1. Introduction
According to the International Energy Agency, global electricity demand is projected to increase in 2025, due to increased production capacity and the development of electric transport. Although the share of renewable electricity sources is projected to increase to 35% in 2035, global coal-fired electricity generation will remain at a relatively high level [
1]—around 30%.
The demand for coal is driven by the ever-growing demand for electricity. For example, for some countries like India and China, coal is a basic resource for electricity generation due to its low cost, making it a key fuel in the energy balance [
1]. Also, for many countries, coal mining remains a vital economic activity, providing jobs and stimulating local economies.
One of the relevant problems of coal power engineering is the use of low-reactivity fuels, characterized by high ignition temperatures, unstable combustion, and a high content of unburned carbon in ash residue [
2]. Trend on reduction in using coal for energy purposes resulted in its major consumption for non-energy applications (chemistry, metallurgy, etc.), thus the low-reactive coal unsuitable for these industries remains as a waste. The mining process for quality coal is impossible without obtaining low-quality, low-reactivity coal. Because its open storage is associated with both economic losses and environmental damage, its utilization is an important problem to be solved.
Coal’s reactivity is the important characteristic of fuel, which in many ways determines technical and ecological performance of coal-fired equipment. For example, higher reactivity allows reducing duration of fuel presence in high temperature area of boiler thus reducing amount of nitrous oxides formed. Increasing the reactivity of coal is possible by using activating additives based on metal oxides or their precursors [
3]. Another way to activate the processes of ignition and combustion of coal is to mix it with highly reactive organic fuels, such as plant biomass [
4] or products of thermal processing of industrial waste [
4,
5]. The use of the latter in coal power engineering can additionally stimulate the waste recycling industry. For example, utilization of waste car tires is a serious environmental problem. One of the methods for their recycling is pyrolysis [
6], the typical target product of which is liquid hydrocarbons (pyrolysis oil).
The liquid hydrocarbons obtained from the pyrolysis of waste tires are characterized by good rheological properties and high combustion heat (40–44 MJ/kg) [
7], as well as the presence of a large number of light and medium fractions (up to 80%wt and 15%wt, respectively) [
8], low pour (T
pp = −30 °C), and flash points (T
fp = 35–55 °C) [
9]. They are also rich in aromatic compounds, alkanes, and alkenes, which makes them suitable for use as fuel in power plants [
10]. The addition of liquid hydrocarbons can increase the reactivity of solid fuel and also act as a binder, improving the mechanical strength and consumer properties of fuel granules [
11]. Additionally, instability of their characteristics due to inherent fluctuations in feedstock properties makes their utilization quite problematic, leaving combustion as feasible option, especially in case of bio-oil being by-product of biochar production [
12,
13]. Moreover, liquid hydrocarbons could be used as modifying fuel additives without preliminary processing and purification [
14].
The problem of combustion of fuel mixtures is still relevant due to a large number of factors, which affect this process [
15]. In [
16], the co-combustion of semi-coke and bituminous coke revealed to have non-linear synergistic effect on the NO emissions and flame position in the burner. In case of mixes of liquid and solid fuels the occurring effects are even more unpredictable. For example, non-additive effects were reported for mixture of bio-oil and biochar during pyrolysis in [
17]. Similar results regarding gaseous pyrolysis products of coal-oil-water slurries are provided by Wan et al. [
18]. Martines et al. [
19] studied the co-pyrolysis of waste tires with biomass and found that liquid hydrocarbons enhance the thermal decomposition of solid fuel, resulting in higher yields of gaseous products and improved reactivity. The results obtained also indicate the possibility of reducing emissions of pollutants such as sulfur oxides and nitrogen oxides, since liquid hydrocarbons help stabilize the combustion. In [
20] authors even mentioned that just the mixture of coal with oil could have unexpected properties.
As long as pyrolysis conditions could occur during some modes of combustion, many unexpected results were reported for this process as well. For example, the results were presented in [
12] for combustion of poultry litter mixed with sweet sorghum bagasse and pyrolysis oil. According to it, even 10%wt of oil allowed to increase flammability index by 30–49% and enhance flame visuals. Another example of synergy is presented in [
13] for coal-oil symbiosis. It was found that 25%wt additive caused activation energy of two oxidation stages to decrease by 89% and 61%, respectively. Additionally, the formation of additional peak in low-temperature area of TG-curve was observed for samples with 15 and 25%wt of oil. Alawa B. [
21] reported that adding liquid hydrocarbons to fuel during its thermal decomposition promotes reduction in greenhouse gas emissions, thereby decreasing the burden on the environment. One of the largest studies in this area [
15], encompassing large number of various liquid and solid wastes, also failed to establish simple dependences between combustion characteristics and fuel properties, indicating complexity of these processes. It is worth mentioning, that despite huge variety of pyrolysis oils used in studied articles [
12,
13,
17,
18,
19,
21], their properties fluctuate in relatively narrow range—calorific value around 40 MJ/kg for dehydrated sample, carbon content—around 80%wt.
The research results of the thermal conversion of liquid hydrocarbons obtained from tires with solid fuel to increase its reactivity presented in [
19] found that mixing liquid hydrocarbons from the pyrolysis of waste tires with solid fuel improves its combustion characteristics, which leads to a decrease in the ignition temperature and an increase in the combustion rate.
Adding liquid hydrocarbons from waste tire pyrolysis to solid fuels may have technological potential in various industries, including power generation, cement production, and metallurgy. Improving the reactivity of solid fuels may improve combustion efficiency and reduce emissions and operating costs. However, further research is needed to optimize the mixing ratio, processing conditions, and combustion parameters to maximize the benefits of this approach, because combustion of such mixture is affected by many factors and thus poorly predictable.
