Biohydrogen Production from Industrial Waste: The Role of Pretreatment Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Inoculation Material
2.2. Agro-Industrial Wastes
2.3. Pretreatment and Hydrolysis of Industry Wastes
2.4. Batch Experiments
2.5. Analytical Methods
2.5.1. Biogas Composition
2.5.2. Volatile Fatty Acids
2.5.3. Carbohydrates
2.5.4. Protein
2.6. Calculations
3. Results and Discussion
3.1. Chemical Characteristics of Waste Materials and Their Susceptibility to Pretreatment
3.2. Batch Experiments
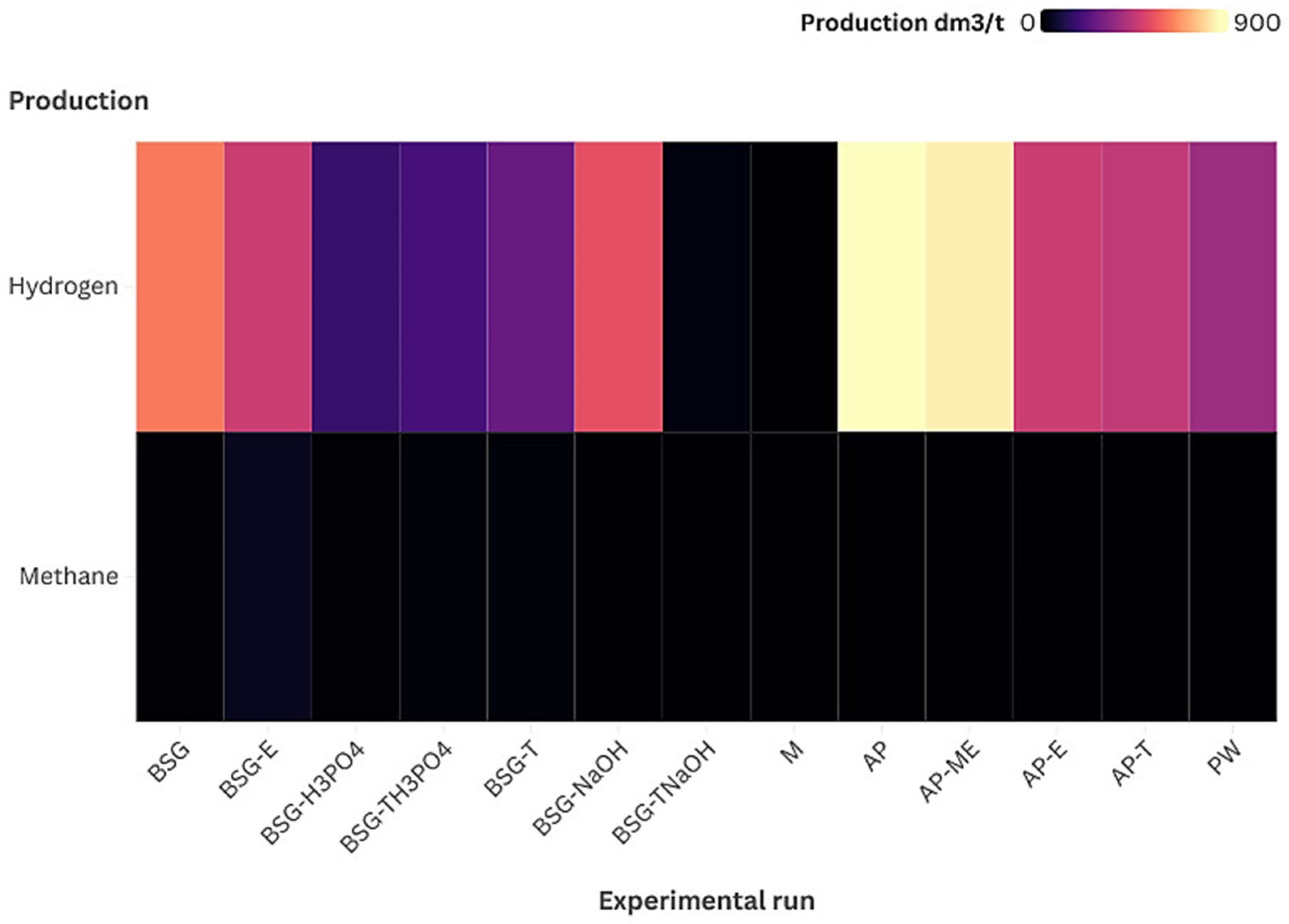
3.2.1. Hydrogen and Methane Production
3.2.2. Biogas Composition and Biogas Yield
3.2.3. Fermentation Dynamics
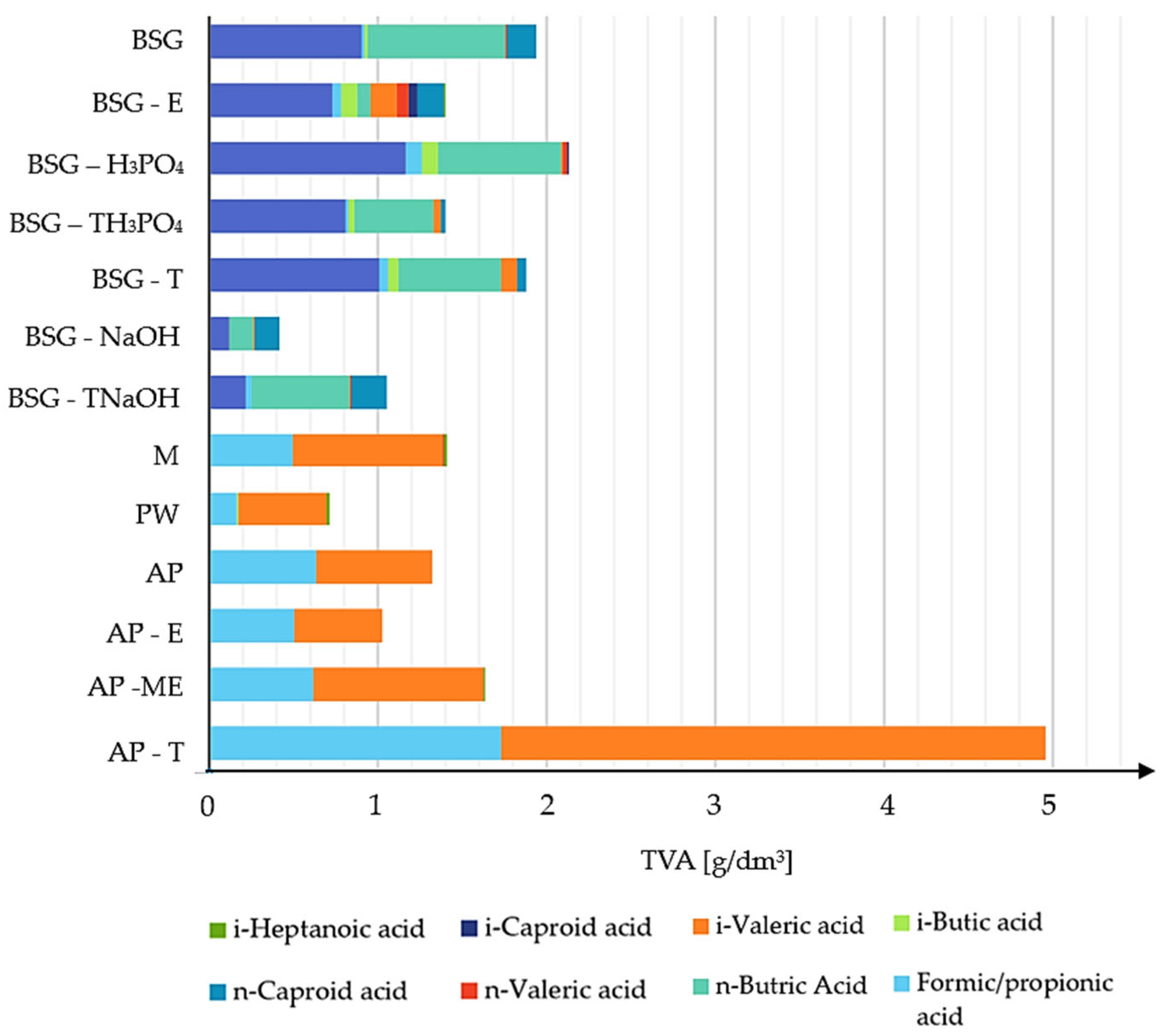
3.2.4. Total Volatile Acids
3.2.5. Energy Yield
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | anaerobic digestion |
| AP | apple pomace |
| AP-E | apple pomace after the enzyme treatment |
| AP-ME | apple pomace after the mixture of enzymes treatment |
| AP-T | apple pomace after thermal treatment |
| AW | agroindustry waste |
| BSG | brewer’s spent grain |
| BSG-E | brewer’s spent grain after the enzyme treatment |
| BSG-T | brewer’s spent grain after thermal treatment |
| BSG-NaOH | brewer’s spent grain after alkali treatment |
| BSG-TNaOH | brewer’s spent grain after alkali and thermal treatment |
| BSG-H3PO4 | brewer’s spent grain after acidic treatment |
| BSG-TH3PO4 | brewer’s spent grain after acidic and thermal treatment |
