Hydrogen Carriers for Renewable Microgrid System Applications
Abstract
1. Introduction
2. Materials and Methods
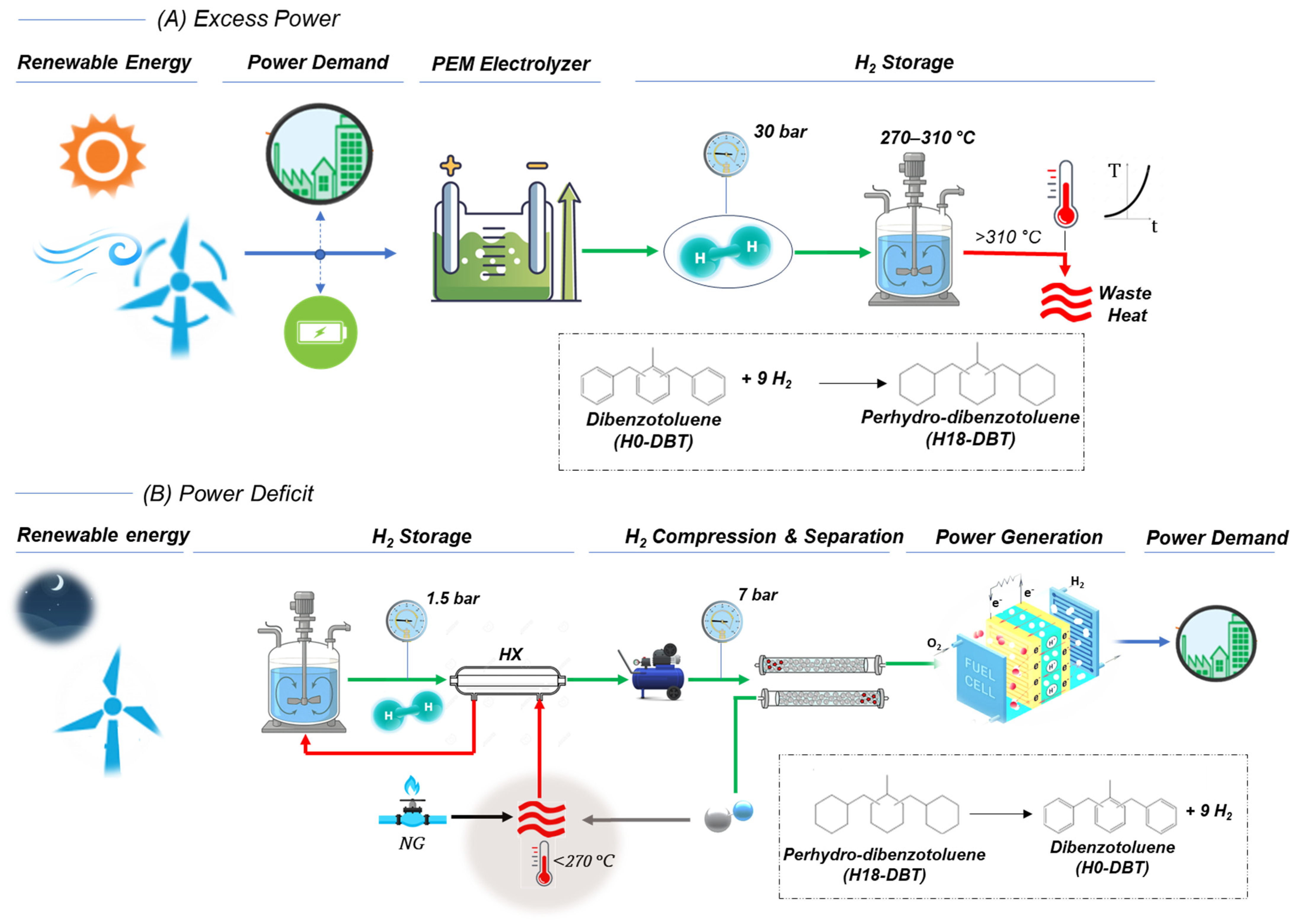
2.1. System Configuration
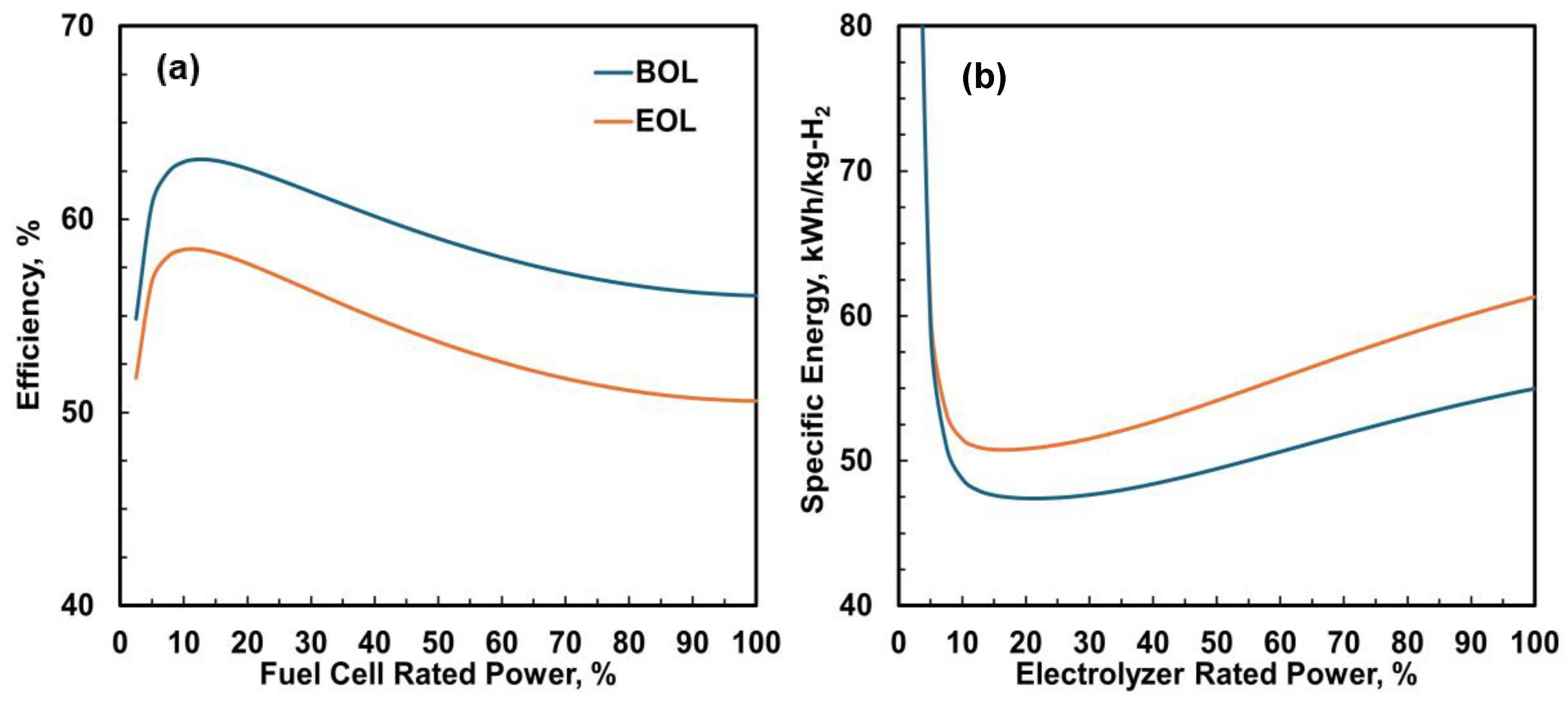
2.2. Fuel Cell, Electrolyzer, and Battery Performance
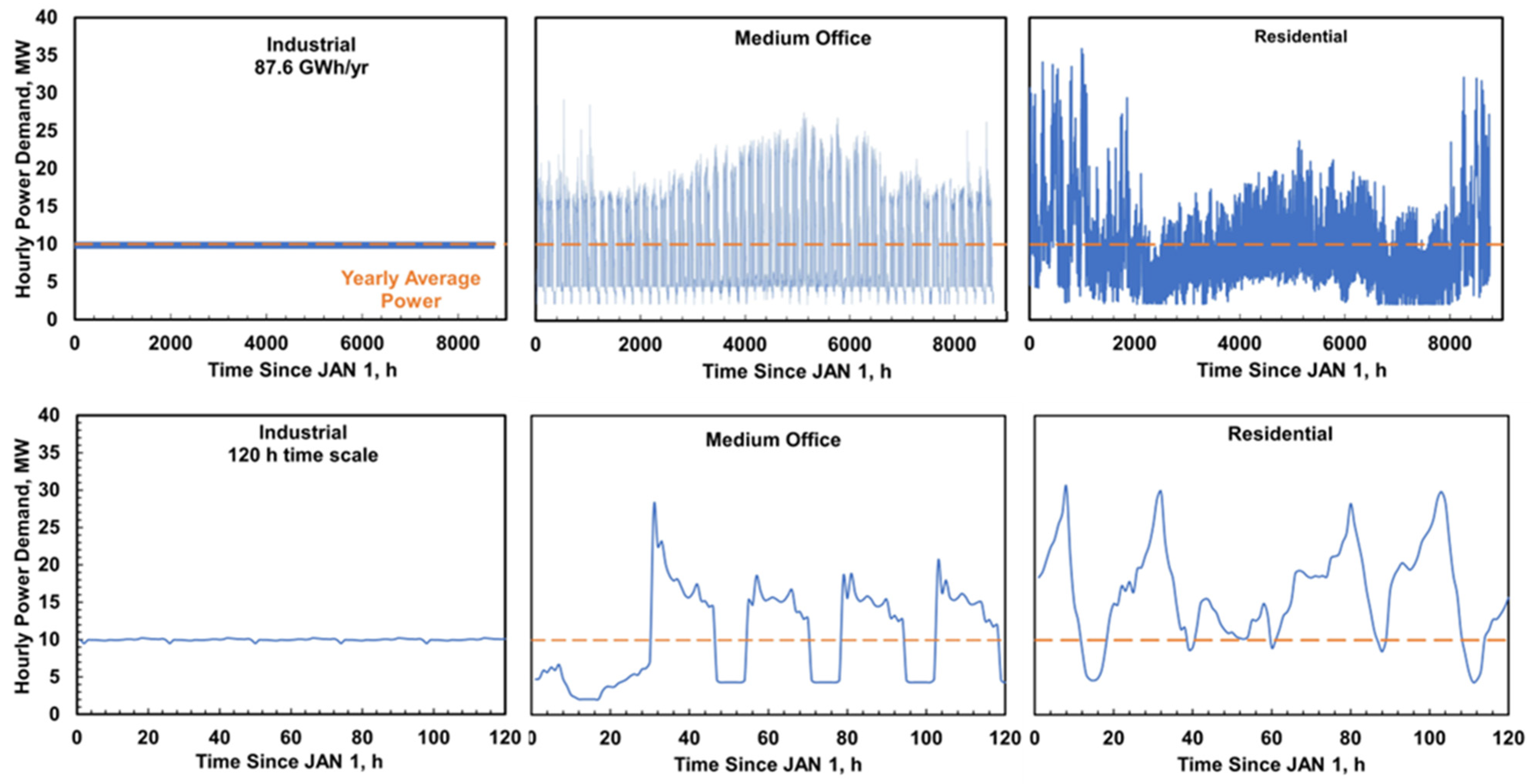
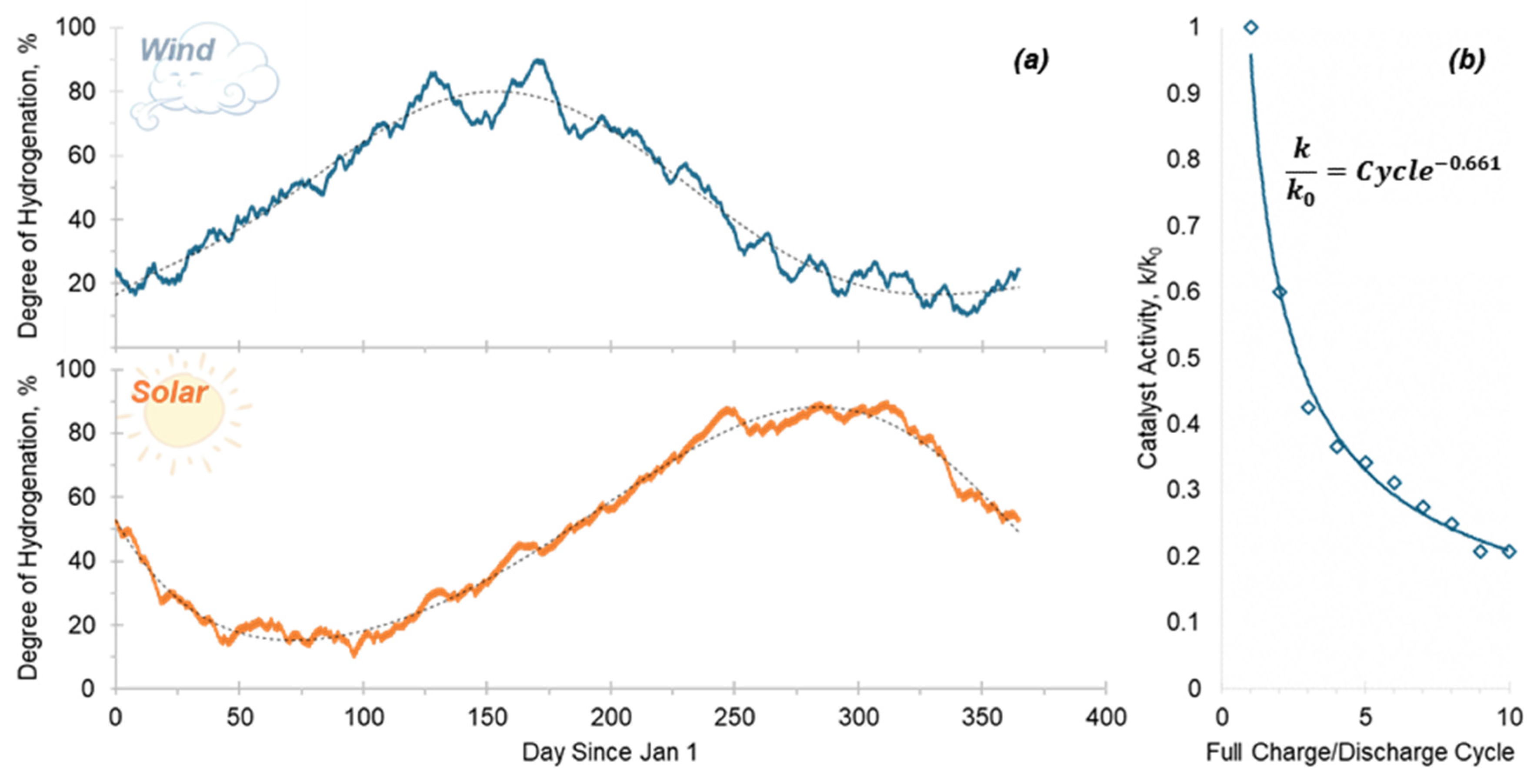
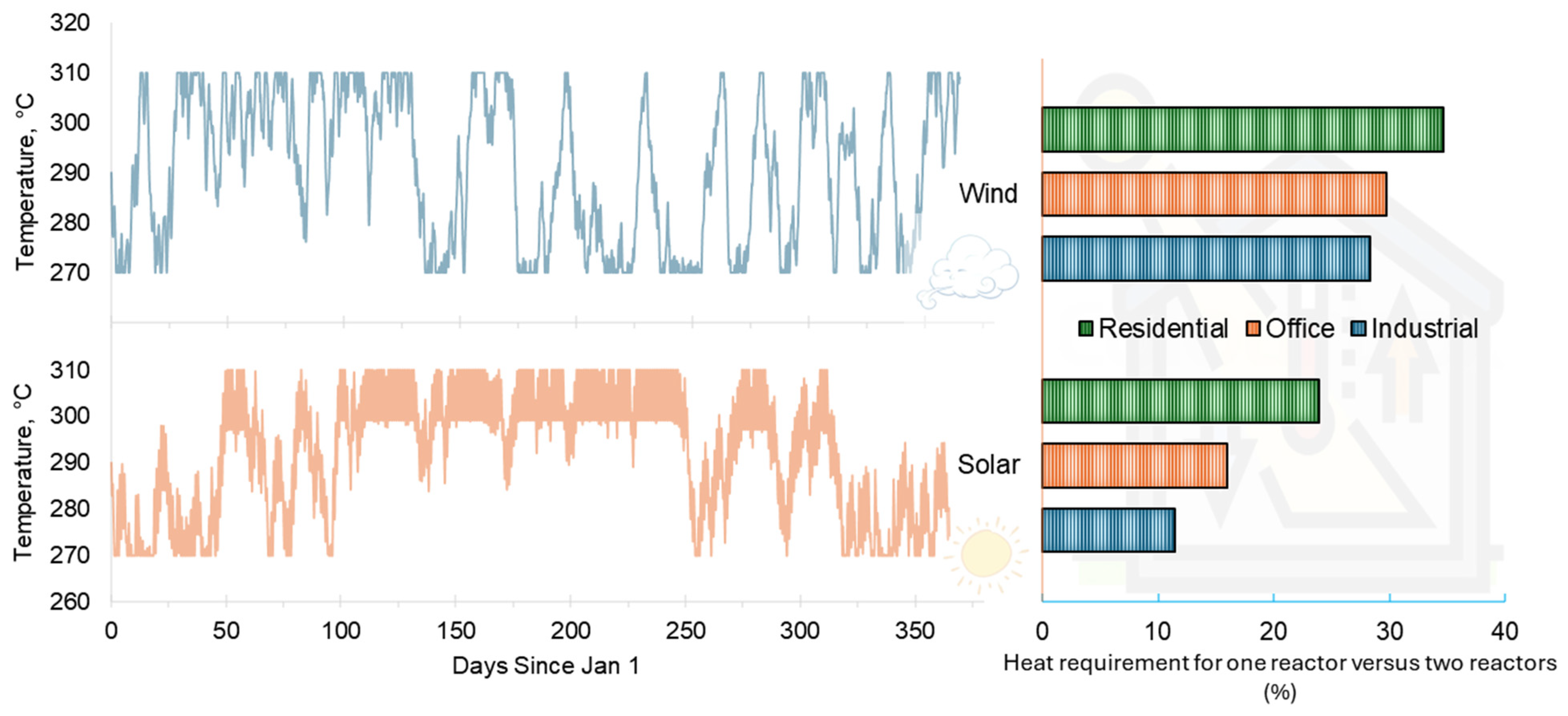
2.3. Duty Cycles