This paper presents the results of a comprehensive study on the fuel reactivity during the oxidation, ignition, and combustion of low-reactivity coal with the addition of liquid hydrocarbons from the pyrolysis of waste tires.
2. Materials and Methods
The solid fuel used was grade T coal from the Kuznetsk coal basin (Kemerovo region, Russian Federation). A coal sample (particle size d = 5–10 mm) was ground in a drum mill using the following grinding mode: the mass ratio of grinding media to material was 1:1, the grinding time was 8 h. The ground sample was then fractionated on sieves with a cell size 80 μm.
Proximate analysis (moisture W, ash A, volatile matter content V, net calorific value NCV) was performed according to standard ISO methods: ISO 589:2008 [
22], ISO 1171:2010 [
23], ISO 562:2010 [
24], and ISO 1928:2009 [
25]. The ultimate analysis was performed using a Vario MICRO cube elemental composition analyzer (Elementar, Hesse, Germany). The results obtained are presented in
Table 1. The particle size distribution was studied using Analysette 22 laser particle sizer (Fritsch, Idar-Oberstein, Germany).
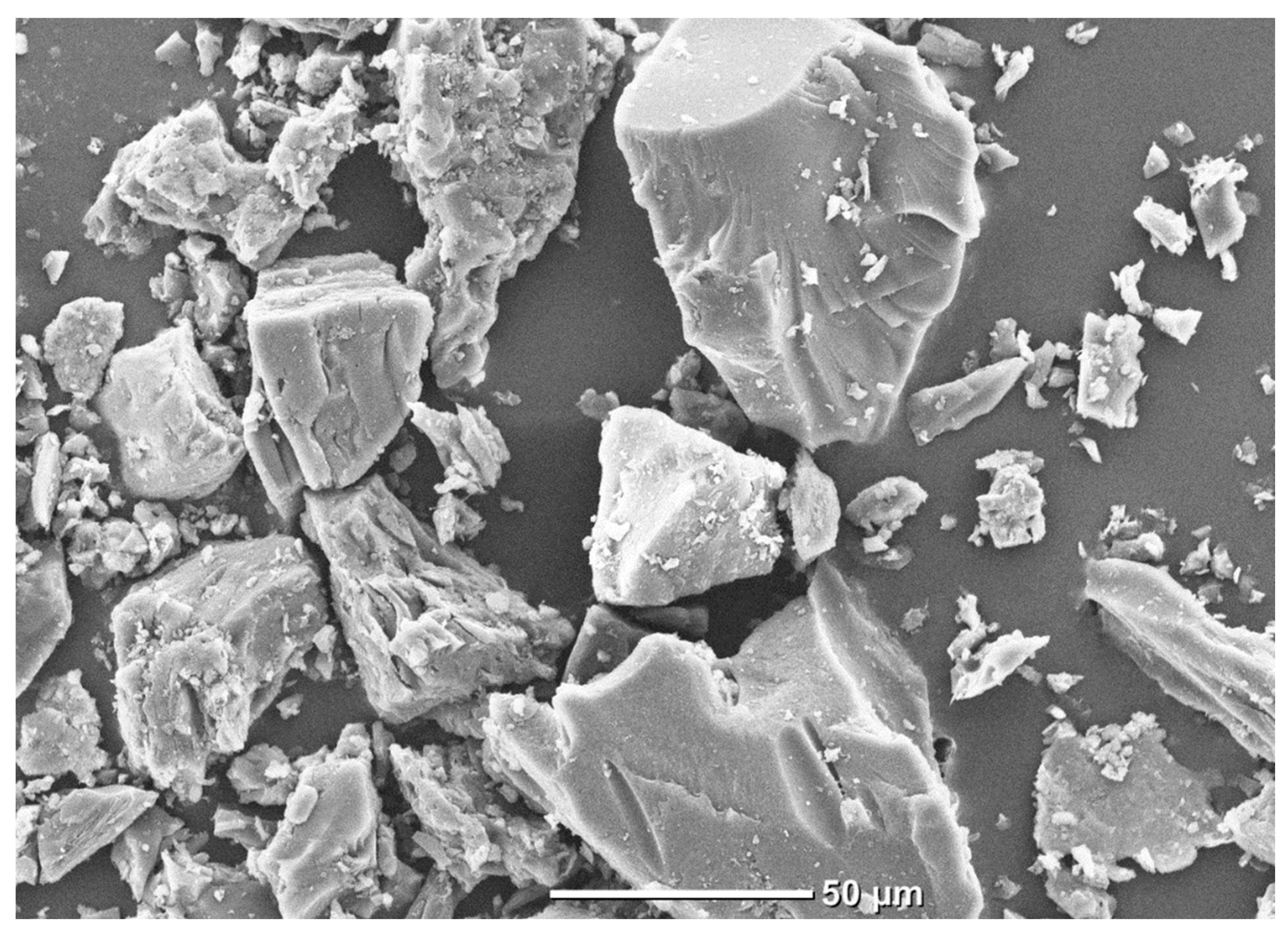
Figure 1 shows an image of particles of the studied coal sample obtained using a JSM-6000 °C scanning electron microscope (JEOL, Tokyo, Japan).
It is evident that the coal particles have an irregular shape and their surface is non-uniform. A number of smaller fragments (less than 5 µm in size) are seen on the surface of the particles.
