A Review on Laminar Burning Velocity of Ammonia Flames
Abstract
1. Introduction
1.1. Characteristics and Advantages of Ammonia Fuel
- (1)
- It is a carbon-free fuel, with no greenhouse gas emissions during its application;
- (2)
- Its energy density is 22.5 MJ/kg, which is comparable to that of conventional fossil fuels. For instance, the calorific value of low-rank coal is approximately 20 MJ/kg;
- (3)
- At room temperature its condensation pressure is only 0.99 MPa, and its boiling point is −33.4 °C under atmospheric pressure. Moreover, the volumetric energy density of liquid ammonia exceeds that of liquid hydrogen by 45% and is virtually identical to that of gasoline, making it a prospective transportation fuel;
- (4)
- Ammonia has a pungent odor. Humans can detect this odor at volume fractions as low as 5 × 10−6, which provides an inherent leak-warning capability;
- (5)
- The large-scale industrial production of ammonia is relatively mature and well-suited to China’s national conditions. China processes abundant renewable energy resources, which can be used to synthesize green ammonia through a fully decarbonized industrial process.
1.2. Challenges in Ammonia Combustion
1.3. Research Value of Laminar Burning Velocity of Ammonia Flames
1.4. Objectives and Structure of This Work
2. Measurement Methods of Laminar Burning Velocity for Ammonia Flames
- (1)
- Steady state: Neither the flame nor the flow field varies with time;
- (2)
- Adiabaticity: Heat released by the flame is solely used to heat unburned substances—no losses by radiation, conduction or convection to the surroundings;
- (3)
- One-dimensional free diffusion: The flow field is unobstructed, with infinitely long spaces both upstream and downstream of the flame.
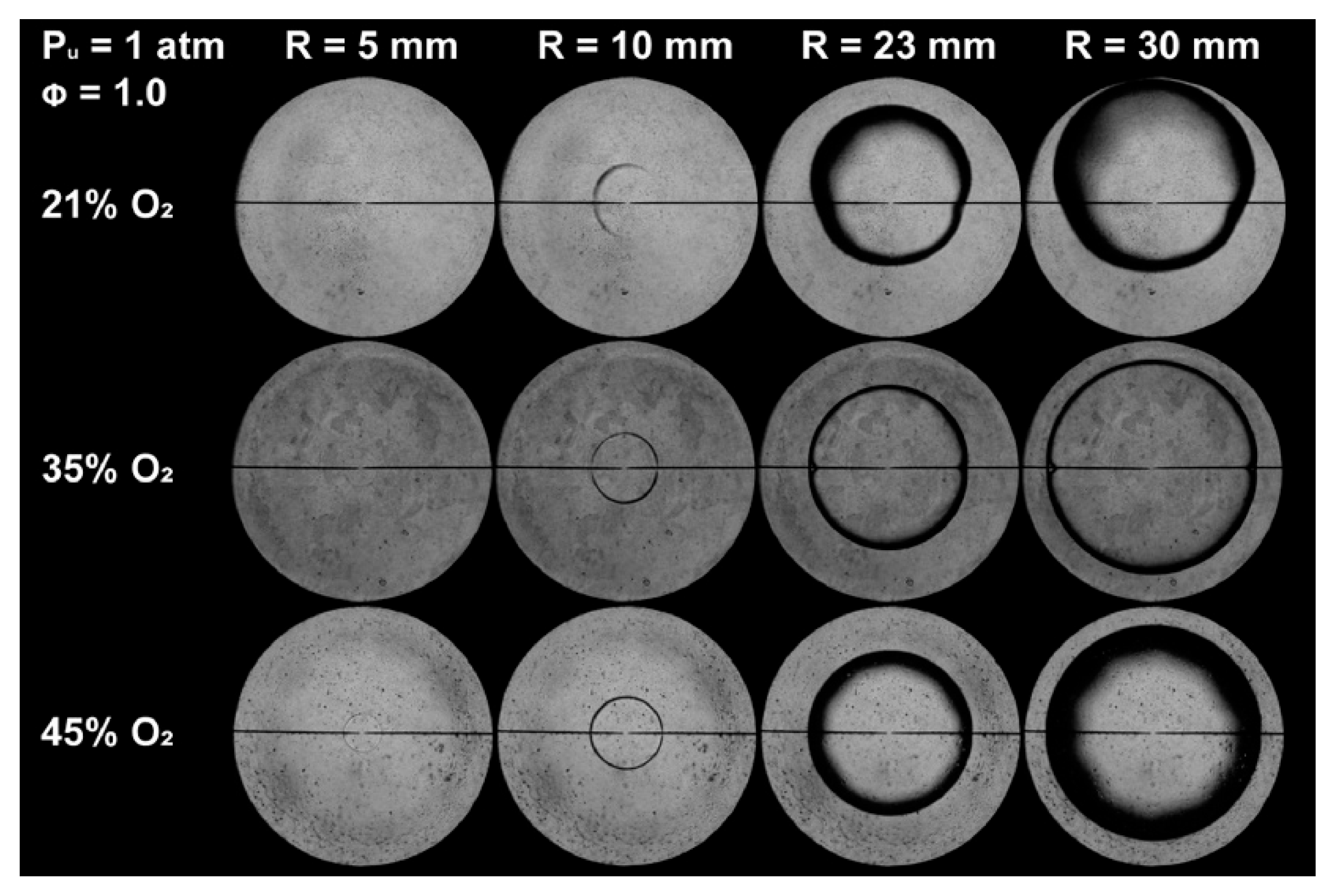
2.1. Spherical Outwardly Propagating Flame Method
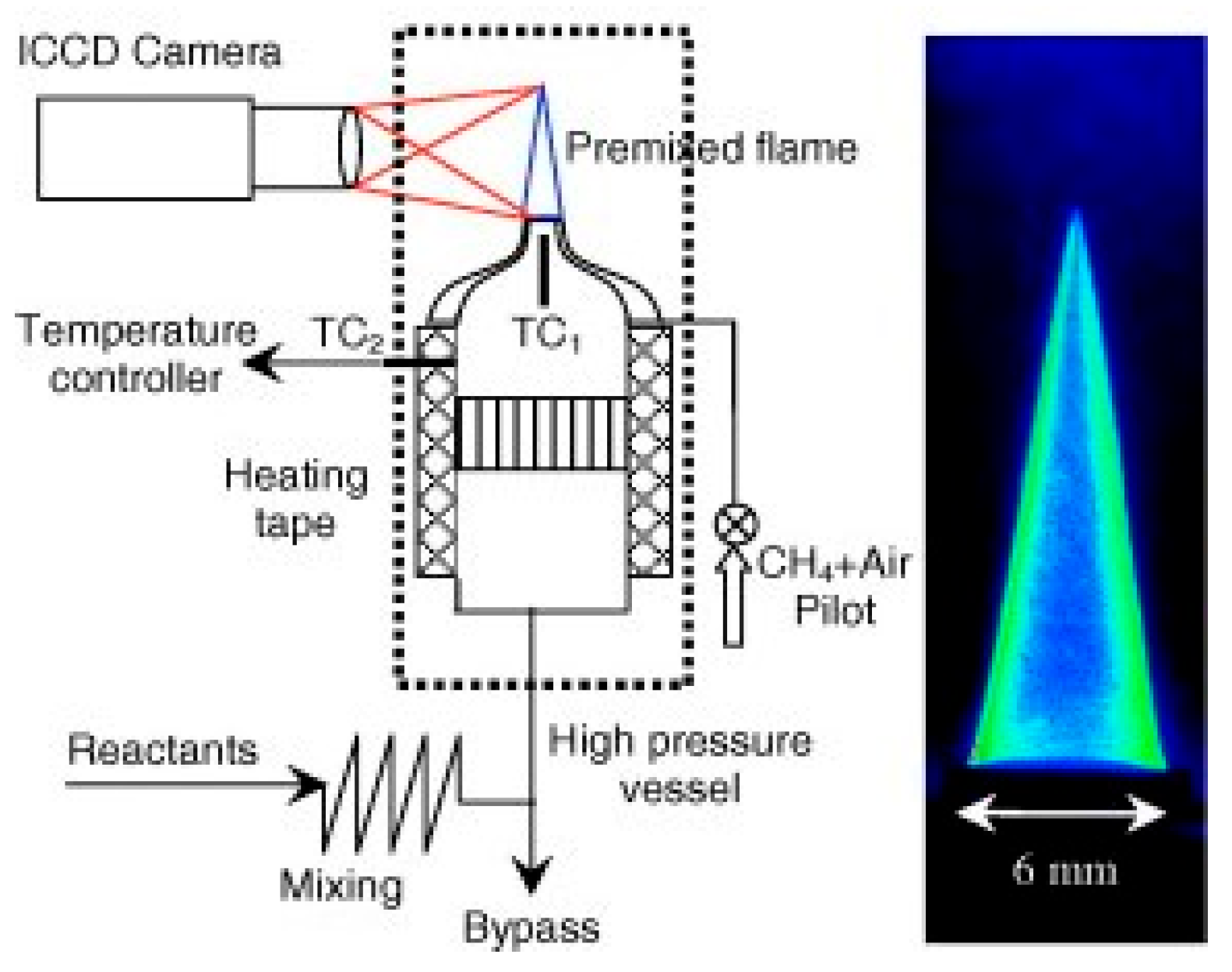
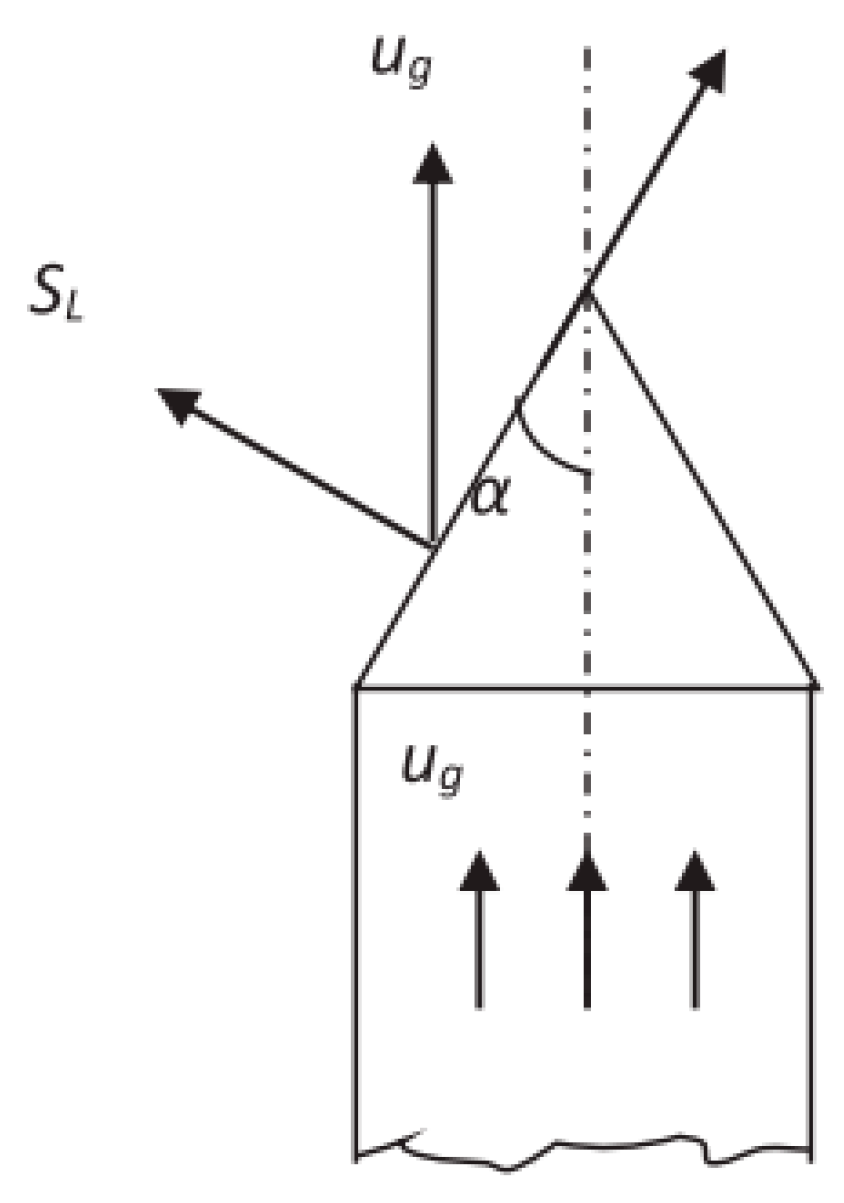
2.2. Bunsen-Burner Method
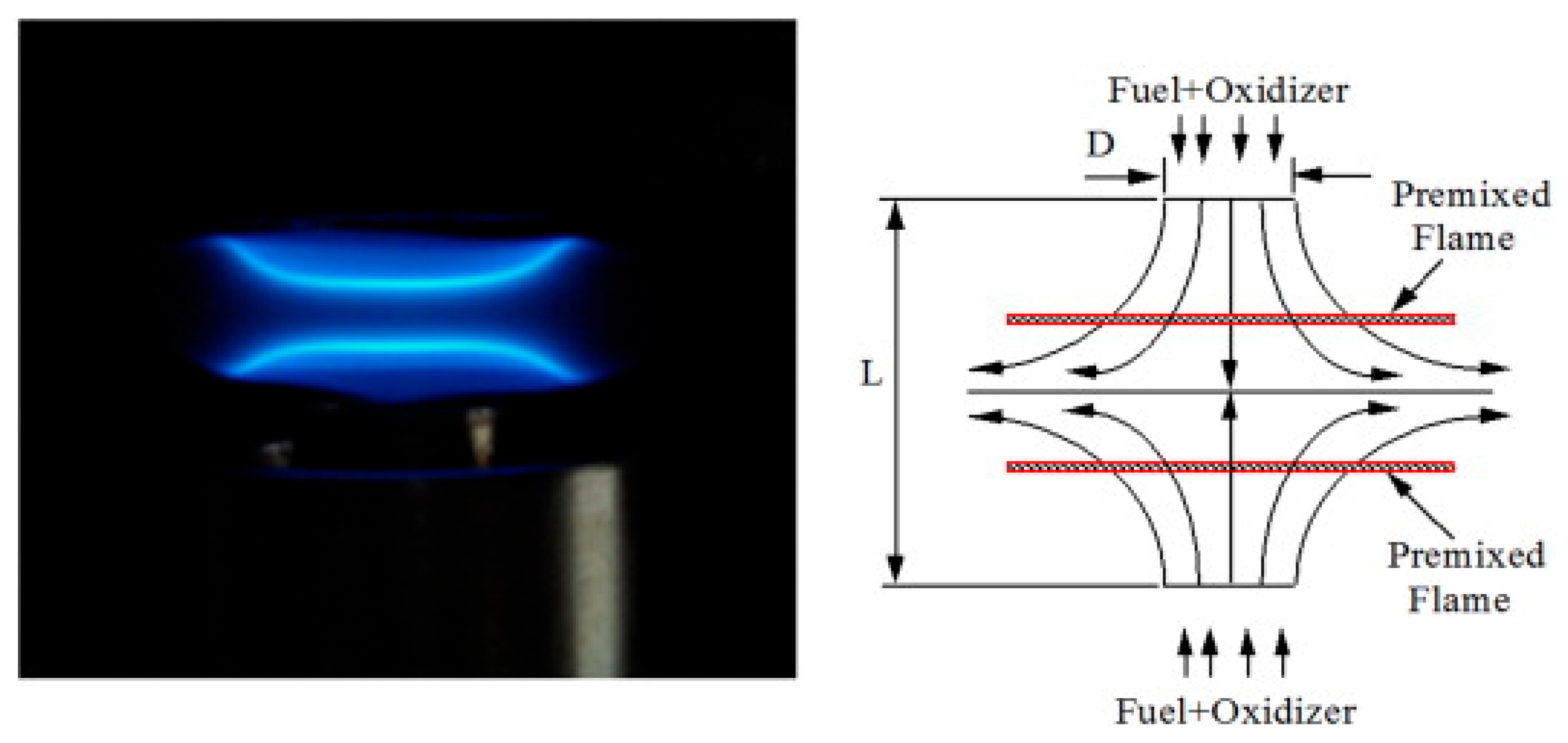
2.3. Counter-Flow Flame and Stagnation Flame Method
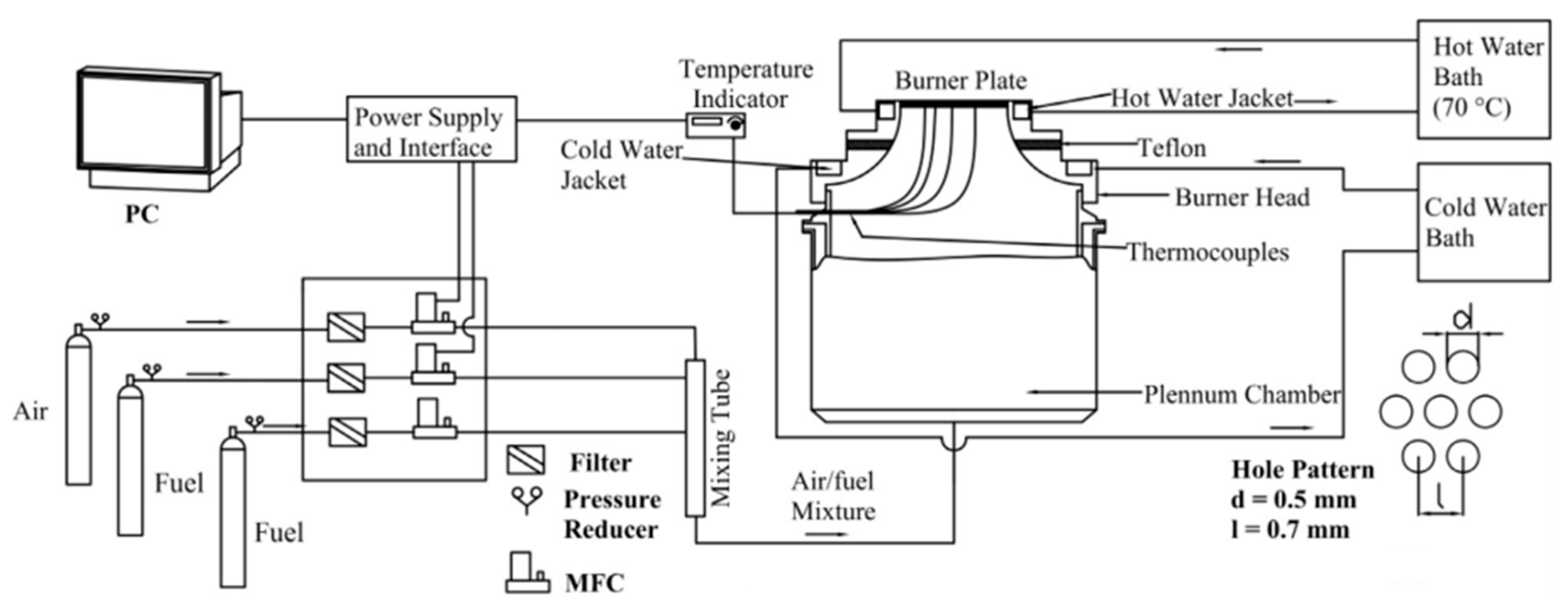
2.4. Heat Flux Method