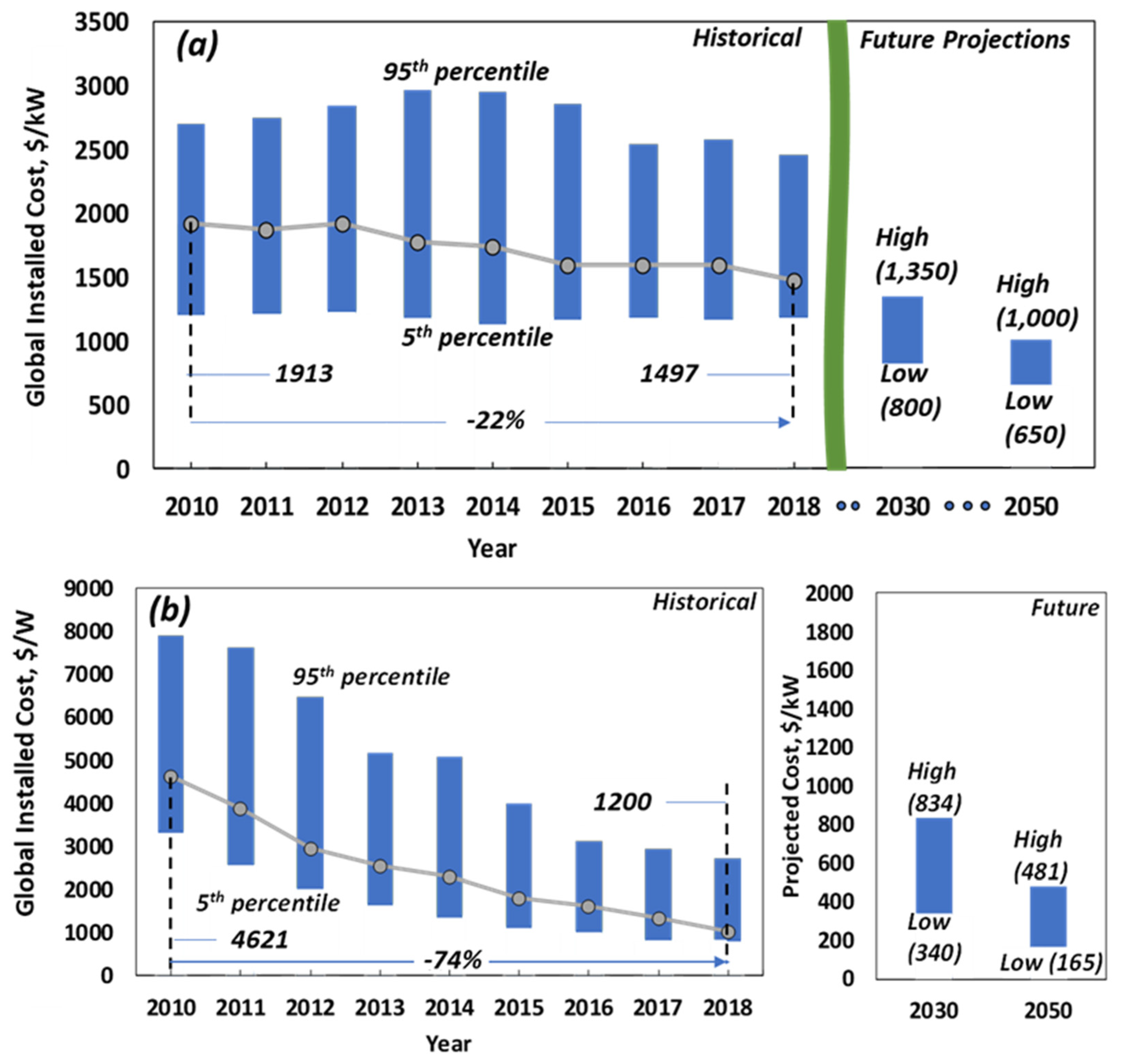
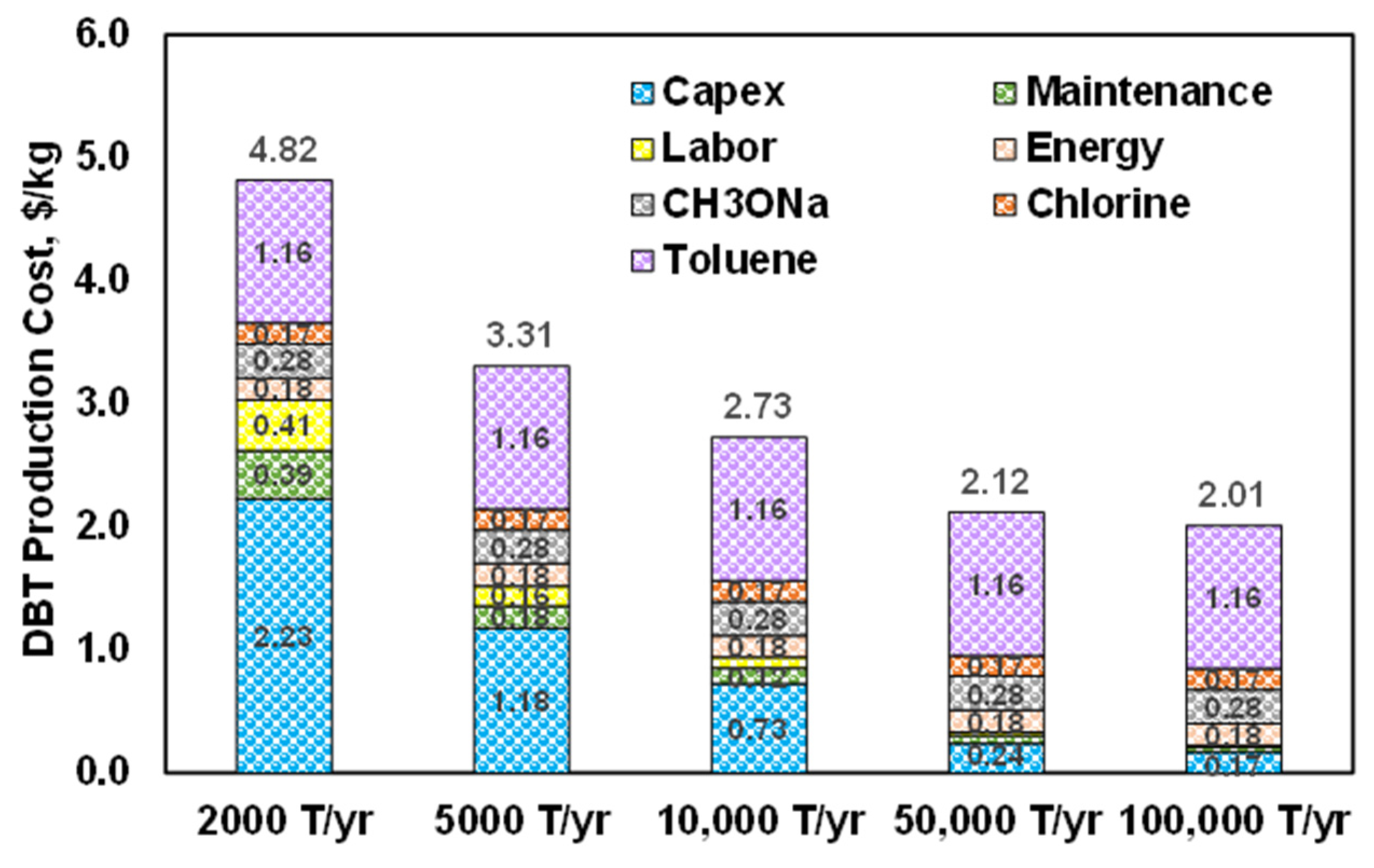
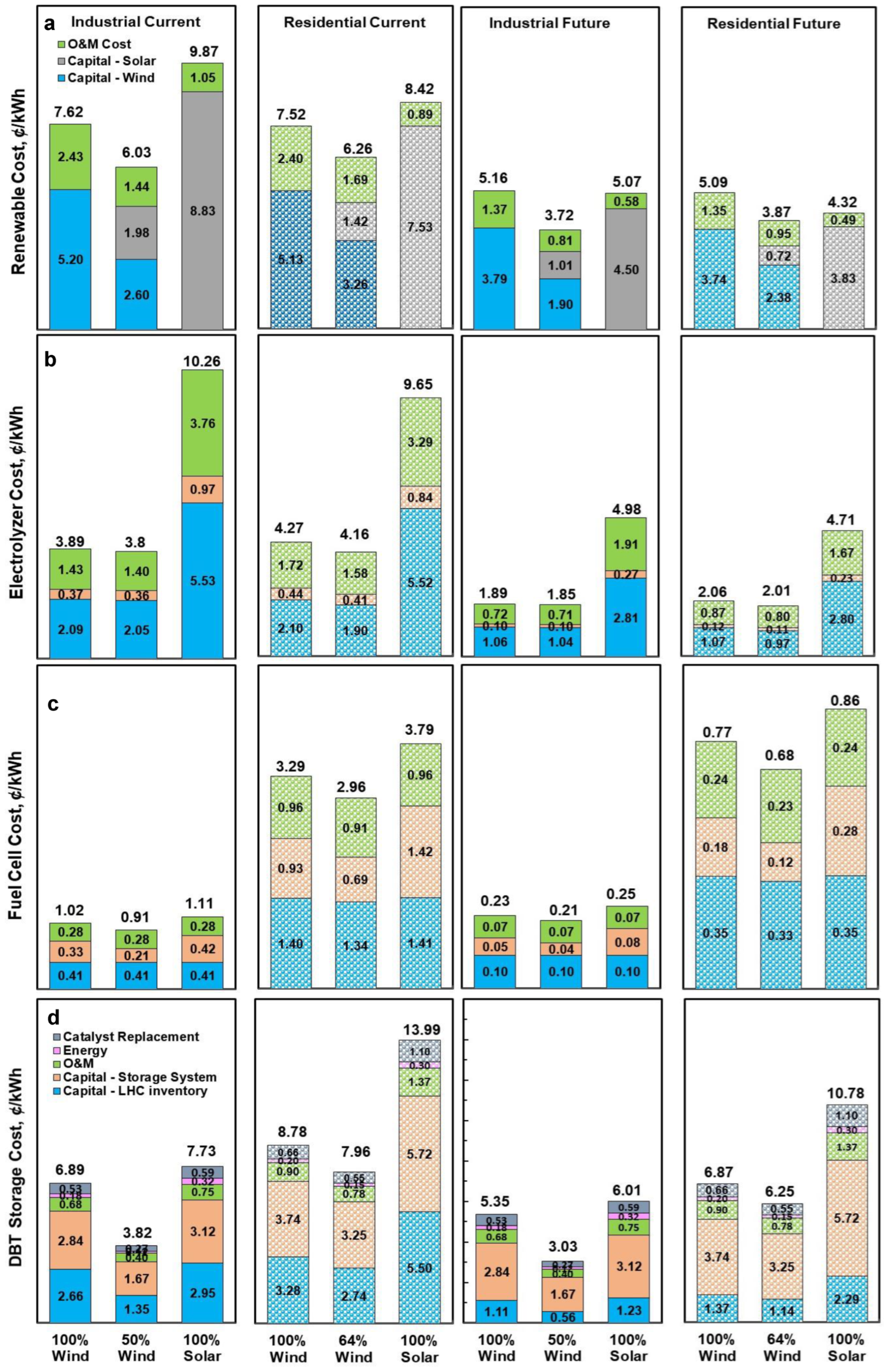
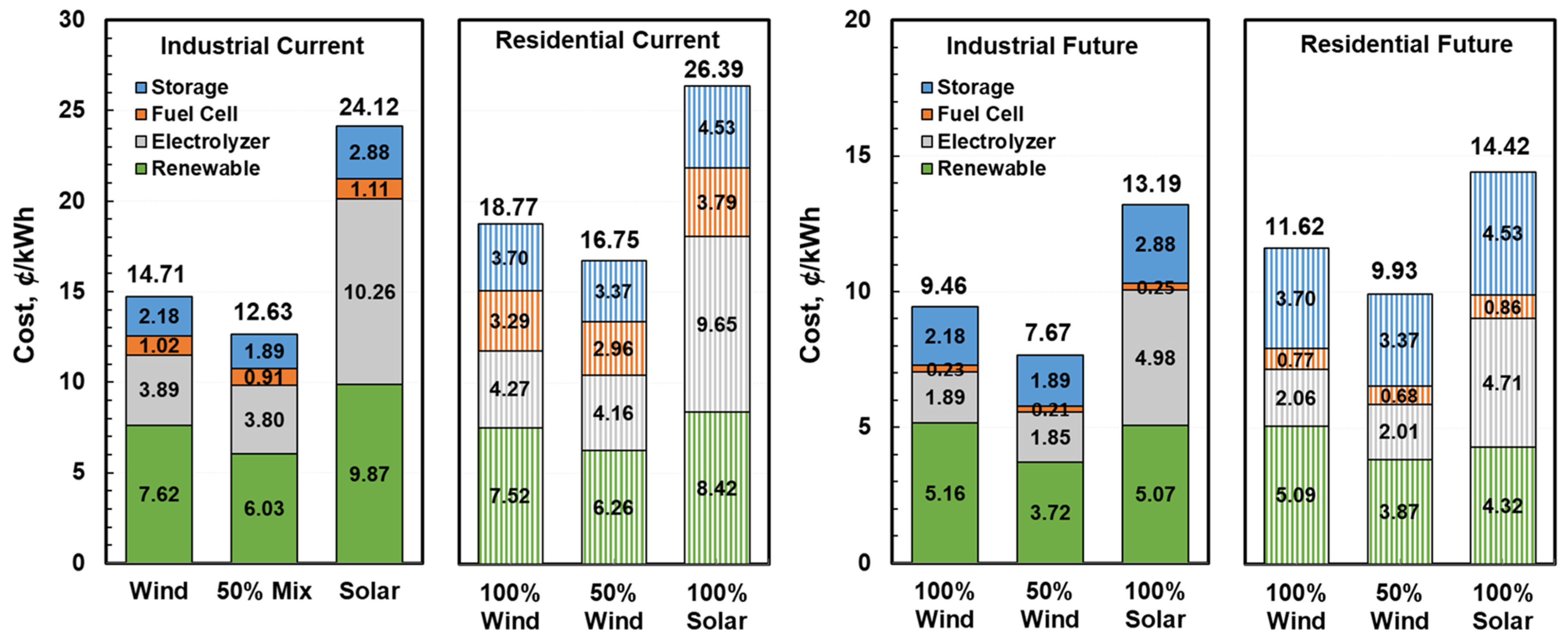
2.4. Economic Assessment
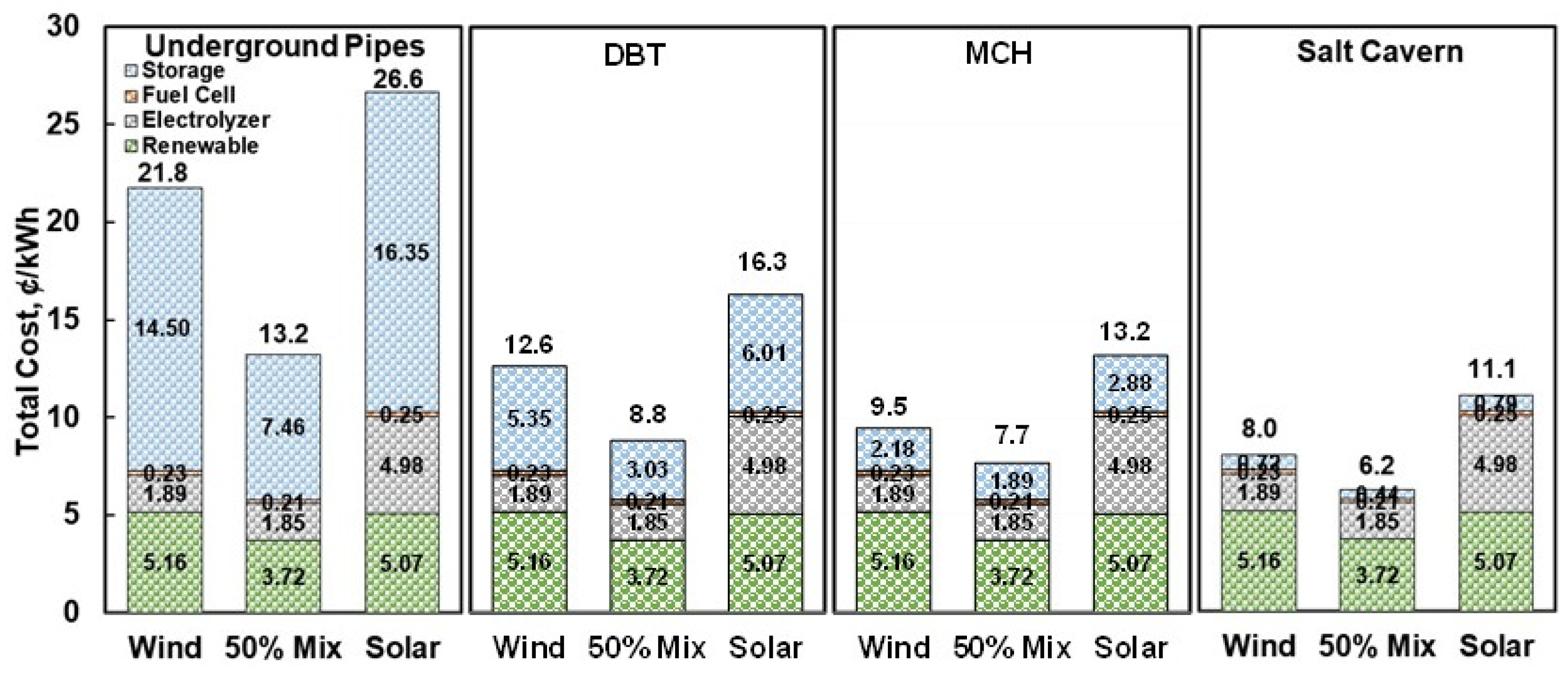
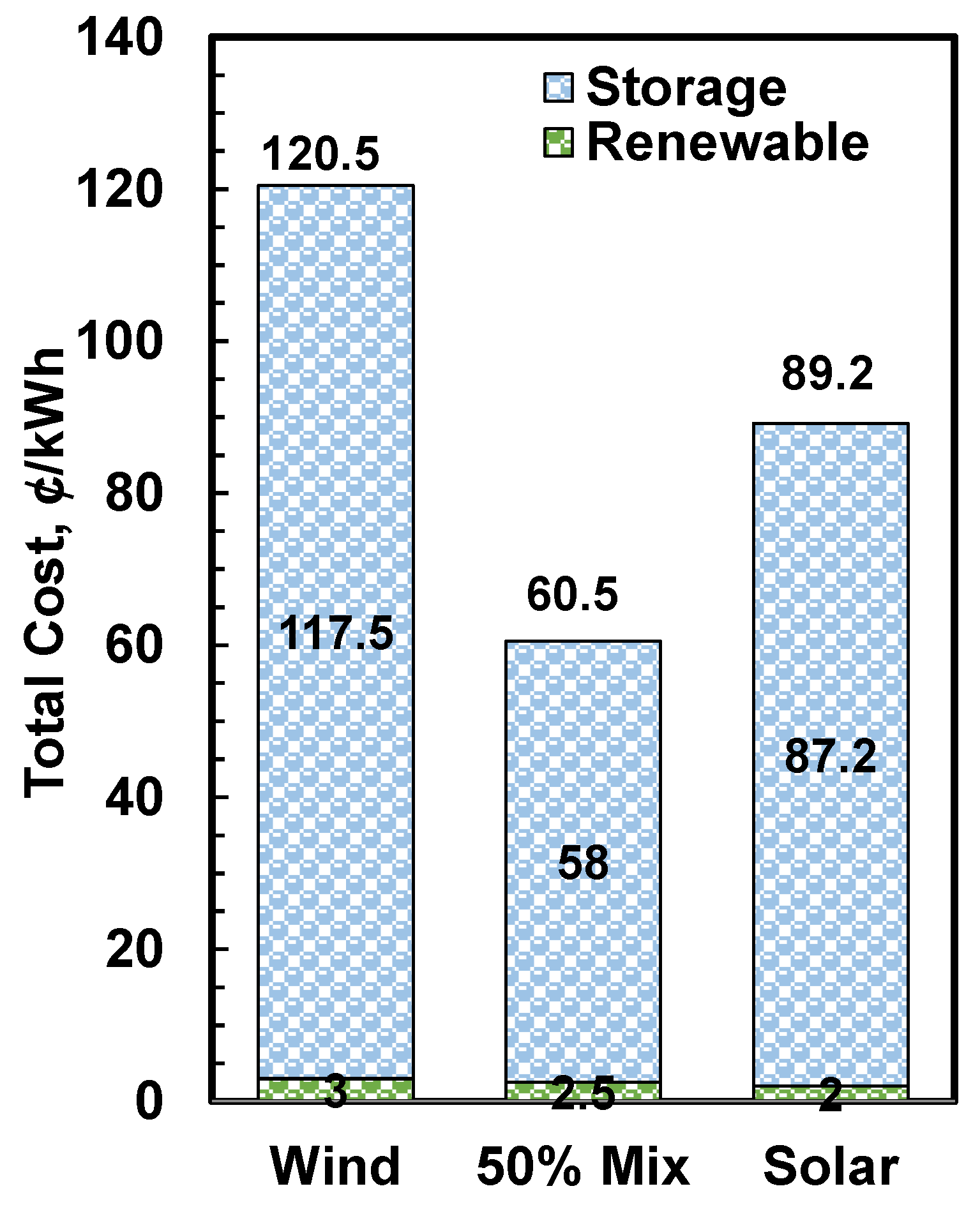
3. Results and Discussion
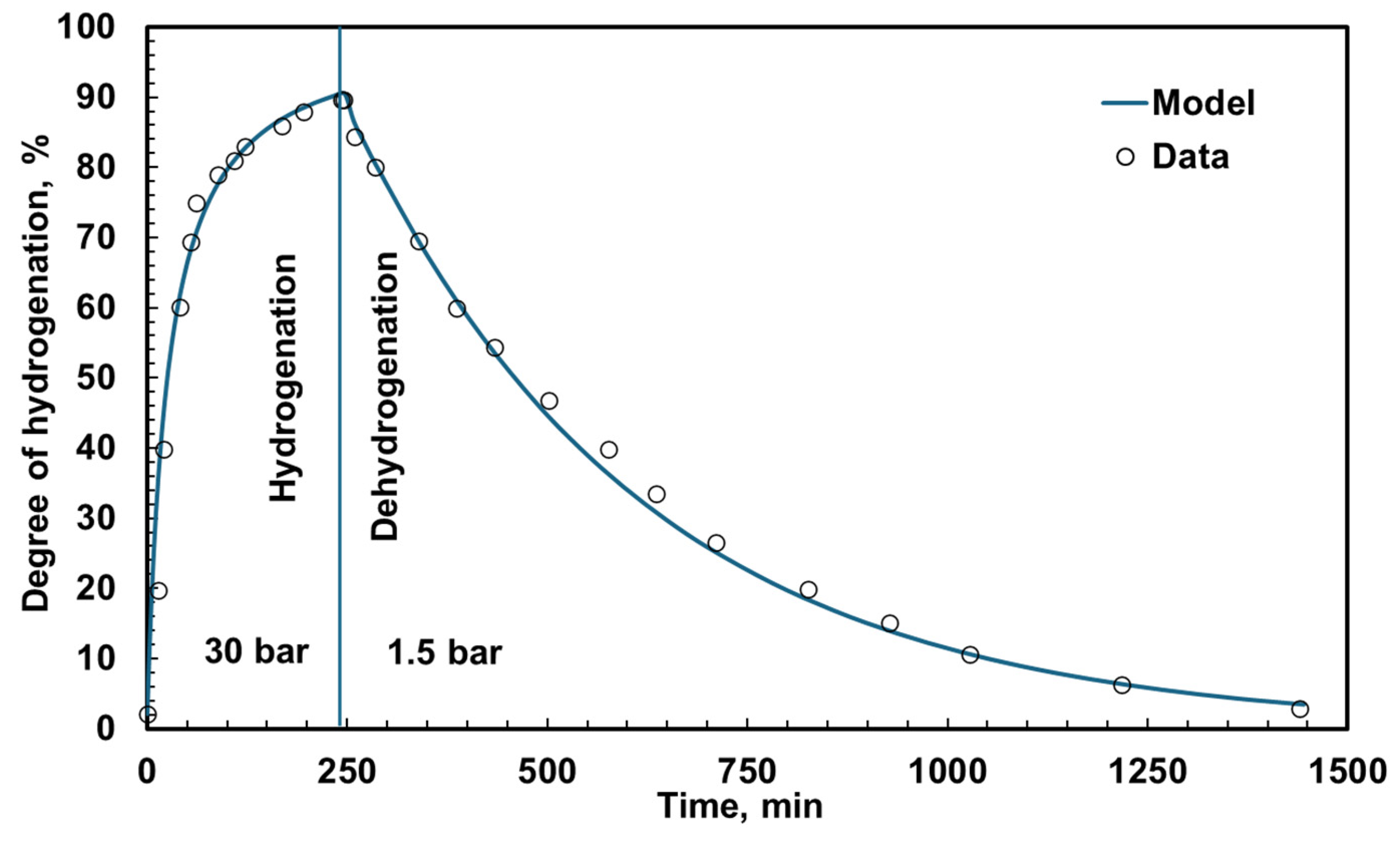
3.1. Reactor Dynamics
3.2. System Performance
3.3. Levelized Cost of Electricity