A sample of liquid hydrocarbons (pyrolysis oil) obtained by steam pyrolysis of waste tires was used as an activating additive [
26]. Steam pyrolysis was carried out in a tubular vertical batch reactor using waste tires of quarry equipment (granules 3–4 mm in size) at a temperature of 500 ± 15 °C with the exposure time of 1 h. The reactor heating rate was about 10 °C/min. The mass of the sample loaded into the reactor was 1 kg. The relative steam consumption was 1 kg per 1 kg of the sample used.
Table 2 shows the physicochemical characteristics of the liquid hydrocarbon sample studied and the methods of their analysis. Before it, the sample was dehydrated using calcium chloride to remove residual water. The mass fraction of water was 1.7%wt.
The modification of the coal sample with liquid hydrocarbons was carried out by mechanical mixing in a mortar. The mixing was carried out for 5 min to obtain homogeneity. The mass fraction of the liquid hydrocarbon additive to the coal sample was 2.5, 5, 10, and 20%wt. Based on the mass content of the additive, the studied samples will be further referred to as C, C2.5, C5, C10, and C20. It is worth noting that samples C2.5, C5, and C10 were powdery (without the formation of large agglomerates), while sample C20 had a dispersed (paste-like) form.
The addition of liquid hydrocarbons obtained as a result of thermal decomposition of waste tires leads to a significant decrease in the ash content of the modified coal fuel and a simultaneous increase in its calorific value. The decrease in ash content occurs, in particular, due to the fact that liquid hydrocarbons practically do not contain mineral impurities typical for original coal (oxides of aluminum, silicon, iron, etc.). When added, the mineral component of the fuel composition is diluted with the organic phase, which leads to a decrease in the total ash content. Liquid hydrocarbons from the pyrolysis of waste tires are characterized by the content of saturated, unsaturated, and aromatic compounds. Mineral components (for example, soot, metal additives) mainly remain in the solid carbon residue of the pyrolysis of waste tires.
The increase in the net calorific value of modified coal fuel occurs as a result of the high calorific value of liquid hydrocarbons—about 43 MJ/kg—which is significantly higher than that of coal (about 30 MJ/kg). Adding even 5–20%wt of liquid hydrocarbons to coal leads to significant increase in its value. In addition, liquid hydrocarbons contain a large number of light volatile substances, which are characterized by high reactivity.
2.1. Thermal Analysis
Thermal analysis was carried out using an STA 449 F3 Jupiter analyzer (NETZSCH, Selb, Germany) in an air environment with a flow of 150 mL/min at four different heating rates (2.5, 5, 10, and 20 °C/min). The study was carried out in temperature range 20–1000 °C. The sample weight was about 10 mg.
The first part of the study was based on thermal analysis of coal samples with different liquid hydrocarbon content (from 2.5 to 20%wt). The heating rate of the samples was constant and equal to 10 °C/min.
The second experimental block of the study was aimed at identifying the dependence of the effect of the heating rate on the oxidation characteristics of the original and modified coal sample with 10%wt of liquid hydrocarbons added. The heating rate was 2.5, 5, 10, and 20 °C/min.
2.2. Ignition and Combustion
The study of ignition and combustion of the studied samples was carried out using a laboratory setup. Its simplified sketch is shown in
Figure 2. It includes the following elements: a combustion chamber—a temperature-controlled muffle furnace (Sputnik, Moscow, Russia) with 2 kW power, a digital temperature controller (thermocouple measurement error ± 3–5 °C), and a volume of 0.003 m
3; a high-speed video camera Mini UX (Photron, San Diego, CA, USA) with a resolution of 1024 × 1024 at a frame rate of 125 frames/s (the parameters are relevant for the experiment); a remotely controlled coordinate mechanism; a flow gas analyzer “Test-203” (BONER, Moscow, Russia).
A cone-shaped fuel sample weighing mass 0.1 g was placed on a horizontal holder (1) of a coordinate mechanism (2). After receiving a signal from a personal computer (3), the coordinate mechanism moved the holder with the sample into the chamber of a temperature-controlled muffle furnace (4), the temperature of which was 700 °C. A comparative assessment of the characteristics of the ignition for the studied samples was carried out by analyzing the ignition delay time and flame combustion time, which was recorded using a high-speed video camera (5). The ignition delay time was considered to be the time from the moment the holder with the fuel filling entered the focus of the video camera lens until the beginning of the formation of a visible glow on the fuel surface, which corresponded to the beginning of the combustion. The flame combustion time was calculated as the difference in time between characteristic video frames displaying the moment of formation of a visible flame and frame of its complete extinction. The diffusion combustion time was determined as duration of visible glow existence at the surface of the sample. The gases released during combustion of the sample (CO, CO2, NOx, and SO2) were analyzed using a flow-through gas analyzer (6).