| COD | chemical oxygen demand |
| DF | dark fermentation |
| FID | flame ionization detector |
| HL | liquid hydrolysates |
| F | Furfural |
| HMF | 5-hydroxymethylfurfural |
| HS | hydrolyzed solids |
| M | molasses |
| SGP | specific gas production |
| SHP | specific hydrogen production |
| SMP | specific methane production |
| PW | potato waste |
| RID | refractive index detector |
| TAN | total ammonium nitrogen |
| TFA | total volatile acids |
| TS | total solids |
| VFA | volatile fatty acids |
| VS | volatile solids |
References
- Anser, M.K.; Usman, M.; Sharif, M.; Bashir, S.; Shabbir, M.S.; Yahya Khan, G.; Lopez, L.B. The dynamic impact of renewable energy sources on environmental economic growth: Evidence from selected Asian economies. Environ. Sci. Pollut. Res. 2022, 29, 3323–3335. [Google Scholar] [CrossRef]
- Akinyele, D.; Olabode, E.; Amole, A. Review of Fuel Cell Technologies and Applications for Sustainable Microgrid Systems. Inventions 2020, 5, 42. [Google Scholar] [CrossRef]
- Hossain Bhuiyan, M.M.; Siddique, Z. Hydrogen as an alternative fuel: A comprehensive review of challenges and opportunities in production, storage, and transportation. Int. J. Hydrogen Energy 2025, 102, 1026–1044. [Google Scholar] [CrossRef]
- Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions—European Environment Agency. Available online: https://www.eea.europa.eu/policy-documents/communication-from-the-commission-to-1 (accessed on 16 September 2025).
