Ionic Liquids and Poly (Ionic Liquids) for CO2 Capture: A Comprehensive Review
Abstract
1. Introduction
2. Overview of Carbon Capture Technologies
3. Properties and Chemistry of ILs and PILs
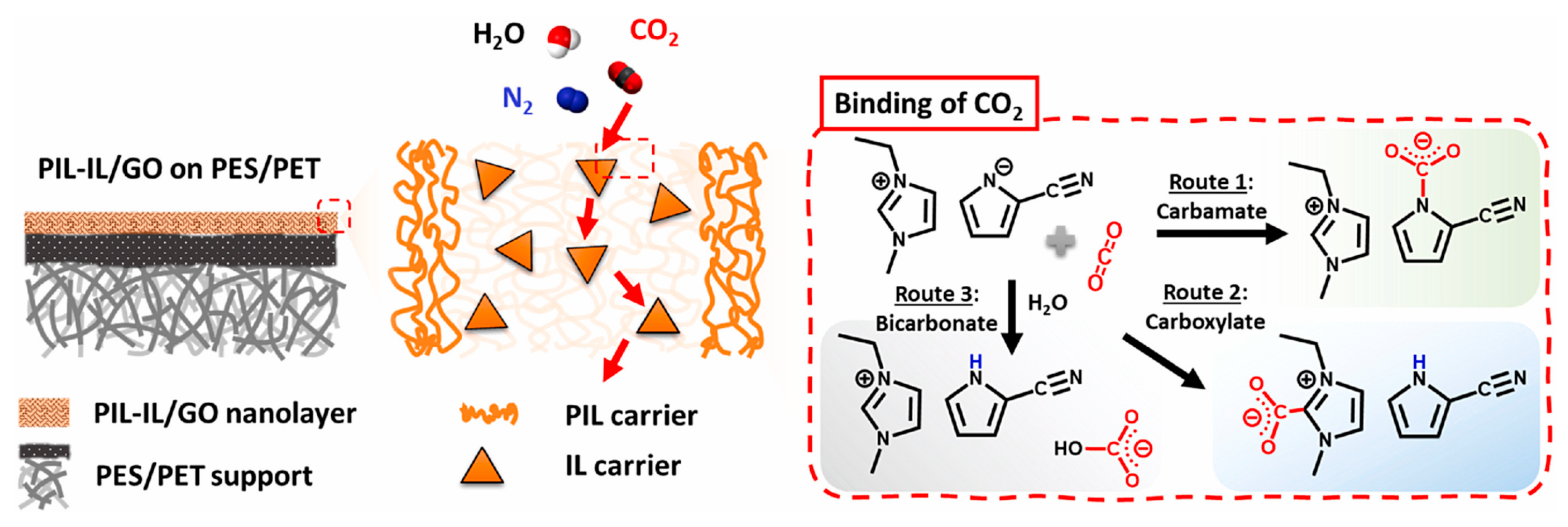
4. Mechanisms of CO2 Capture Using ILs and PILs
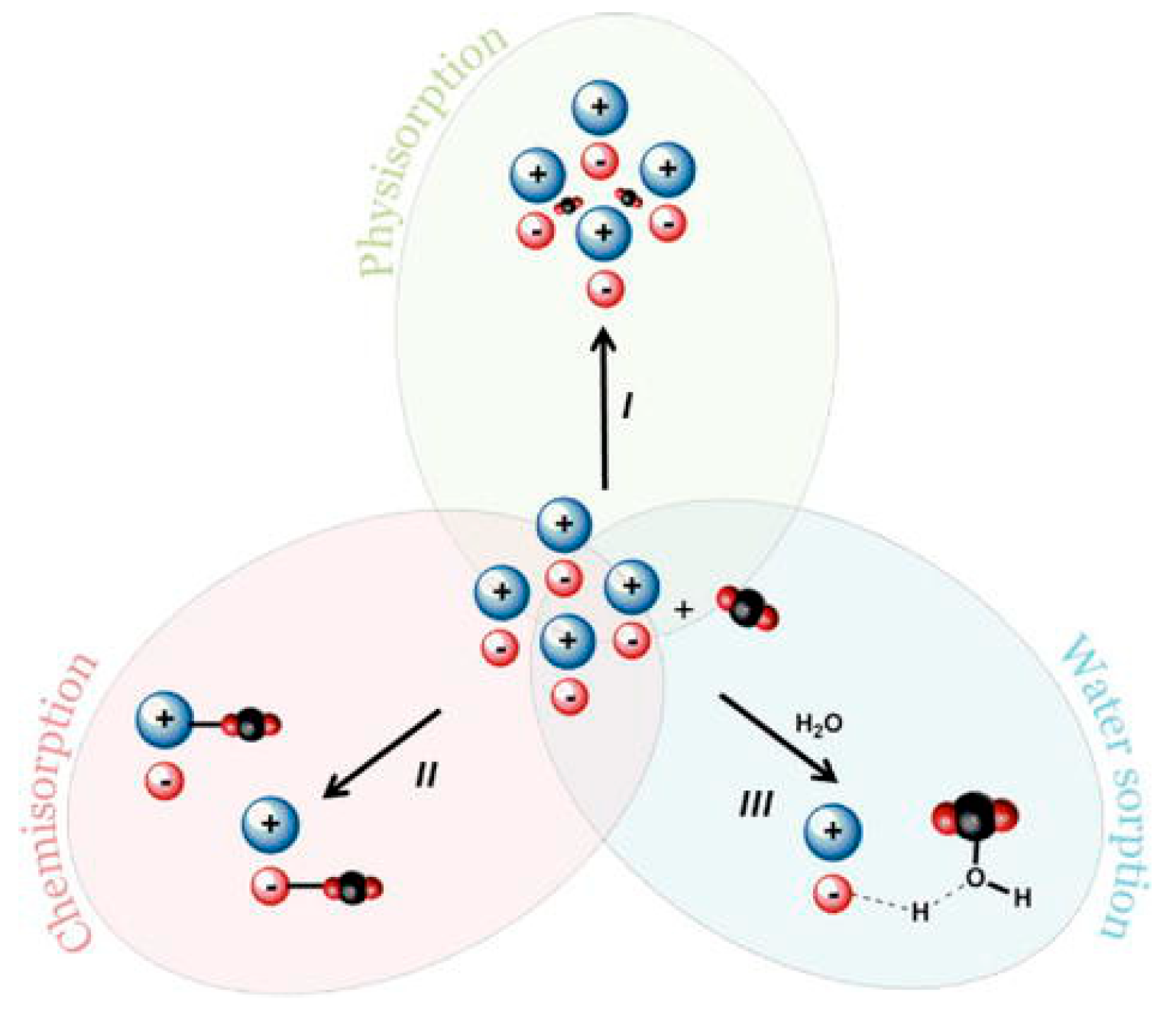
5. Factors Affecting CO2 Sorption
5.1. Effect of Cation
5.2. Effect of Cation Alkyl Chain Length
5.3. Effect of Anion
5.4. Effect of Viscosity
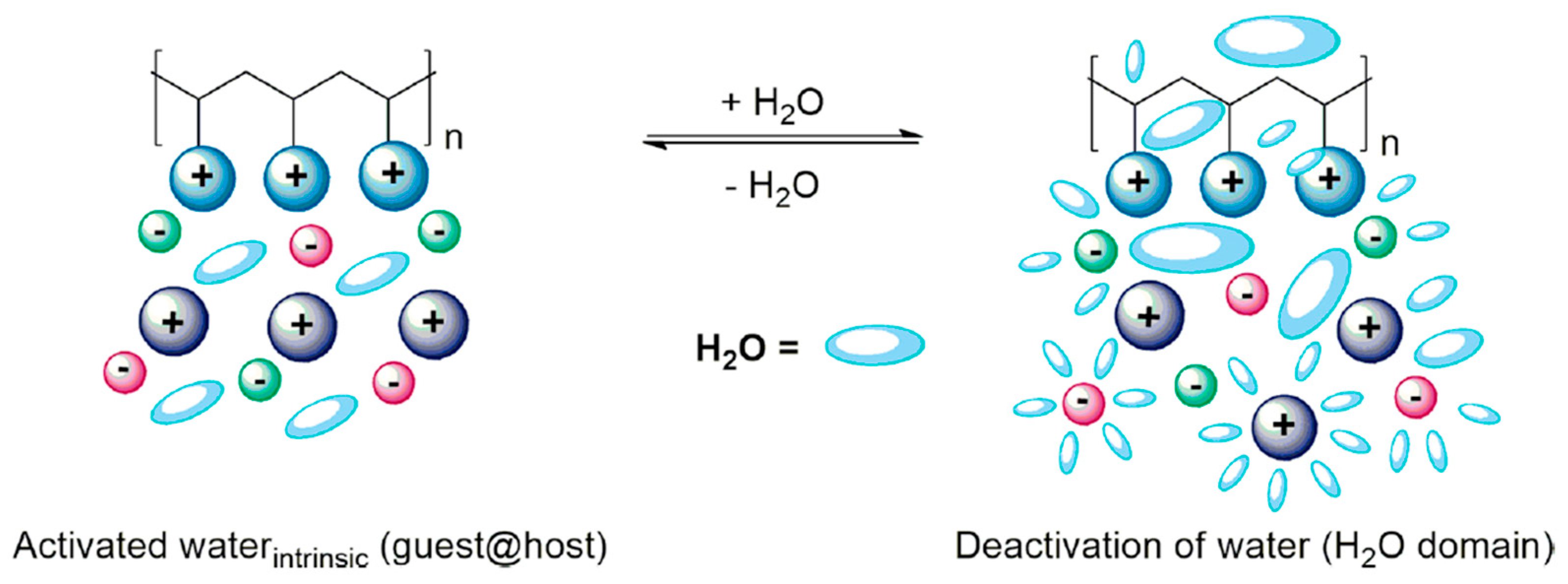
5.5. Effect of Water Content
5.6. Effect of Glass Transition Temperature (Tg)
5.7. Effect of Feed Pressure
6. IL and PIL-Based Composites for Enhanced CO2 Capture
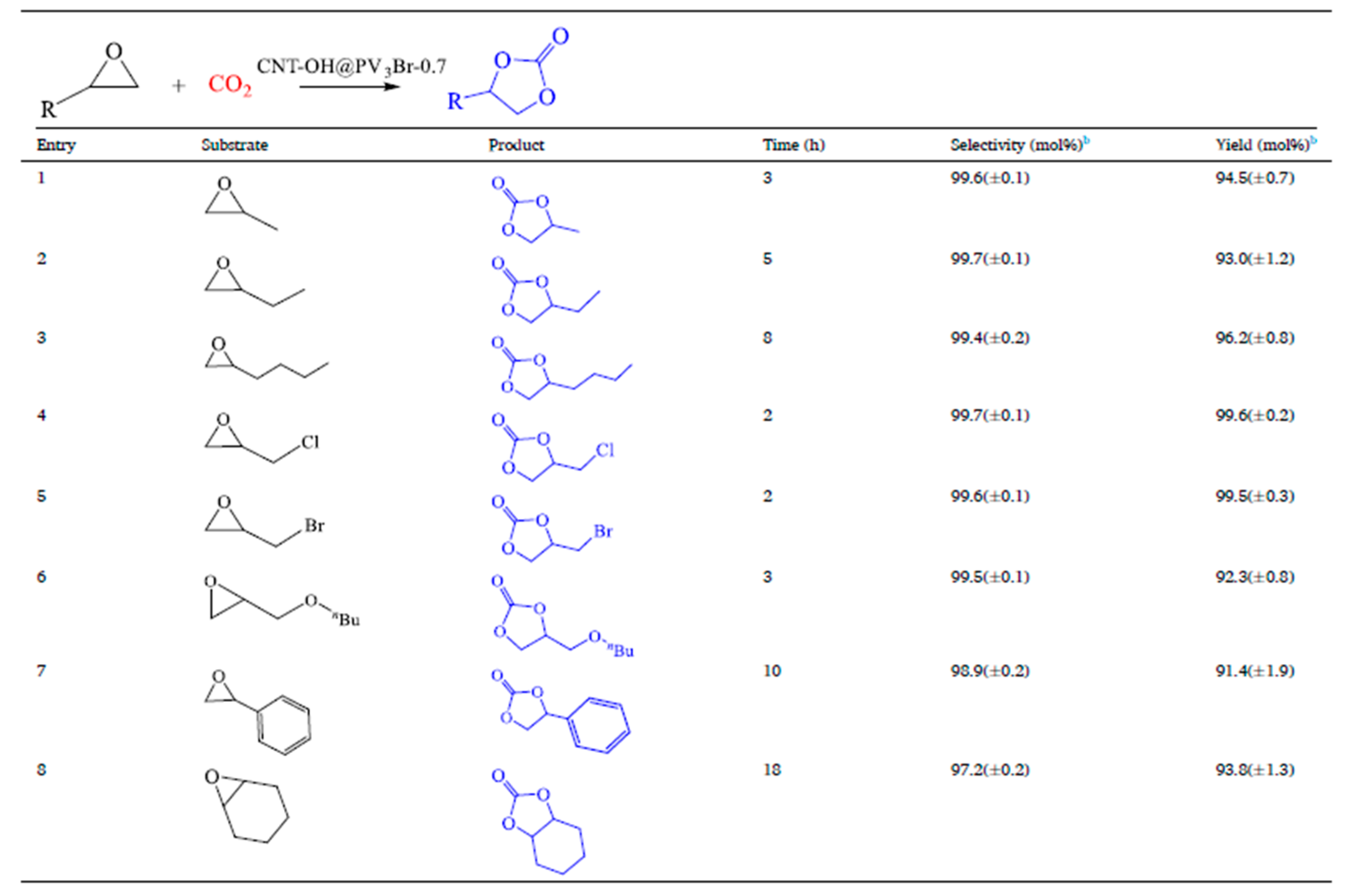
7. PILs as Catalysts for CO2 Conversion
8. Challenges and Future Directions
8.1. Current Challenges
8.2. Future Research Directions
Author Contributions
Funding
Conflicts of Interest
References
- Lindsey, R. Climate Change: Atmospheric Carbon Dioxide. Available online: https://www.climate.gov/news-features/understanding-climate/climate-change-atmospheric-carbon-dioxide (accessed on 11 July 2025).