3. Results and Discussion
3.1. Thermal Analysis of Coal and Liquid Hydrocarbons Samples
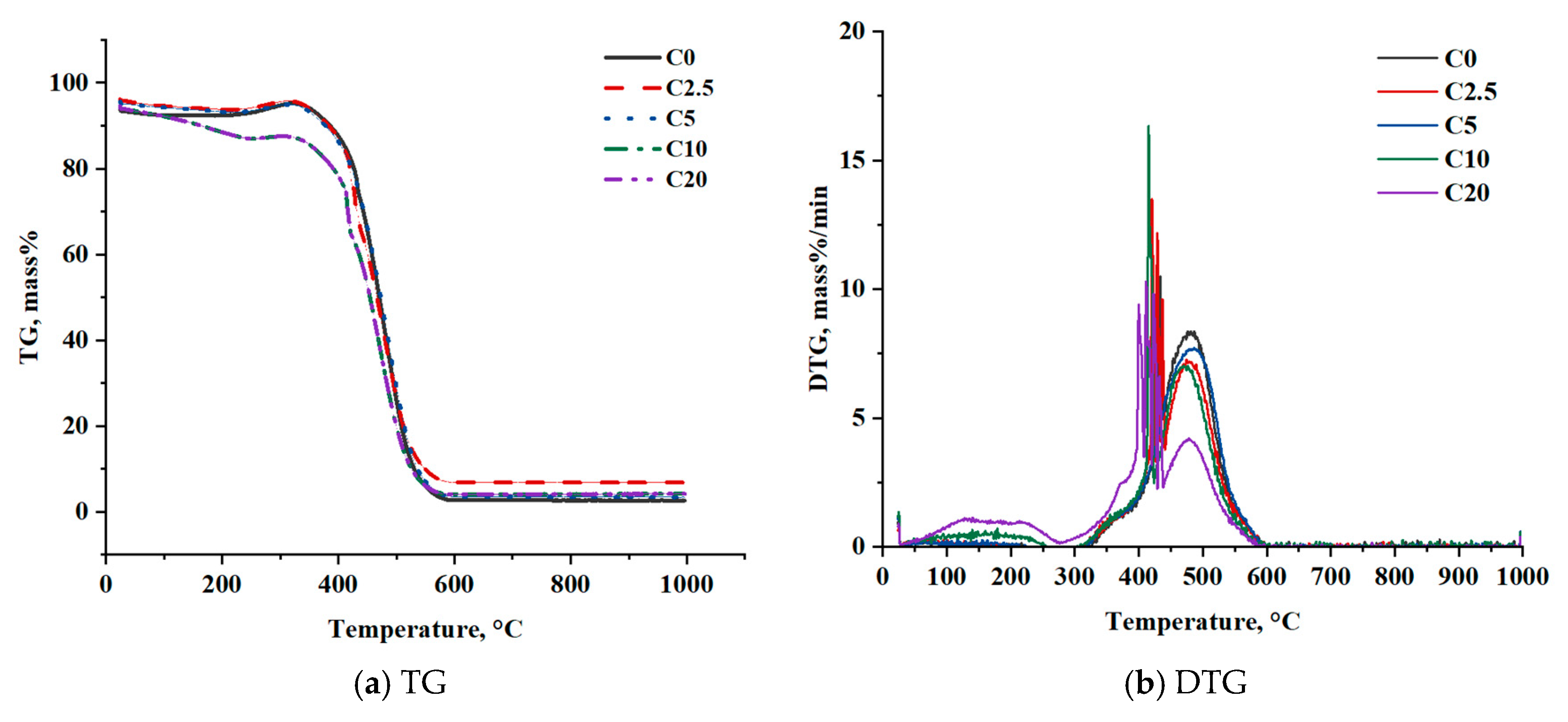
Figure 3 shows the results of a study of the oxidation of coal modified by the addition of liquid hydrocarbons from the pyrolysis of waste tires with different concentrations.
Thermal analysis data show that the oxidation of coal with the addition of liquid hydrocarbons is accompanied by several stages. The first stage is heating to 250–270 °C, during which a slight loss of mass occurs. The second stage of thermal decomposition is characterized by an intensive release of volatile hydrocarbons, including light compounds of the additive used. At the third stage, a significant loss of mass is observed in the temperature range of 350–600 °C, which is associated with char combustion.
According to DTG data (
Figure 3b), for modified samples, an additional peak is formed in the temperature range of 100–250 °C, which is associated with the boiling of gasoline (ambient temperature—180 °C) and diesel (180–330 °C) fractions contained in the liquid hydrocarbons of the pyrolysis of waste tires. Intensity of this peak increases with increasing additive concentration to 10%wt, but decreases for sample with 20%wt of additive. However, trends in reduction in the temperature of the first stage remain throughout all studied range of additive concentration. This is connected to combined effects of evaporation, combustion, and diffusion. The large concentration of additives has very intense evaporation rate and thus low concentration of oxygen in vicinity, this its low combustion rate does not provide enough heat for further promotion of this reaction.