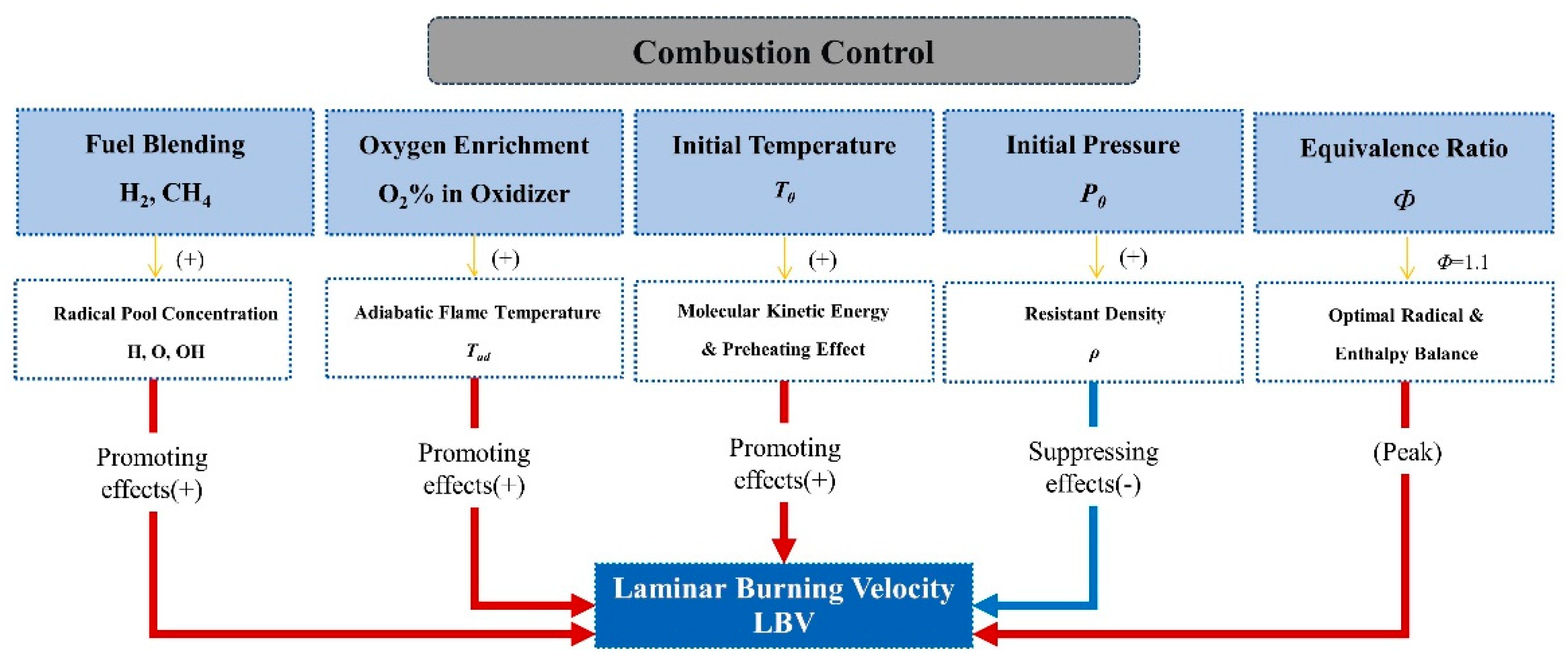
3. Characteristics of Laminar Burning Velocity for Ammonia Flames
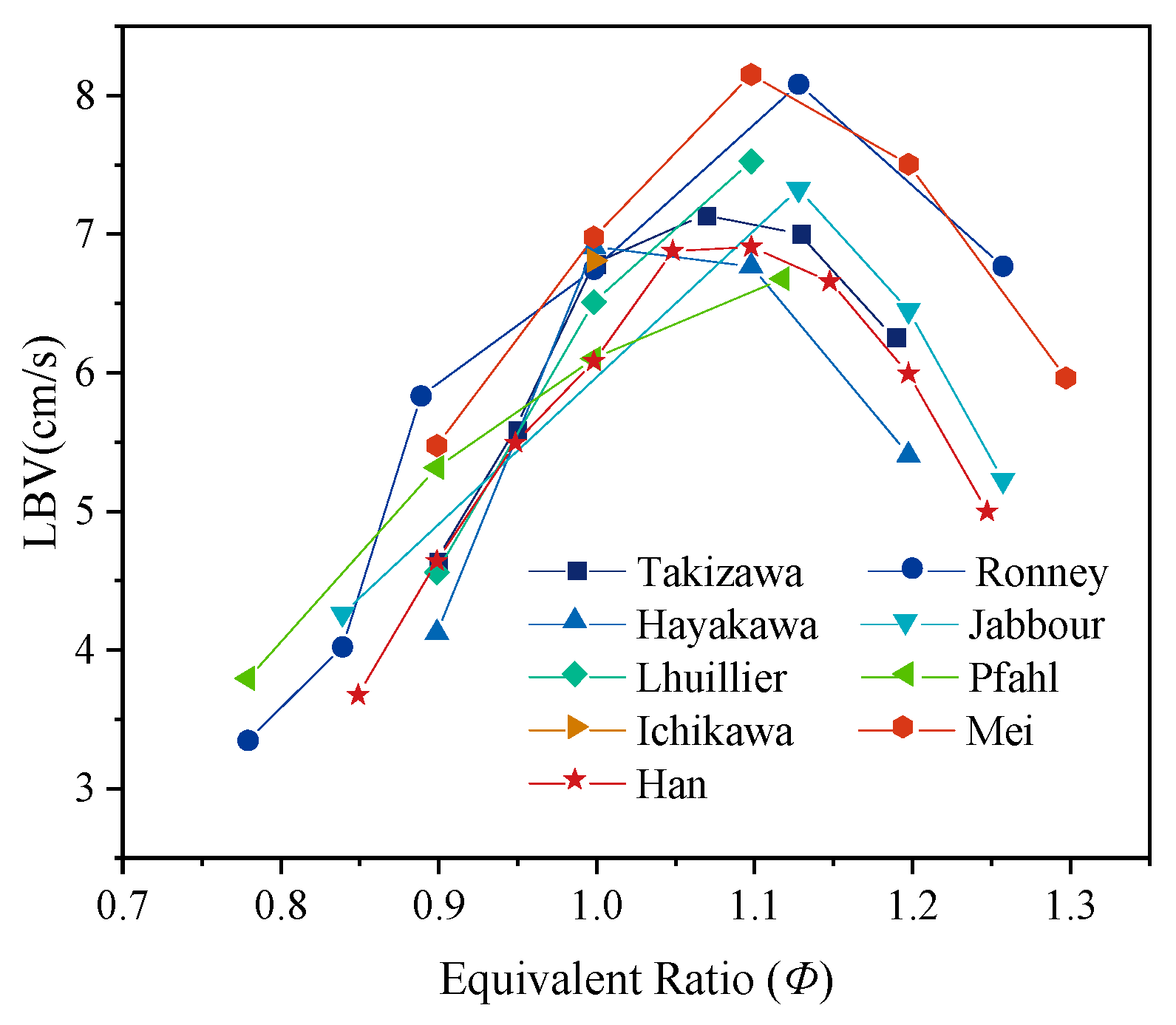
3.1. LBV of Pure Ammonia Flames Under Ambient Temperature and Pressure
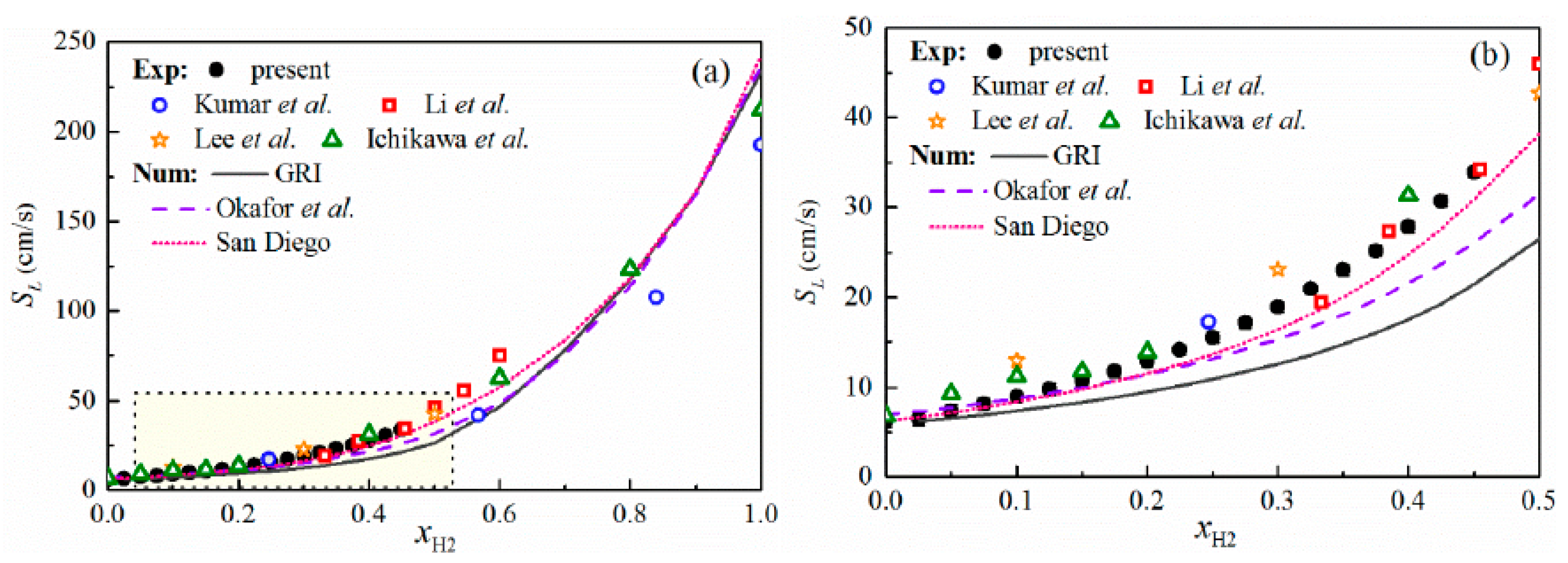
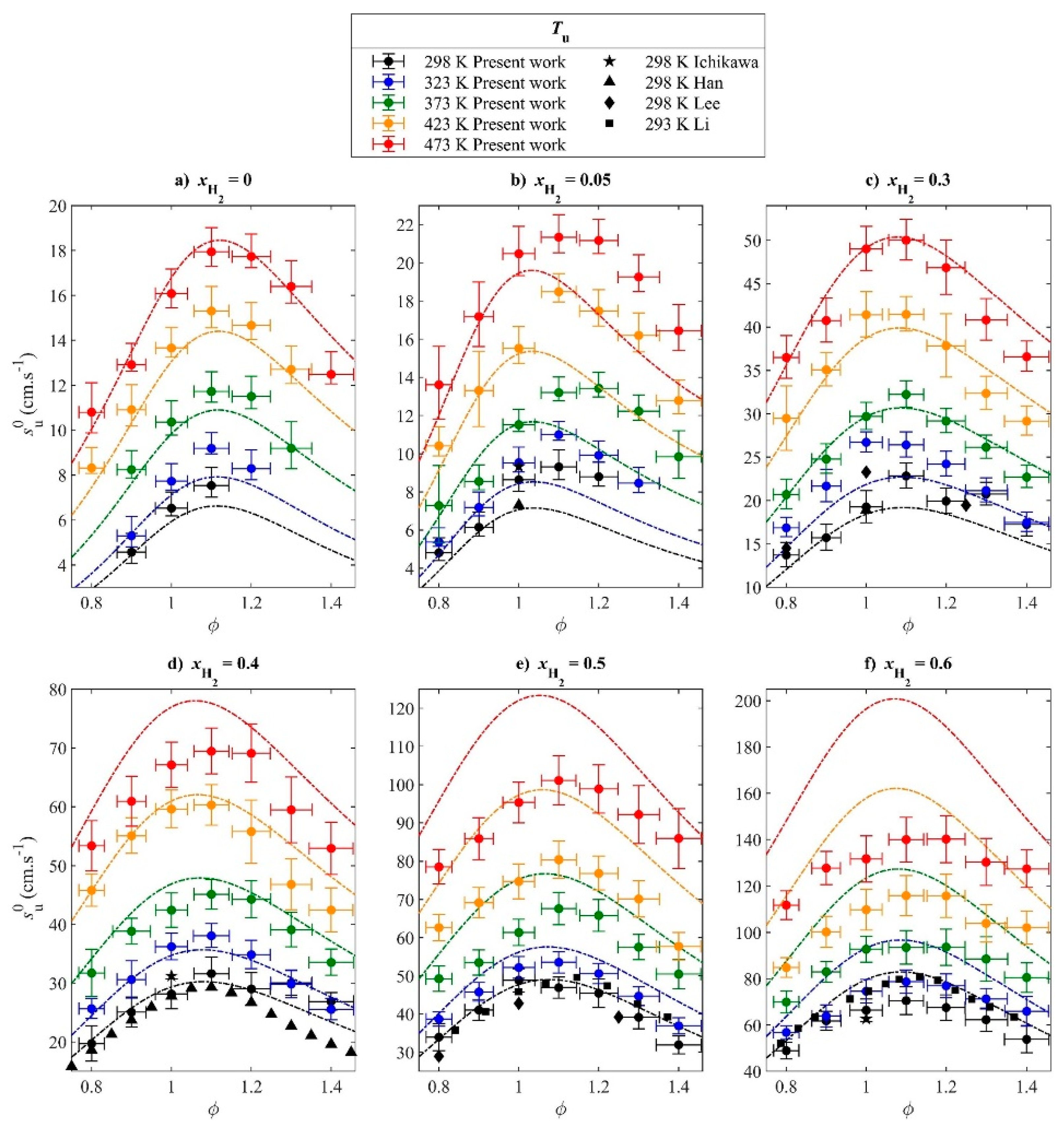
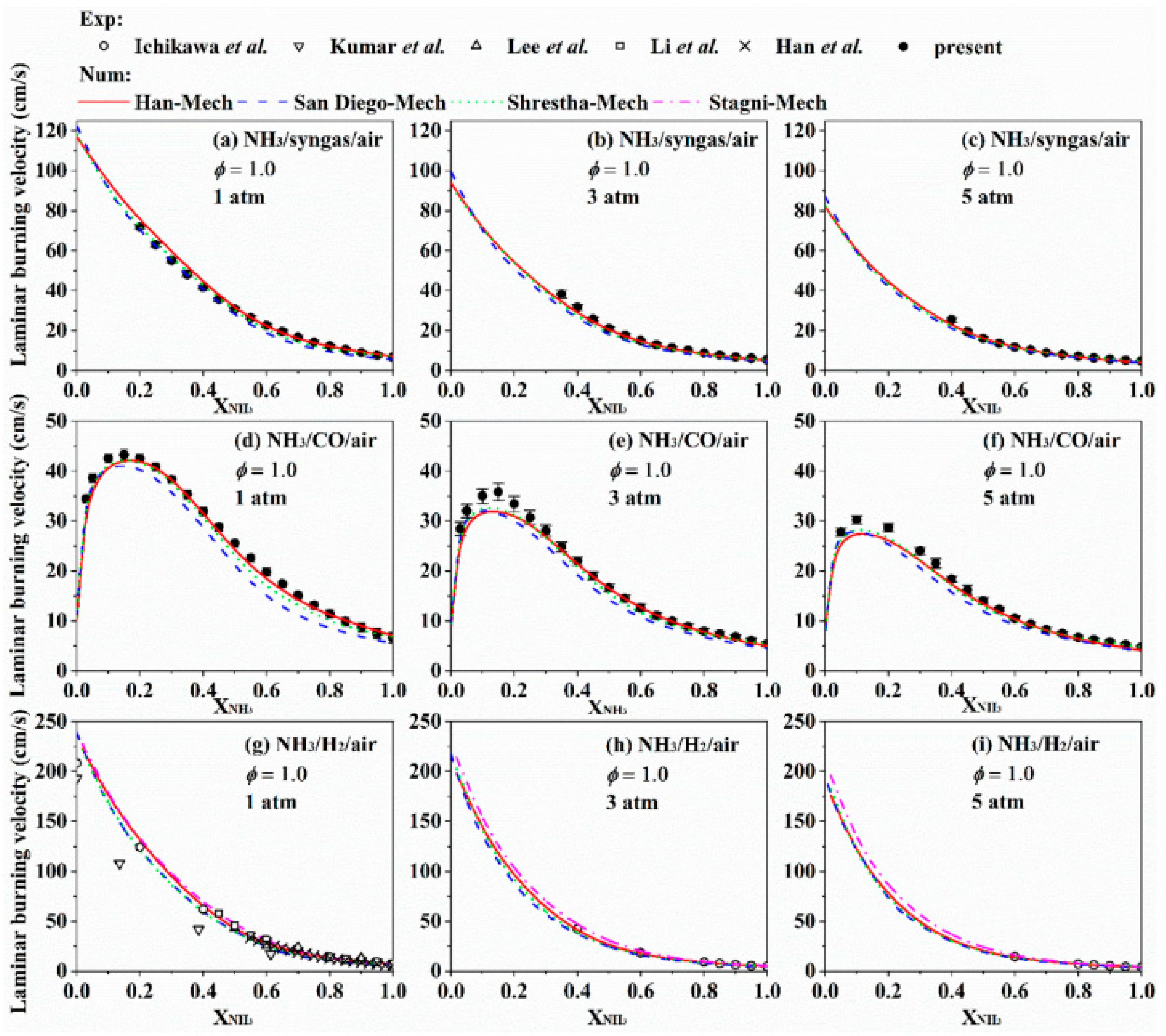
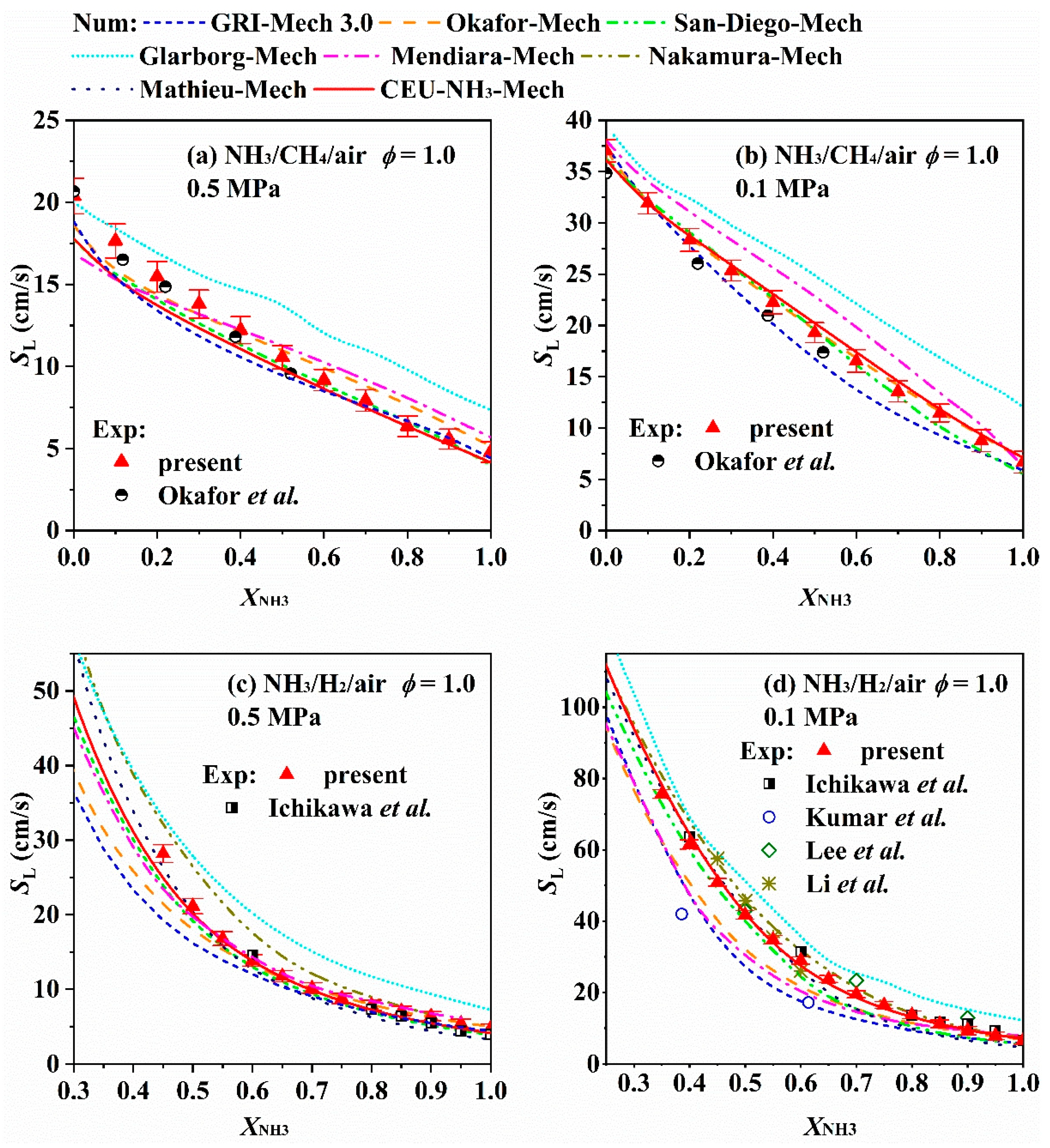
3.2. Effect of Blending High-Reactivity Fuels on the LBV of Ammonia Flames
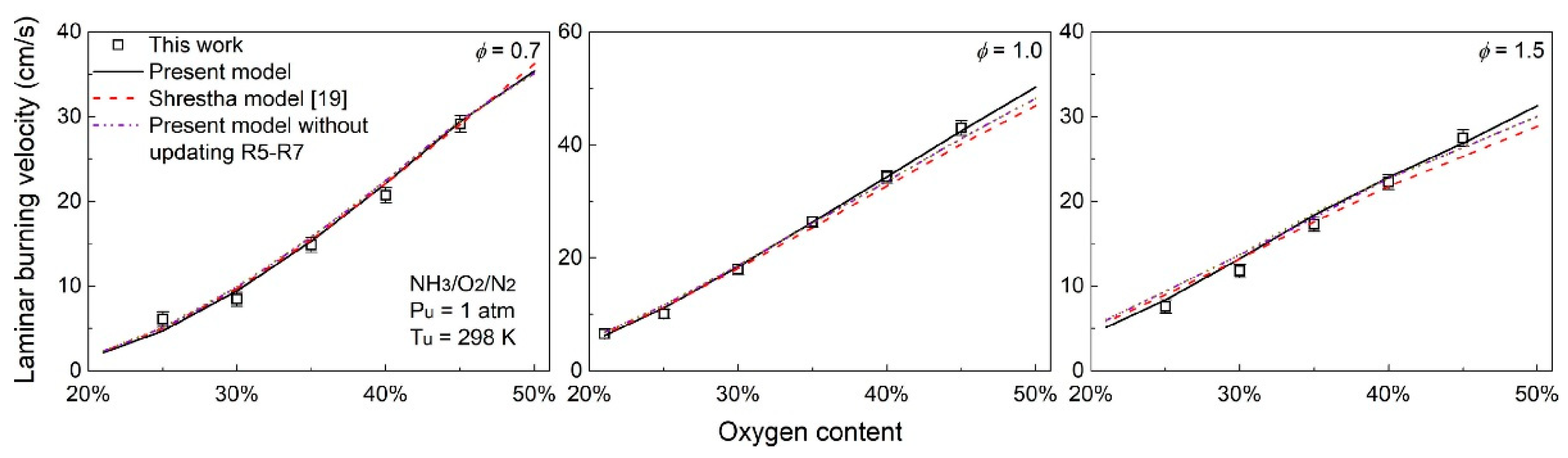
3.3. Effect of Oxygen-Enriched Combustion on the LBV of Ammonia Flames
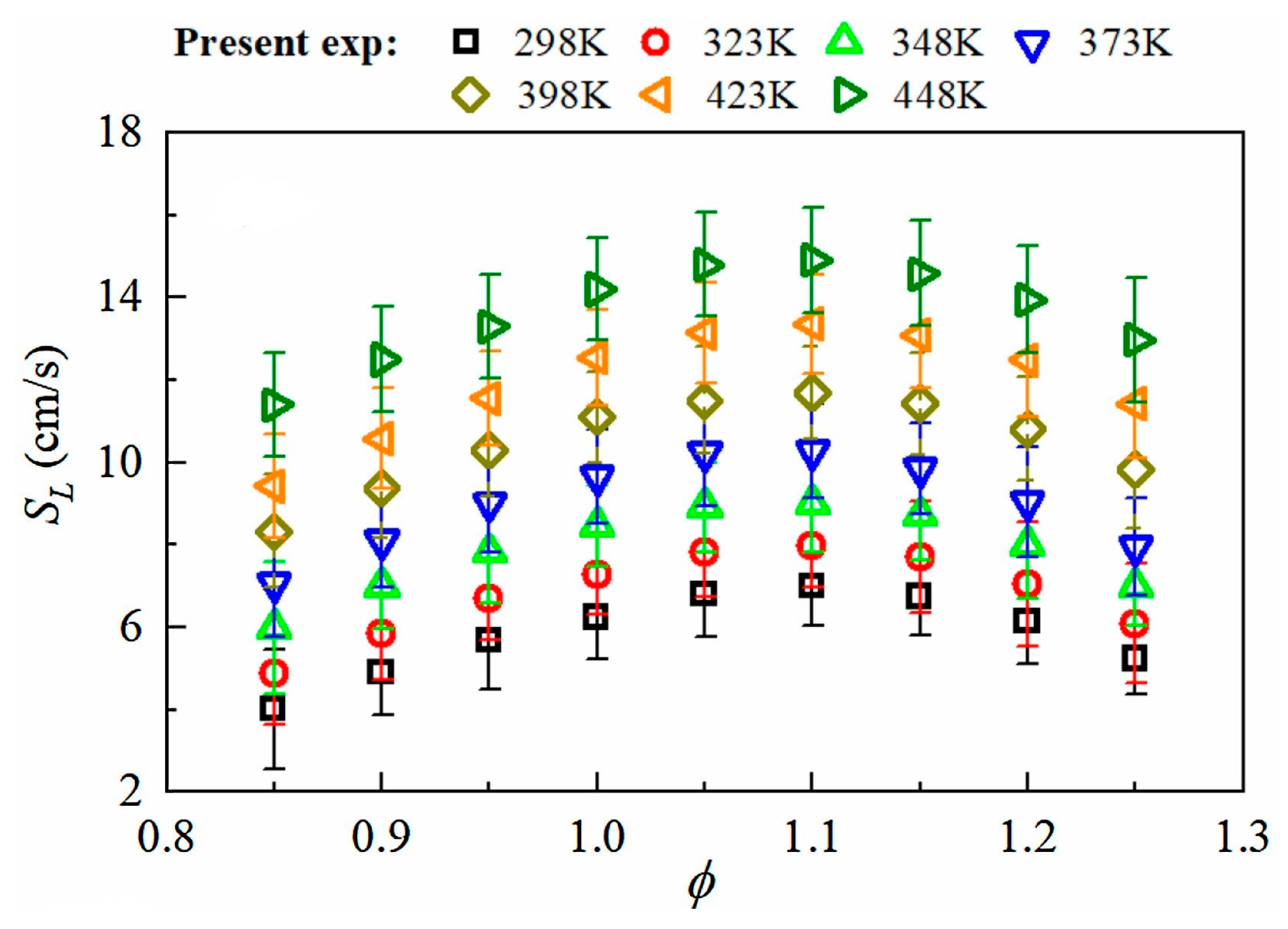
3.4. Effect of Temperature on the LBV of Ammonia Flames
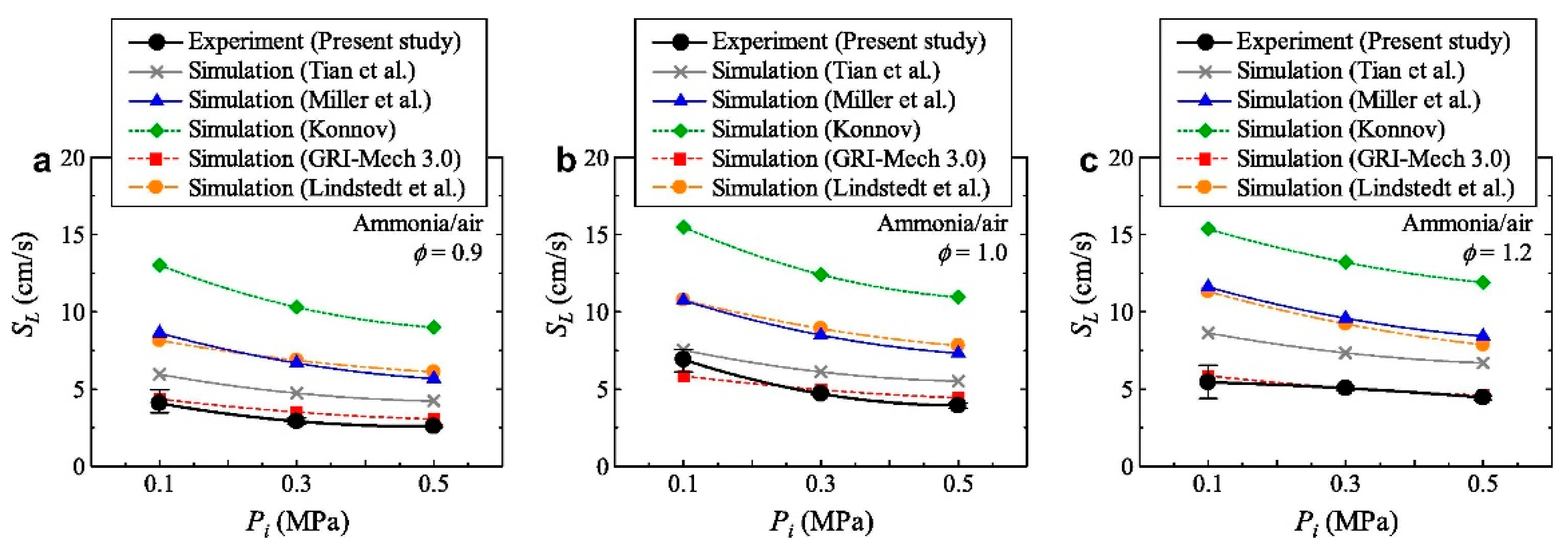
3.5. Effect of Pressure on the LBV of Ammonia Flames
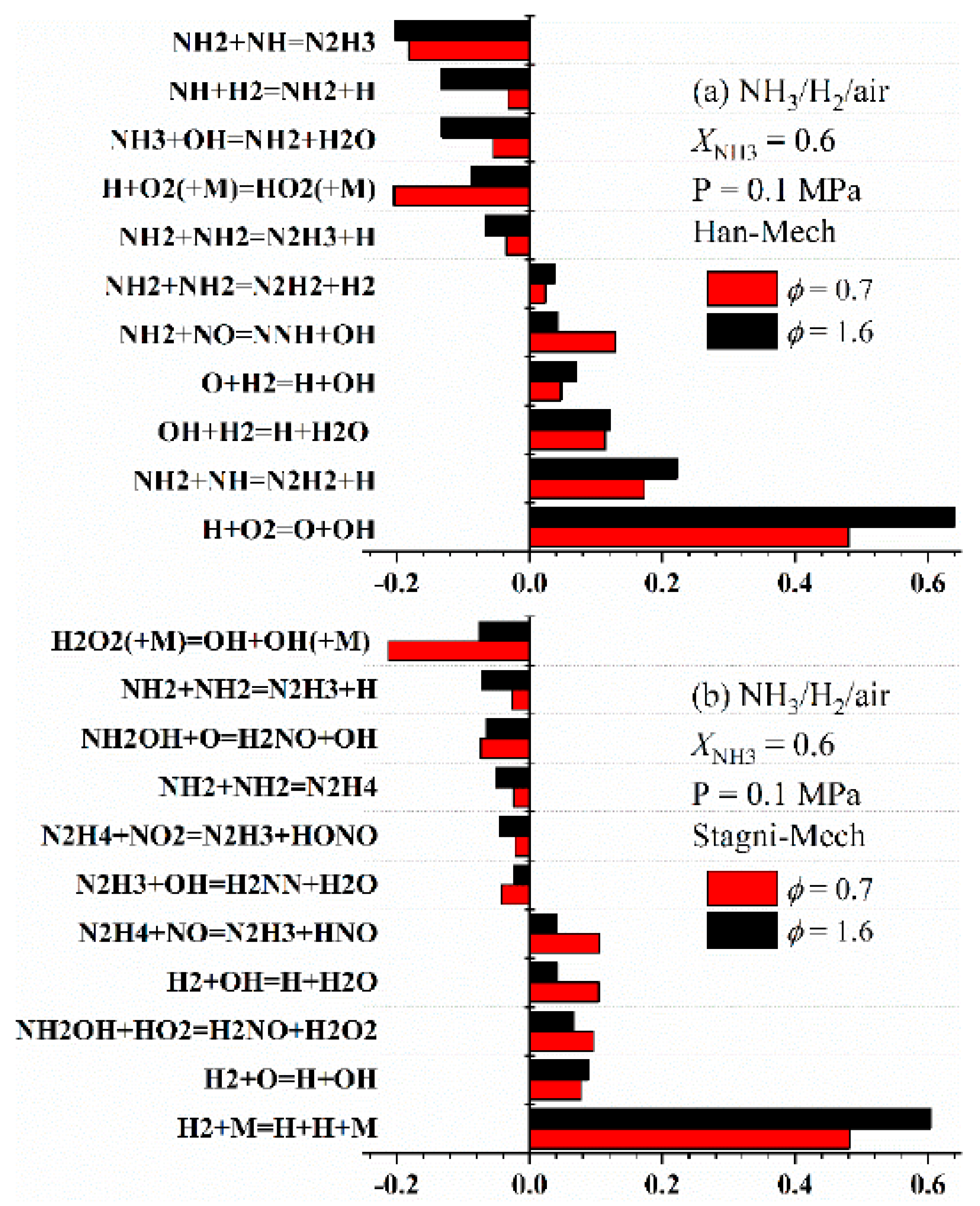
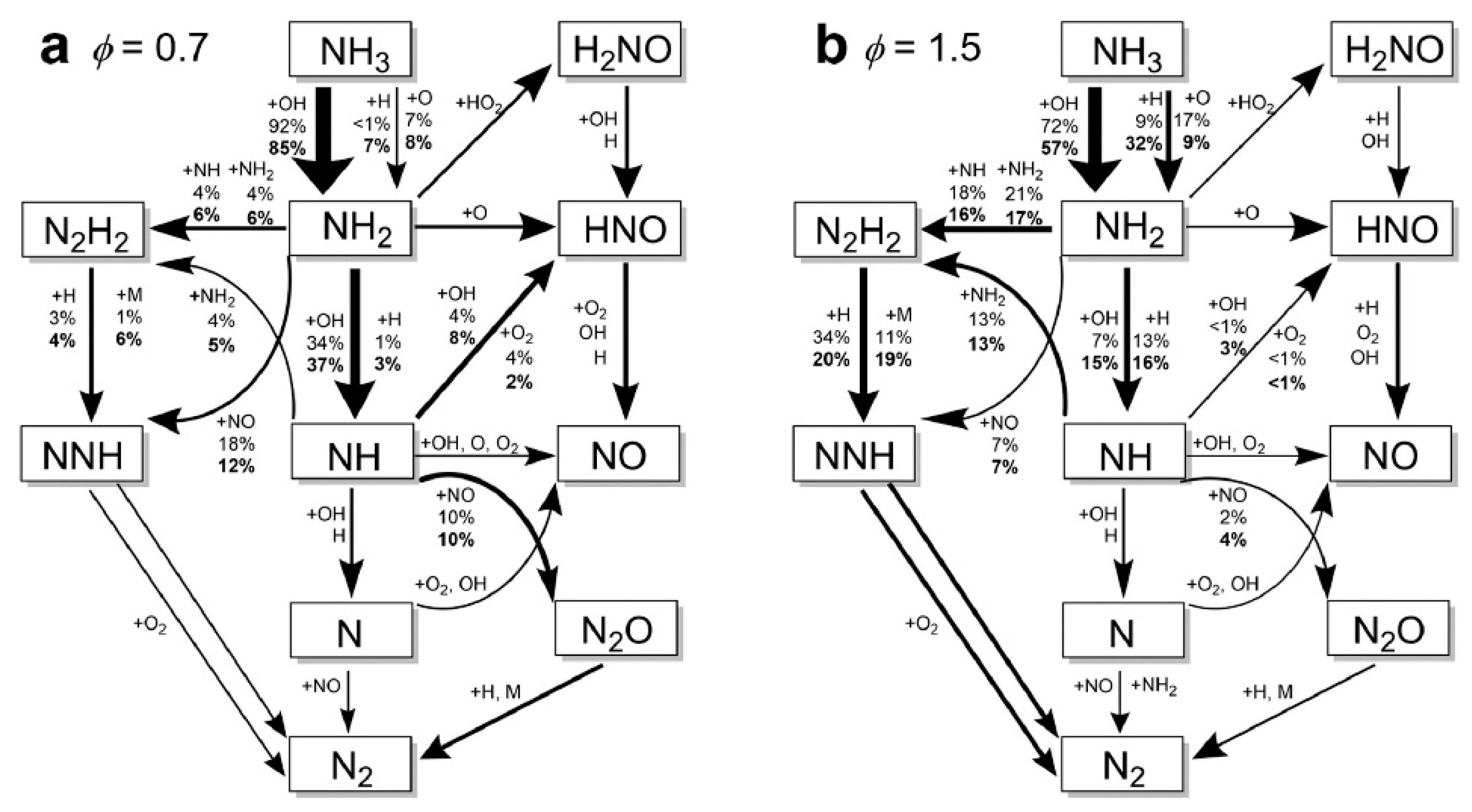
4. Development of Reaction Kinetic Models for Ammonia Combustion
5. Conclusions and Recommendations
- (1)
- The extremely low LBV and flame instability of ammonia impose strict requirements on LBV measurement techniques. The spherical outwardly propagating flame method is significantly affected by buoyancy effects and stretch correction. This influence leads to relatively large measurement errors, especially for pure ammonia flames. The Bunsen-burner method is also unsuitable for measuring the LBV of low-velocity pure ammonia flames due to issues of flame stability and heat dissipation. In contrast, the counter-flow flame method and heat flux method are currently the more reliable choices for measuring the LBV of pure ammonia and ammonia-blended fuels. Each of these two methods leverages distinct advantages: the counter-flow flame method has high stability and low stretch rate, while the heat flux method has quasi-adiabatic characteristics.
- (2)
- The LBV of pure ammonia flames is extremely low (approximately 6–7 cm/s under atmospheric temperature and pressure), which severely limits its direct application in combustion systems. While liquid ammonia spray combustion plays a role in promoting ammonia combustion. Blending high-reactivity fuels (e.g., hydrogen, methane) is