- Friedlingstein, P.; O’Sullivan, M.; Jones, M.W.; Andrew, R.M.; Hauck, J.; Landschützer, P.; Le Quéré, C.; Li, H.; Luijkx, I.T.; Olsen, A.; et al. Global Carbon Budget 2024. Earth Syst. Sci. Data 2025, 17, 965–1039. [Google Scholar] [CrossRef]
- De La Vega, E.; Chalk, T.B.; Wilson, P.A.; Bysani, R.P.; Foster, G.L. Atmospheric CO2 during the Mid-Piacenzian Warm Period and the M2 Glaciation. Sci. Rep. 2020, 10, 11002. [Google Scholar] [CrossRef]
- European Environment Agency. Ocean Acidification. Available online: https://www.eea.europa.eu/en/analysis/indicators/ocean-acidification?activeAccordion=309c5ef9-de09-4759-bc02-802370dfa366 (accessed on 11 July 2025).
- Gunawardene, O.H.P.; Gunathilake, C.A.; Vikrant, K.; Amaraweera, S.M. Carbon Dioxide Capture through Physical and Chemical Adsorption Using Porous Carbon Materials: A Review. Atmosphere 2022, 13, 397. [Google Scholar] [CrossRef]
- Ocean Acidification. Available online: https://www.whoi.edu/know-your-ocean/ocean-topics/how-the-ocean-works/ocean-chemistry/ocean-acidification/ (accessed on 8 February 2025).