3.4. Comparison with Other Storage Systems
4. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chandak, S.; Rout, P.K. The Implementation Framework of a Microgrid: A Review. Int. J. Energy Res. 2021, 45, 3523–3547. [Google Scholar] [CrossRef]
- Ahmad, S.; Shafiullah, M.; Ahmed, C.B.; Alowaifeer, M. A Review of Microgrid Energy Management and Control Strategies. IEEE Access 2023, 11, 21729–21757. [Google Scholar] [CrossRef]
- Abbasi, M.; Abbasi, E.; Li, L.; Aguilera, R.P.; Lu, D.; Wang, F. Review on the Microgrid Concept, Structures, Components, Communication Systems, and Control Methods. Energies 2023, 16, 484. [Google Scholar] [CrossRef]
- Microgrids. Available online: https://www.nrel.gov/grid/microgrids.html (accessed on 29 October 2025).
- Cagnano, A.; De Tuglie, E.; Mancarella, P. Microgrids: Overview and Guidelines for Practical Implementations and Operation. Appl. Energy 2020, 258, 114039. [Google Scholar] [CrossRef]
- Shahgholian, G. A Brief Review on Microgrids: Operation, Applications, Modeling, and Control. Int. Trans. Electr. Energy Syst. 2021, 31, e12885. [Google Scholar] [CrossRef]
- DOE EERE 2021. Available online: https://www.energy.gov/eere/iedo/articles/department-energy-releases-new-tool-tracking-microgrid-installations-united (accessed on 29 October 2025).