- Kapdan, I.K.; Kargi, F. Bio-hydrogen production from waste materials. Enzyme Microb. Technol. 2006, 38, 569–582. [Google Scholar] [CrossRef]
- Younas, M.; Shafique, S.; Hafeez, A.; Javed, F.; Rehman, F. An Overview of Hydrogen Production: Current Status, Potential, and Challenges. Fuel 2022, 316, 123317. [Google Scholar] [CrossRef]
- Singla, S.; Shetti, N.P.; Basu, S.; Mondal, K.; Aminabhavi, T.M. Hydrogen production technologies—Membrane based separation, storage and challenges. J. Environ. Manag. 2022, 302, 113963. [Google Scholar] [CrossRef]
- Srivastava, N.; Srivastava, M.; Abd_Allah, E.F.; Singh, R.; Hashem, A.; Gupta, V.K. Biohydrogen production using kitchen waste as the potential substrate: A sustainable approach. Chemosphere 2021, 271, 129537. [Google Scholar] [CrossRef]
- Pfeifer, K.; Ergal, İ.; Koller, M.; Basen, M.; Schuster, B.; Rittmann, S.K.M.R. Archaea Biotechnology. Biotechnol. Adv. 2021, 47, 107668. [Google Scholar] [CrossRef]
- Hung, C.H.; Chang, Y.T.; Chang, Y.J. Roles of microorganisms other than Clostridium and Enterobacter in anaerobic fermentative biohydrogen production systems—A review. Bioresour. Technol. 2011, 102, 8437–8444. [Google Scholar] [CrossRef]
- Chandran, E.M.; Mohan, E. Sustainable biohydrogen production from lignocellulosic biomass sources—Metabolic pathways, production enhancement, and challenges. Environ. Sci. Pollut. Res. 2023, 30, 102129–102157. [Google Scholar] [CrossRef]
- Yafetto, L.; Odamtten, G.T.; Wiafe-Kwagyan, M. Valorization of agro-industrial wastes into animal feed through microbial fermentation: A review of the global and Ghanaian case. Heliyon 2023, 9, e14814. [Google Scholar] [CrossRef]
- Kour, R.; Singh, S.; Sharma, H.B.; Naik, T.S.S.K.; Shehata, N.N.P.; Ali, W.; Kapoor, D.; Dhanjal, D.S.; Singh, J.; Khan, A.H.; et al. Persistence and remote sensing of agri-food wastes in the environment: Current state and perspectives. Chemosphere 2023, 317, 137822. [Google Scholar] [CrossRef]
- Parsa, A.; Van De Wiel, M.; Schmutz, U.; Taylor, I.; Fried, J. Balancing people, planet, and profit in urban food waste management. Sustain. Prod. Consum. 2024, 45, 203–215. [Google Scholar] [CrossRef]
- Díez, M.P.; Villanueva-Galindo, E.; Moreno-Andrade, I.; Díaz, E.; de la Rubia, M.A.; Mohedano, A.F.; Perez-Rangel, M. Enhanced hydrogen production from food waste via bioaugmentation with Clostridium and Lactobacillus. Biomass Convers. Biorefinery 2025, 1–13. [Google Scholar] [CrossRef]
- Ranjbari, M.; Shams Esfandabadi, Z.; Quatraro, F.; Vatanparast, H.; Lam, S.S.; Aghbashlo, M.; Tabatabaei, M. Biomass and organic waste potentials towards implementing circular bioeconomy platforms: A systematic bibliometric analysis. Fuel 2022, 318, 123585. [Google Scholar] [CrossRef]
- Yu, H.; Xiao, W.; Han, L.; Huang, G. Characterization of mechanical pulverization/phosphoric acid pretreatment of corn stover for enzymatic hydrolysis. Bioresour. Technol. 2019, 282, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Basak, B.; Kumar, R.; Bharadwaj, A.V.S.L.S.; Kim, T.H.; Kim, J.R.; Jang, M.; Oh, S.E.; Roh, H.S.; Jeon, B.H. Advances in physicochemical pretreatment strategies for lignocellulose biomass and their effectiveness in bioconversion for biofuel production. Bioresour. Technol. 2023, 369, 128413. [Google Scholar] [CrossRef]