- Gomez-Coma, L.; Garea, A.; Irabien, A. Carbon Dioxide Capture by [Emim][Ac] Ionic Liquid in a Polysulfone Hollow Fiber Membrane Contactor. Int. J. Greenh. Gas. Control 2016, 52, 401–409. [Google Scholar] [CrossRef]
- Yoon, B.; Voth, G.A. Elucidating the Molecular Mechanism of CO2 Capture by Amino Acid Ionic Liquids. J. Am. Chem. Soc. 2023, 145, 15663–15667. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wang, D.; Krook-Riekkola, A.; Ji, X. MEA-Based CO2 Capture: A Study Focuses on MEA Concentrations and Process Parameters. Front. Energy Res. 2023, 11, 1230743. [Google Scholar] [CrossRef]
- Ochedi, F.O.; Yu, J.; Yu, H.; Liu, Y.; Hussain, A. Carbon Dioxide Capture Using Liquid Absorption Methods: A Review. Environ. Chem. Lett. 2021, 19, 77–109. [Google Scholar] [CrossRef]
- Gao, W.; Liang, S.; Wang, R.; Jiang, Q.; Zhang, Y.; Zheng, Q.; Xie, B.; Toe, C.Y.; Zhu, X.; Wang, J.; et al. Industrial Carbon Dioxide Capture and Utilization: State of the Art and Future Challenges. Chem. Soc. Rev. 2020, 49, 8584–8686. [Google Scholar] [CrossRef]
- El-Nagar, R.A.; Elaraby, A.; Nessim, M.I.; Ghanem, A. Designed Imidazolium-Based Ionic Liquids to Capture Carbon Dioxide from Natural Gas. J. Mol. Liq. 2024, 401, 124708. [Google Scholar] [CrossRef]
- Raja Shahrom, M.S.; Wilfred, C.D.; MacFarlane, D.R.; Vijayraghavan, R.; Chong, F.K. Amino Acid Based Poly(Ionic Liquid) Materials for CO2 Capture: Effect of Anion. J. Mol. Liq. 2019, 276, 644–652. [Google Scholar] [CrossRef]
- Silva, A.; Barrulas, R.V.; Corvo, M.C.; Zanatta, M. Tuning Basic Poly(Ionic Liquid) Solutions towards Atmospheric Pressure CO2 Capture. J. Environ. Chem. Eng. 2023, 11, 110882. [Google Scholar] [CrossRef]
- Yuksel Orhan, O. Effects of Various Anions and Cations in Ionic Liquids on CO2 Capture. J. Mol. Liq. 2021, 333, 115981. [Google Scholar] [CrossRef]
- Zentou, H.; Hoque, B.; Abdalla, M.A.; Saber, A.F.; Abdelaziz, O.Y.; Aliyu, M.; Alkhedhair, A.M.; Alabduly, A.J.; Abdelnaby, M.M. Recent Advances and Challenges in Solid Sorbents for CO2 Capture. Carbon Capture Sci. Technol. 2025, 15, 100386. [Google Scholar] [CrossRef]
- Mahajan, S.; Lahtinen, M. Recent Progress in Metal-Organic Frameworks (MOFs) for CO2 Capture at Different Pressures. J. Environ. Chem. Eng. 2022, 10, 108930. [Google Scholar] [CrossRef]
- Dai, J.; Xie, D.; Liu, Y.; Zhang, Z.; Yang, Y.; Yang, Q.; Ren, Q.; Bao, Z. Supramolecular Metal–Organic Framework for CO2/CH4 and CO2/N2 Separation. Ind. Eng. Chem. Res. 2020, 59, 7866–7874. [Google Scholar] [CrossRef]
- Boer, D.G.; Langerak, J.; Pescarmona, P.P. Zeolites as Selective Adsorbents for CO2 Separation. ACS Appl. Energy Mater. 2023, 6, 2634–2656. [Google Scholar] [CrossRef]
- Song, K.S.; Fritz, P.W.; Coskun, A. Porous Organic Polymers for CO2Capture, Separation and Conversion. Chem. Soc. Rev. 2022, 51, 9831–9852. [Google Scholar] [CrossRef]
- Dai, Z.; Deng, L. Membranes for CO2 Capture and Separation: Progress in Research and Development for Industrial Applications. Sep. Purif. Technol. 2024, 335, 126022. [Google Scholar] [CrossRef]
- Li, X.; Peng, Z.; Pei, Y.; Ajmal, T.; Rana, K.; Aitouche, A.; Mobasheri, R. Oxy-fuel Combustion for Carbon Capture and Storage in Internal Combustion Engines—A Review. Int. J. Energy Res. 2022, 46, 505–522. [Google Scholar] [CrossRef]
- Bera, N.; Sardar, P.; Hazra, R.; Samanta, A.N.; Sarkar, N. Direct Air Capture of CO2 by Amino Acid-Functionalized Ionic Liquid-Based Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2024, 12, 14288–14295. [Google Scholar] [CrossRef]
- Nabais, A.R.; Martins, A.P.S.; Alves, V.D.; Crespo, J.G.; Marrucho, I.M.; Tomé, L.C.; Neves, L.A. Poly(Ionic Liquid)-Based Engineered Mixed Matrix Membranes for CO2/H2 Separation. Sep. Purif. Technol. 2019, 222, 168–176. [Google Scholar] [CrossRef]
- Zhang, R.; Ke, Q.; Zhang, Z.; Zhou, B.; Cui, G.; Lu, H. Tuning Functionalized Ionic Liquids for CO2 Capture. IJMS 2022, 23, 11401. [Google Scholar] [CrossRef]
- Zulfiqar, S.; Sarwar, M.I.; Mecerreyes, D. Polymeric Ionic Liquids for CO2 Capture and Separation: Potential, Progress and Challenges. Polym. Chem. 2015, 6, 6435–6451. [Google Scholar] [CrossRef]
- Adanez, J.; Abad, A.; Garcia-Labiano, F.; Gayan, P.; De Diego, L.F. Progress in Chemical-Looping Combustion and Reforming Technologies. Prog. Energy Combust. Sci. 2012, 38, 215–282. [Google Scholar] [CrossRef]
- Ignatusha, P.; Lin, H.; Kapuscinsky, N.; Scoles, L.; Ma, W.; Patarachao, B.; Du, N. Membrane Separation Technology in Direct Air Capture. Membranes 2024, 14, 30. [Google Scholar] [CrossRef] [PubMed]
- Samanta, A.; Zhao, A.; Shimizu, G.K.H.; Sarkar, P.; Gupta, R. Post-Combustion CO2 Capture Using Solid Sorbents: A Review. Ind. Eng. Chem. Res. 2012, 51, 1438–1463. [Google Scholar] [CrossRef]
- Mondal, M.K.; Balsora, H.K.; Varshney, P. Progress and Trends in CO2 Capture/Separation Technologies: A Review. Energy 2012, 46, 431–441. [Google Scholar] [CrossRef]
- The Integrated Environmental Control Model Team. Amine-Based Post-Combustion CO2 Capture. 2019. Available online: https://www.uwyo.edu/iecm/_bfiles/documentation/201901_iecmtd_amine-based-co2-cap.pdf (accessed on 19 July 2025).
- Yu, Y.; Yang, X.; Zhang, C.; Chen, J.; Lin, W.; Meng, J. A Tough Double-Network Ion Gel Membrane Based on Poly (Ionic Liquid) for Efficient Carbon Capture. Sep. Purif. Technol. 2024, 331, 125591. [Google Scholar] [CrossRef]