an effective way to increase LBV, among which hydrogen exhibits a particularly significant enhancement effect—with LBV showing an exponential growth trend as the hydrogen blending ratio increases. Furthermore, hydrogen blending also facilitates the ignition of liquid ammonia and promotes its spray combustion. Oxygen-enriched combustion can greatly increase the LBV of ammonia flames—when the oxygen concentration exceeds 40%, the LBV can reach the level of conventional hydrocarbon fuels. Increasing the initial temperature significantly promotes LBV, whereas higher pressure generally leads to a decrease in LBV.
- (3)
- Although numerous chemical reaction kinetic models have been developed for systems such as pure ammonia, ammonia/hydrogen, and ammonia/methane, and most of them can well predict LBV under atmospheric pressure and typical blending ratios, there are discrepancies in prediction results between different models under complex conditions (e.g., high pressure, moderate blending ratios), and deviations also exist between model predictions and experimental data. This reveals that significant uncertainties remain in current ammonia combustion kinetic mechanisms, particularly regarding key nitrogen-containing reaction pathways and their pressure-dependent behaviors.
- (1)
- Current experimental data on ammonia flame LBV are mostly concentrated under atmospheric pressure, moderate-to-low temperatures, and moderate-to-low blending ratios. Urgent efforts are needed to systematically perform accurate LBV measurements of ammonia and its blends with diverse fuels (e.g., syngas, alcohols) under extreme operating conditions—such as high pressure (>5 atm), low pressure (<1 atm), high temperature (>1000 K), and high-to-extreme oxygen concentrations (including pure oxygen). Such data will provide critical support for practical application scenarios including internal combustion engines, gas turbines, aerospace, and high-altitude areas.
- (2)