- Cui, M.; Shen, J. Effects of acid and alkaline pretreatments on the biohydrogen production from grass by anaerobic dark fermentation. Int. J. Hydrogen Energy 2012, 37, 1120–1124. [Google Scholar] [CrossRef]
- Chen, J.; Cai, Y.; Wang, Z.; Wang, S.; Li, J.; Song, C.; Zhuang, W.; Liu, D.; Wang, S.; Song, A.; et al. Construction of a Synthetic Microbial Community for Enzymatic Pretreatment of Wheat Straw for Biogas Production via Anaerobic Digestion. Environ. Sci. Technol. 2024, 58, 9446–9455. [Google Scholar] [CrossRef]
- Rodríguez-Valderrama, S.; Escamilla-Alvarado, C.; Rivas-García, P.; Magnin, J.P.; Alcalá-Rodríguez, M.; García-Reyes, R.B. Biorefinery concept comprising acid hydrolysis, dark fermentation, and anaerobic digestion for co-processing of fruit and vegetable wastes and corn stover. Environ. Sci. Pollut. Res. 2020, 27, 28585–28596. [Google Scholar] [CrossRef]
- Xie, Z.; Zou, H.; Zheng, Y.; Fu, S.F. Improving anaerobic digestion of corn straw by using solid-state urea pretreatment. Chemosphere 2022, 293, 133559. [Google Scholar] [CrossRef]
- Ujor, V.C.; Okonkwo, C.C. Microbial detoxification of lignocellulosic biomass hydrolysates: Biochemical and molecular aspects, challenges, exploits and future perspectives. Front. Bioeng. Biotechnol. 2022, 10, 1061667. [Google Scholar] [CrossRef] [PubMed]
- Correia, J.A.d.C.; Júnior, J.E.M.; Gonçalves, L.R.B.; Rocha, M.V.P. Alkaline hydrogen peroxide pretreatment of cashew apple bagasse for ethanol production: Study of parameters. Bioresour. Technol. 2013, 139, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Berlowska, J.; Cieciura, W.; Borowski, S.; Dudkiewicz, M.; Binczarski, M.; Witonska, I.; Otlewska, A.; Kregiel, D. Simultaneous Saccharification and Fermentation of Sugar Beet Pulp with Mixed Bacterial Cultures for Lactic Acid and Propylene Glycol Production. Molecules 2016, 21, 1380. [Google Scholar] [CrossRef] [PubMed]
- Cieciura-Włoch, W.; Borowski, S.; Domański, J. Dark fermentative hydrogen production from hydrolyzed sugar beet pulp improved by nitrogen and phosphorus supplementation. Bioresour. Technol. 2021, 340, 125622. [Google Scholar] [CrossRef]
- Cieciura-Włoch, W.; Borowski, S.; Domański, J. Dark fermentative hydrogen production from hydrolyzed sugar beet pulp improved by iron addition. Bioresour. Technol. 2020, 314, 123713. [Google Scholar] [CrossRef]
- Blasi, A.; Verardi, A.; Lopresto, C.G.; Siciliano, S.; Sangiorgio, P. Lignocellulosic Agricultural Waste Valorization to Obtain Valuable Products: An Overview. Recycling 2023, 8, 61. [Google Scholar] [CrossRef]
- Godoy, C.A.; Valderrama, P.; Furtado, A.C.; Boroski, M. Analysis of HMF and furfural in hydrolyzed lignocellulosic biomass by HPLC-DAD-based method using FDCA as internal standard. MethodsX 2022, 9, 101774. [Google Scholar] [CrossRef]
- Zhao, Y.; Lu, K.; Xu, H.; Zhu, L.; Wang, S. A critical review of recent advances in the production of furfural and 5-hydroxymethylfurfural from lignocellulosic biomass through homogeneous catalytic hydrothermal conversion. Renew. Sustain. Energy Rev. 2021, 139, 110706. [Google Scholar] [CrossRef]