- Xue, Z.; Zhang, Z.; Han, J.; Chen, Y.; Mu, T. Carbon Dioxide Capture by a Dual Amino Ionic Liquid with Amino-Functionalized Imidazolium Cation and Taurine Anion. Int. J. Greenh. Gas. Control 2011, 5, 628–633. [Google Scholar] [CrossRef]
- Numpilai, T.; Pham, L.K.H.; Witoon, T. Advances in Ionic Liquid Technologies for CO2 Capture and Conversion: A Comprehensive Review. Ind. Eng. Chem. Res. 2024, 63, 19865–19915. [Google Scholar] [CrossRef]
- Liu, X.; O’Harra, K.E.; Bara, J.E.; Turner, C.H. Solubility Behavior of CO2 in Ionic Liquids Based on Ionic Polarity Index Analyses. J. Phys. Chem. B 2021, 125, 3665–3676. [Google Scholar] [CrossRef]
- Meli, L.; Miao, J.; Dordick, J.S.; Linhardt, R.J. Electrospinning from Room Temperature Ionic Liquids for Biopolymer Fiber Formation. Green Chem. 2010, 12, 1883–1892. [Google Scholar] [CrossRef]
- Bernard, F.L.; Dos Santos, L.M.; Schwab, M.B.; Polesso, B.B.; Do Nascimento, J.F.; Einloft, S. Polyurethane-based Poly (Ionic Liquid)s for CO2 Removal from Natural Gas. J. Appl. Polym. Sci. 2019, 136, 47536. [Google Scholar] [CrossRef]
- Chen, H.; Elabd, Y.A. Polymerized Ionic Liquids: Solution Properties and Electrospinning. Macromolecules 2009, 42, 3368–3373. [Google Scholar] [CrossRef]
- Kammakakam, I.; Bara, J.E.; Jackson, E.M.; Lertxundi, J.; Mecerreyes, D.; Tomé, L.C. Tailored CO2-Philic Anionic Poly(Ionic Liquid) Composite Membranes: Synthesis, Characterization, and Gas Transport Properties. ACS Sustain. Chem. Eng. 2020, 8, 5954–5965. [Google Scholar] [CrossRef]
- Wu, J.; Yang, Z.; Xie, J.; Zhu, P.; Wei, J.; Jin, R.; Yang, H. Porous Polymer Supported Amino Functionalized Ionic Liquid for Effective CO2 Capture. Langmuir 2023, 39, 2729–2738. [Google Scholar] [CrossRef] [PubMed]
- Philip, F.A.; Henni, A. Enhancement of Post-Combustion CO2 Capture Capacity by Incorporation of Task-Specific Ionic Liquid into ZIF-8. Microporous Mesoporous Mater. 2022, 330, 111580. [Google Scholar] [CrossRef]
- Philip, F.A.; Henni, A. Incorporation of Amino Acid-Functionalized Ionic Liquids into Highly Porous MOF-177 to Improve the Post-Combustion CO2 Capture Capacity. Molecules 2023, 28, 7185. [Google Scholar] [CrossRef]
- Sun, L.; Gao, M.; Tang, S. Porous Amino Acid-Functionalized Poly(Ionic Liquid) Foamed with Supercritical CO2 and Its Application in CO2 Adsorption. Chem. Eng. J. 2021, 412, 128764. [Google Scholar] [CrossRef]
- Zanatta, M.; Lopes, M.; Cabrita, E.J.; Bernardes, C.E.S.; Corvo, M.C. Handling CO2 Sorption Mechanism in PIL@IL Composites. J. CO2 Util. 2020, 41, 101225. [Google Scholar] [CrossRef]
- Lopes, M.M.; Barrulas, R.V.; Paiva, T.G.; Ferreira, A.S.D.; Zanatta, M.; Corvo, M.C. Molecular Interactions in Ionic Liquids: The NMR Contribution Towards Tailored Solvents. In Nuclear Magnetic Resonance; Khaneja, N., Ed.; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Ye, C.-P.; Wang, R.-N.; Gao, X.; Li, W.-Y. CO2 Capture Performance of Supported Phosphonium Dual Amine-Functionalized Ionic Liquids@MCM-41. Energy Fuels 2020, 34, 14379–14387. [Google Scholar] [CrossRef]
- Bernard, F.L.; Duarte, E.A.; Polesso, B.B.; Duczinski, R.B.; Einloft, S. CO2 Sorption Using Encapsulated Imidazolium-Based Fluorinated Ionic Liquids. Environ. Chall. 2021, 4, 100109. [Google Scholar] [CrossRef]
- Gaur, S.S.; Edgehouse, K.J.; Klemm, A.; Wei, P.; Gurkan, B.; Pentzer, E.B. Capsules with Polyurea Shells and Ionic Liquid Cores for CO2 Capture. J. Polym. Sci. 2021, 59, 2980–2989. [Google Scholar] [CrossRef]
- Noorani, N.; Mehrdad, A.; Ahadzadeh, I. CO2 Absorption in Amino Acid-Based Ionic Liquids: Experimental and Theoretical Studies. Fluid Phase Equilibria 2021, 547, 113185. [Google Scholar] [CrossRef]
- Tshemese, Z.; Masikane, S.C.; Mlowe, S.; Revaprasadu, N. Progress in Green Solvents for the Stabilisation of Nanomaterials: Imidazolium Based Ionic Liquids. In Recent Advances in Ionic Liquids; Rahman, M.M., Ed.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Barrera Bogoya, A.; Arnal-Herault, C.; Barth, D.; Mutelet, F.; Belaissaoui, B.; Pinilla Monsalve, L.; Marchal, P.; Tamura, Y.; Nakama, Y.; Hayano, S.; et al. CO2 Sorption of Elastomer Poly(Ionic Liquid)s with Imidazolium Cations Having Different Alkyl Chains: Structure-Morphology-Property Relationships and Thermodynamic Modelling by the PC-SAFT Equation of State. Polymer 2024, 308, 127415. [Google Scholar] [CrossRef]
- Lai, W.-H.; Wang, D.K.; Wey, M.-Y.; Tseng, H.-H. ZIF-8/Styrene-IL Polymerization Hollow Fiber Membrane for Improved CO2/N2 Separation. J. Clean. Prod. 2022, 372, 133785. [Google Scholar] [CrossRef]
- Morozova, S.M.; Shaplov, A.S.; Lozinskaya, E.I.; Mecerreyes, D.; Sardon, H.; Zulfiqar, S.; Suárez-García, F.; Vygodskii, Y.S. Ionic Polyurethanes as a New Family of Poly(Ionic Liquid)s for Efficient CO2 Capture. Macromolecules 2017, 50, 2814–2824. [Google Scholar] [CrossRef]
- Wang, X.; Akhmedov, N.G.; Duan, Y.; Luebke, D.; Li, B. Immobilization of Amino Acid Ionic Liquids into Nanoporous Microspheres as Robust Sorbents for CO2 Capture. J. Mater. Chem. A 2013, 1, 2978–2982. [Google Scholar] [CrossRef]