- Vandoorn, T.L.; De Kooning, J.D.M.; Meersman, B.; Vandevelde, L. Review of Primary Control Strategies for Islanded Microgrids with Power-Electronic Interfaces. Renew. Sustain. Energy Rev. 2013, 19, 613–628. [Google Scholar] [CrossRef]
- Raya-Armenta, J.M.; Bazmohammadi, N.; Avina-Cervantes, J.G.; Sáez, D.; Vasquez, J.C.; Guerrero, J.M. Energy Management System Optimization in Islanded Microgrids: An Overview and Future Trends. Renew. Sustain. Energy Rev. 2021, 149, 111327. [Google Scholar] [CrossRef]
- Bekhradian, R.; Sanaye-Pasand, M. A Comprehensive Survey on Islanding Detection Methods of Synchronous Generator-Based Microgrids: Issues, Solutions and Future Works. IEEE Access 2022, 10, 76202–76219. [Google Scholar] [CrossRef]
- Hossain, M.A.; Pota, H.R.; Hossain, M.J.; Blaabjerg, F. Evolution of Microgrids with Converter-Interfaced Generations: Challenges and Opportunities. Int. J. Electr. Power Energy Syst. 2019, 109, 160–186. [Google Scholar] [CrossRef]
- Abuagreb, M.; Allehyani, M.F.; Johnson, B.K. Overview of Virtual Synchronous Generators: Existing Projects, Challenges, and Future Trends. Electronics 2022, 11, 2843. [Google Scholar] [CrossRef]
- Kebede, A.A.; Kalogiannis, T.; Van Mierlo, J.; Berecibar, M. A Comprehensive Review of Stationary Energy Storage Devices for Large Scale Renewable Energy Sources Grid Integration. Renew. Sustain. Energy Rev. 2022, 159, 112213. [Google Scholar] [CrossRef]
- Al-Shetwi, A.Q.; Hannan, M.A.; Jern, K.P.; Mansur, M.; Mahlia, T.M.I. Grid-Connected Renewable Energy Sources: Review of the Recent Integration Requirements and Control Methods. J. Clean. Prod. 2020, 253, 119831. [Google Scholar] [CrossRef]
- Hossain, M.S.; Madlool, N.A.; Rahim, N.A.; Selvaraj, J.; Pandey, A.K.; Khan, A.F. Role of Smart Grid in Renewable Energy: An Overview. Renew. Sustain. Energy Rev. 2016, 60, 1168–1184. [Google Scholar] [CrossRef]
- Bihari, S.P.; Sadhu, P.K.; Sarita, K.; Khan, B.; Arya, L.D.; Saket, R.K.; Kothari, D.P. A Comprehensive Review of Microgrid Control Mechanism and Impact Assessment for Hybrid Renewable Energy Integration. IEEE Access 2021, 9, 88942–88958. [Google Scholar] [CrossRef]
- García Vera, Y.E.; Dufo-López, R.; Bernal-Agustín, J.L. Energy Management in Microgrids with Renewable Energy Sources: A Literature Review. Appl. Sci. 2019, 9, 3854. [Google Scholar] [CrossRef]
- Arévalo, P.; Ochoa-Correa, D.; Villa-Ávila, E. Optimizing Microgrid Operation: Integration of Emerging Technologies and Artificial Intelligence for Energy Efficiency. Electronics 2024, 13, 3754. [Google Scholar] [CrossRef]
- Juma, S.A.; Ayeng’o, S.P.; Kimambo, C.Z.M. A Review of Control Strategies for Optimized Microgrid Operations. IET Renew. Power Gener. 2024, 18, 2785–2818. [Google Scholar] [CrossRef]
- Grisales-Noreña, L.F.; Vega, H.P.; Montoya, O.D.; Botero-Gómez, V.; Sanin-Villa, D. Cost Optimization of AC Microgrids in Grid-Connected and Isolated Modes Using a Population-Based Genetic Algorithm for Energy Management of Distributed Wind Turbines. Mathematics 2025, 13, 704. [Google Scholar] [CrossRef]
- Punitha, S.; Subramaniam, N.P.; Vimal Raj, P.A.D. A Comprehensive Review of Microgrid Challenges in Architectures, Mitigation Approaches, and Future Directions. J. Electr. Syst. Inf. Technol. 2024, 11, 60. [Google Scholar] [CrossRef]
- Yekini Suberu, M.; Wazir Mustafa, M.; Bashir, N. Energy Storage Systems for Renewable Energy Power Sector Integration and Mitigation of Intermittency. Renew. Sustain. Energy Rev. 2014, 35, 499–514. [Google Scholar] [CrossRef]
- Castillo, A.; Gayme, D.F. Grid-Scale Energy Storage Applications in Renewable Energy Integration: A Survey. Energy Convers. Manag. 2014, 87, 885–894. [Google Scholar] [CrossRef]
- Denholm, P.; O’Connell, M.; Brinkman, G.; Jorgenson, J. Overgeneration from Solar Energy in California. A Field Guide to the Duck Chart; Strategic Energy Analysis Center: Golden, CO, USA, 2015. [Google Scholar]
- Freitas, S.; Brito, M.C. Non-Cumulative Only Solar Photovoltaics for Electricity Load-Matching. Renew. Sustain. Energy Rev. 2019, 109, 271–283. [Google Scholar] [CrossRef]
- Tabassum, S.; Rahman, T.; Islam, A.U.; Rahman, S.; Dipta, D.R.; Roy, S.; Mohammad, N.; Nawar, N.; Hossain, E. Solar Energy in the United States: Development, Challenges and Future Prospects. Energies 2021, 14, 8142. [Google Scholar] [CrossRef]
- Cobos, N.G.; Arroyo, J.M.; Alguacil, N.; Wang, J. Robust Energy and Reserve Scheduling Considering Bulk Energy Storage Units and Wind Uncertainty. IEEE Trans. Power Syst. 2018, 33, 5206–5216. [Google Scholar] [CrossRef]
- van der Linden, S. Bulk Energy Storage Potential in the USA, Current Developments and Future Prospects. Energy 2006, 31, 3446–3457. [Google Scholar] [CrossRef]
- Pickard, W.F.; Shen, A.Q.; Hansing, N.J. Parking the Power: Strategies and Physical Limitations for Bulk Energy Storage in Supply–Demand Matching on a Grid Whose Input Power Is Provided by Intermittent Sources. Renew. Sustain. Energy Rev. 2009, 13, 1934–1945. [Google Scholar] [CrossRef]
- Wadia, C.; Albertus, P.; Srinivasan, V. Resource Constraints on the Battery Energy Storage Potential for Grid and Transportation Applications. J. Power Sources 2011, 196, 1593–1598. [Google Scholar] [CrossRef]
- AL Shaqsi, A.Z.; Sopian, K.; Al-Hinai, A. Review of Energy Storage Services, Applications, Limitations, and Benefits. Energy Rep. 2020, 6, 288–306. [Google Scholar] [CrossRef]
- Abraham, K.M. Prospects and Limits of Energy Storage in Batteries. J. Phys. Chem. Lett. 2015, 6, 830–844. [Google Scholar] [CrossRef]
- Schoenung, S. Economic Analysis of Large-Scale Hydrogen Storage for Renewable Utility Applications; Sandia National Laboratories: Albuquerque, NM, USA; Livermore, CA, USA, 2011. [Google Scholar]
- Environmental and Energy Study Institute. Fact Sheet, Energy Storage (2019). 22 February 2019. Available online: https://www.eesi.org/papers/view/energy-storage-2019 (accessed on 29 October 2025).
- Qi, N.; Huang, K.; Fan, Z.; Xu, B. Long-Term Energy Management for Microgrid with Hybrid Hydrogen-Battery Energy Storage: A Prediction-Free Coordinated Optimization Framework. Appl. Energy 2025, 377, 124485. [Google Scholar] [CrossRef]
- California Hybrid Hydrogen Fuel Cell and Battery Storage Facility Is Now Online. Available online: https://pv-magazine-usa.com/2025/09/30/california-hybrid-hydrogen-fuel-cell-and-battery-storage-facility-is-now-online/ (accessed on 29 October 2025).
- Hybrid Microgrid Combines Hydrogen Fuel Cells with Battery Storage. Available online: https://www.powermag.com/hybrid-microgrid-combines-hydrogen-fuel-cells-with-battery-storage/ (accessed on 29 October 2025).
- Energy Vault, PG&E Launch ‘World’s First’ Ultra-Long Duration Hybrid Battery + Hydrogen Energy Storage Microgrid. Available online: https://www.power-eng.com/hydrogen/energy-vault-pge-launch-worlds-first-ultra-long-duration-hybrid-battery-hydrogen-energy-storage-microgrid/ (accessed on 29 October 2025).