- Esteban, J.; Yustos, P.; Ladero, M. Catalytic Processes from Biomass-Derived Hexoses and Pentoses: A Recent Literature Overview. Catalysts 2018, 8, 637. [Google Scholar] [CrossRef]
- Cieciura-Włoch, W.; Binczarski, M.; Tomaszewska, J.; Borowski, S.; Domański, J.; Dziugan, P.; Witońska, I. The Use of Acidic Hydrolysates after Furfural Production from Sugar Waste Biomass as a Fermentation Medium in the Biotechnological Production of Hydrogen. Energies 2019, 12, 3222. [Google Scholar] [CrossRef]
- Lin, R.; Cheng, J.; Ding, L.; Song, W.; Zhou, J.; Cen, K. Inhibitory effects of furan derivatives and phenolic compounds on dark hydrogen fermentation. Bioresour. Technol. 2015, 196, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.L.; Zhu, M.J. Insight into furfural-tolerant and hydrogen-producing microbial consortia: Mechanism of furfural tolerance and hydrogen production. Bioresour. Technol. 2024, 407, 131141. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.J.; Show, K.Y.; Su, A. Dark fermentation on biohydrogen production: Pure culture. Bioresour. Technol. 2011, 102, 8393–8402. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Li, J.; Liu, F. Continuous biohydrogen production from diluted molasses in an anaerobic contact reactor. Front. Environ. Sci. Eng. China 2011, 5, 140–148. [Google Scholar] [CrossRef]
- Latifi, A.; Avilan, L.; Brugna, M. Clostridial whole cell and enzyme systems for hydrogen production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2019, 103, 567–575. [Google Scholar] [CrossRef]
- Bertoldo, C.; Antranikian, G. Starch-hydrolyzing enzymes from thermophilic archaea and bacteria. Curr. Opin. Chem. Biol. 2002, 6, 151–160. [Google Scholar] [CrossRef]
- Van Zyl, W.H.; Bloom, M.; Viktor, M.J. Engineering yeasts for raw starch conversion. Appl. Microbiol. Biotechnol. 2012, 95, 1377–1388. [Google Scholar] [CrossRef]
- Abouamama, S.; Anis, B.; Abir, S.; Maroua, H.; Sirine, B. Amylolytic and antibacterial activity of filamentous fungi isolated from the rhizosphere of different plants grown in the Tamanghasset region. Heliyon 2023, 9, e14350. [Google Scholar] [CrossRef]
- Toledo-Alarcón, J.; Cabrol, L.; Jeison, D.; Trably, E.; Steyer, J.P.; Tapia-Venegas, E. Impact of the microbial inoculum source on pre-treatment efficiency for fermentative H2 production from glycerol. Int. J. Hydrogen Energy 2020, 45, 1597–1607. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, M.S. Hydrogenases for biological hydrogen production. Bioresour. Technol. 2011, 102, 8423–8431. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Patel, S.K.S.; Lee, J.K.; Kalia, V.C. Extending the limits of Bacillus for novel biotechnological applications. Biotechnol. Adv. 2013, 31, 1543–1561. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, D.; Pérez-Zapatero, E.; Martín-Marroquín, J.M.; Sánchez-Gatón, M.A.; Gómez, M. Comparative Analysis of Additives for Enhanced Biohydrogen Production via Dark Fermentation. JOM 2024, 76, 141–152. [Google Scholar] [CrossRef]
- Sjulander, N.; Kikas, T. Origin, impact and control of lignocellulosic inhibitors in bioethanol production—A review. Energies 2020, 13, 4751. [Google Scholar] [CrossRef]