- Further development is needed for high-precision, low-uncertainty LBV measurement methods highly suitable for ammonia flames—especially under high-pressure and extremely high-oxygen-concentration conditions. Efforts should be directed toward integrating advanced optical measurement techniques and radical imaging technology to improve the accuracy of flame structure identification and LBV extraction.
- (3)
- Current models still exhibit errors in LBV prediction under high-pressure, moderate blending ratio, and high-temperature conditions. In the future, by leveraging sensitivity and pathway analysis, focus should be placed on verifying the rate constants of key elementary reactions (e.g., H + O2 = O + OH, etc.), and developing unified simplified mechanisms highly applicable across a wide range of conditions.
- (4)
- Future research should place greater emphasis on applying optimized kinetic models to CFD simulations of key actual combustion devices (e.g., gas turbines, internal combustion engines). This application will enable cross-scale accurate prediction and optimization. The optimization range covers from fundamental combustion parameters to system-level combustion performance, thereby accelerating the practical application of ammonia fuel.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Zhou, Y. Numerical optimization of the influence of multiple deep air-staged combustion on the NOx emission in an opposed firing utility boiler using lean coal. Fuel 2020, 269, 116996. [Google Scholar] [CrossRef]
- Chen, Z.; You, C.; Wang, H.; Liu, Q. Experimental study on the synergetic removal of fine particles by wet flue gas desulfurization tower with a flow pattern control device. Powder Technol. 2019, 343, 122–128. [Google Scholar] [CrossRef]
- Akihama, K.; Takatori, Y.; Inagaki, K.; Sasaki, S.; Dean, A.M. Mechanism of the Smokeless Rich Diesel Combustion by Reducing Temperature; SAE Technical Paper Series; SAE International: Warrendale, PA, USA, 2001. [Google Scholar]
- Ishida, M.; Yamamoto, S.; Ueki, H.; Sakaguchi, D. Remarkable improvement of NOx–PM trade-off in a diesel engine by means of bioethanol and EGR. Energy 2010, 35, 4572–4581. [Google Scholar] [CrossRef]