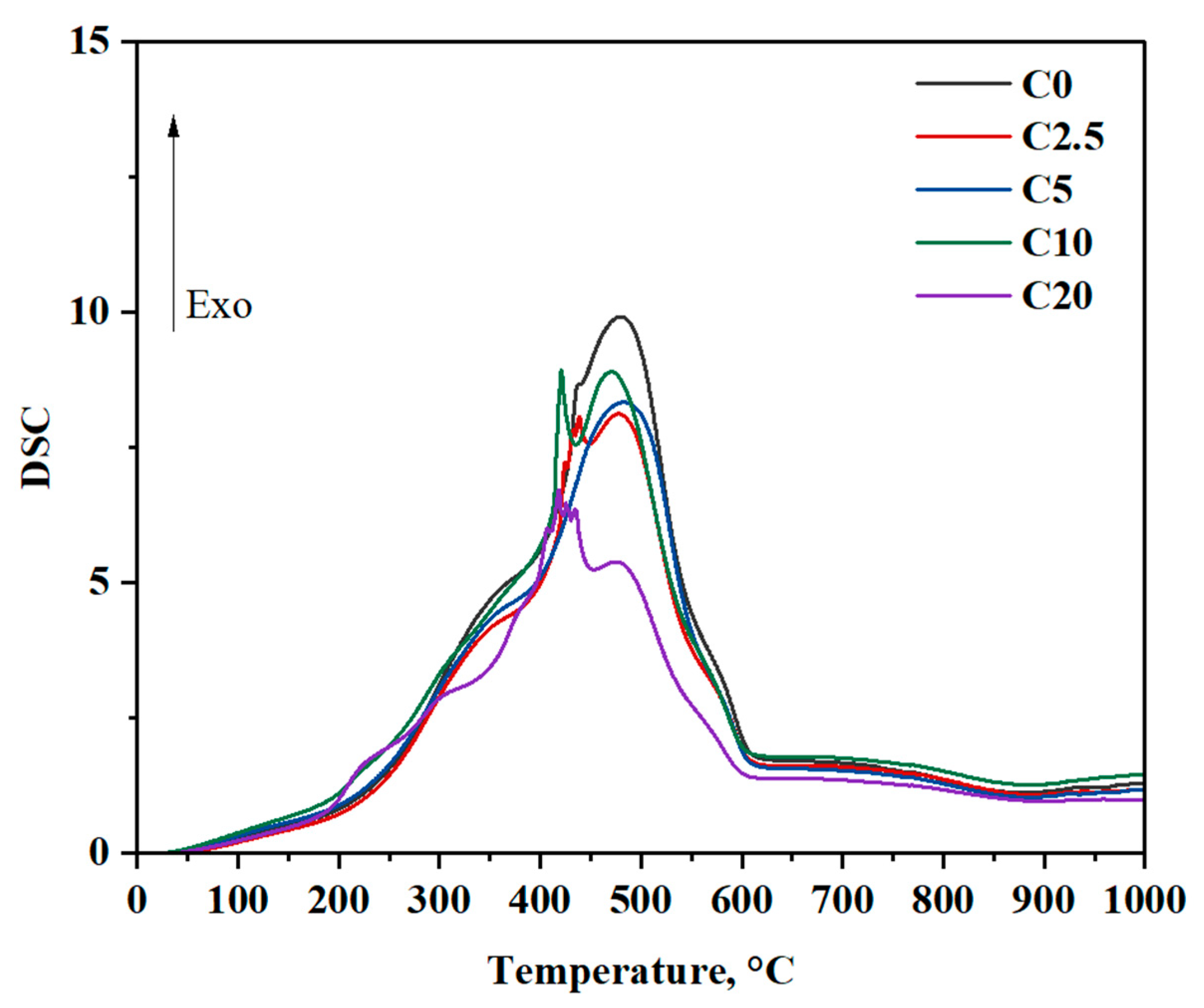
According to DSC data (
Appendix A), the oxidation of coal with the addition of liquid hydrocarbons, regardless of the mass fraction of the additive, occurs in an exothermic mode.
The obtained experimental data correlate with the studies of other authors. In study [
26] similar TG profiles were obtained. An intensive loss of liquid hydrocarbon mass was observed in the temperature range of 300–500 °C.
Thermal analysis data show that an increase in the mass fraction of liquid hydrocarbons in the coal composition to 20%wt leads to a decrease in the temperature of complete fuel oxidation from 592 to 577 °C. Also, with the addition of liquid hydrocarbons, a decrease in the temperature of the intensive oxidation from 341 to 324 °C is observed.
Figure 4 shows the TG and DTG data for the oxidation at different heating rates (2.5–20 °C/min) of the original coal (sample C) and liquid hydrocarbon-modified coal sample (sample C10).
With increasing heating rate of the studied samples, a slight shift in the oxidation to higher temperatures is observed. The initial temperature of intense oxidation increases with increasing heating rate from 300 °C at 2.5 °C/min to 375 °C at 20 °C/min. The temperature of completion of the intense oxidation is determined to be in the range of 510–610 °C. With an increase in the heating rate, an increase in the maximum oxidation rate wmax from 5.4 to 12.5%wt/min is also observed.
It is also important to note the characteristic peaks, which occurred on the DTG profiles in the temperature range of 380–420 °C at heating rates of 10 and 20 °C/min, characterize the intense evaporation of hydrocarbons. At lower heating rates, this process has a significantly lower intensity with evaporation of light and medium fractions at lower temperatures. At a rate of 2.5 °C/min this process takes place at 160–200 °C, at 5 °C/min—at 170–280 °C.
The oxidation process character was different at different heating rates. First of all, at a heating rate of 2.5 °C/min, the addition of liquid hydrocarbons leads to a decrease in the temperature of the onset of active oxidation (from ~350 °C to ~300 °C). The maximum on the DTG profile of the modified sample is shifted to the left (to the area of lower temperatures), while its intensity is higher than for the original coal. This indicates an activating effect of the additive, realized due to the earlier release of volatile hydrocarbon compounds and their oxidation, which, in turn, initiates the decomposition of coal.
At heating rates of 5 °C/min and 10 °C/min, the effect of reducing the reaction temperature is preserved, but becomes less pronounced. With an increase in the heating rate, the difference between the DTG peak for the original and modified coal samples decreases. An intensification of mass loss is noted for the modified sample, especially at 10 °C/min, which indicates a more active decomposition due to the involvement of gas-phase reactions of liquid hydrocarbons.
At a high heating rate (20 °C/min), both samples demonstrate a shift in the active oxidation temperature, which exceeds 500 °C. In this mode, the activating effect of liquid hydrocarbons is almost leveled: the peaks of the DTG profiles almost coincide. At high heating rates, volatile compounds of liquid hydrocarbons do not have time to be released and oxidized before the onset of thermal decomposition of coal. The DTG maximum becomes narrower and more intense, which indicates dominance of kinetic mechanism of oxidation.
3.2. Ignition and Combustion of Samples of Coal and Liquid Hydrocarbons
Figure 5 and
Figure 6 show the ignition delay time values, as well as the time of flame and diffusion combustion of the studied samples at a heating medium temperature of T
g = 700 °C.
