S)2 Solar Cells by Sequential Process. Sol. Energy 2021, 217, 70–77. [Google Scholar] [CrossRef]
- Tu, L.-H.; Tran, N.T.T.; Lin, S.-K.; Lai, C.-H. Efficiency Boost of (Ag0.5,Cu0.5)(In1-x,Gax)Se2 Thin Film Solar Cells by Using a Sequential Process: Effects of Ag-Front Grading and Surface Phase Engineering. Adv. Energy Mater. 2023, 13, 2301227. [Google Scholar] [CrossRef]
- Keller, J.; Sopiha, K.V.; Stolt, O.; Stolt, L.; Persson, C.; Scragg, J.J.S.; Törndahl, T.; Edoff, M. Wide-Gap (Ag,Cu)(In,Ga)Se2 Solar Cells with Different Buffer Materials—A Path to a Better Heterojunction. Prog. Photovolt. Res. Appl. 2020, 28, 237–250. [Google Scholar] [CrossRef]
- Hanket, G.M.; Thompson, C.P.; Larsen, J.K.; Eser, E.; Shafarman, W.N. Control of Ga Profiles in (AgCu)(InGa)Se2 Absorber Layers Deposited on Polyimide Substrates. In Proceedings of the 2012 38th IEEE Photovoltaic Specialists Conference, Austin, TX, USA, 3–8 June 2012; pp. 000662–000667. [Google Scholar]
- Valdes, N.H.; Lee, J.; Shafarman, W.N. Ag Alloying and KF Treatment Effects on Low Bandgap CuInSe2 Solar Cells. IEEE J. Photovolt. 2019, 9, 906–911. [Google Scholar] [CrossRef]
- Kim, G.; Kim, W.M.; Park, J.-K.; Kim, D.; Yu, H.; Jeong, J. Thin Ag Precursor Layer-Assisted Co-Evaporation Process for Low-Temperature Growth of Cu(In,Ga)Se2 Thin Film. ACS Appl. Mater. Interfaces 2019, 11, 31923–31933. [Google Scholar] [CrossRef]
- Prathapani, S.; Gharabeiki, S.; Lauche, J.; Schwiddessen, R.; Reyes-Figueroa, P.; Weinberger, N.; Melchiorre, M.; Schlatmann, R.; Lauermann, I.; Kaufmann, C.A. Impact of Minimal Silver Incorporation on Chalcopyrite Absorbers—Origins for Improved Open-Circuit Voltages in (Ag,Cu)(In,Ga)Se2 Solar Cells. Sol. RRL 2025, 9, 2400863. [Google Scholar] [CrossRef]
- Tom, T.; Lontchi, J.; Rebai, A.; Dufoulon, V.; Guillemoles, J.-F.; Naghavi, N. Ag-Alloying of CIGS Absorber Layers: Impact of the Composition, Rubidium Fluoride Post-Treatment and Bandgap Variations. J. Phys. Energy 2025, 7, 045005. [Google Scholar] [CrossRef]
- Kuo, S.-Y.; Hsieh, M.-Y.; Hsieh, D.-H.; Kuo, H.-C.; Chen, C.-H.; Lai, F.-I. Device Modeling of the Performance of Cu(In,Ga)Se2 Solar Cells with V-Shaped Bandgap Profiles. Int. J. Photoenergy 2014, 2014, 186579. [Google Scholar] [CrossRef]
- Saadat, M.; Moradi, M.; Zahedifar, M. CIGS Absorber Layer with Double Grading Ga Profile for Highly Efficient Solar Cells. Superlattices Microstruct. 2016, 92, 303–307. [Google Scholar] [CrossRef]
- Avancini, E.; Carron, R.; Bissig, B.; Reinhard, P.; Menozzi, R.; Sozzi, G.; Di Napoli, S.; Feurer, T.; Nishiwaki, S.; Buecheler, S.; et al. Impact of Compositional Grading and Overall Cu Deficiency on the Near-Infrared Response in Cu(In, Ga)Se2 Solar Cells. Prog. Photovolt. Res. Appl. 2017, 25, 233–241. [Google Scholar] [CrossRef]
- Jackson, P.; Wuerz, R.; Hariskos, D.; Lotter, E.; Witte, W.; Powalla, M. Effects of Heavy Alkali Elements in Cu(In,Ga)Se2 Solar Cells with Efficiencies up to 22.6%. Phys. Rapid Res. Lett. 2016, 10, 583–586. [Google Scholar] [CrossRef]
- Keller, D.; Buecheler, S.; Reinhard, P.; Pianezzi, F.; Bissig, B.; Carron, R.; Hage, F.; Ramasse, Q.; Erni, R.; Tiwari, A.N. Band Gap Widening at Random CIGS Grain Boundary Detected by Valence Electron Energy Loss Spectroscopy. Appl. Phys. Lett. 2016, 109, 153103. [Google Scholar] [CrossRef]