- Lee, M.J.; Kim, T.H.; Min, B.; Hwang, S.J. Sodium (Na+) concentration effects on metabolic pathway and estimation of ATP use in dark fermentation hydrogen production through stoichiometric analysis. J. Environ. Manage. 2012, 108, 22–26. [Google Scholar] [CrossRef]
- Soares, J.F.; Mayer, F.D.; Mazutti, M.A. Hydrogen production from Brewer’s spent grain hydrolysate by dark fermentation. Int. J. Hydrogen Energy 2024, 52, 352–363. [Google Scholar] [CrossRef]
- Cieciura-Włoch, W.; Borowski, S.; Otlewska, A. Biohydrogen production from fruit and vegetable waste, sugar beet pulp and corn silage via dark fermentation. Renew. Energy 2020, 153, 1226–1237. [Google Scholar] [CrossRef]
- Gupta, S.; Fernandes, A.; Lopes, A.; Grasa, L.; Salafranca, J. Microbes and Parameters Influencing Dark Fermentation for Hydrogen Production. Appl. Sci. 2024, 14, 10789. [Google Scholar] [CrossRef]
- Sganzerla, W.G.; Sillero, L.; Forster-Carneiro, T.; Solera, R.; Perez, M. Determination of Anaerobic Co-fermentation of Brewery Wastewater and Brewer’s Spent Grains for Bio-hydrogen Production. Bioenergy Res. 2023, 16, 1073–1083. [Google Scholar] [CrossRef]
- Zahedi, S.; Solera, R.; Micolucci, F.; Cavinato, C.; Bolzonella, D. Changes in microbial community during hydrogen and methane production in two-stage thermophilic anaerobic co-digestion process from biowaste. Waste Manag. 2016, 49, 40–46. [Google Scholar] [CrossRef]
- Venkata Mohan, S.; Chiranjeevi, P.; Chandrasekhar, K.; Babu, P.S.; Sarkar, O. Acidogenic Biohydrogen Production From Wastewater. In Biohydrogen—Biomass, Biofuels, Biochem, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 279–320. [Google Scholar] [CrossRef]
- Ghimire, A.; Frunzo, L.; Pirozzi, F.; Trably, E.; Escudie, R.; Lens, P.N.L.; Esposito, G. A review on dark fermentative biohydrogen production from organic biomass: Process parameters and use of by-products. Appl. Energy 2015, 144, 73–95. [Google Scholar] [CrossRef]
- Jalil, A.; Yu, Z. Impact of Substrates, Volatile Fatty Acids, and Microbial Communities on Biohydrogen Production: A Systematic Review and Meta-Analysis. Sustainibility 2024, 16, 10755. [Google Scholar] [CrossRef]
- Motte, J.C.; Escudié, R.; Hamelin, J.; Steyer, J.P.; Bernet, N.; Delgenes, J.P.; Dumas, C. Substrate milling pretreatment as a key parameter for Solid-State Anaerobic Digestion optimization. Bioresour. Technol. 2014, 173, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.; Butardo, V.; Sha, G.; Zhang, H.; Wu, X.; Wang, L. Microbial degradation and pollutant control in aerobic composting and anaerobic digestion of organic wastes: A review. Waste Manag. 2025, 204, 114894. [Google Scholar] [CrossRef]
- Barth, M.; Werner, M.; Otto, P.; Richwien, B.; Bahramsari, S.; Krause, M.; Schwan, B.; Abendroth, C. Microwave-assisted organic acids and green hydrogen production during mixed culture fermentation. Biotechnol. Biofuels Bioprod. 2024, 17, 123. [Google Scholar] [CrossRef]
- Kakar, F.L.; Koupaie, E.H.; Hafez, H.; Elbeshbishy, E. Effect of Hydrothermal Pretreatment on Volatile Fatty Acids Production from Source-Separated Organics. Processes 2019, 7, 576. [Google Scholar] [CrossRef]
- Rawoof, S.A.A.; Kumar, P.S.; Vo, D.V.N.; Subramanian, S. Sequential production of hydrogen and methane by anaerobic digestion of organic wastes: A review. Environ. Chem. Lett. 2020, 19, 1043–1063. [Google Scholar] [CrossRef]