Addition of liquid hydrocarbons to coal has a pronounced effect on ignition and the subsequent combustion in general. According to the data presented in
Figure 5, it is evident that an increase in the additive content leads to a significant reduction in the ignition delay time: from 3.9 s for the original sample C to 0.9 s for the sample C20. This is caused by intensification of the release of volatile components and a decrease in the temperature of the onset of fuel oxidation. This dependence has hyperbolic-like form indicating activating character of governing process, likely volatile substances evaporation or combustion. Similar behavior was previously reported in [
33] for wood pyrolysis oil/low-reactivity coal blends.
The additive content affects both stages of the combustion. The flame combustion time fluctuates with trend for increasing from 27.5 s for initial sample to 49.4 s for sample with 20%wt additive. The diffusion combustion time demonstrates similar behavior with more pronounced tendency of increasing. Such growth in both flame and diffusion combustion times could be attributed to diffusion barriers created by evaporating and shielding effect of its combustion products. Ambiguous behavior of combustion times with increasing additive concentration was caused by a variety of occurring processes and their interactions. When the liquid additive is heated, it evaporates and burns in gaseous phase, thus increasing bulk temperature of sample. However, its combustion products reduce oxygen flux to carbon. Therefore, larger concentration of additive on the one hand increases sample temperature, but on the other hand, it reduces oxygen concentration. Thus, balance of these processes determines overall combustion times.
Sample C20 demonstrates the highest values of both flame and diffusion combustion times. This is due to the formation of a stable carbon phase and oxygen barriers due to the excess liquid phase, which prevents effective heat and mass transfer. Thus, the excess of liquid hydrocarbons reduces the reactivity of the fuel and complicates its subsequent combustion. Therefore, the optimal content of liquid hydrocarbons from the point of view of ignition intensification and stable combustion is 5–10%wt. When this value is exceeded, oxidation slows down and combustion time increases.
Analysis of the results (
Figure 5 and
Figure 6) shows that with an increase in the content of liquid hydrocarbons from 2.5 to 10%wt, the ignition and combustion parameters of the fuel (ignition delay time, flame, and diffusion combustion time) change insignificantly. This indicates that thermochemical saturation of the system has been achieved, at which the additive no longer leads to additional intensification of oxidation.