- Szala-Bilnik, J.; Abedini, A.; Crabtree, E.; Bara, J.E.; Turner, C.H. Molecular Transport Behavior of CO2 in Ionic Polyimides and Ionic Liquid Composite Membrane Materials. J. Phys. Chem. B 2019, 123, 7455–7463. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Zhang, C.; Yu, Y.; Hao, T.; Wang, H.; Ding, X.; Meng, J. Tuning the Microstructure of Crosslinked Poly(Ionic Liquid) Membranes and Gels via a Multicomponent Reaction for Improved CO2 Capture Performance. J. Membr. Sci. 2020, 593, 117405. [Google Scholar] [CrossRef]
- Noorani, N.; Mehrdad, A.; Zarei Diznab, R. Thermodynamic Study on Carbon Dioxide Absorption in Vinyl Imidazolium–Amino Acid Ionic Liquids. Fluid Phase Equilibria 2022, 557, 113433. [Google Scholar] [CrossRef]
- Barrera Bogoya, A.; Arnal-Herault, C.; Barth, D.; Mutelet, F.; Belaissaoui, B.; Marchal, P.; Tamura, Y.; Nakama, Y.; Hayano, S.; Jonquieres, A. Influence of Anion and Ionization Ratio on CO2 Sorption of Poly(Ionic Liquid)s with Imidazolium Cations Derived from Polyepichlorohydrin: A Multi-Scale Analysis. Polymer 2025, 323, 128186. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, W.; Ma, L.; Zhou, Y.; Wang, J. Amino Acid Anion Paired Mesoporous Poly(Ionic Liquids) as Metal-/Halogen-Free Heterogeneous Catalysts for Carbon Dioxide Fixation. ACS Sustain. Chem. Eng. 2019, 7, 9387–9398. [Google Scholar] [CrossRef]
- Luo, X.; Guo, Y.; Ding, F.; Zhao, H.; Cui, G.; Li, H.; Wang, C. Significant Improvements in CO2 Capture by Pyridine-Containing Anion-Functionalized Ionic Liquids through Multiple-Site Cooperative Interactions. Angew. Chem. Int. Ed. 2014, 53, 7053–7057. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Z.; Chen, S.; Yu, P.; Luo, Y. CO2 Capture by Imidazolate-Based Ionic Liquids: Effect of Functionalized Cation and Dication. Ind. Eng. Chem. Res. 2013, 52, 6069–6075. [Google Scholar] [CrossRef]
- Cui, G.; Cheng, Y.; Zhang, W.; Shen, X.; Li, L.; Zhang, R.; Chen, Y.; Ke, Q.; Ge, C.; Liu, H.; et al. Highly Efficient CO2 Capture from Open Air and Dilute Gas Streams by Tunable Azolate Ionic Liquids Based Deep Eutectic Solvents. Chem. Eng. J. 2025, 505, 159193. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, Z.; Wang, S.; Jiang, L.; Li, H.; Wang, C. Dual-Tuning Azole-Based Ionic Liquids for Reversible CO2 Capture from Ambient Air. ChemSusChem 2024, 17, e202301951. [Google Scholar] [CrossRef] [PubMed]
- Nisar, M.; Bernard, F.L.; Duarte, E.; Chaban, V.V.; Einloft, S. New Polysulfone Microcapsules Containing Metal Oxides and ([BMIM][NTf2]) Ionic Liquid for CO2 Capture. J. Environ. Chem. Eng. 2021, 9, 104781. [Google Scholar] [CrossRef]
- Yan, J.; Mangolini, F. Engineering Encapsulated Ionic Liquids for Next-Generation Applications. RSC Adv. 2021, 11, 36273–36288. [Google Scholar] [CrossRef]
- Liu, F.; Shen, Y.; Shen, L.; Sun, C.; Chen, L.; Wang, Q.; Li, S.; Li, W. Novel Amino-Functionalized Ionic Liquid/Organic Solvent with Low Viscosity for CO2 Capture. Environ. Sci. Technol. 2020, 54, 3520–3529. [Google Scholar] [CrossRef] [PubMed]
- Molina-Fernández, C.; Chaplier, G.; Deveen, V.; Hartanto, Y.; Luis, P. Poly(Ionic Liquid) Composite Membranes Bearing Different Anions as Biocatalytic Membranes for CO2 Capture. Carbon Capture Sci. Technol. 2024, 13, 100269. [Google Scholar] [CrossRef]
- Hayano, S.; Ota, K.; Ban, H.T. Syntheses, Characterizations and Functions of Cationic Polyethers with Imidazolium-Based Ionic Liquid Moieties. Polym. Chem. 2018, 9, 948–960. [Google Scholar] [CrossRef]
- Osman, A.; Ziyada, A.K.; Khan, A.M.; Rajab, F. Alkylimidazolium-Based Ionic Liquids with Tailored Anions and Cations for CO2 Capture. RSC Adv. 2024, 14, 3985–3995. [Google Scholar] [CrossRef]
- Simons, K.; Nijmeijer, K.; Bara, J.E.; Noble, R.D.; Wessling, M. How Do Polymerized Room-Temperature Ionic Liquid Membranes Plasticize during High Pressure CO2 Permeation? J. Membr. Sci. 2010, 360, 202–209. [Google Scholar] [CrossRef]
- Bernard, F.L.; Duczinski, R.B.; Rojas, M.F.; Fialho, M.C.C.; Carreño, L.Á.; Chaban, V.V.; Vecchia, F.D.; Einloft, S. Cellulose Based Poly(Ionic Liquids): Tuning Cation-Anion Interaction to Improve Carbon Dioxide Sorption. Fuel 2018, 211, 76–86. [Google Scholar] [CrossRef]
- Lee, Y.-Y.; Gurkan, B. Graphene Oxide Reinforced Facilitated Transport Membrane with Poly(Ionic Liquid) and Ionic Liquid Carriers for CO2/N2 Separation. J. Membr. Sci. 2021, 638, 119652. [Google Scholar] [CrossRef]
- Wilson, S.M.W. High Purity CO2 from Direct Air Capture Using a Single TVSA Cycle with Na-X Zeolites. Sep. Purif. Technol. 2022, 294, 121186. [Google Scholar] [CrossRef]
- Zeeshan, M.; Klemm, A.; Damron, J.T.; Unocic, K.A.; Kidder, M.K.; Gurkan, B. Ionic Liquid Functionalizes the Metal Organic Framework for Microwave-Assisted Direct Air Capture of CO2. ACS Mater. Lett. 2024, 6, 3854–3861. [Google Scholar] [CrossRef]
- Wang, K.; Bai, J.; Zhao, Z.; Zhang, Z.; Mao, W.; Jiang, L.; Li, H.; Wang, C. Fine-Tuning of Imidazole-Based Ionic Liquid for Highly Efficient and Reversible Direct Air Capture via Hydrogen Bonding Interaction. Sep. Purif. Technol. 2025, 362, 131790. [Google Scholar] [CrossRef]
- Dorado-Alfaro, S.; Hospital-Benito, D.; Moya, C.; Navarro, P.; Lemus, J.; Palomar, J. Exploiting Process Thermodynamics in Carbon Capture from Direct Air to Industrial Sources: The Paradigmatic Case of Ionic Liquids. Carbon Capture Sci. Technol. 2024, 13, 100320. [Google Scholar] [CrossRef]