- Obiora, N.K.; Ujah, C.O.; Asadu, C.O.; Kolawole, F.O.; Ekwueme, B.N. Production of hydrogen energy from biomass: Prospects and challenges. Green Technol. Sustain. 2024, 2, 100100. [Google Scholar] [CrossRef]
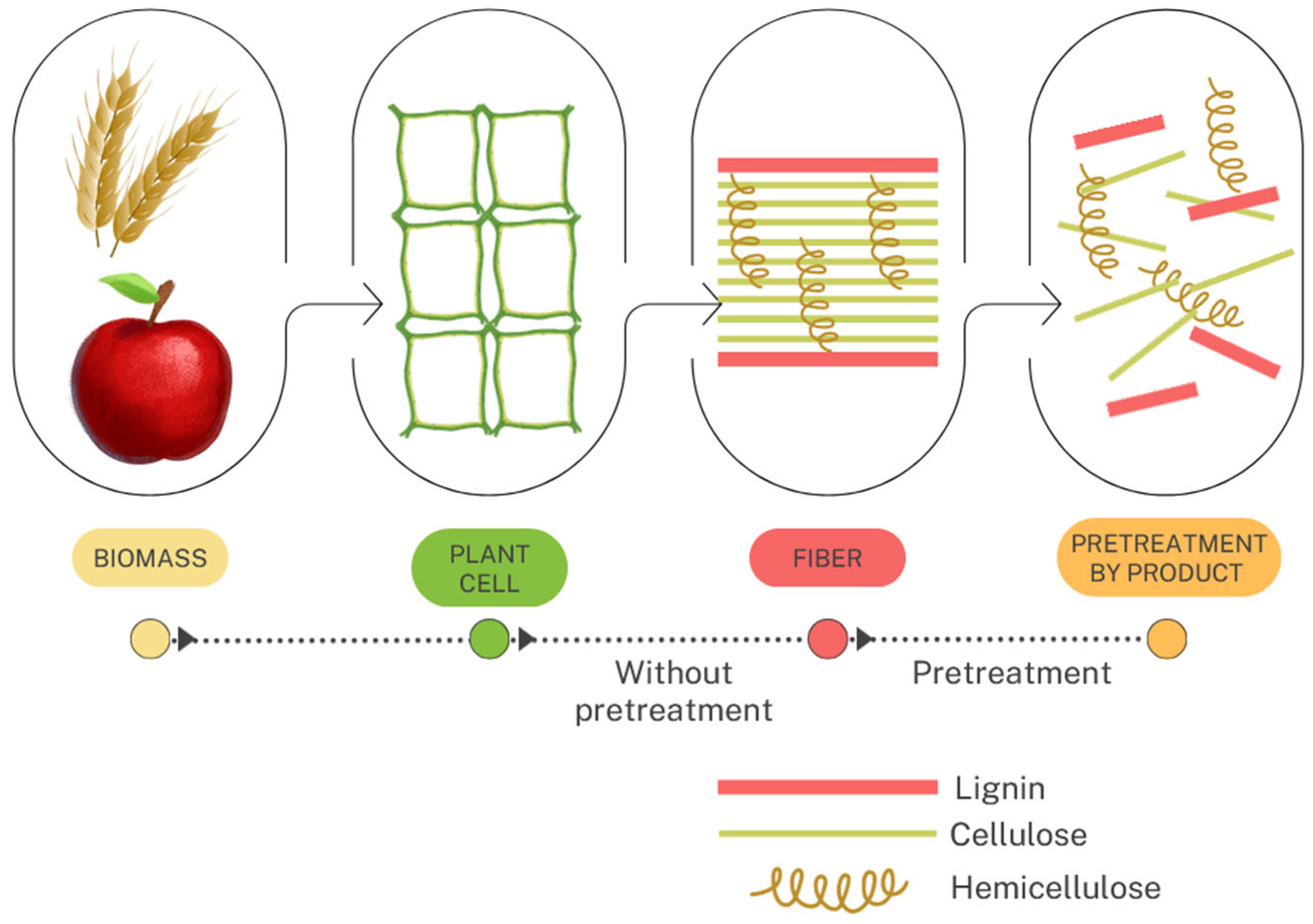


| No. | Agro-Industrial Waste | Hydrolysis | Parameters |
|---|---|---|---|
| 1 | Apple pomace (AP) | thermal | 125 °C, 15 min |
| AP-E | enzymatic | UltraFlo® Max, 45 °C, 8 h | |
| AP-ME | enzymatic | Viscozyme® L, UltraFlo® Max, 45 °C, 8 h | |
| 2 | Spent grain (BSG) | thermal | 125 °C, 15 min |
| BSG-E | enzymatic | Viscozyme® L, 45 °C, 8 h | |
| BSG-H3PO4 | acid | 10% v/v H3PO4, 4 h | |
| BSG-TH3PO4 | thermal-acid | 10% v/v H3PO4, 80 °C, 8 h | |
| BSG-NaOH | alkaline | 10% v/v NaOH, 4 h | |
| BSG-TNaOH | thermal-alkaline | 10% v/v NaOH, 80 °C, 8 h | |
| 3 | Potato powder (PW) | not used | |
| 4 | Molasses (M) | not used | |
| Substrate | Chemical Oxygen Demand [g O2/kg] | Carbohydrates [%] | Protein [%] | Ammonia Nitrogen [mg/g] | Total Nitrogen [mg/g] | Ortho- Phosphates [mg/g] | Total Phosphorus [mg/g] | Iron [mg/g] |
|---|---|---|---|---|---|---|---|---|
| AP | 244.47 ± 3.25 | 18.00 ± 0.25 | 5.20 ± 0.08 | 9.14 ± 0.60 | 7.53 ± 0.25 | 1.78 ± 0.04 | 0.58 ± 0.02 | 24.7 ± 0.02 |
| BSG | 401.70 ± 5.21 | 5.02 ± 0.12 | 24.00 ± 0.10 | 48.21 ± 0.52 | 39.70 ± 0.34 | 10.15 ± 0.05 | 3.31 ± 0.05 | 0.14 ± 0.01 |
| PW | 209.58 ± 1.08 | 20.00 ± 0.15 | 8.00 ± 0.35 | 3.87 ± 0.21 | 3.19 ± 0.12 | 0.80 ± 0.02 | 0.26 ± 0.08 | 0.02 ± 0.00 |
| M | 181.26 ± 0.85 | 75.00 ± 0.22 | 0.00 ± 0.00 | 9.15 ± 0.35 | 7.54 ± 0.32 | 1.24 ± 0.04 | 0.40 ± 0.06 | 0.05 ± 0.01 |
| Substrate | Total Solids [g/kg] | Volatile Solids [g/kg] | Reducing Sugars [g/kg] |
|---|---|---|---|
| AP (raw) | 161.94 ± 1.11 | 159.57 ± 0.97 | 14.10 ± 0.65 |
| AP-E | 98.24 ± 1.63 | 94.22 ± 1.60 | 18.90 ± 0.75 |
| AP-ME | 97.18 ± 1.45 | 93.11 ± 1.08 | 21.20 ± 0.84 |
| AP-T | 135.73 ± 1.97 | 133.65 ± 1.98 | 16.20 ± 0.25 |
| BSG (raw) | 215.75 ± 2.57 | 196.56 ± 1.53 | 1.40 ± 0.08 |
| BSP-E | 162.47 ± 1.85 | 150.45 ± 1.22 | 2.50 ± 0.12 |
| BSG-NaOH | 160.14 ± 2.54 | 109.76 ± 1.67 | 1.40 ± 0.09 |
| BSG-H3PO4 | 209.33 ± 2.42 | 108.85 ± 2.95 | 1.60 ± 0.07 |
| BSG-T | 165.42 ± 2.14 | 148.62 ± 1.76 | 1.90 ± 0.08 |
| BSG-TH3PO4 | 208.02 ± 2.56 | 107.15 ± 1.85 | 2.00 ± 0.11 |
| BSG-TNaOH | 159.25 ± 2.87 | 108.25 ± 1.69 | 1.50 ± 0.09 |
| PW * | 449.33 ± 2.65 | 442.07 ± 3.12 | 9.00 ± 0.21 |
| M * | 854.31 ± 3.28 | 760.22 ± 2.95 | 30.30 ± 1.05 |
| Substrate | SGP [Ncm3/g VS] | SHP [Ncm3/g VS] | SMP [Ncm3/g VS] | Average H2 [%] | Average CH4 [%] | Average CO2 [%] | pH Initial | pH Final | Total VFA [g/dm3] |
|---|---|---|---|---|---|---|---|---|---|
| BSG | 1916.67 ± 20.45 | 59.16 ± 1.32 | 0.96 ± 0.08 | 59.36 ± 1.48 | 0.92 ± 0.15 | 38.73 ± 1.38 | 5.62 | 5.30 | 1.94 |
| BSG-E | 2208.33 ± 25.67 | 60.09 ± 1.58 | 6.33 ± 0.15 | 51.43 ± 1.08 | 5.21 ± 0.24 | 36.56 ± 1.21 | 5.49 | 5.08 | 1.40 |
| BSG-H3PO4 | 621.56 ± 1.93 | 18.56 ± 0.48 | 0.85 ± 0.04 | 50.87 ± 1.02 | 2.32 ± 0.12 | 25.60 ± 1.12 | 6.10 | 5.81 | 2.12 |
| BSG-TH3PO4 | 791.67 ± 2.02 | 22.38 ± 1.45 | 1.55 ± 0.25 | 53.82 ± 0.98 | 3.94 ± 0.28 | 29.54 ± 1.31 | 5.80 | 5.41 | 1.40 |