When the additive content exceeds 5%wt, the reaction zone is saturated with volatile compounds. Their further accumulation does not accelerate ignition, since the oxygen concentration and temperature in the reaction zone become limiting factors. With an increase in the additive content in the composition of the samples, the proportion of heavy fractions (resins, aromatic hydrocarbons) increases, capable of creating diffusion barriers and reducing the access of oxygen to the solid phase. These components, unlike light fractions, do not promote rapid ignition and can interfere by slowing down the initial stage of combustion. Also, a higher additive content worsens heat transfer in the fuel composition. Liquid hydrocarbons from the pyrolysis of waste tires partially absorb heat, reducing the heating rate of coal, slowing down the oxidation reaction. Thus, almost identical values of the ignition and combustion delay time in the range of additive amounts of 2.5–10%wt are explained by the balance of accelerating and inhibiting factors, which start to act with an increase in the content of liquid hydrocarbons beyond 2.5%wt. This indicates the presence of an optimal range of additive concentration (2.5–5%wt), at which the maximum effect of increasing reactivity is achieved, expressed in fuel ignition without deterioration of its thermophysical characteristics and combustion kinetics.
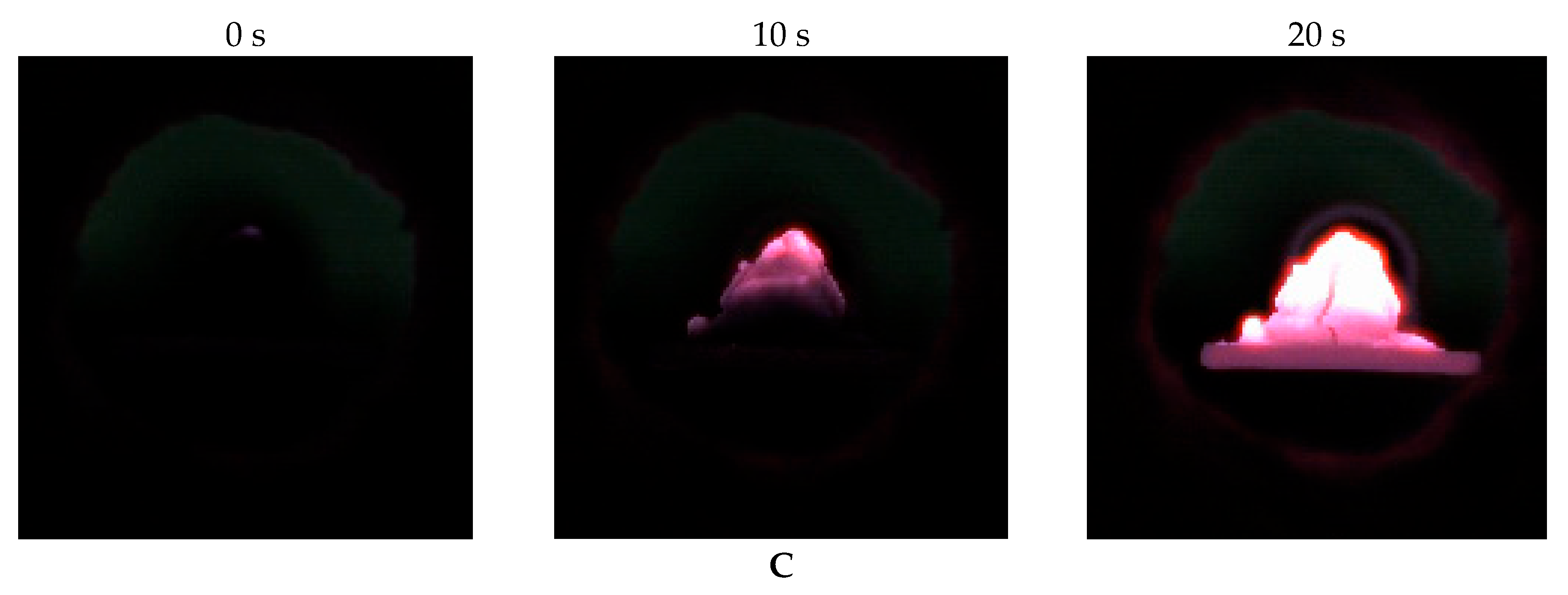
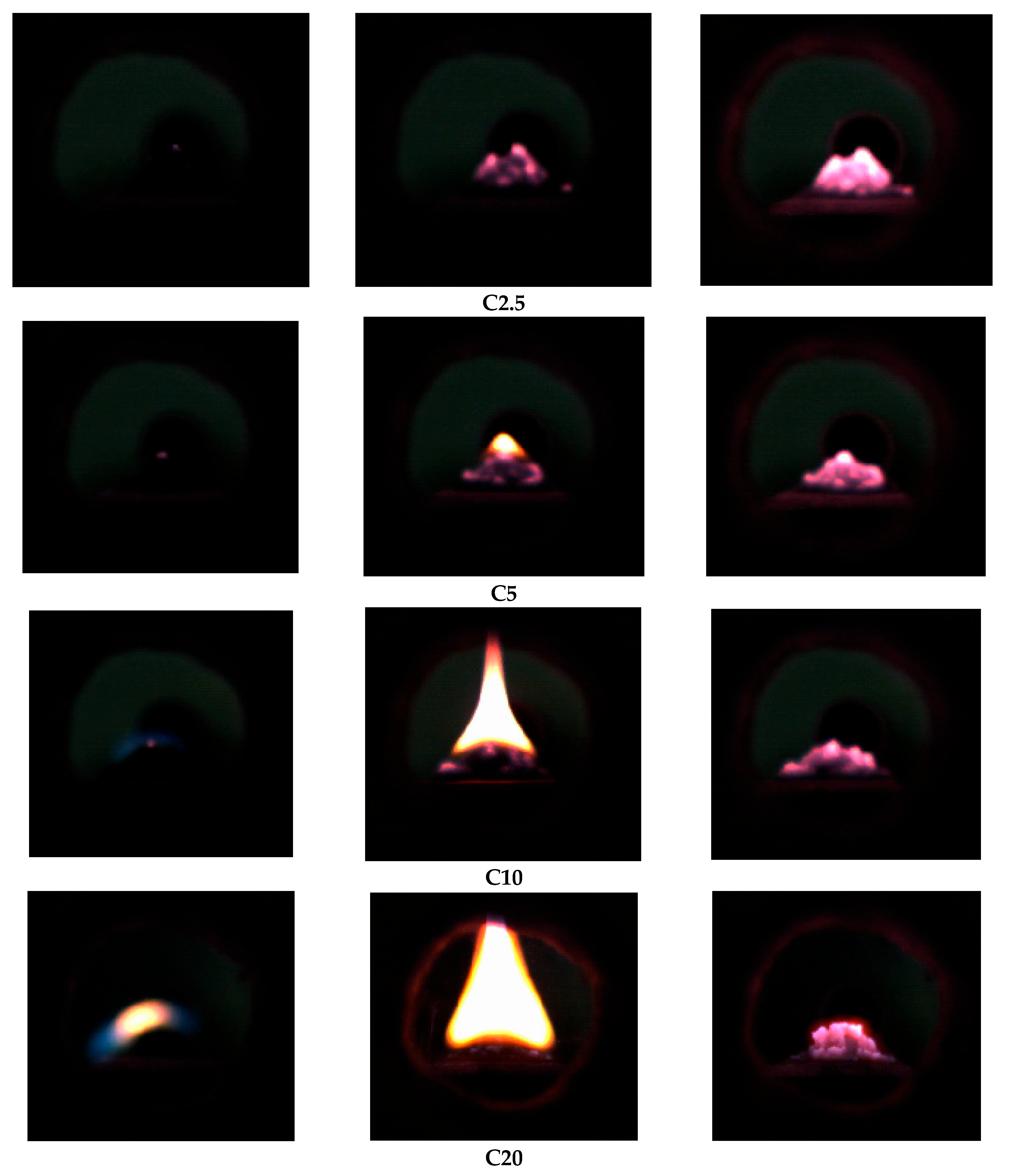
Figure 7 shows typical frames of high-speed video recording of the ignition and combustion of the studied coal samples with different content of liquid hydrocarbons from the pyrolysis of waste tires at a temperature of T
g = 700 °C.
In all cases, the initial ignition occurs in the contact area between flame and the sample surface. In this case, the combustion front moves from the outer surface of the filling to its central part. At the early stages (the first 1–3 s), the front is weak. It is expressed by a dim glow, which indicates the development of preliminary thermal decomposition and initial oxidation zones.
With an increase in the mass fraction of liquid hydrocarbons in the mixture, the flame intensity and the character of the combustion change significantly. The sample with an additive content of 2.5%wt is characterized by a dim, localized glow without a pronounced visible flame. Combustion develops mainly due to the solid phase, and the flame is formed for a short time. Limited front propagation is observed, which is associated with a small number of volatile compounds. In turn, the sample with an additive content of 20%wt demonstrates a pronounced stable visible flame formed at an early stage of the combustion. There is an intensive release of volatile compounds of liquid hydrocarbons, which contribute to the occurrence of combustion with the presence of a visible flame. The front spreads over the entire surface, covering the upper and lateral parts of the sample.