- Guo, X.; Gu, F.; Liu, H.; Yu, Y.; Li, R.; Wang, J. Sustainable PV-Hydrogen-Storage Microgrid Energy Management Using a Hierarchical Economic Model Predictive Control Framework. Energy Inform. 2025, 8, 18. [Google Scholar] [CrossRef]
- Hua, T.Q.; Ahluwalia, R.K.; Peng, J.-K.; Kromer, M.; Lasher, S.; McKenney, K.; Law, K.; Sinha, J. Technical Assessment of Compressed Hydrogen Storage Tank Systems for Automotive Applications. Int. J. Hydrogen Energy 2011, 36, 3037–3049. [Google Scholar] [CrossRef]
- Hassan, I.A.; Ramadan, H.S.; Saleh, M.A.; Hissel, D. Hydrogen Storage Technologies for Stationary and Mobile Applications: Review, Analysis and Perspectives. Renew. Sustain. Energy Rev. 2021, 149, 111311. [Google Scholar] [CrossRef]
- Barthelemy, H.; Weber, M.; Barbier, F. Hydrogen Storage: Recent Improvements and Industrial Perspectives. Int. J. Hydrogen Energy 2017, 42, 7254–7262. [Google Scholar] [CrossRef]
- Zohuri, B. Cryogenics and Liquid Hydrogen Storage. In Hydrogen Energy; Springer International Publishing: Cham, Switzerland, 2019; pp. 121–139. [Google Scholar]
- Valenti, G. Hydrogen Liquefaction and Liquid Hydrogen Storage. In Compendium of Hydrogen Energy; Elsevier: Amsterdam, The Netherlands, 2016; pp. 27–51. [Google Scholar]
- Ezzat, M.F.; Dincer, I. Development and Assessment of a New Hybrid Vehicle with Ammonia and Hydrogen. Appl. Energy 2018, 219, 226–239. [Google Scholar] [CrossRef]
- Inal, O.B.; Zincir, B.; Deniz, C. Investigation on the Decarbonization of Shipping: An Approach to Hydrogen and Ammonia. Int. J. Hydrogen Energy 2022, 47, 19888–19900. [Google Scholar] [CrossRef]
- Cechetto, V.; Di Felice, L.; Gallucci, F. Advances and Perspectives of H2 Production from NH 3 Decomposition in Membrane Reactors. Energy Fuels 2023, 37, 10775–10798. [Google Scholar] [CrossRef]
- Lamb, K.E.; Dolan, M.D.; Kennedy, D.F. Ammonia for Hydrogen Storage; A Review of Catalytic Ammonia Decomposition and Hydrogen Separation and Purification. Int. J. Hydrogen Energy 2019, 44, 3580–3593. [Google Scholar] [CrossRef]
- Engelbrecht, N.; Chiuta, S.; Bessarabov, D.G. A Highly Efficient Autothermal Microchannel Reactor for Ammonia Decomposition: Analysis of Hydrogen Production in Transient and Steady-State Regimes. J. Power Sources 2018, 386, 47–55. [Google Scholar] [CrossRef]
- Papadias, D.D.; Peng, J.-K.; Ahluwalia, R.K. Hydrogen Carriers: Production, Transmission, Decomposition, and Storage. Int. J. Hydrogen Energy 2021, 46, 24169–24189. [Google Scholar] [CrossRef]
- Preuster, P.; Papp, C.; Wasserscheid, P. Liquid Organic Hydrogen Carriers (LOHCs): Toward a Hydrogen-Free Hydrogen Economy. Acc. Chem. Res. 2017, 50, 74–85. [Google Scholar] [CrossRef]
- Hydrogenious LOHC. Available online: https://hydrogenious.net/ (accessed on 29 October 2025).
- Jorschick, H.; Geißelbrecht, M.; Eßl, M.; Preuster, P.; Bösmann, A.; Wasserscheid, P. Benzyltoluene/Dibenzyltoluene-Based Mixtures as Suitable Liquid Organic Hydrogen Carrier Systems for Low Temperature Applications. Int. J. Hydrogen Energy 2020, 45, 14897–14906. [Google Scholar] [CrossRef]
- Held, M.; Rieck, A.; Bajohr, S.; Kolb, T. Hydrogenation of Dibenzyl Toluene—A Competing Side Reaction in Three Phase Methanation. Int. J. Hydrogen Energy 2025, 132, 166–173. [Google Scholar] [CrossRef]
- National Fire Protection Association. Flammable and Combustible Liquids Code. Available online: https://www.nfpa.org/codes-and-standards/nfpa-30-standard-development/30 (accessed on 29 October 2025).
- Modisha, P.M.; Ouma, C.N.M.; Garidzirai, R.; Wasserscheid, P.; Bessarabov, D. The Prospect of Hydrogen Storage Using Liquid Organic Hydrogen Carriers. Energy Fuels 2019, 33, 2778–2796. [Google Scholar] [CrossRef]
- Müller, K.; Stark, K.; Emel’yanenko, V.N.; Varfolomeev, M.A.; Zaitsau, D.H.; Shoifet, E.; Schick, C.; Verevkin, S.P.; Arlt, W. Liquid Organic Hydrogen Carriers: Thermophysical and Thermochemical Studies of Benzyl- and Dibenzyl-Toluene Derivatives. Ind. Eng. Chem. Res. 2015, 54, 7967–7976. [Google Scholar] [CrossRef]
- Müller, K.; Aslam, R.; Fischer, A.; Stark, K.; Wasserscheid, P.; Arlt, W. Experimental Assessment of the Degree of Hydrogen Loading for the Dibenzyl Toluene Based LOHC System. Int. J. Hydrogen Energy 2016, 41, 22097–22103. [Google Scholar] [CrossRef]
- Jorschick, H.; Dürr, S.; Preuster, P.; Bösmann, A.; Wasserscheid, P. Operational Stability of a LOHC-Based Hot Pressure Swing Reactor for Hydrogen Storage. Energy Technol. 2019, 7, 146–152. [Google Scholar] [CrossRef]
- Ali, A.; Kumar G, U.; Lee, H.J. Parametric Study of the Hydrogenation of Dibenzyltoluene and Its Dehydrogenation Performance as a Liquid Organic Hydrogen Carrier. J. Mech. Sci. Technol. 2020, 34, 3069–3077. [Google Scholar] [CrossRef]
- Jorschick, H.; Preuster, P.; Dürr, S.; Seidel, A.; Müller, K.; Bösmann, A.; Wasserscheid, P. Hydrogen Storage Using a Hot Pressure Swing Reactor. Energy Environ. Sci. 2017, 10, 1652–1659. [Google Scholar] [CrossRef]
- Wunsch, A.; Gapp, E.; Peters, T.; Pfeifer, P. Impact of Product Gas Impurities from Dehydrogenation of Perhydro-Dibenzyltoluene on the Performance of a 10 Μm PdAg-Membrane. J. Memb. Sci. 2021, 628, 119094. [Google Scholar] [CrossRef]
- Jorschick, H.; Vogl, M.; Preuster, P.; Bösmann, A.; Wasserscheid, P. Hydrogenation of Liquid Organic Hydrogen Carrier Systems Using Multicomponent Gas Mixtures. Int. J. Hydrogen Energy 2019, 44, 31172–31182. [Google Scholar] [CrossRef]
- Luschtinetz, T.; Sklarow, A.; Gulden, J. Degradation Effects on PEM Fuel Cells Supplied with Hydrogen from a LOHC System. Appl. Mech. Mater. 2016, 839, 165–169. [Google Scholar] [CrossRef]
- Bulgarin, A.; Jorschick, H.; Preuster, P.; Bösmann, A.; Wasserscheid, P. Purity of Hydrogen Released from the Liquid Organic Hydrogen Carrier Compound Perhydro Dibenzyltoluene by Catalytic Dehydrogenation. Int. J. Hydrogen Energy 2020, 45, 712–720. [Google Scholar] [CrossRef]
- Papadias, D.D.; Ahmed, S.; Kumar, R.; Joseck, F. Hydrogen Quality for Fuel Cell Vehicles—A Modeling Study of the Sensitivity of Impurity Content in Hydrogen to the Process Variables in the SMR–PSA Pathway. Int. J. Hydrogen Energy 2009, 34, 6021–6035. [Google Scholar] [CrossRef]
- Woods, D.R. Rules of Thumb in Engineering Practice; Wiley-VCH: Oakville, ON, Canada, 2007; ISBN 9783527312207. [Google Scholar]
- Silla, H. Chemical Process Engineering—Design and Engineering; Heinemann, H., Ed.; Marcel Dekker: New York, NY, USA, 2005. [Google Scholar]
- Ahluwalia, R.K.; Wang, X.; Papadias, D.D.; Star, A.G. Performance and Total Cost of Ownership of a Fuel Cell Hybrid Mining Truck. Energies 2022, 16, 286. [Google Scholar] [CrossRef]
- Borup, R.L.; Kusoglu, A.; Neyerlin, K.C.; Mukundan, R.; Ahluwalia, R.K.; Cullen, D.A.; More, K.L.; Weber, A.Z.; Myers, D.J. Recent Developments in Catalyst-Related PEM Fuel Cell Durability. Curr. Opin. Electrochem. 2020, 21, 192–200. [Google Scholar] [CrossRef]
- Ahluwalia, R.K.; Wang, X.; Peng, J.-K.; Konduru, V.; Arisetty, S.; Ramaswamy, N.; Kumaraguru, S. Achieving 5000-h and 8000-h Low-PGM Electrode Durability on Automotive Drive Cycles. J. Electrochem. Soc. 2021, 168, 044518. [Google Scholar] [CrossRef]
- Wang, X.; Star, A.G.; Ahluwalia, R.K. Performance of Polymer Electrolyte Membrane Water Electrolysis Systems: Configuration, Stack Materials, Turndown and Efficiency. Energies 2023, 16, 4964. [Google Scholar] [CrossRef]
- Ahluwalia, R.K.; Wang, X.; Star, A.G.; Papadias, D.D. Performance and Cost of Fuel Cells for Off-Road Heavy-Duty Vehicles. Int. J. Hydrogen Energy 2022, 47, 10990–11006. [Google Scholar] [CrossRef]
- Ahluwalia, R.K.; Peng, J.-K.; Wang, X.; Papadias, D.; Kopasz, J. Performance and Cost of Fuel Cells for Urban Air Mobility. Int. J. Hydrogen Energy 2021, 46, 36917–36929. [Google Scholar] [CrossRef]
- System Advisor Model. Available online: https://sam.nrel.gov/ (accessed on 29 October 2025).