| BSG-T | 1062.50 ± 15.67 | 29.36 ± 1.62 | 2.16 ± 0.35 | 52.76 ± 0.95 | 3.87 ± 0.32 | 33.46 ± 1.43 | 5.61 | 5.33 | 1.88 |
| BSG-NaOH | 312.89 ± 0.93 | 2.54 ± 0.15 | 0.54 ± 0.06 | 21.41 ± 0.25 | 0.54 ± 0.02 | 18.69 ± 0.16 | 5.98 | 5.48 | 0.42 |
| BSG-TNaOH | 468.33 ± 1.05 | 1.25 ± 0.28 | 0.00 ± 0.00 | 5.21 ± 0.45 | 0.00 ± 0.00 | 14.98 ± 0.45 | 5.85 | 5.51 | 1.05 |
| M | 4083.33 ± 24.94 | 135.31 ± 2.34 | 0.00 ± 0.00 | 63.09 ± 1.12 | 0.00 ± 0.00 | 31.58 ± 1.08 | 5.64 | 4.46 | 1.41 |
| PW | 1208.33 ± 18.65 | 62.95 ± 1.82 | 0.00 ± 0.00 | 47.76 ± 0.89 | 0.00 ± 0.00 | 30.56 ± 1.13 | 5.87 | 5.46 | 0.71 |
| AP | 3333.33 ± 21.85 | 101.06 ± 2.05 | 0.00 ± 0.00 | 57.73 ± 1.43 | 0.00 ± 0.00 | 29.85 ± 1.56 | 5.56 | 5.28 | 1.32 |
| AP-ME | 3593.33 ± 17.39 | 112.69 ± 2.06 | 0.00 ± 0.00 | 59.87 ± 1.23 | 0.00 ± 0.00 | 30.14 ± 1.56 | 5.68 | 5.22 | 1.02 |
| AP-E | 3083.33 ± 19.30 | 74.99 ± 1.54 | 0.61 ± 0.01 | 53.54 ± 1.18 | 0.00 ± 0.00 | 38.01 ± 1.28 | 5.52 | 5.04 | 1.63 |
| AP-T | 3000.00 ± 15.87 | 39.35 ± 1.04 | 0.00 ± 0.00 | 56.41 ± 1.45 | 0.00 ± 0.00 | 25.46 ± 1.37 | 5.56 | 5.11 | 4.96 |
| Substrate | SHP [Ncm3/gVS] | Energy [kJ/kgVS] | Energy [kWh/kgVS] | SMP [Ncm3/gVS] | Energy [kJ/kgVS] | Energy [kWh/kgVS] | Σ Energy |
|---|---|---|---|---|---|---|---|
| BSG | 59.76 | 763.01 | 0.212 | 0.96 | 38.14 | 0.01 | 0.22 |
| BSG-E | 59.09 | 754.45 | 0.210 | 6.33 | 251.5 | 0.07 | 0.28 |
| BSG-H3PO4 | 18.56 | 236.97 | 0.066 | 0.85 | 33.77 | 0.01 | 0.08 |
| BSG-TH3PO4 | 22.38 | 285.74 | 0.079 | 1.55 | 61.58 | 1.71 | 1.79 |
| BSG-T | 29.36 | 374.86 | 0.104 | 2.16 | 85.82 | 0.02 | 0.13 |
| BSG-NaOH | 2.54 | 32.43 | 0.009 | 0.54 | 21.46 | 0.01 | 0.01 |
| BSG-TNaOH | 1.25 | 15.96 | 0.004 | 0.00 | 0.00 | 0.00 | 0.00 |
| M | 135.31 | 1727.62 | 0.480 | 0.00 | 0.00 | 0.00 | 0.48 |
| PW | 62.95 | 803.74 | 0.223 | 0.00 | 0.00 | 0.00 | 0.22 |
| AP | 101.06 | 1290.32 | 0.358 | 0.00 | 0.00 | 0.00 | 0.36 |
| AP-ME | 112.69 | 1438.81 | 0.400 | 0.00 | 0.00 | 0.00 | 0.40 |
| AP-E | 74.99 | 957.46 | 0.266 | 0.61 | 24.24 | 0.01 | 0.27 |
| AP-T | 39.35 | 502.42 | 0.140 | 0.00 | 0.00 | 0.00 | 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cieciura-Włoch, W.; Hajduk, W.; Ikert, M.; Konopski, T.; Khant, M.H.; Domański, J.; Zhang, B.; Kręgiel, D. Biohydrogen Production from Industrial Waste: The Role of Pretreatment Methods. Energies 2025, 18, 5497. https://doi.org/10.3390/en18205497
Cieciura-Włoch W, Hajduk W, Ikert M, Konopski T, Khant MH, Domański J, Zhang B, Kręgiel D. Biohydrogen Production from Industrial Waste: The Role of Pretreatment Methods. Energies. 2025; 18(20):5497. https://doi.org/10.3390/en18205497
Chicago/Turabian StyleCieciura-Włoch, Weronika, Wiktoria Hajduk, Marta Ikert, Tobiasz Konopski, Min Hein Khant, Jarosław Domański, Bolin Zhang, and Dorota Kręgiel. 2025. "Biohydrogen Production from Industrial Waste: The Role of Pretreatment Methods" Energies 18, no. 20: 5497. https://doi.org/10.3390/en18205497
APA StyleCieciura-Włoch, W., Hajduk, W., Ikert, M., Konopski, T., Khant, M. H., Domański, J., Zhang, B., & Kręgiel, D. (2025). Biohydrogen Production from Industrial Waste: The Role of Pretreatment Methods. Energies, 18(20), 5497. https://doi.org/10.3390/en18205497