- Feng, D.; Guo, D.; Zhang, Y.; Sun, S.; Zhao, Y.; Shang, Q.; Sun, H.; Wu, J.; Tan, H. Functionalized construction of biochar with hierarchical pore structures and surface O-/N-containing groups for phenol adsorption. Chem. Eng. J. 2021, 410, 127707. [Google Scholar] [CrossRef]
- Shang, Q.; Feng, D.; Zhang, X.; Cheng, Z.; Wang, S.; Zhao, Y.; Sun, S. Synergistic effects of biomass/plastics and multi-step regulation of H2O in the co-production of H2-CNTs by gasification of biomass and plastic wastes. Appl. Catal. B Environ. Energy 2025, 379, 125695. [Google Scholar] [CrossRef]
- Zou, C.; Xiong, B.; Xue, H.; Zheng, D.; Ge, Z.; Wang, Y.; Jiang, L.; Pan, S.; Wu, S. The role of new energy in carbon neutral. Petrol. Explor. Dev. 2021, 48, 480–491. [Google Scholar] [CrossRef]
- Kojima, Y. Hydrogen storage materials for hydrogen and energy carriers. Int. J. Hydrogen Energy 2019, 44, 18179–18192. [Google Scholar] [CrossRef]
- Kobayashi, H.; Hayakawa, A.; Somarathne, K.D.K.; Okafor, E. Science and technology of ammonia combustion. P. Combust. Inst. 2019, 37, 109–133. [Google Scholar] [CrossRef]
- Murai, R.; Omori, R.; Kano, R.; Tada, Y.; Higashino, H.; Nakatsuka, N.; Hayashi, J.; Akamatsu, F.; Iino, K.; Yamamoto, Y. The radiative characteristics of NH3/N2/O2 non-premixed flame on a 10 kW test furnace. Energy Procedia 2017, 120, 325–332. [Google Scholar] [CrossRef]
- Kurata, O.; Iki, N.; Matsunuma, T.; Inoue, T.; Tsujimura, T.; Furutani, H.; Kobayashi, H.; Hayakawa, A. Performances and emission characteristics of NH3–air and NH3–CH4–air combustion gas-turbine power generations. P. Combust. Inst. 2017, 36, 3351–3359. [Google Scholar] [CrossRef]
- Konnov, A.A.; Mohammad, A.; Kishore, V.R.; Kim, N.I.; Prathap, C.; Kumar, S. A comprehensive review of measurements and data analysis of laminar burning velocities for various fuel+air mixtures. Prog. Energy Combust. Sci. 2018, 68, 197–267. [Google Scholar] [CrossRef]
- Nair, M.R.S.; Gupta, M.C. Burning velocity measurement by bomb method. Combust. Flame 1974, 22, 219–221. [Google Scholar] [CrossRef]
- San Diego Mechanism. Available online: http://combustion.ucsd.edu (accessed on 9 October 2025).