- Barrulas, R.V.; López-Iglesias, C.; Zanatta, M.; Casimiro, T.; Mármol, G.; Carrott, M.R.; García-González, C.A.; Corvo, M.C. The AEROPILs Generation: Novel Poly(Ionic Liquid)-Based Aerogels for CO2 Capture. IJMS 2021, 23, 200. [Google Scholar] [CrossRef]
- Yang, G.; Yu, J.; Peng, S.; Sheng, K.; Zhang, H. Poly(Ionic Liquid)-Modified Metal Organic Framework for Carbon Dioxide Adsorption. Polymers 2020, 12, 370. [Google Scholar] [CrossRef]
- Rocky, K.A.; Pal, A.; Moniruzzaman, M.; Saha, B.B. Adsorption Characteristics and Thermodynamic Property Fields of Polymerized Ionic Liquid and Polyvinyl Alcohol Based Composite/CO2 Pairs. J. Mol. Liq. 2019, 294, 111555. [Google Scholar] [CrossRef]
- Lee, Y.-Y.; Edgehouse, K.; Klemm, A.; Mao, H.; Pentzer, E.; Gurkan, B. Capsules of Reactive Ionic Liquids for Selective Capture of Carbon Dioxide at Low Concentrations. ACS Appl. Mater. Interfaces 2020, 12, 19184–19193. [Google Scholar] [CrossRef]
- Chang, Y.; Wu, Y.; Liu, R.; Jiang, Y.; Zhou, Z.; Wang, T.; Chen, X. A Novel Ionic Liquids Phase Change Absorption System for Carbon Dioxide Capture and Utilization. Fuel 2024, 377, 132832. [Google Scholar] [CrossRef]
- Song, H.; Wang, Y.; Liu, Y.; Chen, L.; Feng, B.; Jin, X.; Zhou, Y.; Huang, T.; Xiao, M.; Huang, F.; et al. Conferring Poly(Ionic Liquid)s with High Surface Areas for Enhanced Catalytic Activity. ACS Sustain. Chem. Eng. 2021, 9, 2115–2128. [Google Scholar] [CrossRef]
- Daryayehsalameh, B.; Nabavi, M.; Vaferi, B. Modeling of CO2 Capture Ability of [Bmim][BF4] Ionic Liquid Using Connectionist Smart Paradigms. Environ. Technol. Innov. 2021, 22, 101484. [Google Scholar] [CrossRef]
- Mohamedali, M.; Ibrahim, H.; Henni, A. Incorporation of Acetate-Based Ionic Liquids into a Zeolitic Imidazolate Framework (ZIF-8) as Efficient Sorbents for Carbon Dioxide Capture. Chem. Eng. J. 2018, 334, 817–828. [Google Scholar] [CrossRef]
- Mohamedali, M.; Ibrahim, H.; Henni, A. Imidazolium Based Ionic Liquids Confined into Mesoporous Silica MCM-41 and SBA-15 for Carbon Dioxide Capture. Microporous Mesoporous Mater. 2020, 294, 109916. [Google Scholar] [CrossRef]
- Cai, K.; Liu, P.; Zhao, T.; Su, K.; Yang, Y.; Tao, D.-J. Construction of Hyper-Crosslinked Ionic Polymers with High Surface Areas for Effective CO2 Capture and Conversion. Microporous Mesoporous Mater. 2022, 343, 112135. [Google Scholar] [CrossRef]
- Zulfiqar, S.; Mantione, D.; El Tall, O.; Ruipérez, F.; Sarwar, M.I.; Rothenberger, A.; Mecerreyes, D. Pyridinium Containing Amide Based Polymeric Ionic Liquids for CO2/CH4 Separation. ACS Sustain. Chem. Eng. 2019, 7, 10241–10247. [Google Scholar] [CrossRef]
- Xie, Y.; Liang, J.; Fu, Y.; Lin, J.; Wang, H.; Tu, S.; Li, J. Poly(Ionic Liquid)s with High Density of Nucleophile/Electrophile for CO2 Fixation to Cyclic Carbonates at Mild Conditions. J. CO2 Util. 2019, 32, 281–289. [Google Scholar] [CrossRef]
- Wan, Y.-L.; Zhang, J.; Wang, L.; Lei, Y.-Z.; Wen, L.-L. Poly(Ionic Liquid)-Coated Hydroxy-Functionalized Carbon Nanotube Nanoarchitectures with Boosted Catalytic Performance for Carbon Dioxide Cycloaddition. J. Colloid Interface Sci. 2024, 653, 844–856. [Google Scholar] [CrossRef]
- Eftaiha, A.F.; Qaroush, A.K.; Hasan, A.K.; Assaf, K.I.; Al-Qaisi, F.M.; Melhem, M.E.; Al-Maythalony, B.A.; Usman, M. Cross-Linked, Porous Imidazolium-Based Poly(Ionic Liquid)s for CO2 Capture and Utilisation. New J. Chem. 2021, 45, 16452–16460. [Google Scholar] [CrossRef]
- Martínez-Izquierdo, L.; Téllez, C.; Coronas, J. Highly Stable Pebax® Renew® Thin-Film Nanocomposite Membranes with Metal Organic Framework ZIF-94 and Ionic Liquid [Bmim][BF4 ] for CO2 Capture. J. Mater. Chem. A 2022, 10, 18822–18833. [Google Scholar] [CrossRef]
- Nellepalli, P.; Tomé, L.C.; Vijayakrishna, K.; Marrucho, I.M. Imidazolium-Based Copoly(Ionic Liquid) Membranes for CO2/N2 Separation. Ind. Eng. Chem. Res. 2019, 58, 2017–2026. [Google Scholar] [CrossRef]
- Fang, X.; Yang, L.; Dai, Z.; Cong, D.; Zheng, D.; Yu, T.; Tu, R.; Zhai, S.; Yang, J.; Song, F.; et al. Poly(Ionic Liquid)s for Photo-Driven CO2 Cycloaddition: Electron Donor–Acceptor Segments Matter. Adv. Sci. 2023, 10, 2206687. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Xu, Y.; Zeng, Z.; Ibrahim, A.-R.; Yang, J.; Yang, S.; Xie, Y.; Hong, Y.; Su, Y.; Wang, H.; et al. Mesoporous Poly(Ionic Liquid)s with Dual Active Sites for Highly Efficient CO2 Conversion. Green Energy Environ. 2023, 8, 478–486. [Google Scholar] [CrossRef]
- Chen, P.; Tang, X.; Meng, X.; Tang, H.; Pan, Y.; Liang, Y. Transition Metal-Free Catalytic Formylation of Carbon Dioxide and Amide with Novel Poly(Ionic Liquid)s. Green Synth. Catal. 2022, 3, 162–167. [Google Scholar] [CrossRef]
- Kulshrestha, A.; Kumar, R.; Sharma, K.P. Efficient Carbon Capture and Mineralization Using Porous Liquids Comprising Hollow Nanoparticles and Enzymes Dispersed in Fatty Acid-Based Ionic Liquids. ACS Sustain. Chem. Eng. 2024, 12, 5799–5808. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, D.; Zhao, Y.; Sun, J. Hydrogen Bond Donor Functionalized Poly(Ionic Liquids)@MIL-101 for the CO2 Capture and Improving the Catalytic CO2 Conversion with Epoxide. J. Colloid Interface Sci. 2022, 618, 22–33. [Google Scholar] [CrossRef]