- Nicoara, N.; Manaligod, R.; Jackson, P.; Hariskos, D.; Witte, W.; Sozzi, G.; Menozzi, R.; Sadewasser, S. Direct Evidence for Grain Boundary Passivation in Cu(In,Ga)Se2 Solar Cells through Alkali-Fluoride Post-Deposition Treatments. Nat. Commun. 2019, 10, 3980. [Google Scholar] [CrossRef]
- Raghuwanshi, M.; Wuerz, R.; Cojocaru-Mirédin, O. Interconnection between Trait, Structure, and Composition of Grain Boundaries in Cu(In,Ga)Se2 Thin-Film Solar Cells. Adv. Funct. Mater. 2020, 30, 2001046. [Google Scholar] [CrossRef]
- Ochoa, M.; Buecheler, S.; Tiwari, A.N.; Carron, R. Challenges and Opportunities for an Efficiency Boost of next Generation Cu(In,Ga)Se2 Solar Cells: Prospects for a Paradigm Shift. Energy Environ. Sci. 2020, 13, 2047–2055. [Google Scholar] [CrossRef]
- Ma, C.; Ding, C.; Guo, H.; Liu, Y.; Chen, N.; Li, Y.; Xiang, C.; Wang, S.; Yan, W.; Xiao, K.; et al. Ag-Alloying Enables Wide-Bandgap (1.69 eV) Chalcopyrite Solar Cell with 11.5% Efficiency and over 900 mV Open Circuit Voltage from Molecular Solution. Nano Res. 2025, 18, 94907972. [Google Scholar] [CrossRef]
- Tom, T.; Lontchi, J.; Rebai, A.; Dufoulon, V.; Cacovich, S.; Raimondi, T.; Guillemoles, J.-F.; Naghavi, N. Unlocking High Efficiency in (Ag,Cu)(In,Ga)Se2 Solar Cells: A Parametric Study on Silver Alloying. In Proceedings of the 2025 IEEE 53rd Photovoltaic Specialists Conference (PVSC), Montreal, QC, Canada, 8–13 June 2025; pp. 0661–0663. [Google Scholar]
- Huang, Z. Silver-Alloyed CIGS Thin-Film Solar Cells on Flexible Stainless-Steel Substrate. Ph.D. Thesis, The City University of New York, New York, NY, USA, 2025. [Google Scholar]
- Li, W.; Chen, L.; Wang, D.; Liang, B.; Shao, X.; Sun, Y.; Yang, C. Defect Engineering via Ag and Na Co-Doping in Wide-Bandgap CIGS: From Interfacial Suppression to Bulk Enhancement. Mater. Futures 2025, 4, 045105. [Google Scholar] [CrossRef]
- Kamikawa, Y.; Nishinaga, J.; Nishida, T.; Ishizuka, S. Highly Efficient Lightweight Flexible Cu(In,Ga)Se2 Solar Cells with a Narrow Bandgap Fabricated on Polyimide Substrates: Impact of Ag Alloying, Cs and Na Doping, and Front Shallow Ga Grading on Cell Performance. Small Sci. 2025, 5, 2400404. [Google Scholar] [CrossRef] [PubMed]




| Voc (mV) | Jsc (mA/cm2) | FF (%) | η (%) | |
|---|---|---|---|---|
| ZSW 2016 | 741 | 37.8 | 80.6 | 22.6 |
| SF 2019 | 734 | 39.6 | 80.4 | 23.35 |
| UU 2023 | 767 | 38.3 | 80.5 | 23.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Tao, S.; Jia, M.; Han, J.; Zhou, J.; Baranova, M.; Gong, Q.; Zhuang, D.; Zhao, M. Bandgap Engineering of CIGS: Active Control of Composition Gradient. Energies 2025, 18, 6089. https://doi.org/10.3390/en18236089
Wu Z, Tao S, Jia M, Han J, Zhou J, Baranova M, Gong Q, Zhuang D, Zhao M. Bandgap Engineering of CIGS: Active Control of Composition Gradient. Energies. 2025; 18(23):6089. https://doi.org/10.3390/en18236089
Chicago/Turabian StyleWu, Zhihao, Shengye Tao, Mengyao Jia, Junsu Han, Jihui Zhou, Maria Baranova, Qianming Gong, Daming Zhuang, and Ming Zhao. 2025. "Bandgap Engineering of CIGS: Active Control of Composition Gradient" Energies 18, no. 23: 6089. https://doi.org/10.3390/en18236089
APA StyleWu, Z., Tao, S., Jia, M., Han, J., Zhou, J., Baranova, M., Gong, Q., Zhuang, D., & Zhao, M. (2025). Bandgap Engineering of CIGS: Active Control of Composition Gradient. Energies, 18(23), 6089. https://doi.org/10.3390/en18236089