- Elbaz, A.M.; Giri, B.R.; Issayev, G.; Shrestha, K.P.; Mauss, F.; Farooq, A.; Roberts, W.L. Experimental and Kinetic Modeling Study of Laminar Flame Speed of Dimethoxymethane and Ammonia Blends. Energy Fuel 2020, 34, 14726–14740. [Google Scholar] [CrossRef]
- Mei, B.; Zhang, X.; Ma, S.; Cui, M.; Guo, H.; Cao, Z.; Li, Y. Experimental and kinetic modeling investigation on the laminar flame propagation of ammonia under oxygen enrichment and elevated pressure conditions. Combust. Flame 2019, 210, 236–246. [Google Scholar] [CrossRef]
- Natarajan, J.; Kochar, Y.; Lieuwen, T.; Seitzman, J. Pressure and preheat dependence of laminar flame speeds of H2/CO/CO2/O2/He mixtures. P. Combust. Inst. 2009, 32, 1261–1268. [Google Scholar] [CrossRef]
- Kumar, P.; Meyer, T.R. Experimental and modeling study of chemical-kinetics mechanisms for H2–NH3–air mixtures in laminar premixed jet flames. Fuel 2013, 108, 166–176. [Google Scholar] [CrossRef]
- Natarajan, J.; Lieuwen, T.; Seitzman, J. Laminar flame speeds of H2/CO mixtures: Effect of CO2 dilution, preheat temperature, and pressure. Combust. Flame 2007, 151, 104–119. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, W.; Yu, B.; Li, X.; Zhang, L.; Zhao, Y.; Sun, S. Experimental and kinetic modeling study on laminar flame speeds and emission characteristics of oxy-ammonia premixed flames. Int. J. Hydrogen Energy 2024, 63, 857–870. [Google Scholar] [CrossRef]
- Simmons, R.F.; Wolfhard, H.G. Some limiting oxygen concentrations for diffusion flames in air diluted with nitrogen. Combust. Flame 1957, 1, 155–161. [Google Scholar] [CrossRef]
- Law, C.K.; Zhu, D.L.; Yu, G. Propagation and extinction of stretched premixed flames. Symp. Combust. 1988, 21, 1419–1426. [Google Scholar] [CrossRef]
- Egolfopoulos, F.N.; Hansen, N.; Ju, Y.; Kohse-Höinghaus, K.; Law, C.K.; Qi, F. Advances and challenges in laminar flame experiments and implications for combustion chemistry. Prog. Energy Combust. Sci. 2014, 43, 36–67. [Google Scholar] [CrossRef]
- Niemann, U.; Seshadri, K.; Williams, F.A. Accuracies of laminar counterflow flame experiments. Combust. Flame 2015, 162, 1540–1549. [Google Scholar] [CrossRef]
- Bosschaart, K.J.; de Goey, L.P.H. The laminar burning velocity of flames propagating in mixtures of hydrocarbons and air measured with the heat flux method. Combust. Flame 2004, 136, 261–269. [Google Scholar] [CrossRef]
- de Goey, L.P.H.; van Maaren, A.; Quax, R.M. Stabilization of Adiabatic Premixed Laminar Flames on a Flat Flame Burner. Combust. Sci. Technol. 1993, 92, 201–207. [Google Scholar] [CrossRef]
- Han, X.; Wang, Z.; Costa, M.; Sun, Z.; He, Y.; Cen, K. Experimental and kinetic modeling study of laminar burning velocities of NH3/air, NH3/H2/air, NH3/CO/air and NH3/CH4/air premixed flames. Combust. Flame 2019, 206, 214–226. [Google Scholar] [CrossRef]
- Han, X.; Wang, Z.; He, Y.; Zhu, Y.; Cen, K. Experimental and kinetic modeling study of laminar burning velocities of NH3/syngas/air premixed flames. Combust. Flame 2020, 213, 1–13. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Z.; Elbaz, A.M.; Han, X.; He, Y.; Costa, M.; Konnov, A.A.; Roberts, W.L. Experimental study and kinetic analysis of the laminar burning velocity of NH3/syngas/air, NH3/CO/air and NH3/H2/air premixed flames at elevated pressures. Combust. Flame 2020, 221, 270–287. [Google Scholar] [CrossRef]
- Wang, Z.; Han, X.; He, Y.; Zhu, R.; Zhu, Y.; Zhou, Z.; Cen, K. Experimental and kinetic study on the laminar burning velocities of NH3 mixing with CH3OH and C2H5OH in premixed flames. Combust. Flame 2021, 229, 111392. [Google Scholar] [CrossRef]
- Ronney, P.D. Effect of Chemistry and Transport Properties on Near-Limit Flames at Microgravity. Combust. Sci. Technol. 1988, 59, 123–141. [Google Scholar] [CrossRef]
- Pfahl, U.J.; Ross, M.C.; Shepherd, J.E.; Pasamehmetoglu, K.O.; Unal, C. Flammability limits, ignition energy, and flame speeds in H2–CH4–NH3–N2O–O2–N2 mixtures. Combust. Flame 2000, 123, 140–158. [Google Scholar] [CrossRef]
- Jabbour, T.; Clodic, D.F. Burning velocity and refrigerant flammability classification. ASHRAE Trans. 2004, 110, 522–533. Available online: https://www.researchgate.net/publication/279588720_Burning_velocity_and_refrigerant_flammability_classification (accessed on 9 October 2025).
- Zakaznov, V.F.; Kursheva, L.A.; Fedina, Z.I. Determination of normal flame velocity and critical diameter of flame extinction in ammonia-air mixture. Combust. Explos. Shock. Waves 1978, 14, 710–713. [Google Scholar] [CrossRef]
- Takizawa, K.; Takahashi, A.; Tokuhashi, K.; Kondo, S.; Sekiya, A. Burning velocity measurements of nitrogen-containing compounds. J. Hazard. Mater. 2008, 155, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, A.; Goto, T.; Mimoto, R.; Arakawa, Y.; Kudo, T.; Kobayashi, H. Laminar burning velocity and Markstein length of ammonia/air premixed flames at various pressures. Fuel 2015, 159, 98–106. [Google Scholar] [CrossRef]
- Ichikawa, A.; Hayakawa, A.; Kitagawa, Y.; Kunkuma Amila Somarathne, K.D.; Kudo, T.; Kobayashi, H. Laminar burning velocity and Markstein length of ammonia/hydrogen/air premixed flames at elevated pressures. Int. J. Hydrogen Energy 2015, 40, 9570–9578. [Google Scholar] [CrossRef]
- Davis, S.G.; Pagliaro, J.L.; Debold, T.F.; van Wingerden, M.; van Wingerden, K. Flammability and explosion characteristics of mildly flammable refrigerants. J. Loss Prev. Process Ind. 2017, 49, 662–674. [Google Scholar] [CrossRef]
- Li, Y.; Bi, M.; Li, B.; Gao, W. Explosion behaviors of ammonia–air mixtures. Combust. Sci. Technol. 2018, 190, 1804–1816. [Google Scholar] [CrossRef]
- Lhuillier, C.; Brequigny, P.; Contino, F.; Mounaïm-Rousselle, C. Experimental study on ammonia/hydrogen/air combustion in spark ignition engine conditions. Fuel 2020, 269, 117448. [Google Scholar] [CrossRef]
- Shrestha, K.P.; Lhuillier, C.; Barbosa, A.A.; Brequigny, P.; Contino, F.; Mounaïm-Rousselle, C.; Seidel, L.; Mauss, F. An experimental and modeling study of ammonia with enriched oxygen content and ammonia/hydrogen laminar flame speed at elevated pressure and temperature. P. Combust. Inst. 2021, 38, 2163–2174. [Google Scholar] [CrossRef]
- Mitu, M.; Movileanu, C.; Giurcan, V. The Laminar Burning Velocities of Stoichiometric Methane-Air Mixture from Closed Vessels Measurements. Energies 2022, 15, 5058. [Google Scholar] [CrossRef]
- Valera-Medina, A.; Marsh, R.; Runyon, J.; Pugh, D.; Beasley, P.; Hughes, T.; Bowen, P. Ammonia–methane combustion in tangential swirl burners for gas turbine power generation. Appl. Energy 2017, 185, 1362–1371. [Google Scholar] [CrossRef]
- Valera-Medina, A.; Morris, S.; Runyon, J.; Pugh, D.G.; Marsh, R.; Beasley, P.; Hughes, T. Ammonia, Methane and Hydrogen for Gas Turbines. Energy Procedia 2015, 75, 118–123. [Google Scholar] [CrossRef]
- Xiao, H.; Howard, M.; Valera-Medina, A.; Dooley, S.; Bowen, P.J. Study on Reduced Chemical Mechanisms of Ammonia/Methane Combustion under Gas Turbine Conditions. Energy Fuel 2016, 30, 8701–8710. [Google Scholar] [CrossRef]
- Xiao, H.; Valera-Medina, A.; Bowen, P.J. Modeling Combustion of Ammonia/Hydrogen Fuel Blends under Gas Turbine Conditions. Energy Fuel 2017, 31, 8631–8642. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, J.H.; Park, J.H.; Kwon, O.C. Studies on properties of laminar premixed hydrogen-added ammonia/air flames for hydrogen production. Int. J. Hydrogen Energy 2010, 35, 1054–1064. [Google Scholar] [CrossRef]
- Li, J.; Huang, H.; Kobayashi, N.; He, Z.; Nagai, Y. Study on using hydrogen and ammonia as fuels: Combustion characteristics and NOx formation. Int. J. Energy Res. 2014, 38, 1214–1223. [Google Scholar] [CrossRef]
- Lhuillier, C.; Brequigny, P.; Lamoureux, N.; Contino, F.; Mounaïm-Rousselle, C. Experimental investigation on laminar burning velocities of ammonia/hydrogen/air mixtures at elevated temperatures. Fuel 2020, 263, 116653. [Google Scholar] [CrossRef]
- Goldmann, A.; Dinkelacker, F. Approximation of laminar flame characteristics on premixed ammonia/hydrogen/nitrogen/air mixtures at elevated temperatures and pressures. Fuel 2018, 224, 366–378. [Google Scholar] [CrossRef]
- Li, J.; Huang, H.; Kobayashi, N.; Wang, C.; Yuan, H. Numerical study on laminar burning velocity and ignition delay time of ammonia flame with hydrogen addition. Energy 2017, 126, 796–809. [Google Scholar] [CrossRef]
- Li, J.; Huang, H.; Deng, L.; He, Z.; Osaka, Y.; Kobayashi, N. Effect of hydrogen addition on combustion and heat release characteristics of ammonia flame. Energy 2019, 175, 604–617. [Google Scholar] [CrossRef]
- da Rocha, R.C.; Costa, M.; Bai, X.-S. Chemical kinetic modelling of ammonia/hydrogen/air ignition, premixed flame propagation and NO emission. Fuel 2019, 246, 24–33. [Google Scholar] [CrossRef]
- Wu, H.; Qian, Y.; Zhang, T.; Zhu, J.; Lu, X. Ignition and flame development of high-pressure liquid ammonia spray combustion with simultaneous high-speed OH* and NH2* chemiluminescence imaging. Combust. Flame 2025, 272, 113899. [Google Scholar] [CrossRef]
- Huang, S.; Wang, Y.; Li, S.; Yi, P.; Chen, R.; Wang, X.; Wu, Z.; Liu, S.; Wang, N.; Li, T. High-pressure ammonia spray combustion in lean premixed hydrogen mixture. Energy 2025, 336, 138520. [Google Scholar] [CrossRef]
- Ishihara, S.; Zhang, J.; Ito, T. Numerical calculation with detailed chemistry on ammonia co-firing in a coal-fired boiler: Effect of ammonia co-firing ratio on NO emissions. Fuel 2020, 274, 117742. [Google Scholar] [CrossRef]
- Zhang, J.; Ito, T.; Ishii, H.; Ishihara, S.; Fujimori, T. Numerical investigation on ammonia co-firing in a pulverized coal combustion facility: Effect of ammonia co-firing ratio. Fuel 2020, 267, 117166. [Google Scholar] [CrossRef]
- Shu, T.; Xue, Y.; Zhou, Z.; Ren, Z. An experimental study of laminar ammonia/methane/air premixed flames using expanding spherical flames. Fuel 2021, 290, 120003. [Google Scholar] [CrossRef]
- Henshaw, P.F.; D’Andrea, T.; Mann, K.R.C.; Ting, D.S.K. Premixed Ammonia-Methane-Air Combustion. Combust. Sci. Technol. 2005, 177, 2151–2170. [Google Scholar] [CrossRef]
- Okafor, E.C.; Naito, Y.; Colson, S.; Ichikawa, A.; Kudo, T.; Hayakawa, A.; Kobayashi, H. Measurement and modelling of the laminar burning velocity of methane-ammonia-air flames at high pressures using a reduced reaction mechanism. Combust. Flame 2019, 204, 162–175. [Google Scholar] [CrossRef]
- Xiao, H.; Valera-Medina, A.; Bowen, P.J. Study on premixed combustion characteristics of co-firing ammonia/methane fuels. Energy 2017, 140, 125–135. [Google Scholar] [CrossRef]
- Grannell, S.; Stack, C.; Gille, D. A Comparison of Combustion Promoters for Ammonia and Two Ways to Run Engines on Ammonia as the Only Fuel. Available online: https://www.ammoniaenergy.org/wp-content/uploads/2021/01/shawngrannellammoniaconfpres2010.pdf (accessed on 9 October 2025).