- Peng, H.; Zhang, Q.; Wang, Y.; Gao, H.; Zhang, N.; Zhou, J.; Zhang, L.; Yang, Q.; Yang, Q.; Lu, Z. Atomically Dispersed Lewis Acid Sites Meet Poly(Ionic Liquid)s Networks for Solvent-Free and Co-Catalyst-Free Conversion of CO2 to Cyclic Carbonates. Appl. Catal. B Environ. 2022, 313, 121463. [Google Scholar] [CrossRef]
- Frade, R.F.; Afonso, C.A. Impact of Ionic Liquids in Environment and Humans: An Overview. Hum. Exp. Toxicol. 2010, 29, 1038–1054. [Google Scholar] [CrossRef] [PubMed]
- Boethling, R.S.; Sommer, E.; DiFiore, D. Designing Small Molecules for Biodegradability. Chem. Rev. 2007, 107, 2207–2227. [Google Scholar] [CrossRef] [PubMed]
- Burner, J.; Schwiedrzik, L.; Krykunov, M.; Luo, J.; Boyd, P.G.; Woo, T.K. High-Performing Deep Learning Regression Models for Predicting Low-Pressure CO2 Adsorption Properties of Metal–Organic Frameworks. J. Phys. Chem. C 2020, 124, 27996–28005. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, K.; Yoo, H.; Lee, Y. Machine Learning-Driven Discovery of Metal–Organic Frameworks for Efficient CO2 Capture in Humid Condition. ACS Sustain. Chem. Eng. 2021, 9, 2872–2879. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Zhang, J.; Gu, J.; Zhang, S.; Li, G.; Shao, J.; He, Y.; Yang, H.; Zhang, S.; et al. Applied Machine Learning to Analyze and Predict CO2 Adsorption Behavior of Metal-Organic Frameworks. Carbon Capture Sci. Technol. 2023, 9, 100146. [Google Scholar] [CrossRef]
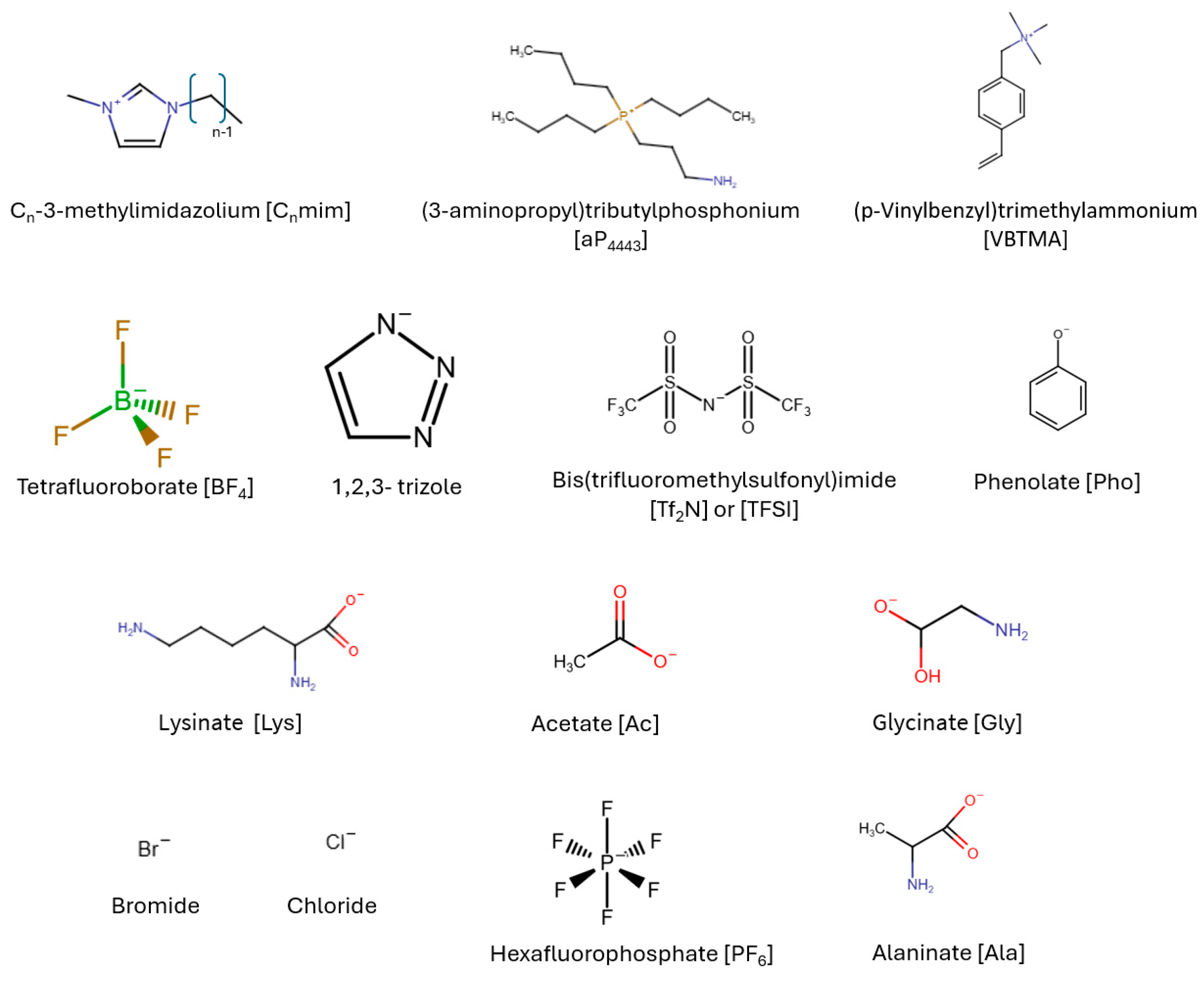


| [aP4443] | (3-aminopropyl) tributylphosphonium |
| [Emim], [C2mim] | 1-Ethyl-3-methylimidazolium |
| [Hmim] | 1-Hexyl-3-methylimidazolium |
| [Bmim] | 1-Butyl-3-methylimidazolium |
| [VEI] | 1-vinyl-3-ethylimidazolium |
| [TEPAH] | tetraethylenepentamine |
| [VBMTA] | (p-Vinylbenzyl) trimethylammonium |
| [C2OHmim] | 1-hydroxyethyl-3-methylimidazole chloride |
| [Tf2N], [TFSI] | Bis(trifluoromethylsulfonyl)imide |
| [Cl] | Chloride |
| [Ac] | Acetate |
| [Gly] | Glycine |
| [Ala] | Alanine |
| [2-MI] | 2-methylimidazolium |
| [Lys] | Lysinate |
| [PF6] | Hexafluorophosphate |
| [BF4] | Tetrafluoroborate |
| [2-Cnpyr] | 2-cyanopyrrolide |
| [aemmim] | 1-aminoethyl-2,3-dimethylimidazolium |
| [tau] | taurine |
| Cation | Anion | CO2 Absorption | Conditions | Processing | Reference |
|---|---|---|---|---|---|
| [aP4443] | 1,2,4-triazole | 4.02 mmol/g | 30 °C | [46] | |
| [aP4443] | 2-hydroxypyridine | 4.44 mmol/g | 30 °C | [46] | |
| [aP4443] | 2-aminopyridine | 5.32 mmol/g | 30 °C | [46] | |
| [aP4443] | 2-aminopyridine | 3.19 mmo/g | 30 °C | 30%IL on MCM-41 | [46] |
| [Emim] | [Tf2N] | 39.5 mg/g | 1 bar 298.15 K | Polysulfone Encapsulated IL | [47] |
| 62.7 mg/g | 10 bar 298.15 K | [47] | |||
| [Bmim] | [Tf2N] | 37 mg/g | 1 bar 298.15 K | [47] | |
| 56.5 mg/g | 10 bar 298.15 K | [47] | |||
| [Hmim] | [Tf2N] | 34.7 mg/g | 1 bar 298.15 K | [47] | |
| 54.6 mg/g | 10 bar 298.15 K | [47] | |||
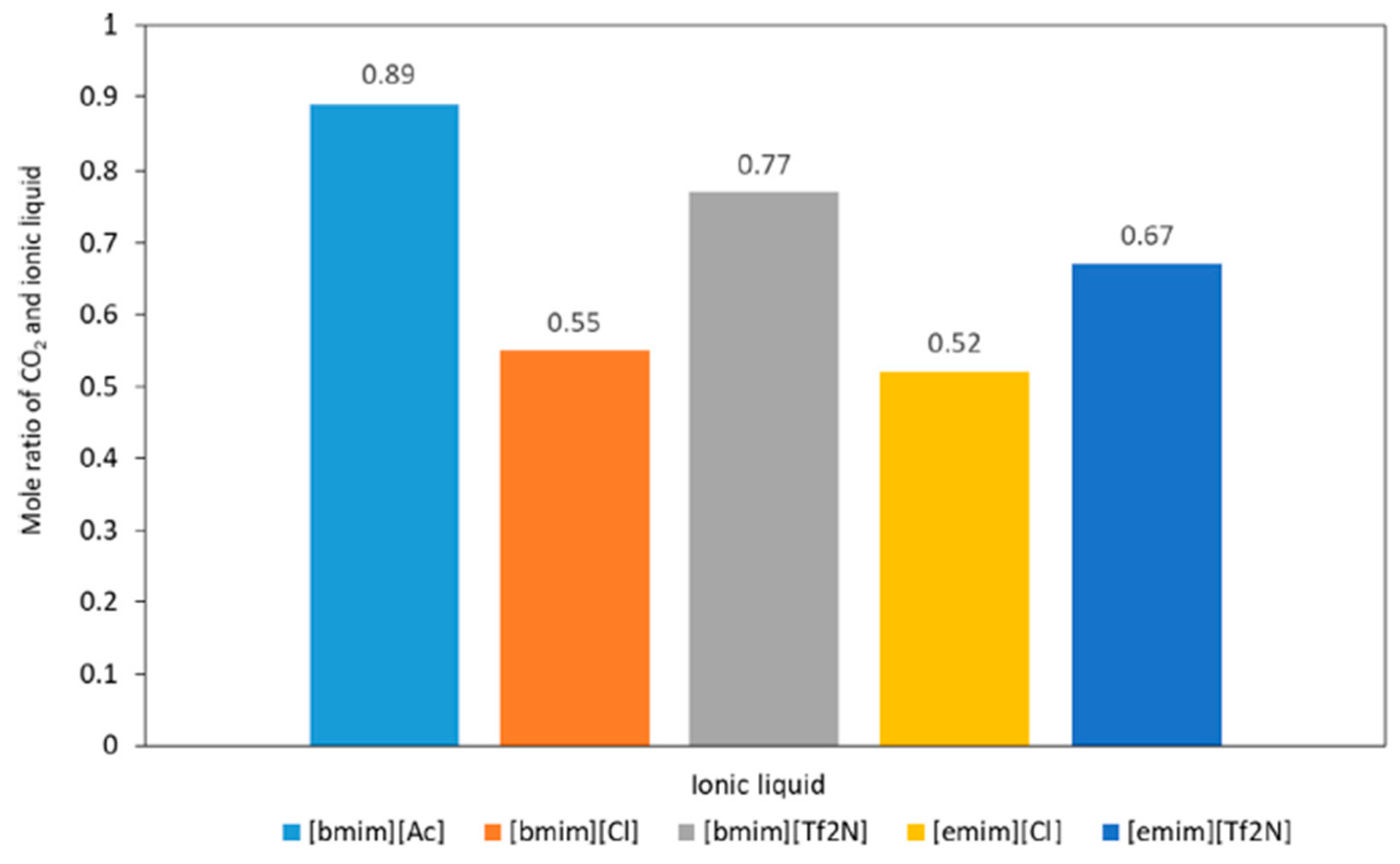
| [Emim] | [Tf2N] | 0.67 mol/mol | 2 bar 303 K | Bend with MEA and 1-hexanol | [15] |
| [Emim] | [Cl] | 0.52 mol/mol | 2 bar 303 K | [15] | |
| [Bmim] | [Tf2N] | 0.77 mol/mol | 2 bar 303 K | [15] | |
| [Bmim] | [Ac] | 0.89 mol/mol | 2 bar 303 K | [15] | |
| [Bmim] | [Cl] | 0.55 mol/mol | 2 bar 303 K | [15] | |
| [Emim] | [Gly] | 0.89 mmol/g | 0.2 bar 303 K | 30% loaded on ZIF-8 | [41] |
| [Emim] | [Ala] | 0.91 mmol/g | 0.2 bar 303 K | [41] | |
| [Emim] | [Gly] | 0.45 mmol/g | 0.2 bar 303 K | 20% loaded on MOF-177 | [42] |
| [Emim] | [Ala] | 0.42 mmol/g | 0.2 bar 303 K | [42] | |
| [Bmim] | [Tf2N] | 46.1 mg/g | 4 bar 45 °C | Polysulfone Encapsulated ILs | [64] |
| [Bmim] | [Tf2N] | 57.4 mg/g | 4 bar 45 °C | Polysulfone Encapsulated + 15 wt% Fe2O3 | [64] |
| [Bmim] | [Tf2N] | 46.1 mg/g | 4 bar 45 °C | Polysulfone Encapsulated + 1 wt% TiO2 | [64] |
| [Bmim] | [Tf2N] | 48.19 mg/g | 4 bar 45 °C | Polysulfone Encapsulated + 20 wt% CuO | [64] |
| [TEPAH] | [2-MI] | 1.72 mol/mol | solution with N-propanol (NPA)/ethylene glycol—8:2 | [66] | |
| 0.53 mol/mol | MEA/water | [66] | |||
| poly [VBMTA] | [Arg] | 1.14 mol/mol | [13] | ||
| [VBMTA] | [Arg] | 0.83 mol/mol | [13] | ||
| poly [VBMTA] | [Lys] | 1.13 mol/mol | [13] | ||
| [VBMTA] | [Lys] | 0.66 mol/mol | [13] | ||
| poly [VBMTA] | [Ala] | 0.56 mol/mol | [13] | ||
| [VBMTA] | [Ala] | 0.29 mol/mol | [13] | ||
| [Bmim] | [PF6] | 0.065 mol/kg | 760 torr 20 °C | oil pickering emulsions stabilized by alkylated graphene oxide nanosheets and polyurea shells. | [48] |
| [Bmim] | [BF4] | 0.8 mol/mol | AI simulation, highest pressure and lowest temperature | [83] | |
| [C2OHmim] | [Lys] | 1.29 mmol/g | 0.1 bar 40 °C | GDX-103 support, 60% IL | [40] |
| [aemmim] | [Tau] | 0.9 mol/mol | 1 bar 303.15 K | [33] | |
| [Bmim] | [Ac] | 0.83 mmol/g | 0.2 bar 303.15 K | Impregnated onto ZIF-30 | [84] |
| [Bmim] | [Ac] | 0.85 mmol/g | 0.2 bar 30 °C | 60% impregnated onto MCM-41 | [85] |
| [CelEt3N] | [PF6] | 38 mg/g | 0.1 MPa 298.15 K | Cationic cellulose-based IL | [71] |
| [CelEt3N] | [PF6] | 168 mg/g | 3 MPa 298.15 K | Cationic cellulose-based IL | [71] |
| PIL | IL | CO2 Absorption | Conditions | Processing | References |
|---|---|---|---|---|---|
| Poly(N-glycidyl-N′-methylimidazolium) (TSFI) | 159.3 mg/g | 10 bar 35 °C | 94.6% ionization ratio | [58] | |
| poly-4,4′-bis(chloromethyl)-1,1′-biphenyl | 100 mg/g | 1 bar 273 K | [86] | ||