- Commercial and Residential Hourly Load Profiles for All TMY3 Locations in the United States. Available online: https://data.openei.org/submissions/153 (accessed on 29 October 2025).
- IRENA. Future of Solar Photovoltaic: Deployment, Investment, Technology, Grid Integration and Socio-Economic Aspects (A Global Energy Transformation: Paper); International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2019; Available online: https://www.irena.org/publications/2019/nov/future-of-solar-photovoltaic (accessed on 29 October 2025).
- IRENA. Future of Wind: Deployment, Investment, Technology, Grid Integration and Socio-Economic Aspects (A Global Energy Transformation Paper); International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2019; Available online: https://www.irena.org/publications/2019/oct/future-of-wind (accessed on 29 October 2025).
- Vignesh, R.; Feldman, D.; Desai, J.; Margolis, R. U.S. Solar Photovoltaic System and Energy Storage Cost Benchmarks: Q1 2021; NREL: Golden, CO, USA, 2021. [Google Scholar]
- 2030 Solar Cost Targets. Available online: https://www.energy.gov/eere/solar/solar-energy-technologies-office-updated-2030-goals-utility-scale-photovoltaics (accessed on 29 October 2025).
- Wiser, R.; Bolinger, M.; Hoen, B.; Millstein, D.; Rand, J.; Barbose, G.; Darghouth, N.; Gorman, W.; Jeong, S.; Mills, A.; et al. Land-Based Wind Market Report: 2021 Edition; U.S. Department of Energy: Alexandria, VA, USA, 2021.
- Stehly, T.; Duffy, P. 2020 Cost of Wind Energy Review; NREL: Golden, CO, USA, 2020. [Google Scholar]
- Badgett, A.; Brauch, J.; Thatte, A.; Rubin, R.; Skangos, C.; Wang, X.; Ahluwalia, R.; Pivovar, B.; Ruth, M. Updated Manufactured Cost Analysis for Proton Exchange Membrane Water Electrolyzers; NREL: Golden, CO, USA, 2024. [Google Scholar]
- Dibenzyl Toluene Market Size. Available online: https://www.researchnester.com/reports/dibenzyl-toluene-market/3348 (accessed on 29 October 2025).
- Tulus, V.; Pérez-Ramírez, J.; Guillén-Gosálbez, G. Planetary Metrics for the Absolute Environmental Sustainability Assessment of Chemicals. Green. Chem. 2021, 23, 9881–9893. [Google Scholar] [CrossRef]
- Zuiderveen, E.A.R.; Caldeira, C.; Vries, T.; Schenk, N.J.; Huijbregts, M.A.J.; Sala, S.; Hanssen, S.V.; van Zelm, R. Evaluating the Environmental Sustainability of Alternative Ways to Produce Benzene, Toluene, and Xylene. ACS Sustain. Chem. Eng. 2024, 12, 5092–5104. [Google Scholar] [CrossRef] [PubMed]
- Modisha, P.; Bessarabov, D. Stress Tolerance Assessment of Dibenzyltoluene-Based Liquid Organic Hydrogen Carriers. Sustain. Energy Fuels 2020, 4, 4662–4670. [Google Scholar] [CrossRef]
- Cho, H.H.; Strezov, V.; Evans, T.J. Life Cycle Assessment of Renewable Hydrogen Transport by Liquid Organic Hydrogen Carriers. J. Clean. Prod. 2024, 469, 143130. [Google Scholar] [CrossRef]
- Straathof, A.J.J.; Bampouli, A. Potential of Commodity Chemicals to Become Bio-based According to Maximum Yields and Petrochemical Prices. Biofuels Bioprod. Biorefining 2017, 11, 798–810. [Google Scholar] [CrossRef]
- IMARC Group. Bio-Based Toluene Manufacturing Plant Project Report. Available online: https://www.imarcgroup.com/bio-based-toluene-manufacturing-plant-project-report (accessed on 29 October 2025).
- Hydrogen Supply and Transportation Using Liquid Organic Hydrogen Carriers (HYSTOC), H2020-JTI FCH-2017-1, Grant Agreement: 779694. 2019. Available online: https://cordis.europa.eu/project/id/779694 (accessed on 29 October 2025).
- DOE H2A Production Analysis. Available online: https://www.hydrogen.energy.gov/h2a_production.html (accessed on 29 October 2025).
- Ba, J.; Gao, R.; Shi, D.; Nessa, H.A.; Shi, C.; Zhang, X.; Pan, L.; Zou, J.-J. Progress in Catalysts for Hydrogen Storage/Release in MBT/DBT Based LOHCs: A Review. Chem. Commun. 2025, 61, 8619–8631. [Google Scholar] [CrossRef]
- Ali, A.; Khan, M.A.; Choi, H. Hydrogen Storage Prediction in Dibenzyltoluene as Liquid Organic Hydrogen Carrier Empowered with Weighted Federated Machine Learning. Mathematics 2022, 10, 3846. [Google Scholar] [CrossRef]
- Niermann, M.; Drünert, S.; Kaltschmitt, M.; Bonhoff, K. Liquid Organic Hydrogen Carriers (LOHCs)—Techno-Economic Analysis of LOHCs in a Defined Process Chain. Energy Environ. Sci. 2019, 12, 290–307. [Google Scholar] [CrossRef]
- Marlotherm Heat Transfer Fluids. Available online: https://info.eastman.com/marlotherm_lh_and_sh.html (accessed on 29 October 2025).
- Energy Storage Costs. Available online: https://www.irena.org/energy-transition/technology/energy-storage (accessed on 29 October 2025).
- Papadias, D.D.; Ahluwalia, R.K. Bulk Storage of Hydrogen. Int. J. Hydrogen Energy 2021, 46, 34527–34541. [Google Scholar] [CrossRef]
- Ji, X.; Louarn, E.; Fache, F.; Vanoye, L.; Bonhommé, A.; Pitault, I.; Meille, V. Analysis of Dibenzyltoluene Mixtures: From Fast Analysis to In-Depth Characterization of the Compounds. Molecules 2023, 28, 3751. [Google Scholar] [CrossRef]


| Hydrogen Carrier Compound | Mol. Wt. | Density | Grav. Cap. | Vol. Cap. | ΔH° | ΔS° |
|---|---|---|---|---|---|---|
| (g mol−1) | (g cm−3) | (wt. %) | (g L−1) | (kJ mol−1) | (JK−1mol−1) | |
| Liquid Hydrogen (20 K) | 2.02 | 0.07 | 100 | 70 | - | - |
| Compressed (350 bar) | 2.02 | 0.026 | 100 | 26 | - | - |
| Two-Way Carriers | ||||||
| H18-DBT (l)/DBT (l) | 290.40 | 0.92 | 6.2 | 57 | 65 | 120 |
| Ethanol (l)/Ethyl acetate (l) | 46.07 | 0.789 | 4.4 | 35 | 36 | 101 |
| Methylcyclohexane/Toluene (l) | 98.19 | 0.77 | 6.2 | 47 | 68 | 119 |
| 1,4-Butanediol (l)/g-butyrolactone (l) | 90.12 | 1.017 | 4.5 | 46 | 43 | 118 |
| 6 M Potassium Formate/Bicarbonate | 84.12 | 1.26 | 0.95 | 12 | 20 | 60 |
| One-Way Carriers | ||||||
| Ammonia NH3 (l)/H2 + N2 | 17.03 | 0.61 | 17.0 | 108 | 46 | 193 |
| Methanol Reforming/H2 + CO2 | 32.04 | 0.792 | 15.7 | 125 | 44 | 136 |
| Formic Acid (l)/H2 + CO2 | 46.03 | 1.22 | 4.4 | 53 | 32 | 213 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papadias, D.D.; Ahluwalia, R.K.; Peng, J.-K.; Valdez, P.; Tbaileh, A.; Brooks, K. Hydrogen Carriers for Renewable Microgrid System Applications. Energies 2025, 18, 5775. https://doi.org/10.3390/en18215775
Papadias DD, Ahluwalia RK, Peng J-K, Valdez P, Tbaileh A, Brooks K. Hydrogen Carriers for Renewable Microgrid System Applications. Energies. 2025; 18(21):5775. https://doi.org/10.3390/en18215775
Chicago/Turabian StylePapadias, Dionissios D., Rajesh K. Ahluwalia, Jui-Kun Peng, Peter Valdez, Ahmad Tbaileh, and Kriston Brooks. 2025. "Hydrogen Carriers for Renewable Microgrid System Applications" Energies 18, no. 21: 5775. https://doi.org/10.3390/en18215775
APA StylePapadias, D. D., Ahluwalia, R. K., Peng, J.-K., Valdez, P., Tbaileh, A., & Brooks, K. (2025). Hydrogen Carriers for Renewable Microgrid System Applications. Energies, 18(21), 5775. https://doi.org/10.3390/en18215775