- Takeishi, H.; Hayashi, J.; Kono, S.; Arita, W.; Iino, K.; Akamatsu, F. Characteristics of ammonia/N2/O2 laminar flame in oxygen-enriched air condition. Trans. JSME 2015, 81, 14-00423. [Google Scholar] [CrossRef]
- Xia, Y.; Hashimoto, G.; Hadi, K.; Hashimoto, N.; Hayakawa, A.; Kobayashi, H.; Fujita, O. Turbulent burning velocity of ammonia/oxygen/nitrogen premixed flame in O2-enriched air condition. Fuel 2020, 268, 117383. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, X.; Huang, J.; Shen, Y.; Zhang, Y.; Liu, Z. The characteristics of flame propagation in ammonia/oxygen mixtures. J. Hazard. Mater. 2019, 363, 187–196. [Google Scholar] [CrossRef]
- Wang, D.; Ji, C.; Wang, Z.; Wang, S.; Zhang, T.; Yang, J. Measurement of oxy-ammonia laminar burning velocity at normal and elevated temperatures. Fuel 2020, 279, 118425. [Google Scholar] [CrossRef]
- Xinlu, H. Research on Fundamental Laminar Combustion Characteristics and Reaction Kinetic Mechanism of Innovative Carbon-Free Ammonia Fuel; Zhe Jiang University: Hangzhou, China, 2021. [Google Scholar]
- Kohansal, M.; Kiani, M.; Masoumi, S.; Nourinejad, S.; Ashjaee, M.; Houshfar, E. Experimental and Numerical Investigation of NH3/CH4 Mixture Combustion Properties under Elevated Initial Pressure and Temperature. Energy Fuel 2023, 37, 10681–10696. [Google Scholar] [CrossRef]
- Karan, A.; Dayma, G.; Chauveau, C.; Halter, F. Experimental study and numerical validation of oxy-ammonia combustion at elevated temperatures and pressures. Combust. Flame 2022, 236, 111819. [Google Scholar] [CrossRef]
- Wang, S. Experimental and Kinetic Study of Gaseous Fuel Combustion Characteristics Under Elevated Pressures Based on Heat Flux Method; Zhejiang University: Hangzhou, China, 2020. [Google Scholar]
- Mitu, M.; Giurcan, V.; Razus, D.; Oancea, D. Inert gas influence on the laminar burning velocity of methane-air mixtures. J. Hazard. Mater. 2017, 321, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Law, C.K. Combustion Physics; Cambridge University: New York, NY, USA, 2006. [Google Scholar]
- Miller, J.A.; Smooke, M.D.; Green, R.M.; Kee, R.J. Kinetic Modeling of the Oxidation of Ammonia in Flames. Combust. Sci. Technol. 2007, 34, 149–176. [Google Scholar] [CrossRef]
- Lindstedt, R.P.; Lockwood, F.C.; Selim, M.A. Detailed Kinetic Modelling of Chemistry and Temperature Effects on Ammonia Oxidation. Combust. Sci. Technol. 1994, 99, 253–276. [Google Scholar] [CrossRef]
- Konnov, A.A. Implementation of the NCN pathway of prompt-NO formation in the detailed reaction mechanism. Combust. Flame 2009, 156, 2093–2105. [Google Scholar] [CrossRef]
- Tian, Z.; Li, Y.; Zhang, L.; Glarborg, P.; Qi, F. An experimental and kinetic modeling study of premixed NH3/CH4/O2/Ar flames at low pressure. Combust. Flame 2009, 156, 1413–1426. [Google Scholar] [CrossRef]
- Smith, G.P.; Golden, D.M.; Frenklach, M.; Moriarty, N.W.; Eiteneer, B.; Goldenberg, M. GRI-Mech 3.0. Available online: http://www.me.berkeley.edu/gri_mech (accessed on 9 October 2025).
- Shrestha, K.P.; Seidel, L.; Zeuch, T.; Mauss, F. Detailed Kinetic Mechanism for the Oxidation of Ammonia Including the Formation and Reduction of Nitrogen Oxides. Energy Fuel 2018, 32, 10202–10217. [Google Scholar] [CrossRef]
- Burke, U.; Somers, K.P.; O’Toole, P.; Zinner, C.M.; Marquet, N.; Bourque, G.; Petersen, E.L.; Metcalfe, W.K.; Serinyel, Z.; Curran, H.J. An ignition delay and kinetic modeling study of methane, dimethyl ether, and their mixtures at high pressures. Combust. Flame 2015, 162, 315–330. [Google Scholar] [CrossRef]
- Stagni, A.; Cavallotti, C.; Arunthanayothin, S.; Song, Y.; Herbinet, O.; Battin-Leclerc, F.; Faravelli, T. An experimental, theoretical and kinetic-modeling study of the gas-phase oxidation of ammonia. React. Chem. Eng. 2020, 5, 696–711. [Google Scholar] [CrossRef]
- Cremer, M.A.; Montgomery, C.J.; Wang, D.H.; Heap, M.P.; Chen, J.-Y. Development and implementation of reduced chemistry for computional fluid dynamics modeling of selective non-catalytic reduction. P. Combust. Inst. 2000, 28, 2427–2434. [Google Scholar] [CrossRef]
- Haynes, B.S. Reactions of ammonia and nitric oxide in the burnt gases of fuel-rich hydrocarbon-air flames. Combust. Flame 1977, 28, 81–91. [Google Scholar] [CrossRef]
- Konnov, A.A.; Ruyck, J.D. A Possible New Route for No Formation Via N2H3. Combust. Sci. Technol. 2001, 168, 1–46. [Google Scholar] [CrossRef]
- Dagaut, P.; Glarborg, P.; Alzueta, M. The oxidation of hydrogen cyanide and related chemistry. Prog. Energy Combust. Sci. 2008, 34, 1–46. [Google Scholar] [CrossRef]
- Klippenstein, S.J.; Harding, L.B.; Glarborg, P.; Miller, J.A. The role of NNH in NO formation and control. Combust. Flame 2011, 158, 774–789. [Google Scholar] [CrossRef]
- Bertolino, A.; Fürst, M.; Stagni, A.; Frassoldati, A.; Pelucchi, M.; Cavallotti, C.; Faravelli, T.; Parente, A. An evolutionary, data-driven approach for mechanism optimization: Theory and application to ammonia combustion. Combust. Flame 2021, 229, 111366. [Google Scholar] [CrossRef]
- Singh, A.S.; Mohapatra, S.; Boyapati, R.; Elbaz, A.M.; Dash, S.K.; Roberts, W.L.; Reddy, V.M. Chemical Kinetic Modeling of the Autoignition Properties of Ammonia at Low–Intermediate Temperature and High Pressure using a Newly Proposed Reaction Mechanism. Energy Fuel 2021, 35, 13506–13522. [Google Scholar] [CrossRef]
- Mathieu, O.; Petersen, E.L. Experimental and modeling study on the high-temperature oxidation of Ammonia and related NOx chemistry. Combust. Flame 2015, 162, 554–570. [Google Scholar] [CrossRef]
- Song, Y.; Hashemi, H.; Christensen, J.M.; Zou, C.; Marshall, P.; Glarborg, P. Ammonia oxidation at high pressure and intermediate temperatures. Fuel 2016, 181, 358–365. [Google Scholar] [CrossRef]
- Otomo, J.; Koshi, M.; Mitsumori, T.; Iwasaki, H.; Yamada, K. Chemical kinetic modeling of ammonia oxidation with improved reaction mechanism for ammonia/air and ammonia/hydrogen/air combustion. Int. J. Hydrogen Energy 2018, 43, 3004–3014. [Google Scholar] [CrossRef]
- Glarborg, P.; Miller, J.A.; Ruscic, B.; Klippenstein, S.J. Modeling nitrogen chemistry in combustion. Prog. Energy Combust. Sci. 2018, 67, 31–68. [Google Scholar] [CrossRef]
- Cai, T.; Zhao, D.; Chan, S.H.; Shahsavari, M. Tailoring reduced mechanisms for predicting flame propagation and ignition characteristics in ammonia and ammonia/hydrogen mixtures. Energy 2022, 260, 125090. [Google Scholar] [CrossRef]
- Zhu, Y.; Curran, H.J.; Girhe, S.; Murakami, Y.; Pitsch, H.; Senecal, K.; Yang, L.; Zhou, C.-W. The combustion chemistry of ammonia and ammonia/hydrogen mixtures: A comprehensive chemical kinetic modeling study. Combust. Flame 2024, 260, 113239. [Google Scholar] [CrossRef]
- Lindstedt, R.P.; Lockwood, F.C.; Selim, M.A. A Detailed Kinetic Study of Ammonia Oxidation. Combust. Sci. Technol. 1995, 108, 231–254. [Google Scholar] [CrossRef]
- Duynslaegher, C.; Contino, F.; Vandooren, J.; Jeanmart, H. Modeling of ammonia combustion at low pressure. Combust. Flame 2012, 159, 2799–2805. [Google Scholar] [CrossRef]
- Nozari, H.; Karabeyoğlu, A. Numerical study of combustion characteristics of ammonia as a renewable fuel and establishment of reduced reaction mechanisms. Fuel 2015, 159, 223–233. [Google Scholar] [CrossRef]
- Nakamura, H.; Hasegawa, S.; Tezuka, T. Kinetic modeling of ammonia/air weak flames in a micro flow reactor with a controlled temperature profile. Combust. Flame 2017, 185, 16–27. [Google Scholar] [CrossRef]
- Zhang, X.; Moosakutty, S.P.; Rajan, R.P.; Younes, M.; Sarathy, S.M. Combustion chemistry of ammonia/hydrogen mixtures: Jet-stirred reactor measurements and comprehensive kinetic modeling. Combust. Flame 2021, 234, 111653. [Google Scholar] [CrossRef]
- Gotama, G.J.; Hayakawa, A.; Okafor, E.C.; Kanoshima, R.; Hayashi, M.; Kudo, T.; Kobayashi, H. Measurement of the laminar burning velocity and kinetics study of the importance of the hydrogen recovery mechanism of ammonia/hydrogen/air premixed flames. Combust. Flame 2022, 236, 111753. [Google Scholar] [CrossRef]
- Skreiberg, Ø.; Kilpinen, P.; Glarborg, P. Ammonia chemistry below 1400 K under fuel-rich conditions in a flow reactor. Combust. Flame 2004, 136, 501–518. [Google Scholar] [CrossRef]
- Mendiara, T.; Glarborg, P. Ammonia chemistry in oxy-fuel combustion of methane. Combust. Flame 2009, 156, 1937–1949. [Google Scholar] [CrossRef]
- Okafor, E.C.; Naito, Y.; Colson, S.; Ichikawa, A.; Kudo, T.; Hayakawa, A.; Kobayashi, H. Experimental and numerical study of the laminar burning velocity of CH4–NH3–air premixed flames. Combust. Flame 2018, 187, 185–198. [Google Scholar] [CrossRef]
- Li, R.; Konnov, A.A.; He, G.; Qin, F.; Zhang, D. Chemical mechanism development and reduction for combustion of NH3/H2/CH4 mixtures. Fuel 2019, 257, 116059. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Z.; Chen, C.; Elbaz, A.M.; Sun, Z.; Roberts, W.L. Applying heat flux method to laminar burning velocity measurements of NH3/CH4/air at elevated pressures and kinetic modeling study. Combust. Flame 2022, 236, 111788. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, S.; Sui, R.; Lu, Q. Impact of ammonia addition on soot and NO/N2O formation in methane/air co-flow diffusion flames. Combust. Flame 2023, 247, 112483. [Google Scholar] [CrossRef]