| poly-1-vinyl-3-ethylimidazolium hydroxide | [Bmim][Ac] | 0.89 mmol/g | 20 bar 298 K | 7 wt% PIL, intrinsic water 13.5 wt% | [44] |
| poly-1-vinyl-3-ethylimidazolium hydroxide | [Bmim][Ac] | 0.35 mmol/g | 20 bar 298 K | 7 wt% PIL, added water 71.2 wt% | [44] |
| [Bmim][Ac] | 0.96 mmol/g | 20 bar 298 K | intrinsic water 9.7 wt% | [44] | |
| Polyurethanes- diquinuclidinium cation (CH3COO) | 18.25 mg/g | 1 bar 273 K | [53] | ||
| Polyurethanes- diquinuclidinium cation (BF4) | 24.76 mg/g | 1 bar 273 K | [53] | ||
| poly(diallyldimethylammonium chloride)-chitosan aerogels | 0.70 mmol/g | 1 bar 25 °C | glutaraldehyde crosslinker | [77] | |
| Methyl-diaminopyridinium bis(trifluoromethanesulfonyl)imide | 8.1 mg/g | 1 bar 273 K | polymerized using isophthaloyl chloride (IPC) | [87] | |
| Methyl-diaminopyridinium bis(trifluoromethanesulfonyl)imide | 13.9 mg/g | 1 bar 273 K | polymerized using 2,6-pyridinedicarbonyl chloride (PDC) | [87] | |
| (1,2,3,4,5,6-hexakis(1-(3-vinylimidazolium) bromide) benzene monomer and bipyridine | 1.44 mmol/g | 1 atm 273.15 K | Free radical copolymerization, post-synthetic metalation with Zn2+ | [88] | |
| vinylimidazolium salt-based PIL | 1.28 mmol/g | 1 bar | Coated on hydroxyl functionalized CNTs | [89] | |
| 3-(3-(phthalimide)propyl)-1-vinylimidazolium bromide | 0.59 mmol/g | 1 bar 25 °C | N-allylphthalimide building blocks, free radical polymerization | [90] |
| PIL | IL | CO2/N2 | CO2/CH4 | CO2/H2 | Conditions | Permeance | Processing | References |
|---|---|---|---|---|---|---|---|---|
| [Emim][Gly] | 28 | 0.1 bar 313 K | 30% loaded on ZIF-8 | [41] | ||||
| 19 | 0.2 bar 313 K | [41] | ||||||
| [Emim][Ala] | 18 | 0.1 bar 313 K | [41] | |||||
| 8 | 0.2 bar 313 K | [41] | ||||||
| [Emim][Gly] | 13 | 0.2 bar 313 K | 20% loaded on MOF-177 | [42] | ||||
| [Emim][Ala] | 11 | 0.2 bar 313 K | [42] | |||||
| polydiallyldimethylammonium 2-cyanopyrrolide | [Emim][2-Cnpyr] | 1180 | Direct Air | 3090 GPU | immobilized within a graphene oxide nanosheet | [72] | ||
| 250 | Cabin Air | 620 GPU | [72] | |||||
| No IL | 27 | CO2/N2 (15/85 cm3 (STP) min−1) | 497 GPU | Pebax® thin film composite | [91] | |||
| [Bmim][BF4] | 29 | CO2/N2 (15/85 cm3 (STP) min−1) | 629 GPU | Pebax® thin film composite, 10%IL | [91] | |||
| [Bmim][BF4] | 25 | CO2/N2 (15/85 cm3 (STP) min−1) | 751 GPU | 15% ZIF-8, Pebax® thin film composite, 10%IL | [91] | |||
| [Bmim][BF4] | 25 | CO2/N2 (15/85 cm3 (STP) min−1) | 891 GPU | 15% ZIF-94, Pebax® thin film composite, 10%IL | [91] | |||
| poly(vinylimidazolium) and poly styrene | [C2mim][Tf2N] | 34.4 | 20 °C, 100 kPa | 24.5 barrer | 30% IL | [92] | ||
| [Emim][NTf2] | 36 | 170 Barrer | polyoxyethylene bis(glycidyl ether) crosslinker | [56] | ||||
| [Emim][NTf2] | 24.6 | 2070 Barrer | trimethylolpropane triglycidyl ether crosslinker, [Emim][NTf2] free IL | [56] | ||||
| 1-Ethyl-3-methylimidazolium 1-{3-(Methacryloyloxy)propylsulfonyl}-(trifluoromethane-sulfonyl)imide | [C2mim][Tf2N] | 86.81 | 118.6 | 4.1 | 3 bar 20 °C | Anion-based polymer, free cation (1-Ethyl-3-methylimidazolium), PEGDA crosslinker | [39] | |
| 1-Ethyl-3-methylimidazolium 1-{3-(Methacryloyloxy)propylsulfonyl}-(p-toluene-sulfonyl)imide | [C2mim][Tf2N] | 51.65 | 61.88 | 3.18 | 3 bar 20 °C | Anion-based polymer, free cation (1-Ethyl-3-methylimidazolium), PEGDA crosslinker | [39] | |
| vinylimidazolium salt-based PIL | 39 | Coated on hydroxyl functionalized CNTs | [89] |
| PIL | Properties | Yield | Reactant | Condition | References |
|---|---|---|---|---|---|
| poly-4,4′-bis(chloromethyl)-1,1′-biphenyl | 99% | Styrene oxide | 373.2 K, CO2 1.0 MPa, 24 h, SO 10 mmol, catalyst 50 mg. | [86] | |
| 1,4-butanediyl-3,3′-bis-1-vinyl imidazolium bromide | imidazolium IL (Vim-COOH), copolymer | 92.70% | Epichlorohydrin | 70 °C 1.0 Mpa | [97] |
| (1,2,4,5-tetrakis(1-((4-vinylphenyl)-_N_, _N_-dimethylamine) bromide) benzene monomer (quaternary ammonium-based monomer) and bipyridine (DVPy) | copolymerization, post-synthetic metalation with Zn2+ | 98.90% | Epichlorohydrin | 50 °C 0.1 bar | [94] |
| (1,2,3,4,5,6-hexakis(1-(3-vinylimidazolium) bromide) benzene monomer and bipyridine | Free radical copolymerization, post-synthetic metalation with Zn2+ | 94.50% | Epoxide | 1 atm 120 °C | [88] |
| vinylimidazolium salt-based PIL | Coated on hydroxyl functionalized CNTs | 94.50% | Epoxide | 0.2 MPa 100 °C | [89] |
| Poly [VEI][Br] and atomically dispersed Al-O-C | In situ polymerization of [VEI][Br] with 1,2-divinylbenzene and 2,2′-azobis-isobutyronitrile | 91% | Epichlorohydrin | 1 bar 80 °C | [98] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kharade, J.; Lozano, K. Ionic Liquids and Poly (Ionic Liquids) for CO2 Capture: A Comprehensive Review. Energies 2025, 18, 4257. https://doi.org/10.3390/en18164257
Kharade J, Lozano K. Ionic Liquids and Poly (Ionic Liquids) for CO2 Capture: A Comprehensive Review. Energies. 2025; 18(16):4257. https://doi.org/10.3390/en18164257
Chicago/Turabian StyleKharade, Jui, and Karen Lozano. 2025. "Ionic Liquids and Poly (Ionic Liquids) for CO2 Capture: A Comprehensive Review" Energies 18, no. 16: 4257. https://doi.org/10.3390/en18164257
APA StyleKharade, J., & Lozano, K. (2025). Ionic Liquids and Poly (Ionic Liquids) for CO2 Capture: A Comprehensive Review. Energies, 18(16), 4257. https://doi.org/10.3390/en18164257