With an increased content of the additive (10–20%wt), the combustion front not only moves faster, but is also accompanied by more pronounced radiation, which indicates a higher temperature and the involvement of gas-phase compounds in the exothermic reactions. At the same time, the duration of combustion increases due to an increase in the diffusion phase, in which combustible gas-phase compounds burn out.
Table 3 presents the qualitative characteristic differences between samples with the addition of 2.5 and 20%wt of liquid hydrocarbons from the pyrolysis of waste tires.
An increase in the content of liquid hydrocarbons leads to a transition of the combustion mode from oxidation to a combined one with the formation of a stable visible flame and pronounced diffusion combustion of the sample. Thus, despite complex character of ignition and combustion of fuel mixtures studied, visually changes are quite straightforward, which makes it more similar to dependence of ignition delay times on additive concentration. However, absence of qualitative changes in combustion behavior with increasing pyrolysis liquid content illustrate same mechanism for all these samples. Still, this trend could significantly change beyond 20%wt, which is already indicated by increase in diffusion and flame combustion times.
3.3. Analysis of the Gas-Phase Combustion Products of Samples of Coal and Liquid Hydrocarbons Samples
Figure 8 shows the dependencies characterizing the change in concentrations of various gas-phase substances (CO, CO
2, NO
x) in the combustion products of the studied mixed samples.
Adding liquid hydrocarbons from the pyrolysis of waste tires to coal leads to a change in the combustion mode due to the presence of highly reactive volatile organic compounds. This could also be seen at the concentration profiles of the emission of gas-phase compounds. Thus, the high content of gasoline (>180 °C) and diesel (180–330 °C) fractions present in the composition of liquid hydrocarbons lead to a lack of oxygen in the area of coal combustion. Thus, incomplete oxidation of carbon occurs, resulting in greater emission of CO. With an increase in the concentration of liquid hydrocarbons in the CO profile for the early stage of the combustion, an additional strong peak is formed. This is due to the sublimation of light compounds of liquid hydrocarbons and the release of volatile substances of coal. In particular, the increase in the concentration of CO is connected to an increase in the carbon content of the fuel. In this case, the increase in CO can be leveled by the technological choice of the optimal combustion mode (excess air coefficient), which helps to promote more complete fuel combustion.
The concentration of carbon dioxide also increases with an increase in the mass fraction of the liquid hydrocarbon additive. This is explained by an increase in the carbon content in the fuel, as well as more intense and complete oxidation of CO. This leads to a reduction in the total time of combustion due to the provision of uniform heat distribution and general intensification of the combustion.
The formation of nitrogen oxides is closely related to the combustion temperature and the nitrogen content in the fuel. The addition of liquid hydrocarbons to the coal composition leads to an increase in the combustion temperature of the fuel, which contributes to the active formation of thermal NOx.
4. Conclusions
The results of an experimental study on fuel reactivity showed that adding liquid hydrocarbons from the pyrolysis of waste tires to bituminous coal significantly affects the combustion characteristics of the obtained mixture.
Due to the high content of light and medium fractions in the tire pyrolysis oil, the fuel’s reactivity increases. This is demonstrated by a reduction in the ignition delay time (by up to 71.6%, from 3.9 s to 0.9 s) and a significant increase in the flame combustion time (by up to 69.5%, from 27.5 s to 49.4 s). This phenomenon is explained by the release of a large number of volatile, combustible compounds from the liquid hydrocarbons during heating. These volatiles create a screening effect around the coal sample, which contributes to a more uniform oxygen supply and stabilizes the combustion temperature.
Thermal analysis showed that adding tire pyrolysis oil to coal significantly lowers the onset temperature for intense oxidation. The temperature ranges of individual oxidation stages also shift slightly toward lower temperatures due to the improved reactivity of the coal mixture. Based on experimental data, a detailed description of bituminous coal combustion with waste tire pyrolysis oil was proposed, and the major influencing factors and mechanisms were identified.
Analysis of the composition of gas-phase combustion products showed that increasing the content of tire pyrolysis oil in the coal leads to higher concentrations of CO and CO2, which is attributed to the increased carbon content. Furthermore, the rise in combustion temperature increases the volume of thermal NOx formed.
The final distribution of combustion products (CO, CO2, NOx) depends on specific process conditions, including flame temperature, oxygen availability in the combustion zone, and the optimal ratio of components. The complex, non-linear trend in the volume of these gas emissions indicates that a large number of factors affect their release. For the first time, the complex effect of waste tire pyrolysis oil on the emission characteristics of coal mixtures was reported. This effect is characterized by, first, a spike in emissions at 2.5 %wt, followed by a decline as the additive content increases to 10 %wt, and another spike at 20 %wt. Optimizing these parameters enables more complete fuel combustion, favoring CO2 formation while minimizing CO and controlling NOx levels. This underscores the need to optimize combustion conditions to achieve complete combustion with minimal harmful emissions.
These findings provide the background to wider adoption of such components in the energy industry. An optimal blend of liquid hydrocarbons from waste tire pyrolysis and low-reactivity coal can improve solid fuel combustion by increasing reactivity and stabilizing the flame. However, using such activating additives with low-reactivity solid fuels at power plants must be accompanied by careful optimization of process parameters to ensure acceptable concentrations of CO and NOx in the combustion products.