- He, X.; Li, M.; Shu, B.; Fernandes, R.; Moshammer, K. Exploring the Effect of Different Reactivity Promoters on the Oxidation of Ammonia in a Jet-Stirred Reactor. J. Phys. Chem. A 2023, 127, 1923–1940. [Google Scholar] [CrossRef]
- Shi, X.X. Research on Combustion Enhancement Method of Ammonia by Cofiring Dimethyl Ether with Insights into Pollutant Formation Mechanism; Shanghai Jiao Tong University: Shanghai, China, 2024. [Google Scholar]


| Thermal Property Parameters | Ammonia | Hydrogen | Methane |
|---|---|---|---|
| Liquefaction temperature at 0.1 MPa (°C) | −33.4 | −252.3 | −161.5 |
| Liquefaction pressure at 298 K (MPa) | 1.03 | 70 | 25 |
| Minimum ignition energy (MJ) | 8.000 | 0.011 | 0.28 |
| Autoignition temperature (K) | 930 | 773–850 | 859 |
| Octane number | ≥130 | ≥130 | 125 |
| Fluid density (kg/L) | 0.674 | 0.071 | 0.422 |
| Vaporization heat (kJ/L) | 923 | 36 | 79 |
| Flammable range (Vol%) | 15–28 | 4–75 | 5–15 |
| Adiabatic flame temperature (°C) | 1750 | 2120 | 1970 |
| Specific heat capacity (KJ/(kg·K)) | 2.19 | 14.3 | 2.48 |
| Stoichiometric air–fuel ratio | 6.06 | 34.33 | 17.17 |
| Lower heating value of fuel (MJ/kg) | 18.6 | 120.0 | 50.0 |
| Higher heating value of fuel (MJ/kg) | 22.5 | 141.9 | 55.5 |
| Gravimetric hydrogen storage density (wt%) | 17.8 | 100.0 | 25.0 |
| Measurement Methods | Sources of Inherent Errors and Uncertainties | Sensitivity to Ammonia Flame Instability | Applicability of Measuring Ammonia-Blended Flames | Applicability Under High-Pressure Conditions |
|---|---|---|---|---|
| Spherical outwardly propagating flame method | Buoyancy effect: It causes flame deformation, which is particularly severe for low-velocity flames. | Extremely high. Pure ammonia flames are susceptible to hydrodynamic instability. | Good to excellent. Blending with high-reactivity fuels can significantly increase the LBV and enhance flame stability. | Excellent. The design of the closed vessel is inherently suitable for measurements in high-pressure environments. |
| Bunsen-burner method | Flame stretch: The stretch rate varies at different points of the conical flame. Flame shape: The actual flame is not an ideal cone. | High. The extremely low LBV makes it difficult for the flame to stabilize at the burner outlet, and flame blow-off is prone to occur. | Moderate to good. It is applicable under the conditions of fuel blending. | Poor. It is difficult to stabilize the flame under high pressure. |
| Counter-flow flame and stagnation flame method | Nozzle boundary effect: It impairs the one-dimensional nature of the flow field. | Low. The flow stretch is a controlled parameter. | Excellent. It is applicable to fuels with various blending ratios and exhibits high stability. | Excellent. It is highly suitable for studying the LBV under high-pressure conditions. |
| Heat flux method | Wall heat flux measurement: It relies on accurate temperature gradient measurement and heat flux measurement. | Extremely low. This method directly establishes an adiabatic and unstretched planar flame. | Excellent. It is suitable for measuring ammonia-blended flames. | Poor. Its applicable pressure range is limited (typically <5 atm). |
| Author | Year | Equivalence Ratio | Initial Temperature | Initial Pressure | Measurement Method | Reference |
|---|---|---|---|---|---|---|
| Zakaznov et al. | 1978 | 0.78–1.03 | 293 K | 1.0 atm | Test tube method | [34] |
| Ronny | 1987 | 0.73–1.125 | 298 K | 0.00665–0.1995 MPa | Spherical outwardly propagating flame method | [31] |
| Pfahl et al. | 2000 | 0.48–1.12 | 295 K | 0.1 MPa | Same as above | [32] |
| Jabbour et al. | 2004 | 0.68–1.3 | 296 K | 1.0 atm | Test tube method | [33] |
| Takizawa et al. | 2007 | 0.9–1.2 | 298 K | 1.05 atm | Spherical outwardly propagating flame method | [35] |
| Hayakawa et al. | 2015 | 0.8–1.2 | 298 K | 0.1–0.5 MPa | Same as above | [36] |
| Ichikawa et al. | 2015 | 1.0 | 298 K | 0.1–0.5 MPa | Same as above | [37] |
| Davis et al. | 2017 | 1.07–1.32 | 298 K | 1.0 atm | Same as above | [38] |
| Li et al. | 2018 | 0.8–1.3 | 300 K | 0.1 MPa | Same as above | [39] |
| Han et al. | 2019 | 0.83–1.25 | 298 K | 1.0 atm | Heat flux method | [27] |
| Mei et al. | 2019 | 0.9–1.3 | 298 K | 1.0 atm | Spherical outwardly propagating flame method | [16] |
| Lhuillier et al. | 2019 | 0.8–1.4 | 298 K–473 K | 0.1 MPa | Same as above | [40] |
| Shrestha et al. | 2020 | 0.8–1.4 | 298 K–473 K | 0.1–0.955 MPa | Same as above | [41] |
| Author | Year | Equivalence Ratio | Initial Temperature and Pressure | Hydrogen Blending Ratio (%) | Measurement Method | Reference |
|---|---|---|---|---|---|---|
| Lee et al. | 2010 | 0.6–1.67 | NPT | 0–50 | Spherical outwardly propagating flame method | [47] |
| Kumar et al. | 2013 | 0.5–1.1 | NPT | 0–20 | Bunsen-burner method | [18] |
| Li et al. | 2014 | 0.6–1.4 | NPT | 0–60 | Same as above | [48] |
| Ichikawa et al. | 2015 | 1.0 | 0.1–0.5 Mpa | 0–100 | Spherical outwardly propagating flame method | [37] |
| Goldmann et al. | 2018 | 0.5–1.7 | 300–1100 K, 0.1–25 MPa | 0–60 | Numerical simulation | [50] |
| Lhuillier et al. | 2020 | 0.8–1.4 | 298–473 K | 0–60 | Spherical outwardly propagating flame method | [49] |
| Han et al. | 2019 | 0.8–1.6 | NPT | 0–50 | Heat flux method | [27] |
| Wang et al. | 2020 | 0.7–1.6 | 0.1–0.5 Mpa | 0–60 | Same as above | [29] |
| Shrestha et al. | 2021 | 0.8–1.4 | 473 K, 1–10 bar | 0–30 | Spherical outwardly propagating flame method | [41] |
| Author | Year | Equivalence Ratio | Initial Temperature and Pressure | Measurement Method | Reference |
|---|---|---|---|---|---|
| Henshaw et al. | 2005 | 0.5–1.5 | NPT | Heat flux method | [59] |
| Xiao et al. | 2017 | 0.6–1.4 | 300–800 K 0.1–2 MPa | Numerical simulation | [61] |
| Okafor et al. | 2019 | 0.7–1.3 | 298 K, 0.1–0.5 MPa | Spherical outwardly propagating flame method | [60] |
| Han et al. | 2019 | 0.6–1.8 | NPT | Heat flux method | [27] |
| Shu et al. | 2021 | 0.6–1.4 | 298 K, 1 atm and 5 atm | Spherical outwardly propagating flame method | [58] |
| Number | Model | Year | Fuel | Number of Species/Reactions | Reference |
|---|---|---|---|---|---|
| 1 | Konnov and Ruyck | 2001 | H/N/O | 31/238 | [83] |
| 2 | Dagaut | 2008 | NH3 | 41/250 | [84] |
| 3 | Klippenstein | 2011 | NH3 | 69/462 | [85] |
| 4 | Bertolino | 2021 | NH3 | 31/203 | [86] |
| 5 | Singh | 2021 | NH3, | 32/259 | [87] |
| 6 | Mathieu | 2015 | NH3, NH3/H2 | 44/269 | [88] |
| 7 | Song | 2016 | NH3, NH3/H2 | 32/204 | [89] |
| 8 | Otomo | 2018 | NH3, NH3/H2 | 32/213 | [90] |
| 9 | Glarborg | 2018 | NH3, NH3/H2 | 151/1397 | [91] |
| 10 | Shrestha | 2018 | NH3, NH3/H2 | 34/250 | [78] |
| 11 | Mei | 2019 | NH3, NH3/H2 | 38/265 | [16] |
| 12 | Stagni | 2020 | NH3, NH3/H2 | 31/203 | [80] |
| 13 | Cai | 2022 | NH3, NH3/H2 | 20/89 | [92] |
| 14 | Zhu | 2024 | NH3, NH3/H2 | 43/312 | [93] |
| Number | Model | Year | Fuel | Number of Species/Reactions | Reference |
|---|---|---|---|---|---|
| 1 | Mathieu | 2015 | NH3, NH3/H2 | 44/269 | [88] |
| 2 | Song | 2016 | NH3, NH3/H2 | 32/204 | [89] |
| 3 | Otomo | 2018 | NH3, NH3/H2 | 32/213 | [90] |
| 4 | Glarborg | 2018 | NH3, NH3/H2 | 151/1397 | [91] |
| 5 | Shrestha | 2018 | NH3, NH3/H2 | 34/250 | [78] |
| 6 | Mei | 2019 | NH3, NH3/H2 | 38/265 | [16] |
| 7 | Stagni | 2020 | NH3, NH3/H2 | 31/203 | [80] |
| 8 | Cai | 2022 | NH3, NH3/H2 | 20/89 | [92] |
| 9 | Zhu | 2024 | NH3, NH3/H2 | 43/312 | [93] |
| 10 | Miller | 1983 | NH3/H2 | 22/98 | [73] |
| 11 | Lindstedt | 1995 | NH3/H2 | 67/419 | [94] |
| 12 | DuynSLaeghe | 2012 | NH3/H2 | 19/80 | [95] |
| 13 | Nozari | 2015 | NH3/H2 | 21/91 | [96] |
| 14 | Nakamura | 2017 | NH3/H2 | 32/224 | [97] |
| 15 | Zhang | 2021 | NH3/H2 | 38/263 | [98] |
| 16 | Gotama | 2022 | NH3/H2 | 26/119 | [99] |
| 17 | Han | 2020 | NH3/CO/H2 | 35/177 | [27] |
| Number | Model | Year | Fuel | Number of Species/Reactions | Reference |
|---|---|---|---|---|---|
| 1 | GRI-Mech 3.0 | 2000 | NH3, NH3/H2, NH3/CH4 | 53/325 | [77] |
| 2 | Skreiberg | 2004 | NH3/CH4/H2/CO | 34/191 | [100] |
| 3 | Mendiara | 2009 | NH3/CH4 | 99/779 | [101] |
| 4 | Tian | 2009 | NH3, NH3/H2, NH3/CH4 | 84/703 | [76] |
| 5 | Konnov | 2009 | NH3, NH3/H2, NH3/CH4 | 129/1231 | [75] |
| 6 | Xiao | 2016 | NH3/CH4 | 48/500 | [45] |
| 7 | Okafor | 2018 | NH3, NH3/H2, NH3/CH4 | 59/356 | [102] |
| 8 | Li | 2019 | NH3/H2/CH4 | 51/420 | [103] |
| 9 | CEU-NH3-Mech-1.1 | 2022 | NH3/CH4, NH3/H2/CO | 91/444 | [104] |
| 10 | Yang | 2023 | NH3/CH4 | 187/1571 | [105] |
| 11 | He | 2023 | NH3/H2/CH4/CH3OH | 180/1406 | [106] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Xiao, Z.; Hu, R.; Feng, D. A Review on Laminar Burning Velocity of Ammonia Flames. Energies 2025, 18, 6000. https://doi.org/10.3390/en18226000
Yang X, Xiao Z, Hu R, Feng D. A Review on Laminar Burning Velocity of Ammonia Flames. Energies. 2025; 18(22):6000. https://doi.org/10.3390/en18226000
Chicago/Turabian StyleYang, Xiao, Zhijian Xiao, Rui Hu, and Dongdong Feng. 2025. "A Review on Laminar Burning Velocity of Ammonia Flames" Energies 18, no. 22: 6000. https://doi.org/10.3390/en18226000
APA StyleYang, X., Xiao, Z., Hu, R., & Feng, D. (2025). A Review on Laminar Burning Velocity of Ammonia Flames. Energies, 18(22), 6000. https://doi.org/10.3390/en18226000








